Abstract
Background
The objective of this study is to investigate the role and experience of early stage non-small cell lung cancer (NSCLC) patient in decision making process concerning treatment selection in the current clinical practice.
Methods
Stage I-II NSCLC patients (surgery 55 patients, SBRT 29 patients, median age 68) were included in this prospective study and completed a questionnaire that explored: (1) perceived patient knowledge of the advantages and disadvantages of the treatment options, (2) experience with current clinical decision making, and (3) the information that the patient reported to have received from their treating physician. This was assessed by multiple-choice, 1–5 Likert Scale, and open questions. The Decisional Conflict Scale was used to assess the decisional conflict. Health related quality of life (HRQoL) was measured with SF-36 questionnaire.
Results
In 19% of patients, there was self-reported perceived lack of knowledge about the advantages and disadvantages of the treatment options. Seventy-four percent of patients felt that they were sufficiently involved in decision-making by their physician, and 81% found it important to be involved in decision making. Forty percent experienced decisional conflict, and one-in-five patients to such an extent that it made them feel unsure about the decision. Subscores with regard to feeling uninformed and on uncertainty, contributed the most to decisional conflict, as 36% felt uninformed and 17% of patients were not satisfied with their decision. HRQoL was not influenced by patient experience with decision-making or patient preferences for shared decision making.
Conclusions
Dutch early-stage NSCLC patients find it important to be involved in treatment decision making. Yet a substantial proportion experiences decisional conflict and feels uninformed. Better patient information and/or involvement in treatment-decision-making is needed in order to improve patient knowledge and hopefully reduce decisional conflict.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-3986-5) contains supplementary material, which is available to authorized users.
Keywords: Cancer patients, Decision-making preferences, Shared decision-making, Surgery, Radiation oncology
Background
Surgical resection is considered the preferred treatment for patients with early-stage non-small cell lung cancer (NSCLC). A less invasive option for patients with comorbidities is stereotactic body radiotherapy (SBRT) [1, 2]. Several studies have demonstrated that SBRT may be as effective as surgery in potentially operable patients, however, randomized trials with larger patient populations and longer follow-up are still lacking [3–5]. In this setting it is important to provide adequate information to allow patients to take an active role in treatment decision.
Shared decision making (SDM) is a process in which physician and patient work together in making a health decision after discussing the options, the benefits and harms, and considering the patients’ values, preferences, and circumstances [6, 7]. SDM is seen as the middle ground between informed choice, where the patient makes the decision based on information received from the physician, and traditional paternalistic decision making, where the physician makes the decision based on best available evidence [8, 9]. Patients who are active participants in the process of their care, for example asking questions, expressing their opinions and preferences, have better health outcomes, more knowledge regarding the disease and they are less anxious than patients who do not participate in the decision making [7, 10–12]. SDM supports patient to understand the disease and weigh advantages and disadvantages of treatment options in their own context, which will result in an informed treatment decision making with patients’ needs and values incorporated. Although SDM has gained increased awareness among the healthcare community, it has not been widely incorporated into routine clinical practice in lung cancer care. This can be explained by the fact that there is lack of familiarity with SDM [13, 14], and also because the care of lung cancer patient can be complex due to multiple treatment types over an extended period of time and often includes a guideline-drive treatment [15]. Furthermore, there are a number of factors that complicate the implementation of SDM in current clinical practice such as guideline based treatments, patient knowledge, time constrains and care settings [16, 17].
This study assesses among Dutch early-stage NSCLC patients: (1) perceived patient knowledge of the advantages and disadvantages of treatment options, (2) experience with current clinical decision-making, and (3) perceived understanding of information regarding their disease and the treatment.
Methods
Patient population
Between December 2012 and December 2014, 155 consecutive patients with stage I or II NSCLC were recruited for this prospective observational study. These patients were subsequently treated surgically or with SBRT at Erasmus University Medical Center, Erasmus MC-Cancer Institute, or Amphia Hospital Breda. Consecutive patients were contacted by telephone to explain the purpose of the study and obtain their consent to receive a questionnaire. Only patients who agreed to participate and provided written informed consent were eligible for the inclusion in this study (n = 84). The overall response rate was 54%. No significant differences were found between responders and non-responders in terms of baseline characteristics. This study was approved by the institutional review board of Erasmus University Medical Center (MEC 2012-462).
Clinical staging of patients treated surgically (n = 55) or with SBRT (n = 29) was done with CT-scan, 18FDG-PET imaging and/or using (minimally invasive) endoscopic techniques when appropriate. Clinical and pathological staging was based on American-Joint-Committee-in-Cancer 7th-edition staging manual [18]. Chronic obstructive pulmonary disease (COPD) was defined according to the GOLD criteria [19]. Comorbidity-scores were recorded using the Charlson-Comorbidity-Index (CCI) [20]. Treatment planning of patients who received SBRT have been described previously [21]. All patients were discussed in a multidisciplinary team meeting before being accepted for treatment.
