Abstract
Background
Gastric cancer (GC) is one of the most common malignant tumors in the world and in China the incidence and mortality rates of gastric cancer are the second highest among all forms of cancer. Annexin A11 (ANXA11) is a member of the annexins family. Previous studies have shown that ANXA11 participates in many cellular functions and has significant influence on ovarian, breast, liver, and colorectal cancer. However, the expression and biological functions of ANXA11 in GC are still unknown.
Material/Methods
A total of 63 paired gastric cancer tissues and matched adjacent mucosa were used to measure the ANXA11 levels and its correlation with clinical characteristics. We carried out the biological functions and underlying mechanism study using SGC-7901and AGS cell lines.
Results
The expression of ANXA11 in cancer tissues was higher than in adjacent mucosa at mRNA and protein levels. In clinicopathological analysis, we found that increased expression of ANXA11 was significantly associated with tumor size, tumor infiltration, local lymph node metastasis, TNM staging, and vascular invasion. Small interfering RNA (siRNA) silencing of ANXA11 inhibits cell proliferation, colony formation, migration, and invasion through the AKT/GSK-3β pathway.
Conclusions
ANXA11 plays a critical role in regulating GC proliferation, migration, and invasion via the AKT/GSK-3β pathway, and can potentially be used as a prognostic factor and therapeutic target for gastric cancer patients.
MeSH Keywords: Annexins, Cell Migration Assays, Cell Proliferation, Glycogen Synthase Kinase 3, Neoplasm Invasiveness, Stomach Neoplasms
Background
Gastric cancer is one of the most common malignant tumors in the world [1] and in China the incidence and mortality rates of gastric cancer are second highest among all cancers [23]. Despite recent developments in the treatment of GC, the prognosis of advanced gastric cancer patients is still unsatisfying, with a 5-year survival rate of about 20–30% and a median survival time of only 11 months [4,5]. Therefore, it is essential for cancer research to find new prognostic markers and novel therapeutic targets for gastric cancer.
Annexins are a multigene family of calcium (Ca2+)-regulated phospholipid-binding proteins [6,7], which are associated with cancer progression, metastasis, apoptosis, cell division, Ca2+ signaling, growth regulation, and membrane traffic and organization [8,9]. ANXA11 is a member of the annexins family and contains a conserved C-terminal structural element with 4 homologous tetrad annexin repeats, in which the Ca2+-binding sites are located and a unique N-terminal domain is rich in glycine, proline, and tyrosine residues [10,11]. Previous studies have shown that ANXA11 is related with cell division, differentiation, apoptosis, signal transduction, and vesicle trafficking [12,13]. ANXA11 has significant influence on ovarian cancer, breast cancer, liver cancer, and colorectal cancer, and is associated with cancer recurrence, metastasis, drug resistance, and lymph node metastasis [14–19]. However, the expression and biological functions of ANXA11 in GC are still unknown. This study aimed to investigate the role and potential mechanism of ANXA11 in gastric cancer.
Material and Methods
Tissue samples
We randomly collected 63 GC Tumor tissues and adjacent mucosa (at least 5 cm away from the edge of the tumor), frozen immediately for qRT-PCR in liquid nitrogen and stored at −80°C until use. For immunohistochemical analysis, samples were routinely processed into 4% formalin-fixed, paraffin-embedded blocks and archived.
qRT-PCR analysis
Total RNA was extracted with Trizol™ reagent (Life technologies, USA), and 2 μg of total RNA was converted to cDNA using the A5000 GoScript Reverse Transcription System (Promega, USA). PCR was conducted using the A6001 GoTaq (R) qRT-PCR Master Mix (Promega, USA). Realtime detection of the emission intensity of SYBR green bound to double-stranded DNA was performed with the ABI PRISM 7500 sequence detection system (Applied Biosystems). PCR primers were: ANXA11 F: 5′-ACGGCTTACGG CAAGGATT-3, R: 5′-ATCAGGCAG GCTTCATCAGT-3′. GAPDH F: 5′-AATCCCATCACCATCTTCCAG-3′, R: 5′-CCTTCTCCATGGTGGTGAAGAC-3′. The relative mRNA expression levels of ANXA11 were analyzed using 2–ΔΔCTmethod.
Immunohistochemistry
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded sections using the labeled horseradish peroxidase (HRP) method. We sectioned 4-μm-thick slides from paraffin blocks mounted on poly-L-lysine-coated slides, deparaffinized and rehydrated in gradient alcohol series and distilled water. The slides were immersed in citrate buffer (0.01 M, pH 6.0) antigen retrieval buffer and boiled for 3 min, followed by slow cooling to room temperature. Endogenous peroxidase activities were quenched in 3% H2O2 for 20 min, followed by washing with PBS. Normal goat serum (5%) was used to block the heterogenetic antigens for 40 min and then they were incubated with monoclonal antibody against ANXA11 (1: 400, Santa Cruz Biotechnology, USA) at 4°C overnight. Negative controls were incubated with PBS only. The sections were then thoroughly washed with PBS 3 times, and incubated with goat anti-rat polyclonal antibody (Zhongshan Golden Bridge Inc; 1: 100 dilution) at 37°C for 30 min, followed by washing 3 times with PBS. Then, the horseradish peroxidase (HRP)-conjugated streptavidin working solution was added and incubated at 37°C for 30 min. Next, the samples were washed with PBS, and DAB substrate-chromogen solution (Zhongshan Golden Bridge Inc.; 1: 100 dilution) was applied to the sample for a color reaction.
