Abstract
AIM
To investigate the neoadjuvant chemotherapy (NAC) effect on the survival of patients with proper stomach cancer submitted to D2 gastrectomy.
METHODS
We proceeded to a review of the literature with PubMed, Embase, ASCO and ESMO meeting abstracts as well as computerized use of the Cochrane Library for randomized controlled trials (RCTs) comparing NAC followed by surgery (NAC + S) with surgery alone (SA) for gastric cancer (GC). The primary outcome was the overall survival rate. Secondary outcomes were the site of the primary tumor, extension of node dissection according to Japanese Gastric Cancer Association (JGCA) performed in both arms, disease-specific (DSS) and disease-free survival (DFS) rates, clinical and pathological response rates and resectability rates after perioperative treatment.
RESULTS
We identified a total of 16 randomized controlled trials comparing NAC + S (n = 1089) with SA (n = 973) published in the period from January 1993 - March 2017. Only 6 of these studies were well-designed, structured trials in which the type of lymph node (LN) dissection performed or at least suggested in the trial protocol was reported. Two out of three of the RCTs with D2 lymphadenectomy performed in almost all cases failed to show survival benefit in the NAC arm. In the third RCT, the survival rate was not even reported, and the primary end points were the clinical outcomes of surgery with and without NAC. In the remaining three RCTs, D2 lymph node dissection was performed in less than 50% of cases or only recommended in the “Study Treatment” protocol without any description in the results of the procedure really perfomed. In one of the two studies, the benefit of NAC was evident only for esophagogastric junction (EGJ) cancers. In the second study, there was no overall survival benefit of NAC. In the last trial, which documented a survival benefit for the NAC arm, the chemotherapy effect was mostly evident for EGJ cancer, and more than one-fourth of patients did not have a proper stomach cancer. Additionally, several patients did not receive resectional surgery. Furthermore, the survival rates of international reference centers that provide adequate surgery for homogeneous stomach cancer patients’ populations are even higher than the survival rates reported after NAC followed by incomplete surgery.
CONCLUSION
NAC for GC has been rapidly introduced in international western guidelines without an evidence-based medicine-related demonstration of its efficacy for a homogeneous population of patients with only stomach tumors submitted to adequate surgery following JGCA guidelines with extended (D2) LN dissection. Additional larger sample-size multicentre RCTs comparing the newer NAC regimens including molecular therapies followed by adequate extended surgery with surgery alone are needed.
Keywords: Gastric cancer, Neoadjuvant chemotherapy, Perioperative chemotherapy, D2 lymphadenectomy, Randomized control trial
Core tip: Neoadjuvant chemotherapy (NAC) for resectable locally advanced gastric cancer has been rapidly introduced in international western guidelines without an evidence-based medicine-related demonstration of its efficacy for a homogeneous population of patients with stomach tumors who received adequate surgery following Japanese Gastric Cancer Association guidelines with an extended (D2) lymph nodes dissection. Additional randomized controlled trials with a larger sample size comparing the newer NAC regimens, including molecular therapies followed by adequate extended surgery with surgery alone are necessary.
INTRODUCTION
Gastric cancer (GC) is the third most common cancer-related cause of death[1]. Worldwide, approximately 1 million new cases of gastric cancer are diagnosed every year[2]. In 2016, nearly 13000 new cases are expected to be diagnosed in Italy with 7400 (56.9%) cases in males and 5300 (40.7%) cases in females[3].
The main prognostic factors for gastric cancer patients who receive radical surgery are the number of metastatic lymph nodes (LN) and the LN-ratio (the ratio between metastatic LN and LN removed).
The 5-year overall survival for resectable GC is approximately 20% to 30% worldwide, but, surprisingly, it is 70% in Japan and in other eastern countries, where the high incidence of the disease is managed with screening programs to find tumors at an earlier stage, and patients receive adequate surgery, including extended LN dissection (D2 gastrectomy)[4].
The therapeutic role of LN dissection has been a matter of discussion for a long period. In eastern countries, D2 lymphadenectomy has been considered the standard procedure for many decades[5-7]. In western countries, a more limited LN dissection has been performed until the last decade due to the low incidence of this tumor and the resulting limited experience with this complex and challenging procedure that has substantial learning curves[8-10].
Nevertheless, at the end of the 90s, the Italian Gastric Cancer Study Group (IGCSG) randomized controlled trial (RCT) showed that when a D2 lymphadenectomy with a pancreas-preserving technique is performed in references centers, it is a reproducible and safe procedure for the radical treatment of GC in western countries as well[11].
Later, Dutch and IGCSG trials also documented a survival benefit of the extended procedure in patients with advanced disease[12,13]. Consequently, the survival rates for western reference centers adopting D2 gastrectomy as the standard procedure for gastric cancer were comparable to the rates reported in Japan and Korea. Therefore, in international western guidelines for the diagnosis and treatment of gastric cancer, D2 gastrectomy is currently the recommended surgical choice for cases of resectable advanced disease.
Although surgery is the treatment of choice for GC, in western countries, prognosis remains poor outside reference centers even after curative resection, especially for the high rate of recurrence and the poor efficacy of adjuvant therapy[14]. Many trials have investigated the impact of adjuvant treatment by comparing surgery alone with surgery followed by adjuvant chemotherapy, but there is no definitive evidence of any survival advantage[15].
Moreover, in the western world, one-third of patients diagnosed with GC have unresectable diseases[16].
Therefore, in the last decade, a multimodal approach of GC has been suggested with the adoption of neoadjuvant (preoperative or perioperative) treatment (NAC). Based on the reason that, at least theoretically, this treatment may reduce tumor volume, improve the R0 resection rate, and kill micro metastases, NAC has been recently introduced in the national guidelines of many countries, especially after publication of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) and the Federation Nationale des Centres de Lutte contre le Cancer (FNCLCC) -Federation Francophone de Cancerologie Digestive (FFCD) RCTs, without a clear and evidence-based medicine (EBM)-related demonstration of a survival benefit compared to controlled surgery with proper enlarged LN dissection in patients with only stomach tumors[15,17,18].
Effectively the MAGIC and FNCLCC-FFCD RCTs have shown a survival advantage of NAC + surgery vs surgery alone, but surgery performed in these patients did not include a proper extended lymphadenectomy in the majority of cases, and many patients had lower esophagus (LE) or esophagogastric junction (EGJ) cancer instead of only GC. Therefore, it is possible that a proper radical surgery (gastrectomy with D2 LN dissection) may nullify the survival benefit of NAC described after incomplete surgery and also that the location of the tumor outside the stomach (EGJ and LE), may amplify the response to preoperative treatment.
To document a possible benefit of NAC after adequate D2 gastrectomy for only stomach cancer, we proceeded to review the literature on neoadjuvant treatment for resectable gastric cancer by mainly investigating these variables.
MATERIALS AND METHODS
Literature search
We proceeded to conduct a complete review of the literature with the support of the Federate Library of Medicine of Turin. The body of evidence for this review was primarily comprised of mature RCT data and meta-analyses of RCTs.
We searched PubMed, Embase, American Society of Clinical Oncology (ASCO) and European society of Medical Oncology (ESMO) meeting abstracts as well as the Cochrane Library by computer for non-randomized and randomized works published from 1993 to March 2017 (Figure 1).
Figure 1.
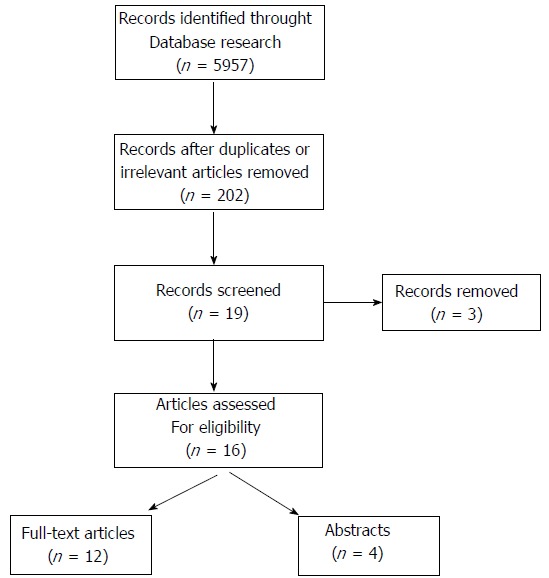
Literature selection flowchart.
Keywords were matched and included “NAC or neoadjuvant chemotherapy or neo-adjuvant chemotherapy or neo-adjuvant chemo-therapy or neoadjuvant chemo-therapy or preoperative chemotherapy or pre-operative chemotherapy or preoperative chemo-therapy or pre-operative chemo-therapy or perioperative chemotherapy or peri-operative chemotherapy or peri-operative chemo-therapy or perioperative chemo-therapy ”, “GC or gastric cancer or stomach cancer or stomach neoplasm or gastric neoplasm or gastric adenocarcinoma or stomach adenocarcinoma”, and “RCT or randomized controlled trial or randomised controlled trial”.
All published trials comparing NAC-containing procedures vs no treatment before surgery in patients with gastric cancer (or gastric cancer + cardia cancer + lower-esophagus cancer) were included earlier in our analysis. Blinding the trial conditions was not necessary. There was no language restriction.
