Abstract
Sialyl Lewisx (SLX) is a carbohydrate ligand for endothelial selectin that participates in cell adhesion, proliferation and scattering. It plays an important role in cancer cell adhesion to vascular endothelial cells, leading to hematogenous metastasis. The prognostic significance of SLX expression level at the invasive front in patients with stage II colorectal cancer (CRC) was examined. A total of 209 patients with stage II CRC curatively resected between 1997 and 2000 were enrolled. The preoperative serum SLX levels measured by radioimmunoassay and SLX immunoexpression levels at the invasive front, and at the non-invasive frontal region determined by tissue microarray were analyzed. SLX expression at the invasive front was positively associated with tumor invasion depth (P=0.007) and tumor budding grade (P=0.038). Disease-free survival curves differed between the high and low SLX-expression groups (5-year survival rates, 77.0 and 89.7%, respectively; P=0.036). Liver cancer recurrence was more frequent in the high-expression group than in the low-expression group (15.9 and 2.4%; P=0.002). Multivariate analysis revealed that its expression (hazard ratio, 5.26; P=0.015) and venous invasion (hazard ratio, 4.14; P=0.040) were independent predictive markers of liver cancer recurrence. Neither the preoperative serum SLX level nor SLX expression at the non-invasive frontal region showed any association with histopathological features or disease-free survival. SLX expression level at the invasive front is a promising marker for identifying patients with stage II CRC with a high risk of liver cancer recurrence.
Keywords: carcinoma, colorectal cancer, Sialyl Lewisx antigen, prognostic factor, tissue array analysis
Introduction
The biological activity of colorectal cancer (CRC) is most accurately reflected by histological characteristics at the invasive front. One parameter of CRC activity is tumor budding, which represents de-differentiation of epithelial cells into more invasive phenotypes in a process known as the epithelial-mesenchymal transition (EMT) (1–3). Tumor budding at the invasive margin has been demonstrated to involve molecules such as Laminin-5, CD44, and L1 cell adhesion molecule (L1CAM) and is associated with poor prognosis in CRC (4–8). Although an association with hematogeneous metastasis has also been documented, the underlying molecular mechanism is not known.
Tissue microarray (TMA) is a technique for high through-put evaluation of protein expression in a large number of archival tissue blocks used for routine histopathological diagnosis. A cohort of tissue core specimens obtained from original tissue blocks are arranged into a single recipient paraffin block (9). TMA analysis efficiently screens for molecular alterations in a large number of cases. Using TMA, we have shown that protein expression levels in CRC are heterogeneous and that the findings from core specimens taken from the invasive front most precisely reflect tumor aggressiveness (6).
Sialyl Lewisx (SLX) is known to be a carbohydrate ligand for endothelial-selectin (E-selectin) expressed on vascular endothelial cells. SLX expression in cancer cells promotes their adhesion to vascular endothelial cells, and several clinical studies show that SLX expression in cancer tissues plays a role in hematogenous metastasis and cancer prognosis (10–13). In previous reports, SLX expression was evaluated by microscopic examination of a slide that was representative of the whole tumor. SLX immunoexpression is shown to be heterogeneous between different areas in a tumor and is higher at the invasive front (12,14,15). Hence, evaluation of tumor cells at the invasive front is considered to be important for revealing the role of SLX expression in CRC metastasis.
This study aims to investigate the association between SLX expression at the invasive front of tumors and the prognosis of Stage II CRC using TMA analysis. In addition, we compare the prognostic significance of SLX expression at the invasive front with that of other tumor regions and examine the prognostic significance of preoperative serum SLX concentrations.
Materials and methods
This study was conducted after obtaining approval from the internal review board of the National Defense Medical College hospital (Tokorozawa, Japan). A consecutive series of 314 patients with Stage II CRC who underwent a potentially curative resection between January 1997 and December 2000 was derived from the files of the Department of Surgery of the National Defense Medical College. Serum SLX levels were measured preoperatively by radioimmunoassay (RIA) in the 209 patients who were enrolled in this study. Serum SLX levels were divided into two categories based on the upper limit of the normal range (38 U/ml): Normal SLX, any value ≤38 U/ml; high SLX, any value >38 U/ml. Clinicopathological features were assessed according to the 2nd edition of the Japanese classification of CRC (16). We defined a focus of tumor budding as an isolated single cancer cell or a cluster composed of fewer than five cancer cells and then classified these cancers based on the number of foci found in a ×200 microscopic field of hematoxylin and eosin (H&E) stained section as follows: G1, 0–4 foci; G2, 5–9 foci; G3, 10 or more foci (2). We showed tumor budding expressing SLX in Fig. 1. Disease-free survival (DFS) was defined as the time from surgery to the first event of either recurrence disease or death. Cancer-specific survival (CSS) was defined as the time from surgery to death from CRC recurrence. The word ‘recurrence’ was used in this report to denote metachronous metastasis at the same site or in another location.
