Abstract
Background and purpose
The associations of individual long-chain n-3 polyunsaturated fatty-acids with incident ischemic stroke and its main subtypes are not well established. We aimed to investigate prospectively the relationship of circulating eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) with risk of total ischemic, atherothrombotic, and cardioembolic stroke.
Methods
We measured circulating phospholipid fatty-acids at baseline in 3 separate US cohorts: The Cardiovascular Health Study (CHS), Nurses' Health Study (NHS), and Health Professionals Follow-Up Study (HPFS). Ischemic strokes were prospectively adjudicated and classified into atherothrombotic (large and small vessel infarctions) or cardioembolic by imaging studies and medical records. Risk according to fatty-acid levels was assessed using Cox proportional-hazards (CHS) or conditional logistic regression (NHS, HPFS) according to study design. Cohort findings were pooled using fixed-effects meta-analysis.
Results
A total of 953 incident ischemic strokes were identified (408 atherothrombotic, 256 cardioembolic, and 289 undetermined subtypes) during median follow-up of 11.2 (CHS) and 8.3 (pooled, NHS and HPFS) years. After multivariable-adjustment, lower risk of total ischemic stroke was seen with higher DPA (highest vs. lowest-quartiles, pooled HR=0.74, 95%CI=0.58-0.92) and DHA (0.80, 0.64-1.00) but not EPA (0.94, 0.77-1.19). DHA was associated with lower risk of atherothrombotic (0.53, 0.34-0.83), and DPA, with lower risk of cardioembolic (0.58, 0.37-0.92) stroke. Findings in each individual cohort were consistent with pooled results.
Conclusions
In 3 large US cohorts, higher circulating levels of DHA were inversely associated with incident atherothrombotic stroke, and DPA, with cardioembolic stroke. These novel findings suggest differential pathways of benefit for DHA, DPA, and EPA.
Keywords: Polyunsaturated fatty acid, atherothrombotic stroke, cardioembolic stroke, epidemiology
Introduction
Stroke is a leading cause of serious long-term disability and death in the United States (1). Based on randomized trials, long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs), which include eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA), reduce major cardiovascular risk factors including hypertension, hyperlipidemia, and endothelial dysfunction (2–4), supporting a potential protective effect against cardiovascular disease (CVD). However, the impact of these fatty acids on ischemic stroke incidence is not well established. For instance, observational studies demonstrate inverse associations of self-reported dietary consumption of n-3 PUFAs with ischemic stroke (5), yet meta-analysis of randomized controlled trials found no effect of n-3 PUFA supplementation on stroke (6). The latter findings were mostly from relatively short-term supplementation trials in high risk patients with pre-existing CVD in whom stroke was not a primary endpoint; thus, these findings may not be generalizable to habitual intake of n-3 PUFAs in primary prevention populations (6). Consequently, the effects of n-3 PUFAs on incidence of ischemic stroke remain controversial.
In addition, ischemic stroke is a heterogeneous disorder with diverse pathophysiological pathways including atherothrombosis and cardioembolism,but most studies have not separately considered etiological subtypes of ischemic stroke (7). Effects of n-3 PUFAs may also differ across these subtypes. For instance, DHA may play a greater role in lowering risk of atherothrombotic stroke by reducing endothelial dysfunction and atherosclerosis (4), whereas EPA and DPA may have a greater impact on cardioembolic stroke risk due to effects on clotting and atrial fibrillation (8,9).
Finally, nearly all long-term studies of n-3 PUFA and stroke risk have estimated dietary n-3 PUFA intake from self-reported questionnaires. This limits the precision of evaluating different individual fatty acids, which are not well separated with dietary questionnaire data. In addition, self-reported dietary estimates might also be limited by random and systematic errors and reliance on accuracy of food composition databases.
To address these key issues, we measured circulating phospholipid levels of n-3 PUFAs and studied their associations with incidence of ischemic stroke, including subtypes of atherothrombotic and cardioembolic stroke, in 3 separate prospective US cohort studies: the Cardiovascular Health Study (CHS), Nurses' Health Study (NHS), and Health Professionals Follow-Up Study (HPFS).
Methods
Population and design
The design, enrollment, and population of CHS, NHS, and HPFS have been described (10,11) (Figure 1). Briefly, CHS is a multi-center, community-based prospective cohort of older US adults. In 1989–90, 5,201 adults age 65 years and older were randomly enrolled from 4 communities. A total of 687 additional black participants were similarly recruited and enrolled in 1992–93. Trained study personnel performed annual clinic evaluations, including: physical examination, diagnostic testing, blood sampling, and questionnaires on health status, medical history, and lifestyle. Among 3,941 participants with available fatty acid measurements performed in 1992-93, we excluded 266 participants with prevalent stroke or transient ischemic attack, resulting in 3,675 participants included in our analysis. Each center's institutional review committee approved the study, and all participants provided informed written consent.
