Abstract
Leiomyosarcomas account for ~24% of all adult sarcomas, and develop predominantly either in the uterus [uterine leiomyosarcoma (ULMS)] or in deep soft tissue or the retroperitoneum [non-uterine leiomyosarcoma (NULMS)]. Leiomyosarcomas are relatively chemoresistant tumors, and the prognosis of patients with leiomyosarcomas is poor. Cancer-testis (CT) antigens are considered promising immunotherapeutic targets because of their restricted expression in normal tissue, except in the testis. Little is known about the expression of CT antigens in leiomyosarcomas. In the present study, the protein expression of the CT antigens MAGE family member A (MAGEA)1, MAGEA3, MAGEA4, G antigen 7 (GAGE7) and cancer/testis antigen 1 (NY-ESO-1) in ULMS and NULMS were investigated using immunohistochemistry (IHC), and their expression profiles compared. In ULMS and NULMS, positive expression was observed in 11/32 (31%) and 1/31 (3%; MAGEA1), 15/32 (47%) and 5/31 (16%; MAGEA3), 11/32 (34%) and 3/31 (10%; MAGEA4), 23/32 (72%) and 11/31 (35%; GAGE7) and 3/32 (9%) and 0/31 (0%; NY-ESO-1), respectively. The ULMSs demonstrated significantly higher positive expression of MAGEA1 (P=0.0034), MAGEA3 (P=0.0141), MAGEA4 (P=0.0319) and GAGE7 (P=0.0054) compared with the NULMSs. The ULMSs also had significantly higher IHC scores for MAGEA1 (P=0.0023), MAGEA3 (P=0.0474), MAGEA4 (P=0.011), GAGE7 (P=0.0319) and NY-ESO-1 (P=0.0437). The results of the present study support the potential utility of MAGEA1, MAGEA3, MAGEA4 and GAGE7 in ULMS and GAGE7 in NULMS as immunotherapeutic targets.
Keywords: leiomyosarcoma, cancer-testis antigen, MAGE family member A1, cancer/testis antigen 1, G antigen 7
Introduction
Soft-tissue sarcomas comprise a rare, complex and heterogeneous group of tumors that account for only 1% of all cancers, and these tumors possess numerous histological subtypes (1). Partially due to the rarity of soft-tissue sarcomas, improvements in their treatment have not been achieved, and novel therapeutic options according to histological subtype are required.
Leiomyosarcomas, defined as malignant tumors with smooth-muscle differentiation, account for 24% of all soft-tissue sarcomas (2). Leiomyosarcomas have the potential to occur in any place in the body, but they develop mainly in the uterus [uterine leiomyosarcoma (ULMS)] or retroperitoneum and deep soft tissue [non-uterine leiomyosarcoma (NULMS)]. ULMSs account for 1–2% of all uterine malignancies (3). Leiomyosarcomas are relatively chemoresistant compared with other sarcoma subtypes, and so their overall prognosis remains poor (4).
Cancer-testis (CT) antigens, which are recognized by specific cytotoxic T lymphocytes (CTLs), are considered promising targets in immunotherapy due to their expression occurring only in tumor tissues of different histological origins and not in normal somatic tissues [except for testis tissue, which has no expression of human leukocyte antigen (HLA) class 1 and is not recognized by CTLs] (5,6). One study noted mRNA expression of CT antigens in several cases of ULMS and NULMS (7), but the available information concerning protein expression of CT antigens in ULMS and NULMS is limited.
In the present study, protein expression of the CT antigens MAGE family member A (MAGEA)1, MAGEA3, MAGEA4, cancer/testis antigen 1 (NY-ESO-1) and G antigen 7 (GAGE7), for which clinical immunotherapy trials are already underway or have been completed, was investigated (8,9). Immunohistochemistry (IHC) was used to validate the potential utility of each of these antigens as a target for immunotherapy in ULMS and NULMS, and comparisons were made between their expression profiles.
Materials and methods
Tissue samples
The paraffin-embedded tissue of 32 ULMS and 31 NULMS cases were obtained from the files of soft-tissue tumors registered at the Department of Anatomic Pathology, Graduate School of Medical Sciences, Kyushu University (Fukuoka, Japan) between April 1988 and December 2014. Each tumor was re-classified according to the World Health Organization classification, by bone and soft-tissue tumor pathologists and gynecological pathologists (10,11). The tissue samples had been obtained from open biopsy specimens or surgically resected tumors. All the 31 NULMS tissues were located at the extremities (24 was located at lower extremities and 7 was at the upper extremities), and no retroperitoneal cases were included. Among the 31 NULMS cases, 11 males and 20 females were included. The mean age of the NULMS patients was 60 years (range, 32–78 years) and the mean age of the ULMS patients was 48 years (range, 29–63 years). In the 32 ULMS cases, 15 patients were receiving chemotherapy and the remaining 17 tissue samples were obtained prior to the patients receiving chemotherapy, including biopsy specimens. In the 31 NULMS cases, 12 patients were receiving chemotherapy and the remaining 19 tissue samples were obtained prior to the patients receiving chemotherapy, including biopsy specimens. The present study was approved by the Ethics Committee of Kyushu University (Fukuoka, Japan) and conducted according to the Ethical Guidelines for Epidemiological Research enacted by the Japanese government.