Figure 1.
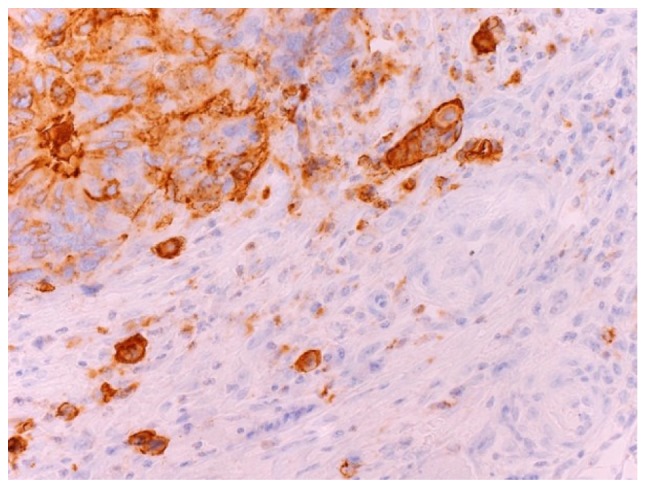
Microscopic appearance of tumor budding cancer cells expressing Sialyl Lewisx under ×400 magnification.
TMA construction and immunohistochemical staining
We first identified two regions of the invasive front (submucosal and subserosal) and two regions of non-invasive frontal lesions (central or superficial tumor area) with viable cancer cells by referring to an H&E stained whole section microscopically. To construct a TMA block, a single tissue core (2-mm diameter) was taken from each region in formalin-fixed paraffin-embedded CRC tissue blocks (‘donor’ blocks) using a Tissue Microarrayer (Beecher Instruments, Silver Spring, MD, USA) and was transferred to the ‘recipient’ blocks (TMA blocks). TMA blocks were cut (4-mm-thick slices) and deparaffinized using standard histological techniques. These sections were deparaffinized with xylene and rehydrated with ethanol. Antigen retrieval was performed in an autoclave (121°C, 15 min). Endogenous peroxidase activity was blocked using 5% H2O2. The sections were incubated in 10% normal goat serum to block nonspecific binding of the antibody and then incubated with an SLX monoclonal antibody (clone KM93; dilution 1:100; Kyowa Medics, Tokyo, Japan) as the primary antibody overnight at 4°C. Subsequently, the sections were incubated with the secondary antibody for 1 h at room temperature, immersed in 0.1% diaminobenzidine tetrahydrochloride (DAB) solution for 5 min, and counterstained with hematoxylin for visualization of the antigen. The negative control was stained using an identical procedure without the primary antibody.
Evaluation of SLX expression
We determined the proportion of stained cancer cells in a whole core of TMA. The distribution of staining was scored as 0 (0–25%), 1 (26–50%), 2 (51–75%), and 3 (76–100%). The sum of the distribution scores from two invasive fronts was used as the final staining score (0–6) for SLX immunoexpression. Final scores of 5 and 6 were considered positive (high SLX expression) because postoperative recurrence and disease-specific mortality rates of patients with a score of 5–6 were extremely high [postoperative recurrence rates, 9.6% (patients with score 0–2), 14.0% (3–4), and 22.7% (5–6); mortality rates, 3.5% (0–2), 6.0% (3–4), and 13.6% (5–6)]. The sum of scores from two non-invasive frontal regions was also used for assessment in the same manner; none of the score ranges had prognostic significance [postoperative recurrence rates: 12.8% (0–2), 15.2% (3–4), and 13.2% (5–6); mortality rates: 11.2% (0–2), 8.7% (3–4), and 7.9% (5–6)]. In addition, we conducted immunohistochemical staining of SLX in 30 standard sections to confirm the integrity of the TMA data. They were classified as either high (≥50%) or low (<50%) grade in terms of the percentage of immunopositive cells among all cancer cells located at the invasive front (the deepest 3 mm width) of the tumor. Immunohistochemical staining was independently evaluated by two observers (MY and ES); in cases of discrepancy, a consensus was reached after re-evaluation.
