Abstract
An increasing number of studies have proven that microRNAs play an important role in the occurrence, development and prognosis of various types of cancer. As a vital gene cluster, the microRNA (miR)-23a/24-2/27a cluster may be an important marker for predicting cancer prognosis and tumor progression. A search was conducted through PubMed, Medline and the Cochrane Library to identify studies investigating the association between the miR-23a/24-2/27a cluster and cancer, and the identified related studies were included in the present meta-analysis. The strength of the association was assessed by hazard ratio (HR) and its 95% confidence interval (95% CI). A total of 21 studies were included in this meta-analysis. The results indicated that a high level of miR-23a exerted a significant effect on overall survival (OS) (HR=2.33, 95% CI: 1.18–4.58; P=0.014), but not on disease-free survival (DFS)/recurrence-free survival (RFS) (HR=1.13, 95% CI: 0.37–3.44; P=0.836). There was an obvious statistically significant association between OS and the expression of miR-24 (HR=2.49, 95% CI: 1.84–3.37; P=0.000), particularly in the digestive system (pooled HR=2.99, 95% CI: 2.17–4.13, P=0.000). In addition, the result suggested a statistically significant association between the expression of miR-27a and OS (pooled HR=1.89, 95% CI: 1.32–2.69; P=0.001), as well as DFS/RFS/progression-free survival (HR=2.19, 95% CI: 1.29–3.70; P=0.003), particularly in renal cell carcinoma (HR=2.30, 95% CI: 1.16–4.67; P=0.017). A subgroup analysis by ethnicity, cancer type and statistical methodology was performed. There was no obvious publication bias. In conclusion, the present study demonstrated that the miR-23a/24-2/27a cluster may be a useful marker for predicting cancer prognosis and tumor progression.
Keywords: microRNA-23a/24-2/27a, cancer, meta-analysis
Introduction
MicroRNAs (miRNAs, miRs) are ~22-nucleotide long, single-stranded non-coding RNA molecules (1). They were first discovered in the nematode Caenorhabditis elegans with the identification of the developmental regulator lin-4 (2). Thus far, there are ~2,588 annotated miRNAs found in the human genome (3). With advances in research, it has been demonstrated that miRNAs may play an important role in various diseases. An increasing number of miRNAs were proven to participate in crucial biological processes, such as cell proliferation, migration, invasion and apoptosis (4–7), which may enable use of the miRNA family in the diagnosis and treatment of disease, due to the extensive alterations in miRNA expression in different diseases.
miR-23a/24-2/27a encodes a ~2,159-nt pri-miRNA transcript, which is located in chromosome 19p13.12 as an intergenic miRNA cluster (8). The profiling analysis suggested that the miR-23a/24-2/27a cluster was significantly upregulated in hepatocellular carcinoma (HCC) (9), pancreatic adenocarcinoma (10) and breast cancer (11). There are several studies on the association of the miR-23a/24-2/27a cluster with various types of cancer. Thus, the present systematic review and meta-analysis were designed to confirm whether miR-23a/24-2/27a may serve as a diagnostic marker for cancer.
Data collection methods
Literature search
Two of the authors (Jing Quan and Suyue Liu) independently conducted a search through PubMed, Medline and the Cochrane Library to identify studies on miR-23a/24-2/27a and cancer. The databases were searched from inception to September 26, 2016. In order to distinguish between miR-24-1 (also referred to as miR-189 and miR-24-1*) and miR-24-2 (also referred to as miR-24 precursor-19 and miR-24-2*), the miRBase was searched. The following search terms were used: (miR-23a or microRNA-23a or has-miR-23a or miR-24 or microRNA-24 or has-miR-24 or miR-27a or microRNA-27a or has-miR-27a) and (cancer or neoplasm or carcinoma or tumor).
Inclusion and exclusion criteria
All the studies met the following criteria: i) They were studies on miR-23a or miR-24 or miR-27a expression in cancer patients; ii) they used tissue samples obtained from surgically resected tumors and neighboring non-cancerous or normal tissues for comparison; iii) the expression of miR-23a or miR-24 or miR-27a was measured by quantitative polymerase chain reaction (qPCR) or reverse transcription qPCR (RT-qPCR) analysis; iv) the association between the expression level and survival outcome was clearly demonstrated.
The exclusion criteria were as follows: i) Duplicated studies; ii) studies without a control group; iii) insufficient data; iv) meetings, reviews and meta-analysis articles on animal and cell studies.
Data extraction and quality assessment
Two independent authors (Jing Quan and Kangfu Dai) extracted the following information from the studies: First author, publication year, country, type of miRNA, type of cancer, role of the gene, validation sample, number of the cases, survival analysis, hazard ratio (HR), months of follow-up and quality scores. The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the selected studies (a score of >5 was considered as high quality).
Statistical analysis
The HR and associated 95% confidence interval (CI) for each study was used to estimate the survival outcome of cancer associated with miR-23a or miR-24 or miR-27a expression. Heterogeneity of combined HRs was assessed by Cochran's Q test and Higgin's I2 statistic. Heterogeneity was considered statistically significant when P<0.05 or I2>50%. In order to evaluate the association between miR-23a or miR-24 or miR-27a expression and survival rate, a fixed-effects or random-effects model was used to calculate the pooled HR. A fixed-effects model (Mantel-Haenszel test) was applied in the absence of between-study heterogeneity (P≥0.05 or I2≤50%), while the random-effects model (Der Simonian and Laird method) was applied if significant heterogeneity was observed (P<0.05 or I2>50%). Stata 14.0 software (StataCorp, College Station, TX, USA) was used to analyze the data from the studies and construct the forest plot. The potential publication bias among the included studies was assessed by Begg's funnel plot and Egger's bias indicator test. P<0.05 in all the two-sided statistical tests was considered to indicate statistically significant differences.