IHC and evaluation
IHC was conducted as previously described (12). Antigen retrieval, the primary antibodies and dilutions used are summarized in Table I. The slides were incubated with the primary antibodies overnight at 4°C. The DAKO™ REAL™ Envision detection system was employed for detection, which contained horseradish peroxidase-conjugated antibodies (undiluted; catalog no. K5007; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), and incubated with slides for 90 min at room temperature. Slides were examined using Olympus light BX 43 microscope (Olympus Corporation, Tokyo, Japan). The IHC results were assessed by three investigators who were blinded to the clinical data of the patients. A consensus assessment was adopted as the IHC result.
Table I.
Antibodies used for immunohistochemical staining.
| Antigen | Clone (catalog no.) | Host species | Dilution | Retrieval | Source |
|---|---|---|---|---|---|
| MAGEA1 | MA454 (sc 20033) | Mouse | 1:1,000 | pH9 Pressure Boiler | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| MAGEA3 | 1H1 (ab140678) | Mouse | 1:500 | pH9 Microwave | OriGene Technologies, Inc., Rockville, MD, USA |
| MAGEA4 | CPTC-MAGEA4-1 (ab2138142) | Mouse | 1:500 | pH6 Microwave | DSHB, University of Iowa, Iowa City, IA, USA |
| GAGE7 | Polyclonal (PA5-26760) | Rabbit | 1:500 | pH9 Microwave | Thermo Fisher Scientific, Inc., Waltham, MA, USA |
| NY-ESO-1 | E978 (sc-53869) | Mouse | 1:100 | pH6 Pressure Boiler | Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
MAGEA, MAGE family member A; GAGE7, (G antigen 7; NY-ESO-1, cancer/testis antigen 1; MA454, anti-MAGE1 antibody; 1H1, anti-EBV antibody; CPTC-MAGEA4-1, melanoma-associated antibody; E978, NY-ESO-1 monoclonal antibody).
The percentage of immunoreactive cells and staining intensity were assessed in the most representative areas. The proportion of immunoreactive cells was scored as 0 to 4 points as follows: 0, < 5%; 1, 5-<25%; 2, 25-<50%; 3, 50-<75%; 4, ≥ 75%, and the intensity was scored as 0 to 3 points as follows: 0, negative; 1, weak staining; 2, moderate staining; 3, strong staining. The total score (proportion score + intensity score) was obtained, and the cases with a total score ≥3 were judged as positive. A total score ≥5 was defined as high expression as previously described (12).
Statistical analysis
Fisher's exact test and the Mann-Whitney U-test were used as appropriate to evaluate the associations between two variables. A two-sided P-value of <0.05 was considered to indicate a significant difference. The data analyses were performed using the JMP statistical software package (v.12.2.0; SAS Institute, Inc., Cary, NC, USA).
Results
IHC results
The IHC results are presented in Tables II and III and Figs. 1–3. MAGEA4, GAGE7 and NY-ESO-1 were localized in the cytoplasm and nuclei, whereas MAGEA1 and MAGEA3 were localized mainly in the cytoplasm. In the ULMS and NULMS samples, positive staining was observed in 10 of 32 (31%) and 1 of 31 (3%) for MAGEA1, 15 of 32 (47%) and 5 of 31 (16%) for MAGEA3, 11 of 32 (34%) and 3 of 31 (10%) for MAGEA4, 23 of 32 (72%) and 11 of 31 (35%) for GAGE7, and 3 of 32 (9%) and 0 of 31 (0%) for NY-ESO-1, respectively (Table II). The positive staining rates for MAGEA1 (P=0.0034), MAGEA3 (P=0.0141), MAGEA4 (P=0.0319) and GAGE7 (P=0.0054) were significantly higher in the ULMSs compared with the NULMSs. The ULMSs tended to have a higher positive staining rate for NY-ESO-1 compared with the NULMSs, but the difference was not significant. No significant difference was observed in the expression of any of the CT antigens between male and female patients (data not presented).
Table II.
Immunohistochemistry results in ULMS and NULMS.
| Positive cases/Total n (positive %) | |||
|---|---|---|---|
| Antigen | ULMS (%) | NULMS (%) | P-value |
| MAGEA1 | 10/32 (31) | 1/31 (3) | 0.0034a |
| MAGEA3 | 15/32 (47) | 5/31 (16) | 0.0141a |
| MAGEA4 | 11/32 (34) | 3/31 (10) | 0.0319a |
| GAGE7 | 23/32 (72) | 11/31 (35) | 0.0054a |
| NY-ESO-1 | 3/32 (9) | 0/31 (0) | 0.2381 |
P<0.05, as assessed using Fisher's exact (test. MAGEA, MAGE family member A; GAGE7, G antigen 7; NY-ESO-1, cancer/testis antigen 1; ULMS, uterine leiomyosarcoma; NULMS, non-uterine leiomyosarcoma).
