Abstract
Maximizing both efficiency and equity are core considerations for population health. These considerations can result in tension in population health science as we seek to improve overall population health while achieving equitable health distributions within populations. Limited work has explored empirically the consequences of different population health intervention strategies on the burden of disease and on within- and between-group differences in disease. To address this gap, we compared the impact of four simulated interventions using data from the National Health and Nutrition Examination Survey. In particular, we focus on assessing how population and high-risk primary prevention and population and high-risk secondary interventions efforts to reduce smoking behavior influence systolic blood pressure (SBP) and hypertension, and how such strategies influence inequalities in SBP by income. The greatest reductions in SBP mean and standard deviation resulted from the population secondary prevention. High-risk primary and secondary prevention and population secondary prevention programs all yielded substantial reductions in hypertension prevalence. The effect of population primary prevention did little to decrease population SBP mean and standard deviation, as well as hypertension prevalence. Both high-risk strategies had a larger impact in the low-income population, leading to the greatest narrowing the income-related gap in disease. The population prevention strategies had a larger impact in the high-income population. Population health approaches must consider the potential impact on both the whole population and also on those with different levels of risk for disease within a population, including those in under-represented or under-served groups.
Keywords: High-risk prevention, Primary prevention, High blood pressure, Smoking, equity, Efficiency, Simulation
Highlights
-
•
A central goal in population health is to maximize overall population health, while minimizing health inequalities within populations.
-
•
A greater understanding of these potential trade-offs can be gained through simulations and sensitivity analyses.
-
•
This study sought to understand the implications of four different strategies on the population prevalence and distribution of high systolic blood pressure.
-
•
The greatest reduction in SBP mean and standard deviation resulted from the population secondary prevention, while high-risk primary and secondary prevention and population secondary prevention all yielded substantial reductions hypertension.
-
•
Efforts to improve overall population health may disadvantage some groups; whether equity or efficiency is preferable is in part a matter of values.
1. Introduction
One of the central goals of population health science is to achieve equitable health distributions within populations, while seeking to maximize overall population health (Keyes & Galea, 2016). However, the impact of health policies and programs is often not distributed equally throughout a population, and health policies and programs may exacerbate health inequalities (Krieger, 2001). While not always the case (McLaren, McIntyre, & Kirkpatrick, 2010), the tension between equity and efficiency means that when resources are finite, there may be a trade-off between maximizing population health while minimizing population health inequity. Numerous scholars have described approaches to improving population health, explicating the differences between focusing on high-risk populations versus populations as a whole (Lalonde, 1974, Rose, 1985). In epidemiology and public health, such explication has been more visible in the work of Geoffrey Rose, for example, in his seminal book, A strategy for preventive medicine (Rose, 1985).
The high-risk approach proposes to intervene for prevention upon those with the strongest likelihood of developing disease (Lalonde, 1974). There are two different ways that prevention may be achieved. Primary prevention strategies identify high-risk individuals based on known risk factors, and intervene to reduce those exposures. The goal of this strategy is to reduce the number of incident cases of disease, or prevent a proportion of disease from ever occurring. Secondary prevention strategies seek to identify high-risk individuals with the disease and reduce disease morbidity, complications, or to decrease the disease prevalence by attenuating disease symptoms to sub-clinical levels. In the case of secondary prevention, the high-risk individuals often represent the most severe cases of disease, especially if risk factors of concern are strong causes of disease, or those with the disease face the greatest barriers to existing health services.
By contrast, rather than focusing on those defined as high-risk, a population approach is based on implementing strategies across the distribution of risk and disease. As with the high-risk approach, the population approach can be designed for both primary and secondary prevention. A population primary prevention strategy seeks to reduce the exposure to a highly prevalent risk factor for disease. A population secondary prevention strategy seeks to disseminate a global treatment strategy throughout an entire population to identify and/or treat cases to reduce disease morbidity or cure a proportion of those with the disease if possible. An overview and examples of each of the four approaches is presented in Table 1.
Table 1.
Summary of high-risk and population primary and secondary prevention approaches.
| Intervention goal | Who is at risk? | Example | |
|---|---|---|---|
| High-risk approach | |||
| Primary prevention | Prevent the exposure in order to reduce the number of incident cases of disease | Individuals with exposures known to significantly increase the risk of disease | Smoking cessation intervention among normotensive smokers (Hjermann, Holme, Byre, & Leren, 1981) |
| Secondary prevention | Treat disease to reduce morbidity and prevalence | Individuals already with the disease, often the most severe cases | Intensive smoking cessation intervention for patients with evidence of cardio-pulmonary distress (Murray, Connett, Rand, Pan, & Anthonisen, 2002) |
| Population approach | |||
| Primary prevention | Reduce exposure to a highly prevalent risk factor for disease | Risk is prevalent throughout the entire population | Community-wide anti-smoking programs (Egger et al., 1983) |
| Secondary prevention | Identify and treat prevalent cases | Disease is prevalent throughout the population | Clinical smoking cessation interventions among hypertensive patients (Jatoi, Jerrard-Dunne, Feely, & Mahmud, 2007) |
In sum, both high-risk and population intervention strategies can be implemented as primary prevention, which seeks to prevent the incidence of disease, and secondary prevention, which seeks to treat or cure those with disease. The main difference between strategies is who is the focus of the intervention. The high-risk strategy is generally implemented to decrease risk or course of disease among those with the greatest potential burden, while the population strategy seeks to maximize the number of individuals reached by an intervention, with less concern for the differential risk that individuals face in developing disease.