Results
Basic information from the included studies
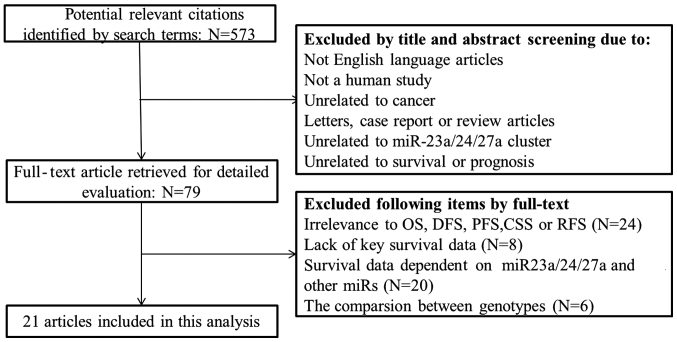
A search through PubMed, Medline and the Cochrane Library identified 572 potentially relevant studies. A total of 79 full-text articles were selected for detailed evaluation following exclusion of studies that were not in English, not human, unrelated to cancer, letters, case reports or review articles, unrelated to miR-23a/24-2/27a cluster, or unrelated to survival or prognosis. Further selection depended on relevance to overall survival (OS), recurrence-free survival (RFS), progression-free survival (PFS), cancer-specific survival, key survival data and survival data on miR-23a/24-2/27a, without any other miRNAs. Finally, 21 studies were included in the analysis. The flow chart of the study selection process is shown in Fig. 1.
Figure 1.
Flow diagram of the identification and selection of studies. OS, overall survival; DFS, disease-free survival; PFS, progression-free survival; CSS, cancer-specific survival; RFS, recurrence-free survival.
The 21 studies included in this meta-analysis included 8 studies on miR-23a, 6 on miR-24 and 8 on miR-27a (12–32). A total of 1,974 cases were included, including diffuse large B-cell lymphoma, laryngeal cancer, non-small-cell lung cancer (NSCLC), prostate cancer, hepatocellular carcinoma (HCC), colorectal cancer and renal cell carcinoma (RCC). The NOS score was used by two independent authors to determine study quality, and the scores of all the studies were >5. The basic information from the 21 studies is summarized in Table I.
Table I.
Basic characteristics of the 21 included studies.
| First author | Year | Country | miR type | Cancer type | Gene role | Validation sample | Sample size | Survival analysis | Hazard ratios (95% CI) | Follow-up (months) | NOS | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wang | 2014 | China | miR-23a | Diffuse large | Oncogene | Tissue | 104 | OS | 3.776 (1.10512.891)M | 34.1 | 7 | (30) |
| B-cell lymphoma | ||||||||||||
| Bao | 2014 | China | miR-23a | HCC | Oncogene | Tissue | 88 | OS, RFS | 2.286 (1.153–3.846)M | 60 | 8 | (12) |
| 2.205 (1.107–3.943)M | ||||||||||||
| Ma | 2014 | China | miR-23a | Gastric cancer | Oncogene | Tissue | 84 | OS | 4.30 (1.5012.33)M | 72 | 6 | (19) |
| Zhang | 2015 | China | miR-23a | Laryngeal cancer | Oncogene | Tissue | 52 | OS | 7.419 (2.561–21.491)U | 60 | 8 | (31) |
| 6.712 (2.076–21.700)M | ||||||||||||
| Qu | 2015 | China | miR-23a | NSCLC | Oncogene | Tissue | 127 | OS | 3.558 (2.982–6.635)M | 60 | 7 | (26) |
| Cai | 2015 | China | miR-23a | Prostate cancer | Anti-oncogene | Tissue | 123 | OS | 1.776 (1.116–2.829)M | 120 | 6 | (13) |
| Qu | 2015 | China | miR-23a | Nasopharyngeal carcinoma | Oncogene | Tissue | 111 | DFS, OS | 0.384 (0.222–0.666)U | 80 | 7 | (25) |
| 0.435 (0.255–0.743)M | ||||||||||||
| 0.357 (0.191–0.667)U | ||||||||||||
| 0.392 (0.214–0.719)M | ||||||||||||
| Liu | 2014 | China | miR-24 | HCC | Oncogene | Tissue | 207 | RFS, OS | 4.75 (2.66–8.47)M | 60 | 7 | (18) |
| 3.58 (2.34–5.46)M | ||||||||||||
| Gao | 2014 | China | miR-24 | Colorectal cancer | Anti-oncogene | Tissue | 95 | OS | 2.552 (1.647–3.956)U | 60 | 7 | (15) |
| 2.767 (1.203–6.364)M | ||||||||||||
| Meng | 2014 | China | miR-24 | HCC | Oncogene | Serum | 72 | OS, DFS | 2.141 (1.158–3.960)M | 60 | 6 | (20) |
| 2.055 (1.114–3.792)M | ||||||||||||
| Zhao | 2015 | China | miR-24 | NSCLC | Oncogene | Tissue | 53 | RFS | 1.77 (0.447.05)M | 20 | 8 | (32) |
| Serum | 1.85 (0.655.08)M | 23 | ||||||||||
| Organista-Nava | 2015 | Mexico | miR-24 | AL | Oncogene | Blood | 36 | OS | 1.83 (0.684.92)M | 72 | 7 | (23) |
| Mori | 2016 | Italy | miR-24 | Head and neck squamous cell carcinomas | Oncogene | Tissue | 108 | OS | 1.80 (1.063.06)M | 72 | 6 | (21) |
| Eitan | 2009 | Israel | miR-23a | Ovarian cancer | Oncogene | Tissue | 26 | RFS | 2.70 (0.65–11.20)M | 60 | 7 | (14) |
| miR-27a | Oncogene | 3.83 (1.06–13.79)M | ||||||||||
| Han | 2011 | China | miR-27a | ALL | Anti-oncogene | Blood | 36 | RFS | 0.76 (0.0226.56)M | 36 | 7 | (16) |
| Huang | 2013 | China | miR-27a | Gastric cancer | Oncogene | Blood | 82 | OS | 1.91 (0.864.23)M | 15 | 7 | (17) |
| Taheriazam | 2015 | Iran | miR-27a | Osteosarcoma | Oncogene | Tissue | 53 | OS | 3.035 (1.731–9.897)M | 108 | 7 | (28) |
| Nakata | 2015 | Japan | miR-27a | ccRCC | Oncogene | Tissue | 183 | CSS, PFS | 1.21 (0.57–2.60)U | 120 | 7 | (22) |
| 2.33 (1.07–5.47)U | ||||||||||||
| 2.71 (1.23–6.42)M | ||||||||||||
| Tang | 2015 | China | miR-27a | Osteosarcoma | Oncogene | Serum | 166 | OS, DFS | 2.17 (1.303.62)M | 100 | 8 | (29) |
| 1.39 (0.464.22)M | ||||||||||||
| Rivera-Daz | 2015 | Puerto | miR-27a | Glioblastoma | Anti- | Tissue | 35 | OS | 0.59 (0.20–1.77)M | 34 | 8 | (27) |
| Rico | multiforme | oncogene | ||||||||||
| Peng | 2015 | China | miR-27a | RCC | Oncogene | Tissue | 133 | OS, RFS | 0.91 (0.14–5.96)M | 36 | 7 | (24) |
| 1.61 (0.47–5.44)M |
HCC, hepatocellular carcinoma; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; AL, acute leukemia; ALL, acute lymphatic leukemia; ccRCC, clearcell renal cell carcinoma; OS, overall survival; RFS, recurrence-free survival; PFS, progressionfree survival; CSS, cancer-specific survival; M, multivariate analysis; U, univariate analysis; NOS, Newcastle-Ottawa Scale; CI, confidence interval.
