Abstract
The aim of the present study was to investigate the effect of Forkhead family transcription factor P3 (Foxp3) knockdown on the function of cluster of differentiation (CD)4+CD25+ regulatory T cell (Tregs) and the tumor growth of a hepatocellular carcinoma (HCC) mouse model. CD4+CD25+ Tregs and CD4+CD25− T cells were sorted from peripheral blood mononuclear cells (PBMCs) of patients with HCC. Then, ultrasound-targeted microbubble destruction (UTMD)-mediated Foxp3-microRNA (miRNA) was transfected into Tregs. Subsequently, CD4+CD25− T cells were co-cultured with PBMC and Tregs without Foxp3-miRNA (Foxp3+Tregs) or Tregs with Foxp3-miRNA (Foxp3−Tregs) and the proliferation-inhibition ratio of CD4+CD25− T cells was detected using a Cell Counting Kit-8. Additionally, HCC mice were treated with UTMD-mediated Foxp3-shRNA, the tumor volume was calculated and the content of CD4+ and CD25+ T cells in the blood were detected using flow cytometry. The content of interferon-γ (IFN-γ), interleukin (IL)-2, IL-10, transforming growth factor-β (TGF-β) and vascular endothelial growth factor (VEGF) in cultural supernatant and serum were detected by ELISA analysis. Foxp3−Tregs significantly reduced the inhibition effect of Foxp3+Tregs on the proliferation of CD4+CD25− T cells (P<0.01). The content of IFN-γ and IL-2 significantly increased, while IL-10 and TGF-β significantly decreased in the co-cultured system of Foxp3−Tregs compared with the co-cultured system of Foxp3+Tregs (P<0.01). Following treatment with Foxp3-shRNA, the average tumor volume, ratio of Tregs/CD4+ T cells and level of IL-10, TGF-β and VEGF significantly decreased, however, the level of IFN-γ and IL-2 significantly increased compared with un-treated HCC mice (P<0.05). Foxp3 knockdown may suppress the tumor growth of HCC mice through relieving the immunosuppressive function of Tregs.
Keywords: ultrasound-targeted microbubble destruction, forkhead family transcription factor P3, cluster of differentiation 4+ cluster of differentiation 25+ regulatory T cells, regulatory T cells
Introduction
Hepatocellular carcinoma (HCC) is one of the most challenging malignant tumors, with a high morbidity and mortality in China (1,2). Although progress has been made in surgery (transplant, embolization or resection); radiation therapy, chemotherapy and ablation, tumor recurrence and metastasis remain difficult to treat (3). Therefore, investigations into the molecular mechanism of recurrence and metastasis of HCC, and less invasive but more effective therapeutic methods for clinical HCC treatment are required.
Immunotherapy has been applied in the treatment of HCC in early clinical trials due to its characteristics: Systemic, nontoxic and long-lived anti-tumor activity (4). Previous studies have demonstrated that the tumor microenvironment may be established by the regulation of lymphocytes, and then serves a vital role in the progression of tumors through immunity and inflammation (5,6). It is well known that cluster of differentiation (CD)4+CD25+ regulatory T cells (Tregs) contribute to the growth of malignant tumors through suppressing immune surveillance (7). Forkhead family transcription factor P3 (Foxp3) is specifically expressed in Tregs and serves an important role in the negative immunoregulatory function of Tregs in mice and humans (8,9). A previous study has indicated that in murine tumor models, downregulated Foxp3 inhibits the tumor growth of CD4+CD25+ Tregs, as observed in leukemia (10). Other studies have demonstrated an increased expression of CD4+CD25+ Tregs in patients with HCC (11,12). However, the effects of a Foxp3 knockdown on the function of CD4+CD25+ Tregs and the development of HCC remain to be elucidated.
Currently, the application of ultrasound-targeted microbubble destruction (UTMD) for gene delivery has become a focus of research due to its low immunogenic, non-invasive, targeted and high-efficiency characteristics (13,14). UTMD has been demonstrated to efficiently deliver genes to cells, myocardium and solid tumors (15–17). In the present study, the expression of Foxp3 was downregulated through UTMD-mediated delivery of Foxp3-microRNA (miRNA) and Foxp3-short hairpin RNA (shRNA) in CD4+CD25+ Tregs to a HCC mice model, respectively. Whether Foxp3 knockdown was able to inhibit the negatively immunoregulatory function of CD4+CD25+ Tregs and suppress the tumor growth of HCC through improving the immune microenvironment was then assessed.
Materials and methods
Patients
The current study recruited a total of 50 patients (28–76 years; male/female, 41/9; 63.77–83.25 kg) with HCC at The First Affiliated Hospital of Harbin Medical University (Harbin, China) from October 2013 to May 2014. The inclusion criteria were as follows: i) All patients were newly diagnosed with TNM stage I–II cancer (18) and did not accept any treatment; ii) patients had no other organic disease with the exception of hepatic pathological changes; and iii) patients had elevated levels of alphafetoprotein and aminotransferase (diagnostic indicators for hepatocellular carcinoma). Approval from the Ethics Committee of The First Affiliated Hospital of Harbin Medical University was obtained and written informed consent was received from all patients.