Figure 1.
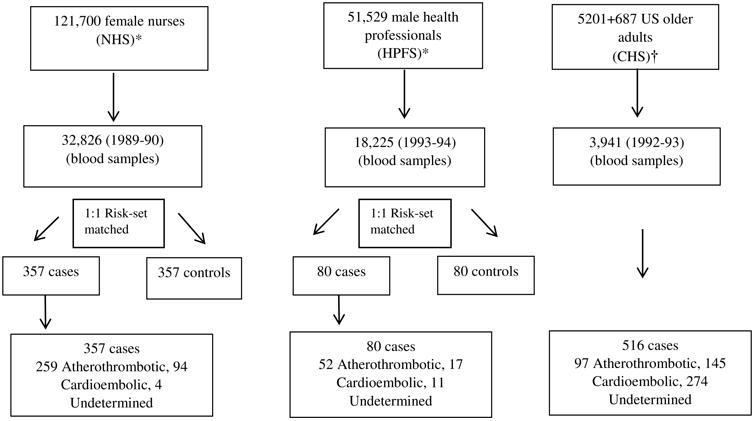
Flow-chart for the recruitment of participants in the cohorts, collection of blood samples and selection of final ischemic stroke events.
*Prospective nested case-control analyses with 1:1 matching (Characteristics Presented for controls). Follow up years: up to 2006 (NHS) and 2004 (HPFS).
†Prospective cohort analysis, Follow up year: up to 2011.
NHS is a prospective US cohort consisting of 121,701 female registered nurses 30–55 years of age enrolled in 1976 in 11 US states; and HPFS is a prospective US cohort consisting of 51,529 U.S. male health professionals 40–75 years of age enrolled in 1986 in the Boston area. In both cohorts, participants were followed through standardized questionnaires every two years on lifestyle, medical characteristics, and incident health outcomes. This analysis is based on a nested case-control study of incident ischemic stroke comprising 357 NHS and 80 HPFS participants (cases) with fatty acid measurements. The Human Subjects Research Committees at Brigham and Women's Hospital and at the Harvard School of Public Health approved the protocols for the studies. The participants' completion and return of the enrollment questionnaire implied informed consent.
Fatty acid measurements
In CHS, fatty acids were measured in stored blood samples from 1992-93, the baseline year for this analysis. Blood was drawn after a 12-hour fast, stored at -70 °C, and shipped on dry ice for long-term storage at -80 °C. Total lipids were extracted from plasma, and phospholipids were separated from neutral lipids by one-dimensional thin layer chromatography. Fatty acid methyl esters (FAMEs) samples were prepared by direct transesterification and separated using gas chromatography (8). Plasma phospholipid fatty acid analysis provided quantitative measurement of fatty acids as a percentage of total fatty acids. Laboratory coefficients of variation (CV) were less than 3% for major fatty acids and also for EPA, DPA, and DHA. Identification, precision, and accuracy were continuously evaluated by using model mixtures of known fatty acid methyl esters and established in-house control samples.No evidence of degradation was observed after 10 years under the blood storage conditions in CHS (12).
In NHS, fatty acids were measured in stored blood samples from 1989-1990, and in HPFS, from 1993-1994—the baseline years for this analysis. In both cohorts, blood samples were centrifuged and stored in aliquots of plasma and red blood cells and buffy coat fractions (13). Samples were placed in liquid nitrogen freezers at -130°C to -196°C until required analysis. Fatty acid concentrations in erythrocyte phospholipids were analyzed by gas-liquid chromatography (14). Detailed methods have been described elsewhere (15). Concentrations of individual circulating fatty acids were expressed as a percentage of total fatty acids. Overall intra-assay CVs for phospholipid fatty acids in the 2 cohorts were 8.7% for EPA, 8.3% for DPA, and 6.9% for DHA. Fatty acid contents in blood samples stored at low temperatures were shown to be stable and had minimal degradation over more than a decade of storage (16).
Follow up and endpoint
In CHS, subjects were followed in alternating study clinic examinations and telephone contacts every 6 months through 2000 and biannual telephone contacts afterwards till 2011. Less than 1% of total person-time were missing and censored early. Incident ischemic was the primary endpoint in our analysis. Suspected cases of incident stroke were evaluated and adjudicated by a centralized events committee using available data from interviews, next of kin, death certificates, and medical records, including clinical work up.
In NHS and HPFS, participants completed biennial follow-up questionnaires to update predisposing factors and report new onset of events including stroke. The biennial follow-up response rate exceeded 90% (95% on average). Participants were followed from baseline (time of blood collection) until the time of development of ischemic stroke. Incident cases of ischemic stroke were matched 1:1 with controls on risk-set sampling based on time of follow up, age, race, smoking status, and time of blood collection, involving a maximum follow-up of 16 years for NHS and 10 years for HPFS with a pooled median follow-up of 8.3 years from baseline till development of incident ischemic stroke. Controls were risk-set sample matched so their median follow-up will be the same as stroke events or cases.