Inclusion criteria
We screened titles and abstracts of all identified papers (5957) and included only trials that satisfied the following criteria: patient populations with gastric cancer (diagnosed and classified according to 6th or 7th TNM and/or Japanese Gastric Cancer Association) without age, gender and racial limitations[19-21]; intervention and a comparative intervention that was clearly documented (NAC + surgery -NAC+S versus surgery alone -SA) for GC regardless of the detailed NAC regimens that were administered, surgical techniques performed (type of gastrectomy and LN dissection), pathological classification, location and stage of the disease. All patients needed to have a history of potentially curative surgery.
NAC could be performed through oral or intravenous (IV), intraperitoneal (IP) and intra-arterial (IA) infusion.
Esophagogastric junction and lower esophagus cancer patients were considered only if they were enrolled together with proper gastric cancer patients in the same study[15,17,18,22]. In these cases, the results of tests for heterogeneity effects according to the site of the primary tumor were documented when reported by the authors.
When results were reported or updated in more than one publication, only the most recent paper was used.
At the end of the process, only RCTs were included in this review (Figure 1).
Exclusion criteria
We included only GC patients according to the theory of site-dependent differences in tumor genomics and biology[23]. Therefore, studies recruiting lower-esophagus and cardia cancers without gastric cancer were excluded. Studies including GC and diseases other than GC (lower esophageal cancer, esophagogastric junction tumor) were also excluded unless analyses were conducted separately.
Studies of preoperative radiotherapy or immunotherapy were excluded if they were not associated with chemotherapy.
We excluded patients with history of prior treatment before entering the trial as well.
Types of interventions
Any chemotherapy regimen performed before resective surgery with or without postoperative adjuvant treatment was included and located in the NAC-arm.
For the surgery alone-arm (SA-arm), we enrolled all types of gastrectomy and lymphadenectomy that were performed (D0, D1, D1plus, D2, D3).
Studies in which GC was associated with another synchronous malignancy or studies containing multivisceral resections were excluded.
Outcomes of interest and definitions
The primary outcomes were the overall survival rates (time from random assignment to the last follow-up or death) and/or disease-free survival rates (DFS) (time from random assignment to tumor recurrence or death) and/or disease-specific survival rates (DSS) (time from random assignment to death from disease). Secondary outcomes included the perioperative response rates, the R0 (margin negative) resection rate after perioperative treatment, and OS or DFS or DSS according to the type of lymph node dissection performed (D1, D2, others) following Japanese Gastric Cancer Treatments Guidelines[21]. Preoperative tumor stages were all recorded following TNM classification of malignant tumors (mostly from the 6th edition[19]).
The objective response to NAC was evaluated as either complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) according to the indications of the Japanese Gastric Cancer Association[21].
Some trials assessed the down-staging effect of NAC by separating out patients with negative nodes from patients with positive nodes on pathological examinations after surgery.
Quality assessment of RCTs
The quality of the included RCTs was assessed using modified Jadad’s scoring system[24] and Cochrane reviewers’ handbook 5.0.1 RCT criteria[25]. The assessment was based on the randomization methods, the report of dropout rates, allocation concealment, the use of intention-to-treat (ITT) analysis, and losses to follow-up, the extent to which valid results were depicted. Based on these criteria, the studies were divided into high quality group (score ≥ 4) and low quality group (score < 4) (Table 1).
Table 1.
Quality assessment of all the 16 randomized controlled trials found in literature and included in this study
| Ref. | Randomization | Allocation concealment | Blind | Withdrawal and dropout | Jadad score | ITT |
| Yonemura et al[29], 1993 | Adequate | Inadequate | Adequate | Well reported | 5 | NR |
| Shchepotin et al[30], 1995 | Unclear | Unclear | Inadequate | NR | 1 | NR |
| Kang et al[31], 1996 | Unclear | Unclear | Inadequate | Well reported | 3 | NR |
| Lygidakis et al[32], 1999 | Inadequate | Unclear | Inadequate | Well reported | 2 | NR |
| Takiguchi et al[33], 2000 | Unclear | Inadequate | inadequate | Well reported | 2 | NR |
| Wang et al[22], 2000 | Unclear | Unclear | Inadequate | Well reported | 3 | NR |
| Kobayashi et al[35], 2000 | Adequate | Adequate | Inadequate | Well reported | 5 | NR |
| Hartgrink et al[4], 2004 | Adequate | Adequate | Inadequate | Well reported | 5 | NR |
| Nio et al[36], 2004 | Inadequate | Inadequate | Inadequate | Well reported | 1 | NR |
| Zhao et al[37], 2006 | unclear | Unclear | Inadequate | Well reported | 3 | NR |
| Cunningham et al[17], 2006 | Adequate | Adequate | Adequate | Well reported | 7 | YES |
| Schuhmacher et al[15], 2010 | Unclear | Unclear | Unclear | Well reported | 4 | YES |
| Imano et al[38], 2010 | unclear | unclear | Unclear | Well reported | 4 | NR |
| Biffi et al[39], 2010 | Unclear | Unclear | Unclear | Well reported | 4 | YES |
| Qu et al[40], 2010 | Adequate | Unclear | Unclear | NR | 4 | NR |
| Ychou et al[18], 2011 | Adequate | Well reported | Unclear | Well reported | 6 | YES |
NR: Not reported; ITT: Intention-to-treat analysis.
RESULTS
Study selection and population
Figure 1 shows the literature trial selection flowchart. In brief, 5957 studies were identified from the literature search. Out of all these studies, a total of 202 relevant papers documenting the results of any comparison of NAC + S vs SA for gastric cancer were selected. Then, we proceeded to a further selection of 19 papers reporting the results of controlled trials that were described as randomized in the title, (6 abstracts and 13 full-text papers). Nevertheless, a more detailed examination showed that in 3 of these studies, patient allocation had in fact not been conducted randomly[26-28]. Therefore, only 16 papers were proper randomized controlled trials that fulfilled our research criteria and were included in this review.
These RCTs were published between 1993 and 2011 with a 24 to 83 mo follow-up period. A total of 2062 patients was included in the analysis with 1089 receiving NAC (52.81%) and 973 (47.18%) undergoing SA.
Characteristics of included studies
In Yonemura’s trial[29] (1993), fifty-five patients with far advanced gastric cancer (TNM stage IV) were enrolled with 29 who received neoadjuvant treatment and 26 who underwent surgery alone (Table 2). NAC patients were treated with a PMUE regimen, a combination of cisplatin (CDDP) 75 mg/m2, mitomycin C (MMC) 10 mg/body, etoposide 150 mg/body, and 400 mg/d UFT. The author reported response rates, resectability rates with curative intent and a survival rate advantage in the NAC group, but, overall, this was not statistically significant. Furthermore, the type of lymph node dissection performed in both arms was not reported.
Table 2.
Positive human epidermal growth factor receptor 2 status by immunohistochemistry and/or fluorescence in situ hybridization for the patients enrolled in the ToGA trial[10,11,27]
| Author Countries | Year, Type of publication | N° patients randomization | Site Stage | Regimen | Response rate | Resectability rate | Survival rate (HR; p) | Node dissection (D1,D2, others) |
| Yonemura et al[29] Japan | 1993 Abstract | 55 Tot 29 NAC + S 26 S | GC IV | PMUE | Adv NAC + S | Adv NAC + S | Adv NAC+S Rates NR | NR |
| Shchepotin et al[30] Ukraine | 1995 Abstract | 146 Tot 50 S 49 IVCH + S 47 IACH + S | GC NR | NR | Adv IACH + S | Adv IACH + S | Adv IACH + S P < 0.001 | NR |
| Kang et al[31] South Korea | 1996 Abstract | 107 Tot 54 S 53 NAC + S | GC III/IV | PEF | NR | Adv NAC + S | No difference P = 0.114 | NR |
| Lygidakis et al[32] Greece | 1999 Paper | 59 Tot 19 S 20 NAC + S + IVCH 20 NAC + S | GC All stages | Mitomycin-C + 5-FU + FA + Farmorubicin | NR | NR | Adv NAC + S + IVCH | NR |
| Takiguchi et al[33] Japan | 2000 Abstract | 262 Tot 139 S 123 NAC + S | GC III/IV | 5FU + CDDP | Adv NAC + S | Adv NAC+S | Adv NAC + S P = 0.0996 | NR |
| Wang et al[22] China | 2000 Paper | 60 Tot 30 S 30 NAC + S | EGJ NR | 5FU | Adv NAC + S | NR | Adv NAC + S P = 0.17 | NR |
| Kobayashi et al[35] Japan | 2000 Paper | 171 Tot 80 S 91 NAC + S | AGC | FUDR | NR | NR | Adv S P = 0.010 | NR |
| Hartgrink et al[4] The Netherlands | 2004 Paper | 59 Tot 30 S 29 NAC + S | Proper AGC (not EGC) | FAMTX | 32% CR or PR | EQUAL | Adv S 34% S vs 21% NAC + S P = 0.017 | At least 15 nodes |