In addition to the potential tradeoff between equity and efficiency due to scarce resources, there are times that the advancement of a population strategy approach may inadvertently worsen health inequalities within a population (Frohlich & Potvin, 2008). Recent theoretical work has been done to develop different high-risk strategies that can be targeted to specific groups depending on the context and goal of the intervention (Benach et al., 2013, Graham, 2004). For example, an intervention may target only those who are the worst-off, or to opt to improve population health through redistribution of health maximizing resources in a population from the most well-off to the least. The goal of these types of approaches is to avoid exacerbating existing inequalities by understanding specific contextual and population concerns.
Building off of prior research outlining population versus high-risk strategies, the purpose of this paper is to assess which approach is optimal for maximizing population health through the use of simulations and sensitivity analyses, while keeping the central focus of all strategies of the tradeoffs between equity and efficiency. While many studies focus on comparisons between population and high-risk interventions, our focus was to compare the impact of four strategies: high-risk primary prevention, high-risk secondary prevention, population primary prevention, and population secondary prevention, simulating versions of each intervention in a U.S. nationally representative sample in order to understand the effects of different strategies on the population prevalence and distribution of disease. In particular, we assessed whether interventions to reduce smoking were associated with lower systolic blood pressure (SBP) and reductions in hypertension prevalence. Hypertension is a highly relevant condition in the US context, as it represents both a disease outcome and is a modifiable risk-factor for many other highly prevalent diseases such as cardiovascular disease (Kannel, 1996) and stroke (Collins et al., 1990). Further, hypertension is a largely symptomless condition, which has implications for intervention strategies. Individuals with chronic asymptomatic conditions are less likely to present in clinical settings and are also less likely to adhere to treatment regimens, compared to those with more perceptible symptoms (Miller, 1997). Therefore, it is critical to understand the impact of high-risk and population prevention strategies on hypertension, as they inform critical public health thinking needed to reverse the incidence and consequences of hypertension in a population. We also compared changes between two sub-samples of the population, those in low- vs. high-income households (less than $35,000 vs. more than $100,000, respectively), groups with well-known differences in hypertension prevalence (Diez-Roux, Link, & Northridge, 2000), to examine the extent to which visible behavioral risk factors were attributable to hypertension inequalities.
2. Methods
2.1. Analytic approach
Using data from the National Health and Nutrition Examination Survey (NHANES) 2011–2012, we modeled the sample distribution of respondents’ SBP. Our analytic sample was limited to respondents for which SBP data were available. SBP was chosen because it is easily measured and is a fairly normally distributed continuous variable for which there is a generally accepted threshold for disease (hypertension) in the US population. Although hypertension is typically defined as SBP > 139 mmHg (Chobanian et al., 2003), we included those with SBP greater than 130 mmHg as hypertensive, in order to avoid unstable results due to small sample sizes. The impact of each intervention was generally similar using the SBP > 139 mmHg threshold for hypertension. We limited the current consideration to SBP rather than both SBP and diastolic blood pressure for purposes of simplicity.
The NHANES sample comprised 7053 individuals who had reported at least one measure of SBP. The average SBP value was recorded among those with multiple measurements. The overall sample mean SBP was 118.8 mmHg and the standard deviation (SD) was 18.4. However, to reduce the influence of extremely high or low SBP measures, we excluded individuals who reported an SBP greater than 2 standard deviations outside of the full sample distribution. Similarly, to avoid the potential selection bias from very young or very old study participants, we limited our analytic sample to those age 25–65. The final analytic sample comprised 3393 individuals. The mean SBP in this sample was 119.6 and the SD was 13.7. The full sample distribution is shown in Supplementary Fig. 1.
2.2. Risk factors
Tobacco smoking is positively associated with increased blood pressure and incident hypertension (Sleight, 1993). Current smoking was defined as self-reported use of tobacco every day or some days over the past 30 days. Smoking was chosen as illustrative because it represents an important health risk and modifiable exposure in the population (Lackland, 2005). That is, there are numerous interventions through which individuals can quit smoking. Both risk factor prevalence and how an intervention is defined are important considerations in guiding intervention strategies (Hernán & Taubman, 2008).
In the analytic sample, 23% (n=765) of respondents reported current smoking. The mean SBP was 120.4 mmHg in smokers and 119.3 mmHg in non-smokers. The prevalence of hypertension was 20.1% to 22.4% in non-smokers vs. smokers. The standard deviation (13.7) was not different between groups.
2.3. Intervention strategies
We simulated the effects of each intervention strategies, based on the hypothetical manipulation of the distributions of smoking and SBP. Of particular interest in this simulation was those individuals in the upper bounds of SBP distribution, indicative of hypertension cases. The interventions were simulated according to the following parameters, which are also summarized in Table 2.
Table 2.
Summary of high-risk and population primary and secondary prevention approaches in the current simulation.
| Primary prevention | Secondary prevention | |
|---|---|---|
| High-risk | Direct efforts to get smokers to quit: | Treat hypertension among smokers: |
| Replace smoker SBP with mean non-smoker SBP | Reduce SBP of hypertension cases by 5%, 10%, or 15% | |
| Population | Passive efforts to get smokers to quit: | Treat hypertension regardless of underlying risk: |
| Impute mean non-smoker SBP for 33% or 50% of smokers | Reduce SBP by 2.5%, 5%, or 10% |
2.3.1. High-risk primary prevention
In the primary prevention strategy, we defined those individuals who reported being current smokers as high-risk. To examine the effects of the high-risk primary prevention strategy, we simulated the change in SBP distribution as a result of hypothetically removing the exposure from the high-risk population (e.g. getting all current smokers to quit smoking). We did this by reducing the SBP of each individual in the high-risk population by the mean difference between the population who reported smoking (SBP=120.4 mmHg) and the population who reported not smoking (SBP=119.3 mmHg).