Ascertainment of ischemic stroke
In CHS, a centralized study panel of neurologists blinded to other participant data reviewed all potential cases of stroke, using hospital records, test results, death certificates, and brain imaging findings (17). Similarly, in NHS and HPFS, potential strokes were identified during follow up using self–reported questionnaires and confirmed by a centralized panel of physicians according to review of medical records, physician questionnaires, death certificates, medical examiner forms, and available brain imaging. In all 3 cohorts, stroke was defined as a neurological deficit of rapid onset lasting more than 24 hours or until death (18), and incident strokes were classified as: (1) ischemic if evidence of a focal brain deficit without evidence of primary hemorrhage; and (2) hemorrhagic if bloody spinal fluid on lumbar puncture or evidence of blood in imaging studies. Ischemic stroke was further classified using standard protocols into subtypes: atherothrombotic (large and small vessel infarctions, carotid ulcerative plaque); cardioembolic (due to cardiac mural thrombi, valvular heart disease, atrial fibrillation, other cardiac sources); or other, including undetermined subtype. In CHS, patients with incomplete findings, negative findings or two or more likely causes of stroke were classified as undetermined ischemic stroke. In NHS and HPFS, a definite diagnosis of ischemic stroke subtype was made when computed tomographic (CT) scan, magnetic resonance imaging (MRI), angiography, echocardiogram, or autopsy confirmed the lesion. Detailed information on classification of ischemic stroke subtypes in CHS (19) and NHS/ HPFS (20) has been reported.
Ascertainment of covariates
In CHS, information on medical history, lifestyle, and other risk factors was collected during annual clinical visits using standardized interviews, physical examinations, and laboratory testing (10,21), including on smoking, alcohol use, hypertension, diabetes, family history of CVD, and body mass index. Atrial fibrillation was identified on the basis of 12-lead resting electrocardiograms performed as a part of the baseline examination (22). Physical activity was assessed by using the modified Minnesota Leisure Time Activities scale (23). Usual dietary habits were assessed using a food frequency questionnaire validated against six detailed 24-h diet recall interviews spaced approximately one month apart (24).
In NHS and HPFS, data on covariates were obtained via validated questionnaires including on demographics, medical history, lifestyle, family history, and major CVD risk factors including smoking (25), hypertension and diabetes (26). Height and weight were self-reported but showed high age-adjusted correlation coefficients with technician-measured values (27). Physical activity and calculated metabolic equivalents (MET-hours/week) were assessed using validated questionnaires based on self-reported average time spent on leisure time activities (28). Usual dietary habits were measured using a semi-quantitative food frequency questionnaire that was validated in a sub-sample of participants (29).
Statistical analysis
In each cohort, long chain n-3 PUFA levels, measured as percent of total fatty acids, were evaluated in quartiles as indicator categories. Linear test for trends were performed by assigning the median value of the quartile to each category and entering this variable into the model as a continuous variable. Multivariable models were adjusted for potential confounders based on biologic relevance, clinical interest, or association with the exposure or outcome, including age, sex, race, smoking, physical activity, alcohol intake, hypertension, family history of CVD and diabetes, menopausal status, BMI, aspirin use, and intakes of fruits, vegetables, and meats. In CHS, multivariable adjusted Cox proportional hazards analyses assessed the prospective relationship of circulating n-3 PUFAs and incident ischemic stroke and its subtypes, with time at risk from time of blood draw to the first stroke, death or lost to follow up. The proportional-hazards assumption was not violated based on Schoenfeld residuals. In NHS and HPFS, conditional logistic regression models evaluated the corresponding prospective associations of circulating n-3 PUFAs and incident ischemic stroke and its subtypes. Given risk-set sampling in these two cohorts, coefficients from these models are directly analogous to Cox proportional hazards and estimate hazard ratios. Estimates were pooled across the three cohorts using fixed-effect meta-analysis. Multiple imputations (10-fold) were used for missing data for continuous variables, and missing indicator variables, for missing categorical variables. Statistical significance was defined as two-tailed α=0.05. Analyses were performed using SAS 9.3 (SAS, Cary, North Carolina).
Results
At baseline, participants in CHS were older (75.1±4.8) than participants in NHS (61.0±6.1) or HPFS (67.6±7.8) (Table 1). Most participants were white: 88% in CHS, 98% in NHS and 94% in HPFS. The 3 cohorts included broad mixtures of lifestyle, smoking, and dietary habits. Diabetes and hypertension were more prevalent in CHS at baseline. As expected, cases and controls in NHS and HPFS were similar in matching factors including age, race/ethnicity and smoking. Median follow-up was 11.2 years in CHS and 8.3 years in NHS and HPFS (pooled). During follow-up, 953 incident ischemic strokes were identified, including 408 atherothrombotic, 256 cardioembolic, and 289 ischemic strokes with insufficient information for subclassification.