| Nio et al[36] Japan | 2004 Paper | 295 Tot 193 S 102 NAC + S | GC All stages > 50% stage I | UFT | NR | NR | Overall No Adv. NAC + S P = 0.6878 stage II/III -pN + Adv. NAC + S P = 0.0486 | D2 48% S 56% NAC + S |
| Zhao et al[37] China | 2006 Paper | 60 Tot 20 5’-DFUR 20 5FU + CF 20 S | GC All stages | 5’-DFUR Or 5FU+CF | 5’-DFUR and 5FU + CF increase AI and reduce PI | NR | NR | NR |
| Cunningham et al[17] United Kingdom | 2006 Paper | 503 Tot 250 S 253 NAC + S | GC, EGJ, LE All stages | Epirubicin Cisplatinum 5-FU | Diameter 5 cm vs 3 cm P < 0.001 T1 + T2 stages > NAC + S P = 0.002 | NR | Adv NAC + S OS/DFS 23% S 36.3%/NAC+S HR 0.75/0.66 P = 0.009/0.001 more evident for EGJ | D2 40% S 42% NAC + S |
| Schumacher et al[15] Germany | 2010 Paper | 282/144 Tot 72 S 72 NAC + S | GC, EGJ (Siewert II, III) stages III, IV | Cisplatinum + FF | Adv in S Tumor length, thickness and width and P Stages more | R0 67% S 81.9% NAC + S P = 0.036 LN + 76.5% S 61.4% NAC + S P = 0.018 | No Adv NAC + S 52 ms S 64 ms NAC + S HR = 0.84 P = 0.46 | D2 94% 92.6% S 95.7% NAC + S |
| Imano et al[38] Japan | 2010 Paper | 63 Tot 16 S 15 CDDP 16 5-FU 16 5-FU + CDDP | GC NR | 5FU or CDDP or 5F +CDDP | 5-FU + CDDP Increases AI Reduces PI | NR | No differences in 4 arms | D2 in all arms |
| Biffi et al[39] Italy | 2010 Paper | 240/70 Tot 35 S 34 NAC + S | EGJ (Siewert II, III), AG | TCF | 65% CR + PR | Adv NAC + S (P value NR) | not evaluated premature interruption for low accrual | D2 in almost all cases |
| Qu et al[40] China | 2010 Paper | 78 Tot 39 S 39 NAC + S | AGC | Paclitaxel and FOLFOX4 | Adv NAC + S P = 0.001 | Adv NAC + S P = 0.025 | Adv NAC+S P = 0.006 at 2 yr | NR |
| Ychou et al[18] France | 2011 Paper | 224 Tot 111 S 113 NAC + S | LE,EGJ,GC All stages | CDDP + 5FU | Adv NAC + S P = 0.054 | Adv NAC + S P = 0.04 | OS (NAC + S/S) = 38/24 HR = 0.69 P = 0.02 DFS (NAC + S/S) = 34/19 HR= 0.65 P = 0.003 more evident for EGJ | D2 recommended No data on the effect of D2 vs other LN dissection |
GC: Gastric cancer; NAC: Neoadjuvant chemotherapy; S: Surgery; EGC: Early gastric cancer; AGC: Advanced gastric cancer; EGJ: Esophagogastric junction; LE: Lower esophagus; OS: Overall survival; DFS: Disease free survival; LN: Lymph node; D2: D2 lymphadenectomy; FA: Folinic acid; CR: Complete response; PR: Partial response; 5-FU: 5-fluorouracil; PMUE: Cisplatin + mitomycin C + etoposide +1-(2-tetrahydrofuryl)- + uracil; IVCH: Intravenous (systemic) chemotherapy; IACH: Super selective intra-arterial chemotherapy; PEF: Low dose cisplatinum + epirubicin + 5-fluorouracil; CDDP: Low dose cisplatinum; 5'-DFUR: 5'-deoxy-5-fluorouridine; FAMTX: 5-fluorouracil + doxorubicin + methotrexate; UFT: Tegafur + 5-fluorouracil; FF: d-L-folinic acid+fluouracile; TCF: Docetaxel + cisplatin + fluorouracil; FOLFOX4: 5-fluorouracil + leucovorin + oxaliplatin; Adv: Advantage; NR: Not reported; Tot: Total; AI: Apoptosis index; PI: Cell proliferation index; HR: Hazard ratio.
In 1995, Shchepotin et al[30] included 146 GC patients in a RCT in which the tumor stage was not specified (Table 2). Fifty patients were treated with surgery alone, and 96 patients were submitted to NAC. Out of these patients, 49 received intravenous (systemic) chemotherapy (IVCH) and 47 received super selective intra-arterial chemotherapy (IACH). The chemotherapy regimen was not mentioned. Only IACH + S showed a response rate and a survival rate advantage over surgery alone, and this survival benefit was statistically significant (P < 0.01). Patient selection and the type of lymph node dissection performed were not reported.
The Kang et al[31] trial enrolled 107 patients with operable gastric cancer at TNM stages III and IV. Fifty-four patients received surgery alone and 53 received NAC with a PEF regimen followed by surgery. The response rate was not specified. The curative resection rate was higher in the NAC + S group than in the SA group. However, there was no significant difference in overall survival between the two arms (P = 0.114). The type of lymph node dissection that was performed was not reported (Table 2).
Lygidakis et al[32] recruited 58 patients with resectable gastric cancer at all stages. Patients were randomly assigned to 3 groups. Group A (No. 20) patients received preoperative hypoxic upper abdominal chemotherapy using mitomycin-C, 5-fluorouracil, leucovorin, and farmorubicin combined with adjuvant systemic chemotherapy; Group B (No 19) patients received preoperative hypoxic upper abdominal chemotherapy without systemic chemotherapy; and Group C (No 19) patients received surgery alone. Improved survival was reported by combining hypoxic upper abdominal perfusion NAC with surgery compared to surgery alone. Response rate, resectability rate and type of LN dissection were not documented (Table 2).
Two-hundred-sixty-two patients with resectable GC mostly with serosal invasion were treated by Takiguchi et al[33] with either SA (239 patients) or NAC (123) between May 1993 and May 1998. NAC patients received 5-FU (F group, n = 39) until the day before surgery or 5-FU combined with CDDP (FP Group, n = 84). NAC with 5-FU combined with low-dose CDDP was reported to reduce the rate of intraperitoneal positive cytology, increase the rate of successful resections and improve OS without statistical significance. The extension of the lymph node dissection was not described (Table 2).
In 2000, Wang et al[22] published the results of an RCT that included sixty patients with gastric cardia cancer that were randomly assigned to surgery alone (No. 30) or to NAC with 5-FU (No. 30). As Siewert III cancers are nowdays considered as proper gastric cancers, this study was included in the study despite our inclusion criteria[34]. The 5-year OS rate was improved in the NAC group but this survival benefit was not statistically significant. The resectability rate and type of node dissection performed in both groups were not reported (Table 2).
Kobayashi et al[35] performed a multicenter randomized clinical trial by recruiting 171 patients with advanced gastric cancer (a more detailed TNM tumor stage was not specified). These patients were randomized either to NAC with oral 5’-DFUR (No 91) followed by surgery and adjuvant therapy with iv MMC and oral 5’-FUDR or for surgery alone (No. 80) followed by the same adjuvant therapy. This clinical trial failed to demonstrate any survival benefit of preoperative chemotherapy over surgery alone. Response rate, resectability rate and the type of node dissection performed in both arms were not documented (Table 2).
In 2004, Hartgrink et al[4] reported the long-term results of the Dutch randomized FAMTX trial. In this trial, 59 AGC patients were recruited with no further details about the stage of the disease. Of these patients, 29 were allocated to the FAMTX regimen prior to surgery, while 30 patients received surgery alone. The resectability rates were equal for both groups. A complete or partial response was registered in 32% of the FAMTX group. The median survival since randomization was 18 mo in the FAMTX group vs 30 mo in the SA group (P = 0.17). Therefore, the trial could not show a beneficial effect of preoperative FAMTX. The standard surgical procedure included a limited (D1) lymphadenectomy. The authors concluded that surgery alone is the best treatment for operable gastric cancer (Table 2).
In 2004, Nio et al[36] published the results of a RCT in which 295 patients with GC of all stages were included (more than 50% were stage I). Overall, 193 patients were randomly allocated to the SA-arm and 102 were allocated to the NAC-arm with the UFT regimen (Tegafur + 5-FU). The response rate to NAC was 33.3%. The resectability rate was not reported. There were no significant differences in the OS rates between the NAC and SA groups. A survival benefit of NAC over SA was documented only in stages 2 and 3 and was higher in stage 2 and 3 patients with a complete or partial response to NAC compared to non-responders. The type of LN dissection performed was D2 only in 48% in the SA arm and 56% in the NAC-arm.
Zhao et al[37] studied the apoptosis induced by preoperative oral 5’-FUDR administration in GC and its mechanism of action. Sixty patients with gastric cancer of all TNM stages were randomly assigned to 3 groups (20 each group) before surgery. Group one patients received a NAC treatment with 5’-DFUR oral administration; group two patients received 5-FU + CF by venous drip for 3-5 d; and group three patients received surgery alone. The authors documented a significant increase in the apoptosis of gastric carcinoma cells and a decrease in the tumor cell proliferation index in group one patients after oral 5’-FUDR administration compared to groups 2 and 3. Nevertheless, preoperative NAC did not improve patient prognosis. The resectability rate and type of lymph node dissection were not reported.