Cell lines and cell culture
Human gastric cell lines SGC-7901, BGC-823, MGC-803, AGS, and GES1 were obtained from the Committee of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 (Gibco, USA) medium supplemented with 10% fetal bovine serum (BI, USA) in a humidified incubator (Thermal, USA) at 37.0°C with 5% CO2.
RNA interference
Small interfering RNA oligonucleotides targeting ANXA11 and a negative control were designed and purchased from RiboBIO Company. Transfection of ANXA11-specific siRNA and the negative control was performed with Lipofectamine™ 2000 (Invitrogen, USA). According to the manufacturer’s protocol, 24 h before transfection, 5×105 AGS and SGC-7901 cells per well were seeded into a 6-well plate with 2 mL of RPMI-1640 cultured in a humidified environment. After 24 h and when the cells were 70% confluent, transfection was performed. siRNA specifically targeting ANXA11 and a negative control were transfected into AGS and SGC-7901 cells. At 24 h or 48 h after transfection, cells were harvested for subsequent experiments.
Western blot analysis (WB)
Proteins were extracted using RIPA lysis buffer (Solarbio, Beijing, China) supplemented with 1× protease inhibitor cocktail (Roche, Mannheim, Germany). The supernatants were collected by centrifugation at 12000 rpm for 15 min at 4°C. Protein concentrations were determined by bicinchoninic acid (BCA) protein assay (Thermal, USA). Then, equal amounts of protein samples were boiled for 10 min in loading buffer (Solarbio, Beijing, China), separated by 10–12% SDS-PAGE and transferred onto PVDF membranes (Millipore, MA). After blocking in 5% skim milk (in TBS) for 1 h at room temperature, the PVDF membrane was incubated overnight with primary antibodies at 4°C. The secondary antibody was incubated at room temperature for 1 h. Protein bands were detected using the Odyssey system (LI-COR Biosciences, USA).
Cell proliferation assay
We used Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) assay to examine the proliferation of GC cells, according to the manufacturer’s instructions. Briefly, about 2×103 AGS and SGC-7901 cells per well were plated in triplicate in 96-well plates and treated with ANXA11-siRNA, negative control (NC), and without treatment (CON). At each of the desired time points (4, 48, 72, and 96 h after transfection), CCK-8 solution was added (10 UL/well) to the cells and incubated for 2 h at 37°C, followed by measurement of absorbance at 450 nm with a microplate reader (Thermal, USA) for quantifying relative cell density.
Cell cycle analysis
Cells transfected with ANXA11-siRNA, negative control, and without treatment for 48 h were harvested and fixed with 70% pre-cooled ethanol at 4°C overnight. Afterwards, the cells were washed with PBS and stained with propidium iodide (PI) probe solution (BD Biosciences, San Jose, CA) for 30 min in the dark. DNA contents were assessed by flow cytometric analysis using a BD FACS Calibur device (Becton-Dickinson, San Diego, USA).
Cell colony formation assay
Cells were collected at 24 h after transfection, counted, and plated at 2×103 viable cells per well into 6-well plates. Seven days after plating, cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet, and colonies were counted under a light microscope. The experiment was performed in 3 replicates.
Wound-healing experiments
Cells (5×105) were seeded in 24-well plates. After transfection, when the cell confluence reached ≥80%, scratch-wounds were made by scraping the cell layer across each culture plate using a 0.2-ml micropipette tip. To ensure recording the same region, marks were made on the wells across the wounded area. The medium was substituted with serum-free medium and contain 1 mM mitomycin to inhibit cell division. Phase-contrast images were recorded (0, 12, 24, and 48 h after scratching) under an inverted microscope. All experiments were performed in triplicate.
Transwell invasion assay
Transwell invasion assay was performed using 24-well Transwell plates (Corning Costar, Cambridge, MA, USA) invasion assays. The bottom of Transwell plates were coated with a thin layer of Matrigel Basement Membrane Matrix (BD Biosciences, Bedford, MA), 1×105 AGS and SGC-7901 cells were loaded into the upper membrane chamber (24-well insert; 8-mm pore size) with serum-free medium, and 600 ul medium with 10% FBS was added to the lower chamber as a chemo-attractant. After 20-h incubation, cells on the upper surface of the membrane were removed using a cotton swab, and cells which penetrated to the lower surface of the membrane were stained with crystal violet. The numbers of cells penetrating through the membrane was counted under an inverted microscope from 5 randomly selected fields. Dates were obtained from 3 independent experiments.
Statistical analysis
All the experimental data are expressed as the mean ±SD of at least 3 independent experiments. All statistical analyses were performed with SPSS (version 21.0) software (IBM, Armonk, NY, USA). One-way analysis of variance and t tests were performed for comparisons. Chi-square analyses was used to analyze categorical variables. Values with P<0.05 were considered statistically significant, and all statistical tests were 2-sided.