Followup
All 209 patients received regular follow-up care at our outpatient clinic. Physical examination, serum carcinoembryonic antigen (CEA) levels, and carbohydrate antigen (CA) 19-9 levels were monitored every 3 months. Contrast computed tomography (CT) scan was performed every 6 months, and colonoscopy was performed biannually. Whenever any findings suggestive of cancer relapse did not appear after 5 years, the follow-up procedure was changed to an annual physical without any other detailed examinations. At the date of the last followup, 32 patients had died, with a median time from surgery to death of 44.5 months (range, 13.5–121.6 months). Of these, 19 died from CRC recurrence, 5 died from other carcinomas, and 8 died from other reasons or from unclear causes. The median follow-up period for the survivors (n=177) was 62.9 months (range, 38.8–133.3 months). Adjuvant chemotherapy was administered to 13% of all patients (27/209) after curative surgery.
Statistical analysis
Comparisons between groups were performed using the χ2 test or Fisher's exact method. Survival was analyzed according to the Kaplan-Meier product limit method. The significance of differences was determined using the log-rank test. Covariates with trend-significant effects (P<0.10) on univariate analysis were selected for multivariate analysis of the factors for survival using Cox's proportional hazard model and for postoperative recurrence using a logistic regression model. Statistical analysis used JMP version 11, and statistical significance was considered as P<0.05. The degree of interobserver agreement for the evaluation of immunoreactivity was measured using the generalized κ test for two or more observers. In accordance with the criteria of Landis and Koch (17), κ values were assigned a strength of agreement score of poor (<0.00), slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and near perfect (0.81–1.00).
Results
Interobserver agreement
Characteristic microscopic appearances of SLX-positive and SLX-negative TMA specimens of CRCs are shown in Fig. 2, respectively. The level of interobserver agreement for evaluation of SLX immunostaining was substantial [82.3% (κ=0.63)]. We showed the association of pair scores of SLX expression at two invasive frontal regions in Table I. SLX expression had heterogeneity even at the invasive frontal region. The proportion of the cases with the gap of 2 or more score was 24%. SLX expression at the invasive front in TMA was in good agreement with that in standard section in 26 of 30 cases (87%).
Figure 2.
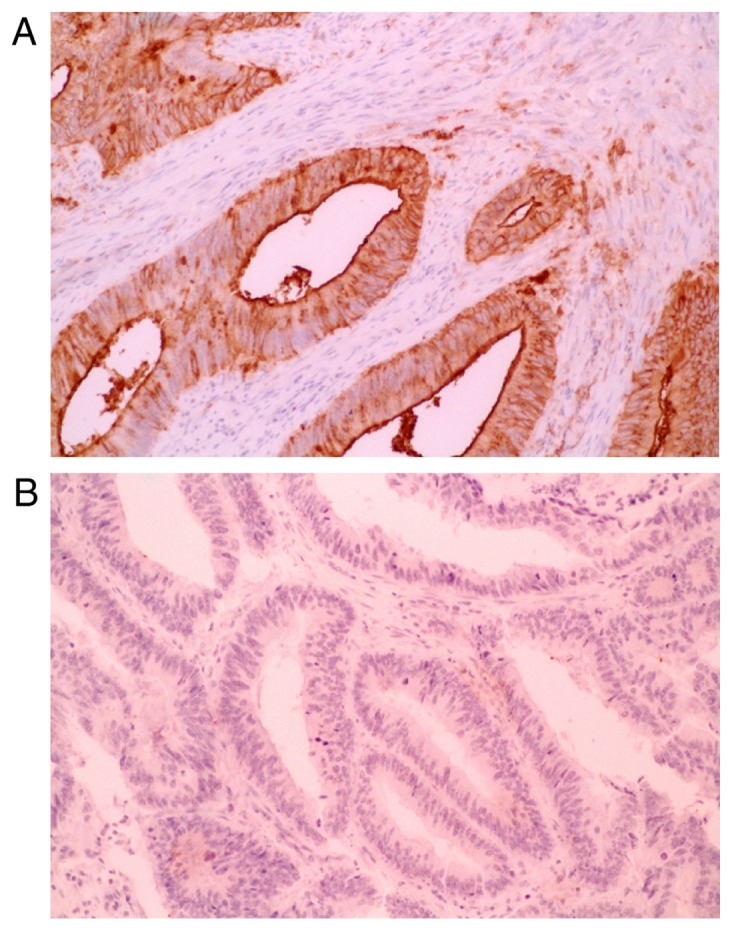
Sialyl Lewisx expression in colorectal tumors under ×200 magnification. A tumor with (A) positive and (B) negative cytoplasmic staining.