Data collection
Baseline characteristics of patients were collected by reviewing the patients’ medical records and hospital information system. After the treatment decision was made but before the actual start of the treatment, patients completed a questionnaire. The aim of this questionnaire is to investigate: (1) perceived patient knowledge of the advantages and disadvantages of treatment options, (2) experience with current clinical decision-making (this includes the preferences, patient experience and involvement in treatment decision-making using Decisional Conflict Scale (DCS) and Control Preferences Scale (CPS), and (3) perceived understanding of information regarding their disease and the treatment. These components are measured at baseline using multiple-choice questions, a 1–5 Likert Scale, and open questions. Health-related-quality-of-life (HRQoL) was measured before the treatment, 6 months and 12 months after the treatment using the Short-Form 36-Item Health Survey (SF-36). For details regarding the questionnaire see Additional file 1.
Control preference scale
The patients’ preferred decisional role was assessed using a modified version of the CPS. The CPS is an instrument that assesses preferences regarding patient participation in health care decisions. Patients were asked to select one of the five statements on roles in treatment decision-making; (A) the physician makes the decision about the treatment alone, (B) the physician makes the decision after considering the patient’s opinion, (C) the patient makes the decision together with the clinician, (D) the patient makes the decision after considering the doctor’s opinion, and (E) the patient makes the decision about the treatment alone [22–24]. This scale has been widely used in previous studies [25, 26]. To investigate the potential association between education level and CPS patients were asked to indicate their educational attainment.
Decisional conflict scale
The DCS was used to assess the level of ‘decisional conflict’ that patients experience while making health care decisions. This scale has been extensively validated and has been widely used. The DCS measures decision uncertainty that leads to decision delay, and quantifies modifiable factors which contribute to uncertainty. It contains 16 items, each using a five-point Likert response format (i.e. completely agree, agree, neither agree nor disagree, disagree, completely disagree). These items are combined to form total score and five subscales (i.e. uncertainty, informed, values clarity, support, and effective decision subscore). Scores lower than 25 are associated with implementing decisions and scores exceeding 37.5 are associated with delay or feeling unsure about implementation [27, 28]. In case of missing values (<6%) we used a multiple imputation technique to impute missing values in order to avoid them being depicted as ‘unknown’ in incomplete observations. We have used 5-fold multiple imputation using SPSS for Windows version 21 [29]. In the surgery group 32 and 19 patients were alive at 6 and 12 months without tumor progression, respectively. In the SBRT group this was 9 and 4 patients at 6 and 12 months, respectively. Due to the low response rates at 6 and 12 months we could not explore decisional conflict over time.
Health related quality of life assessment
HRQoL was measured with the SF-36. The SF-36 is the most extensively used and evaluated health outcomes measure and has shown to be valid and reliable in multiple populations. The SF-36 assess eight self-reported aspects of HRQoL (i.e. physical functioning, role physical functioning, role emotional functioning, mental health, vitality, social functioning, bodily pain, and general health). It also yields physical (PCS) and mental (MCS) health summary measures. Scale scores are obtained by summing the items together within a domain, dividing this outcome by the range of scores and then transforming the scores to a scale from 0 to 100 [30]. The mean score of the PCS and MCS is 50 with a standard deviation of 10 and wherein a higher score means a better health status. Furthermore, a higher score on the SF-36 subdomains represents a better functioning; a high score on the bodily pain scale indicates the absence of pain. The scale has good reliability, with Cronbach α ranging from 0.65 to 0.96 for all subscales [31]. We used the Dutch adaptation of the SF-36 health status scale [32]. Patients were asked to complete the SF-36 form after treatment decision was made but before the treatment (baseline), at 6 and 12 months to all surviving patients. In case of missing values we applied simple imputation [33, 34]. HRQoL was assessed in 84 patients at baseline (surgery = 55, SBRT = 29). Due to the low response rates at 6 and 12 months (surgery group 32 and 19 patients were alive at 6 and 12 months and this was in the SBRT group 9 and 4 patients, respectively) the effect of time could not be analyzed.
Local control and the presence of metastases were defined according to the guidelines of ACCP and STS [35]. Twelve patients were diagnosed with tumor recurrence after the treatment, four of these patients had both loco-regional and distant recurrence.
Statistical analysis
Continuous data are reported as mean ± SD or median with range, and categorical data are reported as proportions. Normally distributed continuous variables were compared by using Student t tests, and not normally distributed (Kolmogorov-Smirnov) data were compared by using the Mann-Whitney-U-test. Discrete variables were compared by using the Chi-Square test or the Fisher Exact test where appropriate. Aim 1 and 3 of this manuscript were analyzed using simple statistics by counting the ‘yes’ and ‘no’ answers. Components measured with 1–5 Likert-scale were not categorized.
A general linear model (GLM) with the bootstrap method was used to assess the association between HRQoL measured at baseline and 1) patient experience with involvement in treatment selection, 2) patient preferences for SDM, and 3) patients’ preferred decisional role in treatment decision-making (assessed with CPS). The purpose behind the use of bootstrapping is to account for skewed distribution of residuals of SF-36 variables [36, 37] and to obtain valid and reliable p-values.
All statistical tests were two-tailed and a p-value of <0.05 was regarded as statistical significant. The statistical software package SPSS for Windows version 21 (SPSS Inc., Chicago, IL) was used for data analysis. GraphPad Prism5.00 for Windows (GraphPad software, San Diego, CA) was used to obtain graphs of QoL.