Separation of peripheral blood mononuclear cells, CD4+ CD25+ Tregs and CD4+CD25− T cells
Initially, venous blood (40–60 ml) was collected aseptically from patients with HCC, and diluted with the same volume of phosphate buffered saline. The diluted blood samples were slowly added on the liquid surface of lymphocyte isolation medium (Shaanxi Haoyang Biological Technology Co., Ltd., Xi'an, China) at a ratio of 1:1, and peripheral blood mononuclear cells (PBMCs) were separated by centrifugation (700 × g for 25 min at 25°C). Subsequently, CD4+CD25+ Tregs and CD4+CD25− T cells were sorted from PBMCs using CD4+CD25+ Tregs magnetic bead separation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) following the manufacturer's protocol. The sorted CD4+CD25+ Tregs or CD4+CD25− T cells (1×107/ml, 0.1 ml) were co-stained with anti-CD4 fluorescein isothiocyanate (FITC)-conjugated and anti-CD25 phycoerythrin (PE)-conjugated antibodies (10 µg/ml; cat. nos. 555346 and 555432; BD Biosciences, Franklin Lakes, NJ, USA) for 30–40 min at room temperature away from light. Cells were washed with PBS, and samples were fixed in 1% formaldehyde solution at 4°C for 1 h and detected by FACSCria (BD Biosciences). Finally, the data were analyzed by FACSDiva software (version 4.1; BD Biosciences), and the purity of the CD4+CD25+ Tregs and CD4+CD25− T cells was detected at >90%.
Construction of Foxp3-miRNA and Foxp3-shRNA expression plasmids
A specific miRNA sequence targeted to human Foxp3 (GenBank Accession No. NM_50943; https://www.ncbi.nlm.nih.gov/gene/?term=50943) and a control miRNA sequence (Table I) were designed and chemically synthesized by Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Double-stranded DNA oligonucleotides corresponding to miRNA were obtained by the annealing of two corresponding oligomeric single-strand DNA at 95°C for 5 min and inserted into pcDNA6.2-GW/EmGFP-miR vector (Invitrogen; Thermo Fisher Scientific, Inc.). A specific shRNA sequence targeted to mouse Foxp3 (Table I) was designed by Shanghai GenePharma Co., Ltd. (Shanghai, China) and cloned into the pGPU6/GFP/Neo vector (Invitrogen; Thermo Fisher Scientific, Inc.). Ultimately, the recombinant plasmid Foxp3-miRNA and Foxp3-shRNA were successfully constructed.
Table I.
miRNA and shRNA sequences of Foxp3.
| RNA type | Sequence (5′-3′) |
|---|---|
| miRNA-Foxp3 | F: TGCTGCACAGATGAAGCCTTGGTCAGGTTTTGGCCACTGACTGACCTGACCAACTTCATCTGTG |
| R: CCTGCACAGATGAAGTTGGTCAGGTCAGTCAGTGGCCAAAACCTGACCAAGGCTTCATCTGTGC | |
| miRNA-Foxp3-NC | F: TGCTGAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT |
| R: CCTGAAATGTACTGCGTGGAGACGTCAGTCAGTGGCCAAAACGTCTCCACGCGCAGTACATTTC | |
| shRNA-Foxp3 | F: CACCGAGGCAGAGGACACTCAATGATTCAAGAGATCATTGAGTGTCCTCTGCCTCTTTTTTG |
| R: AATTCAAAAAAGAGGCAGAGGACACTCAATGATCTCTTGAATCATTGAGTGTCCTCTGCCTCG | |
| shRNA-Foxp3-NC | F: CACCGTTCTCCGAACGTGTCACGTCAAGAGATTACGTGACACGTTCGGAGAATTTTTTG |
| R: AATTCAAAAAAGTTCTCCGAACGTGTCACGCTCTTGAATTACGTGACACGTTCGGAGAACG |
miRNA, microRNA; shRNA, short hairpin RNA; Foxp3, forkhead family transcription factor P3; F, forward; R, reverse; NC, negative scrambled control.
Preparation of microbubble
SonoVue powder (Bracco, Milan, Italy) was dissolved in 5 ml normal saline. Following 5 min oscillation, microbubbles were evenly distributed. Microbubbles with a density of 2×108-5×108/ml, diameter of 2.5–6.0 µm and concentration of 5 mg/ml were observed under a light microscope (Olympus Corporation, Tokyo, Japan).