In the most known RCT on NAC (MAGIC Trial) published in 2006 in the New England Journal of Medicine (NEJM), Cunningham et al[17] studied the effects of preoperative treatment on 503 recruited patients with all stages of resectable adenocarcinoma of the stomach, esophagogastric junction, or the lower esophagus (Table 2). Patients were randomly assigned to either a perioperative treatment (250 patients) followed by surgery or surgery alone (253 patients). Perioperative chemotherapy consisted of three preoperative and three postoperative cycles of i.v. epirubicin (50 mg/m2) and cisplatin (60 mg/m2) on day 1 as well as a continuous i.v. infusion of fluorouracil (200 mg/m2 per day) for 21 d. The resected tumors were significantly smaller and less advanced in the perioperative-chemotherapy group (P < 0.001), and among patients undergoing resection, there was a greater proportion of stage T1 and T2 tumors in the perioperative-chemotherapy arm compared to the surgery alone arm (P = 0.002). Compared with the surgery alone arm, the perioperative chemotherapy arm had a higher likelihood of overall survival (hazard ratio for death, 0.75; 95 percent confidence interval, 0.60 to 0.93; P = 0.009; five-year survival rate, 36% vs 23%) and progression-free survival (hazard ratio for progression, 0.66; 95 percent confidence interval, 0.53 to 0.81; P < 0.001). Nevertheless, the tumor was located in the stomach in 74% of cases in the NAC- arm and 73.9% in the surgery alone-arm; the tumor originated from the lower esophagus or from the esophago-gastric junction in the ramaining cases. D2 lymphadenectomy was performed only in 40% of patients in the SA arm and in 42% of patients in the NAC arm. Furthermore, according to tests for heterogeneity of the treatment effects related to the baseline characteristics of patients, the survival benefit of perioperative chemotherapy was stronger for the esophagogastric junction and the lower esophagus site of the primary tumor compared to the proper stomach site. Resectability rates were not significantly different among the two groups.
Schuhmacher et al[15] in 2010 reported the preliminary results of the European Organisation for Research and Treatment of Cancer ( EORTC) randomized trial Nr. 40954 that compared NAC to SA for locally advanced cancer of the GC and EGJ (Table 2). The original trial design required that 360 patients with locally advanced (UICC stages III and IV cM0) adenocarcinoma of the stomach or esophagogastric junction (Sievert II and III) were randomly assigned to preoperative chemotherapy with cisplatin, d-L-folinic acid and fluorouracil after surgery or surgery alone. This trial was stopped for poor accrual after 144 patients were enrolled, including 72 patients in the surgery alone arm and 72 in the perioperative-chemotherapy treatment arm. More than half of patients had tumors located in the proximal third of the stomach, including EGJ Siewert type II and III. The trial showed a significantly increased R0 resection and pN0 rates in the NAC arm (P = 0.036) compared to the SA arm, but failed to demonstrate a survival benefit of perioperative treatment. In this trial, adequate surgery with D2 lymph node dissection was performed in 92.6% of SA arm patients and in 95.7% of NAC + S arm patients. The survival rates at 2 years were 72.7% (95%CI: 60.7%-81.7%) and 69.9% (95%CI: 57.7%-79.2%) in the neoadjuvant and surgery-only arms, respectively. The better outcome than expected after surgery alone was ascribed to the high quality of surgery and nodal dissection that was performed.
Imano et al[38] in 2010 reported the results of a four-arm randomized trial designed to evaluate the effects of short-term NAC (chemotherapy performed for 72 h before surgery) on the proliferative ability of cancer cells in gastric cancer. Sixty-three patients with gastric cancer were randomly assigned to 4 groups as follows: Group F, which had a single administration of 5-FU (16 patients); Group C, which had a single administration of CDDP (15 patients); Group FC, which had administration of both 5-FU and CDDP; and the Control Group, which had surgery alone (16 patients). Gastric cancer TNM or UICC stages were not reported. The D2 lymph node dissection was performed in all patients.The authors found that the combination of CDDP and 5-FU reduced proliferative ability and increased cellular apoptosis in gastric cancer cells. Nevertheless, there were no differences in overall survival rates between each group. Resectability and R0 resection rates were not reported (Table 2).
A group from the European Institute of Oncology in Milan[39] published the data of a multicenter RCT in which the objective was the non-inferiority of preoperative NAC with TCF regimen followed by surgery (Arm A) compared to surgery followed by the same chemotherapy regimen (Arm B) on clinical outcomes (morbidity and mortality of surgery) in GC patients (Table 2). Based on the initial design, a target sample size of 240 patients with locally advanced GC was required. Nevertheless, this trial was prematurely stopped at 69 randomized patients due to insufficient accrual with 34 in Arm A and 35 in Arm B, D2 lymphadenectomy was performed in almost all cases in both arms. The resectability rate was greater in the Arm A compared to the Arm B. In conclusion, preoperative TCF was safe and had similar morbidity compared to surgery followed by a TCF regimen. Furthermore, due to its early discontinuation for slow accrual, this study did not provide information about the efficacy of preoperative TCF, and no survival results were reported.
Qu et al[40] enrolled 78 patients with cTNM stage III or IV (M0) gastric cancer (Table 2). Thirty-nine of these patients were randomized to the NAC arm (paclitaxel combined with FOLFOX4 regimen) and 39 to the surgical arm. The clinical response rate was 66% in the NAC arm. The R0 resection rate was significantly higher, and the number of lymph node metastases was significantly lower in the NAC arm compared to the SA arm. In addition, the 2-year survival rate was higher in NAC- than in the surgery alone-arm. The location of the disease and type of lymph node dissection performed were not described. The sample size was very small.
The results of the FNCLCC and FFCD multicenter Phase III trial[18] were published in the Journal of Clinical Oncology in 2011 (Table 2). In this trial, 224 patients with resectable adenocarcinoma of the lower esophagus, gastroesophageal junction (EGJ), or stomach were randomly assigned to either perioperative chemotherapy with cisplatin and a fluorouracil regimen (NAC arm, n = 113) followed by surgery or surgery alone (SA arm, n =111). The curative resection rate was significantly higher in the NAC arm compared to that in the SA arm (84% vs 73%, P = 0.04). A non-significant decrease of lymph node metastases in the NAC arm compared to that of the SA arm was reported (67% vs 80%; P = 0.54). Compared to the SA group, the NAC group showed a better OS (5-year rate 38% vs 24%; HR for death 0.69; P = 0.02) and a better disease-free survival (5-year rate: 34% vs 19%; HR for death 0.65; P = 0.003). Nevertheless, in this trial, only 25% of patients had a tumor located in the stomach, while in 64% of cases, the tumor site was the esophagogastric junction, and another 11% of patients had cancer of the lower esophagus. While an extended lymphadenectomy (D2) was recommended in the “Study Treatment” protocol, the lymph node dissections performed in both arms were not described in the results. The authors concluded that compared to surgery alone, perioperative chemotherapy using a combination of cisplatin and fluorouracil significantly improved the curative resection rate, OS and DFS of patients with adenocarcinoma of the lower esophagus, EGJ and stomach. Nevertheless, this strong conclusion is an evident contrast to their previous comment reported in the “RESULTS” section of this paper where they stated that although the multivariable analysis showed the two significant prognostic factors for OS were the administration of a preoperative chemotherapy (P = 0.01) and tumor site (P < 0.01), the chemotherapy effect was only significant in the esophagogastric junction subgroup, which included around two-thirds of the patients. The two other subgroups (lower esophagus and stomach) were too small to distinguish between no effect and a small effect. Should these comments have been emphasized in the conclusion of the paper as well?
DISCUSSION
Actually, curative resection (R0) with D2 lymphadenectomy is the recommended standard procedure for advanced gastric cancer according to the Japanese[21], Korean[41], British[42], German[43], and Italian[44] guidelines, as well as the guidelines of the European Society for Medical Oncology (ESMO)[45] and the joint ESMO- ESSO (European Society of Surgical Oncology) ESTRO (European Society of Radiotherapy and Oncology)[46]. Recently, the National Comprehensive Cancer Network (NCCN) recommended D2 gastrectomy in the United States as well[47].
Following the results of the MAGIC[17] and FNCLCC-FFCD[18] trials, which documented an overall survival and a disease free survival benefit after NAC compared to surgery alone (from 23% to 36%, P = 0.009 and from 24% to 38%, P = 0.02, respectively), the same reported national and international guidelines suggested the adoption of preoperative or perioperative chemotherapy, mostly in cases in which lymph node metastasis is clinically suspected (cN+) or there is a clinical TNM stage 3 or higher ( cT3+). In some experiences NAC has been employed for earlier T stage as well (cT2+)[47].
We deeply believe that the conclusions drawn after the publication and circulation of those two trials have been too strong in influencing the decision of several medical-oncology societies to adopt unconditionally preoperative or perioperative chemotherapy regimens for locally advanced resectable gastric cancer. We have tried to demonstrate that the survival benefits reported in MAGIC and FNCLCC-FFCD trials are significant in those series, but the 5-year OS rate of NAC-arms in both trials is even lower than OS rates reported in series from other eastern and western reference centers for gastric cancer treatment after only adequate curative surgery with extended lymph node dissection[48-52]. In fact, these reported benefits come from unbalanced series regarding the extent of nodal dissection. Furthermore, subsite analysis of both study populations clearly shows that chemotherapy effects on patient survival claimed in the published reports are mostly related to the site of tumor. In both trials, the chemotherapy effect is strongly evident in the EGJ subgroup.
Consequently, we did a massive literature search to investigate the eventual demonstration of any survival benefit of preoperative or preoperative chemotherapy in patient populations with tumors properly located only in the stomach and treated with adequate surgery, including complete D2 lymph node dissection as described by JGCA guidelines and recommended by international guidelines.