Table III.
Results of immunohistochemistry, determining high and low expression levels.
| Uterine leiomyosarcoma | Non-uterine leiomyosarcoma | |||
|---|---|---|---|---|
| Antigen | Low, N (%) | High, N (%) | Low, N (%) | High, N (%) |
| MAGEA1 | 8 (25) | 2 (6) | 1 (3) | 0 (0) |
| MAGEA3 | 14 (31) | 1 (3) | 3 (10) | 2 (6) |
| MAGEA4 | 7 (22) | 4 (13) | 2 (6) | 1 (3) |
| GAGE7 | 19 (59) | 4 (13) | 9 (29) | 2 (6) |
| NY-ESO-1 | 2 (6) | 1 (3) | 0 (0) | 0 (0) |
MAGEA, MAGE family member A; GAGE7, G antigen 7; NY-ESO-1, cancer/testis antigen 1.
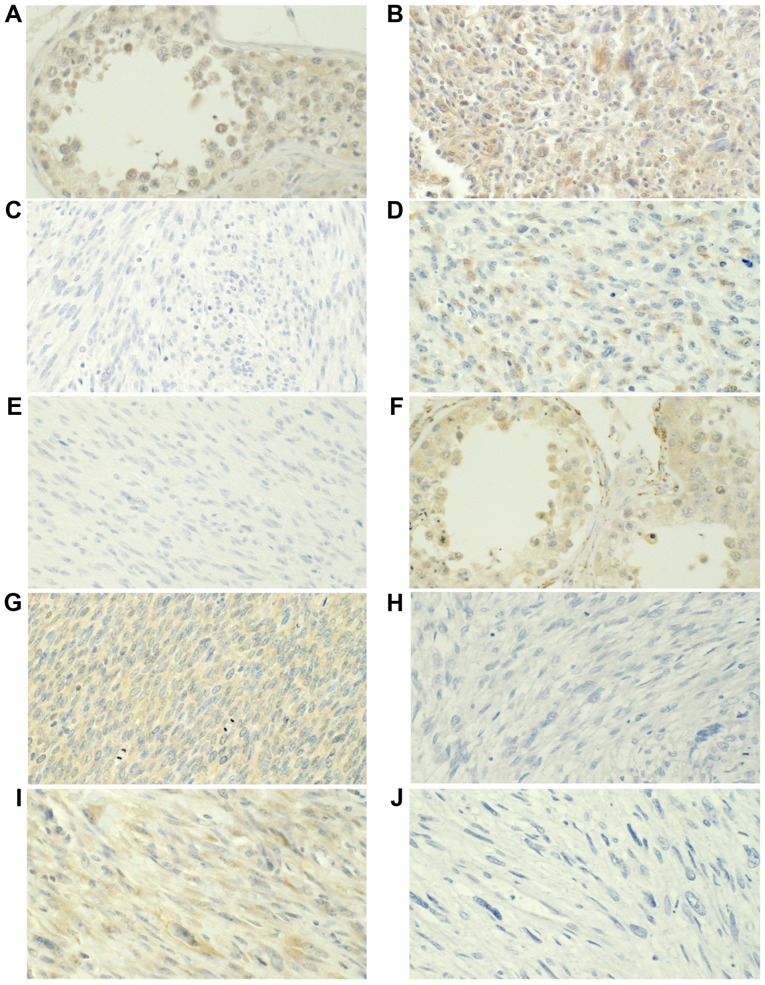
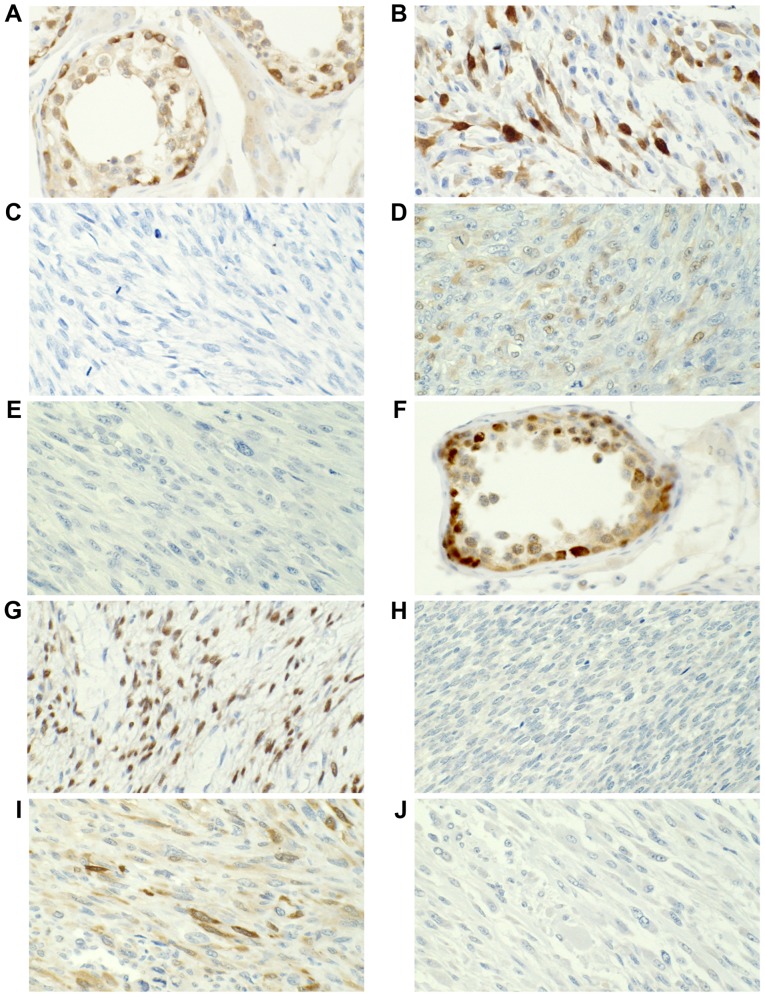
Figure 1.