Results
ANXA11 was up-regulated in GC tissues and cell lines
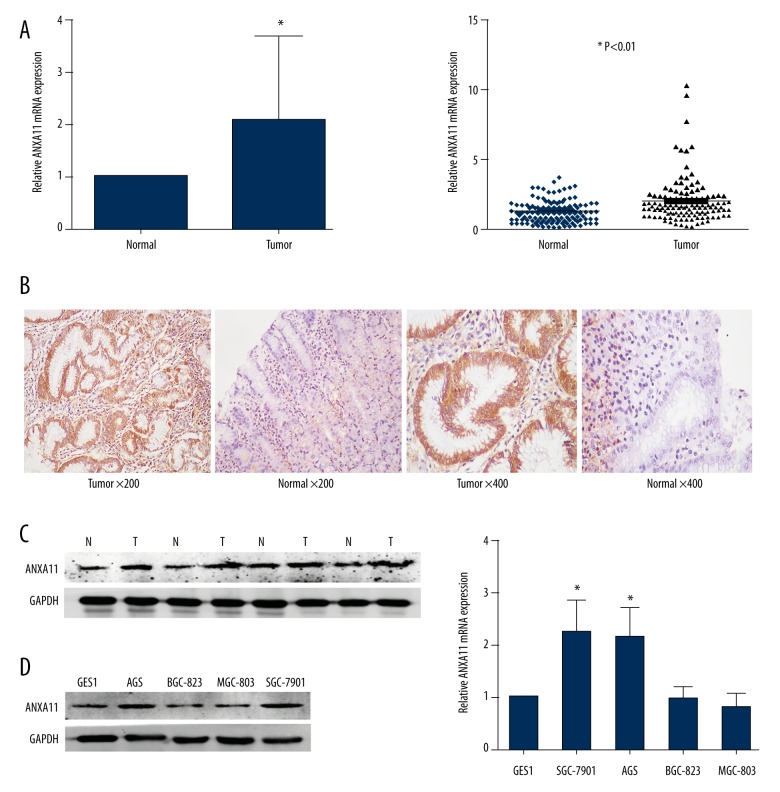
To evaluate the expression of ANXA11 in gastric cancer tissues, qRT-PCR, WB, and immunohistochemistry were used to detect the expression levels in 63 pairs of tumor tissues and their matched adjacent mucosa. Compared with the adjacent mucosa, ANXA11 was up-regulated in 76.19% (48/63) of the tumor samples. The expression of ANXA11 in gastric cancer tissues was significantly higher than in the matched adjacent mucosa (Figure 1A–1C). Additionally, qRT-PCR and Western blotting assay were performed to measure the relative expression of ANXA11 in GES1, SGC-7901, BGC-823, MGC-803, and AGS cell lines. The relative expression levels of ANXA11 in SGC-7901 and AGS were higher than in GES1, while BGC-823 and MGC-803 cell lines were reduced. Thus, AGS and SGC-7901 were selected as the target cell lines for further study (Figure 1D, 1E).
Figure 1.
ANXA11 expression in GC samples and cell lines. (A) ANXA11 mRNA expression levels were measured in 63 paired (tumor and adjacent mucosa) gastric cancer tissues by qRT-PCR. (B) Expression of ANXA11 was detected by immunohistochemistry in gastric cancer tissues and adjacent mucosa. (C) Expression of ANXA11 protein in primary GC tissues (T) and the paired adjacent mucosa (N) from the same patient by Western blotting. (D, E) Protein and mRNA expression level of ANXA11 in GC cell lines. * P<0.05.
Correlation between ANXA11 expression and clinical pathological variables of 63 GC cases
We confirmed that ANXA11 exhibited higher expression in gastric cancer tissues compared with matched adjacent mucosa using qRT-PCR, WB, and immunohistochemistry. We assessed the relationship between ANXA11 and clinical pathological parameters and found that increased expression of ANXA11 was significantly associated with tumor size, tumor infiltration, local lymph node metastasis, TNM staging, and vascular invasion (Table 1).
Table 1.
Correlation between ANXA11 expression and clinical pathological variables of 63gastric cancer cases.