2.3.2. High-risk secondary prevention
Because secondary prevention usually implies clinical treatment, here we defined the high-risk population as smokers with a SBP of 130 mmHg or higher. To simulate the effect of treating high-risk individuals using clinical approaches, we measured changes in SBP distribution as a result of treatment to reduce the SBP of high-risk hypertensive individuals by 5%, 10%, and 15% to simulate an intervention that focuses on targeted decreases based on an individual's baseline SBP and smoking risk. Three levels were chosen both to examine the sensitivity of intervention intensiveness by simulating a range of SBP reduction goals that might be feasible policy recommendations, as well as to incorporate some of the differential uptake of the intervention within individuals who received the intervention.
2.3.3. Population primary prevention
In this approach, we simulated changes in the SBP distribution assuming a hypothetical reduction of smoking throughout the entire sample. We modeled a reduction in the prevalence of smoking by 33% and 50%. For primary prevention of smoking, we randomly selected 33% of the population who were current smokers and imputed their adjusted SBP as the mean SBP of the non-smoking population. We repeated these steps with 50% of the smoking population. Under this strategy, all reductions were made randomly in the sample, independent of baseline SBP that individuals reported.
2.3.4. Population secondary prevention
We simulated the changes in the distribution of SBP if hypertensive individuals in the general population were targeted, regardless of risk factors, to achieve reductions of 2.5%, 5%, and 10%. As in the high-risk treatment approaches, we used different levels of reduction to simulate varying levels of intensiveness of the intervention targets, and to model differential uptake of the intervention within individuals who received the intervention.
For each intervention, we calculated the mean, standard deviation, the prevalence and absolute number of cases of hypertension in low- and high-income groups in order to investigate differing effects of each strategy. Comparing changes in the sample mean, given smoking status, highlighted the overall population effect of each intervention. By calculating the percent change in prevalence of hypertension, we were able to assess the relative clinical effectiveness of one intervention strategy versus others. We also calculated the number of cases that fell below the clinical definition of hypertension after each intervention as an absolute measure of the change in disease prevalence in the population. The standard deviation, combined with the changes in hypertension prevalence demonstrated the effect of each intervention in decreasing the population SBP inequalities.
As a way to visually examine the absolute effects of each intervention, and the effects relative to other interventions, we standardized the SBP values for each individual to a normal distribution using the resulting sample mean and standard deviation after each intervention. We plotted the SBP distributions comparing the effects of high-risk primary and secondary prevention strategies in Fig. 1, and population primary and secondary prevention strategies in Fig. 2.
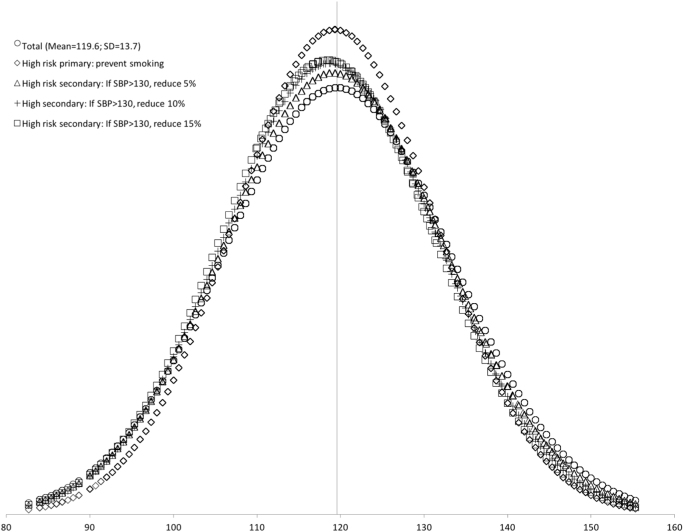
Fig. 1.
SBP distribution in the general population, the high-risk population, and subsequent changes resulting from high-risk primary and secondary prevention strategies. Note: High-risk intervention A targeted only those at high-risk, defined as those individuals who reported current smoking (n=765). High-risk secondary intervention targeted only those at high-risk, defined as those individuals whose SBP>130 (n=719).
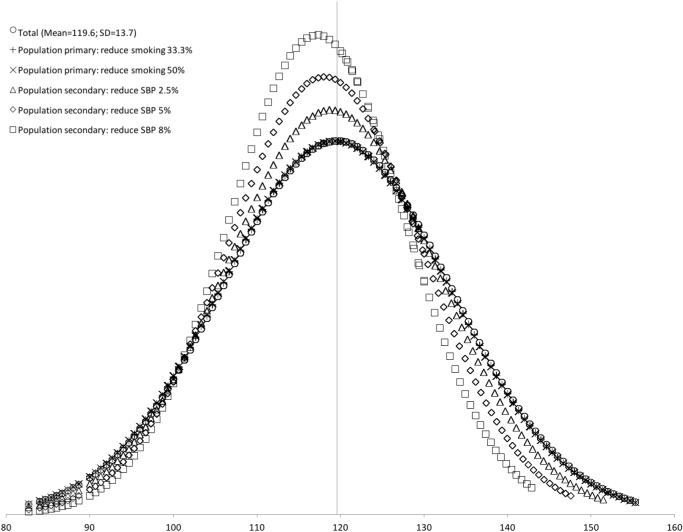
Fig. 2.
SBP distribution in the general population, showing changes as a result of population primary and secondary prevention strategies1. 1Population intervention strategies targeted all individuals in the population, regardless of individual risk factors.