Results
The baseline characteristics of all 84 patients are listed in Table 1. In 55 patients surgical treatment was chosen (median age = 65), in 29 patients SBRT (median age = 73). In this cohort of patients the education level was in accordance with the education level of the general Dutch population [38].
Table 1.
Patient characteristics
| Characteristics | Total (n = 84) | Surgery (n = 55) | Radiotherapy (n = 29) | P-value |
|---|---|---|---|---|
| Sex | 0.406 | |||
| -Male (%) | 44 (52) | 27 (49) | 17 (59) | |
| -Female (%) | 40 (48) | 28 (51) | 12 (41) | |
| Age, median (range) | 68 (50–87) | 65 (50–81) | 73 (52–87) | 0.001 |
| Education level (%): | 0.875 | |||
| -Primary education | 12 (14) | 8 (15) | 4 (14) | |
| -Secondary education | 21 (55) | 29 (53) | 17 (59) | |
| -Higher education | 46 (27) | 15 (27) | 8 (27) | |
| -Other | 3 (4) | 3 (5) | – | |
| Smoking habits | ||||
| -Nonsmoker (%) | 3 (4) | 2 (4) | 1 (3) | 0.588 |
| -Current or former smoker (%) | 60 (71) | 38 (69) | 22 (76) | |
| -Unknown, n (%) | 21 (25) | 15 (27) | 6 (21) | |
| FEV1% mean ± SDa | 80 (24) | 87 (20) | 67 (26) | 0.001 |
| -Unknown, n (%) | 3 (4) | 2 (4) | 1 (3) | |
| DLCO (%) mean ± SDb | 76 (24) | 83 (22) | 61 (22) | <0.001 |
| COPD (%)c | 0.001 | |||
| -No COPD | 38 (45) | 31 (56) | 7 (24) | |
| -GOLD I | 17 (20) | 10 (18) | 7 (24) | |
| -GOLD II | 19 (23) | 13 (24) | 6 (21) | |
| -GOLD III | 8 (10) | 1 (2) | 7 (24) | |
| -GOLD IV | 2 (2) | – | 2 (7) | |
| Charlson comorbidity index (%) | 0.026 | |||
| - ≤ 1 | 47 (56) | 33 (60) | 14 (48) | |
| -2–3 | 26 (31) | 17 (31) | 9 (32) | |
| -4 | 6 (7) | 3 (5) | 3 (10) | |
| - ≥ 5 | 5 (6) | 2 (4) | 3 (10) | |
| Clinical stage (%) | 0.001 | |||
| -IA | 47 (56) | 22 (40) | 25 (86) | |
| -IB | 14 (17) | 12 (22) | 2 (7) | |
| -IIA | 17 (20) | 15 (27) | 2 (7) | |
| -IIB | 6 (7) | 6 (11) | ||
| Pathological stage (%) | ||||
| -IA | 17 (31) | 17 (31) | – | |
| -IB | 18 (33) | 18 (33) | – | |
| -IIA | 9 (16) | 9 (16) | – | |
| -IIB | 7 (13) | 7 (13) | – | |
| -IIIA/B | 4 (7) | 4 (7) | – | |
| Histology (%) | 0.262 | |||
| -Squamous cell carcinoma | 18 (21) | 14 (26) | 4 (14) | |
| -Adenocarcinoma | 21 (25) | 15 (27) | 6 (21) | |
| -Large cell carcinoma | 8 (10) | 6 (11) | 2 (7) | |
| -NSCLC | 37 (44) | 20 (36) | 17 (58) | |
| Clinical tumor diameter (mm), median (range) | 25 (7–130) | 29 (7–130) | 22 (9–41) | <0.001 |
| -Unknown, n (%) | 11 (5) | – | ||
| Pathological tumor diameter (mm), median (range) | 28 (1–90) | 28 (1–90) | – |
aFEV1%: Forced expiratory volume in 1 s expressed as a percent of predicted
bDiffusion capacity of the lung for carbon monoxide
cCOPD: chronic obstructive pulmonary disease
Perceived patient knowledge regarding the treatment
Self- reported lack of knowledge about the advantages and disadvantages of the treatment options was present in 18% of patients in the surgery group and in 22% of patients in the SBRT group. Self-reported lack of knowledge about the treatment risks was present in 6% of patients in the surgery group and in 21% of patients in the SBRT group.
Experience with current clinical decision-making
Patient preferences for SDM
The majority (85%) of patients agreed that ideally decision-making should be done together with the physician. Twelve percent of patients wanted to leave the decision about the appropriate treatment to their treating physician and 3% indicated that the decision should be done mainly by patients. No association was found between the education level and the control preference scale.
Experience in treatment decision-making
On average, patients in this cohort discussed their treatment with three physicians. The majority of patients in the surgery and SBRT group involved a family member in making the choice for a treatment, 75 and 68%, respectively. Most of patients thought that they had enough time to make an informed decision (80% in the surgery group and 79% in the SBRT group). Patients indicated that several subjects were discussed during the conversation with their treating physician. Two percent of patients in the surgery group had the feeling that not every aspect of the treatment was discussed during the conversation with their treating physician. This was 11% in the SBRT group.
