Abstract
Purpose
To devise an uninvasive selection system for human embryos with high developmental potential after a single oocyte retrieval cycle by comparing the in vitro and in vivo effectiveness of first division synchrony against subsequent embryonic developmental stages.
Methods
The effects of using assisted reproductive technology on 948 embryos that were produced in 137 cycles were examined by dividing the embryos into “early cleavage” (first division within 25.90 hours) and “late cleavage” (first division at or after 25.90 hours) groups and comparing the blastocysts and good‐quality blastocyst formation rates between the two groups. These two groups were each divided further into “high synchrony” (first division synchrony within 3.96 hours) and “low synchrony” (first division synchrony at or after 3.96 hours) groups. The blastocysts, good‐quality blastocyst formation rates, and pregnancy rates were compared among these four groups.
Results
Both the blastocysts and good‐quality blastocyst formation rates were significantly higher in the early‐cleavage groups than in the late‐cleavage groups. The blastocyst formation rate of the latter was also significantly increased in the high‐synchrony, compared with the low‐synchrony, group.
Conclusion
First division synchrony in a single oocyte retrieval cycle could be a useful assessment of the blastocyst formation rate that enables the selection of viable embryos at an early stage of culture.
Keywords: blastocyst, embryo, first division, synchrony, time‐lapse incubator
1. INTRODUCTION
The EmbryoScope (Vitrolife, Göteborg, Sweden) time‐lapse incubator has attracted widespread interest in the scientific community in recent years.1, 2, 3, 4, 5, 6 This embryo‐monitoring system includes an incubator with a built‐in microscope and charge‐coupled device camera. Using this instrument, embryos and embryonic development can be assessed and monitored at all times without removal from the incubator, eliminating the risks that are posed by external stressors, such as temperature change, light exposure, high‐level oxygen exposure, and pH changes in the culture medium. In this manner, more information can be obtained by analyzing embryonic growth at various time points, rather than morphological observation at a single time point. The concurrent application of a single‐step culture medium enables a continuous blastocyst culture system to be established. Eliminating the need for the direct observation or exchange of culture medium guarantees undisturbed growth conditions and increased success of embryogenesis.
Most importantly, the continuous observation of embryo development in a time‐lapse system allows the optimal selection and prioritization of the order of embryos to be transferred. Recent assisted reproductive technology (ART) includes improvements in cryopreservation techniques7, 8, 9, 10 that have increased the priority for the culture and transfer of blastocysts, compared with fresh embryo transfer.11, 12, 13 Limiting embryo transfer to blastocysts alone is not always recommended, as low blastocyst formation rates can terminate the embryo transfer process. Therefore, the most reliable indicator of embryo quality is whether or not embryos reach the blastocyst stage.
Embryos need to be cultured for 5‐6 days to reach the blastocyst stage. In early‐stage embryo transfer (day 2 or 3), the selection of good embryos during the early stage of culture is crucial in order to determine the optimal culture duration and timing for embryo transfer. Previous studies have demonstrated the methods for selecting good embryos, with a focus on the division speed and morphology of early‐stage embryos.11, 14, 15, 16 Although the early‐stage transfer of embryos that are selected by using a time‐lapse system does not increase pregnancy rates, blastocyst transfer does so.17 Thus, the use of a time‐lapse system is important in order to determine whether embryos have reached the blastocyst stage at an early stage of culture.
Various studies attempting to identify the indicators of the blastocyst formation rate have found: correlations between the time from fertilization to the first division and the number of blastomeres and embryo implantation potential on day 2;18 an association between the times of first and second division and embryo implantation potential on day 3;19 relationships between synchrony of the second and third divisions and the rate of formation of good‐quality blastocysts;20 and correlations between synchrony of the second division and the potential of embryos to develop into blastocysts.21 Several other studies also have found that the time until the first division is an important factor for blastocyst formation rates.22, 23, 24, 25, 26, 27 From this perspective, it was examined whether synchrony and the average time until the first division were indicators of blastocyst formation rates in order to determine the characteristics of the embryos that developed into transferable blastocysts during the early culture period.