| Clinical pathological parameters | ANXA11 expression | χ2 | P value | |
|---|---|---|---|---|
| High | Low | |||
| All | 48 | 15 | ||
| Age (years) | ||||
| <55 | 21 | 4 | 1.393 | 0.238 |
| ≥55 | 27 | 11 | ||
| Gender | ||||
| Male | 38 | 12 | 0.005 | 0.945 |
| Female | 10 | 3 | ||
| Tumor size | ||||
| <5 cm | 12 | 10 | 8.731 | 0.003* |
| ≥5 cm | 36 | 5 | ||
| Tumor infiltration | ||||
| T1 | 3 | 2 | 12.429 | 0.014* |
| T2 | 4 | 4 | ||
| T3 | 5 | 4 | ||
| T4a | 29 | 5 | ||
| T4b | 7 | 0 | ||
| Local lymph node metastasis | ||||
| N0 | 10 | 6 | 12.582 | 0.006* |
| N1 | 5 | 6 | ||
| N2 | 12 | 2 | ||
| N3 | 21 | 1 | ||
| Distant metastasis | ||||
| M0 | 45 | 15 | 0.984 | 0.321 |
| M1 | 3 | 0 | ||
| TNM staging | ||||
| I | 4 | 4 | 14.766 | 0.002* |
| II | 8 | 8 | ||
| III | 33 | 3 | ||
| IV | 3 | 0 | ||
| Vascular invasion | ||||
| Yes | 32 | 4 | 7.467 | 0.006* |
| No | 16 | 11 | ||
All cases were classified according to the World Health Organization’s (2010) pathological classification of gastric cancer. Tumor infiltration: T1 includes T1a and T1b, T4 includes T4a and T4b. Local lymph node metastasis: N3 includes N3a and N3b. TNM grade I includes Ia and Ib, grade II includes IIa and IIb, grade III includes IIIa, IIIb and IIIc.
Statistically significant (P<0.05).
ANXA11 is down-regulated in ANXA11-siRNA cell lines
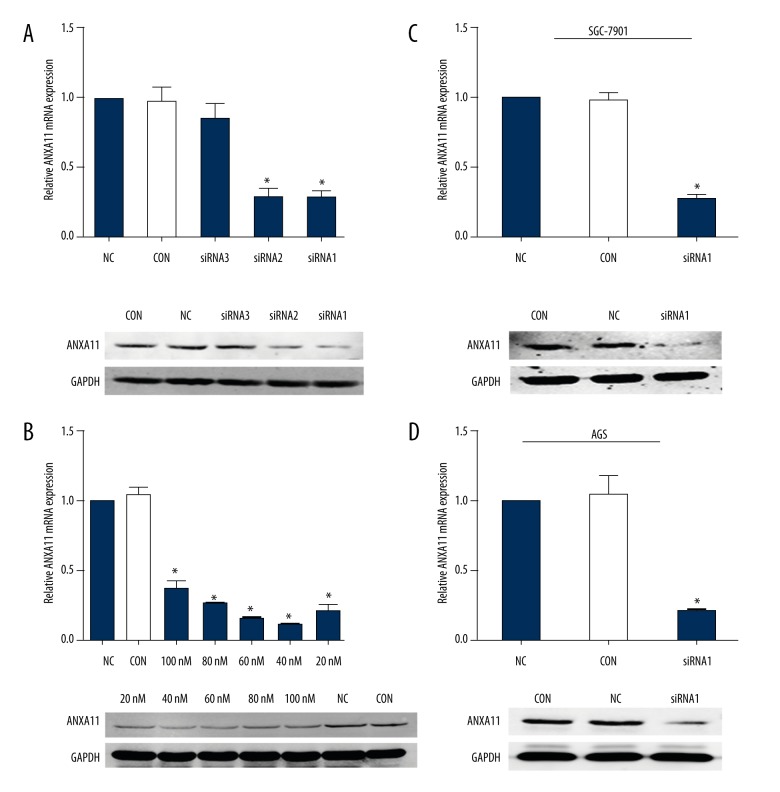
qRT-PCR and WB assays revealed that ANXA11 was stably down-regulated in AGS and SGC-7901 cell lines by ANXA11-siRNA1 and ANXA11-siRNA2, and NC-siRNA interfering showed no effect on the ANXA11 expression at mRNA and protein levels (Figure 2A, 2C, 2D). Through WB and qRT-PCR analysis, we also found a dose-dependent silencing effect of ANXA11 expression in the ANXA11-siRNA1-treated SGC-7901 cell line at concentrations ranging from 20 to 100 nM (Figure 2B).
Figure 2.
Knockdown of ANXA11 expression in GC cell lines. (A) Effect of silencing of ANXA11 using different siRNA. GC cell lines were treated with ANXA11-siRNA1, ANXA11-siRNA2, ANXA11-siRNA3, and NC-siRNA at the concentration of 40 nM or without treatment (Con) for 24 h or 48 h. (B) Dose-dependent silencing of ANXA11 by SGC-7901 cells were treated with ANXA11-siRNA1 at the indicated concentrations of 100 nM, 80 nM, 60 nM, 40 nM, 20 nM, or NC at the concentration of 40 nM or without treatment. (C, D) WB and qRT-PCR were performed to check the ANXA11 expression levels in AGS and SGC-7901 cell lines with ANXA11-siRNA at the concentration of 40 nM. * P<0.05.