Cell transfection and grouping
Tregs with a concentration of 1×106/ml were resuspended in serum-free RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), and 1.5–2×105 cells were seeded into each well of a 96-well plate (Corning Incorporated, Corning, NY, USA). Then, the cells were divided into 7 groups as follows: i) Control group, 90 µl medium + 10 µl control plasmid; ii) SonoVue microbubbles + Foxp3-miRNA plasmid (MB + P) group, 80 µl medium + 20 µl mixture of microbubbles and Foxp3-miRNA plasmid (1:1); iii) ultrasound + Foxp3-miRNA plasmid (US + P) group, 90 µl medium + 10 µl Foxp3-miRNA plasmid with ultrasonic irradiation; iv) ultrasound + SonoVue microbubbles + Foxp3-miRNA plasmid (US + MB + P) group, 80 µl medium + 20 µl mixture of microbubbles and Foxp3-miRNA plasmid (1:1) with ultrasonic irradiation; v) Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) + Foxp3-miRNA plasmid (L + P) group, 90 µl medium + 10 µl Foxp3-miRNA plasmid + 1 µl Lipofectamine® 2000; vi) Ultrasound + Lipofectamine2000 + Foxp3-miRNA plasmid (US + L + P) group, 90 µl medium + 10 µl Foxp3-miRNA plasmid + 1 µl Lipofectamine2000 with ultrasonic irradiation; vii) Ultrasound + SonoVue microbubbles + Lipofectamine 2000 + Foxp3-miRNA plasmid (US + MB + L + P) group, 80 µl medium + 20 µl mixture of microbubbles and Foxp3-miRNA plasmid (1:1) + 1 µl Lipofectamine® 2000 with ultrasonic irradiation. The groups with ultrasonic irradiation were exposed to irradiation conditions with (MI=1.4; exposure time=150 sec) using IU22 ultrasonic equipment (Philips Healthcare, DA Best, The Netherlands). Following transfection for 24 h, the transfection efficiency was observed under fluorescence microscope (Olympus Corporation) and detected by FACSCalibur flow cytometer (BD Biosciences) as previously described (19). Results were analyzed using FACSComp (version5.1) software (BD Biosciences).
Detection of Tregs activity
Following 24 h transfection, 10 µl Cell Counting Kit (CCK)8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added into each well and cultured at 37°C for 4 h. Then, the absorbance was read at 460 nm using a microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA). The cell survival rate was calculated as optical density (OD)test group-blank group/ODcontrol group-blank group.
Inhibition effect of Tregs on CD4+CD25− T cells
Two healthy volunteers (one 40-year-old male weighing 75 kg and one 45-year-old female weighing 63 kg) were recruited at The First Affiliated Hospital of Harbin Medical University in May 2014. Written informed consent was received from healthy volunteers and fasting peripheral blood samples were collected. PBMCs were separated from the peripheral blood. Tregs in the control group (90 µl medium + 10 µl control plasmid) and optimal transfection group (US + MB + L + P) were collected and co-cultured with CD4+CD25− T cells and PBMCs. CD4+CD25− T cell activity was detected using the CCK8 assay according to the manufacturer's protocol. The proliferation-inhibition ratio of CD4+CD25− T cells was calculated as ODcells without Tregs-cells with Tregs/ODcells without Tregs. Supernatant was collected to detect the contents of interferon-γ (IFN-γ) and interleukin-2 (IL-2) secreted by CD4+CD25− T cells in addition to IL-10 and transforming growth factor-β (TGF-β) secreted by Tregs using the corresponding ELISA kits (IFN-γ, E0110345; IL-2, E0110308; IL-10, E0110023; TGF-β, E01T0058; Shanghai BlueGene Biotech Co., Ltd., Shanghai, China) according to the manufacturer's protocol.
Animal model and grouping
Ethical approval was obtained from the Ethics Committee of the Animal Laboratory Center of the First Affiliated Hospital of Harbin Medical University. A total of 21 healthy male C57BL/6 mice (8 weeks old; 18–22 g) were purchased from the Animal Laboratory Center of the first affiliated hospital of Harbin medical university, and acclimatized to a 12 h light/dark cycle at 21°C with 60–70% relative humidity and given free access to food and water for a week prior to the trial. For the HCC mice model, 200 µl Hepa1-6 cells (5×107/ml; Shanghai Cell Bank of Chinese Academy of Sciences, Shanghai, China) were transplanted subcutaneously into the right flanks of mice in the model group (n=18). The other 3 mice were treated with normal saline as a control. Following 7 days, the 18 mice in the model group were randomly and equally assigned to 2 groups (n=9): HCC and treatment groups. Mice in the treatment group were treated with microbubbles and Foxp3-shRNA plasmid, 200 µl intravenously and 300 µl directly into tumors. Mice in HCC group were injected with an equal dose of normal saline in the same method. Following injection, tumors were immediately irradiated by ultrasound for 300 sec. The treatment was repeated every 3 days, for 21 days, a total of 7 times.
