Abstract
Sjögren’s syndrome (SjS) is a complex chronic autoimmune disease of multifactorial etiology that results in eventual loss of secretory function in the exocrine glands. The challenges towards finding a therapeutic prevention or treatment for SjS are due primarily to a lack of understanding in the pathophysiological and clinical progression of the disease. In order to circumnavigate this problem, there is a need for appropriate animal models that resemble the major phenotypes of human SjS and deliver a clear underlying biological or molecular mechanism capable of defining various aspects for the disease. Here, we present an overview of SjS mouse models that are providing insight into the autoimmune process of SjS and advance our focus on potential diagnostic and therapeutic targets.
Keywords: Sjögren’s Syndrome, Mouse Models, Autoimmunity, Spontaneous, Transgenic, Experimentally Induced
Introduction
The immune system is regulated by the dynamic interaction of innate and adaptive immune responses. As efficient as the innate immune response is designed to function, its effectiveness is often limited against an ever-changing microenvironment [1]. Adaptive immunity elicits the protective immunological response via the delicate interaction of professional antigen presenting cells (APC), natural killer (NK), T and B cells. Unlike innate responses, adaptive immunity is dependent on antigenic presentation by the major histocompatibility complex (MHC) structures, together with T cell receptors (TCR) and B cell receptors (BCR) that co-evolve with the antigenic repertoires of the organism [2]. The disequilibrium between the pathogenicity of the invading microbes and the immune response or presentation of self-antigens and the immune activation result in rampant infection or exacerbate autoimmunity, respectively. Presently approximately 80 clinically distinct autoimmune diseases have been identified, which include the more commonly studied rheumatoid arthritis (RA), multiple sclerosis (MS), systemic lupus erythematosus (SLE), insulin-dependent type 1 diabetes mellitus (T1D), and Sjögren’s syndrome (SjS) [3–6]. Two forms of SjS have been defined: primary SjS (pSjS) and secondary SjS (sSjS) in the latter of which patients suffer additional autoimmune processes, especially connective tissue disorders such as RA and SLE [7].
SjS has a prevalence of about 0.5% with an incidence of 3–6 per 100,000 per year. More than 90% of those diagnosed are females, and are primarily diagnosed in the 4th–6th decade of life [8,9]. SjS mainly targets the exocrine glands, especially salivary (SG) and lacrimal glands (LG), resulting in dry eyes (keratoconjunctivitis) and dry mouth (xerostomia). Other clinical phenotypes consist of glandular lymphocytic infiltration, presence of autoantibodies to Ro (SSA) or La (SSB), antinuclear antibodies (ANAs), anti-acetylcholine muscarinic type 3 receptor (anti-M3R) and hypergammaglobulinemia. Additionally, extraglandular manifestations are often observed in SjS patients including vasculitis [10], fatigue [11,12], abnormal organ function [13], and various neuropathies. Prior to the past few years, the recognized neural symptoms of SjS included peripheral neuropathy [10,14] and loss of concentration or memory [15] however, more recently, reports have been published focusing on other novel neural symptoms. Similar to the SLE headache, evidence is surfacing that there is a chronic tension-type headache associated with SjS independent of autoantibodies or any other neurological symptoms (fatigue, depression, etc.) [16,17]. In these cases, there is no physiological cause detectable by MRI, which may indicate an immunological variable.
The major challenge encountered in studying human autoimmune diseases, in particular SjS, is the lack of understanding regarding the autoimmune process occurring prior to the clinical symptoms. It is difficult to examine the temporal changes in disease phenotypes or genetic contribution since patients are often diagnosed at the later stages of disease. Additionally, the heterogeneity of the human population might undermine the interpretation of the analysis due to the lack of appropriate comparative controls. As a result, to fully comprehend the complete spectrum of the autoimmune process, one must employ the study of animal models that encompass the comprehensive disease process mimicking human disease. To be an appropriate or ideal animal model for SjS, this hypothetical model must recapitulate many of the objective human disease phenotypes defined by the classification criteria, specifically lymphocyte infiltration in the exocrine glands and presence of serum anti-SSA/SSB or rheumatoid factor (RF) and ANA. In addition, an increase in the expression of pro-inflammatory cytokines, production of target specific autoantibodies such as anti-M3R and carbonic anhydrase (CA), as well as unregulated glandular apoptosis. More importantly, this hypothetical model must address the neuropathy and extraglandular manifestations associated with SjS. Lastly, clinical manifestation specifically secretory dysfunction in the LG and SG must also develop. Currently, there is no particular animal model that completely recapitulates the whole spectrum of human SjS, however there are various models that replicate different biological and immunological aspects of the disease. A number of elegantly written reviews describe the use and importance of different SjS animal models [18–20]. This review provides the latest facets on some of the representative models with special focus on disease characteristics and application in solving human SjS disease.
Spontaneous/Genetic affectation models
Non-obese diabetic (NOD) mouse model
The inter-breeding of an outbred ICR mouse strain, which is cataract prone, gave rise to the NOD mouse model, mostly for their characteristics resembling SjS and T1D. The appearance of autoimmune diabetes prior to autoimmune exocrinopathy (AEC) in the NOD mouse suggests that it is an excellent model of secondary, but not pSjS [21]. Human SjS pathology exhibits lymphocytic infiltration, an active decrease in the salivary flow rates, and total protein concentrations increased/decreased in saliva and tears as a result of exocrine gland dysfunction. These symptoms are observed in both male and female NOD mice. In addition, NOD mice also show increases in secretory levels of autoantibodies like SSB (anti-La) [22–24]. A lymphocytic infiltration in SG and LG shows a greater number of T-cells rather than B-cells justifying predominance of CD4+ to that of CD8+ lymphocytes in NOD mice [25]. Van Blokland et al. [26] demonstrated that macrophages and dendritic cells were first to infiltrate into the glands that subsequently develop lymphocytic foci (LF) around 8 weeks (wks) of age. The vast majority of inflammatory infiltrates in the SG were CD4+ Vβ8+ and CD4+ Vβ6+ T cells, whereas CD8+ T cells and B220+ B cells were fewer in numbers [27]. Intercellular adhesion molecule-1 (ICAM-1) is one of the immunoglobulin superfamily proteins expressed in SjS patients. It was observed that an over expression of ICAM-1 also occurred in SG of NOD mice[28]. Roescher et al. [28] demonstrated that blocking ICAM-1 interaction by systemic administration of soluble ICAM-1 can be an effective therapy for autoimmune diabetes in the NOD mouse. It was also shown that treatment at an early stage led to a modest decrease in autoimmune inflammation of the SG [28].
Numerous cytokine gene knockout (KO) mice with the NOD background have been explored for the role of various cytokines in the pathogenesis of SjS. During lymphocytic infiltration to the target tissues, a considerable amount of pro-inflammatory cytokines, e.g., interferon gamma (IFN-γ), tumour necrosis factor-beta (TNF-β), interleukins (IL-1β, 2, 6, 12 and 18), were observed. In order to portray the role of specific immunological factors in disease, a number of gene KO NOD mice have been studied. NOD.Ifnγ−/− and NOD.IfnγR−/− have been used to investigate the role of INF-γ in SjS pathogenesis [29]. Surprisingly, NOD.Ifnγ−/− and NOD.IfnγR−/− mice fail to develop pre-disease or overt SjS disease. This observation, together with the fact that IFN-γ appears prior to the early immune phase independent of effector functions of immune cells [29], suggests that the appearance of physiological events precede overt disease onset. As alluded, SjS is a B cell mediated disease where autoantibodies contribute a significant role in both disease etiology and diagnoses. This concept is substantially supported by examining the NOD.Igu−/− mouse. Abolishing functional B cells and autoantibodies completely restored normal saliva flow despite the presence of lymphocytic foci and pathophysiological abnormalities. Furthermore, infusion of serum fractions from parental NOD mice or pSjS patients to NOD.Igu−/− mice resulted in inhibition of normal secretory function. Further examination of the pathogenic serum fractions revealed the presence of anti-M3R autoantibodies with IgG1 isotypes, which has a direct negative effect on saliva secretion [30–32]. Consistent with NOD.Igu−/− mice, elimination of il4 gene in NOD.IL4−/− and NOD.B10-H2b.IL4−/− mice clearly support a critical function of B cells by interfering with the proliferation and isotypic IgG1 pathogenic autoantibodies mediated by IL-4 cytokine [33,34]. The translational aspect of this concept is remarkable with the use of biologics targeting B cells such as anti-CD20 monoclonal antibody to deplete B cells [35]. The consistency in patient response, long-term benefit, and potential side effects of anti-CD20 monoclonal antibody therapy (Rituximab) are still under investigation. Clinical studies have shown that B-cell depletion can restore salivary gland function, reduce inflammatory lesions in the glands, and decrease rheumatoid factor with no changes in SSA/SSB antibodies [36–39]. More importantly, B-cell depletion therapy has some positive effects on neurological-associated symptoms and extraglandular manifestations in some patient cohorts [40,41].