Table 1.
Baseline characteristics of participants.
| Characteristics | NHS* | HPFS* | CHS† |
|---|---|---|---|
| Age, year | 61.0±6.1 | 67.6±7.8 | 75.1±4.8 |
| White, % | 99 | 94 | 88 |
| Male, % | 0 | 100 | 40 |
| BMI, kg/m2 | 25.4±4.8 | 26.2±3.2 | 26.6±4.57 |
| Education, (>12y), % | 100 | 100 | 47 |
| Currentsmoker, % | 16.6 | 5 | 9 |
| Pastsmoker, % | 40 | 53 | 43 |
| Neversmoker, % | 44 | 42 | 49 |
| Physicalactivity, MET-h/wk (kcal/wk in CHS) ** | 16.8 ±18.8 | 35.8 ±44.6 | 1063±1450 |
| Alcohol, drinks/wk | 0.45±0.84 | 0.80±1.21 | 2.02±6.06 |
| Hypertension, % | 22 | 22 | 48 |
| Diabetes, % | 7.26 | 8.33 | 14.30 |
| Atrial fibrillation, % | 4.3 | 0.42 | 5.0 |
| Aspirin use, % | 45.9 | 42.5 | 36.0 |
| Processed meat, servings/day | 0.22±0.30 | 0.41±0.65 | 0.35±0.4 |
| Unprocessed meat, servings/day | 0.91±0.48 | 0.98±0.52 | 0.45±0.4 |
| Fruit, servings/day | 1.49±0.91 | 1.90±1.48 | 2.10±1.10 |
| Vegetables, servings/day | 1.24±0.64 | 3.89±2.25 | 2.50±1.35 |
| Fish, servings/day | 0.31±0.27 | 0.24±0.23 | 0.22±0.19 |
| No of incident cases during follow up: | |||
| Ischemic stroke | 357 | 80 | 516 |
| Atherothrombotic | 259 | 52 | 97 |
| Cardioembolic | 94 | 17 | 145 |
| Undetermined | 4 | 11 | 274 |
Values are mean±SD for continuous variables and percent for categorical variables.
Prospective nested case-control analyses with 1:1 matching (Characteristics presented for controls).
Prospective cohort analysis.
Leisure-time physical activity, kcal/week
Phospholipid n-3 PUFAs and risk of ischemic stroke
After adjustment for age, sex, race, smoking, physical activity, alcohol intake, family history of CVD and diabetes, hypertension, menopausal status, BMI, aspirin use, and intakes of fruits, vegetables, and meats, lower risk of incident ischemic stroke was seen with higher circulating levels of DHA (comparing the top to bottom quartile, pooled HR=0.80, 95%CI: 0.64-1.00, P-trend=0.05) and DPA (pooled HR=0.74, 95%CI: 0.58-0.92, P-trend=0.03) but not EPA (pooled HR=0.94, 95%CI: 0.77-1.19, P-trend=0.68) (Table 2).
Table 2.
Risk of incident ischemic stroke, atherothrombotic stroke and cardioembolic stroke according to phospholipid n-3 polyunsaturated fatty acid biomarkers in CHS, NHS, and HPFS.
| Cohort-specific fatty acid quartiles | |||||
|---|---|---|---|---|---|
| I | II | III | IV | P for trend | |
|
Total Ischemic Stroke (N=953 cases) |
|||||
| EPA | Reference | 0.95 (0.77, 1.18) | 0.93 (0.74, 1.16) | 0.94 (0.77, 1.19) | 0.68 |
| DPA | Reference | 0.90 (0.74, 1.14) | 0.95 (0.78, 1.19) | 0.74 (0.58, 0.92) | 0.03 |
| DHA | Reference | 0.93 (0.75, 1.14) | 0.85 (0.71, 1.10) | 0.80 (0.63, 1.00) | 0.05 |
|
Atherothrombotic Stroke (N=408 cases) |
|||||
| EPA | Reference | 1.03 (0.70, 1.52) | 0.97 (0.65, 1.44) | 1.06 (0.71, 1.59) | 0.29 |
| DPA | Reference | 1.05 (0.72, 1.53) | 1.02 (0.69, 1.53) | 0.98 (0.64, 1.53) | 0.90 |
| DHA | Reference | 0.88 (0.60, 1.16) | 0.72 (0.48, 1.07) | 0.53 (0.34, 0.83) | 0.001 |
|
Cardioembolic Stroke (N=256 cases) |
|||||
| EPA | Reference | 1.10 (0.72, 1.66) | 0.96 (0.62, 1.50) | 0.94 (0.61, 1.45) | 0.80 |
| DPA | Reference | 0.75 (0.50, 1.14) | 0.83 (0.55, 1.35) | 0.59 (0.38, 0.93) | 0.01 |
| DHA | Reference | 1.25 (0.83, 1.90) | 1.07 (0.69, 1.65) | 1.09 (0.69, 1.72) | 0.70 |
Values are pooled hazard ratios (95% CIs) adjusted for age (years), race (white, nonwhite), sex (in CHS), smoking status (never, former, current, missing), physical activity (METS/week and Leisure-time physical activity, kcal/week for CHS), alcohol (servings/day), hypertension, family history of diabetes (yes, no, missing), parental history of cardiovascular disease (yes, no, missing), menopausal status in NHS (pre, post), postmenopausal hormone use in NHS (no, yes, missing), body mass index (kg/m2), regular aspirin use (yes, no, missing), and consumption of processed meats (servings/day), unprocessed meats (servings/day), fruits (servings/day) and vegetables (servings/day).
