Abstract
Background
Reproductive research is quintessential in understanding not only the cause of infertility, but also for creating family planning tools. The knockout (KO) system approach is conducive to discovering genes that are essential for fertility in mice. However, in vivo research has been limited due to its high cost and length of time needed to establish KO mice.
Methods
The mechanisms behind the CRISPR/Cas9 system and its application in investigating male fertility in mice are described by using original and review articles.
Results
The CRISPR/CAS9 SYSTEM has enabled researchers to rapidly, efficiently, and inexpensively produce genetically modified mice to study male fertility. Several genes have been highlighted that were found to be indispensable for male fertility by using the CRISPR/Cas9 system, as well as more complicated gene manipulation techniques, such as point mutations, tag insertions, and double knockouts, which have become easier with this new technology.
Conclusion
In order to increase efficiency and usage, new methods of CRISPR/Cas9 integration are being developed, such as electroporation and applying the system to embryonic stem cells. The hidden mysteries of male fertility will be unraveled with the help of this new technology.
Keywords: CRISPR/Cas9, fertility, gene editing, reproduction, spermatozoa
1. INTRODUCTION
Reproduction is the basis of life on this planet. Through reproductive research, scientists can unlock the mechanisms behind fertility, leading to cures for infertility and increased family planning tools, such as contraception. The world's population is slated to reach 7.6 billion by 20201 and, as resources are finite, an increase in food scarcity, economic turmoil, and increased climate change could be seen. Although there are many tools to help infertile couples, the lack of diversity in contraception, especially for men, is detrimental to society, for the burden of contraception becomes heavily weighted on women.
The CRISPR/Cas9 system has radically transformed the reproduction field. As it is difficult to differentiate spermatozoa in vitro, the knockout (KO) system approach at an individual level is conducive to discovering genes that are essential for fertility. However, the cost and the amount of time that are associated with conventional KO methods are high. With the discovery of CRISPR/Cas9, it is now possible to quickly and easily knock out genes and understand their effect in vivo.
Mice are superior for reproductive research due to their fast reproduction cycle and their genes being highly homologous to those of humans. Although much of the reproductive research has been conducted using non‐mammalian species, such as nematodes, sea urchins, ascidians, and frogs, 99% of the mouse genes have direct counterparts in humans.2 This can be used to elucidate the function of genes that are similar to those in humans by genetically modifying mice. Furthermore, methods have been established to analyze the phenotypes in mice, such as in vitro fertilization, intracytoplasmic sperm injection, seminiferous tubule transplantation, and embryo transfer. In this review, the mechanisms behind the CRISPR/Cas9 system and how it has been applied to investigating male fertility in mice will be discussed.
2. ANALYSIS OF ESSENTIAL GENES FOR MALE FERTILITY BY USING THE CONVENTIONAL KNOCKOUT APPROACH
Gene disruption has been pivotal in studying gene function in vivo. The most common KO method uses homologous recombination in embryonic stem (ES) cells.3 This conventional method requires transfecting the targeting vectors that include a drug cassette, such as a neomycin resistance gene, in addition to the target sequence into ES cells (Figure 1A). These pluripotent ES cells are genetically manipulated in a culture4 and then the surviving cells that undergo intended homologous recombination would be injected into preimplantation embryos (Figure 1B). The ES cell contribution in the resulting chimeric animal can be recognized easily due to the transmission of coat colors. By mating chimeric mice with wild‐type mice, heterozygous mice can be obtained through germline transmission. Even if the procedure is successful, this method can take about 1 year to complete.
Figure 1.
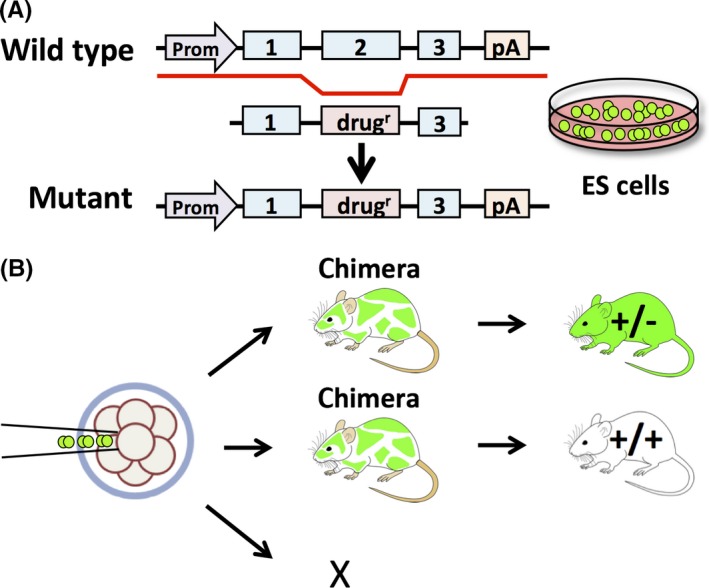
Conventional method of gene disruption by using homologous recombination in embryonic stem (ES) cells. A, Design and construction of a mutant vector transfected into ES cells; those cells carrying the mutation were screened. B, The injection of ES cells carrying the mutation into embryos. The chimeric mice are bred and the heterozygous mice are obtained through germ‐line transmission. The process takes 1‐2 years until completion. drugr, drug resistance gene; pA, polyadenylation signal; Prom, promoter
Throughout the history of reproductive research, there have been many in vitro analyzed genes that were thought to be essential, but were later found to be dispensable after in vivo KO studies.5 For example, the protease enzyme, acrosin, was thought to be necessary for zona pellucida (ZP) penetration, but spermatozoa that lack the active protease were still able to penetrate the ZP and the KO mice were fertile.6 Postacrosomal WW domain‐binding protein (PAWP) was thought to be necessary for the resumption of meiosis and pronuclear development during egg activation,7 as the sperm‐borne oocyte‐activating factor,8, 9 but through conventional KO analysis, PAWP was found to have no effect on the development of embryos nor was any difference found in the calcium oscillations that are necessary for egg activation in mice.10 As indicated in these examples, the KO approach is critical in understanding whether or not genes are essential for male fertility.