NOD-derived C57BL/6.NOD-Aec1Aec2
The main limitations of the NOD and NOD-derived mouse models are the lack of comparative normal control and lengthy effort of generating transgenic strains. Therefore, it is imperative to identify SjS-susceptible gene(s) or loci that could be transferred on the commonly used C57BL/6 background. Early works by a number of laboratories determined that breeding individual insulin-dependent diabetes (Idd) susceptibility intervals, such as Idd1, Idd3, Idd5, Idd9, and Idd13 loci, into the non-autoimmune C57BL/6J mouse did not recapitulate development of SjS in these mouse lines. Interestingly, by breeding the combination of Idd3 and Idd5 loci derived from the NOD mouse strain on the C57BL/6 background, the resulting line, referred to as C57BL/6.NOD-Aec1Aec2 (where Aec1 corresponds to Idd3 and Aec2 corresponds to Idd5), fully recapitulated the SjS phenotype [42,43]. This double congenic strain exhibited lymphocytic infiltrations of the SG and LG at 12–16 wks of age, and production of autoantibodies to nuclear antigens (SSA/Ro, SSB/La) and M3R as illustrated in Figure 1. By 20 wks of age, fully developed LF show predominantly CD4+ T cell with lesser numbers of B cells. A few numbers of CD8+ T cells, macrophages and dendritic cells were present in the exocrine glands accompanied by a loss in production of saliva and tears. Similar to SjS patients, significant up-regulation of IL-17 and IL-23 were subsequently observed both locally in the glands and systemically [44]. A recent study using C57BL/6.NOD-Aec1Aec2 mice has suggested a potential mechanism by innate immunity in initiating pSjS in which the authors determined that polyinosinic:polycytidylic acid (poly I:C) can induce IL-7 expression in the SG [45]. In addition, C57BL/6.NOD-Aec1Aec2 mice have been shown to exhibit high numbers of self-reactive B cell repertoires against SG antigens using single-cell analysis of the spleens and cervical lymph nodes [46].
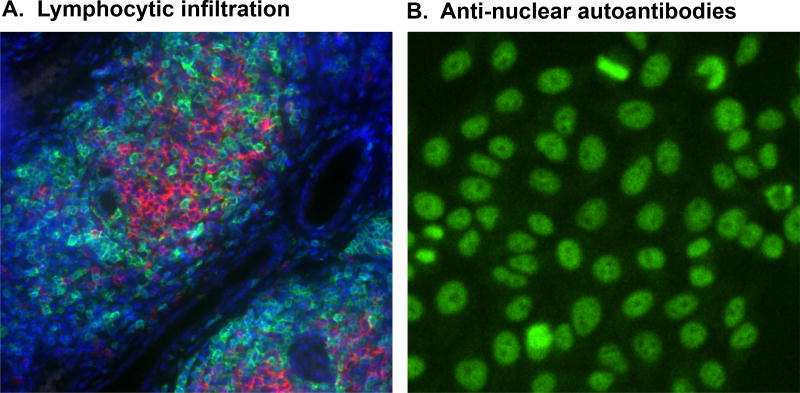
Figure 1. Main clinical features of SjS.
A. Lymphocytic infiltration in the salivary glands of C57BL/6.NOD-Aec1Aec2 mice composed of mainly of CD3+ T cells (green) and B220+ B cells (red) with nuclei stained with 4',6-diamidino-2-phenylindole (DAPI:blue). B. Anti-nuclear autoantibodies (ANA) depicting nuclear speckled staining using HEp2 cells of sera diluted 1/40 prepared from C57BL/6.NOD-Aec1Aec2 mice at 200X magnification.
One critical advantage of the C57BL/6.NOD-Aec1Aec2 mouse model is the ability to dissect the genetic contribution of individual genes or sets of genes of the two loci (idd3 and idd5) in different biological and immunological contexts of SjS. Development of the recombinant inbred line C57BL/6.NOD.Aec1R1Aec2, possessing a shortened genetic region of Aec1 (19.2cM compared to 48.5 cM) generated by crossing C57BL/6.NOD.Aec1Aec2 with C57BL/6 mice [47], yet maintaining the SjS-like disease phenotype, produced an observed sexual dimorphism in disease. SG infiltrations could be observed as early as 10wks of age in males compared to 19wks in females, yet females presented with more severe sialoadenitis and larger infiltrations in the submandibular gland by 22wks of age with no dacryoadenitis. Males exhibited significantly higher levels of dacryoadenitis. Homogeneous nuclear ANA patterns were observed in males as early as 5wks of age, but not until 10wks in females. Both male and female mice showed a significant loss of saliva flow rate (35– 40%), but only males displayed a loss of LG secretory function. Similar approaches were taken to decipher the genetic elements in the Aec2 region attributed to SjS. Shortening the Aec2 locus to approximately 10 +/− 5 cM around position 79 cM distal to the centromere uncovered several potential SjS-susceptible genes. One of the potential candidate genes is the tumor necrosis factor (ligand) superfamily member 4 (TNFSR4 or Ox40 ligand), encoding a product whose biological functions correlate with both physiological homeostasis (lipid, lipoprotein, and fatty acid metabolism) and immune interactions between regulatory T cell (Treg) and pathogenic T helper 17 (Th17) cell populations [48]. This observation was confirmed in a study using large pSjS patient cohorts from Sweden and Norway in which the authors have found polymorphism in TNFSF4 susceptible gene in the immune system is strongly associated with the development of pSjS [49], however only weak association was found in Chinese Han population [50].
Murphy Roths Large (MRL) and lymphoproliferation (MRL/lpr) mice
The MRL strain is an established model for SLE, but it also manifests similar phenotypes observed in SjS [51]. Mice carrying the lpr mutation have defects in the Fas antigen gene [52]. Mice homozygous for the lpr gene develop many of the autoimmune phenotypes, specifically infiltration of mononuclear cells in the SG [53]. Interestingly, severity of the disease depends on the genetic background and environmental factors [54]. MRL-Zpr/Zpr mice spontaneously develop similar autoimmune profiles with features resembling the pathogenesis of SjS, particularly sialoadenitis and lacrimitis [55,56]. Jonsson R. et al. have determined that MRL/Mp-lpr/lpr mice express high levels of IL-2 receptor (IL-2R) and elevated production of IFN-γ similar to human patients [57]. Staining for cellular composition of the SG at varying stages of disease revealed infiltrations with focal inflammation at 9wks to 23wks of age in submandibular glands [58]. In addition, these mouse strains produced local immunoglobulins (IgA, IgG, IgM) and rheumatoid factor (RF) in SG, lymph nodes, and spleen. Levels of IgG were determined to be higher than IgM and IgA in SG and lymph nodes but not spleen. Furthermore, this group has shown that the levels of IgG secretion were positively correlated with disease severity of the SG [59].
NFS/sld mice
NFS/sld mutant mice are characterized by an autosomal recessive gene with sublingual gland differentiation arrest (sld), providing a model in which aberrant immune responses against α-fodrin are elicited [60]. A 120-kilodalton (kD) organ-specific autoantigen was purified from SG tissues in this mouse model, identical to that of the human cytoskeletal protein α-fodrin with which some SjS patients have been identified [61]. It was noted that when thymectomy was performed, T cells dominated the experimental autoimmune sialoadenitis (EAS) phenotype in NFS/sld mice 3 days after birth in SG and LG and resulted in development of inflammatory lesions in other organs [62]. However, it remains to be determined whether there is a strong association between antibodies to α-fodrin and SjS [63]. Regardless, NFS/sld mice have provided important insight regarding the role of apoptosis mediated by α-fodrin proteolysis and Fas/FasL in SjS. NFS/sld mice have been shown to express high levels of FasL and Fas in the majority of infiltrating lymphocytes of the SG and epithelial duct cells, respectively [64]. Vanags et al. [65] have shown that 240 kDa membrane-associated cytoskeleton α-fodrin is cleaved into three fragments of 150, 120 and 23 kDa by caspase-3 activation. Since α-fodrin is involved in membrane function and contraction, proteolysis of α-fodrin is detrimental to epithelial cells of SG. Functioned as an autoantigen, purified 120 kDa fragments of α-fodrin have been shown to stimulate T cell proliferation and IL-2 and IFN-γ production in vitro [66]. In addition, specific autoantibodies against α-fodrin have been detected in NOD mice, and these antibodies correlated with the levels of sialadenitis. Witte et al. [67] have identified IgA antibodies reactive with α-fodrin are detectable in 64% of patients with pSjS, 47% of patients with sSjS and SLE, and 86% of patients with sSjS and RA, compared to only 1 in 160 sera obtained from normal controls. Similarly, the prevalence of IgG antibodies reactive with α-fodrin was significantly higher in sera obtained from patients with pSjS and sSjS compared to sera from normal controls.
IQI/Jic mice
IQI/Jic is an inbred strain generated from ICR mice in which the previously mentioned NOD was originated. Similar to the NOD mouse model, IQI/Jic mice developed sialadenitis in the SG and LG at 3 months of age with mostly B220+ cells in the larger lesions and CD4+T cells in smaller foci. Female mice appeared to have progressively severe infiltrate with age while male mice developed less severe and stable lymphocytic foci regardless of age. Secretory function has never been examined, but the animals were positive for nuclear speckled IgG antibody with no anti-SSA/B [68]. Aged IQI/Jic also developed extraglandular manifestations specifically inflammatory lesions in the lung, pancreas and kidney [69]. Further study has demonstrated that the animals developed autoantibodies to serine proteinase kallikrein (Klk)-1 and 13. Interestingly, only Klk-13 was able to induce splenic T cell proliferation, therefore it is considered one of the autoantigens in SjS [70]. Although, both Klk-1 and Klk-13 autoantibodies have not been substantiated in human patients, anti-Klk-11 has been shown to be elevated in SjS patients with dry eye disease [71].