Table I.
Association of pair scores of Sialyl Lewisx expression at two invasive frontal regions.
| Distribution score (subserosal) | ||||
|---|---|---|---|---|
| Distribution score (submucosal) | 0 (%) | 1 (%) | 2 (%) | 3 (%) |
| 0 | 48 (23) | 12 (6) | 10 (5) | 7 (3) |
| 1 | 13 (6) | 7 (3) | 6 (3) | 10 (5) |
| 2 | 6 (3) | 7 (3) | 21 (10) | 2 (1) |
| 3 | 7 (3) | 11 (5) | 3 (2) | 39 (19) |
Relationship to survival
The serum SLX level was high in 9% of all subjects, whereas high tissue expression of SLX at the invasive front and non-invasive frontal region were observed in 21 and 18%, respectively.
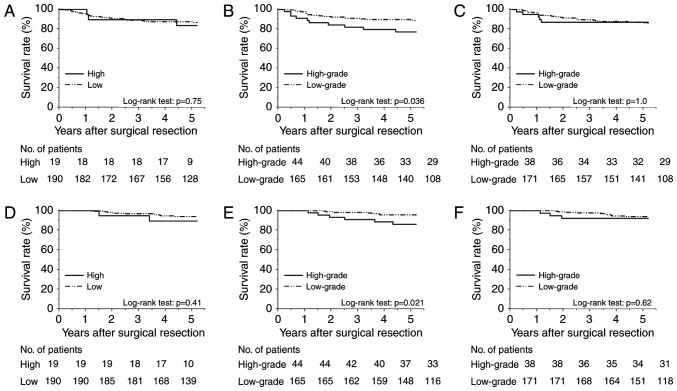
Fig. 3A-C show the Kaplan-Meier DFS curves, and Fig. 3D-F display the CSS curves according to the preoperative serum SLX levels, SLX expression levels at the invasive front, and those at non-invasive frontal regions, respectively. The DFS and CSS of the high serum-SLX group did not differ significantly from those of the low serum-SLX group (5-year DFS rates, 83.5 and 87.3%; P=0.75; 5-year CSS rates, 89.5 and 94.0%; P=0.41). In contrast, the group with high SLX expression at the invasive front had poorer prognoses than did the low-expression group (5-year DFS rates, 77.0 and 89.7%; P=0.036; 5-year CSS rates, 86.1 and 95.6%; P=0.021). High SLX expression at non-invasive frontal regions did not correlate with shorter survivals (5-year DFS rates, 86.8 and 86.2%; P=1.0; 5-year CSS rates, 92.1 and 93.9%; P=0.62). These data indicate the superiority of the invasive front for clinically relevant evaluation of SLX expression.
Figure 3.
Kaplan-Meier survival curves for 209 patients with Stage II colorectal cancer. Disease-free survival curves stratified by (A) preoperative serum Sialyl Lewisx (SLX) level, (B) SLX expression at the invasive front, and (C) SLX expression at the non-invasive frontal regions. Cancer specific survival curves stratified by (D) preoperative serum SLX level, (E) SLX expression at the invasive front, and (F) SLX expression at the non-invasive frontal regions.
Univariate analyses of DFS indicate that lymphatic invasion (P<0.001), venous invasion (P=0.023), tumor budding (P=0.001), serum CEA level (P=0.022), SLX expression at the invasive front (P=0.036), the number of retrieved lymph nodes (P=0.073), and the depth of tumor invasion (P=0.090) are prognostically significant or marginally significant. Using these factors as variables, multivariate analysis revealed that lymphatic invasion (hazard ratio [HR] 2.8; P=0.028) and tumor budding (HR 2.6; P=0.021) are independent prognostic factors.