2. MATERIALS AND METHODS
All the study's participants were required to provide written informed consent and the study design was approved by the appropriate ethics review boards. Ovarian stimulation followed a short protocol that used the gonadotropin‐releasing hormone analog, buserelin acetate (Fuji Pharmaceutical Company, Ltd., Tokyo, Japan), together with follicle‐stimulating hormone and human menopausal gonadotropin (ASUKA Pharmaceutical Company, Ltd., Tokyo, Japan). Human chorionic gonadotropin (hCG; Fuji Pharmaceutical Company, Ltd.) or leuprolide was administered when the maximum diameter of two or more follicles had reached 18 mm. Cumulus‐oocyte complexes were retrieved by ultrasound‐guided transvaginal follicle aspiration at approximately 36 hours after hCG injection. Between April, 2014 and June, 2016, the outcomes of 137 patients for 137 cycles were examined. The fertilization of the oocytes was performed by intracytoplasmic sperm injection (ICSI) using standard techniques. The retrieved oocytes were precultured for 3 hours in a 10% serum‐added human tubal fluid (HTF; NAKA Medical, Tokyo, Japan). After preculture, the oocytes were denuded by pipetting in 80 U/mL hyaluronidase solution (NAKA Medical). A single sperm was immobilized in 7% polyvinylpyrrolidone solution (NAKA Medical) and ICSI then was performed in a drop of 20% serum‐added 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid‐HTF (NAKA Medical) by using the IM‐11‐2 injector (Narishige, Tokyo, Japan) and K‐MPIP‐3130 injection pipettes (Cook Medical, Bloomington, IN, USA). All the embryos were transferred to 30 μL of ONESTEP medium (NAKA Medical), covered with sterile mineral oil, and incubated at 37°C under 5% CO2, 5% O2, and 90% N2 for a maximum of 6 days. All the oocytes were cultured in the EmbryoScope immediately after microinjection. Cryopreservation of the blastocysts was performed by established methods9, 10 by using a Cryotop® (Kitazato, Shizuoka, Japan). The blastocysts in which both the inner cell mass and trophectoderm were graded as B or higher were regarded as “good quality,”28 whereas the blastocysts that were graded as C were discarded.
2.1. Experimental designs
The treatment cycles in which four or more fertilized oocytes were cultured in the EmbryoScope were targeted. The time until division was measured from the point at which ICSI was performed. The time lag of the first division was measured by subtracting the time that the first embryo divided from the subsequent time of division of each embryo. The average time lag of the first division time was defined as the degree of synchrony of the first division (Figure 1). The smaller the time lag, the greater the synchrony. The study used 25.90 hours as a reference value for the definition of early‐cleavage embryos in the first division, according to the authors’ previous study.17 The average time of synchrony of the first division in a single oocyte retrieval cycle was 3.96 hours for 137 cycles.
Figure 1.
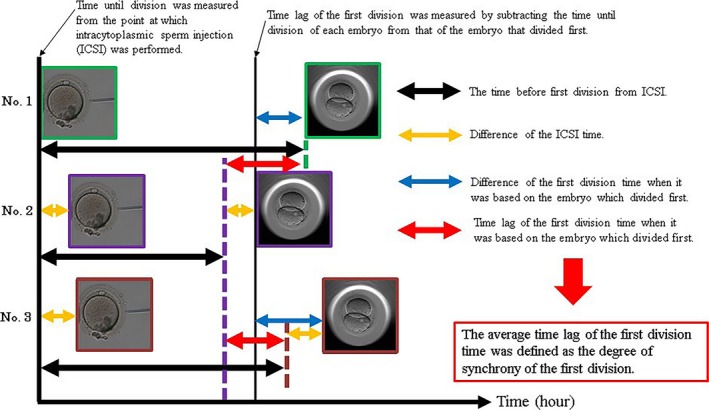
A detailed view of synchrony observed in embryos undergoing first division
2.1.1. Experiment I
The embryos were divided into two groups: those in which the first division occurred within 25.90 hours (early‐cleavage group) and those in which the first division occurred at or after 25.90 hours (late‐cleavage group). The blastocyst and good‐quality blastocyst formation rates were compared between these two groups.
2.1.2. Experiment II
Using the average time of synchrony of the first division (3.96 hours) as a reference value, the embryos that displayed a time of synchrony of the first division of within 3.96 hours were defined as “high‐synchrony” and those that displayed a time of synchrony of the first division at or after 3.96 hours were defined as “low‐synchrony” (Figure2). These four groups were compared for blastocyst formation rates, good‐quality blastocyst formation rates, and pregnancy rates. Group A was defined as the embryos in which the first division occurred within 25.90 hours and the average time of synchrony was within 3.96 hours, group B was defined as those embryos in which the first division occurred within 25.90 hours and the average time of synchrony was ≥3.96 hours, group C was defined as the embryos in which the first division occurred at or after 25.90 hours and the average time of synchrony was within 3.96 hours, and group D was defined as those embryos in which the first division occurred at or after 25.90 hours and the average time of synchrony was ≥3.96 hours.