3. Results
3.1. High-risk prevention strategies
First, we examined the effects of the high-risk prevention strategy. The mean SBP among the high-risk sample was 120.4 mmHg, nearly one point higher than that of the total sample mean (119.6). The effect of primary prevention decreased the SBP mean and standard deviation to 119.3 ± 12.1 mmHg and reduced the hypertension prevalence by 23.6%. As a result of the high-risk secondary prevention, the population mean SBP decreased by 0.4 mmHg for each additional 5% reduction in SBP in the high-risk population; the most intensive (15%) reduction yielded a mean SBP of 118.4 ± 12.9. Compared to the general population, the prevalence of hypertension decreased by 10.4%, 17.9%, and 22.6%, as a result of 5%, 10%, and 15% reductions in SBP among high-risk hypertensive individuals.
Overall, the high-risk primary prevention led to the largest decreases in the population SBP SD, as well as in cases of hypertension. The high-risk secondary prevention strategies led to the largest decrease in SBP mean and led to a similar decrease in hypertension prevalence as the primary prevention strategy. All high-risk strategy results are presented in Table 3 and graphically in Fig. 1.
Table 3.
Mean systolic blood pressure (SBP), standard deviation (SD SBP) and changes in cases under simulated high-risk intervention scenarios.
| Total N (%) | Mean SBP (mmHg) | SD SBP (mmHg) | SBP>130, N (%) | Change in casesa vs. population, N (%) | |
|---|---|---|---|---|---|
| Population SBP | 3393 | 119.6 | 13.7 | 719 (21.2) | – |
| Smokers | 765 (23.0) | 120.4 | 13.7 | 171 (22.4) | (5.7) |
| Non-smokers | 1941 (77.0) | 119.3 | 13.7 | 548 (20.1) | (-5.2) |
| High-risk, primary preventionb | |||||
| Prevent smoking | 3393 | 119.3 | 12.1 | 548 (16.2) | –171 (–23.6) |
| High-risk, secondary preventionc | |||||
| Reduce SBP 5% | 3393 | 119.2 | 13.2 | 643 (19.0) | –76 (–10.4) |
| Reduce SBP 10% | 3393 | 118.8 | 13.0 | 590 (17.4) | –129 (–17.9) |
| Reduce SBP 15% | 3393 | 118.4 | 12.9 | 556 (16.4) | –163 (–22.6) |
Cases of hypertension defined as those with SBP>130.
The intervention targeted only those at high-risk, defined as those individuals who reported current smoking (n=765).
The intervention targeted only those at high-risk, defined as those individuals whose SBP>130 (n=719).
3.2. Population prevention strategies
Next we examined the effects of both population prevention strategies. In the primary prevention strategies, a reduction of smoking levels by 33% and 50%, had a negligible impact. Mean SBP decreased to 119.4 and 119.3 mmHg respectively, and neither strategy level reduced the sample standard deviation. The impact of reducing the sample of smokers by 50% prevented only 1.4% of the hypertension prevalence in the population. The secondary prevention strategy achieved substantial decreases in the sample SBP mean, SD, and hypertension prevalence. An 8% reduction in population SBP decreased the overall mean SBP by 4.8 mmHg to 114.8 ± 9.7. The prevalence of hypertension decreased by 25.0%, 44.3%, and 64.2%, as a result of 2.5%, 5%, and 8% changes from population secondary prevention strategies.
Overall, population primary prevention had little impact per the assumptions of our model, while secondary prevention led to substantial decreases in systolic blood pressure mean, standard deviation, and prevalence of hypertension in the general population. All population prevention strategy results are presented in Table 4 and Fig. 2.
Table 4.
Mean systolic blood pressure (SBP), standard deviation (SD) and changes in cases under hypothetical population intervention scenarios.
| Mean SBP (mmHg) | SD SBP (mmHg) | SBP>130, N (%) | Change in casesa vs. population, N (%) | |
|---|---|---|---|---|
| Population SBP | 119.6 | 13.7 | 719 (21.2) | – |
| Population primary preventionb | ||||
| Reduce smoking 33% | 119.4 | 13.7 | 712 (21.0) | –7 (–0.9) |
| Reduce smoking by 50% | 119.3 | 13.7 | 710 (20.9) | –9 (–1.4) |
| Population secondary preventionb | ||||
| Reduce SBP 2.5% | 118.1 | 12.3 | 540 (15.9) | –179 (–25.0) |
| Reduce SBP 5% | 116.6 | 11.1 | 401 (11.8) | –318 (–44.3) |
| Reduce SBP 8% | 114.8 | 9.7 | 257 (7.6) | –462 (–64.2) |
Cases of hypertension defined as those with SBP>130.
The intervention targeted all individuals in the population with SBP>130, regardless of risk factors.
To summarize the effects of all four strategies, the high-risk primary prevention strategies led to substantial decreases in the population standard deviation and hypertension prevalence, while the population primary prevention had little impact on any measure. The secondary prevention strategies both led to substantial decreases in SBP mean, standard deviation, and hypertension prevalence; the greatest impacts were seen in the most intensive level of the population secondary prevention strategy.
3.3. The effects of prevention strategies in low versus high household income populations
We examined inequalities in SBP distribution in a sample stratified by low-income (n=1358) and high-income (n=662) individuals. The prevalence of smoking was 31.7% in the low-income group and 12.8% in the high-income group. The mean SBP was 2 mmHg lower in the high-income group (120.3 and 118.3 mmHg, respectively). The prevalence of hypertension was 22.5% and 17.8% among low- and high-income groups.
We simulated the interventions in each income group, in order to examine the impact of each strategy in different socio-economic contexts. While the high-risk primary prevention strategy had a negligible impact on SBP means, the low-income sample achieved a much greater reduction than the high-income population in the prevalence of hypertension (30.7% vs. 2.8%) and SBP standard deviation (11.4 mmHg vs. 12.4 mmHg). Relative to group baseline values, the high-risk secondary prevention strategy yielded greater relative decreases in SBP means (–1.6 vs. –0.6 mmHg) and standard deviations (–1.1 vs. –0.4 mmHg) within the low- vs. high-income group. At the most intensive intervention level, the hypertension prevalence decreased by 28.9% in the low-income group compared to 15.2% in the high-income group. Also, there was a slight narrowing of inequalities in the SBP means, which decreased from 2.0 to 0.9 mmHg at the level of a 15% reduction. The narrowing was similar for standard deviations.