Relationship to postoperative recurrence
Of the entire cohort, 28 patients (13.4%) had postoperative recurrence. The overall recurrence rate for the group with high SLX expression at the invasive front was higher than that of the group with low SLX expression (22.7 vs. 10.9%; P=0.041) (Table II). Regarding sites of recurrence, liver cancer recurrence was more frequent among patients with high SLX expression at the invasive front than among those with low SLX expression (15.9 vs. 2.4%; P=0.002). We observed no statistically significant differences between groups with respect to primary site, lung, or peritoneal recurrence. Univariate analyses revealed that liver cancer recurrence is associated with venous invasion, tumor budding, serum CEA level, and SLX expression at the invasive front (Table III). Further, multivariate analysis revealed that venous invasion and SLX expression at the invasive front are independent factors for postoperative liver cancer recurrence. Patients with both moderate to severe venous invasion and high SLX expression at the invasive front had a significantly higher liver cancer recurrence rate (25.0 vs. 3.6%; P=0.006) and a significantly poorer prognosis (5-year DFS, 62.5 vs. 89.1%; P<0.001) compared with the other patients.
Table II.
Correlation between postoperative recurrence and degree of SLX staining at the invasive front.
| Degree of SLX staining, no (%) | ||||
|---|---|---|---|---|
| Recurrence | Total (n=209) | High expression (n=44) | Low expression (n=165) | P-value |
| Overall | 28 (13.4) | 10 (22.7) | 18 (10.9) | 0.041 |
| Primary recurrence sitea | ||||
| Local | 11 (5.3) | 4 (9.1) | 7 (4.2) | 0.25 |
| Liver | 11 (5.3) | 7 (15.9) | 4 (2.4) | 0.002 |
| Lung | 10 (4.8) | 4 (9.1) | 6 (3.6) | 0.22 |
| Peritoneum | 3 (1.4) | 2 (4.6) | 1 (0.6) | 0.11 |
| Other organs | 3 (1.4) | 1 (2.3) | 2 (1.2) | 0.51 |
Primary recurrence not limited to a single organ. SLX, Sialyl Lewisx.
Table III.
Univariate and multivariate analyses of risk factors influencing liver recurrence in patients with colorectal cancer.
| Univariate analysis | Mutivariate analysis by logistic regression model | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Comparison | Hazard ratio | 95% confidence interval | P-value | Hazard ratio | 95% confidence interval | P-value |
| Depth of tumor invasion | |||||||
| T4 | T3 | 2.49 | 0.62–8.68 | 0.18 | |||
| Histologic type | |||||||
| Por/Muca | Wel/Modb | 0.78 | 0.06–18.15 | 1.00 | |||
| Lymphatic invasion | |||||||
| Moderate to severe | No or minimal | 1.87 | 0.27–7.90 | 0.47 | |||
| Venous invasion | |||||||
| Moderate to severe | No or minimal | 4.67 | 1.31–16.58 | 0.016 | 4.14 | 1.07–18.15 | 0.040 |
| Tumor buddingc | |||||||
| G3 | G1/G2 | 3.51 | 1.02–12.11 | 0.036 | 1.56 | 0.35–6.27 | 0.54 |
| Serum CEA level | |||||||
| >5.0 ng/ml | ≤5.0 ng/ml | 4.91 | 1.38–17.47 | 0.013 | 3.20 | 0.81–13.94 | 0.097 |
| The number of sampled lymph nodes | |||||||
| <12 | ≥12 | 2.06 | 0.52–7.15 | 0.28 | |||
| SLX expression at the invasive front | |||||||
| High | Low | 7.61 | 2.12–27.37 | 0.002 | 5.26 | 1.39–22.27 | 0.015 |
Well to moderately differentiated tubular adenocarcinoma.
Poorly differentiated adenocarcinoma or mucinous carcinoma.
Grade of tumor budding: G1, 0–4 foci; G2, 5–9 foci; and G3, 10 or more foci (per ×200 microscopic field). SLX, Sialyl Lewisx.
Relationship to clinicopathological findings (Table IV)
Table IV.