MAGEA1 and MAGEA3 protein expression was assessed using immunohistochemistry. MAGEA1 expression in (A) the testis, (B) positive and (C) negative staining in ULMS, and (D) positive and (E) negative staining in NULMS. MAGEA3 expression in (F) the testis, (G) positive and (H) negative staining in ULMS, and (I) positive and (J) negative staining in NULMS. Magnification, ×400. MAGEA, MAGE family member A; ULMS, uterine leiomyosarcoma; NULMS, non-uterine leiomyosarcoma.
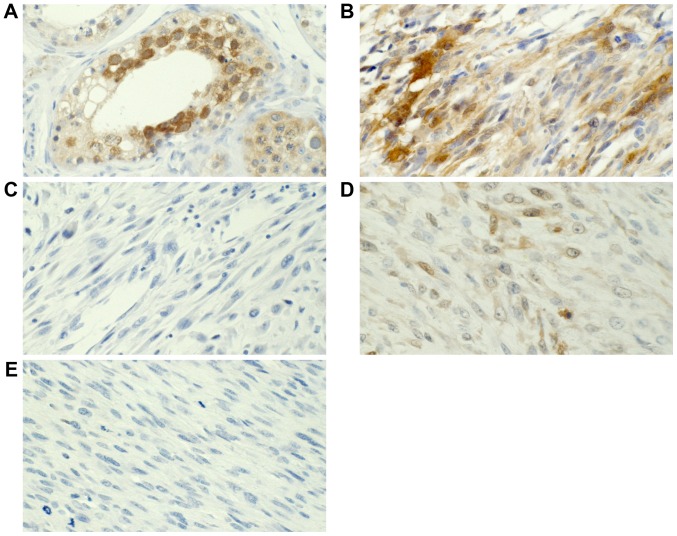
Figure 3.
NY-ESO-1 expression was analyzed using immunohistochemistry. (A) NY-ESO-1 expression in the testis. (B) NY-ESO-1-positive and (C) -negative staining in ULMS. (D) NY-ESO-1-positive and (E) -negative staining in NULMS. Magnification, ×400. NY-ESO, cancer/testis antigen 1; ULMS, uterine leiomyosarcoma; NULMS, non-uterine leiomyosarcoma.
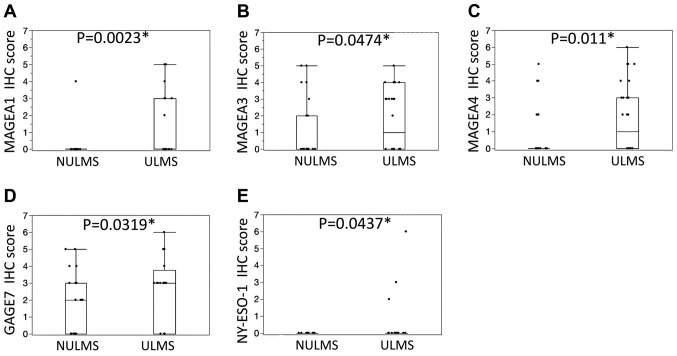
In addition, the immunohistochemical scores of the ULMS cases were significantly higher for MAGEA1 (P=0.0023), MAGEA3 (P=0.0474), MAGEA4 (P=0.011), GAGE7 (P=0.0319) and NY-ESO-1 (P=0.0437) compared with the NULMS (Fig. 4). In the ULMS and NULMS samples, high expression was observed in 2 of 32 (6%) and 0 of 31 (0%) samples for MAGEA1, 1 of 32 (3%) and 2 of 31 (6%) for MAGEA3, 4 of 32 (13%) and 1 of 31 (3%) for MAGEA4, 4 of 32 (13%) and 2 of 31 (6%) for GAGE7, and 1 of 32 (3%) and 0 of 31 (0%) for NY-ESO-1, respectively. No significant association was observed between the expression of any of the CT antigens (data not presented).