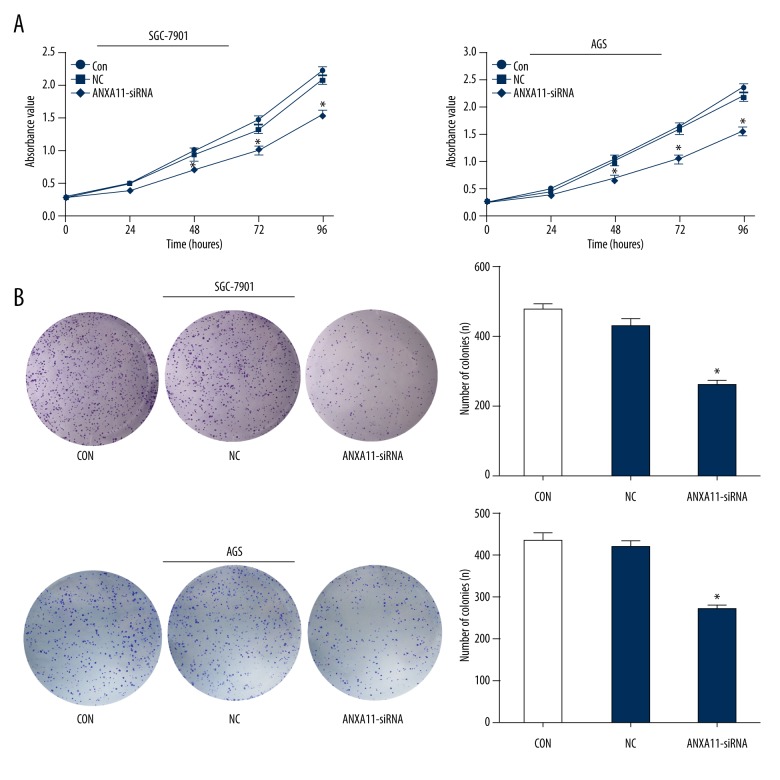
ANXA11 downregulation inhibits cell proliferation and colony formation
To investigate the biological effects of ANXA11, we transfected AGS and SGC-7901 cell lines with ANXA11-siRNA, assessed by CCK-8 kit and colony formation assay. The transfection of ANXA11 siRNA significantly inhibited the proliferation and colony formation capacity of AGS and SGC-7901 cell lines compared with the negative control. These findings indicate that ANXA11 has an important role in cell proliferation in gastric cancer (Figure 3A, 3B).
Figure 3.
ANXA11 downregulation inhibits GC cell lines proliferation and clone formation. (A) cck-8 assays. (B) Colony formation assays. * P<0.05.
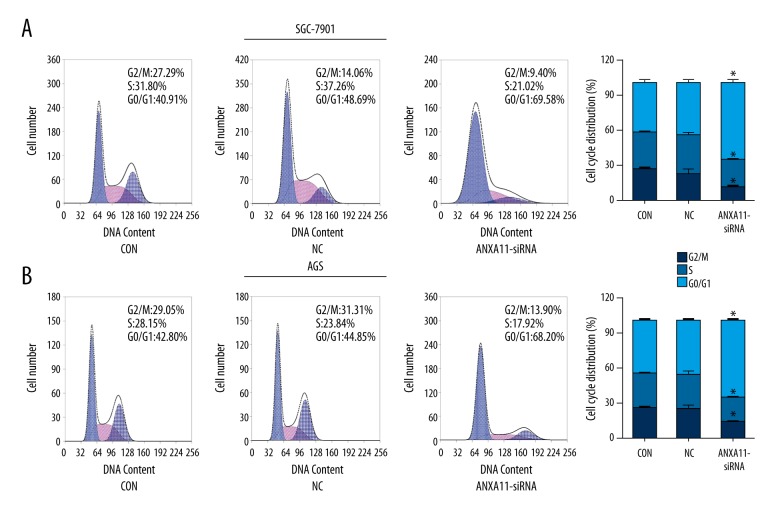
ANXA11 downregulation causes cell cycle arrest
Cell cycle distribution in AGS and SGC-7901 cells was assessed by flow cytometric analysis. The results showed that down-regulation of ANXA11 in AGS and SGC-7901 cell lines led to significant cell accumulation in the G0/G1 phase and a decreased percentage of cells in the S phase when compared to the NC groups (Figure 4A, 4B).
Figure 4.