Association of OS with the expression of miR-23a
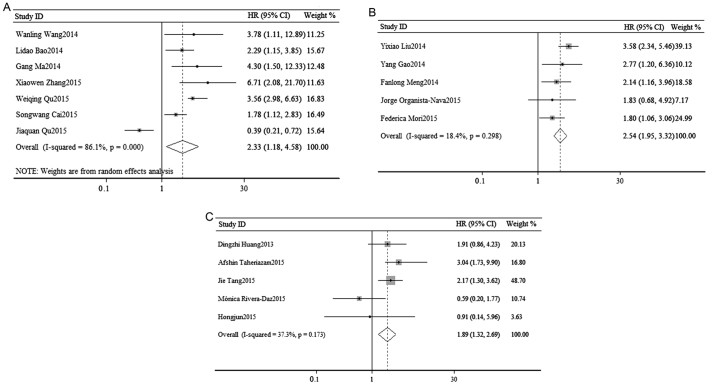
In order to elucidate the association between OS and the expression of miR-23a, a survival analysis was performed. As shown in Fig. 2A, a random-effects analysis was used to calculate the pooled HR and its 95% CI due to the relatively high heterogeneity in 7 cohorts (I2=86.1%, P=0.000). However, the result indicated that there was a statistically significant association between OS and the expression of miR-23a (pooled HR=2.33, 95% CI: 1.18–4.58; P=0.014).
Figure 2.
Forest plots on the association of overall survival with (A) miR-23a, (B) miR-24 and (C) miR-27a. miR, microRNA; HR, hazard ratio; CI, confidence interval.
To further elucidate this association, a stratified analysis was performed (Table II). In the subgroup analysis by cancer type, the result predicted that a high expression level of miR-23a was associated with poorer OS in digestive system cancers (pooled HR=2.69, 95% CI: 1.57–4.63; P=0.034) by a random-effects model (I2=53.6%, P=0.142). However, it failed to predict OS in respiratory system cancers (pooled HR=2.03, 95% CI: 0.38–10.80; P=0.408) by a random-effects model (I2=95%, P=0.000). In the subgroup analysis by statistical methodology, there was a statistically significant association between OS and the expression of miR-23a in the multivariate analysis (pooled HR=2.33, 95% CI: 1.18–4.58; P=0.014) by a random-effects model (I2=86.1%, P=0.000), while there was no statistically significant association between OS and the expression of miR-23a in the univariate analysis (pooled HR=1.58, 95% CI: 0.08–30.81; P=0.76) by a random-effects model (I2=95.7%, P=0.000). When stratified by dominant ethnicity, a significant association was observed in Asians (random-effects model; pooled HR=2.33, 95% CI: 1.18–4.58; P=0.014).
Table II.
Subgroup analysis and heterogeneity analysis of miR-23a/24-2/27a.
| Test of association | Test of heterogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified analysis | No. of studies | Pooled HR (95% CI) | Z | P-value | Model | X2 | P-value | I2 (%) | ||
| miR-23a | ||||||||||
| OS | Overall | 7 | 2.33 (1.18–4.58) | 2.45 | 0.014 | R | 43.15 | 0.000 | 86.1 | |
| Cancer types | ||||||||||
| Digestive system | 2 | 2.46 (0.98–6.21) | 2.12 | 0.034 | R | 1.04 | 0.307 | 4.0 | ||
| Respiratory system | 3 | 2.03 (0.38–10.80) | 0.83 | 0.408 | R | 39.85 | 0.000 | 95 | ||
| Statistical methodology | ||||||||||
| Univariate analysis | 2 | 1.58 (0.08–30.81) | 0.30 | 0.76 | R | 23.23 | 0.000 | 95.7 | ||
| Multivariate analysis | 7 | 2.33 (1.18–4.58) | 2.45 | 0.014 | R | 43.15 | 0.000 | 86.1 | ||
| Ethnicity | ||||||||||
| Asian | 7 | 2.33 (1.18–4.58) | 2.45 | 0.014 | R | 43.15 | 0.000 | 86.1 | ||
| DFS/RFS | Overall | 3 | 1.13 (0.37–3.44) | 0.21 | 0.836 | R | 13.18 | 0.000 | 84.8 | |
| miR-24 | ||||||||||
| OS | Overall | 5 | 2.49 (1.84–3.37) | 6.91 | 0.000 | F | 4.90 | 0.298 | 18.4 | |
| Sample | ||||||||||
| Tissue | 3 | 2.74 (2.02–3.73) | 6.43 | 0.000 | F | 3.94 | 0.139 | 49.3 | ||
| Blood | 2 | 2.05 (1.22–3.45) | 2.60 | 0.000 | F | 0.07 | 0.792 | 0.0 | ||
| Cancer types | ||||||||||
| Digestive system | 3 | 2.99 (2.17–4.13) | 6.68 | 0.000 | F | 1.86 | 0.394 | 0.0 | ||
| Ethnicity | ||||||||||
| Asian | 3 | 2.99 (2.17–4.13) | 6.68 | 0.000 | F | 1.86 | 0.394 | 0.0 | ||
| Caucasian | 2 | 1.81 (1.13–2.88) | 2.48 | 0.013 | F | 0.00 | 0.977 | 0.0 | ||
| DFS/RFS | Overall | 4 | 2.85 (1.96–4.14) | 5.47 | 0.000 | F | 5.22 | 0.157 | 42.5 | |
| Sample | ||||||||||
| Tissue | 2 | 4.10 (2.40–7.00) | 5.18 | 0.000 | F | 1.66 | 0.198 | 39.7 | ||
| Blood | 2 | 2.00 (1.18–3.38) | 2.58 | 0.01 | F | 0.03 | 0.863 | 0.0 | ||
| MiR-27a | ||||||||||
| OS | Overall | 5 | 1.89 (1.32–2.69) | 3.48 | 0.001 | F | 6.38 | 0.173 | 37.3 | |