The impact of population primary prevention was negligible for both income groups. Population secondary prevention led to substantial reductions in SBP means and standard deviations for both groups, but the decrease in hypertension prevalence was slightly greater in the high-income population. Again, inequalities decreased in the SBP means, from 2.0 to 1.4 mmHg at the level of an 8% reduction, and for standard deviations. Full simulation results and key differences in interventions between income groups are summarized below in Table 5, Table 6.
Table 5.
Mean systolic blood pressure (SBP), standard deviation (SD) and changes in hypertension cases under hypothetical high-risk and population intervention scenarios, stratified by income status.
|
Low household income (less than $35,000/year) |
High household income (more than $100,000/year) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean SBP | SD SBP | SBP> 130, N (%) | Change in casesavs. population, N (%) | N (%) | Mean SBP | SD SBP | SBP>130, N (%) | Change in casesavs. population, N (%) | |
| All SBP | 1358 | 120.3 | 13.8 | 305 (22.5) | – | 662 | 118.3 | 13.2 | 118 (17.8) | – |
| Smokers | 430 (31.7) | 120.2 | 14.0 | 93 (21.6) | (-4.0) | 85 (12.8) | 119.8 | 12.4 | 18 (21.2) | (19.1) |
| Non-smokers | 928 (68.3) | 120.3 | 13.8 | 212 (22.8) | (1.3) | 577 (87.2) | 118.1 | 13.3 | 100 (17.3) | (-2.8) |
| High-risk, primary preventionb | ||||||||||
| Prevent smoking | 1358 | 120.3 | 11.4 | 212 (15.6) | -70 (-30.7) | 662 | 118.2 | 12.4 | 100 (15.1) | –18 (–2.8) |
| High-risk, secondary preventionc | ||||||||||
| Reduce SBP 5% | 1358 | 119.8 | 13.2 | 262 (19.3) | -43 (-14.2) | 662 | 118.1 | 12.9 | 107 (16.2) | –11 (–9.0) |
| Reduce SBP 10% | 1358 | 119.2 | 12.8 | 241 (17.7) | -64 (-21.3) | 662 | 118.0 | 12.8 | 102 (15.4) | –16 (–13.5) |
| Reduce SBP 15% | 1358 | 118.7 | 12.7 | 217 (16.0) | -88 (-28.9) | 662 | 117.8 | 12.8 | 100 (15.1) | –18 (–15.2) |
| Population primary preventiond | ||||||||||
| Reduce smoking 33% | 1358 | 120.1 | 13.8 | 300 (22.1) | -5 (-1.8) | 662 | 118.2 | 13.2 | 118 (17.8) | 0 (0) |
| Reduce smoking 50% | 1358 | 120.0 | 13.8 | 299 (22.0) | -6 (-2.2) | 662 | 118.2 | 13.2 | 117 (17.7) | –1 (–0.6) |
| Population secondary preventiond | ||||||||||
| Reduce SBP 2.5% | 1358 | 119.5 | 12.8 | 228 (16.8) | –77 (–25.3) | 662 | 117.7 | 12.2 | 85 (12.8) | –33 (–28.1) |
| Reduce SBP 5% | 1358 | 118.7 | 11.8 | 168 (12.4) | –137 (–44.9) | 662 | 117.1 | 11.4 | 64 (9.7) | –54 (–45.5) |
| Reduce SBP 8% | 1358 | 117.8 | 10.7 | 116 (8.5) | –189 (–62.2) | 662 | 116.4 | 10.5 | 41 (6.2) | –77 (–65.2) |
Cases of hypertension defined as those with SBP>130.
The intervention targeted only those at high-risk, defined as those individuals who reported both smoking and high BMI.
The intervention targeted only those at high-risk, defined as those individuals whose SBP>130.
The intervention targeted all individuals in the population, regardless of risk.
Table 6.
Summary of effects of hypothetical intervention strategies among high and low household earners.
| Low household income (less than $35,000/year) | High household income (more than $100,000/year) | Overall | |
|---|---|---|---|
| High-risk | |||
| Primary prevention | Larger decrease in standard deviation and hypertension prevalence | No significant reductions in mean SBP for either group | |
| Secondary prevention | Larger decrease in mean SBP | Larger decrease in hypertension prevalence | Reductions in standard deviation not different by income group |
| Population | |||
| Primary prevention | No significant reductions in SBP mean, standard deviation, or hypertension prevalence | ||
| Secondary prevention | Larger decrease in mean SBP | Larger decrease in SBP standard deviation and hypertension prevalence | |
Overall, the lowest reductions in mean SBP occurred among the high-income sample, though this is partially because the baseline SBP was lower than the low-income group. Relative to baseline levels, the greatest SBP reductions were seen in the low-income group. The impact of high-risk primary prevention led to greater decreases in hypertension prevalence among the low-income group, while the impact of population secondary prevention led to slightly greater decreases in hypertension prevalence among the high income sample.
4. Discussion
Each of the four different intervention strategies modeled had a distinct impact on the SBP mean, distribution, and prevalence of hypertension in the study sample. The greatest overall reductions in mean SBP, standard deviation, and hypertension prevalence resulted from the population secondary prevention strategy (i.e. directly intervening to reduce SBP regardless of smoking status), though the high-risk primary prevention strategy led to substantial decreases in the population standard deviation and hypertension prevalence as well. The effect of reducing the prevalence of current smoking in the population primary prevention strategy did little to decrease sample mean and standard deviation, at the levels of smoking reduction that we modeled.