In the surgery group, 40% of patients experienced decisional conflict (score > 25), and 22% to such an extent that they felt unsure about their decision (score > 37.5). Thirty-two percent felt uncertain about the best choice, and 39% felt uninformed. Twenty-nine percent felt unclear about personal values for benefits and side effects of the treatment. Twenty-one percent felt unsupported in decision-making, and 21% of patients were not satisfied with their decision.
In the SBRT group, 48% of patients experienced decisional conflict, and 7% to such an extent that they felt unsure about their decision. Thirty-five percent felt uncertain about the best choice, and 29% felt uninformed. Thirty-two percent felt unclear about personal values for benefits and side effects of the treatment. Fourteen percent felt unsupported in decision-making, and 7% of patients were not satisfied with their decision. Subscores on feeling uninformed and on uncertainty contributed the most to decisional conflict. Scores exceeding 37.5 are described here, details of the total score and five subscales for the two treatment groups are illustrated in Fig. 1.
Fig. 1.
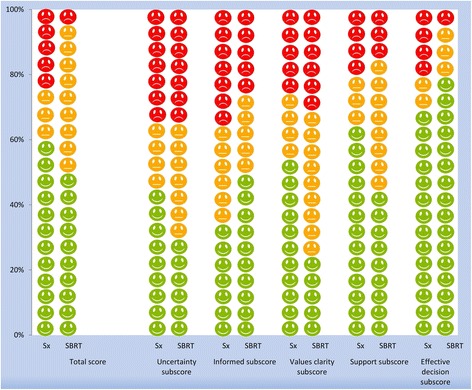
Decisional conflict in patients treated surgically or with stereotactic body radiotherapy (SBRT). Scores <25 (green smiley) are associated with implementing decisions and scores <37.5 (red smiley) are associated with delay or feeling unsure about implementation. Orange smiley represent scores between 25 and 37.5
Involvement in treatment decision-making
Seventy-four percent of patients felt that they were sufficiently involved in decision-making by their physician, 73% felt that they had a choice between different treatment options, 81% found it important to be involved in decision-making, 6% reported that alternative treatment options and complementary treatments were not discussed during the conversation about their treatment. Patients mentioned immunotherapy, diet and vitamin supplements as an example. Involvement in treatment decision-making for the two treatment groups can be found in Table 2.
Table 2.
Involvement in treatment decision making for the two treatment groups
| Involvement in decision making | Surgery (%) | Radiotherapy (%) |
|---|---|---|
| - Felt sufficiently involved | 78 | 68 |
| - Found important to be involved | 78 | 89 |
| - Having a choice | 71 | 79 |
| - Not having a choice | 18 | 7 |
Perceived understanding of information regarding the disease and the treatment
Patients were asked to report which topics were discussed during the conversation about their treatment. Figure 2 illustrates that the minority of patients who undergone surgery or radiation therapy received information about the survival, 24 and 18%, respectively.
Fig. 2.
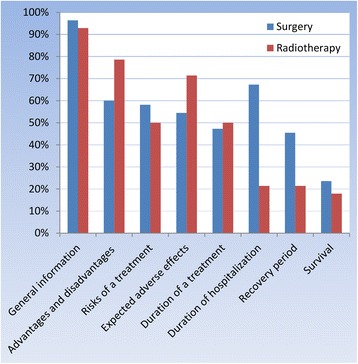
Information that the patient received during the consultation
Health related quality of life assessment
At baseline, patients in the surgery group scored higher on physical component summary (mean 42.4 ± 12.3) than patients in the SBRT group (mean 34.4 ± 10.1), Fig. 3. No major differences could be found between the HRQoL in the surgery and SBRT group for the other measured SF-36 scales, except for physical functioning and general health (Fig. 4). Recurrence rates and death rates are illustrated in Table 3.
Fig. 3.
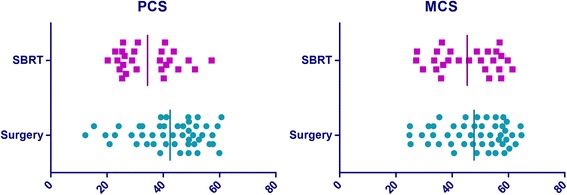
Scatterplot of physical component summary (PCS) and mental component summary (MCS) at baseline in the surgery and stereotactic body radiotherapy (SBRT) group
Fig. 4.
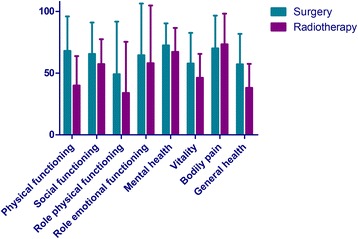
Eight self-reported aspects of HRQoL measured at baseline. The scores are expressed as the mean score with a standard deviation stratified by treatment group. A high score indicates better HRQoL, with a high score on bodily pain representing absence of pain
Table 3.