| Sample | ||||||||||
| Tissue | 3 | 1.50 (0.79–2.69) | 1.24 | 0.214 | R | 5.60 | 0.061 | 64.3 | ||
| Blood | 2 | 2.09 (1.36–3.22) | 3.36 | 0.001 | F | 0.07 | 0.792 | 0.0 | ||
| Cancer types | ||||||||||
| Osteosarcoma | 2 | 2.37 (1.52–3.68) | 3.82 | 0.000 | F | 0.42 | 0.515 | 0.0 | ||
| Ethnicity | ||||||||||
| Asian | 3 | 2.01 (1.32–3.05) | 3.25 | 0.001 | F | 0.79 | 0.675 | 0.0 | ||
| Caucasian | 2 | 1.60 (0.81–3.17) | 1.36 | 0.175 | R | 5.29 | 0.021 | 81.1 | ||
| DFS/RFS/PFS | Overall | 5 | 2.19 (1.29–3.70) | 2.92 | 0.003 | F | 2.21 | 0.698 | 0.0 | |
| Sample | ||||||||||
| Tissue | 3 | 2.58 (1.41–4.72) | 3.07 | 0.002 | F | 0.95 | 0.623 | 0.0 | ||
| Blood | 2 | 1.32 (0.46–3.80) | 0.51 | 0.608 | F | 0.10 | 0.753 | 0.0 | ||
| Cancer types | ||||||||||
| RCC | 2 | 2.30 (1.16–4.57) | 2.39 | 0.017 | F | 0.48 | 0.49 | 0.0 | ||
| Ethnicity | ||||||||||
| Asian | 4 | 1.95 (1.10–3.47) | 2.28 | 0.022 | F | 1.33 | 0.698 | 0.0 | ||
OS, overall survival; RFS, recurrence-free survival; PFS, progressionfree survival; CSS, cancer-specific survival; F, fixedeffects model; R, randomeffects model; RCC, renal cell carcinoma; HR, hazard ratio; CI, confidence interval.
Association of OS with the expression of miR-24
A survival analysis was performed to elucidate the association between OS and the expression of miR-24. As shown in Fig. 2B, a fixed-effects analysis was used to calculate the pooled HR and its 95% CI in 5 cohorts (I2=18.4%, P=0.298). The result indicated that the dysregulation of miR-24 in various cancers may predict a poorer OS (pooled HR=2.49, 95% CI: 1.84–3.37). The association was statistically significant (P=0.000).
As shown in Table II, further stratified analysis by detected samples suggested that a poorer OS was associated with high expression level of miR-24 in tissues (fixed-effects model, pooled HR=2.74, 95% CI: 2.02–3.73; P=0.000) as well as in the blood (fixed-effects model, pooled HR=2.05, 95% CI: 1.22–3.45; P=0.000) sample. An obvious statistically significant association was observed between OS and the expression of miR-24 in digestive system cancers (pooled HR=2.99, 95% CI: 2.17–4.13; P=0.000) by a fixed-effects model (I2=0.0%, P=0.394). In the subgroup analysis of dominant ethnicity, a significant association was observed in Asians (fixed-effects model, pooled HR=2.99, 95% CI: 2.17–4.13; P=0.000) as well as Caucasians (fixed-effects model, pooled HR=1.81, 95% CI: 1.13–2.88; P=0.013).
Association of OS with the expression of miR-27a
A total of 5 studies evaluated OS and miR-27a. Due to the relatively low significant heterogeneity, a fixed-effects model was used to calculate the pooled HR and its 95% CI (I2=37.3%, P=0.173). The result suggested that the association between OS and the expression of miR-27a was statistically significant (pooled HR=1.89, 95% CI: 1.32–2.69; P=0.001; Fig. 2C).
Subgroup analyses failed to demonstrate a significant association between poorer OS and high level of miR-27a expression in the tissue subgroup (pooled HR=1.50, 95% CI: 0.79–2.69, P=0.214) by a random-effects model (I2=37.3%, P=0.173), but revealed that a high level of miR-27a in the blood was a significant predictor of poor OS (pooled HR=2.09, 95% CI: 1.36–3.22; P=0.001) by a fixed-effects model (I2=0.0%, P=0.792). In addition, there was an obvious statistically significant association between OS and the expression of miR-24 in osteosarcoma (fixed-effects model, pooled HR=2.37, 95% CI: 1.52–3.68; P=0.000). In the subgroup analysis by dominant ethnicity, a significant association was observed in Asians (fixed-effects model, pooled HR=2.01, 95% CI: 1.32–3.05; P=0.001), but not in Caucasians (random-effects model, pooled HR=1.60, 95% CI: 0.81–3.17; P=0.175; Table II).