Among the income-stratified population, the lowest reductions in mean SBP were seen among the high-income group, though this is partially because the baseline SBP was lower than the low-income group. Relative to baseline levels, the greatest SBP reductions were seen in the low-income group. Further, the impact of high-risk primary and secondary prevention strategies led to greater decreases in hypertension prevalence among the low-income group, thereby narrowing the income-related gap in disease. By contrast, the population secondary prevention led to slightly greater decreases in hypertension prevalence among the high-income group.
The simulation illustrates the effect that small changes in ubiquitous causes will result in more substantial change in the health of populations than will larger changes in rarer causes. This is illustrated by the greater impact of high-risk primary prevention in the low-income group, where smoking prevalence was nearly three times higher than within the high-income group (31.7% vs. 12.8%).
As expected, the baseline mean SBP values were substantially higher in the low-income group. Further, there were no differences in SBP means by smoking status only among the low-income group, while the mean SBP was 1.7 mmHg higher in smokers vs. non-smokers in the high-income group. This suggests that SBP inequalities between income groups are not wholly attributable to the observed smoking status. Low-income populations have a historically greater risk of chronic health conditions due to well-known factors that reinforce the income gradient in health, such as fewer material and informational resources to prevent disease (Link & Phelan, 1995), and greater psychosocial stressors (Siegrist & Marmot, 2004). A thorough investigation of income inequalities and health is beyond the scope of this paper, but we mention it to emphasize the point that, a researcher or policy maker who chooses to define a high-risk population based solely on the prevalence of “modifiable” risk factors (e.g., smoking status) rather than more distal social definitions of risk, would overlook significant causes of inequalities in health outcomes. This issue was raised by Rose himself, when he warned that, by ignoring underlying causes of illness, any intervention will do little to address health inequalities in the long-term (Rose, 1985). An additional benefit of a structural focus is that it reduces the potential for victim blaming and stigmatization that can occur when individuals are identified as high-risk based on presumed behavioral characteristics. This was a prominent critique of early formulations of high-risk intervention strategies (Labonté, 1994).
A high-risk population can be defined by any number of criteria. One may choose a definition based on socio-demographic groups such as age, sex, race/ethnicity, income, or neighborhood; or high-risk may be defined using “modifiable” risk behaviors, such as smoking, physical activity, substance use, or other comorbid conditions. There is not always a clear distinction between what is or is not modifiable (e.g., social isolation (Berkman et al., 2003, Pantell et al., 2013). For these reasons, it is important to consider the assumptions and implications of how we define who is at high-risk. Focusing on what is modifiable may allow for a more well-defined intervention, but this may lead one to address the most proximate causes of disease, rather than thinking about risk in terms of macrosocial or structural determinants (Krieger and Davey Smith, 2016, Schwartz et al., 2015). In fact, structural determinants that are unaccounted for may affect compliance or adherence with an intervention, as has been posited as an explanation for inconsistent and unexpected findings in several large intervention studies (Multiple Risk Factor Intervention Trial Research Group, 1982, Orr et al., 2003). The behavior of individuals is affected by the political, economic, and cultural contexts in which they live, and this must be taken into account for any intervention to be successful.
A more general limitation of the high-risk approach, as discussed by Rose (1985), is the difficulty that researchers face in predicting individual risk for disease. In our simulations, despite modeling a well-established risk factor for hypertension, the act of reducing the prevalence of individual exposure to smoking did little to reduce the overall population mean SBP, especially when primary prevention was attempted for the entire population. As the underlying risk in the high-risk sample increased, so too did the efficacy of the primary prevention strategies. It seems that the effect of the high-risk primary prevention strategy was driven mainly by decreases within the low-income cases. The efficacy of these strategies for an individual is limited by our ability to predict the individual risk of disease, which is in part dependent on the prevalence of the causal factors that interact with that exposure to cause disease. In other words, the magnitude of the effect of an exposure on disease is dependent on the prevalence of the causal factors that interact with that exposure. Though we are able to predict health in populations with much more certainty than we can predict health in individuals, we will improve our ability to predict an individual's risk for disease by understanding how multiple risk factors interact to cause disease. Our predictive ability might also increase by defining our high-risk population in more narrow terms (e.g., individuals “exposed” to both smoking and low-income status), but do so knowing that the absolute number of cases we can prevent will likely decrease as the population becomes smaller. For example, the primary prevention strategy decreased hypertension among low-income individuals by 30.7% vs. 23.6% in the general population. However, the general population in this example was comprised of 3393 individuals, so a decrease of 23.6% prevented 171 cases of hypertension, while the low-income population is comprised of 1358 individuals, so a decrease of 30.7% prevented only 70 cases of hypertension. Additionally, high-risk strategies imply that high-risk individuals must be identified and consent to participating in an intervention, both of which may be difficult and expensive.
By contrast, population strategies focus on universal strategies that affect all individuals, regardless of baseline risk of developing disease. This universality also has advantages and disadvantages. A population strategy typically seeks to decrease population-level risk of disease, which is why vaccination programs strive for herd immunity by maximizing population coverage of vaccines. At the individual level, most individuals would never get the disease with or without the vaccination. Rose referred to this as the “prevention paradox” (Rose, 1985, p. 432). This can make the implementation of a population-level intervention very difficult, especially in the short-term. Indeed, recent calls to increase the tax on sugar sweetened beverages in New York City were met with staunch resistance and ultimately defeated, in part because opponents believed that beverage choice was an individual decision, and the risk conferred by high fructose corn syrup is not perceived as worth the increased cost by the individual consumer (Brownell et al., 2009, Gollust et al., 2014). When they do succeed, however, population strategies can be powerfully sustainable drivers of healthy behavior change. For example, workplace smoking bans have been shown to encourage smokers to quit or to reduce tobacco consumption (Fichtenberg & Glantz, 2002).