Figure 2.

Embryos showing synchrony of the first division. (A) The embryos that displayed synchrony within 3.96 hours were recognized as “high synchrony” and (B) the embryos that displayed synchrony at or after 3.96 hours were recognized as “low synchrony”
2.1.3. Experiment III
Blastocysts were transferred to 92 patients for 134 cycles after the next hormone replacement therapy cycle. After thawing, a single blastocyst transfer was performed, and 3 weeks after transfer, the clinical pregnancy rates were determined by using ultrasound to detect the presence of a gestational sac.
2.2. Statistical analysis
The statistical analysis was performed by using the chi‐square test with continuity correction (experiment I) or Bonferroni corrected chi‐square test with continuity correction (experiments II and III). P< .05 was considered to be statistically significant.
3. RESULTS
Age, maturation, fertilization, blastocyst formation and good‐quality blastocyst formation rates, and the average synchrony time are shown in Table 1.
Table 1.
Embryological outcomes of intracytoplasmic sperm injection with respect to the study's parameters
| Characteristic | Value |
|---|---|
| Patient per cycle | 137 patients for 137 cycles |
| Age (years)a | 37.1±4.0 |
| No. of retrieved oocytes | 1286 |
| Mature oocytes: N (%)b | 1127 (87.6) |
| Fertilized oocytes: N (%)c | 948 (84.1) |
| Cultured embryos (N)d | 918 |
| Blastocysts: N (%)e | 600 (65.4) |
| Good‐quality blastocysts: N (%)f | 344 (37.5) |
| Average synchrony time (hours)a | 3.96±2.15 |
Data are presented as the mean ± standard deviation.
The percentage of retrieved oocytes
The percentage of mature oocytes.
Thirty fertilized oocytes were cryopreserved for transfer on day 1, 2, or 3.
Percentage per cultured embryo.
Good‐quality blastocysts were defined as those scoring B or higher for both inner cell mass and trophectoderm grade.
3.1. Experiment I
As shown in Table 2, the blastocyst formation rate in the satisfactory group (74.1%) was significantly higher (P<.01) than in the unsatisfactory group (56.1%). The good‐quality blastocyst formation rate in the satisfactory group (47.5%) was also significantly higher (P<.01) than in the unsatisfactory group (26.8%).
Table 2.
Effect of first division times on the subsequent embryonic development
| Criteriona | Cultured embryos (N) | Blastocysts: N (%)b | |
|---|---|---|---|
| Total | Good quality | ||
| Satisfactory | 474 | 351 (74.1)c | 225 (47.5)c |
| Unsatisfactory | 444 | 249 (56.1)c | 119 (26.8)c |
Embryos that completed the first divisions within 25.9 hours after culture were regarded as satisfactory embryos.
The percentage per cultured embryo.
Values with different superscript letters significantly different (P<.01) within each column.
3.2. Experiment II
As shown in Table 3, the blastocyst formation rate in group A (75.6%) was significantly higher (P<.01) than in groups C (62.9%) and D (49.3%), while the blastocyst formation rates in groups B and C (70.3 and 62.6%, respectively) were significantly higher (P<.01) than in group D (49.3%). The good‐quality blastocyst formation rates in group A (49.1%) and group B (43.5%) were also significantly higher (P<.01) than in groups C (30.0%) and D (23.5%).
Table 3.
Effect of first division synchrony on the blastocyst formation of human embryos
| First division | Cultured embryos (N) | Blastocysts: N (%)c | |||
|---|---|---|---|---|---|
| Group | Timea | Synchronyb | Total | Good quality | |
| A | + | + | 336 | 254 (75.6)d | 165 (49.1)d |
| B | + | – | 138 | 97 (70.3)d,d | 60 (43.5) d |
| C | – | + | 227 | 142 (62.6)d | 68 (30.0) d |
| D | – | – | 217 | 107 (49.3)d | 51 (23.5) d |
Embryos in which the first division occurred within 25.90 hours (+); embryos in which the first division occurred at or after 25.90 hours (–).
The average time of synchrony was within 3.96 hours (+); the average time of synchrony was ≥3.96 hours (–).
The percentage per cultured embryo.
Values with different superscript letters significantly different (P<.01) within each column.