Association of tumor progression [disease-free survival (DFS)/recurrence-free survival (RFS)] with the expression of miR-23a
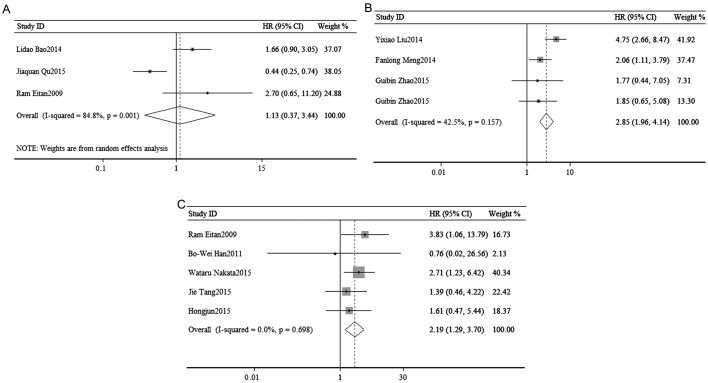
To access the association of tumor progression with miR-23a expression, disease recurrence and metastasis were assessed. As shown in the Fig. 5, a random-effects model was used to calculate the pooled HR and its 95% CI in 3 cohorts (I2=84.8%, P=0.001), but failed to show a significant association between the expression of miR-23a and poor DFS/RFS (pooled HR=1.13, 95% CI: 0.37–3.44; P=0.836; Fig. 3A).
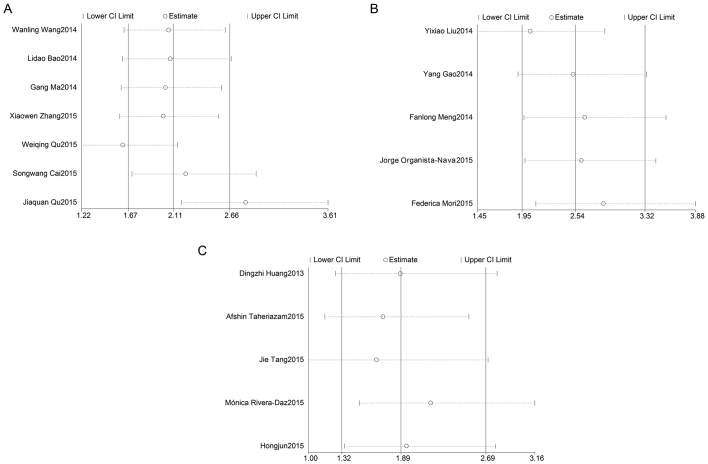
Figure 5.
Sensitivity analysis. Meta-analysis estimates, given named study is omitted. (A) Effect of individual studies on the pooled hazard ratio (HR) for overall survival (OS) associated with miR-23a expression; (B) effect of individual studies on the pooled HR for OS associated with miR-24 expression; (C) effect of individual studies on the pooled HR for OS associated with miR-27a expression. CI, confidence interval.
Figure 3.
Forest plots on the association of disease-free survival/recurrence-free survival/progression-free survival with (A) miR-23a, (B) miR-24 and (C) miR-27a. HR, hazard ratio; CI, confidence interval.
Association of tumor progression (DFS/RFS) with the expression of miR-24
Disease recurrence and metastasis were used to access the association of tumor progression with miR-24 expression. Due to the low heterogeneity, the pooled HR and its 95% CI were calculated by a fixed-effects model (I2=42.5%, P=0.157) and the meta-analysis result suggested that high expression of miR-24 was significantly associated with poor DFS/RFS (pooled HR=2.85, 95% CI: 1.96–4.14; P=0.000; Fig. 3B).
As shown in Table II, further stratified analysis indicated that tumor progression was associated with a high expression level of miR-24 in tissue samples (fixed-effects model, pooled HR=4.10, 95% CI: 2.40–7.00; P=0.000), as well as in the blood (fixed-effects model, pooled HR=2.00, 95% CI: 1.18–3.38; P=0.01).
Association of tumor progression [DFS/RFS/progression-free survival (PFS)] with the expression of miR-27a
The association of tumor progression with miR-27a expression was analyzed to combine disease recurrence and metastasis. A total of 6 studies included a DFS/RFS/PFS analysis, with significant heterogeneity (I2=0.00%, P=0.698), and demonstrated a significant association between the expression of miR-27a and poor DFS/RFS/PFS (pooled HR=2.19, 95% CI: 1.29–3.70; P=0.003) (Fig. 3C).
In the subgroup analysis of the association of high expression of miR-27a and tumor progression, a significant association was observed for tissue samples (fixed-effects model, pooled HR=2.58, 95% CI: 1.41–4.72; P=0.002). However, no significant association was observed between tumor progression and high expression of miR-27a in blood samples (fixed-effects model, pooled HR=1.32, 95% CI: 0.46–3.80; P=0.608). Moreover, we found that high expression of miR-27a was significantly associated with poor DFS/RFS/PFS in RCC (fixed-effects model, pooled HR=2.30, 95% CI: 1.16–4.67; P=0.017). No obvious heterogeneity was observed (I2=0.00%, P=0.49) and the fixed-effects model was used. A significant association was also observed in Asian patients (fixed-effects model; pooled HR=1.95, 95% CI: 1.10–3.47; P=0.022).
Heterogeneity analysis result
To assess OS for miR-23a (I2=86.1%), miR-24 (I2=18.4%) and miR-27a (I2=37.3%), as well as DFS/RFS/PFS for miR-23a (I2=84.8%), miR-24 (I2=42.5%) and miR-27a (I2=0.00%), heterogeneity was analyzed among studies. In the subgroup analysis by cancer type and miR-23a expression, significant heterogeneity was observed in respiratory system cancers (I2=95%). In the stratified analysis by statistical methodology and miR-23a expression, significant heterogeneity was also observed in the univariate analysis (I2=95.7%) as well as in the multivariate analysis (I2=86.2%). However, in a subgroup analysis, there was also significant heterogeneity among Asians (OS for miR-23a: I2=86.1%), as well as Caucasians (OS for miR-27a: I2=81.1%). In addition, significant heterogeneity was observed in the subgroup of tissue samples (OS for miR-27a: I2=64.3%), while no significant heterogeneity was observed in other sample subgroups.