Figure 4.
Immunohistochemical scores in NULMS and ULMS. The ULMSs demonstrated significantly higher scores for (A) MAGEA1, (B) MAGEA3, (C) MAGEA4, (D) GAGE7 and (E) NY-ESO-1 compared with the NULMS cases. ULMS, uterine leiomyosarcoma; NULMS, non-uterine leiomyosarcoma; MAGEA, MAGE family member A; GAGE7, G antigen 7; NY-ESO, cancer/testis antigen 1.
Discussion
As previously discussed, CT antigens are considered to be a promising target for immunotherapy due to their restricted expression in normal tissues (except in the testes, which do not express HLA molecules). MAGEA1 expression was detected in myxoid liposarcoma (11.0%) (13), medulloblastoma (4.0%) (14), head and neck squamous cell carcinoma (13.0%) (15), seminoma (16.6%) (16) and esophageal squamous cell carcinoma (38.8%) (17) as demonstrated by IHC. MAGEA3 expression was detected in gastric carcinoma (45.0%) (18), head and neck squamous cell carcinoma (45.0%) (19), invasive intrahepatic cholangiocarcinoma (47.0%) (20), and prostate cancer (85.8%) (21) as demonstrated by IHC. MAGEA4 expression was detected in uterine endometrioid adenocarcinomas (12.0%), uterine papillary serous carcinomas (63.0%), uterine carcinosarcoma (91.0%) (22), urinary squamous cell carcinoma (45.5%) (23), cervical squamous cell carcinoma (33.0%) (24), breast cancer (74.0%) (25) and oral squamous cell carcinoma (56.5%) (26). NY-ESO-1 expression was detected in invasive ductal carcinoma of the breast (11.2%) (27), metastatic malignant melanoma (32.0%) (28), head and neck squamous cell carcinoma (4.3%) (29) and non-small cell lung cancer (25.0%) (30). GAGE7 expression was detected in spermatocytic seminoma (67.0%), seminomas (4.0%) (31) and advanced breast carcinoma (43.9%) (32).
Clinical trials of immunotherapeutic targets for CT antigens have been actively performed. NY-ESO-1, MAGEA3 and MAGEA4 are of particular interest to researchers and clinicians, and developments in immunotherapeutic techniques including peptide vaccines, dendritic cell vaccines and T cell receptor gene transduced T lymphocytes for these CT antigens are currently underway (33–35).
However, little is known about the expression of CT antigens in sarcomas, and even less is known about their expression in leiomyosarcoma. Previously, NY-ESO-1 expression in various sarcomas had been analyzed using IHC and revealed that leiomyosarcomas demonstrated negligible NY-ESO-1 protein expression (36,37). Another study surveyed the mRNA expression of CT antigens in several ULMS and NULMS cases and reported that ULMS demonstrated relatively higher mRNA expression of CT antigens compared with NULMS (38). The results of the present study coincide with these results. However, there appear to be no other studies that evaluate the protein expression of CT antigens other than NY-ESO-1 in ULMS or NULMS. The present investigation therefore appears to be the first to determine the protein expression of CT antigens in ULMS and NULMS. The results of the present study revealed that the expression of CT antigens was dominant in ULMS compared with NULMS. Additionally, considering the rate of positivity and high expression, GAGE7 and MAGEA4 may be potential targets for immunotherapy in ULMS cases.
Although the functions of CT antigens remain unclear, it has been reported that MAGEA1 may act as a transcriptional repressor of genes required for differentiation (39). MAGEA4 was demonstrated to promote apoptosis in non-small cell lung cancers (40), and additionally was revealed to induce growth by inhibiting apoptosis in normal oral keratinocytes (41). The functions of NY-ESO-1, MAGA3 and GAGE7 are poorly understood. The expressions of these CT antigens were reported to be regulated by hypomethylation of the promoter region (42,43). Further studies on the functions and epigenetic regulation of these CT antigens in ULMS and NULMS are needed to elucidate the reason why expression of CT antigens is increased in ULMS compared with NULMS.
The oncofetal protein U3 small nucleolar ribonucleoprotein (IMP3) has been revealed to be expressed in ULMS (7 of 15, 47%) and NULMS (36 of 67, 54%) (44). Oncofetal protein may potentially be a promising target for immunotherapy because it is highly expressed in fetal tissue and malignant tumors but rarely observed in adult benign tissues (45,46). Similarly to the results of the IMP3 study, the results of the present study support the potential utility of MAGEA1, MAGEA3, MAGEA4 and GAGE7 as targets for immunotherapy for ULMS. GAGE7 may also be a potential immunotherapeutic target for NULMS.