Recurrence rate of patients treated surgically or with SBRT. Four patients had both loco-regional recurrence and distant recurrence
| Surgery (%) | Radiotherapy (%) | |
|---|---|---|
| All recurrence | 9 (16) | 3 (10) |
| Time till all recurrence(mean ± SD) | 1.1 ± 0.7 months | 0.4 ± 0.06 months |
| Local recurrence | 1 (2) | – |
| Loco-regional recurrence | 4 (7) | 1 (3) |
| Distant recurrence | 9 (16) | 2 (7) |
| Death | 5 (9) | 8 (28) |
SDM and HRQoL at baseline
No significant association could be found between HRQoL and patient experience with involvement in treatment selection (PCS p-value = 0.398, MCS p-value = 0.341), patient preferences for SDM (PCS p-values = 0.439, MCS p-value = 0.580), and final decision in lung cancer treatment selection (PCS p-value = 0.402, MCS p-value = 0.662).
Discussion
This study illustrate that in the current clinical practice lung cancer patients experience decisional conflict and suboptimal information provision regarding the treatment and survival which highlights the need of improvement of information conveyance, and involvement of patients with early-stage NSCLC in treatment decision-making.
Perceived patient knowledge regarding the treatment and communication with the patient
Up to one-fifth of patients reported lack of knowledge about the advantages and disadvantages of the treatment options and one-tenth of patients reported lack of knowledge about the treatment risks. These results illustrate that providing information needs to improve, particularly in an early stage of diagnosis and treatment because lung cancer patients are emotionally unstable and could be overloaded with information about their disease [39]. Numerous studies explored different strategies to improve and adopt SDM in clinical practice [40]. One of the main topics of improving cancer communication is ‘health literacy’ which involves the ability of the patient to read, understand, and use health information to make an appropriate decision. In order to achieve an effective communication it is essential to describe health state in language that is accessible to the patient and discuss the benefits and risks of treatment options in a balanced way [41, 42]. In the field of breast cancer it is illustrated that by deciding on a cancer treatment without fully understanding the associated risks and benefits could lead to overuse or underuse of cancer treatments [43, 44].
Additionally, the majority of patients felt sufficiently involved in treatment decision-making and indicated that they had enough time to make an informed decision. It was interesting to see that the minority of patients reported to have received information on survival. It is crucial to discuss survival and prognosis with the patient in a way that the patient will understand this information because previous studies have shown that the cancer patients overestimate their life expectancy and probabilities of cure when compared to their physicians’ perspective [45–47]. This will lead to unrealistic high expectations about the medical treatment which is a common phenomenon in oncology patients [48, 49].
Experience with current clinical decision-making
The majority of patients had a strong desire to participate in treatment decision-making and preferred the decision to be the outcome of a SDM-process. This is in line with the previous studies showing that more patients preferred to participate rather than delegate decisions [50]. One of the challenges of SDM is knowing how much involvement a patient wants and needs. It is even more difficult when patients vary in the amount of control that they prefer to have over the treatment decision-making at the time of diagnosis [26]. Using tools such as decision aids prior to the consultation or during the visit will improve the communication between the patient and physician and there will be more time for the patient to absorb health care information and ask questions during the consultation [51, 52].
Forty percent of patients experienced decisional conflict, and one in five patients to such an extent that it made them feel unsure about the decision. Decisional conflict was most evident in the uncertainty and informed subscale, suggesting that improvement of patient uncertainty and better informing the patient before the treatment will improve the quality of decision-making [27]. The same rates has been reported by patients treated for other type of cancer [53, 54]. Various factors can play a role in high levels of decisional conflict in cancer patients. First, most cancer patients want as much information as possible, however, they could be overloaded with information when it is offered ‘all at once’ or when the information is not provided to the patients’ family [55]. As we have illustrated in this study, an inadequate level of perceived information contributes the most to decisional conflict. Second, periodic assessment of cancer patient’s information requirements is also crucial, considering the complexity of cancer care. Finally, in our previous study we have illustrated that patients who receive SBRT differ significantly from the surgical patients [56]. It is important to appreciate these differences and realize that SBRT patients do not always have a choice between treatment options.
Although decisional conflict is about what patients go through when confronted with a difficult decision, the idea of decisional conflict is also to help patients to think about participation in decision-making and motivate them to engage in treatment decision-making [57]. Furthermore, these scales also illustrate how patients are informed and where the improvements are needed.
Health related quality of life and shared decision making
In general, lung cancer patients have poor HRQoL compared to the general population or patients without lung cancer [58, 59]. In this study, patients in the SBRT group scored at baseline lower on physical component summary compared to patients treated surgically. No differences could be found regarding the mental component summary. An explanation for the observed differences in HRQoL between the two groups could be the significant differences in baseline characteristics [2, 56]. No association could be found between HRQoL and different aspect of SDM meaning that in this study HRQoL was not positively or negatively influenced by patient experiences with SDM. Our findings are comparable with a number of studies concluding that there is weak evidence that aspects of SDM are positively or negatively associated with QoL outcomes [60].
Strengths and limitations
The present study is a prospective observational cohort study allowing for new insights into the process of SDM and information conveyance in lung cancer patients. Although many articles have been written on SDM and patient participation in treatment decision-making in cancer patients, to our knowledge little research has been done on the role of early-stage lung cancer patients -treated surgically or with SBRT- in treatment decision-making and patients experiences and preferences regarding SDM. Also, the lung cancer patients were surveyed after diagnosis but before the treatment which allow us to investigate the unbiased perception of the patient regarding the treatment decision-making.