Transgenic models
Cytokine overexpression
IL-2 Transgenic
IL-2 is a critical cytokine regulating the proliferation of T cells and more importantly it influences the homeostasis and induction of regulatory T cells (Treg). The immunological balance between effector T cells and Treg cells provides natural immunologic self-tolerance, which prevents the initiation of autoimmunity [72]. Loss of IL-2 in il-2 KO mice resulted in severe immunopathology including lymphoadenopathy, splenomegaly, T cell infiltration of the bone marrow and loss of B cells [73]. Sharma et al. [74] have demonstrated that IL-2−/− mice, which are partially deficient in Treg, displayed impaired SG function and lymphocytic infiltration in the SG and LG. Similar disease phenotypes were also noticed in IL-2rα−/− mice. Interestingly, Scurfy mice with foxp3 mutation, which lacks CD4+CD25+ Treg cells, did not develop any clinical signs of SjS. However, transfer of lymph node cells from Scurfy mice to RAG-1 KO recipients rapidly and effectively induced inflammation and loss of function in the SG. An early study by Fox RI et al. [75] has determined that SG lymphocytes and not peripheral blood lymphocytes of SjS patients produced high levels of IL-2. A number of studies have indicated a clear deficiency in the frequencies of Treg cells in SjS patients [76,77]. As a result, IL-2 KO is an appealing mouse model in which cell-based therapy involved Treg cells are used.
IL-10 Transgenic
IL-10 cytokine is produced primarily by monocytes and functions in immune-regulation and inflammation. While it downregulates Th1 cytokines, it has been demonstrated to enhance B cell survival, proliferation, and antibody production [78]. These B cell association functions of IL-10 indicate its important role in the development of SjS. The development of a transgenic mouse model of C57Bl/6 mice utilizing IL-10 under regulation by the human salivary amylase promoter showed marked Fas/FasL mediated destruction of the SG [79]. Interestingly when IL-10 is delivered to a spontaneous SjS model, NOD, by recombinant adeno-associated viral vector the result was an increase in saliva flow rates [80]. In patients with SjS salivary IL-10 levels were found to be significantly increased as compared to healthy controls and was also shown to correlate with symptom severity for xerophthalmia and xerostomia [81]. Additionally, significant increases in systemic levels of IL-10 (and IL-4) have been demonstrated to correlate with SjS disease [82,83]. The supposition that there may be a genetic predisposition to SjS led to the evaluation IL-10 promoter polymorphisms. However, here the data is conflicting as early reports suggested that there was a correlation with certain promoter polymorphisms [84,85] while later work contradicted that data demonstrating no correlation [86].
IL-12 Transgenic
IL-12 is produced in response to bacterial products and functions in the differentiation, proliferation and maintenance of T cells to Th1 cells. Uncontrolled upregulation of IL-12 can lead to damaging inflammation from cellular infiltrate [87]. Specifically to study the effect of lung inflammation, IL-12 transgenic mice bred to wild-type CBA mice demonstrated an increase in lymphocytic infiltrate as well as a significant decrease over time in the presence of NK cells [88]. This data helps to explain a decreased NK activity in patients with certain viral infections. The effects of IL-12 have also been studied in terms of its synergistic relationship with IL-18. Injection of recombinant IL-12 and/or IL-18 induced damage to the LG and SG through the induction of IFN-y and nitric oxide and not through lymphocyte infiltration, which was absent in this model [89]. Increases of both of these cytokines have been noted in patients with pSjS and have been shown to be specifically expressed within the minor SG of both patients with pSjS and sSjS [90,91].
IL-14α Transgenic
IL-14 is involved in activation of B cell proliferation in particular germinal center B cells. IL-14α and IL-14β are two isoforms of il-14 gene in which IL-14α is produced by plus strand of the gene. IL-14α transgenic mice demonstrated hypergammaglobulinemia at 13 wks followed by autoantibody production with active lymphocytic infiltration of exocrine gland by 27 wks of age. IL-14α transgenic mice expressed high levels of lymphotoxin-α (LTA) transcript and protein in the SG [92] in which a similar observation was demonstrated in the NOD mice [93]. Genetic KO of lta gene in IL-14α transgenic mice restored normal SG flow rate with no lymphocytic infiltration in the SG [92]. A few unique features of IL-14α transgenic mice, which have not been investigated in other mouse models, are the high frequency of large B cell lymphoma at 12–20 months old and immune-complex mediated nephritis [94]. Upon evaluation of IL-14α expression levels in pSjS and sSjS patient peripheral blood lymphocytes, significantly increased levels of cytokine were present [94]. Recent study by Altorok et al. [95] using genome wide DNA methylation has revealed that lta gene is hypomethylated in naïve T cells of pSjS. Furthermore, single-nucleotide polymorphisms (SNP) analysis has demonstrated a strong correlation between several SNP in the LTA/LTB/TNFα locus and pSjS [96]. The classical signs of SjS with high incidence of B cell lymphoma and nephritis suggest that IL-14α transgenic mice could be an ideal animal model for SjS especially with strong correlation with human patients.
Thrombospondin-1
Thrombospondin-1 (TSP-1) is a glycoprotein that mediates cell-cell and cell-matrix interactions. It has also been shown to be a ligand of latent transforming growth factor-beta (TGF-β) complex, which upon activation is an important regulator of Th17 cells and Foxp3+ Tregs. TSP-1 deficient mice were found to display ocular surface disease similar to SjS patients, which was attributed to the development of anti-SSA/SSB in the serum as well as lymphocyte infiltration [97]. Further analysis revealed that TSP-1 deficient mice demonstrated increased Th1 and Th17 cytokines and their transcription factors in the conjunctiva and draining lymph nodes [98].
BAFF transgenic and Act 1 deficient
B cell survival and maturation is regulated by the B-lymphocyte stimulator known as B-cell-activating factor (BAFF) [99]. BAFF transgenic strains exhibited LF with marginal zone (MZ)-like B cells in the SG. It’s postulated that excessive BAFF expression contributes to differentiation of autoreactive B cells which leads to the loss of saliva flow [100]. In addition, BAFF transgenic strains develop anti-DNA antibodies and immunoglobulin deposition in the kidneys [101]. Unexpectedly, disrupting TNF-α in BAFF transgenic mice failed to protect mice against autoimmunity [102]. Interestingly, adaptor molecule activator 1 (Act1), which exerts survival of B cells by acting as a negative regulator of BAFF and CD40, showed similar dominancy of MZ B cells. Skin lesions were observed at an early age of 3 wks, inflammation of the SG around 27 wks and decreased levels of saliva [103,104]. A similar study was conducted in Act1-deficient mice. The results showed a similar dominancy of MZ B cells as well as the presence of skin lesions at just 3 wks of age. Additionally, inflammation in the SG was noted around 27 wks and coincided with decreased SG flow [105]. Similar to human patients, autoantibodies specific to Ro and La antigens were also detected in this mouse model [105,106].
RbAP48 transgenic
As mentioned, the primary targets for SjS are the SG and LG. The underlying mechanism for the organ specificity is not well established. A study by Ishimaru et al. [107] in which transgenic mouse model overexpressing retinoblastoma associated protein 48 (RbAP48) in the exocrine gland was developed to examine AEC. RbAP48 transgenic mice developed lymphocytic infiltration in the glands composed mostly of Thy1.2+ CD4+ T cells by 24 wks of age and more severe at 30–50 wks old. Saliva and tear flow rates were significantly reduced by 30 wks of age with high titers of autoantibodies against SSA, SSB, and 120-kD α-fodrin. Furthermore, SG cells of RbAP48 transgenic mice functioned efficiently as antigen presentation cells producing high levels of IFN-γ. Interestingly, high levels of IFN-γ are negatively impacted by the treatment of 17β-estradiol. This observation is consistent with previous study, which demonstrated that estrogen-deficient mice exhibited elevated expression of RbAP48 directly caused tissue specific apoptosis in the exocrine glands via p52 apoptotic pathway [108]. These studies have clearly indicated the role of tissue specific antigen and the influence of hormone on the immune response in SjS.
Experimentally induced/Extrinsic factor models
Murine cytomegalovirus
The role of viral infections remains controversial in autoimmunity, particularly for SjS, which is known to exhibit a strong IFN-signature. Several studies have suggested an association between SjS and hepatitis C virus, as well as members of the herpes family of viruses including Epstein-Barr virus (EBV) and/or cytomegalovirus (CMV) [109]. Sialoadenitis and production of anti-Ro and anti-La autoantibodies has been recorded in the genetically modified C57BL/6 mice when injected with CMV [110]. It was suggested that the histopathological changes were observed because of the inability of the immune system to clear infected murine CMV attributed to the modifications of Fas or TNFR1-mediated apoptosis [110]. A chronic sialoadenitis was observed in Fas (Fas/CD95)-mutant C57BL/6 (B6)-lpr/lpr mice when infected with murine CMV [110]. An apoptosis of ductal and acinar cells with high levels of Fas was found during CMV infection; encouragingly, the intervention using FasL gene therapy resulted in significant reductions in the number of inflammatory foci and the degree of tissue destruction in the SG [110].
Human T-cell leukemia virus 1 (HTLV-1)
HTLV-1 transgenic mice were originally established as a mouse model for neurofibromas, a benign cancer of the nerves in the peripheral nervous system [111]. Interestingly, detailed examination of the exocrine glands revealed proliferation of ductal epithelial cells at several wks after birth. The unregulated ductal cell proliferation resulted in abnormal glandular structure. Subsequently, lymphocytic infiltration including plasma can be detected adjacent to the proliferating epithelial cells. Transgenic mice aged from 6–8 months exhibited severe lymphocytic infiltration resulting in acinar cell destruction and thickening of basement membranes [112], a common feature often observed in human SjS [113,114]. The development of autoimmune exocrinopathy in the HTLV-1 transgenic mice is most likely due to resistance of Fas-mediated apoptosis by self-reactive T cells [115] and hyperproliferation/activation of B cells with enhanced IgM secretion [116]. It is not known which self-reactive T cells escaped the immunological selection, however the autoimmune T cell population is not specific to SjS since this mouse model has been shown to develop chronic inflammatory arthropathy which resembles rheumatoid arthritis in humans [117].