One limitation of the present study was that sample-size of the study was too small to be conclusive. Another limitation was that only IHC expression was studied, thus further studies including western blotting or reverse transcription-quantitative polymerase chain reaction may be beneficial.
In conclusion, the analysis in the present study indicated that the CT antigens MAGEA1, MAGEA3, MAGEA4 and GAGE7 are frequently expressed in ULMS, and the expressions of these proteins was were higher in ULMS compared with NULMS. GAGE7 and MAGEA4 have potential use as immunotherapeutic targets in ULMS.
Figure 2.
MAGEA4 and GAGE7 protein expression was analyzed using immunohistochemistry. MAGEA4 expression in (A) the testis, (B) positive and (C) negative staining in ULMS, and (D) positive and (E) negative staining in NULMS. GAGE7 expression in (F) the testis, (G) positive and (H) negative staining in ULMS, and (I) positive and (J) negative staining in NULMS. Magnification, ×400. MAGEA, MAGE family member A; ULMS, uterine leiomyosarcoma; NULMS, non-uterine leiomyosarcoma; GAGE7, G antigen 7.
Acknowledgements
The authors would like to thank the Research Support Center, Graduate School of Medical Sciences, Kyushu University for the technical support. The present study was supported by JSPS KAKEN (grant no. 25293088).
References
- 1.Tierney JF, Mosseri V, Stewart LA, Souhami RL, Parmar MK. Adjuvant chemotherapy for soft-tissue sarcoma: Review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995;72:469–475. doi: 10.1038/bjc.1995.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 3.D'Angelo E, Prat J. Uterine sarcomas: A review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, Mouridsen H, Crowther D, Somers R, Verweij J, Santoro A, Buesa J, Tursz T. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: An analysis of 2,185 patients treated with anthracycline-containing first-line regimens-a European organization for research and treatment of cancer soft tissue and bone S. J Clin Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 5.Juretic A, Spagnoli GC, Schultz-Thater E, Sarcevic B. Cancer/testis tumour-associated antigens: Immunohistochemical detection with monoclonal antibodies. Lancet Oncol. 2003;4:104–109. doi: 10.1016/S1470-2045(03)00982-3. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda H, Lethé B, Lehmann F, van Baren N, Baurain JF, de Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/S1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 7.Ayyoub M, Taub RN, Keohan ML, Hesdorffer M, Metthez G, Memeo L, Mansukhani M, Hibshoosh H, Hesdorffer CS, Valmori D. The frequent expression of cancer/testis antigens provides opportunities for immunotherapeutic targeting of sarcoma. Cancer Immun. 2004;4:7. [PubMed] [Google Scholar]
- 8.Saito T, Wada H, Yamasaki M, Miyata H, Nishikawa H, Sato E, Kageyama S, Shiku H, Mori M, Doki Y. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine. 2014;32:5901–5907. doi: 10.1016/j.vaccine.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, Lucas KG. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. 2015;64:1251–1260. doi: 10.1007/s00262-015-1731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christopher DM, Fletcher JA, Pancras CW, Hogendoorn FM. WHO Classification of tumor of Tissue and Bone. Int. Agency Res. Cancer. 2013:33–43. [Google Scholar]
- 11.Kurman RJ, Carcangiu ML, Herrington S, Young RH. WHO classification of tumours Female Reproductive Organs. Int. Agency Res. Cancer. 2014:139–141. [Google Scholar]
- 12.Iura K, Kohashi K, Hotokebuchi Y, Ishii T, Maekawa A, Yamada Y, Yamamoto H, Iwamoto Y, Oda Y. Cancer-testis antigens PRAME and NY-ESO-1 correlate with tumour grade and poor prognosis in myxoid liposarcoma. J Pathol Clin Res. 2015;1:144–159. doi: 10.1002/cjp2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemminger JA, Toland AE, Scharschmidt TJ, Mayerson JL, Guttridge DC, Iwenofu OH. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2 and CTAG2 in myxoid and round cell liposarcoma. Mod Pathol. 2014;9:1238–1245. doi: 10.1038/modpathol.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oba-Shinjo SM, Caballero OL, Jungbluth AA, Rosemberg S, Old LJ, Simpson AJ, Marie SK. Cancer-testis (CT) antigen expression in medulloblastoma. Cancer Immun. 2008;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Kienstra MA, Neel HB, Strome SE, Roche P. Identification of NY-ESO-1, MAGE-1 and MAGE-3 in head and neck squamous cell carcinoma. Head Neck. 2003;25:457–463. doi: 10.1002/hed.10223. [DOI] [PubMed] [Google Scholar]