When we evaluated atherothrombotic stroke in similarly adjusted analyses, participants in the highest compared to the lowest quartile of DHA had 47% lower risk (pooled HR=0.53, 95%CI: 0.34-0.83, P-trend=0.001). In contrast, neither DPA nor EPA were significantly associated with atherothrombotic stroke. When we evaluated cardioembolic stroke, participants in the highest compared to the lowest quartile of DPA had 42% lower risk (pooled HR=0.58, 95%CI: 0.37-0.92, P-trend=0.01). Neither EPA nor DHA were significantly associated with cardioembolic stroke (Figure 2). Results for each cohort are presented in Supplemental Tables I-III.
Figure 2.
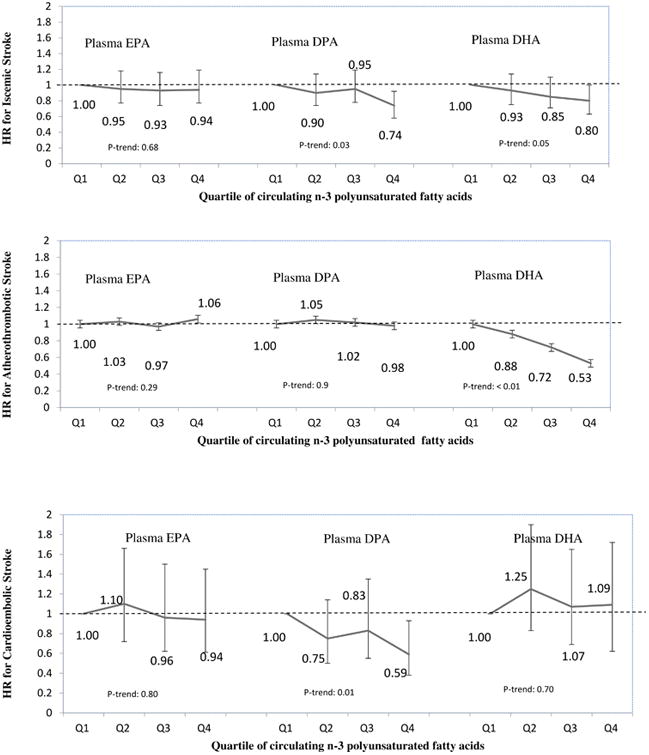
Risk of incident ischemic stroke, atherothrombotic stroke and cardioembolic stroke according to circulating phospholipid n-3 polyunsaturated fatty acid biomarkers in CHS, NHS, and HPFS. HR: Hazard ratio; Q: Quartile. Adjusted for age, sex, race, smoking, physical activity, alcohol intake, hypertension, family history of CVD and diabetes, menopausal status, BMI, aspirin use, and intakes of fruits, vegetables, and meats.
Discussion
In three large, independent prospective U.S. cohorts, we observed an inverse relationship between circulating levels of DPA and DHA and incident ischemic stroke. Among ischemic stroke subtypes, DHA was inversely associated with atherothrombotic stroke, and DPA, with cardioembolic stroke. These associations remained significant after adjustment for demographic, lifestyle, and vascular risk factors. In comparison, EPA was not associated with total ischemic, atherothrombotic, or cardioembolic stroke. These findings support the hypothesis that individual serum fatty acids have differential associations with total ischemic, atherothrombotic, and cardioembolic stroke.