ANXA11 downregulation causes cell cycle arrest at the G0/G1 phase. (A) SGC-7901 cell cycle distribution. (B) AGS cell cycle distribution. * P<0.05
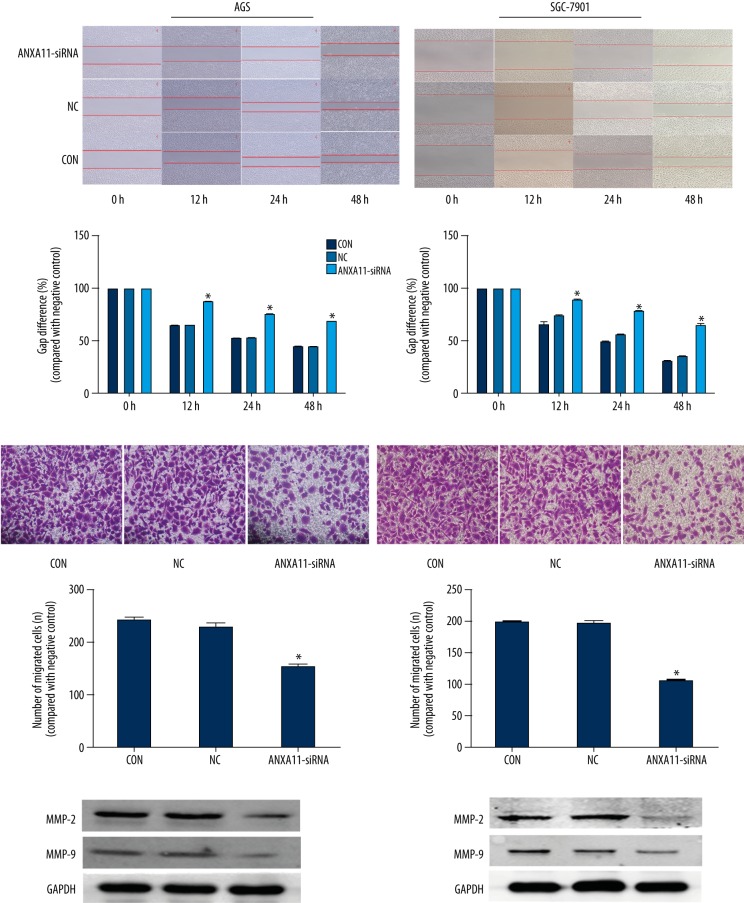
ANXA11 downregulation inhibits cell migration and invasion
The wound-healing experiment and invasion assay were used to observe the effect of down-regulated ANXA11 in AGS and SGC-7901 cell lines. As shown in Figure 5B, down-regulation of ANXA11 significantly inhibits the invasion activity of AGS and SGC-7901 cell lines. We also investigated the influence of the migration effect using a wound-healing experiment, showing that ANXA11 downregulation inhibits AGS and SGC-7901 cell lines migration (Figure 5A). To analyze the potential mechanism underlying blockage of ANXA11-specifc siRNA-mediated invasion and migration, MMP2 and MMP9 protein expression was examined. As shown in Figure 5C, MMP2 and MMP9 protein levels were reduced in AGS and SGC-7901 cell lines with downregulation of ANXA11.
Figure 5.
ANXA11 downregulation inhibits GC cell lines migration and invasion. (A) Cell migration assays. (B) Invasion assays. (C) Changes in MMP2 and MMP9 protein levels were assessed by WB assays. * P<0.05.
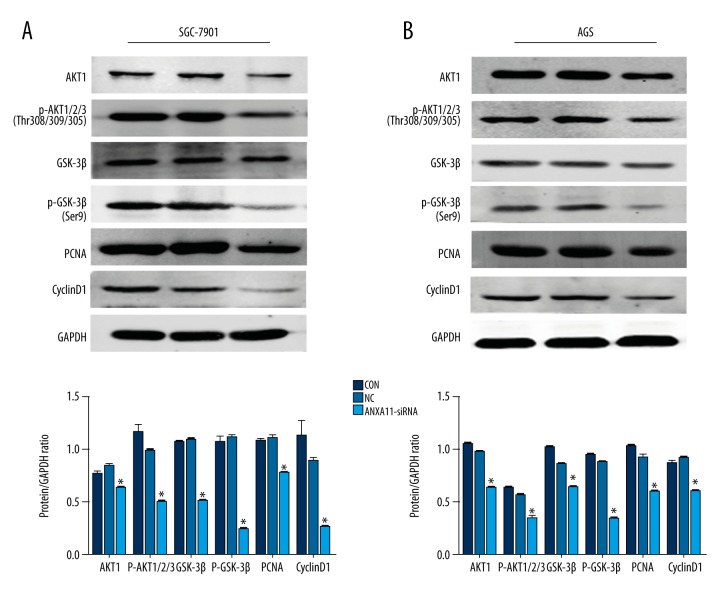
ANXA11 downregulation inhibits the AKT/GSK-3β signaling pathway
To explore the underlying molecular basis of ANXA11 in regulating AGS and SCG-7901 cell proliferation, migration, and invasion, Western blot analysis was performed to analyze the changes in proteins related to the AKT/GSK-3β pathway. The expression of several proteins, including AKT1, phosphor-Akt1/2/3Thr308/309/305, GSK-3β, phosphor-GSK-3βSer9, CyclinD1, and PCNA, were examined by WB. The results show that the protein expression levels of these markers were significantly decreased at 48 h after transfection with ANXA11-siRNA in AGS and SGC-7901 cell lines in comparison with the NC cell line (Figure 6A, 6B).
Figure 6.
ANXA11 downregulation inhibits the AKT/GSK-3β signaling pathway in GC cell lines. (A, B) The protein expression levels of AKT1, phosphor-Akt1/2/3Thr308/309/305, GSK-3β, phosphor-GSK-3βSer9, CyclinD1, and PCNA were examined by WB in AGS and SGC-7901 cell lines, and the expression levels of AKT1, phosphor-Akt1/2/3Thr308/309/305, GSK-3β, phosphor-GSK-3βSer9, CyclinD1, and PCNA were significantly decreased in the ANXA11-siRNA group compared to the NC group. * P<0.05.