Clinicopathological features and their correlations with degree of SLX staining at the invasive front.
| Degree of SLX staining, no. (%) | ||||
|---|---|---|---|---|
| Characteristic | Total (n=209) | High expression (n=44) | Low expression (n=165) | P-value |
| Age, years | ||||
| <70 | 128 | 31 (24) | 97 (76) | 0.15 |
| ≥70 | 81 | 13 (16) | 68 (84) | |
| Sex | ||||
| Male | 125 | 27 (22) | 98 (78) | 0.81 |
| Female | 84 | 17 (20) | 67 (80) | |
| Tumor location | ||||
| Colon | 152 | 29 (19) | 123 (81) | 0.25 |
| Rectum | 57 | 15 (26) | 42 (74) | |
| Tumor size | ||||
| <45 mm | 86 | 20 (23) | 66 (77) | 0.51 |
| ≥45 mm | 123 | 24 (20) | 99 (80) | |
| Depth of tumor invasion | ||||
| T3 | 168 | 29 (17) | 139 (83) | 0.007 |
| T4 | 41 | 15 (37) | 26 (63) | |
| Histologic type | ||||
| Wel/Moda | 199 | 42 (21) | 157 (79) | 1.00 |
| Por/Mucb | 10 | 2 (20) | 8 (80) | |
| Lymphatic invasion | ||||
| No or minimal | 186 | 36 (19) | 150 (81) | 0.087 |
| Moderate to severe | 23 | 8 (35) | 15 (65) | |
| Venous invasion | ||||
| No or minimal | 148 | 28 (19) | 120 (81) | 0.24 |
| Moderate to severe | 61 | 16 (26) | 45 (74) | |
| Tumor buddingc | ||||
| G1/G2 | 166 | 30 (18) | 136 (82) | 0.038 |
| G3 | 43 | 14 (33) | 29 (67) | |
| Number of retrieved lymph nodes | ||||
| ≥12 | 162 | 30 (19) | 132 (81) | 0.095 |
| <12 | 47 | 14 (30) | 33 (70) | |
| Serum CEA level | ||||
| Low (≤5.0 ng/ml) | 150 | 22 (14) | 128 (86) | <0.001 |
| High (>5.0 ng/ml) | 59 | 22 (37) | 37 (63) | |
| Serum SLX level | ||||
| Low (≤38 U/ml) | 190 | 34 (18) | 156 (82) | <0.001 |
| High (>38 U/ml) | 19 | 10 (33) | 9 (67) | |
| SLX at non-invasive frontal region | ||||
| Low expression | 171 | 18 (41) | 153 (93) | <0.001 |
| High expression | 38 | 26 (59) | 12 (7) | |
Well to moderately differentiated tubular adenocarcinoma.
Poorly differentiated adenocarcinoma or mucinous carcinoma.
Grade of tumor budding: G1, 0–4 foci; G2, 5–9 foci; and G3, 10 or more foci (per ×200 microscopic field). SLX, Sialyl Lewisx.
Tumors from the group with high SLX expression at the invasive front showed more aggressive properties than did those from the low SLX-expression group with respect to the depth of tumor invasion (pT4, 34 vs. 16%; P=0.007), grade of tumor budding (G3, 32 vs. 18%; P=0.038), and the serum CEA level (CEA >5.0 ng/ml, 50 vs. 22%; P<0.001). A strong correlation was observed between the serum SLX concentration and the level of tissue SLX protein expression at the invasive front (P<0.001).
Discussion
This study indicates that SLX expression at the tumor invasive front is significantly associated with the depth of tumor invasion, the grade of tumor budding, and the CEA level. Univariate analysis of DFS revealed that the SLX expression level is a significant prognostic factor. With respect to the site of postoperative recurrence, SLX expression level associates strongly with liver cancer recurrence but not recurrence in the primary site, lung, or peritoneum. Thus, SLX expression might be a specific predictive marker of postoperative liver cancer recurrence in Stage II CRC.