- 16.Cheville JC, Roche P. MAGE-1 and MAGE-3 tumor rejection antigens in human germ cell tumors. Mod Pathol. 1999;12:974–978. [PubMed] [Google Scholar]
- 17.Haier J, Owzcareck M, Guller U, Spagnoli GC, Bürger H, Senninger N, Kocher T. Expression of MAGE-A cancer/testis antigens in esophageal squamous cell carcinomas. Anticancer Res. 2006;26:2281–2287. [PubMed] [Google Scholar]
- 18.Sadanaga N, Nagashima H, Tahara K, Yoshikawa Y, Mori M. The heterogeneous expression of MAGE-3 protein: Difference between primary lesions and metastatic lymph nodes in gastric carcinoma. Oncol Rep. 1999;6:975–977. doi: 10.3892/or.6.5.975. [DOI] [PubMed] [Google Scholar]
- 19.Lee KD, Chang HK, Jo YK, Kim BS, Lee BH, Lee YW, Lee HK, Huh MH, Min YG, Spagnoli GC, Yu TH. Expression of the MAGE 3 gene product in squamous cell carcinomas of the head and neck. Anticancer Res. 1999;19:5037–5042. [PubMed] [Google Scholar]
- 20.Tsuneyama K, Sasaki M, Shimonishi T, Nakanuma Y. Expression of MAGE-A3 in intrahepatic cholangiocarcinoma and its precursor lesions. Pathol Int. 2004;54:181–186. doi: 10.1111/j.1440-1827.2003.01605.x. [DOI] [PubMed] [Google Scholar]
- 21.Hudolin T, Juretic A, Spagnoli GC, Pasini J, Bandic D, Heberer M, Kosicek M, Cacic M. Immunohistochemical expression of tumor antigens MAGE-A1, MAGE-A3/4 and NY-ESO-1 in cancerous and benign prostatic tissue. Prostate. 2006;66:13–18. doi: 10.1002/pros.20312. [DOI] [PubMed] [Google Scholar]
- 22.Resnick MB, Sabo E, Kondratev S, Kerner H, Spagnoli GC, Yakirevich E. Cancer-testis antigen expression in uterine malignancies with an emphasis on carcinosarcomas and papillary serous carcinomas. Int J Cancer. 2002;101:190–195. doi: 10.1002/ijc.10585. [DOI] [PubMed] [Google Scholar]
- 23.Kocher T, Zheng M, Bolli M, Simon R, Forster T, Schultz-Thater E, Remmel E, Noppen C, Schmid U, Ackermann D, et al. Prognostic relevance of MAGE-A4 tumor antigen expression in transitional cell carcinoma of the urinary bladder: A tissue microarray study. Int J Cancer. 2002;100:702–705. doi: 10.1002/ijc.10540. [DOI] [PubMed] [Google Scholar]
- 24.Sarcevic B, Spagnoli GC, Terracciano L, Schultz-Thater E, Heberer M, Gamulin M, Krajina Z, Oresic T, Separovic R, Juretic A. Expression of cancer/testis tumor associated antigens in cervical squamous cell carcinoma. Oncology. 2003;64:443–449. doi: 10.1159/000070305. [DOI] [PubMed] [Google Scholar]
- 25.Bandić D, Juretić A, Sarcević B, Separović V, Kujundzić-Tiljak M, Hudolin T, Spagnoli GC, Cović D, Samija M. Expression and possible prognostic role of MAGE-A4, NY-ESO-1 and HER-2 antigens in women with relapsing invasive ductal breast cancer: Retrospective immunohistochemical study. Croat Med J. 2006;47:32–41. [PMC free article] [PubMed] [Google Scholar]
- 26.Montoro JR, Mamede RC, Neder Serafini L, Saggioro FP, Figueiredo DL, Silva WA, Jr, Jungbluth AA, Spagnoli GC, Zago MA. Expression of cancer-testis antigens MAGE-A4 and MAGE-C1 in oral squamous cell carcinoma. Head Neck. 2012;34:1123–1128. doi: 10.1002/hed.21880. [DOI] [PubMed] [Google Scholar]
- 27.Chen YT, Ross DS, Chiu R, Zhou XK, Chen YY, Lee P, Hoda SA, Simpson AJ, Old LJ, Caballero O, Neville AM. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS One. 2011;6:e17876. doi: 10.1371/journal.pone.0017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velazquez EF, Jungbluth AA, Yancovitz M, Gnjatic S, Adams S, O'Neill D, Zavilevich K, Albukh T, Christos P, Mazumdar M, et al. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)-correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 29.Laban S, Atanackovic D, Luetkens T, Knecht R, Busch CJ, Freytag M, Spagnoli G, Ritter G, Hoffmann TK, Knuth A, et al. Simultaneous cytoplasmic and nuclear protein expression of melanoma antigen-A family and NY-ESO-1 cancer-testis antigens represents an independent marker for poor survival in head and neck cancer. Int J Cancer. 2014;135:1142–1152. doi: 10.1002/ijc.28752. [DOI] [PubMed] [Google Scholar]