Discussion
Due to lack of effective treatment methods and screening tools, the mortality of GC has not obviously declined in the past decade. Many gastric cancer patients present at advanced stage due to absence of specific symptoms. Patients diagnosed at early stage have overall survival rates of over 80%, and those diagnosed at advanced stage have only 24% survival [20]. Therefore, it is urgent for cancer research to find new prognostic markers and novel therapeutic targets for gastric cancer.
The annexins are a multigene family of proteins that bind Ca2+ and phospholipids. Functions of the annexins include cancer progression, metastasis, apoptosis, cell division, Ca2+ signaling, growth regulation, and membrane traffic and organization [8,9]. Some of the annexins have been well characterized and show a cell- and tissue-specific pattern of expression. The correlations of this pattern are intriguing and changes in the expression levels of certain annexins may influence some patterns of cellular behavior, such as migration, invasion, proliferation, and apoptosis [6,7,9]. As such, annexins may yet prove to have therapeutic and prognostic potential in malignant disease.
ANXA11 is a member of the annexins family, and increased expression of ANXA11 has been reported in several malignancies and implicated in influencing a number of cellular processes, including cancer recurrence, metastasis, drug resistance, lymph node metastasis, and poor survival, in many cancers [14–19]. But so far, there is no report on the role of ANXA11 in gastric cancer. In our study, fist we assessed ANXA11 expression in GC tumor tissues and their adjacent mucosa by using qRT-PCR, WB, and immunohistochemistry in gastric cancer patients, and then we analyzed the high expression of ANXA11 and the biological characteristics of gastric cancer. We also investigated the effect of ANXA11 on cell proliferation, migration, and invasion by in vitro experiments with gastric cancer cell lines.
In gastric cancer patient tissues, ANXA11 expression was widely increased in cancer tissues compared with adjacent mucosa. The clinicopathological analysis found that increased expression of ANXA11 was significantly associated with tumor size, tumor infiltration, local lymph node metastasis, TNM staging, and vascular invasion. These results suggest that ANXA11 might be involved in gastric cancer progression and could serve as a potential independent prognostic factor.
To further elucidate the tumor oncogene role of ANXA11 in GC, we investigated the effect of ANXA11 on the viability of GC cell lines in vitro. Recent evidence has shown that down-regulation of ANXA11 in ovarian cancer and liver cancer cell lines inhibits cell proliferation and induces apoptosis in vitro and in vivo [15,16,19]. In our study, the results agree with previous research. Downregulation of ANXA11 resulted in a slower rate of cell growth, colony formation, and induction of cell cycle at the G0/G1 phase in AGS and SGC-7901 cell lines. PCNA is a critical eukaryotic replication accessory factor [21], and downregulation of PCNA expression suggests cellular proliferation is markedly inhibited. These results further confirm that ANXA11 plays an important role in cell proliferation in gastric cancer cell lines.
Invasion and migration are the main characteristics of tumor cells, which can acquire the ability to degrade the basement membranes and extracellular matrix and invade blood vessels [22]. The Transwell invasion assay and wound-healing experiments performed in the present study show that ANXA11 knockdown can suppress the invasion and migration ability of human gastric cancer cells. MMP-9 and MMP-2, 2 members of the matrix metalloproteinases (MMP) family, are required for tumor invasion and metastasis [23,24]. We found that ANXA11 knockdown decreased the MMP-9 and MMP-2 expression in AGS and SGC-7901 cell lines, indicating that the inhibition of gastric cancer cell invasion and migration by ANXA11 may be partly mediated by the downregulation of MMP-2 and MMP-9 expression.
Glycogen synthase kinase-3β (GSK-3β) was initially identified as an enzyme that regulates glycogen synthesis in response to insulin [25]. But later research found that GSK-3β is a ubiquitously expressed serine/threonine protein kinase and a critical downstream element of the PI3Kinase/AKT cell survival pathway [26,27], which has been proposed to affect cell differentiation, proliferation, migration, invasion, and apoptosis [28–30]. In the present study, we found that expression of AKT1, phosphor-Akt1/2/3 Thr308/309/305, phosphor-GSK-3βSer9, and CyclinD1 were decreased when cells were treated with ANXA11-siRNA. These findings suggest that ANXA11 acts as an oncogene in gastric cancer and exerts its role at least partly via the AKT/GSK-3β pathway.
The limitations of this study include its small sample size, lack of long-term survival observations, and lack of assessment of effects of overexpression of ANXA11 in gastric cancer cell lines. Therefore, further studies were needed.
Conclusions
In conclusion, this study has shown that ANXA11 expression was widely increased in cancer tissues compared with adjacent mucosa. The clinicopathological analysis found that increased expression of ANXA11 was associated with tumor size, tumor infiltration, local lymph node metastasis, TNM staging, and vascular invasion significantly. Moreover, ANXA11 plays a critical role in regulating GC cell line proliferation, migration, and invasion by the AKT/GSK-3β pathway, and is a potential prognostic factor and therapeutic target for gastric cancer patients.