In this literature search, we found only 16 randomized controlled trials that compared neoadjuvant chemotherapy followed by surgery vs surgery alone in gastric cancer. Ten of these 16 papers were low sample-size single-center trials in which the extension of LN dissection was not described at all (Table 2). When reported, there was very little evidence of the effects of NAC on clinical and pathological responses. Very often, the eventual chemotherapy effect on those responses was not accomplished by any survival advantage, and in cases of survival benefit, this was not significant, or its level of significance was decreased by the low sample size of the study population.
Only 6 articles accurately described the extension of LN dissection performed on the patient population (Table 3).
Table 3.
Characteristics and outcomes of randomized controlled trial describing the type of lymph node dissection and the site of primary tumor in both arms
| Ref. | No. patients randomization | Site of primary tumor | Node dissection (D1, D2, others) | Survival rate (HR, p) | HR for site of primary tumor |
| Nio et al[36], 2004 | 295 Tot 193 S 102 NAC + S | Stomach | D2 in less than 50% overall 48% S 56% NAC + S | Overall No Adv NAC + S P = 0.6878 stage II/III -pN+ Adv NAC + S P = 0.0486 | NR |
| Cunningham et al[17], 2006 | 503 Tot 250 S 253 NAC + S | Stomach 73.9% EGJ 11.5% LE 14.6% | D2 in less than 50% 40% S 42% NAC + S | Adv NAC + S OS/DFS 5 yr-SR 23%/NR S 36.3%/NR NAC + S HR = 0.75/0.66 P = 0.009/0.001 | HR LE 0.7 EGJ: 0.5 Stomach: 0.8 |
| Schumacher et al[15], 2010 | 282/144 Tot 72 S 72 NAC + S | Stomach 47.2% EGJ 52.8% (Siewert II, III) | Proper D2 in 94% overall 92.6% S 95.7% NAC+S | No adv NAC + S 52 ms S 64 ms NAC + S HR = 0.84 P = 0.46 | NR |
| Imano et al[38], 2010 | 63 Tot 16 S 15 CDDP 16 5-FU 16 5-FU + CDDP | Stomach | Proper D2 in all patients of both arms | No differences for all arms | NR |
| Biffi et al[39], 2010 | 240/70 Tot 35 S 34 NAC + S | Stomach 59% EGJ 41% (Siewert II, III) | Proper D2 in almost all cases | not evaluated premature interruption for low accrual | NR |
| Ychou et al[18], 2011 | 224 Tot 111 S 113 NAC + S | LE 11%, EGJ 64%, Stomach 25% | D2 recommended No data on LND performed | OS (NAC + S/S) 38/24 HR = 0.69 P = 0.02 DFS (NAC + S/S) 34/19 HR = 0.65 P = 0.003 | HR LE 1.14 EGJ 0.57 Stomach 0.92 |
S: Surgery; NAC: Neoadjuvant chemotherapy; Adv: Advantage; LND: Lymph node dissection; OS: Overall survival; DFR: Disease free survival; HR: Hazard ratio; LN+: Lymph node positive; D2: D2 lymph node dissection; NR: Not reported; LE: Lower esophagus; EGJ: Esophagogastric junction; pN+: Positive lymph nodes at pathological examinations.
A proper D2 lymph node dissection was documented in only 3 published RCTs[15,38,39]. Two of these trials with complete D2 dissection performed in almost all cases did not show any survival benefit in the NAC arm[15,38]. In the third RCT with adequate surgery and extended lymph node dissection, the primary end points were morbidity and mortality of surgery. Furthermore, due to low accrual with early discontinuation of the trial, no information on the efficacy of preoperative chemotherapy was provided, and no survival results were reported[39].
In the remaining 3 RCTs[17,18,36], a proper D2 nodal dissection was performed in less than 50% of patients or was recommended in the “Study Treatment” protocol but not described in the results.
In one of these trials[18], subsite analysis according to the site of the tumor showed that the favourable chemotherapy effect on OS reported in the conclusion of the trial for all groups of tumor site (lower esophagus, EGJ, stomach) was actually strongly evident only for EGJ cancers, which represented 64% of all cancers of the study population, while the other two subgroups (lower esophagus and stomach, 11% and 25%, respectively) were too small to distinguish between no effect and a small effect. Furthermore, the 5-year OS and DFS rates reported in the trial for the SA arm were 24% and 19%, respectively and were too low compared to rates commonly reported in other series from either eastern or western reference centers for gastric cancer care and research[13,48,50,51,54].
In the randomized trial published by Nio et al[36], there were no significant differences in overall survival rates between the NAC and SA groups in the whole study population. A survival benefit of NAC over surgery alone was documented only in subsite analysis according to the UICC stage of disease. This benefit was reported for UICC stage II and III, and it was higher in stage II and III patients with complete or partial response to NAC compared to non-responders.
Finally, the MAGIC trial[17] also contains several critical points. Despite the reported significant OS benefit for the NAC arm, more than one-fourth of patients had a cancer located outside the stomach (i.e., lower esophagus or esophagogastric junction) and D2 lymphadenectomy was performed in only 40.4% of patients of the SA group and in only 42.5% of patients of the perioperative-chemotherapy group. Furthermore, non-resectional surgery was performed in 16.8 and 13.2% of patients in the SA and NAC groups, respectively. As a result of the inadequacy of surgical treatment and lymph node dissection administered to patients, the rate of overall survival and progression-free survival at 5 years after the operation was extremely low in the SA group (23% and 18%, respectively), and in the NAC group the chemotherapy effect on prognosis produced survival rates that remained lower than those reported in worldwide reference centers after adequate extended surgery without any neoadjuvant treatment. Furthermore, although non statistically significant, tests for heterogeneity effects according to the site of the primary tumor showed that the perioperative chemotherapy effect was much more prevalent for esophagogastric junction cancer (HR for mortality 0.5) and much smaller for stomach cancer (HR for mortality 0.8).
Therefore, did preoperative or perioperative chemotherapy make up for all shortcomings of incomplete surgery that did not respect the adequacy of cure and extension of nodal dissection? Could neoadjuvant treatment be a valid support for inadequate surgery alone in those centers where it is performed without a complete D2 lymph node dissection? Furthermore, could neoadjuvant treatment combined with adequate surgery increase survival rates to the rates found by eastern authors?
The only well-designed clinical trials with controlled D2 surgery performed in almost all cases in both arms were prematurely ended for low accrual[15,39]. One of the studies did not even consider survival as a primary end point, and the objective of the study was the effect of preoperative drugs on clinical outcomes of surgery (morbidity and mortality) and on pathological response[39]. The EORTC trial effectively aimed to detect an improvement in median survival after administration of a neoadjuvant treatment, but when it was stopped for poor accrual, despite a significant increase in curative resection, it failed to demonstrate a survival benefit[15]. The authors themselves concluded that the high quality of surgery performed with extended resection of regional lymph nodes outside the perigastric area was responsible for the better than expected outcome after radical surgery alone. In fact, the survival rates at 2 and 5 years were surprisingly high at approximately 70 and 50%, respectively, without any differences in the two arms of the study.
Survival results after surgery alone in reference centers
As already reported above, several studies from either eastern or western countries have reported survival rates after adequate surgery with extended nodal dissection that were significantly higher than rates reported both in the MAGIC[17] and FNCLCC-FFCD[18] trials after neoadjuvant treatment followed by incomplete surgery. Furthermore, these studies consider only cancer arising from the stomach, while the two RCTs included the lower esophagus and EGJ cancers as well (Table 4).
Table 4.
Survival results after surgery alone in reference centers
| Study, country | Period | Setup | No. of patients | Node dissection | 5-yr OS rates |
| Cunningham et al[17] United Kingdom | 1994-2002 | MC RCT | 503 | D2 in 40% S/42% NAC | NAC + S 36.3% Surgery alone 23% |
| Ychou et al[18] France | 1995-2003 | MC RCT | 224 | No data on type of LND | NAC + S 38% Surgery alone 24% |
| Maruyama et al[48] Japan | 1991-2009 | MC Retr | 11261 | D2 | AJCC Stage II 73.1% AJCC stage III 44.5% |
| Wu et al[53] Taiwan | 1993-1999 | MC RCT | 110/111 | D1 vs D3 | D1 53.6%/ D3 59.5% |
| Kim et al[49] South Korea | 2009-2011 | MC Retr | 1561 | D2 | AJCC Stage II 86.5% AJCC Stage III 63.7% |
| Siewert et al[50] Germany | 1986-1989 | MC Prosp | 1096 | D2 | 46.60% |
| Sue-Ling et al[51] United Kingdom | 1970-1989 | SI Prosp | 207 | D2 | 55% |
| Viste et al[54] Norway | 1980-1990 | SI Retr | 105 | D2 | 47% |
| Robertson et al[55] Hong Kong | 1987-1991 | SI RCT | 25/30 | D1 vs D2 | D1 45%/D2 35% |
| Dent et al[56] South Africa | 1982-1985 | SS RCT | 22/21 | D1 vs D2 | D1 69%/D2 67% |
| Bonenkamp et al[57] The Netherlands | 1989-1993 | MC RCT | 380/331 | D1 vs D2 | D1/D2: 45%/47% D2, pT2: 44% D2, pT3: 22% D2, LN-/LN+: 69%/30% |
| Degiuli et al[13] Italy | 1998-2006 | MC RCT | 133/134 | D1 vs D2 | D1 /D2: 66.5%/64.2% D2 pT2-T4: 59% D2 pT2-pT4 N+: 51% |
MC: Multicenter; RCT: Randomized control trial; Retr: Retrospective study; NAC: Neoadjuvant chemoterapy; S: Surgery; Prosp: Prospective study; SI: Single institution; SS: Single surgeon; LND: Lymph node dissection; OS: Overall survival; AJCC: American Joint Committee on Cancer; LN+: Lymph node positive; LN-: Lymph node negative; D1: D1 lymph node dissection; D2: D2 lymph node dissection; D3: D3 lymph node dissection; NR: Not reported; pT2: Pathological TNM T2 stage; pT3: Pathological TNM T3 stage.