Detection index
Prior to each treatment, the long diameters (a) and short diameters (b) of the tumor were detected and tumor volume was calculated as follows: 1/6πa2b. Tumor growth curves were constructed according to the tumor volume. Following 1 week of treatment, all mice were sacrificed using 1.5% pentobarbital (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with a dose of 375 mg/kg and the tumors were weighed. The tumor inhibition rate=(mean tumor weight in control group-mean tumor weight in experimental group)/mean tumor weight in control group. In addition, following treatment for 1 week, blood samples were collected. The content of CD4+ and CD25+ T cells were detected by staining with CD4-FITC and CD25-Allophycocyanin conjugated antibodies (1:50; cat. nos. 553047 and 557192; BD Biosciences), respectively, using FACSCria (BD Biosciences). Briefly, the cells (1×107 cells/ml, 0.1 ml) were stained with CD4-FITC or CD25-Allophycocyanin conjugated antibodies for 30–40 min at room temperature in the dark. Cells were washed with PBS and samples were fixed in 1% formaldehyde solution at 4°C for 1 h and detected by FACSCria (BD Biosciences). Finally, the data were analyzed by FACSDiVa software (version 4.1; BD Biosciences). The content of vascular endothelial growth factor (VEGF), IL-10, TGF-β, IFN-γ and IL-2 in serum was detected by the corresponding ELISA kits (VEGF, E03V0010; IFN-γ, E03I0345; IL-2, E03I0308; IL-10, E03I0023; TGF-β, E03T0058; Shanghai BlueGene Biotech Co. Ltd.) according to the manufacturer's protocol.
Western blot analysis
Cells and HCC tissue were collected and homogenized in pre-cooled RIPA lysis buffer. Supernatant was acquired by centrifugation at 11,347 × g for 15 min at 4°C. Subsequently; the protein concentration was detected by the BCA Protein Quantitative Assay (Sangon Shanghai Biotech Co., Ltd., Shanghai, China). A total of 40 µg protein per lane sample was separated by 10% SDS-PAGE and transferred onto polyvinylidene fluoride membranes, which were blocked in 5% non-fat milk for 1 h at room temperature. The membranes were incubated with mouse anti-human and rabbit anti-mouse GAPDH antibodies (1:1,000; cat. nos. ab10901 and ab37168; Abcam, Cambridge, MA, USA), mouse anti-human (1:1,000; cat. no. ab10901, Abcam) and rabbit anti-mouse (1:1,000; cat. no. TA346949; Beijing Zhongshan Jingqiao Biotechnology Co., Beijing, China) Foxp3 polyclonal antibody overnight at 4°C and subsequently incubated with goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (HRP) or goat anti-rabbit IgG-HRP (1:5,000; cat. nos. ZB-2305 and ZB-2301; Zhongshan Jingqiao Biotechnology Co.) for 2 h at room temperature. Ultimately, proteins were detected with enhanced chemiluminescence reagent (EMD Millipore, Billerica, MA, USA).
Statistical analysis
Statistical analysis was performed by SPSS 12.0 statistical analysis software (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation and analyzed by one-way analysis of variance followed by a least significance differences test. P<0.05 was considered to indicate statistically significant differences.
Results
Comparison of transfection efficiency between groups
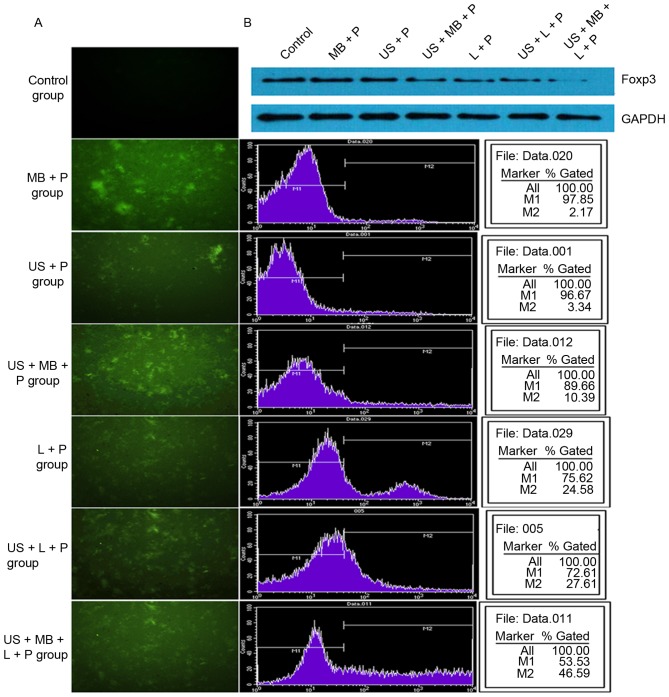
Following 24 h of transfection, fluorescence measurements indicated that no green fluorescent cells were present in the control group, whereas different numbers of green fluorescent cells were observed in the other six groups. US + MB + L + P group had more green fluorescent cells than the other five groups (Fig. 1A). In addition, flow cytometry analysis demonstrated that transfection efficiency was 0, 2.17±0.57, 3.34±0.43, 10.39±1.65, 24.58±2.48, 27.61±3.40 and 46.59±4.10% in the MB + P, US + P, US + MB + P, L + P, US + L + P, and US + MB + L + P groups, respectively (Fig. 1B), which were consistent with fluorescence measurements. In addition, western blot analysis demonstrated that the expression of Foxp3 was inhibited following transfection with the Foxp3-miRNA plasmid (Fig. 1B). Compared with the control group, following transfection with Foxp3-miRNA plasmid for 24 h, the survival rate of Tregs was significantly inhibited in the other groups (P<0.01, Table II) with the exception of the US + P group. All these results suggested that UTMD or Lipofectamine® 2000 may effectively transfect Foxp3-miRNA into Tregs, and the combination of UTMD with Lipofectamine® 2000 enhanced the transfection efficiency.