There were many genes found that were essential for male fertility at various stages of spermatogenesis and fertilization by using conventional gene‐disruption methods. Please refer to the following reviews for detailed gene disruption discoveries.5, 11, 12
3. GENOME EDITING USING the CRISPR/CAS9 SYSTEM
3.1. Genome editing
Genome editing is a mechanism that cleaves double‐stranded DNA at a targeted region by using a site‐specific nuclease and modifies that region by using genetic repair (Figure 2A). There are two main types of DNA repair: non‐homologous end‐joining (NHEJ) and homologous recombination (HR). Although NHEJ is a pathway that repairs cleaved DNA ends,13 this is an imperfect process, as errors such as indels (insertions and/or deletions) of several bases can occur. In contrast, HR repairs the cleavage site by using a homologous sequence as a template.14 Similar to the chromosomal crossover event during meiosis, HR uses a single‐stranded DNA (ssDNA) or a double‐stranded DNA (dsDNA) that has homology with the target region and can guide DNA repair.
Figure 2.
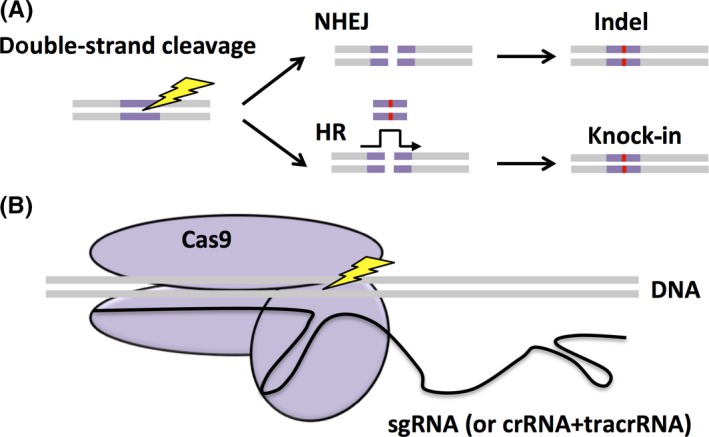
Genome editing after double‐strand cleavage by using the CRISPR/Cas9 complex. A, A double‐strand cleavage event occurs at a targeted region. There are two main DNA repair pathways, non‐homologous end‐joining (NHEJ), resulting in indel mutations, and homologous recombination (HR), resulting in a knock‐in homologous to a co‐transfected template strand. B, The single‐guide RNA (sgRNA) (black) and the Cas9 endonuclease (purple) form the CRISPR/Cas9 complex. It binds to the complementary sequence on the DNA and the Cas9 endonuclease cleaves the DNA. crRNA, CRISPR RNA; tracrRNA, transactivating crRNA
There are two main types of site‐specific nucleases that are used for genome editing: (i) artificial nucleases, such as the zinc finger nuclease (ZFN) and the transcription activator‐like effector nuclease (TALEN); and (ii) RNA‐inducible nucleases, such as the clustered, regularly interspaced, short palindromic repeats (CRISPR)/CRISPR‐associated 9 (Cas9) system.15 The ZFNs and TALENs are composed of a DNA‐binding domain that recognizes the target sequence and the FokI nuclease.16, 17 When the complex binds to the target site, the FokI dimer cleaves the genomic DNA. In contrast, the CRISPR/Cas9 system recognizes the target sequence from the RNA and cleaves the genomic DNA with the Cas9 nuclease.18 The ZFN and TALEN systems require complex vector constructions to create fusion proteins for each target sequence. However, the CRISPR/Cas9 system can target a new sequence solely by changing the RNA sequence. In addition to being easy to use and cost‐effective, the CRISPR/Cas9 system has an efficiency equal to or greater than the TALENs, especially in mammalian cells.19 Furthermore, it can be used to manipulate a wide variety of animals and plants.20
3.2. CRISPR/Cas9 basics
The CRISPR/Cas system was found to be important for adaptive immunity in bacteria. They were first discovered in 198721 but were not functionally identified until 20 years later.22 There are three types of CRISPR systems, but CRISPR type II is most commonly used for genome editing (Figure 2B).23 In this type, the DNA of an infecting bacteriophage is cleaved into small pieces and then integrated into the CRISPR locus. Then, when this sequence is transcribed to small CRISPR RNA (crRNA), it forms a complex with the endonuclease, Cas9, assisted by transactivating crRNA (tracrRNA). This complex then targets non‐self DNA by using the crRNA to locate, bind to, and cleave the target site. As more research on this system was conducted, it was found that the original bacteriophage DNA contained a “protospacer‐adjacent motif” (PAM) sequence, characterized by a NGG sequence—any nucleotide (N) and two guanines (GG)—after about 20 bp of non‐self DNA,24 and the cleavage site was almost always 3 bp before the PAM sequence. After the Cas9 complex cleaves both DNA strands, the double‐stranded breaks are repaired via a mechanism that is mediated by the NHEJ pathway. However, often the repair is imperfect, leading to indels. This disruption can cause a frameshift mutation and the subsequent mistranslation of the protein. Researchers then began to construct different methods to use this unique system for gene modification in model animals, including mice.