3.3. Experiment III
As shown in Table 4, there was no significant difference in the pregnancy (36.0‐63.6%) and miscarriage (11.1‐35.7%) rates among groups.
Table 4.
Effect of first division synchrony on the pregnancy rate of human embryos
| First division | Transferred embryos (N) | Pregnancies: N (%)c | Miscarriages: N (%)d | Average age of the patients at the oocyte retrieval cycle (years)e | ||
|---|---|---|---|---|---|---|
| Group | Timea | Synchronyb | ||||
| A | + | + | 66 | 32 (48.5) | 6 (18.8) | 36.1±3.5 |
| B | + | – | 25 | 9 (36.0) | 1 (11.1) | 36.5±4.0 |
| C | – | + | 21 | 11 (52.4) | 2 (18.2) | 36.9±4.5 |
| D | – | – | 22 | 14 (63.6) | 5 (35.7) | 37.0±2.6 |
Embryos in which the first division occurred within 25.90 hours (+); embryos in which the first division occurred at or after 25.90 hours (–).
The average time of synchrony was within 3.96 hours (+); the average time of synchrony took ≥3.96 hours (–).
The percentage per transferred embryo.
The percentage per pregnancy.
The data are presented as the mean ± standard deviation.
4. DISCUSSION
The blastocyst and good‐quality blastocyst formation rates were high for the embryos that exhibited an early first division (before 25.90 hours). Even for the embryos that displayed a later first division, those featuring high synchrony of the first division in a single oocyte retrieval cycle had a high probability of reaching the blastocyst stage. Moreover, the embryos that displayed an early first division and shorter average time of synchrony showed the highest blastocyst formation rate of all the embryo groups that were examined in this study. Therefore, it has been hypothesized that high synchrony of the first division in a single oocyte retrieval cycle was a useful criterion for predicting the blastocyst formation rates. However, the embryos that had a late first division and shorter average time of synchrony showed lower blastocyst and good‐quality blastocyst formation rates than the embryos that exhibited an early first division and either a shorter‐ or longer‐than‐average time of synchrony. These results suggested that the first division time (25.90 hours) was a more effective indicator of blastocyst formation, compared with synchrony of the first division.
Previous reports have shown that early‐cleavage embryos had strong developmental potential.22, 23, 24, 25, 26 Some studies classified all embryos into “early‐cleavage” and “non‐early‐cleavage” groups and compared them.23, 24, 25, 26, 27 Other studies also grouped the embryos into those that ultimately resulted in pregnancy and those that did not.22, 26 In contrast, this study compared the embryos that were retrieved from a single oocyte retrieval cycle and measured the synchrony of the first division, thereby predicting the subsequent embryo development more accurately than the retardation time until first division. The stronger correlation for synchrony, rather than the retardation time until division with the blastocyst formation rate, might be attributed to the high synchrony of the first division in a single oocyte retrieval cycle and therefore a more homogeneous quality of retrieved oocytes.
Previous studies also have revealed various morphological factors that are associated with the oocyte quality. Typical examples are the lipofuscin body29 and the smooth endoplasmic reticulum cluster,30 where only a small percentage of embryos with such morphological abnormalities reached the blastocyst stage. The presence of a lipofuscin body, smooth endoplasmic reticulum cluster, centrally located cytoplasmic granularity, or vacuoles in embryos reduced the rate of pregnancy.31 The oocyte quality with these factors was considered to be inhomogeneous, resulting in large variations in division speed, which reduced the blastocyst formation rate. In the current study, the good‐quality blastocyst formation rate was not improved by the selection of embryos that displayed a late first division with an early synchrony to first division, possibly as more of these embryos featured morphological abnormalities. As the average speed of the first division was not an accurate indicator of such variations, the synchrony of the first division showed a stronger correlation with the blastocyst formation rate. However, the high‐synchrony embryos showed an improved blastocyst formation rate for the embryos where the first division occurred at or after 25.90 hours. Therefore, synchrony also can be an indicator of the blastocyst formation rate.