Publication bias and sensitivity analysis
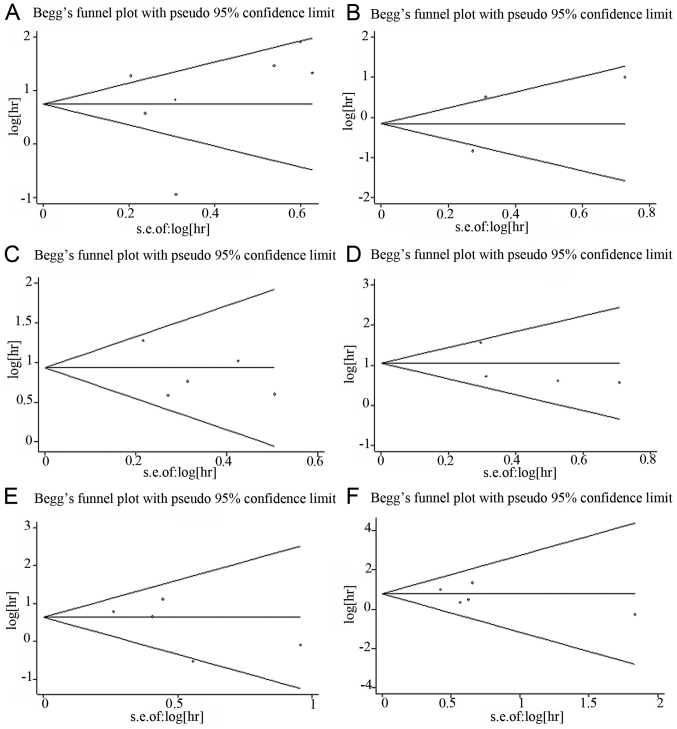
Begg's funnel plot and Egger's test were used to assess the potential publication bias. For miR-23a, 7 cohorts evaluating OS and 3 cohorts evaluating DFS/RFS were included. No obvious asymmetry was observed in the Begg's funnel plot (Fig. 4A and B) and Egger's test indicated no potential publication bias (OS: t=0.30, P=0.778; DFS/RFS: t=0.23, P=0.854). For miR-24, 5 and 4 cohorts evaluating OS and DFS/RFS, respectively, were included. There was no obvious asymmetry in the Begg's funnel plot (Fig. 4C and D) and Egger's test also indicated no potential publication bias (OS: t=−0.99, P=0.395; DFS/RFS: t=−0.94, P=0.448). To evaluate OS and DFS/RFS/PFS for miR-27a, 5 and 4 cohorts were included, respectively. No obvious asymmetry was observed in the Begg's funnel plot (Fig. 4E and F) and Egger's test also indicated no potential publication bias (OS: t=−1.21, P=0.312; DFS/RFS/PFS: t=−0.82, P=0.472).
Figure 4.
Begg's funnel plots of publication bias test. (A) Association of overall survival (OS) with miR-23a; (B) Association of disease-free survival/recurrence-free survival (DFS/RFS) with miR-23a; (C) Association of OS with miR-24; (D) Association of DFS/RFS with miR-24; (E) Association of OS with miR-27a; (F) Association of DFS/RFS/progression-free survival with miR-27a.
In order to assess the effect of any individual study on the stability of the overall result, the sensitivity analysis was used by omitting each study at a given time. As shown in Fig. 5A, the result of sensitivity analysis for miR-23a with OS was affected due to the results of 2 studies: Qu et al (26) and Qu et al (25). The result of the sensitivity analysis for miR-24 with OS (Fig. 5B) reflected the stability of the studies, as did the result of the sensitivity analysis for miR-27a with OS (Fig. 5C). Due to lack of a sufficient number of studies, sensitivity analyses for miR-23a, miR-24 and miR-27a with DFS/RFS were not preformed.
Discussion
To the best of our knowledge, this is the first meta-analysis to investigate the association between the miR-23a/24-2/27a cluster and various cancers. The miR-23a/24-2/27a cluster exists in the vertebrate genome, and has been confirmed to play an important role in cancer progression (33,34). This cluster must be distinguished from the miR-23b/24-1/27b cluster, as the latter is located in close proximity on the human chromosome 9q22.32 region and also plays an important role in cancer (35).
In the present meta-analysis, it was demonstrated that a high expression level of miR-23a, as well as miR-24 and miR-27a, was associated with worse OS in various types of cancers. Particularly in cancers of the digestive system, high expression of miR-23a and miR-24 indicated a worse prognosis. For cancers of the respiratory system, there was no statistical significance in the overall study sample, but significant relevance was observed in certain individual studies. Therefore, more studies are required to prove the association between high expression of miR-23a and OS in respiratory system cancers. Furthermore, due to the lack of studies on the association of high expression of miR-23a and OS in Caucasians, the association of high expression level of miR-23a with worse OS was only investigated in Asians. For miR-24, a significant association was observed in Asians as well as Caucasians. Unlike miR-23a and miR-24, a significant association between a high expression level of miR-27a and OS was observed in Asians, but not in Caucasians. A stratified analysis by detected sample suggested that poorer OS was associated with high expression levels of miR-24 and miR-27a in tissue as well as in blood samples. And the results by tissue were more sensitive compared with the results by blood for miR-24 and miR-27a. Moreover, there was a statistically significant association between OS and the expression of miR-24 in osteosarcoma.
In addition, there was a significant association between the expression of miR-24, as well as miR-27a, and tumor progression. However, there was no obvious association between the expression of miR-23a and poor DFS/RFS. Further stratified analysis indicated that a high expression level of miR-24, as well as miR-27a, in tissue samples was associated with tumor progression. The association persisted for miR-24, while no significant association was observed for miR-27a expression in the blood.