4. GENERATION OF GENE‐MODIFIED MICE BY USING CRISPR/CAS9
There are many ways that researchers are producing genetically modified mice by using the CRISPR/Cas9 system.
4.1. Injection into zygotes
One method of CRISPR/Cas9 introduction involves co‐injecting a single‐guide RNA (sgRNA, a chimera of crRNA and tracrRNA) and the messenger (m)RNA encoding the Cas9 endonuclease into the cytoplasm of zygotes (cytoplasmic injection method [eg RNA]) (Figure 3A).25 A plasmid‐based delivery system also was developed, in which a plasmid vector (pX330 [addgene No. 42230]) expressing sgRNA and Cas9 are injected into the pronucleus of the fertilized eggs (pronuclear injection method [eg DNA]) (Figure 3B).26 DNA has a lower decomposition rate and is easier to handle than RNA.27 In addition, there is little difference between the mutation rates. The injected DNA can be integrated into the genome, but this risk can be decreased with the use of a circular plasmid, such as pX330.
Figure 3.
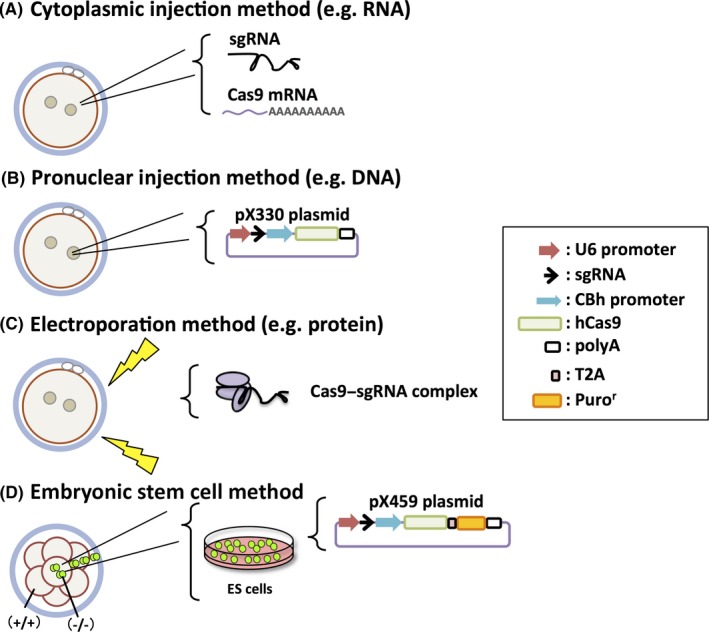
Four methods of the CRISPR/Cas9 system to generate gene‐modified mice. A, Cytoplasmic injection method (eg RNA): co‐injection of a single‐guide RNA (sgRNA) and the Cas9 messenger (m)RNA into the cytoplasm of zygotes. B, Pronuclear injection method (eg, DNA): plasmid‐based delivery system (a plasmid expressing sgRNA and Cas9 is injected into the pronucleus of the fertilized eggs). C, Electroporation method (eg protein): a pulse of high‐voltage electricity opens the oocyte's membrane pore to incorporate the Cas9 protein–RNA complex. D, Embryonic stem (ES) cell method: a modified plasmid with a puromycin resistance cassette (Puror) is transfected into ES cells and drug‐selected. The mutant ES cells are injected into eight‐cell‐stage embryos or blastocysts. CBh promoter, chicken β‐actin hybrid promoter; hCas9, humanized Cas9; polyA, polyadenylation signal; T2A, Thosea asigna virus 2A peptide; U6 promoter, commonly used for driving small RNA expression
The Cas9 protein cleaves the target sequence after matching it with the sgRNA, resulting in founder mice with mutations that are potentially in both alleles if successful. However, it is also possible that F0 mice exhibit mosaicism of several mutations due to the CRISPR/Cas9 complex functioning even after the cleavage stage. It was thought that mosaicism is obtained solely by injecting DNA because the transcription and translation of Cas9 from the plasmid takes much time, but injecting RNA also causes mosaicism in F0 mice.28, 29 Therefore, more reliable results can be obtained when the F0 mice are mated and the phenotypes in the next generations are analyzed.