Potential limitations need to be addressed regarding the present study. First, the conceptual design of this study was not built on a specific theory. We explicitly chose to include all patients with stage I or II NSCLC who were planned for a surgical treatment or SBRT. We wanted to illustrate the patient participation in treatment decision-making, since there is little research about the role of early-stage lung cancer patients -treated surgically or with SBRT- in treatment decision-making. Second, overall response rate was 54% thus making the sample size of this study small. The non-responders were contacted to ask why they would not be part of the study. The following major reasons were given: 1) they were shocked by the diagnosis and therefore they did not want to complete the questionnaire; 2) they were too preoccupied with their illness and therefore they had no time for the questionnaire; 3) the questionnaire was too confrontational. However, no significant differences were found between responders and non-responders in terms of baseline characteristics. Third, we are aware of the shortcomings of using GLM. By using the bootstrap method we have tried to account for this inadequacy. However, no differences were observed between the results of GLM and results of GLM with bootstrapping. Finally, the response rate at 6 and 12 months was low due to recurrences rates and death rates in both treatment groups making analyses of HRQoL at 6 and 12 months difficult.
Conclusions
Shared-decision-making (SDM), where patients are involved as active partners with the physician in treatment decisions, is an important part of patient-centered cancer care as it weighs the pros and cons of treatment options while taking patients values and preferences into account.
Dutch early-stage NSCLC patients find it important to be involved in treatment decision-making. The majority of patients in this study found it important to be involved in decision-making and reported that they felt sufficiently involved by their treating physician. Yet a substantial proportion of patients experiences decisional conflict and feels uninformed. HRQoL was not influenced by patient experiences with SDM. Better patient information, and patient involvement in treatment decision-making is needed in order to improve patient knowledge and hopefully reduce decisional conflict.
Acknowledgments
The authors thank Laixi Xue for her support in data collection.
Funding
This work had no specific funding and there are no financial disclosures from any authors.
Availability of data and materials
The raw data is available upon request from the corresponding author.
Abbreviations
- ACCP
American College of Chest Physicians
- CCI
Charlson-Comorbidity-Index
- COPD
Chronic obstructive pulmonary disease
- CPS
Control Preferences Scale
- DCS
Decisional Conflict Scale
- GLM
General linear model
- HRQoL
Health related quality of life
- MCS
Mental component summary
- NSCLC
Non-small cell lung cancer
- PCS
Physical component summary
- SBRT
Stereotactic body radiotherapy
- SDM
Shared-decision-making
- SF-36
Short-Form 36-Item Health Survey
- STS
Society of Thoracic Surgeons
Additional file
Questionnaire used in the study. Description of data: Questionnaire used in the study. (DOC 50 kb)
Authors’ contributions
The idea for this paper originated from the conjoined experience of SM, JN, JA, MdeM, AM, OB, HT and AB. All authors conceived and designed the questionnaire including correcting the questionnaire at different stages of the design. JN, JA, MdeM, AM, OB recruited actively patients from clinical practice. SM did the statistical analysis and wrote the paper together with HT and AB. SM, JT and AB revised the work critically for important intellectual content. All authors were responsible for the final approval of this paper ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the manuscript.
Ethics approval and consent to participate
This study was approved by the institutional review board of Erasmus University Medical Center (MEC 2012-462). Only patients who agreed to participate and provided written informed consent were eligible for the inclusion in this study. Consent regarding publication of individual patient data was waived by Ethics committee of Erasmus MC.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-018-3986-5) contains supplementary material, which is available to authorized users.