A growing body of evidence from basic science and experimental research in the past decade indicates that DHA may exert protection against atherosclerosis through multiple pathways (2). Atherothrombotic stroke is a consequence of atherosclerotic changes of the vascular surface and subsequent platelet activation at the site of vascular lesion. DHA lowers plasma triglycerides by reducing fatty acid availability for triglyceride synthesis due to decreased de novo lipogenesis, and may also reduce insulin resistance and glucose intolerance (30). Evidence also suggests that DHA, but not EPA, can lower resting heart rate and systolic and diastolic blood pressure by affecting cardiac electrophysiological pathways, as well as dilator and constrictor responses in microcirculation (4,31). Other mechanisms that may suggest a cause-effect association between DHA and atherothrombotic stroke include reduction of oxidative stress, inflammation and endothelial dysfunction (2,32). In a Japanese cohort, DHA but not EPA was inversely associated with carotid intima-media thickness, consistent with our findings for atherothrombotic stroke and suggesting anti-atherogenic effects of DHA. DHAis also the precursor toneuroprotectin D1, an anti-inflammatory docosanoid derivative which downregulates apoptosis and promotes cell survival in models of ischemic stroke(33). Accumulation of DHA in the brain has been associated with reduced neuroinflammation (34) and activation of anti-apoptotic pathways. Collectively, this evidence suggests that DHA may confer a protective effect on ischemic stroke, in particular atherosclerosis and atherothrombosis of the arteries feeding the brain.
In contrast to at herothrombotic stroke, cardioembolic stroke is mainly caused by several underlying pathophysiological mechanisms associated with arrhythmia, cardiac wall or chamber abnormality, or valvular disease (35). Atrial fibrillation is the most important source of cardioembolic stroke, followed by left ventricular thrombus associated with myocardial infarction or congestive heart failure (36). In vitro and animal studies, and some human studies have suggested that n-3 PUFAs, including DPA, can prevent arrhythmia and reduce the onset of atrial fibrillation by directly influencing atrial and ventricular myocyte electrophysiology, as well as modulations in autonomic tone, or anti-inflammatory, or anti-fibrotic effects (2). In a prospective evaluation of plasma n-3 PUFA biomarkers within CHS, total n-3 PUFA and DHA levels were significantly associated with lower risk of incident atrial fibrillation (37), while the semi-parametric analyses of DPA suggested possible trends toward protective associations at higher levels of this fatty acid. However, findings from randomized controlled trials (RCTs) have been mixed, mostly focusing on the recurrence or postoperative atrial fibrillation after cardiac surgery (2,38), with a comprehensive meta-analysis (2687 patients from 8 RCTs) showing that n-3 PUFA therapy reduces the incidence of postoperative atrial fibrillation (pooled OR=0.84, 95%CI: 0.71-0.99, P=0.03) using a fixed model (39).
Cardiac emboli may also originate in peripheral circulation (paradoxical emboli), consisting of embolic debris such as platelet aggregates, thrombus, and platelet-thrombi. Experimental studies demonstrate differential inhibitory effects of n-3 PUFAs on cyclooxygenase (COX)-1 and COX-2 metabolism. In platelets, DPA is metabolized into 11- and l4-hydroxy docosapentaenoic acids by the lipoxygenase pathway. When platelets were incubated with DPA, this inhibited the COX enzyme thereby reducing the thromboxane A2 production from arachidonic acid (40). In these experiments, DPA was the most potent inhibitor of COX-1 activity, possibly 10 times more potent than EPA in inhibiting platelet aggregation (41,42). Of note, DPA is mainly derived from endogenous elongation from EPA, and DPA can also undergo conversion to EPA; therefore, our findings may reflect this biomarker of metabolism, rather than a direct cause-effect relationship. In comparison, in a controlled trial of 59 diabetic subjects, randomized to 4 gram/day of EPA, DHA or olive oil (placebo) for 6 weeks, DHA, but not EPA, reduced ex vivo collagen-stimulated platelet aggregation, but neither DHA nor EPA significantly affected platelet aggregation in response to platelet activating factor or fibrinolytic markers including plasminogen activator inhibitor-1 antigen or tissue plasminogen activator antigen (43). Accordingly, well designed randomized trials are required to assess the observed relationships of DPA with ischemic stroke.
Few studies have evaluated associations of individual n-3 PUFA biomarkers with ischemic stroke subtypes. In a South Korean retrospective case-control study of 248 stroke patients (104 with cerebral atherosclerotic stenosis and 144 without cerebral atherosclerotic stenosis) and 215 non-stroke controls, phospholipid DHA was associated with lower risk of ischemic stroke associated with cerebral atherosclerotic stenosis (odds ratio 0.59, 95% CI: 0.35-0.99) (44). In a prospective nested case-control study (964 matched pairs) of postmenopausal US women, circulating DHA and DPA, but not EPA, were inversely associated with incidence of total ischemic stroke (5). DHA was associated with atherothrombotic stroke, but no significant association was confirmed between DPA and cardioembolic stroke (N=209 events), with wide CIs that included the possibility of benefit. Likewise, in a Korean retrospective case-control study of 120 subjects, a low level of total long-chain n-3 PUFAs and DHA in erythrocytes was a risk factor for ischemic stroke, particularly small artery occlusion subtype, but this study was underpowered to draw conclusions on cardioembolic stroke, including only 40 patients with ischemic stroke (45).