In addition to injecting DNA or RNA, it is possible to inject the Cas9 protein and sgRNA (or crRNA and tracrRNA) complex into the cytoplasm or pronucleus of zygotes to obtain KO mice.30, 31, 32 As the protein–RNA complex can cause immediate DNA cleavage after injection, it is thought that this method leads to high cleavage efficiency and a lower rate of mosaicism.
The CRISPR/Cas9 system also can assist with other manipulation techniques. Point mutations, tag insertions, such as a FLAG‐tag, and knock‐ins (KIs) can be performed via HR by introducing the CRISPR/Cas9 complex with a ssDNA or dsDNA that is homologous to the target sequence into a fertilized egg. In these cases, however, the level of HR efficiency is lower than that of NHEJ, so it is necessary to generate many mice.33
4.2. Electroporation
The KO mice can be created efficiently via microinjecting the CRISPR/Cas9 complex into the cytoplasm or pronucleus of fertilized eggs, but microinjection is a difficult technical procedure that needs much skill to efficiently perform. Recently, it has become possible to incorporate the Cas9 mRNA and sgRNA34, 35 or protein–RNA complex36 through electroporation, a physical transfection method that uses a pulse of high‐voltage electricity to briefly open the cell membrane pores (electroporation method [eg. protein]) (Figure 3C). As electroporation makes it possible to treat many zygotes at once in a short period of time and without the need for skilled injection,37 this method might be used more commonly, especially by using a protein–RNA complex that causes immediate DNA cleavage after introduction.
4.3. Embryonic stem cell method
In order to increase the efficiency of KO and KI mice production, a combination of the CRISPR/Cas9 system and ES cell gene editing method can be used (Figure 3D).33 When the pX330 plasmid and a plasmid carrying a drug resistance gene cassette are simultaneously transfected into ES cells and subjugated to short‐term drug selection, the Cas9 protein cleaves most of the cells. To make the process simple and easy, a modified pX330 plasmid containing a puromycin resistance cassette was created (pX459: Addgene, No. 62988). After this plasmid was transfected, 90% of the ES cells had an indel mutation in at least one allele and 80% of them had indel mutations in both alleles.33 Furthermore, large (mega base) deletions are possible if different sgRNAs are simultaneously introduced. If the entire gene is excised, instead of the introduction of indels to form premature stop codons, then it is sure that the protein is not translated.38 Not only that, it is even possible to create KI mice if a dsDNA template that has a homology region of ~1 kbp is cotransfected as a circular plasmid.33 Although the efficiencies of these types of manipulation depends on the target, ES cells are more suitable for complicated genome editing, rather than zygotes, because mutant ES cell clones of interest can be obtained at a higher rate. Usually, one clone with the intended mutation can be obtained from several checked clones.
Wild‐type embryos are injected with the selected mutant ES cells to generate chimeric mice. The chimeric mice can be mated to obtain F1 mice that have the desired mutation. In the zygote injection method, many mice without the desired mutation are born. There is much effort required to genotype and store these mice for little return. However, because chimeric mice are created by using ES cell clones that already have been screened for the desired mutation, the amount of mice that are bred and the associated costs can be reduced while guaranteeing mutant mice production. Not only that, by using green fluorescent protein (GFP)‐labeled ES cells39 and selecting clones that have mutations in both alleles, a phenotypic analysis of the F0 generation can be performed by observing the green cells in the chimeric mouse directly or by sorting the GFP‐positive cells.33
4.4. Benefits
The simplicity of this gene manipulation tool gives rise to many advantages. It is extremely fast to make transgenic mutant animals by using this system. Ideally, homozygous mutants can be obtained in <2 months.40 In comparison, the conventional ES cell method can take 1‐2 years to obtain the mutant mouse lines. The CRISPR/Cas9 system is also extremely sustainable and efficient, with relatively low costs. With these benefits, the CRISPR/Cas9 system revolutionizes research about not only somatic cells, but also germ cells.
4.5. Off‐target effects
With all emerging technologies, there are always complications and the CRISPR/Cas9 system is not immune to these. Off‐target cleavage is a risk that is posed by this system. Although sgRNAs are designed to be specific to the target gene, there is still a possibility that the complex could bind to and cut an off‐target area that is similar to the original on‐target sequence. Most sgRNAs carry ~20 bp before the PAM sequence. Current research shows that anything with >13 bp matches before the PAM sequence has a high risk of off‐target cuts.41 However, further research shows that, out of 382 targeted sites, only three (0.8%) had off‐target cleavage when KO mice were produced using zygote injections.40 In contrast to cancer cells that might have a lower ability to repair DNA, zygotes, ES cells, and induced pluripotent stem cells seem to have a low risk of off‐target cuts.42, 43, 44 However, there is still the possibility of off‐target cuts when producing KO mice. One way to confirm low off‐target sequences is through software, such as CRISPRdirect.45
Another method to reduce off‐target effects is by introducing a nickase Cas9 to the system.46 As the CRISPR/Cas9 system can target only 20 bases, the specificity of this recognition sequence is low. In comparison, ZFN or TALEN recognize a target sequence of ~40 bases. Therefore, using a nickase Cas9 and using two sgRNAs might increase the specificity of the recognition sequence. Furthermore, mutated Cas9s that have more specificity have been developed recently. To counter off‐target cleavage, researchers made a Cas9 that “enhances specificities,” which was created after analyzing the crystal structure of Cas9.47, 48 These updates to Cas9 could lead to more precise gene editing.