Figure 1.
Transfection efficiency in different treatment groups. (A) Fluorescence measurements and flow cytometry analysis indicated that the transfection efficiency was gradually increased in the control, MB + P, US + P, US + MB + P, L + P, US + L + P and US + MB + L + P groups and the (B) western blot analysis demonstrated a gradual decrease in Foxp3 expression. Foxp3, forkhead family transcription factor P3; MB, SonoVue microbubbles; P, Foxp3-microRNA plasmid; US, ultrasound; L, Lipofectamine® 2000.
Table II.
Survival rate of Tregs in treatment groups.
| Group | Survival rate, % |
|---|---|
| Control | 100 |
| MB + P | 88.610±2.864a |
| US + P | 96.552±2.330 |
| US + MB + P | 82.772±5.256a |
| L + P | 77.510±2.661a |
| US + L + P | 86.548±3.208a |
| US + MB + L + P | 80.026±1.264a |
P<0.01 vs. control. Data are presented as mean ± standard deviation. Treg, regulatory T cell; MB, SonoVue microbubbles; P, Foxp3-microRNA plasmid; US, ultrasound; L, Lipofectamine2000.
Effect of UTMD-mediated Foxp3-miRNA on the function of Tregs
The present study demonstrated that when CD4+CD25− T cells were co-cultured with PBMCs and Tregs without Foxp3-miRNA (Foxp3+Tregs), the proliferation-inhibition ratio of CD4+CD25− T cells was significantly decreased (P<0.01; Table III) compared with cells co-cultured with PBMCs and Tregs with Foxp3-miRNA (Foxp3−Tregs), suggesting that Foxp3−Tregs may influence the inhibition effect of Foxp3+Tregs on CD4+CD25− T cells proliferation.
Table III.
Effect of Tregs with or without Foxp3-miRNA on the proliferation of CD4+CD25− T cells.
| Index | CD4+CD25− T cells group | CD4+CD25− T cells + PBMCs group | CD4+CD25− T cells + PBMCs + Foxp3+Tregs group | CD4+CD25− T cells + PBMCs + Foxp3+Tregs group |
|---|---|---|---|---|
| OD value | 0.870±0.095 | 1.544±0.138a | 0.976±0.119b | 1.254±0.114c |
| Proliferation rate, % | – | 77.806±5.770 | 12.114±3.360b | 44.606±8.974c |
| Proliferation-inhibition ratio, % | – | – | 36.870±3.385 | 18.608±5.643c |
P<0.01 vs. CD4+CD25−T cells group
P<0.01 vs. CD4+CD25− T cells + PBMCs group
P<0.01 vs. CD4+CD25− T cells + PBMCs + Foxp3+Tregs group. Data are presented as mean ± standard deviation. Treg, regulatory T cell; Foxp3, forkhead family transcription factor P3; miRNA, microRNA; CD, cluster of differentiation; Foxp3+Tregs, Tregs without Foxp3-miRNA; Foxp3−Tregs, Tregs with Foxp3-miRNA; OD, optical density; PBMC, peripheral blood mononuclear cell.
Effect of UTMD-mediated Foxp3-miRNA on the levels of IFN-γ, IL-2, IL-10 and TGF-β in vitro
ELISA analysis indicated that the contents of IFN-γ and IL-2 secreted by CD4+CD25− T cells significantly increased (P<0.01), whereas the content of IL-10 and TGF-β secreted by Tregs significantly decreased in the co-cultured system of Foxp3−Tregs compared with the co-cultured system of Foxp3+Tregs (P<0.01; Table IV). These results suggested that Foxp3−Tregs influenced the levels of IFN-γ and IL-2 secreted by CD4+CD25− T cells in addition to the level of IL-10 and TGF-β secreted by Tregs.
Table IV.
The effect of Tregs with or without Foxp3-miRNA on the levels of IFN-γ, IL-2, IL-10 and TGF-β.
| Index | CD4+CD25− T cells + PBMCs + Foxp3+Tregs group | CD4+CD25− T cells + PBMCs + Foxp3+Tregs group |
|---|---|---|
| CD4+CD25− T cells, pg/ml | ||
| Level of IFN-γ | 163.99±15.43 | 276.38±16.83a |
| Level of IL-2 | 88.57±7.57 | 151.88±11.06a |
| Tregs, pg/ml | ||
| Level of IL-10 | 124.25±11.33 | 82.24±10.42a |
| Level of TGF-β | 159.93±9.19 | 97.79±12.08a |
P<0.01 vs. CD4+CD25− T cells + PBMCs + Foxp3+Tregs group. Data are presented as mean ± standard deviation. Treg, regulatory T cell; Foxp3, forkhead family transcription factor P3; miRNA, microRNA; IFN-γ, interferon-γ; IL-2, interleukin-2; TGF-β, transforming growth factor-β; CD, cluster of differentiation; PBMC, peripheral blood mononuclear cell.