Contributor Information
S. Mokhles, Phone: +31-10-7034378, Email: s.mokhles@erasmusmc.nl
J. J. M. E. Nuyttens, Email: j.nuyttens@erasmusmc.nl
M. de Mol, Email: MdeMol1@amphia.nl
J. G. J. V. Aerts, Email: j.aerts@erasmusmc.nl
A. P. W. M. Maat, Email: a.p.w.m.maat@erasmusmc.nl
Ö. Birim, Email: o.birim@erasmusmc.nl
A. J. J. C. Bogers, Email: a.j.j.c.bogers@erasmusmc.nl
J. J. M. Takkenberg, Email: j.j.m.takkenberg@erasmusmc.nl
References
- 1.Vansteenkiste J, Crino L, Dooms C, Douillard JY, Faivre-Finn C, Lim E, Rocco G, Senan S, Van Schil P, Veronesi G, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol. 2014;25(8):1462–1474. doi: 10.1093/annonc/mdu089. [DOI] [PubMed] [Google Scholar]
- 2.Mokhles S, Verstegen N, Maat AP, Birim O, Bogers AJ, Mokhles MM, Lagerwaard FJ, Senan S, Takkenberg JJ. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer. 2015;87(3):283–289. doi: 10.1016/j.lungcan.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJM, McRae SE, Widder J, Feng L, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solda F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol. 2013;109(1):1–7. doi: 10.1016/j.radonc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Treasure T, Rintoul RC, Macbeth F. SABR in early operable lung cancer: time for evidence. Lancet Oncol. 2015;16(6):597–598. doi: 10.1016/S1470-2045(15)70225-1. [DOI] [PubMed] [Google Scholar]
- 6.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Cording E, Tomson D, Dodd C, Rollnick S, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368(1):6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 8.Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. JAMA. 1992;267(16):2221–2226. doi: 10.1001/jama.1992.03480160079038. [DOI] [PubMed] [Google Scholar]
- 9.Jordan JL, Ellis SJ, Chambers R. Defining shared decision making and concordance: are they one and the same? Postgrad Med J. 2002;78(921):383–384. doi: 10.1136/pmj.78.921.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler FJ, Jr, Gallagher PM, Drake KM, Sepucha KR. Decision dissonance: evaluating an approach to measuring the quality of surgical decision making. Jt Comm J Qual Patient Saf. 2013;39(3):136–144. doi: 10.1016/S1553-7250(13)39020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan J, Sysko J. The contingency of patient preferences for involvement in health decision making. Health Care Manag Rev. 2007;32(1):30–36. doi: 10.1097/00004010-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Murray E, Pollack L, White M, Lo B. Clinical decision-making: Patients’ preferences and experiences. Patient Educ Couns. 2007;65(2):189–196. doi: 10.1016/j.pec.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci. 2006;1:16. doi: 10.1186/1748-5908-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedberg MW, Van Busum K, Wexler R, Bowen M, Schneider EC. A demonstration of shared decision making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood) 2013;32(2):268–275. doi: 10.1377/hlthaff.2012.1084. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann TC, Montori VM, Del Mar C. The connection between evidence-based medicine and shared decision making. JAMA. 2014;312(13):1295–1296. doi: 10.1001/jama.2014.10186. [DOI] [PubMed] [Google Scholar]
- 16.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. doi: 10.1016/j.pec.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Mokhles S, Maat A, Aerts J, Nuyttens J, Bogers A, Takkenberg JJM. Opinions of lung cancer clinicians on shared decision making in early-stage non-small-cell lung cancerdagger. Interact Cardiovasc Thorac Surg. 2017;25:278–284. doi: 10.1093/icvts/ivx103. [DOI] [PubMed] [Google Scholar]
- 18.Goldstraw P. IASLC staging manual in thoracic oncology. 1. Orange Park: Editorial Rx Press; 2009. [Google Scholar]
- 19.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Nuyttens JJ, van de Pol M. The CyberKnife radiosurgery system for lung cancer. Expert Rev Med Devices. 2012;9(5):465–475. doi: 10.1586/erd.12.35. [DOI] [PubMed] [Google Scholar]
- 22.Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 23.Salkeld G, Solomon M, Short L, Butow PN. A matter of trust—patient’s views on decision-making in colorectal cancer. Health Expect. 2004;7(2):104–114. doi: 10.1111/j.1369-7625.2004.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient-physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091–3098. doi: 10.1200/JCO.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 25.Wallberg B, Michelson H, Nystedt M, Bolund C, Degner LF, Wilking N. Information needs and preferences for participation in treatment decisions among Swedish breast cancer patients. Acta Oncol. 2000;39(4):467–476. doi: 10.1080/028418600750013375. [DOI] [PubMed] [Google Scholar]
- 26.Mallinger JB, Shields CG, Griggs JJ, Roscoe JA, Morrow GR, Rosenbluth RJ, Lord RS, Gross H. Stability of decisional role preference over the course of cancer therapy. Psychooncology. 2006;15(4):297–305. doi: 10.1002/pon.954. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor AM. Validation of a decisional conflict scale. Med Decis Mak. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 28.Koedoot N, Molenaar S, Oosterveld P, Bakker P, de Graeff A, Nooy M, Varekamp I, de Haes H. The decisional conflict scale: further validation in two samples of Dutch oncology patients. Patient Educ Couns. 2001;45(3):187–193. doi: 10.1016/S0738-3991(01)00120-3. [DOI] [PubMed] [Google Scholar]
- 29.Rubin DB. Multiple imputation for non-response in surveys. New York: Wiley; 1997. [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Smith HJ, Taylor R, Mitchell A. A comparison of four quality of life instruments in cardiac patients: SF-36, QLI, QLMI, and SEIQoL. Heart. 2000;84(4):390–394. doi: 10.1136/heart.84.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, Sprangers MA, te Velde A, Verrips E. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–1068. doi: 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 33.Bandayrel K, Johnston BC. Recent advances in patient and proxy-reported quality of life research. Health Qual Life Outcomes. 2014;12(1):110. doi: 10.1186/s12955-014-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coste J, Quinquis L, Audureau E, Pouchot J. Non response, incomplete and inconsistent responses to self-administered health-related quality of life measures in the general population: patterns, determinants and impact on the validity of estimates - a population-based study in France using the MOS SF-36. Health Qual Life Outcomes. 2013;11:44. doi: 10.1186/1477-7525-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donington J, Ferguson M, Mazzone P, Handy J, Jr, Schuchert M, Fernando H, Loo B, Jr, Lanuti M, de Hoyos A, Detterbeck F, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142(6):1620–1635. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 36.Efron B. Better bootstrap confidence-intervals. J Am Stat Assoc. 1987;82(397):171–185. doi: 10.1080/01621459.1987.10478410. [DOI] [Google Scholar]
- 37.Efron B. An introduction to the bootstrap method. New York: Chapmann and Hall/CRC; 1993. [Google Scholar]
- 38.Dutch population better educated. https://www.cbs.nl/en-gb/news/2013/40/dutch-population-better-educated.