5. CRISPR/CAS9 AND REPRODUCTION
5.1. Essential genes for male fertility that have been found by the CRISPR/Cas9 system
The introduction of the CRISPR/Cas9 system into the genome editing world led to the rapid creation of KO mice. For example, by utilizing the CRISPR/Cas9 system in addition to other KO methods, >80 KO mice of genes that are predominantly expressed in the testis and conserved in both mice and humans were generated. Surprisingly, 54 of those KO male mouse lines were fertile, meaning that the importance of these genes cannot be based solely on their tissue expression profile.49 In contrast, there are several genes that were found to be indispensable for male fertility by using the CRISPR/Cas9 system, such as Ppp3r2, Ccdc63, and Cabyr.
Ppp3r2 is a component of calcineurin, the calcium‐dependent phosphatase, and is expressed predominantly in the testis.50 It was knocked out by using a pronuclear injection of the pX330 plasmid, resulting in a 372 bp‐deleted founder mouse.51 These KO male mice were infertile, and after close analysis, it was found that the spermatozoa had inflexible midpieces, resulting in the loss of ZP‐penetrating ability. This has a profound implication for contraception, as calcineurin inhibitors were found to temporarily cause sperm midpiece rigidity and male infertility.
Another example is Ccdc63, a homolog of Chlamydomonas DC2 that is part of the outer dynein arm‐docking complex in the flagellar axoneme.52 It also was knocked out by using a pronuclear injection of the pX330 plasmid, resulting in a 1 bp insertion.53 The KO male mice were infertile because their spermatozoa exhibited truncated flagella.
Within the principal piece of sperm flagella, which largely control proper flagellar beating, is the fibrous sheath. Localized in the fibrous sheath is a protein called Ca2+‐binding tyrosine‐phosphorylation‐regulated protein (CABYR) that is phosphorylated during capacitation.54 In vitro research showed that CABYR might play a role in capacitation.54, 55 However, CRISPR‐Cas9‐generated Cabyr KO male mice were severely subfertile due to significantly reduced sperm motility and electron microscopy of the KO spermatozoa showed disorganized fibrous sheaths.56
There are ~1000 genes that are predominantly expressed in the mouse testis49 and many of these genes still need to be analyzed with the KO approach. The genes that are essential for male fertility will be revealed more rapidly with the CRISPR/Cas9 system.
5.2. Point mutation
The CRISPR/Cas9 system can be used for not only generating KO mice rapidly but also for more complicated gene manipulations. Point mutations are useful genetic modifications that cause a single nucleotide base insertion, deletion, or substitution (Figure 4A). Through the use of the CRISPR/Cas9 system, point mutations can be made to understand the specific influence that one amino acid has on a protein. For example, DNAIC1 is a component of the dynein outer arm in the cilia and sperm flagella.57 Serine 124 and 127 in DNAIC1 are phosphorylated during sperm maturation58, 59, 60; thus, these serine residues were substituted with alanines by using the CRISPR/Cas9 system in order to understand their reproductive role.53 The generated mutant male mice were fertile, indicating that the phosphorylation of these two serine residues is not essential for male fertility.
Figure 4.
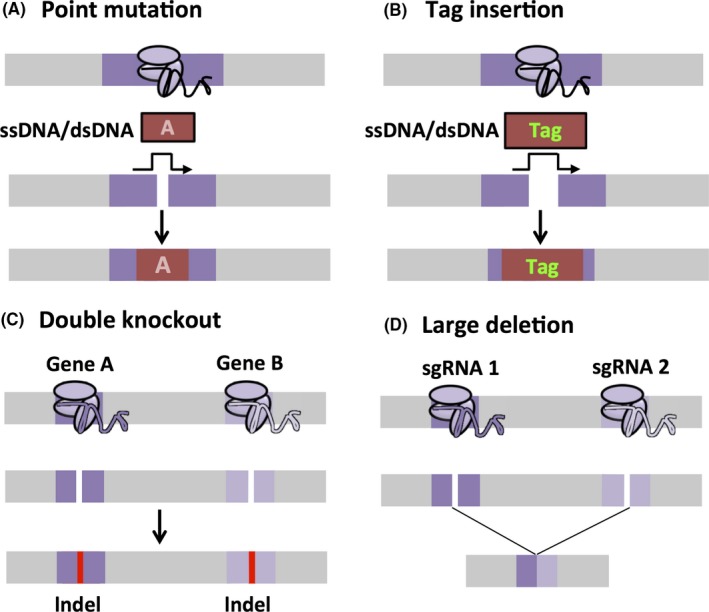
Complicated gene manipulation techniques. A, Point mutation: Co‐injecting a single‐stranded DNA (ssDNA) or double‐stranded DNA (dsDNA) can cause a single nucleotide base insertion, deletion, or substitution through homologous recombination (HR). B, Tag insertion: tags, such as a FLAG‐tag, can be inserted similarly to point mutations by using ssDNA or dsDNA and HR. Reporter genes, such as enhanced green fluorescent protein (EGFP), can also be knocked in. C, Double knockout: by using a combination of different sgRNAs, Cas9 can cleave multiple target genes to generate complex KO mice. D, Large deletion: the CRISPR/Cas9 system can mediate large genomic deletions by transfecting two or more sgRNAs
Another example of inserting a point mutation into the genome by using the CRISPR/Cas9 system is in the case of IZUMO1 and understanding its intracellular domain. IZUMO1 is an immunoglobulin superfamily protein, is found on the acrosomal membrane of the acrosome‐intact spermatozoa, and is essential for sperm–egg fusion.61 During the acrosome reaction, IZUMO1 diffuses from the acrosomal membrane to the sperm surface62 and the intracellular domain of IZUMO1 could be involved in this localization change.59 In order to understand the function of the intracellular domain, mice that have a point mutation in this region were created by using the CRISPR/Cas9 system.63 In this point mutation, a serine was replaced with a stop codon and the intracellular region of 55 amino acids was shortened to three amino acids. Although the amount of IZUMO1 that was present in the spermatozoa decreased due to the point mutation, the localization change of IZUMO1 that is associated with the acrosome reaction was normal. The reproductive ability of these mutant mice was also normal and it was concluded that the intracellular region of IZUMO1 is not essential for localization change and fertility.