Effect of UTMD-mediated Foxp3-shRNA on the tumor growth in HCC model of mice
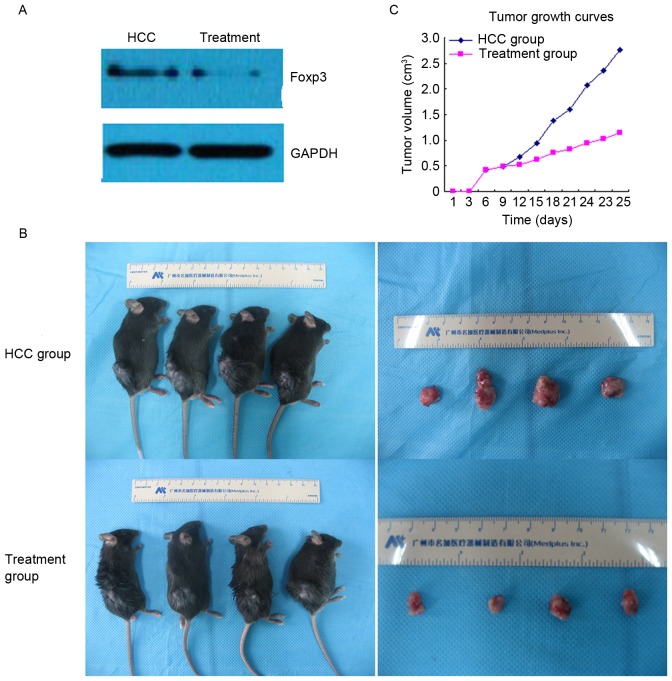
Western blot analysis indicated that the expression of Foxp3 was reduced in the treatment group compared with the HCC group (Fig. 2A). In addition, the present study demonstrated that the mean tumor volume and tumor weight were markedly lower in the treatment group compared with the HCC group (1.251±0.244 vs. 2.742±0.221 cm3 and 1.328±0.163 vs. 3.086±0.227 g; P<0.01; data not shown). Tumor growth curves also indicated that the average tumor volume was markedly inhibited by UTMD-mediated Foxp3-shRNA (Fig. 2B and C).
Figure 2.
Effect of Foxp3 knockdown on the HCC model of mice. Following treatment with Foxp3-short hairpin RNA, (A) western blot analysis indicated a reduced expression of Foxp3; (B) tumor volume was reduced and (C) tumor growth curves demonstrated a trend of decreased tumor volume compared with the HCC group. Foxp3, forkhead family transcription factor P3; HCC, hepatocellular carcinoma.
Effect of UTMD-mediated Foxp3-shRNA on Tregs and CD4+ T cells in HCC model of mice
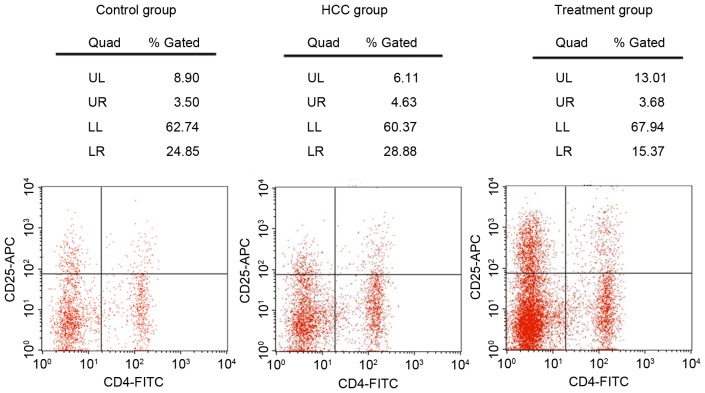
Flow cytometry analysis (Fig. 3) indicated that compared with the control group, the ratio of Tregs/CD4+ T cells was increased in the HCC group (4.89±0.81 vs. 3.11±0.65%; P<0.05; data not shown); however, once treated with Foxp3-shRNA, the ratio of Tregs/CD4+ T cells decreased, to below the HCC group (3.28±0.75 vs. 4.89±0.81%; P<0.05; data not shown).
Figure 3.
Content of regulatory T cells and CD4+ T cells in the blood of mice, by flow cytometry analysis for the control, HCC and treatment group. CD, cluster of differentiation; HCC, hepatocellular carcinoma; FITC, fluorescein isothiocyanate; APC, allophycocyanin; UL, upper left; UR, upper right; LL, lower left; LR, lower right.