Ro peptides
Autoantigens like Ro52, Ro60 and La48 are highly up-regulated in SjS in both humans and some animal models. BALB/c mice immunized with short peptides from the 60-kDa Ro showed lymphocytic infiltrates in the SG and a subsequent decline in saliva flow. Similar to human patients, immunized animals developed an immune response directed against the entire Ro/La ribonucleoproteins [118]. Oral feeding of Ro or Ro peptides abolished the susceptibility of BALB/c mice to SjS disease induction [119] as opposed to an enhanced production of proinflammatory cytokines in Ro gene KO (Ro52−/−) mice [120]. Recent work evaluated the immunization of several different mouse strains (Balb/c, DBA-2, PL/J, SJL/J, and C57BL/6) with Ro60 peptide. Differences were vast between the various strains with SJL/J mice showing no immune response to Ro60 whereas the others had various degrees of epitope spreading or lymphocytic infiltration presenting genetically controllable preclinical model [121].
Muscarinic acetylcholine type-3 receptor (M3R) peptides
M3R is the major muscarinic acetylcholine receptor in the SG [122]. When murine M3R peptides were used to immunize C57BL/6.M3r−/− mice and their splenocytes injected into M3r−/− / Rag1−/− double KO mice, these recipient mice showed high serum levels of anti-M3R antibodies and low saliva volumes [123]. When extracellular peptides of M3R were used to vaccinate immuno-deficient C57BL/6.M3r−/− mice, they triggered the development of marked mononuclear cell inflammation in the exocrine glands accompanied by SG hypofunction suggesting the role of M3R [123]. In addition, CD3+ T cells from the double KO mice when injected into C57BL/6.Rag−/− mice also resulted in a marked cell infiltration in the SG, suggesting that M3R reactive T cells play a crucial role in the development of sialoadenitis in C57Bl/6.M3r−/−/Rag1−/− mice [123]. Studies have shown that antibodies to M3R have been detected in the saliva of patients with pSjS. The presence of these antibodies was correlated to salivary flow rates and was shown to be significantly higher than in patients with SLE [124]. New evidence in patients with SjS as compared to health controls suggests that autoantibodies specific to the second extracellular loop of M3R may play an important role in salivary dysfunction [125].
Carbonic anhydrase (CA)
CA is a metalloenzyme containing zinc ion important for the regulation of acid-base status with a wide evolutionary distribution. A subset of patients with autoimmune diseases, including patients with SjS, produces autoantibodies against CA II [126]. Intradermal immunization of PL/J (H-2u) mice with human CAII in adjuvant containing monophosphoryl lipid A and trehalosediorynomycolatein showed lymphocytic infiltration in the SG compared with mice immunized with adjuvant alone and untreated mice with minimal lymphocytic infiltrations in the pancreas and kidney [127]. Forty-five percent of patients, who met the criteria for SjS, did not have antibodies to Ro or La were found to be positive for anti-CA 6 (as well as SG Protein 1 and parotid secretory protein) [128]. This evidence suggests a potential application of anti-CA as one of the diagnostic biomarkers.
Expert commentary
SjS is a complex and multifactorial autoimmune disease in which an intricate relationship between genetics and environmental factors must be considered. As detailed in Table 1, a wide range of different mouse models has been developed to study different aspects of the underlying etiology of the disease. These mouse models provide valuable knowledge on the temporal progression of the SjS autoimmune process that currently is impossible to examine in human patients. Furthermore, these mouse models enhance our understanding of genetic factors such as individual gene or genes that are associated with susceptibility to SjS which we can modulate in-vivo. Several of the current models available allow us to evaluate particular aspects of SjS, like the induction of Ro and La antibodies in the induced models using Ro 60 peptide or M3R immunization to examine disease-specific autoantigens. Viral-associated elements have been postulated to be involved in the development of many autoimmune diseases in particular SjS, therefore having the CMV or HTLV-1 transgenic mice allow us to study the role of viruses in autoimmunity. Spontaneous mouse models like the NOD and C57BL/6.NOD-Aec1Aec2 develop a variety of symptoms and autoantibodies, which are found in human patients. These different but specific etiological mouse models may lead us to an answer on the type of system that is most beneficial for studying this disorder. One reason so many different models exist for SjS (besides the fact that no one model completely mimics overt and covert disease process of human SjS) is because there is some debate over the disease “trigger.” The question is whether the trigger is environmental or genetic or some combination of both. Perhaps in the latter we find an answer that would evaluate SjS more efficiently. Using a genetically susceptible model that is then induced virus, peptide, or cytokine may provide answers that could not be attained by either model individually.
Table 1.
Selected mouse models' phenotypes in relation to human Sjögren's syndrome symptoms
| Autoantibodies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dacryo- adenitis |
Sial- adenitis |
Pro- inflammatory cytokine |
Anti- Ro/ SSA |
Anti- La/ SSB |
ANA | Anti-α- fodrin |
Anti- β-AR5 |
Anti- M3R6 |
Decreased SFR7 |
Stomatitis sicca |
Altered Protein in saliva |
Decreased Amylase & EGF activity |
References | |
|
|
||||||||||||||
| Human Sjögren’s syndrome Patients | Yes | Yes | Yes | Yes1 | Yes1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | [129–137] |
| Mouse Model Phenotypes | ||||||||||||||
| Spontaneous Models | ||||||||||||||
|
| ||||||||||||||
| NOD | Yes | Yes | Yes | No | Yes | No | Yes | ND4 | Yes | Yes | Yes | Yes | Yes | [138,139] |
| C57BL/6.NOD-Aec1Aec2 | Yes | Yes | Yes | Yes | Yes | Yes | ND | ND | ND | Yes | Yes | Yes | No | [42,43] |
| MRL | No | Yes | Yes | ND | ND | ND | ND | ND | ND | No | ND | ND | ND | [55,56] |
| MRL/lpr | Yes | Yes | Yes | Yes | Yes2 | ND | ND | ND | ND | No | ND | ND | ND | [140–143] |
| NFS/sld | Yes | Yes | Yes | ND | ND | ND | Yes | ND | ND | Yes3 | ND | ND | ND | [60,61] |
| IQI/Jic | Yes | Yes | ND | No | No | Yes | ND | ND | ND | ND | ND | ND | ND | [69,70] |
| Knockout models | ||||||||||||||
|
| ||||||||||||||
| NOD.IFNγ−/− | Yes | No | ND | ND | ND | No | ND | ND | No | No | No | No | No | [29] |
| NOD.IL4−/− | ND | No | ND | ND | ND | Yes | ND | ND | No | No | ND | Yes | No | [33,34] |
| BALB/c.Act1−/− | No | Yes | Yes | Yes | Yes | Yes | ND | ND | ND | Yes | Yes | ND | ND | [105,106] |
| TSP-1 | Yes | ND | Yes | Yes | Yes | ND | ND | ND | ND | ND | ND | ND | ND | [97] |
| Transgenic Models | ||||||||||||||
|
| ||||||||||||||
| C57BL/6 IL-14α Tg | ND | Yes | Yes | Yes1 | Yes1 | Yes1 | ND | ND | ND | Yes | Yes | ND | ND | [94] |
| BAFF Tg | ND | Yes | Yes | No | No | Yes | ND | ND | ND | Yes | ND | ND | ND | [100,101] |
| RbAP48 Tg | Yes | Yes | Yes | Yes | Yes | ND | Yes | ND | ND | Yes | ND | ND | ND | [107] |
| Injected models | ||||||||||||||
|
| ||||||||||||||
| Fas-mutant C57BL/6-lpr/lpr + CMV | ND | Yes | ND | Yes | Yes | ND | ND | ND | ND | ND | ND | ND | ND | [109,110] |
| BALB/c + 60-kDA Ro Ag peptides | ND | Yes | ND | Yes | Yes | ND | ND | ND | No | Yes | ND | ND | ND | [118,121] |
| PL/J (H-2u) + CAII | ND | Yes | Yes | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | [126] |
Variable,
Low levels,
Thought to be due to factors other than autoimmune response,
Several variables have not yet been examined in these mouse models and are therefore given the designation ND (no data).
Adrenergic Receptor,
Type III Muscarinic Receptor,
Saliva Flow Rate
Five years view
While the current mouse models have aided in our understanding of SjS, its etiology, symptoms, and its pathogenesis, there is still much to be elucidated. With the mouse models currently available, it is difficult to ascertain the complete mechanism of disease progression. For example, the current mouse models are limited in expression of Ro and La autoantibodies, a hallmark feature and diagnostic marker of disease. Additionally, the sheer number of “accepted” models means that there will be differences in attained results based on the models selected. Whether results attained from one model would be maintained in another would corroborate one’s results but may not be possible due to the variation in model development. This leads to the key questions to be answered when dealing with SjS, what is the etiology and are there multiple factors which affect either generation of the disease or its severity? Continued extensive study in this area is critical for further evaluation of SjS.
Another area that is of critical importance is therapy development. To date, the clinical therapies available are primarily dedicated to treat symptoms and not the disorder. The available mouse models cover a wide array of stages of disease allowing specific stages to be studied independently, but proper development of novel therapeutics will require development of a comprehensive mouse model. This could be attained through the use of genome-wide association (GWA) studies, which allow the comparison of a number of genetic variants to determine commonality. One major area that has not been addressed in many and possibly all the current mouse models is a lack of experimentation has been performed on the neuropathies associated with SjS. While some symptoms, such as headache, are not easily measurable in mice, others (e.g. fatigue, memory-loss, etc.) are. Moving forward it will become necessary to either generate or determine which of the already existing mouse models exhibit a more complete representation of SjS, to allow development of treatments focused on treating the disease as a whole.