Not only that, but many human diseases are caused by small mutations, such as single‐point mutations. With the CRISPR/Cas9 system, human infertility can be mimicked and point mutations can be made in order to generate disease‐model mice.
5.3. Tag insertion
In order to study protein localization and functions, a set of tags has been created. Using the CRISPR/Cas9 system, these tags, such as a FLAG‐tag, can be inserted into the endogenous locus more easily than by using the conventional method (Figure 4B). T‐Complex‐associated‐testis‐expressed 1 (TCTE1) is an evolutionarily conserved protein that localizes to the nexin–dynein regulatory complex of the flagellar axoneme. Thus, Tcte1 KO mice were generated by using the conventional ES method and they were infertile because of impaired sperm motility.64 As several attempts to generate the TCTE1 antibody failed, the CRISPR/Cas9 system was used to insert a FLAG‐tag into the C‐terminus in order to identify the localization of TCTE1.64 The obtained homozygous KI male mice were fertile and exhibited normal sperm motility, suggesting that the FLAG‐tag insertion did not impair TCTE1 function. Using both Western blot and immunofluorescence analyses with the FLAG antibody, TCTE1 was shown to be localized in the sperm flagella. The FLAG‐tag can be used for immunoprecipitation analysis as well, but unfortunately, TCTE1 was not extracted with a mild lysis buffer that is compatible with immunoprecipitation.
5.4. Double knockout
Compensation is a big issue within genome research. Knocking out one gene might not lead to a phenotype due to compensation from a homolog, which could be the reason why 54 KO mouse lines were fertile, as mentioned above.49 Producing double‐knockout (DKO) mice by using the conventional method is difficult to do in one step because there is a very low possibility of obtaining the desired recombination events in both regions in one attempt. The DKO creation would require multiple cross‐breedings with no guarantee of recombination if the genes are linked on the same chromosome. However, by using the CRISPR/Cas9 system, it was possible to target multiple genes solely by injecting a combination of sgRNAs and Cas9 (Figure 4C). For example, if one wanted to mutate two seemingly separate genes, then one would only have to create two sgRNAs that are specific to those two genes. Then, after injection, or electroporation, or transfection, Cas9 would snip those sites, rendering both genes non‐functional.
An example of this mechanism using the CRISPR/Cas9 system can be seen in research that was done on the Y chromosome genes, Zfy1 and Zfy2, in which the single KO mice did not have a significant phenotype but the DKO mice were infertile because of abnormal sperm morphology and motility.65 It should be noted, however, that if two sgRNAs are located closely together in the same chromosome, large deletions could occur (Figure 4D).
6. CONCLUSION
Before the development of the CRISPR/Cas9 system, in vivo gene research was extremely tedious. It took years to make the same KO animals that the CRISPR/Cas9 system now produces in merely months. This technology also has allowed researchers to perform complicated gene modifications, such as point mutations, tag insertions, DKOs, and large deletions. Researchers are trying to fully understand the details of this technology in order to increase its efficiency. For example, crystal structure analyses of the Cas9 protein can lead researchers to understand how it cuts the target sequence and potentially mutate it to cut the target more specifically and even cut other sites with different PAM sequences. Quickly, the hidden mysteries of male fertility will be unraveled with the help of the CRISPR/Cas9 system.
DISCLOSURES
Conflict of interest: The authors declare no conflict of interest. Human and Animal Rights: This article does not contain any study with human or animal participants that have been performed by any of the authors.
ACKNOWLEDGEMENTS
F. A. was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Scholarship. This work also was supported in part by MEXT KAKENHI grant no. JP25112007 (M. I.).