Effect of UTMD-mediated Foxp3-shRNA on the levels of IL-10, TGF-β, IFN-γ, IL-2 and VEGF in serum of HCC mouse model
ELISA analysis demonstrated that the level of IL-10, TGF-β and VEGF increased, whereas the levels of IFN-γ and IL-2 decreased in HCC group, compared with the control group (P<0.05; Table V). However, compared with the HCC group, the level of IL-10, TGF-β and VEGF decreased, while the level of IFN-γ and IL-2 increased in the treatment group (P<0.05; Table V).
Table V.
Effect of Foxp3 knockdown on the level of IL-10, TGF-β, IFN-γ, IL-2 and VEGF in serum in vivo.
| Factor, pg/ml | Control group (n=3) | HCC group (n=9) | Treatment group (n=9) |
|---|---|---|---|
| IL-10 | 111.09±6.19 | 132.99±7.15b | 102.39±4.82d |
| TGF-β | 124.06±8.12 | 159.68±7.88b | 124.26±5.99d |
| IFN-γ | 46.18±8.85 | 21.78±3.56a | 45.25±10.36c |
| IL-2 | 61.25±6.62 | 37.88±4.88b | 59.09±4.96d |
| VEGF | 48.88±8.11 | 68.35±4.69a | 44.11±5.79d |
P<0.05
P<0.01 vs. control
P<0.05
P<0.01 vs. HCC group. Data are presented as the mean ± standard deviation. Foxp3, forkhead family transcription factor P3; IL, interleukin; TGF-β, transforming growth factor-β; IFN-γ, interferon-γ; VEGF, vascular endothelial growth factor; HCC, hepatocellular carcinoma.
Discussion
UTMD-mediated gene therapy has been applied both in vitro and in vivo (17). In contrast with UTMD, Lipofectamine® 2000 is a common non-viral vector characterized by a low immunogenicity and high safety but low efficiency, non-targeting and increased cytotoxicity (20,21). The present study demonstrated that UTMD or Lipofectamine® 2000 may effectively transfect Foxp3-miRNA into Tregs, with maximum transfection efficiency observed in the US + MA + L + P group. These results suggested that the combination of UTMD with Lipofectamine® 2000 may enhance transfection efficiency.
Tregs was a major factor in the suppression of the tumor-specific immune response in patients with HCC, and the elimination or suppression of Tregs function may effectively enhance the antitumor immune response (22). Foxp3 has been demonstrated to regulate the immunosuppressive function of Tregs (23). Previous studies have indicated that the immune responses induced by CD4+CD25− T cells may be inhibited by CD4+CD25+ Tregs in vitro through cytokine-independent mechanisms (24,25). Liyanage et al (26) demonstrated that Tregs from patients with pancreas or breast adenocarcinoma secreted IL-10 and TGF-β; however, when Tregs were co-cultured with CD4+25− T cells, Tregs were able to inhibit the proliferation in addition to the secretion of IFN-γ and IL-2 in CD4+25− T cells. Furthermore, Foxp3 is able to suppress the production of IFN-γ and IL-2 secreted by effector T cells, while upregulating the level of IL-10 and TGF-β secreted by Tregs (27). Furthermore, Foxp3+Tregs is able to suppress the activation of T cells and inhibit the proliferation of effector CD4+ T cells (28). Notably, Foxp3 knockdown may enhance tumor immunity (29). Consistently, the current study demonstrated that Foxp3−Tregs reduced the inhibition effect of Foxp3+Tregs from patients with HCC on the proliferation of CD4+CD25− T cells. In addition, Foxp3−Tregs may also increase the level of IFN-γ and IL-2 secreted by CD4+CD25− T cells but decrease the level of IL-10 and TGF-β secreted by Tregs. These results indicate that the downregulation of Foxp3 may mitigate the immunosuppressive function of Tregs.
It has been suggested that the immunosuppressive function of Tregs, at least partially, contributed to the proliferation of tumor cells (30). A previous study indicated that a depletion of Tregs may enhance tumor immunity and inhibit tumor growth in patients with HCC (12). Notably, the present study revealed that UTMD-mediated Foxp3-shRNA inhibited the tumor growth in HCC model mice, suggesting that UTMD-mediated Foxp3-shRNA may inhibit the function of Tregs. In addition, the current study also indicates that the downregulation of Foxp3 reduced the ratio of Tregs/CD4+ T cells. Following treatment with Foxp3-shRNA, the level of IL-10 and TGF-β decreased, while the level of IFN-γ and IL-2 increased, in the current study. These results indicate that the downregulation of Foxp3 enhanced the immunologic function and anti-tumor effect. Furthermore, the present study also demonstrated that UTMD-mediated Foxp3-shRNA reduced the level of VEGF. It has been demonstrated that VEGF promotes the formation of tumor angiogenesis and inhibits the apoptosis of tumor cells (31). These results indicate that UTMD-mediated Foxp3-shRNA may partially inhibit the tumor growth in HCC mice through suppressing the production of VEGF.