Further, it is important to consider the differences in effects of primary vs. secondary prevention, both in terms of equity, cost-effectiveness, and timing (McLeod, Blakely, Kvizhinadze, & Harris, 2014). Primary prevention programs may be more cost-effective in the long-term as individuals are prevented from ever getting disease. Since secondary prevention programs seek to treat those with disease, it may seem as though they are more cost-effective in the shorter-term. The impact on health equity may also vary by strategy, and the most equitable interventions likely include a combination of primary prevention supplemented with secondary treatment (Blakely et al., 2015).
There are several limitations to these simulations. First, the parameters we set for our strategies and their impact were likely based on overly optimistic assumptions. In reality, the targets of such interventions would likely be more modest. As such, we are presenting the maximum impact of intervention rather than the actualized impact. Also, we assumed that the changes would be permanent, namely that no smokers would relapse. Further, we lowered the threshold for high SBP and removed outliers, thus results may not generalize to the broader population, or to individuals with high SBP diagnosed in clinical care. Overall, while our approach led to a simplification of individual's health behavior, our goal was to contrast different strategies under an explicit set of assumptions. We believe that the results of these comparisons should serve to further stimulate discussion about the differing impacts of health interventions. Additionally, part of the decision to impute smoker's SBP independent of their baseline SBP was to account for some of the unintended consequences of removing this exposure; for some individuals quitting smoking may lead to other health outcomes, which themselves may be risk factors for hypertension (e.g., weight gain (Williamson et al., 1991)). Also, part of the decrease in SD from the interventions was due to the use of mean imputation (Donders, van der Heijden, Stijnen, & Moons, 2006). These methods could be improved with the integration of multiple imputation techniques for modeling a more realistic impact of these interventions in the population. While many of the assumptions and parameters we presented are subject to debate, the purpose of this paper was to illustrate what questions and considerations might be necessary for practitioners to address as they begin to plan an intervention.
Finally, the analysis did not account for financial considerations of these strategies. Cost-effectiveness is a significant consideration in planning health interventions, and is often what gets the most attention in these types of analyses. We argue that questions about maximizing both efficiency and equity should also be core considerations that inform how we may develop and implement population health interventions. In fact, a consideration of intervention effects within various population subgroups should be included in cost-effectiveness models (McLeod et al., 2014). It is critical to consider the impact of an intervention on health equity, including a thoughtful and transparent consideration of when subgroup differences in health indicators (e.g., life expectancy) may be appropriate to use, and when differences may reflect the consequences of discrimination. In addition, there are numerous other decisions that policy developers must make as they consider evidence to inform interventions, each of which rests on value-laden assumptions. For example, choosing to measure relative vs. absolute health inequalities can lead to very different interpretations and implication (Mechanic, 2002). Also influential is the use and estimation of statistical weights to represent those population sub-groups in large population surveys (Pearcy & Keppel, 2002).
A growing body of literature has developed to formally present these assumptions and discuss their implications for policy makers (Harper et al., 2010). In that spirit, the present analysis seeks to show that efforts to improve overall population health may disadvantage some groups; whether equity or efficiency is preferable is a matter of values. Our collective biases, judgments, and goals are influential throughout the process of health inequalities research and policy making, and an increased attention to these assumptions will promote a greater understanding of population health research methods and will prove useful to improve the quality of policies to understand and address health inequalities.
Acknowledgements
Funding was provided by the National Institute of Mental Health (5T32MH1304343, JMP).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ssmph.2016.11.002.
Appendix A. Supplementary material
Supplementary material
.
References
- Benach J., Malmusi D., Yasui Y., Martínez J.M. A new typology of policies to tackle health inequalities and scenarios of impact based on Rose's population approach. Journal of Epidemiology and Community Health. 2013;67(3):286–291. doi: 10.1136/jech-2011-200363. [DOI] [PubMed] [Google Scholar]
- Berkman L.F., Blumenthal J., Burg M., Carney R.M., Catellier D., Cowan M.J., Enhancing Recovery in Coronary Heart Disease Patients Investigators (ENRICHD) Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- Blakely T., Cobiac L.J., Cleghorn C.L., Pearson A.L., van der Deen F.S., Kvizhinadze G., Wilson N. Health, health inequality, and cost impacts of annual increases in tobacco tax: Multistate life table modeling in New Zealand. PLoS Med. 2015;12(7):e1001856. doi: 10.1371/journal.pmed.1001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell K.D., Farley T., Willett W.C., Popkin B.M., Chaloupka F.J., Thompson J.W., Ludwig D.S. The public health and economic benefits of taxing sugar-sweetened beverages. New England Journal of Medicine. 2009;361(16):1599–1605. doi: 10.1056/NEJMhpr0905723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo Jr J.L., Wright Jr J.T. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. Jama. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Collins R., Peto R., MacMahon S., Godwin J., Qizilbash N., Hebert P., Fiebach N. Blood pressure, stroke, and coronary heart disease: Part 2, short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. The Lancet, 335(8693) 1990:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- Diez-Roux A.V., Link B.G., Northridge M.E. A multilevel analysis of income inequality and cardiovascular disease risk factors. Social Science Medicine. 2000;50(5):673–687. doi: 10.1016/s0277-9536(99)00320-2. [DOI] [PubMed] [Google Scholar]