Abbasi F, Miyata H, Ikawa M. Revolutionizing male fertility factor research in mice by using the genome editing tool, CRISPR/Cas9. Reprod Med Biol. 2018;17:3‐10. https://doi.org/10.1002/rmb2.12067
REFERENCES
- 1. Castaneda J, Matzuk MM. Toward a rapid and reversible male pill. Science. 2015;350:385‐386. [DOI] [PubMed] [Google Scholar]
- 2. Gunter C, Dhand R. Human biology by proxy. Nature. 2002;420:509. [Google Scholar]
- 3. Hall B, Limaye A, Kulkarni AB. Overview: generation of gene knockout mice. Curr Protoc Cell Biol. 2009;19:19.12.1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Limaye A, Hall B, Kulkarni AB. Manipulation of mouse embryonic stem cells for knockout mouse production. Curr Protoc Cell Biol. 2009;19:19.13.1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okabe M. Mechanism of fertilization: a modern view. Exp Anim. 2014;63:357‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baba T, Azuma S, Kashiwabara S, Toyoda Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem. 1994;269:31845‐31849. [PubMed] [Google Scholar]
- 7. Wu AT, Sutovsky P, Manandhar G, et al. PAWP, a sperm‐specific WW domain‐binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem. 2004;282:12164‐12175. [DOI] [PubMed] [Google Scholar]
- 8. Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development. 1990;110:1295‐1302. [DOI] [PubMed] [Google Scholar]
- 9. Aarabi M, Balakier H, Bashar S, et al. Sperm‐derived WW domain‐binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J. 2014;28:4434‐4440. [DOI] [PubMed] [Google Scholar]
- 10. Satouh Y, Nozawa K, Ikawa M. Sperm postacrosomal WW domain‐binding protein is not required for mouse egg activation. Biol Reprod. 2015;93:94. [DOI] [PubMed] [Google Scholar]
- 11. Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest. 2010;120:984‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang HH, Pannunzio NR, Adachi N, Lieber MR. Non‐homologous DNA end joining and alternative pathways to double‐strand break repair. Nat Rev Mol Cell Biol. 2017;18:495‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Heyer W. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas‐based methods for genome engineering. Trends Biotechnol. 2013;31:397‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636‐646. [DOI] [PubMed] [Google Scholar]
- 17. Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2012;14:49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual‐RNA‐guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR‐Cas9. Science. 2014;346:1258096. [DOI] [PubMed] [Google Scholar]
- 20. Sander JD, Joung JK. CRISPR‐Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169:5429‐5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barrangou R, Fremaux C, Deveau H, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709‐1712. [DOI] [PubMed] [Google Scholar]
- 23. Makarova KS, Haft DH, Barrangou R, et al. Evolution and classification of the CRISPR–Cas systems. Nat Rev Microbiol. 2011;9:467‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA‐guided endonuclease Cas9. Nature. 2014;507:62‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Yang H, Shivalila CS, et al. One‐step generation of mice carrying mutations in multiple genes by CRISPR/Cas‐mediated genome engineering. Cell. 2013;153:901‐918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep. 2013;3:3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fujihara Y, Ikawa M. CRISPR/Cas9‐based genome editing in mice by single plasmid injection. Methods Enzymol. 2014;546:319‐336. [DOI] [PubMed] [Google Scholar]
- 28. Yen S, Zhang M, Deng JM, et al. Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev Biol. 2014;393:3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oliver D, Yuan S, Mcswiggin H, Yan W. Pervasive genotypic mosaicism in founder mice derived from genome editing through pronuclear injection. PLoS ONE. 2015;10:e0129457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sung YH, Kim JM, Kim HT, et al. Highly efficient gene knockout in mice and zebrafish with RNA‐guided endonucleases. Genome Res. 2014;24:125‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aida T, Chiyo K, Usami T, et al. Cloning‐free CRISPR/Cas system facilitates functional cassette knock‐in in mice. Genome Biol. 2015;16:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Shao Y, Guan Y, et al. Large genomic fragment deletion and functional gene cassette knock‐in via Cas9 protein mediated genome editing in one‐cell rodent embryos. Sci Rep. 2015;5:17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oji A, Noda T, Fujihara Y, et al. CRISPR/Cas9 mediated genome editing in ES cells and its application for chimeric analysis in mice. Sci Rep. 2016;6:31666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qin W, Dion SL, Kutny PM, et al. Efficient CRISPR/Cas9‐mediated genome editing in mice by zygote electroporation of nuclease. Genetics. 2015;200:423‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hashimoto M, Takemoto T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9‐based genome editing. Sci Rep. 2015;5:11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hashimoto M, Yamashita Y, Takemoto T. Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non‐mosaic mutants in the mouse. Dev Biol. 2016;418:1‐9. [DOI] [PubMed] [Google Scholar]
- 37. Kaneko T. Genome editing in mouse and rat by electroporation. Methods Mol Biol. 2017;1630:81‐89. [DOI] [PubMed] [Google Scholar]