Recently, a number of studies indicated that the miR-23a/24-2/27 cluster plays an important role in the occurrence and development of cancer. Huang et al demonstrated that a high expression level of miR-23a/24/27a decreased transforming growth factor-β-induced tumor-suppressive activity in a Smad-dependent manner in HCC (9) and lung cancer (36). Furthermore, the cluster was also found to be involved in altering lymphoid cell differentiation via the transcription factor PU.1 (37). An increasing number of studies demonstrated that several miRNA clusters may regulate the occurrence and development of cancer by cooperating with the c-Myc oncogene (38–40). The association of miR-23a/24-2/27a with c-Myc has also been investigated and the findings suggested that the miR-23a/24-2/27a cluster was upregulated in breast cancer and was correlated with cancer cell metastasis, migration and invasion via c-Myc (34).
There were some limitations to this meta-analysis. First, all the included studies were published in English; therefore, English language bias may exist in this meta-analysis. Second, the number of eligible studies was not sufficient, despite the fact that no significant publication bias was detected in this meta-analysis; furthermore, the subgroup analysis was limited by the sample size, compromising the validity of the results. Third, some HRs were estimated from survival curves and data were extracted according to the Tierney's method (41). Finally, certain aspects were not uniform among studies, including clinical characteristics and follow-up time, which may lead to estimation errors.
In summary, the present meta-analysis demonstrated that high expression levels of miR-23a, miR-24-2 and miR-27a were associated with poor survival in various types of cancer. The miR-23a/24-2/27a cluster may prove useful for monitoring the progression and prognosis of cancer in clinical practice. However, to verify the association between the expression of the miR-23a/24-2/27a cluster and cancer, a larger number of relevant studies are required.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81101922), the Science and Technology Development Fund Project of Shenzhen (grant nos. JCYJ20150403091443329 and JCYJ20170307111334308), the fund of ‘San-Ming’ project of medicine in Shenzhen and funds from the Guangdong Key Medical Subject.
References
- 1.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvitz HR, Sulston JE. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics. 1980;96:435–454. doi: 10.1093/genetics/96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: MicroRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hua K, Jin J, Zhang H, Zhao B, Wu C, Xu H, Fang L. MicroRNA-7 inhibits proliferation, migration and invasion of thyroid papillary cancer cells via targeting CKS2. Int J Oncol. 2016;49:1531–1540. doi: 10.3892/ijo.2016.3660. [DOI] [PubMed] [Google Scholar]
- 5.Nip H, Dar AA, Saini S, Colden M, Varahram S, Chowdhary H, Yamamura S, Mitsui Y, Tanaka Y, Kato T, et al. Oncogenic microRNA-4534 regulates PTEN pathway in prostate cancer. Oncotarget. 2016;7:68371–68384. doi: 10.18632/oncotarget.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin L, Li Y, Liu J, Yang S, Gui Y, Mao X, Nie G, Lai Y. Tumor suppressor miR-149-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Mol Med Rep. 2016;13:5386–5392. doi: 10.3892/mmr.2016.5205. [DOI] [PubMed] [Google Scholar]
- 7.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 8.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, He X, Ding J, Huang S, He X, Ding J, Liang L, Zhao Y, Zhang Z, Yao X, Pan Z, Zhang P, Li J, et al. Upregulation of miR-23a approximately 27a approximately 24 decreases transforming growth factor-beta-induced tumor-suppressive activities in human hepatocellular carcinoma cells. Int J Cancer. 2008;123:972–978. doi: 10.1002/ijc.23580. [DOI] [PubMed] [Google Scholar]
- 10.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattie MD, Benz CC, Bowers J, Sensinger K, Wong L, Scott GK, Fedele V, Ginzinger D, Getts R, Haqq C. Optimized high-throughput microRNA expression profiling provides novel biomarker assessment of clinical prostate and breast cancer biopsies. Mol Cancer. 2006;5:24. doi: 10.1186/1476-4598-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao L, Zhao J, Dai X, Wang Y, Ma R, Su Y, Cui H, Niu J, Bai S, Xiao Z, et al. Correlation between miR-23a and onset of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2014;38:318–330. doi: 10.1016/j.clinre.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Cai S, Chen R, Li X, Cai Y, Ye Z, Li S, Li J, Huang H, Peng S, Wang J, et al. Downregulation of microRNA-23a suppresses prostate cancer metastasis by targeting the PAK6-LIMK1 signaling pathway. Oncotarget. 2015;6:3904–3917. doi: 10.18632/oncotarget.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eitan R, Kushnir M, Lithwick-Yanai G, David MB, Hoshen M, Glezerman M, Hod M, Sabah G, Rosenwald S, Levavi H. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol. 2009;114:253–259. doi: 10.1016/j.ygyno.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Liu Y, Du L, Li J, Qu A, Zhang X, Wang L, Wang C. Down-regulation of miR-24-3p in colorectal cancer is associated with malignant behavior. Med Oncol. 2015;32:362. doi: 10.1007/s12032-014-0362-4. [DOI] [PubMed] [Google Scholar]
- 16.Han BW, Feng DD, Li ZG, Luo XQ, Zhang H, Li XJ, Zhang XJ, Zheng LL, Zeng CW, Lin KY, et al. A set of miRNAs that involve in the pathways of drug resistance and leukemic stem-cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALL. Hum Mol Genet. 2011;20:4903–4915. doi: 10.1093/hmg/ddr428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang D, Wang H, Liu R, Li H, Ge S, Bai M, Deng T, Yao G, Ba Y. miRNA27a is a biomarker for predicting chemosensitivity and prognosis in metastatic or recurrent gastric cancer. J Cell Biochem. 2014;115:549–556. doi: 10.1002/jcb.24689. [DOI] [PubMed] [Google Scholar]