Recently, Maruyama et al[48] reported an overall 5-year survival rate of 70.1% among all UICC stages of the disease (data from Japanese Nationwide Registry of 11.261 gastric patients over the period 1991-2009) after surgery alone with accurate D2 lymph node dissection. Removing data for Stage I patients, which usually do not have indications for preoperative treatment, the rates referring to UICC stage II and III, which are commonly submitted to NAC according to the current guidelines, were 73% and 44.5, respectively and were still significantly higher with respect to figures reported in the neoadjuvant settings discussed above (Table 4).
In a recent RCT from Taiwan, Wu et al[53] observed an overall 5-year survival of 59.5% after extended surgery, and the rate of T1 patients in this study population was 26% (Table 4).
In 2017, Kim et al[49] published survival data as observed in high volume centers in Korea. Overall, the 5-year survival was surprisingly very high at approximately 80% in all patients, but AJCC stage Irepresented 63% of the study population. Anyway, the 5-year survival rates for stage II and III were still 86.5% and 63.7%, respectively (Table 4).
Survival rates after SA higher than those published in MAGIC and FNCLCC-FFCD neoadjuvant settings have been reported also in the western world, both in non-randomized and randomized studies on D2 gastrectomy. In historical non-randomized trials published in the 80s and 90s in Germany[50], England[51] and Norway[54], the 5-year OS rates coming from reference centers after extended procedures were reported as 46.5, 55% and 47%, respectively. In all these study populations, T1 cancers represented less than 10% of all patients (Table 4).
In historical randomized trials from Honk-Kong[55] and South Africa[56], despite evident limitations due to the low accrual, OS after D2 gastrectomy were reported as 35% and 67%, respectively (Table 4).
Additionally, in more recent RCTs coming from Europe, the Dutch[57] and the IGCSG[13] trials, the rates of overall survival observed after proper D2 gastrectomy alone were at least comparable to the results reported by Cunningham and Ychou in the MAGIC and FNCLCC-FFCD RCTs after chemotherapy followed by incomplete surgery. The results of the Dutch trials were first published in the NEJM in 1999. The 5-year OS rate after extended nodal dissection was 47% in all patients. It was 77%, 44% and 22% in T1, T2 and T3 patients, respectively, and 69% and 30% in lymph node-negative or lymph node-positive patients, respectively[57] (Table 4).
The survival results of the IGCSG RCT were published in 2014 in the British Journal of Surgery[13]. The overall 5-year survival and the 5-year disease-specific survival rates were 64.2% and 72.6%, respectively, for the whole cohort of patients submitted to D2 lymphadenectomy. Tumors were stratified by the depth of invasion and by lymph node involvement, and both the OS and DSS were calculated for pT2-pT4 patients and for pT2-pT4 with node-positive patients. In these two subsites of patients (which represent a common indication for neoadjuvant treatments), the 5-year OS and DSS were 59% and 69%, respectively. The observed morbidity and mortality were extremely low (24% and 2.2%, respectively), and therefore, they did not negatively impact the effects of extended surgery on survival, in contrast to what was described previously by Cuschieri et al[58] and Bonenkamp et al[57] in their studies. This trial is a clear demonstration that adequate surgery with proper extended nodal dissection with low complications and mortality can provide impressive survival results that are even significantly higher than those reported in trials adopting neoadjuvant treatments in a similar patient population (Table 4).
On the other hand, due to the high rates of morbidity and mortality, the MAGIC trial designed by Cuschieri to demonstrate the superiority of the extended gastrectomy over the standard D1 procedure in Great Britain failed to document a survival advantage[58,59].
In fact, the OS reported by Cuschieri 5 years after surgery was low with 33% in the D2 arm and 35% in the D1 arm, which was only comparable to the results observed in the MAGIC and FNCLCC-FFCD trials when taking all patients into consideration. It was even lower if we removed AJCC Stage I patients. In fact, in the remaining AJCC stage II and stage IIIpatients, it was 31% and 11%, respectively. These poor survival rates have been related to a combination of the observed severe morbidity and mortality with the inadequacy of the procedures performed, including many protocol violations, especially due to high rates of noncompliance (e.g., non-removal of several Ln stations that were expected to be removed during lymph node dissections). A few years later, the positive results of the MAGIC trial on NAC do seem a direct consequence of this lack of quality and adequacy of surgical treatment provided to gastric cancer patients in many upper GI centers from Great Britain until the revolutionary centralization of treatments adopted since recent years. The chemotherapy effect was more evident when the quality of surgery was lower.
In conclusion, neoadjuvant (preoperative or perioperative) chemotherapy for GC has been rapidly introduced in international western guidelines without evidence-based medicine related demonstrations of its efficacy for proper stomach cancer in patients who receive a complete extended (D2) lymph node dissection. The currently available data support the adoption of NAC procedures in several national guidelines based on low-volume study populations with mixed sites of the primary disease (lower esophagus, esophagogastric junction and stomach), and statistical tests have often shown evidence of the heterogeneity of chemotherapy effects according to the site of the primary tumor. In both MAGIC and FNCLCC-FFCD trials, tests for heterogeneity showed that perioperative chemotherapy effects were much more evident for esophagogastric junction cancer and much less for stomach cancer. Furthermore, in both these RCTs supporting the adoption of NAC, surgery provided to patients was incomplete because extended nodal dissection was performed in less than 50% of patients or not described, and no results on the LN yield were documented to warrant its adequacy. The only well-designed RCT with extended surgery performed in almost all cases was prematurely ended for poor accrual, but at the moment of its closure, it failed to demonstrate a survival benefit for NAC.
Moreover, in national reference centers for gastric cancer care and research, OS and DSS rates of subsites of patients theoretically fit for NAC submitted to surgery alone with complete D2 dissection and performed for cancers located in the stomach only, are even higher than those reported after NAC followed by incomplete surgery provided to a patient population with mixed sites of the primary tumor.
We think additional high volume sample-size multicenter (and eventually multinational) RCTs comparing newer treatment regimens of neoadjuvant settings (capecitabine, oxaliplatin, docetaxel) combined with molecular therapies (epidermal growth factor receptor inhibitors or antiangiogenic agents) followed by controlled D2 surgery with controlled D2 surgery alone are necessary to investigate the survival effects of modern preoperative chemotherapy in patients with only stomach cancer who receive proper extended surgery.
ARTICLE HIGHLIGHTS
Research background
Actually, curative surgery with complete D2 lymph node dissection is the treatment of choice for gastric cancer (GC). In high-volume reference centers surgery alone gives excellent survival rates. Anyway, in western countries, prognosis remains poor outside reference centers even after curative resection, especially for the high rate of recurrence mainly due to incomplete nodal dissection. Many trials have investigated the impact of adjuvant treatment by comparing surgery alone with surgery followed by adjuvant chemotherapy, but there is no definitive evidence of any survival advantage.
Therefore, in the last decade, a multimodal approach of GC has been suggested with the adoption of preoperative or perioperative treatment (NAC). The problem is that NAC has been introduced in the national guidelines of many countries, especially after publication of MRC and French RCTs, without a clear and evidence-based medicine (EBM)-related demonstration of a survival benefit compared to controlled surgery with proper enlarged LN dissection in patients with only stomach tumors.
In fact, MAGIC and FNCLCC-FFCD RCTs have shown a survival advantage of NAC + surgery vs surgery alone, but surgery performed in these patients did not include a proper extended lymphadenectomy in the majority of cases, and many patients had lower esophageal or cardia cancer instead of only GC. Therefore, it is possible that a proper radical surgery (gastrectomy with D2 LN dissection) may nullify the survival benefit of NAC described after incomplete surgery and also that the location of the tumor outside the stomach (cardia and lower esophagus), may amplify the response to preoperative treatment.
To document a possible benefit of NAC after adequate D2 gastrectomy for only stomach cancer, we proceeded to review the literature on neoadjuvant treatment for resectable gastric cancer by mainly investigating these variables.
Research motivation
Neoadjuvant (pre- or peri-operative) chemotherapy has been recently introduced in the national guidelines of many countries mainly after publication of MRC and French RCTs, without a clear and evidence-based medicine (EBM)-related demonstration of a survival benefit compared to controlled surgery with proper enlarged LN dissection in patients with only stomach tumors. The advantage reported after NAC seems mostly related to incomplete nodal dissection performed in both arms of these trials and to heterogeneous recruitment of lower esophagus and esophago-gastric junction cancer together with proper stomach cancer. Are there any trials among RCTs available in Literature investigating the effect of NAC over an homogeneous population of patients with only-stomach cancer treated with complete D2 lymph node dissection in both arms? Otherwise we could not exclude that: 1) a proper radical surgery (gastrectomy with D2 LN dissection) may nullify the survival benefit of NAC described after incomplete surgery and 2) the location of the tumor outside the stomach (esophago-gastric junction and lower esophagus) may amplify the response to preoperative treatment. In other words, NAC could be unuseful in proper gastric cancer submitted to curative complete D2 dissection.