- 38. Makino S, Fukumura R, Gondo Y. Illegitimate translation causes unexpected gene expression from on‐target out‐of‐frame alleles created by CRISPR‐Cas9. Sci Rep. 2016;6:39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fujihara Y, Kaseda K, Inoue N, Ikawa M, Okabe M. Production of mouse pups from germline transmission‐failed knockout chimeras. Transgenic Res. 2012;22:195‐200. [DOI] [PubMed] [Google Scholar]
- 40. Mashiko D, Young SA, Muto M, et al. Feasibility for a large scale mouse mutagenesis by injecting CRISPR/Cas plasmid into zygotes. Dev Growth Differ. 2013;56:122‐129. [DOI] [PubMed] [Google Scholar]
- 41. Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fu Y, Foden JA, Khayter C, et al. High‐frequency off‐target mutagenesis induced by CRISPR‐Cas nucleases in human cells. Nat Biotechnol. 2013;31:822‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Cao J, Xiong M, et al. Engineering human stem cell lines with inducible gene knockout using CRISPR/Cas9. Cell Stem Cell. 2015;17:233‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park C, Kim D, Son J, et al. Functional correction of large factor VIII gene chromosomal inversions in hemophilia A patient‐derived iPSCs using CRISPR‐Cas9. Cell Stem Cell. 2015;17:213‐220. [DOI] [PubMed] [Google Scholar]
- 45. Naito Y, Hino K, Bono H, Ui‐Tei K. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off‐target sites. Bioinformatics. 2014;31:1120‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ran F, Hsu P, Lin C, et al. Double nicking by RNA‐guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kleinstiver BP, Prew MS, Tsai SQ, et al. Engineered CRISPR‐Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2015;351:84‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyata H, Castaneda JM, Fujihara Y, et al. Genome engineering uncovers 54 evolutionarily conserved and testis‐enriched genes that are not required for male fertility in mice. Proc Natl Acad Sci USA. 2016;113:7704‐7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu L, Zhang J, Yuan J, et al. Characterization of a human regulatory subunit of protein phosphatase 3 gene (PPP3RL) expressed specifically in testis. Mol Biol Rep. 2005;32:41‐45. [DOI] [PubMed] [Google Scholar]
- 51. Miyata H, Satouh Y, Mashiko D, et al. Sperm calcineurin inhibition prevents mouse fertility with implications for male contraceptive. Science. 2015;350:442‐445. [DOI] [PubMed] [Google Scholar]
- 52. Takada S. The outer dynein arm‐docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol Biol Cell. 2002;13:1015‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Young S, Miyata H, Satouh Y, et al. CRISPR/Cas9‐mediated rapid generation of multiple mouse lines identified Ccdc63 as essential for spermiogenesis. Int J Mol Sci. 2015;16:24732‐24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Naaby‐Hansen S, Mandal A, Wolkowicz MJ, et al. CABYR, a novel calcium‐binding tyrosine phosphorylation‐regulated fibrous sheath protein involved in capacitation. Dev Biol. 2002;242:236‐254. [DOI] [PubMed] [Google Scholar]
- 55. Li YF, He W, Kim YH, et al. CABYR isoforms expressed in late steps of spermiogenesis bind with AKAPs and ropporin in mouse sperm fibrous sheath. Reprod Biol Endocrinol. 2010;8:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Young SA, Miyata H, Satouh Y, Aitken RJ, Baker MA, Ikawa M. CABYR is essential for fibrous sheath integrity and progressive motility in mouse spermatozoa. J Cell Sci. 2016;129:4379‐4387. [DOI] [PubMed] [Google Scholar]
- 57. Ostrowski LE, Yin W, Rogers TD, et al. Conditional deletion of DNAIC1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010;43:55‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Platt MD, Salicioni AM, Hunt DF, Visconti PE. Use of differential isotopic labeling and mass spectrometry to analyze capacitation‐associated changes in the phosphorylation status of mouse sperm proteins. J Proteome Res. 2009;8:1431‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baker MA, Hetherington L, Weinberg A, et al. Analysis of phosphopeptide changes as spermatozoa acquire functional competence in the epididymis demonstrates changes in the post‐translational modification of Izumo1. J Proteome Res. 2012;11:5252‐5264. [DOI] [PubMed] [Google Scholar]
- 60. Baker MA, Smith ND, Hetherington L, et al. Label‐free quantitation of phosphopeptide changes during rat sperm capacitation. J Proteome Res. 2010;9:718‐729. [DOI] [PubMed] [Google Scholar]
- 61. Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234‐238. [DOI] [PubMed] [Google Scholar]
- 62. Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm–egg fusion and dynamic localization of IZUMO1. J Cell Sci. 2012;125:4985‐4990. [DOI] [PubMed] [Google Scholar]
- 63. Young SA, Miyata H, Satouh Y, et al. CRISPR/Cas9‐mediated mutation revealed cytoplasmic tail is dispensable for IZUMO1 function and male fertility. Reproduction. 2016;152:665‐672. [DOI] [PubMed] [Google Scholar]
- 64. Castaneda JM, Hua R, Miyata H, et al. TCTE1 is a conserved component of the dynein regulatory complex and is required for motility and metabolism in mouse spermatozoa. Proc Natl Acad Sci USA. 2017;114:E5370‐E5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nakasuji T, Ogonuki N, Chiba T, et al. Complementary critical functions of Zfy1 and Zfy2 in mouse spermatogenesis and reproduction. PLoS Genet. 2017;13:e1006578. [DOI] [PMC free article] [PubMed] [Google Scholar]