Key issues.
Treatment and/or prevention of SjS are currently impeded by a lack of understanding pre-disease events and subsequent disease progression.
While each mouse model of SS mimics particular disease symptoms or phenotypes, the characteristics vary across models. To date, there is no single mouse model that comprehensively replicates the full breadth of human SjS.
The combination of methods for development of mouse model (ie spontaneous combined with immunization) may mimic disease more thoroughly demonstrating a possible role for both genetic and environmental disease induction.
Future research should be directed towards continued development of diagnostic and therapeutic strategies and should include a more comprehensive mouse model perhaps designed and built based on on-going GWA studies.
Acknowledgments
This study was supported in part by PHS grants R00 DE018958 grant (CQ Nguyen) from the NIDCR and funds from the Center for Orphan Autoimmune Disorders.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
* Of interest
** Of considerable interest
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10(5):351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 3.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen CQ, Cha SR, Peck AB. Sjögren’s syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Frontiers in Bioscience. 2007;12:1767–1789. doi: 10.2741/2187. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavragani CP, Moutsopoulos HM. Sjogren’s Syndrome. Annu Rev Pathol. 2013 doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 7.Talal N. Overview of Sjögren’s syndrome. J Dent Res. 1987;66:672–674. doi: 10.1177/00220345870660s111. [DOI] [PubMed] [Google Scholar]
- 8.Whitacre CC. Sex differences in autoimmune disease. Nature immunology. 2001;2(9):777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan DA. Sex hormones and Sjogren’s syndrome. J. Rheumatol. Suppl. 1997;50:17–32. [PubMed] [Google Scholar]
- 10.Mellgren SI, Conn DL, Stevens JC, Dyck PJ. Peripheral neuropathy in primary Sjogren’s syndrome. Neurology. 1989;39(3):390–394. doi: 10.1212/wnl.39.3.390. [DOI] [PubMed] [Google Scholar]
- 11.Meijer JM, Meiners PM, Huddleston Slater JJ, et al. Health-related quality of life, employment and disability in patients with Sjogren’s syndrome. Rheumatology (Oxford, England) 2009;48(9):1077–1082. doi: 10.1093/rheumatology/kep141. [DOI] [PubMed] [Google Scholar]
- 12.Mengshoel AM, Norheim KB, Omdal R. Primary Sjogren’s syndrome - Fatigue is an ever-present, fluctuating and uncontrollable lack of energy. Arthritis care & research. 2013 doi: 10.1002/acr.22263. [DOI] [PubMed] [Google Scholar]
- 13.Malladi AS, Sack KE, Shiboski SC, et al. Primary Sjogren’s syndrome as a systemic disease: a study of participants enrolled in an international Sjogren’s syndrome registry. Arthritis care & research. 2012;64(6):911–918. doi: 10.1002/acr.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vrethem M, Lindvall B, Holmgren H, Henriksson KG, Lindstrom F, Ernerudh J. Neuropathy and myopathy in primary Sjogren’s syndrome: neurophysiological, immunological and muscle biopsy results. Acta neurologica Scandinavica. 1990;82(2):126–131. doi: 10.1111/j.1600-0404.1990.tb01601.x. [DOI] [PubMed] [Google Scholar]
- 15.Malinow KL, Molina R, Gordon B, Selnes OA, Provost TT, Alexander EL. Neuropsychiatric dysfunction in primary Sjogren’s syndrome. Ann Intern Med. 1985;103(3):344–350. doi: 10.7326/0003-4819-103-3-344. [DOI] [PubMed] [Google Scholar]
- 16.Gokcay F, Oder G, Celebisoy N, Gokcay A, Sirin H, Kabasakal Y. Headache in primary Sjogren’s syndrome: a prevalence study. Acta neurologica Scandinavica. 2008;118(3):189–192. doi: 10.1111/j.1600-0404.2008.00997.x. [DOI] [PubMed] [Google Scholar]
- 17.Tjensvoll AB, Harboe E, Goransson LG, et al. Headache in primary Sjogren’s syndrome: a population-based retrospective cohort study. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013;20(3):558–563. doi: 10.1111/ene.12033. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie TN, Lee BH, Nguyen CQ. Current concepts: mouse models of Sjogren’s syndrome. J Biomed Biotechnol. 2011;2011:549107. doi: 10.1155/2011/549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byung Ha Lee AEG, Pauley Kaleb M, Park Yun-Jong, Cha Seunghee. Animal models in autoimmune diseases: lessons learned from mouse models for sjogren syndrome. Clin Rev Allerg Immunol. 2012;42:35–44. doi: 10.1007/s12016-011-8288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delaleu N, Nguyen CQ, Peck AB, Jonsson R. Sjögren’s syndrome: studying the disease in mice. Arthritis Res Ther. 2011;13:217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Robinson CP, Yamachika S, Bounous DI, et al. A novel NOD-derived murine model of primary Sjögren’s syndrome. Arthritis Rheum. 1998;41:150–156. doi: 10.1002/1529-0131(199801)41:1<150::AID-ART18>3.0.CO;2-T. First study to suggest that T1D-susceptible MHC I-A(g7) is not require for SjS and SjS occurs independently of T1D. [DOI] [PubMed] [Google Scholar]
- 22.Delaleu N. Biomarker profiles in serum and saliva of experimental sjogren syndrome: associations with specific autoimmune manifestations. Arthritis Res Ther. 2008;10:R22. doi: 10.1186/ar2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Seunghee Cha HN, Brown Vinette B, Peck Ammon B, Humphreys Beher Michael G. Two NOD IDD-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (sjogren syndrome) on a healthy murine background. Arthritis & Rheumatism. 2007;46(5):1390–1398. doi: 10.1002/art.10258. This study identified two SjS-susceptible loci that are directly contributed to SjS. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Nakagawa Y, Purushotam KR, Humphreys-Beher MG. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263:E607–E614. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- 25.Robinson CP, Cornelius J, Bounous DE, Yamamoto H, Humphreys-Beher MG, Peck AB. Characterization of the changing lymphocyte populations and cytokine expression in the exocrine tissues of autoimmune NOD mice. Autoimmunity. 1998;27:29–44. doi: 10.3109/08916939809008035. [DOI] [PubMed] [Google Scholar]
- 26.van Blokland SC, van Helden-Meeuwsen CG, Wierenga-Wolf AF, et al. Two different types of sialoadenitis in the NOD- and MRL/lpr mouse models for Sjogren’s syndrome: a differential role for dendritic cells in the initiation of sialoadenitis? Lab Invest. 2000;80:575–585. doi: 10.1038/labinvest.3780062. [DOI] [PubMed] [Google Scholar]
- 27.Yanagi K, Haneji N, Ishimaru N, Saito I, Hayashi Y. Analysis of T cell receptor Vbeta usage in the autoimmune sialadenitis of non-obese diabetic (NOD) mice. Clinical and experimental immunology. 1997;110:440–446. doi: 10.1046/j.1365-2249.1997.4271445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roescher N, Vosters JL, Yin H, Illei GG, Tak PP, Chiorini JA. Effect of soluble ICAM-1 on a sjogren syndrome-like phenotype in NOD mice is disease stage dependent. PLoS One. 2011;6:e19962. doi: 10.1371/journal.pone.0019962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cha S, Brayer J, Gao J, et al. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand. J. Immunol. 2004;60(6):552–565. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 30*.Robinson CPBJ, Yamachika S, Esch TR, Peck AB, Stewart CA, Peen E, Jonsson R, Humphreys-Beher MG. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjögren’s syndrome. Proc Natl Acad Sci U S A. 1998;95(13):7538–7843. doi: 10.1073/pnas.95.13.7538. This is the first study which demonstrated that pathogenic antibodies from SjS patients and NOD mice were capable of shutting down saliva flow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Cha S, Jonsson R, Opalko J, Peck AB. Detection of anti-type 3 muscarinic acetylcholine receptor autoantibodies in the sera of Sjogren’s syndrome patients by use of a transfected cell line assay. Arthritis Rheum. 2004;50(8):2615–2621. doi: 10.1002/art.20371. [DOI] [PubMed] [Google Scholar]
- 32.Cha S, Singson E, Cornelius J, Yagna JP, Knot HJ, Peck AB. Muscarinic acetylcholine type-3 receptor desensitization due to chronic exposure to Sjogren’s syndrome-associated autoantibodies. J. Rheumatol. 2006;33(2):296–306. [PubMed] [Google Scholar]
- 33.Brayer JB, Cha S, Nagashima H, et al. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD.IL-4-gene knockout mouse model of Sjogren’s syndrome. Scand. J. Immunol. 2001;54(1–2):133–140. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 34.Gao J, Killedar S, Cornelius JG, Nguyen C, Cha S, Peck AB. Sjogren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J. Autoimmun. 2006;26(2):90–103. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Bowman S, Barone F. Biologic treatments in Sjogren’s syndrome. Presse Med. 2012;41(9 Pt 2):e495–509. doi: 10.1016/j.lpm.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Devauchelle-Pensec V, Pennec Y, Morvan J, et al. Improvement of Sjogren’s syndrome after two infusions of rituximab (anti-CD20) Arthritis and rheumatism. 2007;57(2):310–317. doi: 10.1002/art.22536. [DOI] [PubMed] [Google Scholar]