In ART, several factors, such as hormone levels (eg, anti‐Müllerian hormone), endometrial thickness, and semen quality, are examined in order to determine an effective treatment strategy before oocyte retrieval. For example, early examination will determine whether in vitro fertilization or ICSI should be performed, the stage at which the embryo transfer should be performed, and whether all the embryos should be cryopreserved. However, embryo transfer might need to be avoided if the number of retrieved oocytes is less than expected and/or the cultured embryos fail to develop properly. When embryos show high synchrony of the first division and sufficient numbers are likely to develop into blastocysts, all the blastocysts should be cryopreserved, instead of early embryo transfer. Cryopreserved embryo transfer,17 which provides higher pregnancy rates, can be performed after the next cycle. In the present study, cryopreserved‐thawed blastocyst transfer achieved good pregnancy rates for all groups, suggesting that this was an effective transfer technique. Once the embryos reach the blastocyst stage, a certain pregnancy rate can be expected. Therefore, it is important to determine whether the embryos will reach the blastocyst stage. The synchrony of the first division, as well as the first division time, can be indicators of whether the embryos reach the blastocyst stage. In cases in which the embryos show poor synchrony of the first division and are unlikely to reach the blastocyst stage, alternatives such as early embryo transfer or the cancellation of embryo transfer should be adopted as early as possible, if cryopreservation of all the blastocysts was chosen as a treatment modality. The decision to perform an early embryo transfer must be taken by at least day 2 of culture, as a delay in starting luteal support can reduce the pregnancy rate.32 If synchrony of the first division, which is observed on day 1 of culture, is used as an indicator of subsequent embryo development, luteal support through oral administration, injection, or transvaginal administration can be started on day 1. This enables the treatment to be changed, if necessary. This method also required a shorter observation time than those that were used in previous studies to determine whether embryos have high developmental potential.
In conclusion, synchrony of the first division in a single oocyte retrieval cycle was found to be a useful indicator of the blastocyst formation rate. In the future, the authors intend to use this method in order to determine the optimal culture duration and to effectively time the embryo transfer process.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: All the procedures were followed in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments. Informed consent was obtained from all the patients in the study. Additionally, all the procedures that involved human participants were carried out in accordance with the ethical standards of the Institutional Review Board of the AIIKUKAI Medical Corporation, Kagoshima, Japan. This article does not contain any study with animal participants that was performed by any of the authors.
ACKNOWLEDGEMENTS
The authors would like to thank the doctors and nursing staff in the embryology department at the Aiiku Ladies Clinic (Kagoshima, Japan) for their assistance and technical expertise in helping to prepare this manuscript.
Mizobe Y, Tokunaga M, Oya N, et al. Synchrony of the first division as an index of the blastocyst formation rate during embryonic development. Reprod Med Biol. 2018;17:64‐70. https://doi.org/10.1002/rmb2.12070
REFERENCES
- 1. Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Human embryonic development after blastomere removal: a time‐lapse analysis. Hum Reprod. 2012;27:97‐105. [DOI] [PubMed] [Google Scholar]
- 2. Kirkegaard K, Agerholm IE, Ingerslev HJ. Time‐lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277‐1285. [DOI] [PubMed] [Google Scholar]
- 3. Cruz M, Gadea B, Garrido N, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time‐lapse imaging. J Assist Reprod Genet. 2011;28:569‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubio I, Galán A, Larreategui Z, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertil Steril. 2014;102:1287‐1294. [DOI] [PubMed] [Google Scholar]
- 5. Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time‐lapse imaging without PGS. Reprod Biomed Online. 2013;27:140‐146. [DOI] [PubMed] [Google Scholar]
- 6. Mizobe Y, Akiyoshi T, Minami S, et al. Effect of a time‐lapse incubator (EmbryoScope®) on in vitro culture of human embryos. J Mamm Ova Res. 2014;31:40‐44. [Google Scholar]
- 7. Mukaida T, Nakamura S, Tomiyama T, et al. Vitrification of human blastocysts using cryoloops: clinical outcome of 223 cycles. Hum Reprod. 2003;18:384‐391. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi K, Mukaida T, Oka C. Perinatal outcome of blastocyst transfer with vitrification using cryoloop: a 4‐year follow‐up study. Fertil Steril. 2005;84:88‐92. [DOI] [PubMed] [Google Scholar]