- 30.John T, Starmans MH, Chen YT, Russell PA, Barnett SA, White SC, Mitchell PL, Walkiewicz M, Azad A, Lambin P, et al. The role of Cancer-Testis antigens as predictive and prognostic markers in non-small cell lung cancer. PLoS One. 2013;8:e67876. doi: 10.1371/journal.pone.0067876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao CS, Badve SS, Ulbright TM. The utility of immunostaining for NUT, GAGE7 and NY-ESO-1 in the diagnosis of spermatocytic seminoma. Histopathology. 2014;65:35–44. doi: 10.1111/his.12365. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, Yang F, Zhang T, Zhuang R, Sun Y, Fang L, Zhang C, Ma Y, Huang G, Ma F, et al. Heterogeneous expression of CT10, CT45 and GAGE7 antigens and their prognostic significance in human breast Carcinoma. Jpn J Clin Oncol. 2013;43:243–250. doi: 10.1093/jjco/hys236. [DOI] [PubMed] [Google Scholar]
- 33.Oshita C, Takikawa M, Kume A, Miyata H, Ashizawa T, Iizuka A, Kiyohara Y, Yoshikawa S, Tanosaki R, Yamazaki N, et al. Dendritic cell-based vaccination in metastatic melanoma patients: Phase II clinical trial. Oncol Rep. 2012;28:1131–1138. doi: 10.3892/or.2012.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kageyama S, Ikeda H, Miyahara Y, Imai N, Ishihara M, Saito K, Sugino S, Ueda S, Ishikawa T, Kokura S, et al. Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancer. Clin Cancer Res. 2015;21:2268–2277. doi: 10.1158/1078-0432.CCR-14-1559. [DOI] [PubMed] [Google Scholar]
- 35.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai JP, Robbins PF, Raffeld M, Aung PP, Tsokos M, Rosenberg SA, Miettinen MM, Lee CC. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: Significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol. 2012;25:854–858. doi: 10.1038/modpathol.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endo M, de Graaff MA, Ingram DR, Lim S, Lev DC, Briaire-de Bruijn IH, Somaiah N, Bovée JV, Lazar AJ, Nielsen TO. NY-ESO-1 (CTAG1B) expression in mesenchymal tumors. Mod Pathol. 2015;28:587–595. doi: 10.1038/modpathol.2014.155. [DOI] [PubMed] [Google Scholar]
- 38.Ayyoub M, Taub RN, Keohan ML, Hesdorffer M, Metthez G, Memeo L, Mansukhani M, Hibshoosh H, Hesdorffer CS, Valmori D. The frequent expression of cancer/testis antigens provides opportunities for immunotherapeutic targeting of sarcoma. Cancer Immun. 2004;4:7. [PubMed] [Google Scholar]
- 39.Laduron S, Deplus R, Zhou S, Kholmanskikh O, Godelaine D, De Smet C, Hayward SD, Fuks F, Boon T, De Plaen E. MAGE-A1 interacts with adaptor SKIP and the deacetylase HDAC1 to repress transcription. Nucleic Acids Res. 2004;32:4340–4350. doi: 10.1093/nar/gkh735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peikert T, Specks U, Farver C, Erzurum SC, Comhair SA. Melanoma antigen A4 is expressed in non-small cell lung cancers and promotes apoptosis. Cancer Res. 2006;66:4693–4700. doi: 10.1158/0008-5472.CAN-05-3327. [DOI] [PubMed] [Google Scholar]
- 41.Bhan S, Chuang A, Negi SS, Glazer CA, Califano JA. MAGEA4 induces growth in normal oral keratinocytes by inhibiting growth arrest and apoptosis. Oncol Rep. 2012;28:1498–1502. doi: 10.3892/or.2012.1934. [DOI] [PubMed] [Google Scholar]
- 42.Woloszynska-Read A, Mhawech-Fauceglia P, Yu J, Odunsi K, Karpf AR. Intertumor and intratumor NY-ESO-1 expression heterogeneity is associated with promoter-specific and global DNA methylation status in ovarian cancer. Clin Cancer Res. 2008;14:3283–3290. doi: 10.1158/1078-0432.CCR-07-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang SJ, Soria JC, Wang L, Hassan KA, Morice RC, Walsh GL, Hong WK, Mao L. Activation of melanoma antigen tumor antigens occurs early in lung cancer carcinogenesis. Cancer Res. 2001;61:7959–7963. [PubMed] [Google Scholar]
- 44.Cornejo K, Shi M, Jiang Z. Oncofetal protein IMP3: A useful diagnostic biomarker for leiomyosarcoma. Hum Pathol. 2012;43:1567–1572. doi: 10.1016/j.humpath.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Mueller-Pillasch F, Pohl B, Wilda M, Lacher U, Beil M, Wallrapp C, Hameister H, Knöchel W, Adler G, Gress TM. Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech Dev. 1999;88:95–99. doi: 10.1016/S0925-4773(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–1270. doi: 10.1128/MCB.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]