- 39.Jensen JD, Carcioppolo N, King AJ, Scherr CL, Jones CL, Niederdieppe J. The cancer information overload (CIO) scale: establishing predictive and discriminant validity. Patient Educ Couns. 2014;94(1):90–96. doi: 10.1016/j.pec.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Legare F, Ratte S, Stacey D, Kryworuchko J, Gravel K, Graham ID, Turcotte S. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2010;5:CD006732. doi: 10.1002/14651858.CD006732.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Katz SJ, Belkora J, Elwyn G. Shared decision making for treatment of cancer: challenges and opportunities. J Oncol Pract. 2014;10(3):206–208. doi: 10.1200/JOP.2014.001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorne S, Oliffe JL, Stajduhar KI. Communicating shared decision-making: cancer patient perspectives. Patient Educ Couns. 2013;90(3):291–296. doi: 10.1016/j.pec.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Katz SJ, Hawley ST. From policy to patients and back: surgical treatment decision making for patients with breast cancer. Health Aff (Millwood) 2007;26(3):761–769. doi: 10.1377/hlthaff.26.3.761. [DOI] [PubMed] [Google Scholar]
- 44.Bickell NA, Weidmann J, Fei K, Lin JJ, Leventhal H. Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust. J Clin Oncol. 2009;27(31):5160–5167. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackillop WJ, Stewart WE, Ginsburg AD, Stewart SS. Cancer-patients perceptions of their disease and its treatment. Br J Cancer. 1988;58(3):355–358. doi: 10.1038/bjc.1988.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weeks JC, Cook EF, O’Day SJ, Petersen LM, Wenger N, Reding D, Harrell FE, Kussin P, Dawson NV, Connors AF, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279(21):1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 47.Reuben DB, Naeim A. Perspectives, preferences, care practices, and outcomes in late-stage cancer patients: connecting the dots. J Clin Oncol. 2004;22(24):4869–4871. doi: 10.1200/JCO.2004.09.960. [DOI] [PubMed] [Google Scholar]
- 48.Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, Schrag D. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg SM, Tracy MS, Meyer ME, Sepucha K, Gelber S, Hirshfield-Bartek J, Troyan S, Morrow M, Schapira L, Come SE, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. 2013;159(6):373–381. doi: 10.7326/0003-4819-159-6-201309170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9–18. doi: 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, Entwistle VA, Fiset V, Holmes-Rovner M, Khangura S, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;3:CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Elwyn G, O’Connor AM, Bennett C, Newcombe RG, Politi M, Durand MA, Drake E, Joseph-Williams N, Khangura S, Saarimaki A, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi) PLoS One. 2009;4(3):e4705. doi: 10.1371/journal.pone.0004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sim JA, Shin JS, Park SM, Chang YJ, Shin A, Noh DY, Han W, Yang HK, Lee HJ, Kim YW, et al. Association between information provision and decisional conflict in cancer patients. Ann Oncol. 2015;26(9):1974–1980. doi: 10.1093/annonc/mdv275. [DOI] [PubMed] [Google Scholar]
- 54.Taylor BA, Hart RD, Rigby MH, Trites J, Taylor SM, Hong P. Decisional conflict in patients considering diagnostic thyroidectomy with indeterminate fine needle aspirate cytopathology. J Otolaryngol Head Neck Surg. 2016;45:16. doi: 10.1186/s40463-016-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hope S, Williams AE, Lunn D. Information provision to cancer patients: a practical example of identifying the need for changes in practice from the Dorset cancer Centre. Eur J Cancer Care (Engl) 2000;9(4):238–242. doi: 10.1046/j.1365-2354.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 56.Mokhles S, Nuyttens JJ, Maat AP, Birim O, Aerts JG, Bogers AJ, Takkenberg JJ. Survival and treatment of non-small cell lung cancer stage I-II treated surgically or with stereotactic body radiotherapy: patient and tumor-specific factors affect the prognosis. Ann Surg Oncol. 2015;22(1):316–323. doi: 10.1245/s10434-014-3860-x. [DOI] [PubMed] [Google Scholar]
- 57.Janis IL. Decision making:a psycological analysis of conflict, choice, and commitment. New York: Free Press; 1977. [Google Scholar]
- 58.Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81(1):11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Myrdal G, Valtysdottir S, Lambe M, Stahle E. Quality of life following lung cancer surgery. Thorax. 2003;58(3):194–197. doi: 10.1136/thorax.58.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kashaf MS, McGill E. Does shared decision making in cancer treatment improve quality of life? A systematic literature review. Med Decis Mak. 2015;35(8):1037–1048. doi: 10.1177/0272989X15598529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data is available upon request from the corresponding author.