Research objectives
The main objective was to investigate whether a possible survival benefit (OS and/or DSS and/or DFS) of NAC after adequate D2 gastrectomy for only stomach cancer was documented in all RCTS available in Literature till now. Secondary outcomes included the perioperative response rates, the R0 (margin negative) resection rate, and OS or DFS or DSS according to the type of lymph node dissection performed (D1, D2, others) after neoadjuvant treatment. We realized that no RCTs available till now could document a survival benefit of NAC over surgery alone for homogeneous population of patients with tumor of the only stomach treated with complete D2 gastrectomy and that a further large volume randomized trial is needed.
Research methods
We proceeded to conduct a complete review of the literature with the support of the Federate Library of Medicine of Turin. The body of evidence for this review was primarily comprised of mature randomized controlled trial (RCT) data. All published randomized trials comparing NAC-containing procedures vs no treatment before surgery in patients with gastric cancer (or gastric cancer + cardia cancer + lower-esophagus cancer) were included in our analysis. Blinding the trial conditions was not necessary. There was no language restriction. Esophagogastric junction and lower esophageal cancer patients were considered only if they were enrolled together with proper gastric cancer patients in the same study. The reason for considering also esophago-gastric junction and lower esophageal cancers in the recruitment process was that these locations represented a consistent part of the study population of the main RCTs actually claimed to be the basis for NAC treatment in gastric cancer.
At the end of the process, only RCTs were included in this review. As we wanted to include all RCTS available in Literature at the moment, any chemotherapy regimen performed before resective surgery with or without postoperative adjuvant treatment was included and located in the NAC-arm as well as in the surgery alone-arm (SA-arm) we enrolled all types of gastrectomy and lymphadenectomy that were performed (D0, D1, D1plus, D2, D3). The quality of the included RCTs was assessed usin nodified JADAD’s scoring system and Cochrane reviewers’ andbook 5.0.1 RCT criteria.
Research results
The currently available data support the adoption of NAC procedures in several national guidelines based on low-volume study populations with mixed sites of the primary disease (lower esophagus, esophago-gastric junction and stomach). Statistical tests have often shown evidence of the heterogeneity of chemotherapy effects according to the site of the primary tumor. In fact, in both Magic and FNCLCC-FFCD trials, tests for heterogeneity showed that perioperative chemotherapy effects were much more evident for esophago-gastric junction cancer and much less for stomach cancer.
Furthermore, in both these RCTs supporting the adoption of NAC, surgery provided to patients was incomplete because extended nodal dissection was performed in less than 50% of patients or not described, and no results on the LN yield were documented to warrant its adequacy. The only well-designed RCT with extended surgery performed in almost all cases was prematurely ended for poor accrual, but at the moment of its closure, it failed to demonstrate a survival benefit for NAC.
Moreover, in national reference centers for gastric cancer care and research, OS and DSS rates of subsites of patients theoretically fit for NAC submitted to surgery alone with complete D2 dissection and performed for cancers located in the stomach only, are even higher than those reported after NAC followed by incomplete surgery provided to a patient population with mixed sites of the primary tumor.
Research conclusions
This study documented that the adoption of NAC procedures in several national guidelines is actually based on low-volume study populations with mixed sites of the primary disease (lower esophagus, esophago-gastric junction and stomach) and with inadequate surgery as concern the extent of nodal dissection (incomplete D2 dissection). In fact, statistical tests reported in these trials have shown evidence of the heterogeneity of chemotherapy effects according to the site of the primary tumor, documented a major effect mainly over esophagogastric junction cancer. Moreover, survival rates reported in Surgery-Alone arm by main RCTs claimed to be fundamental for supporting the adoption of NAC ( MRC and French trials) are really too low as compared to rates documented in reference centers study population after proper D2 gastrectomy without preoperative chemotherapy. Effectively, in these surgical series coming from high volume referral centres, survival rates after extended surgery without NAC are even higher than those reported after NAC followed by incomplete surgery.
Our hypothesis is that, with current data available it ‘s not possible to exclude that: (1) The location of the tumor outside the stomach (esophago-gastric junction and lower esophagus) may amplify the response to preoperative treatment. (2) A proper radical surgery (gastrectomy with D2 LN dissection) may nullify the survival benefit of NAC described after incomplete surgery. (3) On the contrary, NAC effect on survival is more likely to be evident after incomplete surgery. In other words, NAC could be unuseful in cancer of the only stomach submitted to curative complete D2 dissection.
Research perspectives
We think additional high volume sample-size multicenter (and eventually multinational) RCTs comparing newer treatment regimens of neoadjuvant settings (capecitabine, oxaliplatin, docetaxel) combined with molecular therapies (epidermal growth factor receptor inhibitors or antiangiogenic agents) followed by controlled D2 surgery with controlled D2 surgery alone are necessary to investigate the survival effects of modern preoperative chemotherapy in patients with gastric cancer only who receive proper extended surgery. This is the ony way to investigate if neoadjuvant therapy can positively impact survival also in homogeneous population of gastric cancer patients without any biases related to location of the tumor and adequacy of surgery administered.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
Conflict-of-interest statement: All authors declare they have no conflicts of interest related to the work submitted for publication.
Peer-review started: November 19, 2017
First decision: November 30, 2017
Article in press: December 20, 2017
P- Reviewer: Aoyagi K, Fujita T, Fukuchi M, Petrucciani N S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ
Contributor Information
Rossella Reddavid, Surgical Oncology and Digestive Surgery, Department of Oncology, University of Turin, San Luigi University Hospital, Orbassano, Turin 10049, Italy.
Silvia Sofia, Surgical Oncology and Digestive Surgery, Department of Oncology, University of Turin, San Luigi University Hospital, Orbassano, Turin 10049, Italy.
Paolo Chiaro, Department of Surgical Sciences, Digestive and Oncological Surgery, University of Turin, Molinette Hospital, Turin 10126, Italy.
Fabio Colli, Department of Surgical Sciences, Digestive and Oncological Surgery, University of Turin, Molinette Hospital, Turin 10126, Italy.
Renza Trapani, Surgical Oncology and Digestive Surgery, Department of Oncology, University of Turin, San Luigi University Hospital, Orbassano, Turin 10049, Italy.
Laura Esposito, Surgical Oncology and Digestive Surgery, Department of Oncology, University of Turin, San Luigi University Hospital, Orbassano, Turin 10049, Italy.
Mario Solej, Surgical Oncology and Digestive Surgery, Department of Oncology, University of Turin, San Luigi University Hospital, Orbassano, Turin 10049, Italy.
Maurizio Degiuli, Surgical Oncology and Digestive Surgery, Department of Oncology, University of Turin, San Luigi University Hospital, Orbassano, Turin 10049, Italy. maurizio.degiuli@unito.it.