- 37.Pers JO, Devauchelle V, Daridon C, et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjogren’s syndrome. Arthritis and rheumatism. 2007;56(5):1464–1477. doi: 10.1002/art.22603. [DOI] [PubMed] [Google Scholar]
- 38.Pijpe J, Meijer JM, Bootsma H, et al. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjogren’s syndrome. Arthritis and rheumatism. 2009;60(11):3251–3256. doi: 10.1002/art.24903. [DOI] [PubMed] [Google Scholar]
- 39.Meijer JM, Pijpe J, Vissink A, Kallenberg CG, Bootsma H. Treatment of primary Sjogren syndrome with rituximab: extended follow-up, safety and efficacy of retreatment. Annals of the rheumatic diseases. 2009;68(2):284–285. doi: 10.1136/ard.2008.092601. [DOI] [PubMed] [Google Scholar]
- 40.Seror R, Sordet C, Guillevin L, et al. Tolerance and efficacy of rituximab and changes in serum B cell biomarkers in patients with systemic complications of primary Sjogren’s syndrome. Annals of the rheumatic diseases. 2007;66(3):351–357. doi: 10.1136/ard.2006.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottenberg JE, Guillevin L, Lambotte O, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Annals of the rheumatic diseases. 2005;64(6):913–920. doi: 10.1136/ard.2004.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brayer J, Lowry J, Cha S, et al. Alleles from chromosomes 1 and 3 of NOD mice combine to influence Sjogren’s syndrome-like autoimmune exocrinopathy. J Rheumatol. 2000;27(8):1896–1904. [PubMed] [Google Scholar]
- 43.Cha S, Nagashima H, Brown VB, Peck AB, Humphreys-Beher MG. Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjogren’s syndrome) on a healthy murine background. Arthritis. Rheum. 2002;46(5):1390–1398. doi: 10.1002/art.10258. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren’s syndrome: findings in humans and mice. Arthritis & Rheumatism. 2008;58(3):734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin JO, Shinohara YQY. Innate Immune Signaling Induces Interleukin-7 Production from Salivary Gland Cells and Accelerates the Development of Primary Sjögren’s Syndrome in a Mouse Model. PLoS One. 2013;8(10):e77605. doi: 10.1371/journal.pone.0077605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen CQ, Ogunniyi AO, Karabiyik A, JC L. Single-cell analysis reveals isotype-specific autoreactive B cell repertoires in Sjögren’s syndrome. PLoS One. 2013;8(3):e58127. doi: 10.1371/journal.pone.0058127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen C, Singson E, Kim JY, et al. Sjögren’s syndrome-like disease of C57BL/6.NOD-Aec1 Aec2 mice: gender differences in keratoconjunctivitis sicca defined by a cross-over in the chromosome 3 Aec1 locus. Scandinavian Journal of Immunology. 2006;64(3):295–307. doi: 10.1111/j.1365-3083.2006.01828.x. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen CQ, Cornelius JG, Cooper L, et al. Identification of possible candidate genes regulating Sjogren’s syndrome-associated autoimmunity: a potential role for TNFSF4 in autoimmune exocrinopathy. Arthritis Res Ther. 2008;10(6):R137. doi: 10.1186/ar2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordmark G, Kristjansdottir G, Theander E, et al. Association of EBF1, FAM167A(C8orf13)-BLK and TNFSF4 gene variants with primary Sjögren’s syndrome. Genes Immun. 2011;12(2):100–109. doi: 10.1038/gene.2010.44. [DOI] [PubMed] [Google Scholar]
- 50.Sun F, Li P, Chen H, et al. Association studies of TNFSF4, TNFAIP3 and FAM167A–BLK polymorphisms with primary Sjogren’s syndrome in Han Chinese. J Hum Genet. 2013;58(7):475–479. doi: 10.1038/jhg.2013.26. [DOI] [PubMed] [Google Scholar]
- 51.Hang L, Theofilopoulos AN, Dixon FJ. A spontaneous rheumatoid arthritis-like disease in MRL/l mice. J Exp Med. 1982;155:1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 53.Skarstein K, Johannessen AC, Holmdahl RRJ. Effects of sialadenitis after cellular transfer in autoimmune MRL/lpr mice. Clin Immunol Immunopathol. 1997;84(2):177–184. doi: 10.1006/clin.1997.4387. [DOI] [PubMed] [Google Scholar]
- 54.Fleck M, Kern ER, Zhou T, Lang B, JD M. Murine cytomegalovirus induces a Sjögren’s syndrome-like disease in C57Bl/6-lpr/lpr mice. Arthritis Rheumatol Int. 1998;41:2175–2184. doi: 10.1002/1529-0131(199812)41:12<2175::AID-ART12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 55.Hang L, Theofilopoulos AN, Dixon FJ. A spontaneous rheumatoid arthritis-like disease in MRL/l mice. J Exp Med. 1982;155(6):1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoffman RW, Alspaugh MA, Waggie KS, Durham JB, Walker SE. Sjogren’s syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984;27(2):157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- 57.Jonsson R, Holmdahl R. Infiltrating mononuclear cells in salivary glands and kidneys in autoimmune MRL/Mp-lpr/lpr mice express IL-2 receptor and produce interferon-gamma. J Oral Pathol Med. 1990;19:330–334. doi: 10.1111/j.1600-0714.1990.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 58.Jonsson R, Tarkowski A, Backman K, Holmdahl R, Klareskog L. Sialadenitis in the MRL-l mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987;60:611–616. [PMC free article] [PubMed] [Google Scholar]
- 59.Jonsson R, Pitts A, Mestecky J, Koopman W. Local IgA and IgM rheumatoid factor production in autoimmune MRL/lpr mice. Autoimmunity. 1991;10:7–14. doi: 10.3109/08916939108997142. [DOI] [PubMed] [Google Scholar]
- 60.Haneji N, Hamano H, Yanagi K, Hayashi Y. A new animal model for primary Sjogren’s syndrome in NFS/sld mutant mice. J. Immunol. 1994;153(6):2769–2777. [PubMed] [Google Scholar]
- 61*.Haneji N, Nakamura T, Takio K, et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science. 1997;276(5312):604–607. doi: 10.1126/science.276.5312.604. Elegant study which showed the role of α-fodrin autoantigen in SjS. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi YHN, Hamano H, Yanagi K, Takahashi M, Ishimaru N. Effector mechanism of experimental autoimmune sialadenitis in the mouse model for primary Sjögren’s syndrome. Cell Immunol. 1996;171:217–225. doi: 10.1006/cimm.1996.0196. [DOI] [PubMed] [Google Scholar]
- 63.Zandbelt MMVJ, Van De Putte LB, Van Venrooij WJ, Van Den Hoogen FH. Anti-α-fodrin antibodies do not add much to the diagnosis of Sjögren’s syndrome. Arthritis Res Ther. 2004;6:R33–R38. doi: 10.1186/ar1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saegusa K, Ishimaru N, Yanagi K, et al. Prevention and induction of autoimmune exocrinopathy is dependent on pathogenic autoantigen cleavage in murine Sjogren’s syndrome. Journal of immunology. 2002;169(2):1050–1057. doi: 10.4049/jimmunol.169.2.1050. [DOI] [PubMed] [Google Scholar]
- 65.Vanags DM, Porn-Ares MI, Coppola S, Burgess DH, Orrenius S. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. The Journal of biological chemistry. 1996;271(49):31075–31085. doi: 10.1074/jbc.271.49.31075. [DOI] [PubMed] [Google Scholar]
- 66.Yanagi K, Ishimaru N, Haneji N, Saegusa K, Saito I, Hayashi Y. Anti-120-kDa alpha-fodrin immune response with Th1-cytokine profile in the NOD mouse model of Sjogren’s syndrome. Eur J Immunol. 1998;28(10):3336–3345. doi: 10.1002/(SICI)1521-4141(199810)28:10<3336::AID-IMMU3336>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 67.Witte T, Matthias T, Arnett FC, et al. IgA and IgG autoantibodies against alpha-fodrin as markers for Sjogren’s syndrome. Systemic lupus erythematosus. J Rheumatol. 2000;27(11):2617–2620. [PubMed] [Google Scholar]
- 68.Saegusa J, Kubota H. Sialadenitis in IQI/Jic mice: a new animal model of Sjogren’s syndrome. J Vet Med Sci. 1997;59(10):897–903. doi: 10.1292/jvms.59.897. [DOI] [PubMed] [Google Scholar]
- 69.Takada K, Takiguchi M, Konno A, Inaba M. Spontaneous development of multiple glandular and extraglandular lesions in aged IQI/Jic mice: a model for primary Sjogren’s syndrome. Rheumatology (Oxford) 2004;43(7):858–862. doi: 10.1093/rheumatology/keh209. [DOI] [PubMed] [Google Scholar]
- 70.Takada K, Takiguchi M, Konno A, Inaba M. Autoimmunity against a tissue kallikrein in IQI/Jic Mice: a model for Sjogren’s syndrome. J. Biol. Chem. 2005;280(5):3982–3988. doi: 10.1074/jbc.M410157200. [DOI] [PubMed] [Google Scholar]
- 71.El Annan J, Jiang G, Wang D, Zhou J, Foulks GN, Shao H. Elevated immunoglobulin to tissue KLK11 in patients with Sjogren syndrome. Cornea. 2013;32(5):e90–93. doi: 10.1097/ICO.0b013e31826a1e2e. [DOI] [PubMed] [Google Scholar]