No randomized trial has been conducted to investigate the effect of n-3 PUFAs in primary prevention of stroke. Prior randomized trials of n-3 PUFA supplementation have focused on coronary heart disease or total CVD in secondary prevention or high risk populations (46). These trials also may be limited by insufficient duration of follow up (often 1-3 years) to detect effects on development of chronic diseases such as stroke. No prior trial has focused on stroke in a primary prevention setting. Our findings suggest that specific n-3 PUFA may benefit certain subtypes of ischemic stroke, supporting the need for additional well conducted, prospective observational studies, mechanistic intervention studies, and controlled trials in primary prevention populations and with longer follow up time.
When interpreting our findings, potential limitations should be considered. Fatty acids were measured at baseline, and random changes in exposure over time would cause misclassification and attenuate findings toward the null. Systematic changes in exposure might also be possible. Caution should be used when interpreting associations of phospholipid fatty acids because these may reflect multiple factors, such as metabolism and selective incorporation into lipoproteins, in addition to intake. Because they are measured as percentage of fatty acids, their levels are influenced by other fatty acids, and the specific N-3 fatty acids may be measured with different degrees of precision. Consistent with clinical experiences, a subset of ischemic strokes, mainly in CHS, could not be reliably classified as atherothrombotic or cardioembolic, reducing statistical power. This was largely due to lack of records or full work up at the time of the ischemic stroke event. Variations in etiological classification systems of ischemic stroke across cohorts may have resulted in the dilution of effect and bias towards null for the association of fatty acids with each subtype of ischemic stroke. However, this would not affect the significance of our observed associations. We cannot exclude residual confounding by unknown or unmeasured factors. However, findings remained significant after adjustment for major risk factors and the specificity of findings between certain n-3 PUFA and specific ischemic stroke subtypes, as well as biologic plausibility based on experimentally observed effects of n-3 PUFA, suggest that confounding may not be the sole explanation for our findings. Our cohorts included mostly white Americans, which may limit generalizability to other ethnicities or races. Yet, we have little reason to believe that biologic effects of n-3 PUFA might vary according to race.
Several strengths should be considered. Information on demographics, risk factors, and lifestyle were prospectively collected in a well-established multicenter cohort with little loss to follow-up, thus, minimizing selection bias. We were able to evaluate each long-chain n3-PUFA separately, while adjusting for multiple covariates, minimizing confounding.Consistency of our findings among the three cohorts increases their validity and generalizability.
In conclusion, we found that circulating DHA and DPA, but not EPA, are associated with lower risk of ischemic stroke, with differential associations with atherothrombotic vs. cardioembolic stroke.
Supplementary Material
Acknowledgments
We thank the participants and staff of CHS, NHS and HPFS.
Funding sources: This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) (grants R01-HL085710and 3R01 ES014433-03S1).The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and HL102214 from NHLBI, with additional contributions from the National Institute of Neurological Disorders and Stroke and from the National Institute of Aging (AG-023629, AG-15928, AG-20098, and AG-027058). NHS and HPFS are funded through the National Institute of Environmental Health Sciences and the National Institutes of Health (NIH), R01-ES014433 and ES013692, and NIH research grants HL-60712, HL-034594, HL-088521, HL-35464, DK-58845, CA-186107, CA-49449, CA-87969, CA-55075, and CA-167552. Dr. Yakoob was supported by Harvard University Scholarship, Harvard Lown Cardiovascular Research Foundation Scholarship, and Founders Affiliate American Heart Association Pre-Doctoral Training Fellowship.
Footnotes
Disclosures: Dr. Mozaffarian reports reports ad hoc honoraria or consulting from Boston Heart Diagnostics, Haas Avocado Board, Astra Zeneca, GOED, DSM, and Life Sciences Research Organization; chapter royalties from UpToDate; and scientific advisory board, Elysium Health. Harvard University has been assigned patent US8889739 B2, listing Dr. Mozaffarian as one of three co-inventors, for use of trans-palmitoleic acid in identifying and treating metabolic disease.