References
- 1.Inghelmann R, Grande E, Francisci S, Verdecchia A, Micheli A, Baili P, Capocaccia R, De Angelis R. Regional estimates of stomach cancer burden in Italy. Tumori. 2007;93:367–373. doi: 10.1177/030089160709300407. [DOI] [PubMed] [Google Scholar]
- 2.Russo A, Li P, Strong VE. Differences in the multimodal treatment of gastric cancer: East versus west. J Surg Oncol. 2017;115:603–614. doi: 10.1002/jso.24517. [DOI] [PubMed] [Google Scholar]
- 3.Pinto C, Mangone L. [Epidemiology of cancer in Italy: from real data to the need for cancer networks.] Recenti Prog Med. 2016;107:505–506. doi: 10.1701/2454.25696. [DOI] [PubMed] [Google Scholar]
- 4.Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, de Vries JE, Wils JA, van der Bijl J, van Krieken JH; Cooperating Investigators of The Dutch Gastric Cancer Group. Neo-adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol. 2004;30:643–649. doi: 10.1016/j.ejso.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 5.de Steur WO, Dikken JL, Hartgrink HH. Lymph node dissection in resectable advanced gastric cancer. Dig Surg. 2013;30:96–103. doi: 10.1159/000350873. [DOI] [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association Registration Committee, Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, Arai K, Kodera Y, Nashimoto A. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 7.Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Modern surgery for gastric cancer--Japanese perspective. Scand J Surg. 2006;95:232–235. doi: 10.1177/145749690609500404. [DOI] [PubMed] [Google Scholar]
- 8.Jiang L, Yang KH, Chen Y, Guan QL, Zhao P, Tian JH, Wang Q. Systematic review and meta-analysis of the effectiveness and safety of extended lymphadenectomy in patients with resectable gastric cancer. Br J Surg. 2014;101:595–604. doi: 10.1002/bjs.9497. [DOI] [PubMed] [Google Scholar]
- 9.Giuliani A, Miccini M, Basso L. Extent of lymphadenectomy and perioperative therapies: two open issues in gastric cancer. World J Gastroenterol. 2014;20:3889–3904. doi: 10.3748/wjg.v20.i14.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verlato G, Giacopuzzi S, Bencivenga M, Morgagni P, De Manzoni G. Problems faced by evidence-based medicine in evaluating lymphadenectomy for gastric cancer. World J Gastroenterol. 2014;20:12883–12891. doi: 10.3748/wjg.v20.i36.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degiuli M, Sasako M, Ponti A, Soldati T, Danese F, Calvo F. Morbidity and mortality after D2 gastrectomy for gastric cancer: results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. J Clin Oncol. 1998;16:1490–1493. doi: 10.1200/JCO.1998.16.4.1490. [DOI] [PubMed] [Google Scholar]
- 12.Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 13.Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G, Morino M; Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Beeharry MK, Liu W, Yan M, Zhu Z. Preoperative Chemotherapy for Gastric Cancer: Personal Interventions and Precision Medicine. Biomed Res Int. 2016;2016:3923585. doi: 10.1155/2016/3923585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210–5218. doi: 10.1200/JCO.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenlee RT, Howe HL. County-level poverty and distant stage cancer in the United States. Cancer Causes Control. 2009;20:989–1000. doi: 10.1007/s10552-009-9299-x. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 18.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 19.Sobin LH, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours. 6. New York: Wiley;; 2002. [Google Scholar]
- 20.Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumours. 7. New York: Wiley-Liss;; 2010. [Google Scholar]
- 21.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XL, Wu GX, Zhang MD, Guo M, Zhang H, Sun XF. A favorable impact of preoperative FPLC chemotherapy on patients with gastric cardia cancer. Oncol Rep. 2000;7:241–244. [PubMed] [Google Scholar]
- 23.Shah MA, Khanin R, Tang L, Janjigian YY, Klimstra DS, Gerdes H, Kelsen DP. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693–2701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Green S. 2008. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.1 (Updated September 2008). e Cochrane Collaboration. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 26.Masuyama M, Taniguchi H, Takeuchi K, Miyata K, Koyama H, Tanaka H, Higashida T, Koishi Y, Mugitani T, Yamaguchi T. [Recurrence and survival rate of advanced gastric cancer after preoperative EAP-II intra-arterial infusion therapy] Gan To Kagaku Ryoho. 1994;21:2253–2255. [PubMed] [Google Scholar]
- 27.Nishioka B, Ouchi T, Watanabe S, Umehara M, Yamane E, Yahata K, Muto F, Kojima O, Nomiyama S, Sakita M, et al. [Follow-up study of preoperative oral administration of an antineoplastic agent as an adjuvant chemotherapy in stomach cancer] Gan To Kagaku Ryoho. 1982;9:1427–1432. [PubMed] [Google Scholar]
- 28.Zhang CW, Zou SC, Shi D, Zhao DJ. Clinical significance of preoperative regional intra-arterial infusion chemotherapy for advanced gastric cancer. World J Gastroenterol. 2004;10:3070–3072. doi: 10.3748/wjg.v10.i20.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonemura Y, Sawa T, Kinoshita K, Matsuki N, Fushida S, Tanaka S, Ohoyama S, Takashima T, Kimura H, Kamata T. Neoadjuvant chemotherapy for high-grade advanced gastric cancer. World J Surg. 1993;17:256–61; discussion 261-2. doi: 10.1007/BF01658939. [DOI] [PubMed] [Google Scholar]
- 30.Shchepotin I, Evans S, Chorny V, Ugrinov O, Osinsky S, Galakchin K, Troitsky I, Buras R, Shabahang M, Nauta R. Preoperative superselective intraarterial chemotherapy in the combined treatment of gastric-carcinoma. Oncol Rep. 1995;2:473–479. doi: 10.3892/or.2.3.473. [DOI] [PubMed] [Google Scholar]
- 31.Kang YK, Choi DW, Im YH, Kim CM, Lee JI, Moon NM, Lee JO. 1996. A phase III randomized comparison of neoadjuvant che- motherapy followed by surgery versus surgery for locally ad- vanced stomach cancer. Abstract 503 presented at the ASCO Annual Meeting. [Google Scholar]
- 32.Lygidakis NJ, Sgourakis G, Aphinives P. Upper abdominal stop-flow perfusion as a neo and adjuvant hypoxic regional chemotherapy for resectable gastric carcinoma. A prospective randomized clinical trial. Hepatogastroenterology. 1999;46:2035–2038. [PubMed] [Google Scholar]
- 33.Takiguchi N, Oda K, Suzuki H, Wakatsuki K, Nunomora M, Kouda K, Saito N, Nakajima N. Neoadjuvant chemotherapy with 5- uorouracil (5-FU) or low dose cis-platinum (CDDP) + 5-FU in the treatment of gastric carcinoma with serosal invasion. Proc Am Soc Clin Oncol. 2000;19:A1178. [Google Scholar]
- 34.Brierley JD, Gospodarwicz MK, Wittekind C, Amin MB. TNM classification of maligant tumours. 8th ed. Oxford: Wiley Blackwell;; 2017. [Google Scholar]
- 35.Kobayashi T, Kimura T. [Long-term outcome of preoperative chemotherapy with 5’-deoxy-5-fluorouridine (5’-DFUR) for gastric cancer] Gan To Kagaku Ryoho. 2000;27:1521–1526. [PubMed] [Google Scholar]
- 36.Nio Y, Koike M, Omori H, Hashimoto K, Itakura M, Yano S, Higami T, Maruyama R. A randomized consent design trial of neoadjuvant chemotherapy with tegafur plus uracil (UFT) for gastric cancer--a single institute study. Anticancer Res. 2004;24:1879–1887. [PubMed] [Google Scholar]
- 37.Zhao WH, Wang SF, Ding W, Sheng JM, Ma ZM, Teng LS, Wang M, Wu FS, Luo B. Apoptosis induced by preoperative oral 5’-DFUR administration in gastric adenocarcinoma and its mechanism of action. World J Gastroenterol. 2006;12:1356–1361. doi: 10.3748/wjg.v12.i9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imano M, Itoh T, Satou T, Sogo Y, Hirai H, Kato H, Yasuda A, Peng YF, Shinkai M, Yasuda T, et al. Prospective randomized trial of short-term neoadjuvant chemotherapy for advanced gastric cancer. Eur J Surg Oncol. 2010;36:963–968. doi: 10.1016/j.ejso.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Biffi R, Fazio N, Luca F, Chiappa A, Andreoni B, Zampino MG, Roth A, Schuller JC, Fiori G, Orsi F, et al. Surgical outcome after docetaxel-based neoadjuvant chemotherapy in locally-advanced gastric cancer. World J Gastroenterol. 2010;16:868–874. doi: 10.3748/wjg.v16.i7.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu JJ, Shi YR, Liu FR, Ma SQ, Ma FY. [A clinical study of paclitaxel combined with FOLFOX4 regimen as neoadjuvant chemotherapy for advanced gastric cancer] Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:664–667. [PubMed] [Google Scholar]
- 41.Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87–104. doi: 10.5230/jgc.2014.14.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–1472. doi: 10.1136/gut.2010.228254. [DOI] [PubMed] [Google Scholar]
- 43.Meyer HJ, Hölscher AH, Lordick F, Messmann H, Mönig S, Schumacher C, Stahl M, Wilke H, Möhler M. [Current S3 guidelines on surgical treatment of gastric carcinoma] Chirurg. 2012;83:31–37. doi: 10.1007/s00104-011-2149-x. [DOI] [PubMed] [Google Scholar]
- 44.De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei MA, Pacelli F, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. 2017;20:20–30. doi: 10.1007/s10120-016-0615-3. [DOI] [PubMed] [Google Scholar]
- 45.Okines A, Verheij M, Allum W, Cunningham D, Cervantes A; ESMO Guidelines Working Group. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v50–v54. doi: 10.1093/annonc/mdq164. [DOI] [PubMed] [Google Scholar]
- 46.Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO) Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi57–vi63. doi: 10.1093/annonc/mdt344. [DOI] [PubMed] [Google Scholar]
- 47.Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 48.Maruyama K, Katai H. Surgical treatment of gastric cancer in Japan, trend from standardization to individualization. Chirurgia (Bucur) 2014;109:722–730. [PubMed] [Google Scholar]
- 49.Kim EY, Song KY, Lee J. Does Hospital Volume Really Affect the Surgical and Oncological Outcomes of Gastric Cancer in Korea? J Gastric Cancer. 2017;17:246–254. doi: 10.5230/jgc.2017.17.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siewert JR, Böttcher K, Roder JD, Busch R, Hermanek P, Meyer HJ. Prognostic relevance of systematic lymph node dissection in gastric carcinoma. German Gastric Carcinoma Study Group. Br J Surg. 1993;80:1015–1018. doi: 10.1002/bjs.1800800829. [DOI] [PubMed] [Google Scholar]
- 51.Sue-Ling HM, Johnston D, Martin IG, Dixon MF, Lansdown MR, McMahon MJ, Axon AT. Gastric cancer: a curable disease in Britain. BMJ. 1993;307:591–596. doi: 10.1136/bmj.307.6904.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Degiuli M, Sasako M, Ponti A, Calvo F. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer. 2004;90:1727–1732. doi: 10.1038/sj.bjc.6601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, Lui WY, Whang-Peng J. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–315. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 54.Viste A, Svanes K, Janssen CW Jr, Maartmann-Moe H, Søreide O. Prognostic importance of radical lymphadenectomy in curative resections for gastric cancer. Eur J Surg. 1994;160:497–502. [PubMed] [Google Scholar]
- 55.Robertson CS, Chung SC, Woods SD, Griffin SM, Raimes SA, Lau JT, Li AK. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176–182. doi: 10.1097/00000658-199408000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110–112. doi: 10.1002/bjs.1800750206. [DOI] [PubMed] [Google Scholar]
- 57.Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, Meyer S, Plukker JT, Van Elk P, Obertop H, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 58.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, Cook P. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]