- 9. Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300‐308. [DOI] [PubMed] [Google Scholar]
- 10. Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: The Cryotop method. Theriogenology. 2007;67:73‐80. [DOI] [PubMed] [Google Scholar]
- 11. Milki AA, Hinckley MD, Fisch JD, Dasig D, Behr B. Comparison of blastocyst transfer with day 3 embryo transfer in similar patient populations. Fertil Steril. 2000;73:126‐129. [DOI] [PubMed] [Google Scholar]
- 12. Pribenszky C, Mátyás S, Kovács P, Losonczi E, Zádori J, Vajta G. Pregnancy achieved by transfer of a single blastocyst selected by time‐lapse monitoring. Reprod Biomed Online. 2010;21:533‐536. [DOI] [PubMed] [Google Scholar]
- 13. Zuh D, Zhang J, Cao S, et al. Vitrified‐warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles—time for a new embryo transfer strategy? Fertil Steril. 2011;95:1691‐1695. [DOI] [PubMed] [Google Scholar]
- 14. Dennis SJ, Thomas MA, Williams DB, Robins JC. Embryo morphology score on day 3 is predictive of implantation and live birth rates. J Assist Reprod Genet. 2006;23:171‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee MJ, Lee RK, Lin MH, Hwu YM. Cleavage speed and implantation potential of early‐cleavage embryos in IVF or ICSI cycles. J Assist Reprod Genet. 2012;29:745‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conaghan J, Chen AA, Willman SP, et al. Improving embryo selection using a computer‐automated time‐lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertil Steril. 2013;100:412‐419. [DOI] [PubMed] [Google Scholar]
- 17. Mizobe Y, Oya N, Iwakiri R, et al. Effects of early cleavage patterns of human embryos on subsequent in vitro development and implantation. Fertil Steril. 2016;106:348‐353. [DOI] [PubMed] [Google Scholar]
- 18. Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time‐lapse recordings of IVF/ICSI‐fertilized oocytes. Reprod Biomed Online. 2008;17:385‐391. [DOI] [PubMed] [Google Scholar]
- 19. Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658‐2671. [DOI] [PubMed] [Google Scholar]
- 20. Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high‐potential embryos by culture in poly(dimethylsiloxane) microwells and time‐lapse imaging. Fertil Steril. 2012;97:332‐337. [DOI] [PubMed] [Google Scholar]
- 21. Cruz M, Garrido N, Herrero J, Pérez‐Cano I, Muñoz M, Meseguer M. Timing of cell division in human cleavage‐stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online. 2012;25:371‐381. [DOI] [PubMed] [Google Scholar]
- 22. Kirkegaard K, Sundvall L, Erlandsen M, Hindkjær JJ, Knudsen UB, Ingerslev HJ. Timing of human preimplantation embryonic development is confounded by embryo origin. Hum Reprod. 2016;31:324‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shoukir Y, Campana A, Farley T, Sakkas D. Early cleavage of in‐vitro fertilized human embryos to the 2‐cell stage: a novel indicator of embryo quality and viability. Hum Reprod. 1997;12:1531‐1536. [DOI] [PubMed] [Google Scholar]
- 24. Sakkas D, Shoukir Y, Chardonnens D, Bianchi PG, Campana A. Early cleavage of human embryos to the two‐cell stage after intracytoplasmic sperm injection as an indicator of embryo viability. Hum Reprod. 1998;13:182‐187. [DOI] [PubMed] [Google Scholar]
- 25. Fenwick J, Platteau P, Murdoch AP, Herbert M. Time from insemination to first cleavage predicts developmental competence of human preimplantation embryos in vitro. Hum Reprod. 2002;17:407‐412. [DOI] [PubMed] [Google Scholar]
- 26. Salumets A, Hydén‐Granskog C, Mäkinen S, Suikkari AM, Tiitinen A, Tuuri T. Early cleavage predicts the viability of human embryos in elective single embryo transfer procedures. Hum Reprod. 2003;18:821‐825. [DOI] [PubMed] [Google Scholar]
- 27. Giorgetti C, Hans E, Terriou P, et al. Early cleavage: an additional predictor of high implantation rate following elective single embryo transfer. Reprod Biomed Online. 2007;14:85‐91. [DOI] [PubMed] [Google Scholar]
- 28. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155‐1158. [DOI] [PubMed] [Google Scholar]
- 29. Otsuki J, Nagai Y, Chiba K. Lipofuscin bodies in human oocytes as an indicator of oocyte quality. J Assist Reprod Genet. 2007;24:263‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591‐1597. [DOI] [PubMed] [Google Scholar]
- 31. Otsuki J. Intracytoplasmic morphological abnormalities in human oocytes. J Mamm Ova Res. 2009;26:26‐31. [Google Scholar]
- 32. Williams SC, Oehninger S, Gibbons WE, van Cleave WC, Muasher SJ. Delaying the initiation of progesterone supplementation results in decreased pregnancy rates after in vitro fertilization: a randomized, prospective study. Fertil Steril. 2001;76:1140‐1143. [DOI] [PubMed] [Google Scholar]