- 72.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nature reviews. Immunology. 2010;10(12):849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kramer S, Schimpl A, Hunig T. Immunopathology of interleukin (IL) 2-deficient mice: thymus dependence and suppression by thymus-dependent cells with an intact IL-2 gene. The Journal of experimental medicine. 1995;182(6):1769–1776. doi: 10.1084/jem.182.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma R, Deshmukh US, Zheng L, Fu SM, Ju ST. X-linked Foxp3 (Scurfy) mutation dominantly inhibits submandibular gland development and inflammation respectively through adaptive and innate immune mechanisms. Journal of immunology. 2009;183(5):3212–3218. doi: 10.4049/jimmunol.0804355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox RI, Theofilopoulos AN, Altman A. Production of interleukin 2 (IL 2) by salivary gland lymphocytes in Sjogren’s syndrome. Detection of reactive cells by using antibody directed to synthetic peptides of IL 2. Journal of immunology. 1985;135(5):3109–3115. [PubMed] [Google Scholar]
- 76.Szodoray P, Papp G, Horvath IF, et al. Cells with regulatory function of the innate and adaptive immune system in primary Sjogren’s syndrome. Clin Exp Immunol. 2009;157(3):343–349. doi: 10.1111/j.1365-2249.2009.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Qian L, Wang G, et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjogren’s syndrome. The Journal of rheumatology. 2007;34(12):2438–2445. [PubMed] [Google Scholar]
- 78.Mauri C, Bosma A. Immune regulatory function of B cells. Annual review of immunology. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 79.Saito IHK, Shimuta M, Inoue H, Sakurai H, Yamada K, Ishimaru N, Higashiyama H, Sumida T, Ishida H, Suda T, Noda T, Hayashi Y, Tsubota K. Fas ligand-mediated exocrinopathy resembling Sjögren’s syndrome in mice transgenic for IL-10. J Immunol Methods. 1999;162:2488–2494. [PubMed] [Google Scholar]
- 80.Kok MRYS, Lodde BM, Wang J, Couwenhoven RI, Yakar S, Voutetakis A, Leroith D, Schmidt M, Afione S, Pillemer SR, Tsutsui MT, Tak PP, Chiorini JA, Baum BJ. Local adeno-associated virus-mediated interleukin 10 gene transfer has disease-modifying effects in a murine model of Sjögren’s syndrome. Hum Gene Ther. 2003;14:1605–1618. doi: 10.1089/104303403322542257. [DOI] [PubMed] [Google Scholar]
- 81.Bertorello R, Cordone MP, Contini P, et al. Increased levels of interleukin-10 in saliva of Sjogren’s syndrome patients. Correlation with disease activity. Clin Exp Med. 2004;4(3):148–151. doi: 10.1007/s10238-004-0049-9. [DOI] [PubMed] [Google Scholar]
- 82.Perrier S, Serre AF, Dubost JJ, et al. Increased serum levels of interleukin 10 in Sjogren’s syndrome; correlation with increased IgG1. The Journal of rheumatology. 2000;27(4):935–939. [PubMed] [Google Scholar]
- 83.Giron-Gonzalez JA, Baturone R, Soto MJ, et al. Implications of immunomodulatory interleukins for the hyperimmunoglobulinemia of Sjogren’s syndrome. Cell Immunol. 2009;259(1):56–60. doi: 10.1016/j.cellimm.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Font J, Garcia-Carrasco M, Ramos-Casals M, et al. The role of interleukin-10 promoter polymorphisms in the clinical expression of primary Sjogren’s syndrome. Rheumatology. 2002;41(9):1025–1030. doi: 10.1093/rheumatology/41.9.1025. [DOI] [PubMed] [Google Scholar]
- 85.Origuchi T, Kawasaki E, Ide A, et al. Correlation between interleukin 10 gene promoter region polymorphisms and clinical manifestations in Japanese patients with Sjogren’s syndrome. Annals of the rheumatic diseases. 2003;62(11):1117–1118. doi: 10.1136/ard.62.11.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Willeke P, Gaubitz M, Schotte H, Becker H, Domschke W, Schluter B. The role of interleukin-10 promoter polymorphisms in primary Sjogren’s syndrome. Scandinavian journal of rheumatology. 2008;37(4):293–299. doi: 10.1080/03009740801910353. [DOI] [PubMed] [Google Scholar]
- 87.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 88.McGrath-Morrow S, Laube B, Tzou SC, et al. IL-12 overexpression in mice as a model for Sjogren lung disease. American journal of physiology. Lung cellular and molecular physiology. 2006;291(4):L837–846. doi: 10.1152/ajplung.00134.2006. [DOI] [PubMed] [Google Scholar]
- 89.Kimura-Shimmyo AKS, Ueda H, Ikeda T, Kanno S, Akira S, Nakanishi K, Mimura O, Okamura H. Cytokine-induced injury of the lacrimal and salivary glands. J Immunother. 2002;25(suppl 1):S42–S51. doi: 10.1097/00002371-200203001-00007. [DOI] [PubMed] [Google Scholar]
- 90.Bombardieri M, Barone F, Pittoni V, et al. Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjogren’s syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res Ther. 2004;6(5):R447–456. doi: 10.1186/ar1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Horiuchi M, Yamano S, Inoue H, et al. Possible involvement of IL-12 expression by Epstein-Barr virus in Sjogren syndrome. J Clin Pathol. 1999;52(11):833–837. doi: 10.1136/jcp.52.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen L, Suresh L, Wu J, et al. A role for lymphotoxin in primary Sjogren’s disease. Journal of immunology. 2010;185(10):6355–6363. doi: 10.4049/jimmunol.1001520. [DOI] [PubMed] [Google Scholar]
- 93.Delaleu N, Immervoll H, Cornelius J, Jonsson R. Biomarker profiles in serum and saliva of experimental Sjogren’s syndrome: associations with specific autoimmune manifestations. Arthritis Res Ther. 2008;10(1):R22. doi: 10.1186/ar2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen L, Suresh L, Li H, et al. IL-14 alpha, the nexus for primary Sjogren’s disease in mice and humans. Clinical immunology. 2009;130(3):304–312. doi: 10.1016/j.clim.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 95.Altorok N, Coit P, Hughes T, et al. Genome-wide DNA methylation patterns in naive CD4 T cells from patients with primary Sjogren’s syndrome. Arthritis and rheumatism. 2013 doi: 10.1002/art.38264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bolstad AI, Le Hellard S, Kristjansdottir G, et al. Association between genetic variants in the tumour necrosis factor/lymphotoxin alpha/lymphotoxin beta locus and primary Sjogren’s syndrome in Scandinavian samples. Annals of the rheumatic diseases. 2012;71(6):981–988. doi: 10.1136/annrheumdis-2011-200446. [DOI] [PubMed] [Google Scholar]
- 97.Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjogren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175(3):1136–1147. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Contreras-Ruiz L, Regenfuss B, Mir FA, Kearns J, Masli S. Conjunctival Inflammation in Thrombospondin-1 Deficient Mouse Model of Sjogren’s Syndrome. PLoS One. 2013;8(9):e75937. doi: 10.1371/journal.pone.0075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mackay FSP. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 100*.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J. Clin. Invest. 2002;109(1):59–68. doi: 10.1172/JCI14121. Presented clear evidence on how BAFF is involved in the development of SjS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Batten MFC, Ng LG, Groom J, Wheway J, Laabi Y, Xin X, Schneider P, Tschopp J, Mackay CR, Mackay F. J Immunol. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. 2004;172:812–822. doi: 10.4049/jimmunol.172.2.812. [DOI] [PubMed] [Google Scholar]
- 103.Qian YGN, Xiao J, Wang Y, Tian J, Han S, Scott M, Carter R, Jorgensen TN, Li X. Deficiency of Act1, a critical modulator of B cell function, leads to development of Sjögren’s syndrome. Eur J Immunol. 2008;38:2219–2228. doi: 10.1002/eji.200738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.X L. Act1 modulates autoimmunity through its dual functions in CD40L/ BAFF and IL-17 signaling. Cytokine Growth Factor Rev. 2008;41:105–113. doi: 10.1016/j.cyto.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 105.Qian Y, Giltiay N, Xiao J, et al. Deficiency of Act1, a critical modulator of B cell function, leads to development of Sjogren’s syndrome. Eur J Immunol. 2008;38(8):2219–2228. doi: 10.1002/eji.200738113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson AC, Davison LM, Giltiay NV, Vareechon C, Li X, Jorgensen TN. Lack of T cells in Act1-deficient mice results in elevated IgM-specific autoantibodies but reduced lupus-like disease. Eur J Immunol. 2012;42(7):1695–1705. doi: 10.1002/eji.201142238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ishimaru N, Arakaki R, Yoshida S, Yamada A, Noji S, Hayashi Y. Expression of the retinoblastoma protein RbAp48 in exocrine glands leads to Sjogren’s syndrome-like autoimmune exocrinopathy. The Journal of experimental medicine. 2008;205(12):2915–2927. doi: 10.1084/jem.20080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ishimaru N, Arakaki R, Omotehara F, et al. Novel role for RbAp48 in tissue-specific, estrogen deficiency-dependent apoptosis in the exocrine glands. Mol Cell Biol. 2006;26(8):2924–2935. doi: 10.1128/MCB.26.8.2924-2935.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fleck M, Kern ER, Zhou T, Lang B, Mountz JD. Murine cytomegalovirus induces a Sjogren’s syndrome-like disease in C57Bl/6-lpr/lpr mice. Arthritis and rheumatism. 1998;41(12):2175–2184. doi: 10.1002/1529-0131(199812)41:12<2175::AID-ART12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 110.Fleck M, Zhang HG, Kern ER, Hsu HC, Muller-Ladner U, Mountz JD. Treatment of chronic sialadenitis in a murine model of Sjogren’s syndrome by local fasL gene transfer. Arthritis and rheumatism. 2001;44(4):964–973. doi: 10.1002/1529-0131(200104)44:4<964::AID-ANR154>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 111.Hinrichs SH, Nerenberg M, Reynolds RK, Khoury G, Jay G. A transgenic mouse model for human neurofibromatosis. Science. 1987;237(4820):1340–1343. doi: 10.1126/science.2888191. [DOI] [PubMed] [Google Scholar]