In conclusion, the current study demonstrated that the combination of UTMD with Lipofectamine® 2000 may be the optimal choice to enhance the transfection efficiency in gene therapy. Furthermore, UTMD-mediated Foxp3-miRNA/shRNA may relieve the immunosuppressive function of Tregs in patients with HCC in vitro, and partially inhibit the tumor growth in HCC mice through enhancing immunologic function and suppressing the production of VEGF. The present study is not without limitations; the long-term effects of UTMD-mediated Foxp3-miRNA/shRNA on immunologic function and tumor growth remain unclear. Therefore, the safety and efficacy of UTMD-mediated Foxp3-miRNA/shRNA should be further investigated in the future.
Acknowledgements
The present study was supported by General Projects of National Natural Science Foundation of China (grant no. 81171346), the Research Foundation Of The Talent Of Scientific And Technical Innovation of Harbin City (grant no. 2016RAQXJ148), General Projects of Heilongjiang Province Natural Science Foundation of China (grant no. H2017026) and the Scientific Research Innovation Fund of The First Affiliated Hospital of Harbin Medical University.
References
- 1.Han KH, Kudo M, Ye SL, Choi JY, Poon RP, Seong J, Park JW, Ichida T, Chung JW, Chow P, Cheng AL. Asian consensus workshop report: Expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81(Suppl 1):S158–S164. doi: 10.1159/000333280. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 4.Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: Unique challenges and clinical opportunities. Oncoimmunology. 2012;1:48–55. doi: 10.4161/onci.1.1.18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998;58:4673–4681. [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 8.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: Rregulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 10.Tsai BY, Suen JL, Chiang BL. Lentiviral-mediated Foxp3 RNAi suppresses tumor growth of regulatory T cell-like leukemia in a murine tumor model. Gene Ther. 2010;17:972–979. doi: 10.1038/gt.2010.38. [DOI] [PubMed] [Google Scholar]
- 11.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 12.Yang XH, Yamagiwa S, Ichida T, Matsuda Y, Sugahara S, Watanabe H, Sato Y, Abo T, Horwitz DA, Aoyagi Y. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. doi: 10.1016/j.jhep.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 13.Walton CB, Anderson CD, Boulay R, Shohet RV. Introduction to the ultrasound targeted microbubble destruction technique. J Vis Exp. 2011;52:e2963. doi: 10.3791/2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geis NA, Katus HA, Bekeredjian R. Microbubbles as a vehicle for gene and drug delivery: Current clinical implications and future perspectives. Curr Pharm Design. 2012;18:2166–2183. doi: 10.2174/138161212800099946. [DOI] [PubMed] [Google Scholar]
- 15.Li YS, Davidson E, Reid CN, McHale AP. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: Potential applications for gene therapy of cancer. Cancer Lett. 2009;273:62–69. doi: 10.1016/j.canlet.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Fujii H, Sun Z, Li SH, Wu J, Fazel S, Weisel RD, Rakowski H, Lindner J, Li RK. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc Imaging. 2009;2:869–879. doi: 10.1016/j.jcmg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Carson AR, McTiernan CF, Lavery L, Hodnick A, Grata M, Leng X, Wang J, Chen X, Modzelewski RA, Villanueva FS. Gene therapy of carcinoma using ultrasound-targeted microbubble destruction. Ultrasound Med Biol. 2011;37:393–402. doi: 10.1016/j.ultrasmedbio.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kee KM, Wang JH, Lin CY, Wang CC, Cheng YF, Lu SN. Validation of the 7th edition TNM staging system for hepatocellular carcinoma: An analysis of 8,828 patients in a single medical center. Dig Dis Sci. 2013;58:2721–2728. doi: 10.1007/s10620-013-2716-8. [DOI] [PubMed] [Google Scholar]
- 19.Saayman SM, Lazar DC, Scott TA, Hart JR, Takahashi M, Burnett JC, Planelles V, Morris KV, Weinberg MS. Potent and targeted activation of latent HIV-1 using the CRISPR/dCas9 activator complex. Mol Ther. 2016;24:488–498. doi: 10.1038/mt.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewert KK, Ahmad A, Bouxsein NF, Evans HM, Safinya CR. Gene Therapy Protocols. Springer; New York, NY: 2008. Non-viral gene delivery with cationic liposome-DNA complexes; pp. 159–175. [DOI] [PubMed] [Google Scholar]
- 21.Karmali PP, Chaudhuri A. Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Med Res Rev. 2007;27:696–722. doi: 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HH, Mei MH, Fei R, Liao WJ, Wang XY, Qin LL, Wang JH, Wei L, Chen HS. Regulatory T cell depletion enhances tumor specific CD8 T-cell responses, elicited by tumor antigen NY-ESO-1b in hepatocellular carcinoma patients, in vitro. Int J Oncol. 2010;36:841–848. doi: 10.3892/ijo_00000561. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+) CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shevach EM. CD4+ CD25+ suppressor T cells: More questions than answers. Nature Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 26.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 27.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, Nomura T, Miyachi Y, Tsukada T, Sakaguchi S. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 28.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+ CD25+ Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 29.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 31.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]