References
- 1.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 3.Nobili V, Bedogni G, Alisi A, Pietrobattista A, Risé P, Galli C, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 4.Geleijnse JM, Giltay EJ, Grobbee DE, Donders ART, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Yaemsiri S, Sen S, Tinker LF, Robinson WR, Evans RW, Rosamond W, et al. Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke. 2013;44:2710–2717. doi: 10.1161/STROKEAHA.111.000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA American Medical Association. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 7.Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, et al. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, et al. Circulating long-chain ω-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155:160–170. doi: 10.1059/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Wu JHY. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 12.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. n-3 Polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003;77:319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 13.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87:1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 14.Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–381. doi: 10.1093/aje/kwi213. [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr. 2007;86:929–937. doi: 10.1093/ajcn/86.4.929. [DOI] [PubMed] [Google Scholar]
- 16.Zeleniuch-Jacquotte A, Chajès V, Van Kappel AL, Riboli E, Toniolo P. Reliability of fatty acid composition in human serum phospholipids. Eur J Clin Nutr. 2000;54:367–372. doi: 10.1038/sj.ejcn.1600964. [DOI] [PubMed] [Google Scholar]
- 17.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 18.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 2016;12:I13–144. [PubMed] [Google Scholar]
- 19.Longstreth WT, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 20.Iso H, Hennekens CH, Stampfer MJ, Rexrode KM, Colditz GA, Speizer FE, et al. Prospective study of aspirin use and risk of stroke in women. Stroke. 1999;30:1764–1771. doi: 10.1161/01.str.30.9.1764. [DOI] [PubMed] [Google Scholar]
- 21.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 22.Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med. 1990;29:362–374. [PubMed] [Google Scholar]
- 23.Taylor HL, Jacobs DR, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 24.Kumanyika SK, Tell GS, Shemanski L, Martel J, Chinchilli VM. Dietary assessment using a picture-sort approach. Am J Clin Nutr. 1997;65:1123S–1129S. doi: 10.1093/ajcn/65.4.1123S. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Curhan GC, Hu FB, Rimm EB, Forman JP. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care. 2011;34:892–897. doi: 10.2337/dc10-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ascherio A, Rimm EB, Giovannucci EL, Colditz GA, Rosner B, Willett WC, et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86:1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 27.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 30.Landi G, Cella E, Boccardi E, Musicco M. Lacunar versus non-lacunar infarcts: pathogenetic and prognostic differences. J Neurol Neurosurg Psychiatry. 1992;55:441–445. doi: 10.1136/jnnp.55.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 32.Leaf A, Weber PC. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988;318:549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- 33.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 34.Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs. 2008;9:735–743. [PubMed] [Google Scholar]
- 35.Hart RG, Halperin JL. Atrial Fibrillation and Stroke. Stroke. 2001;32:803–808. doi: 10.1161/01.str.32.3.803. [DOI] [PubMed] [Google Scholar]
- 36.Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6:150–161. doi: 10.2174/157340310791658730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu JHY, Lemaitre RN, King IB, Song X, Sacks FM, Rimm EB, et al. Association of Plasma Phospholipid Long-Chain Omega-3 Fatty Acids With Incident Atrial Fibrillation in Older Adults: The Cardiovascular Health Study. Circulation. 2012;125:1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martino A, Pezzi L, Magnano R, Salustri E, Penco M, Calo' L. World J Cardiol. Vol. 8. Baishideng Publishing Group Inc; 2016. Omega 3 and atrial fibrillation: Where are we? pp. 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali-Hassan-Sayegh S, Mirhosseini SJ, Rezaeisadrabadi M, Dehghan HR, Sedaghat-Hamedani F, Kayvanpour E, et al. Antioxidant supplementations for prevention of atrial fibrillation after cardiac surgery: an updated comprehensive systematic review and meta-analysis of 23 randomized controlled trials. Interact Cardiovasc Thorac Surg. 2014;18:646–654. doi: 10.1093/icvts/ivu020. [DOI] [PubMed] [Google Scholar]
- 40.Careaga MM, Sprecher H. Synthesis of two hydroxy fatty acids from 7,10,13,16,19-docosapentaenoic acid by human platelets. J Biol Chem. 1984;259:14413–14417. [PubMed] [Google Scholar]
- 41.Norris PC, Dennis EA. Omega-3 fatty acids cause dramatic changes in TLR4 and purinergic eicosanoid signaling. Proc Natl Acad Sci U S A. 2012;109:8517–8522. doi: 10.1073/pnas.1200189109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akiba S, Murata T, Kitatani K, Sato T. Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull. 2000;23:1293–1297. doi: 10.1248/bpb.23.1293. [DOI] [PubMed] [Google Scholar]
- 43.Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, et al. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93. doi: 10.1016/s0021-9150(02)00307-6. [DOI] [PubMed] [Google Scholar]
- 44.Kim YJ, Kim OY, Cho Y, Chung JH, Jung YS, Hwang GS, et al. Plasma phospholipid fatty acid composition in ischemic stroke: importance of docosahexaenoic acid in the risk for intracranial atherosclerotic stenosis. Atherosclerosis. 2012;225:418–424. doi: 10.1016/j.atherosclerosis.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Park Y, Park S, Yi H, Kim HY, Kang SJ, Kim J, et al. Low level of n-3 polyunsaturated fatty acids in erythrocytes is a risk factor for both acute ischemic and hemorrhagic stroke in Koreans. Nutr Res. 2009;29:825–830. doi: 10.1016/j.nutres.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Larsson SC, Orsini N, Wolk A. Long-chain omega-3 polyunsaturated fatty acids and risk of stroke: a meta-analysis. Eur J Epidemiol. 2012;27:895–901. doi: 10.1007/s10654-012-9748-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