Acknowledgements
The authors would like to thank Dr. Xin Tian for his valuable suggestions.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CancerJ Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Li ZX, Kaminishi M. A comparison of gastric cancer between Japan and China. Gastric Cancer. 2009;12:52–53. doi: 10.1007/s10120-008-0495-2. [DOI] [PubMed] [Google Scholar]
- 5.Bolke E, Peiper M, Budach W. Capecitabine and oxaliplatin for advanced esophagogastric cancer. New Engl J Med. 2008;358:1965. doi: 10.1056/NEJMc080178. author reply 1965. [DOI] [PubMed] [Google Scholar]
- 6.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–71. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 7.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–61. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 8.Moss SE, Morgan RO. The annexins. Genome Biol. 2004;5:219. doi: 10.1186/gb-2004-5-4-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monastyrskaya K, Babiychuk EB, Hostettler A, et al. Annexins as intracellular calcium sensors. Cell Calcium. 2007;41:207–19. doi: 10.1016/j.ceca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Mizutani A, Watanabe N, Kitao T, et al. The long amino-terminal tail domain of annexin XI is necessary for its nuclear localization. Arch Biochem Biophys. 1995;318:157–65. doi: 10.1006/abbi.1995.1216. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Ando Y, Tokumitsu H, et al. Binding site of annexin XI on the calcyclin molecule. Biochem Biophys Res Commun. 1993;196:1376–82. doi: 10.1006/bbrc.1993.2405. [DOI] [PubMed] [Google Scholar]
- 12.Williams LH, McClive PJ, Van Den Bergen JA, et al. Annexin XI co-localises with calcyclin in proliferating cells of the embryonic mouse testis. Dev Dyn. 2005;234:432–37. doi: 10.1002/dvdy.20548. [DOI] [PubMed] [Google Scholar]
- 13.Shibata H, Kanadome T, Sugiura H, et al. A new role for annexin A11 in the early secretory pathway via stabilizing Sec31A protein at the endoplasmic reticulum exit sites (ERES) J Biol Chem. 2015;290:4981–93. doi: 10.1074/jbc.M114.592089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Madrid F, Tang N, Alansari H, et al. Autoantibodies to Annexin XI-A and other autoantigens in the diagnosis of breast cancer. Cancer Res. 2004;64:5089–96. doi: 10.1158/0008-5472.CAN-03-0932. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Guo C, Wang J, et al. ANXA11 regulates the tumorigenesis, lymph node metastasis and 5-fluorouracil sensitivity of murine hepatocarcinoma Hca-P cells by targeting c-Jun. Oncotarget. 2016;7:16297–310. doi: 10.18632/oncotarget.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Wang J, Guo C, et al. Annexin A11 knockdown inhibits in vitro proliferation and enhances survival of Hca-F cell via Akt2/FoxO1 pathway and MMP-9 expression. Biomed Pharmacother. 2015;70:58–63. doi: 10.1016/j.biopha.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Roh SA, Park IJ, Yoon YS, et al. Feasibility of novel PPP1R15A and proposed ANXA11 single nucleotide polymorphisms as predictive markers for bevacizumab regimen in metastatic colorectal cancer. J Cancer Res Clin Oncol. 2016;142:1705–14. doi: 10.1007/s00432-016-2177-5. [DOI] [PubMed] [Google Scholar]
- 18.Song J, Shih Ie M, Salani R, et al. Annexin XI is associated with cisplatin resistance and related to tumor recurrence in ovarian cancer patients. Clin Cancer Res. 2007;13:6842–49. doi: 10.1158/1078-0432.CCR-07-0569. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Shih I-M, Chan DW, et al. Suppression of Annexin A11 in ovarian cancer: Implications in chemoresistance. Neoplasia. 2009;11:605–15. doi: 10.1593/neo.09286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend R, Jr, Beauchamp BD, et al. Sabiston textbook of surgery: the biological basis of modern surgical practic. Canada: Elsevier, Inc.; 2015. [Google Scholar]
- 21.Park SY, Jeong MS, Han CW, et al. Structural and functional insight into proliferating cell nuclear antigen. J Microbiol Biotechnol. 2016;26:637–47. doi: 10.4014/jmb.1509.09051. [DOI] [PubMed] [Google Scholar]
- 22.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277–83. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shay G, Lynch CC, Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44–46:200–6. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sani IK, Marashi SH, Kalalinia F. Solamargine inhibits migration and invasion of human hepatocellular carcinoma cells through down-regulation of matrix metalloproteinases 2 and 9 expression and activity. Toxicol In Vitro. 2015;29:893–900. doi: 10.1016/j.tiv.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Welsh GI, Wilson C, Proud CG. GSK3: A SHAGGY frog story. Trends Cell Biol. 1996;6:274–79. doi: 10.1016/0962-8924(96)10023-4. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava AK, Pandey SK. Potential mechanism(s) involved in the regulation of glycogen synthesis by insulin. Mol Cell Biochem. 1998;182:135–41. [PubMed] [Google Scholar]
- 27.Cross DA, Alessi DR, Cohen P, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–89. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 28.Kockeritz L, Doble B, Patel S, et al. Glycogen synthase kinase-3 – an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–88. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 29.Neth P, Ciccarella M, Egea V, et al. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892–903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- 30.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]