- Donders A.R.T., van der Heijden G.J., Stijnen T., Moons K.G. Review: A gentle introduction to imputation of missing values. Journal of Clinical Epidemiology. 2006;59(10):1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Fichtenberg C.M., Glantz S.A. Effect of smoke-free workplaces on smoking behaviour: Systematic review. Bmj. 2002;325(7357):188. doi: 10.1136/bmj.325.7357.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich K.L., Potvin L. Transcending the known in public health practice: The inequality paradox: The population approach and vulnerable populations. American Journal of Public Health. 2008;98(2):216–221. doi: 10.2105/AJPH.2007.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollust S.E., Barry C.L., Niederdeppe J. Americans' opinions about policies to reduce consumption of sugar-sweetened beverages. Preventive Medicine. 2014;63:52–57. doi: 10.1016/j.ypmed.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Graham H. Tackling inequalities in health in England: Remedying health disadvantages, narrowing health gaps or reducing health gradients? Journal of Social Policy. 2004;33(1):115–131. [Google Scholar]
- Harper S., King N.B., Meersman S.C., Reichman M.E., Breen N., Lynch J. Implicit value judgments in the measurement of health inequalities. Milbank Quarterly. 2010;88(1):4–29. doi: 10.1111/j.1468-0009.2010.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán M.A., Taubman S.L. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. International Journal of Obesity. 2008;32:S8–S14. doi: 10.1038/ijo.2008.82. [DOI] [PubMed] [Google Scholar]
- Kannel W.B. Blood pressure as a cardiovascular risk factor: Prevention and treatment. Jama. 1996;275(20):1571–1576. [PubMed] [Google Scholar]
- Keyes K.M., Galea S. Oxford University Press; 2016. Population Health Science. [Google Scholar]
- Krieger N. Theories for social epidemiology in the 21st century: An ecosocial perspective. International Journal of Epidemiology. 2001;30(4):668–677. doi: 10.1093/ije/30.4.668. [DOI] [PubMed] [Google Scholar]
- Krieger N., Davey Smith G. The tale wagged by the DAG: Broadening the scope of causal inference and explanation for epidemiology. International Journal of Epidemiology https://doi org/ 2016 doi: 10.1093/ije/dyw114. [DOI] [PubMed] [Google Scholar]
- Labonté R. WB Saunders; Toronto, Ontario: 1994. Death of program, birth of metaphor: The development of health promotion in Canada. Health Promotion in Canada. Provincial, National & International Perspectives; pp. 72–90. [Google Scholar]
- Lackland D.T. Population strategies to treat hypertension. Current Treatment Options in Cardiovascular Medicine. 2005;7(4):253–258. doi: 10.1007/s11936-005-0036-9. [DOI] [PubMed] [Google Scholar]
- Lalonde M. Office of the Canadian Minister of National Health and Welfare; 1974. A new perspective on the health of Canadians. [Google Scholar]
- Link B.G., Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior. 1995:80–94. [PubMed] [Google Scholar]
- McLaren L., McIntyre L., Kirkpatrick S. Rose's population strategy of prevention need not increase social inequalities in health. International Journal of Epidemiology. 2010;39(2):372–377. doi: 10.1093/ije/dyp315. [DOI] [PubMed] [Google Scholar]
- McLeod M., Blakely T., Kvizhinadze G., Harris R. Why equal treatment is not always equitable: The impact of existing ethnic health inequalities in cost-effectiveness modeling. Population Health Metrics. 2014;12(1):1. doi: 10.1186/1478-7954-12-15. (JOUR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic D. Disadvantage, inequality, and social policy. Health Affairs. 2002;21(2):48–59. doi: 10.1377/hlthaff.21.2.48. [DOI] [PubMed] [Google Scholar]
- Miller N.H. Compliance with treatment regimens in chronic asymptomatic diseases. The American Journal of Medicine. 1997;102(2):43–49. doi: 10.1016/s0002-9343(97)00467-1. [DOI] [PubMed] [Google Scholar]
- Multiple Risk Factor Intervention Trial Research Group Multiple risk factor intervention trial: Risk factor changes and mortality results. JAMA. 1982;248(12):1465–1477. [PubMed] [Google Scholar]
- Orr L., Feins J., Jacob R., Beecroft E., Sanbonmatsu L., Katz L.F., Kling J.R. . U.S. Department of Housing and Urban Development; 2003. Moving to opportunity: Interim impacts evaluation [Google Scholar]
- Pantell M., Rehkopf D., Jutte D., Syme S.L., Balmes J., Adler N. Social isolation: A predictor of mortality comparable to traditional clinical risk factors. American Journal of Public Health. 2013;103(11):2056–2062. doi: 10.2105/AJPH.2013.301261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy J.N., Keppel K.G. A summary measure of health disparity. Public Health Reports. 2002;117(3):273. doi: 10.1016/S0033-3549(04)50161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. Sick individuals and sick populations. International Journal of Epidemiology. 1985;14(1):32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Prins S.J., Campbell U.B., Gatto N.M. Is the “well-defined intervention assumption” politically conservative? Social Science Medicine. 2015;166:254–257. doi: 10.1016/j.socscimed.2015.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist J., Marmot M. Health inequalities and the psychosocial environment—two scientific challenges. Social Science Medicine. 2004;58(8):1463–1473. doi: 10.1016/S0277-9536(03)00349-6. [DOI] [PubMed] [Google Scholar]
- Sleight P. Smoking and hypertension. Clinical and Experimental Hypertension. 1993;15(6):1181–1192. doi: 10.3109/10641969309037104. [DOI] [PubMed] [Google Scholar]
- Williamson D.F., Madans J., Anda R.F., Kleinman J.C., Giovino G.A., Byers T. Smoking cessation and severity of weight gain in a national cohort. New England Journal of Medicine. 1991;324(11):739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material