- 18.Liu YX, Long XD, Xi ZF, Ma Y, Huang XY, Yao JG, Wang C, Xing TY, Xia Q. MicroRNA-24 modulates aflatoxin B1-related hepatocellular carcinoma prognosis and tumorigenesis. Biomed Res Int. 2014;2014:482926. doi: 10.1155/2014/482926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma G, Dai W, Sang A, Yang X, Gao C. Upregulation of microRNA-23a/b promotes tumor progression and confers poor prognosis in patients with gastric cancer. Int J Clin Exp Pathol. 2014;7:8833–8840. [PMC free article] [PubMed] [Google Scholar]
- 20.Meng FL, Wang W, Jia WD. Diagnostic and prognostic significance of serum miR-24-3p in HBV-related hepatocellular carcinoma. Med Oncol. 2014;31:177. doi: 10.1007/s12032-014-0177-3. [DOI] [PubMed] [Google Scholar]
- 21.Mori F, Ferraiuolo M, Santoro R, Sacconi A, Goeman F, Pallocca M, Pulito C, Korita E, Fanciulli M, Muti P, et al. Multitargeting activity of miR-24 inhibits long-term melatonin anticancer effects. Oncotarget. 2016;7:20532–20548. doi: 10.18632/oncotarget.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakata W, Uemura M, Sato M, Fujita K, Jingushi K, Ueda Y, Kitae K, Tsujikawa K, Nonomura N. Expression of miR-27a-3p is an independent predictive factor for recurrence in clear cell renal cell carcinoma. Oncotarget. 2015;6:21645–21654. doi: 10.18632/oncotarget.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Organista-Nava J, Gómez-Gómez Y, Illades-Aguiar B, Del Carmen Alarcón-Romero L, Saavedra-Herrera MV, Rivera-Ramírez AB, Garzón-Barrientos VH, Leyva-Vázquez MA. High miR-24 expression is associated with risk of relapse and poor survival in acute leukemia. Oncol Rep. 2015;33:1639–1649. doi: 10.3892/or.2015.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng H, Wang X, Zhang P, Sun T, Ren X, Xia Z. miR-27a promotes cell proliferation and metastasis in renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:2259–2266. [PMC free article] [PubMed] [Google Scholar]
- 25.Qu JQ, Yi HM, Ye X, Li LN, Zhu JF, Xiao T, Yuan L, Li JY, Wang YY, Feng J, et al. MiR-23a sensitizes nasopharyngeal carcinoma to irradiation by targeting IL-8/Stat3 pathway. Oncotarget. 2015;6:28341–28356. doi: 10.18632/oncotarget.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu WQ, Liu L, Yu Z. Clinical value of microRNA-23a upregulation in non-small cell lung cancer. Int J Clin Exp Med. 2015;8:13598–13603. [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera-Diaz M, Miranda-Roman MA, Soto D, Quintero-Aguilo M, Ortiz-Zuazaga H, Marcos-Martinez MJ, Vivas-Mejía PE. MicroRNA-27a distinguishes glioblastoma multiforme from diffuse and anaplastic astrocytomas and has prognostic value. Am J Cancer Res. 2015;5:201–218. [PMC free article] [PubMed] [Google Scholar]
- 28.Taheriazam A, Bahador R, Karbasy SH, Jamshidi SM, Torkaman A, Yahaghi E, Shakeri M. Down-regulation of microRNA-26a and up-regulation of microRNA-27a contributes to aggressive progression of human osteosarcoma. Diagn Pathol. 2015;10:166. doi: 10.1186/s13000-015-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Tang J, Zhao H, Cai H, Wu H. Diagnostic and prognostic potentials of microRNA-27a in osteosarcoma. Biomed Pharmacother. 2015;71:222–226. doi: 10.1016/j.biopha.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 30.Wang WL, Yang C, Han XL, Wang R, Huang Y, Zi YM, Li JD. MicroRNA-23a expression in paraffin-embedded specimen correlates with overall survival of diffuse large B-cell lymphoma. Med Oncol. 2014;31:919. doi: 10.1007/s12032-014-0919-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XW, Liu N, Chen S, Wang Y, Zhang ZX, Sun YY, Qiu GB, Fu WN. High microRNA-23a expression in laryngeal squamous cell carcinoma is associated with poor patient prognosis. Diagn Pathol. 2015;10:22. doi: 10.1186/s13000-015-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao G, Liu L, Zhao T, Jin S, Jiang S, Cao S, Han J, Xin Y, Dong Q, Liu X, Cui J. Upregulation of miR-24 promotes cell proliferation by targeting NAIF1 in non-small cell lung cancer. Tumour Biol. 2015;36:3693–3701. doi: 10.1007/s13277-014-3008-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters; Proc Natl Acad Sci USA; 2011; pp. 8287–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Liu X, Xu W, Zhou P, Gao P, Jiang S, Lobie PE, Zhu T. c-MYC-regulated miR-23a/24-2/27a cluster promotes mammary carcinoma cell invasion and hepatic metastasis by targeting Sprouty2. J Biol Chem. 2013;288:18121–18133. doi: 10.1074/jbc.M113.478560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto Y, Kojima S, Nishikawa R, Enokida H, Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T, Seki N. The microRNA-23b/27b/24-1 cluster is a disease progression marker and tumor suppressor in prostate cancer. Oncotarget. 2014;5:7748–7759. doi: 10.18632/oncotarget.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao M, Seike M, Soeno C, Mizutani H, Kitamura K, Minegishi Y, Noro R, Yoshimura A, Cai L, Gemma A. MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. Int J Oncol. 2012;41:869–875. doi: 10.3892/ijo.2012.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong KY, Owens KS, Rogers JH, Mullenix J, Velu CS, Grimes HL, Dahl R. MIR-23A microRNA cluster inhibits B-cell development. Exp Hematol. 2010;38:629–640 e1. doi: 10.1016/j.exphem.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta A, Mann M, Zhao JL, Marinov GK, Majumdar D, Garcia-Flores Y, Du X, Erikci E, Chowdhury K, Baltimore D. The microRNA-212/132 cluster regulates B cell development by targeting Sox4. J Exp Med. 2015;212:1679–1692. doi: 10.1084/jem.20150489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang CM, Chiba T, Brill B, Delis N, von Manstein V, Vafaizadeh V, Oellerich T, Groner B. Expression of the miR-302/367 cluster in glioblastoma cells suppresses tumorigenic gene expression patterns and abolishes transformation related phenotypes. Int J Cancer. 2015;137:2296–2309. doi: 10.1002/ijc.29606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]