- 112.Green JE, Hinrichs SH, Vogel J, Jay G. Exocrinopathy resembling Sjogren’s syndrome in HTLV-1 tax transgenic mice. Nature. 1989;341(6237):72–74. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- 113.Molina C, Alliende C, Aguilera S, et al. Basal lamina disorganisation of the acini and ducts of labial salivary glands from patients with Sjogren’s syndrome: association with mononuclear cell infiltration. Annals of the rheumatic diseases. 2006;65(2):178–183. doi: 10.1136/ard.2004.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Velozo J, Aguilera S, Alliende C, et al. Severe alterations in expression and localisation of {alpha}6{beta}4 integrin in salivary gland acini from patients with Sjogren syndrome. Annals of the rheumatic diseases. 2009;68(6):991–996. doi: 10.1136/ard.2008.089607. [DOI] [PubMed] [Google Scholar]
- 115.Kishi S, Saijyo S, Arai M, et al. Resistance to fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-I) transgenic mice with autoimmune arthropathy. The Journal of experimental medicine. 1997;186(1):57–64. doi: 10.1084/jem.186.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peebles RS, Maliszewski CR, Sato TA, et al. Abnormal B-cell function in HTLV-I-tax transgenic mice. Oncogene. 1995;10(6):1045–1051. [PubMed] [Google Scholar]
- 117.Iwakura Y, Saijo S, Kioka Y, et al. Autoimmunity induction by human T cell leukemia virus type 1 in transgenic mice that develop chronic inflammatory arthropathy resembling rheumatoid arthritis in humans. Journal of immunology. 1995;155(3):1588–1598. [PubMed] [Google Scholar]
- 118*.Scofield RH, Asfa S, Obeso D, Jonsson R, Kurien BT. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren’s syndrome. J. Immunol. 2005;175(12):8409–8414. doi: 10.4049/jimmunol.175.12.8409. Described for the first time immunization using Ro peptides resulted in SjS. [DOI] [PubMed] [Google Scholar]
- 119.Kurien BT, Asfa S, Li C, Dorri Y, Jonsson R, RH S. Induction of oral tolerance in experimental Sjögren’s syndrome autoimmunity. Scand J Immunol Lett. 2005;61:418–425. doi: 10.1111/j.1365-3083.2005.01593.x. [DOI] [PubMed] [Google Scholar]
- 120*.Espinosa A, Dardalhon V, Brauner S, et al. Loss of the lupus autoantigenRo52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med. 2009;206:1661–1671. doi: 10.1084/jem.20090585. This study was the first to provide direct evidence that defective Ro52 antigen can lead to systemic autoimmunity via IL-23-Th17 pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kurien BT, Dsouza A, Igoe A, et al. Immunization with 60 kD Ro peptide produces different stages of preclinical autoimmunity in a Sjogren’s syndrome model among multiple strains of inbred mice. Clin Exp Immunol. 2013;173(1):67–75. doi: 10.1111/cei.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Yea. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000;97:9579e9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123*.Iizuka M, Wakamatsu E, Tsuboi H, et al. Pathogenic role of immune response to M3 muscarinic acetylcholine receptor in Sjögren’s syndrome-like sialoadenitis. J Autoimmun. 2010;35:383–389. doi: 10.1016/j.jaut.2010.08.004. This study provided a clear evidence on the role of M3R in SjS. [DOI] [PubMed] [Google Scholar]
- 124.He J, Qiang L, Ding Y, et al. The role of muscarinic acetylcholine receptor type 3 polypeptide (M3RP205-220) antibody in the saliva of patients with primary Sjogren’s syndrome. Clin Exp Rheumatol. 2012;30(3):322–326. [PubMed] [Google Scholar]
- 125.Tsuboi H, Iizuka M, Asashima H, Sumida T. Anti-M3 muscarinic acetylcholine receptor antibodies and Sjogren’s syndrome. Nihon Rinsho Meneki Gakkai Kaishi. 2013;36(2):77–85. doi: 10.2177/jsci.36.77. [DOI] [PubMed] [Google Scholar]
- 126.Inagaki Y, Jinno-Yoshida Y, Hamasaki Y, Ueki H. A novel autoantibody reactive with carbonic anhydrase in sera from patients with systemic lupus erythematosus and Sjogren’s syndrome. J Dermatol Sci. 1991;2(3):147–154. doi: 10.1016/0923-1811(91)90060-b. [DOI] [PubMed] [Google Scholar]
- 127.Nishimori I, Bratanova T, Toshkov I, Caffrey T, Mogaki M, Shibata Y. Hollingsworth MA: Induction of experimental autoimmune sialoadenitis by immunization of PL/J mice with carbonic anhydrase II. J Immunol Methods. 1995;154:4865–4873. [PubMed] [Google Scholar]
- 128.Shen L, Suresh L, Lindemann M, et al. Novel autoantibodies in Sjogren’s syndrome. Clinical immunology. 2012;145(3):251–255. doi: 10.1016/j.clim.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 129.Dawson L, Tobin A, Smith P, Gordon T. Antimuscarinic antibodies in Sjogren’s syndrome: where are we, and where are we going? Arthritis. Rheum. 2005;52(10):2984–2995. doi: 10.1002/art.21347. [DOI] [PubMed] [Google Scholar]
- 130.Delaleu N, Jonsson R, Koller MM. Sjogren’s syndrome. Eur. J. Oral Sci. 2005;113(2):101–113. doi: 10.1111/j.1600-0722.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 131.Fox PC, Speight PM. Current concepts of autoimmune exocrinopathy: immunologic mechanisms in the salivary pathology of Sjogren’s syndrome. Crit. Rev. Oral Biol. Med. 1996;7(2):144–158. doi: 10.1177/10454411960070020301. [DOI] [PubMed] [Google Scholar]
- 132.Fox RI, Kang HI. Pathogenesis of Sjogren’s syndrome. Rheum. Dis. Clin. North Am. 1992;18(3):517–538. [PubMed] [Google Scholar]
- 133.Gordon TP, Bolstad AI, Rischmueller M, Jonsson R, Waterman SA. Autoantibodies in primary Sjogren’s syndrome: new insights into mechanisms of autoantibody diversification and disease pathogenesis. Autoimmunity. 2001;34(2):123–132. doi: 10.3109/08916930109001960. [DOI] [PubMed] [Google Scholar]
- 134.Hansen A, Lipsky PE, Dorner T. New concepts in the pathogenesis of Sjogren syndrome: many questions, fewer answers. Curr. Opin. Rheumatol. 2003;15(5):563–570. doi: 10.1097/00002281-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 135.Hansen A, Lipsky PE, Dorner T. Immunopathogenesis of primary Sjogren’s syndrome: implications for disease management and therapy. Curr. Opin. Rheumatol. 2005;17(5):558–565. doi: 10.1097/01.bor.0000172801.56744.c3. [DOI] [PubMed] [Google Scholar]
- 136.Jonsson R, Haga HJ, Gordon TP. Current concepts on diagnosis, autoantibodies and therapy in Sjogren’s syndrome. Scand. J. Rheumatol. 2000;29(6):341–348. doi: 10.1080/030097400447525. [DOI] [PubMed] [Google Scholar]
- 137.Manthorpe R, Bredberg A, Henriksson G, Larsson A. Progress and regression within primary Sjogren’s syndrome. Scand. J. Rheumatol. 2006;35(1):1–6. doi: 10.1080/03009740500537945. [DOI] [PubMed] [Google Scholar]
- 138.Doyle ME, Boggs L, Attia R, et al. Autoimmune dacryoadenitis of NOD/LtJ mice and its subsequent effects on tear protein composition. Am J Pathol. 2007;171(4):1224–1236. doi: 10.2353/ajpath.2007.070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Humphreys-Beher MG, Peck AB. New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model. Arch Oral Biol. 1999;(44 Suppl. 1):S21–25. doi: 10.1016/s0003-9969(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 140.Bloom DD, St Clair EW, Pisetsky DS, Clarke SH. The anti-La response of a single MRL/Mp-lpr/lpr mouse: specificity for DNA and VH gene usage. Eur J Immunol. 1994;24(6):1332–1338. doi: 10.1002/eji.1830240614. [DOI] [PubMed] [Google Scholar]
- 141.Jie G, Jiang Q, Rui Z, Yifei Y. Expression of interleukin-17 in autoimmune dacryoadenitis in MRL/lpr mice. Current eye research. 2010;35(10):865–871. doi: 10.3109/02713683.2010.497600. [DOI] [PubMed] [Google Scholar]
- 142.St Clair EW, Kenan D, Burch JA, Jr, Keene JD, Pisetsky DS. Anti-La antibody production by MRL-1pr/1pr mice. Analysis of fine specificity. Journal of immunology. 1991;146(6):1885–1892. [PubMed] [Google Scholar]
- 143.Wahren M, Skarstein K, Blange I, Pettersson I, Jonsson R. MRL/lpr mice produce anti-Ro 52,000 MW antibodies: detection, analysis of specificity and site of production. Immunology. 1994;83(1):9–15. [PMC free article] [PubMed] [Google Scholar]