Hematogenous metastasis of CRC is known to be a multistep process. Membrane-type 1 matrix metalloproteinase (MT1-MMP) is a membrane-anchored zinc-binding endopeptidase that is expressed at the leading edge of various invasive carcinomas. MT1-MMP degrades the basement membrane, and once in direct contact with the stroma, cleaves pro-MMP-2 made by stromal cells, converting it to an active protease. Subsequently, the activated MMP-2 dissolves the collagen I network and creates a channel in front of the carcinoma cell that allows it to invade more deeply into the stroma (18). Furthermore, it has been revealed that cancer cells in the invasive front acquire the cell motility by activating proteins like Rho-family and are prone to invade blood vessels (19). Through these steps, the cancer cells at the invasive front show migration across the stroma and approaching blood vessels. When cancer cells overexpressing SLX in the invasive front invade a blood vessel, they circulate to the liver, lung, and other distant organs. Ligand-receptor interactions between CAs and cell adhesion molecules of the selectin family are proposed to play a role in the preparation of metastatic foci at distant organs. Overexpressed SLX on cancer cells gives rise to their weak adhesion to selectins on endothelial cells and prepares their stronger adhesion and transmigration outside the blood vessel through integrin, CD44, CXCR4, and their receptor systems (20–22). Accordingly, circulating cancer cells overexpressing SLX increase the likelihood of forming a metastatic lesion. This study reveals that SLX expression at the invasive front is an independent risk factor for postoperative liver cancer recurrence of Stage II CRC. This finding is similar to that for the venous invasion level, which well supports the theory that hematogenous metastasis occurs through the adhesion of cancer-expressing SLX to E-selectin on the surface of endothelial cells.
Our results are consistent with a previous report by Akamine et al (11) showing that CRC patient prognosis is rarely associated with serum SLX level but significantly associated with SLX expression level in tumor tissues. Nakagoe et al (23,24) reported that the level of serum SLX from the drainage vein of a tumor was significantly higher than that of a peripheral vein, and the concentration of SLX in the tumor's drainage vein but not the peripheral vein is associated with CRC prognosis. We propose that the measurement of SLX through a typical blood test is of little meaning for predicting postoperative recurrence during the surveillance period.
A number of meta-analyses indicate that intensive follow-up examinations are associated with a favorable prognosis in CRC patients (25). Early detection and treatment of postoperative recurrences likely contribute to better survival. As for CRC, liver cancer recurrence occurs frequently, and 29% of patients will develop liver metastases within 3 years of diagnosis (26). Meanwhile, patient survival after curative resection of liver cancer recurrence has improved dramatically, with 5-year survival ranging from 25 to 74% (27,28). Consequently, it is worthwhile to identify the patients at highest risk of postoperative liver cancer recurrence and to perform intensive examinations during surveillance of these patients. Increasing evidence indicates that unfavorable features such as T4 lesions, poorly differentiated histology, the number of sampled lymph nodes (<12), venous invasion, lymphatic invasion, perineural invasion, ileus or perforation as an initial symptom, and high serum CEA level (>5.0 ng/ml) are risk factors for postoperative recurrence in Stage II CRC (29–31). Although information on perineural invasion and initial symptoms could not be collected here, this study reveals that the levels of SLX expression at the invasive front and venous invasion are key predictive factors of postoperative liver cancer recurrence. Thus, we recommend intensive followup for patients whose tumors show evidence of these two factors.
This study has several potential limitations. First, the TMA method is not generally used in routine pathological diagnosis; thus, it is necessary to determine whether similar results are obtained from examinations using standard sections. Second, as this was a retrospective study with limited cases, the verification of our results requires a large-scale prospective study. Nonetheless, this is the first report to clearly show that SLX expression in the invasive front of CRC is a predictor of hematogeneous metastasis, especially liver recurrence.
In conclusion, the present study demonstrates the important role of SLX expression at the invasive front as an independent liver recurrence predictor in Stage II CRC. The level of SLX expression also correlates with the serum SLX concentration, the depth of tumor invasion, and the grade of tumor budding. The clinical application of these findings could identify patients at high risk for postoperative liver cancer recurrence, allowing for the selection of patients for whom intensive followup is most beneficial.
Acknowledgements
This study was supported in part by the Japan Society for the Promotion of Science KAKENHI (grant nos. 23501302, 25462074, and 26462032).
Glossary
Abbreviations
- SLX
Sialyl Lewisx
- E-selectin
endothelial selectin
- CRC
colorectal cancer
- TMA
Tissue microarray
- RIA
radioimmunoassay
- H&E
hematoxylin and eosin
- DAB
diaminobenzidine tetrahydrochloride
- CEA
carcinoembryonic antigen
- CA
carbohydrate antigen
- CT
computed tomography
- DFS
disease-free survival
- CSS
cancer-specific survival
- HR
hazard ratio
- MT1
membrane-type 1
- MMP
matrix metalloproteinase
References
- 1.Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004;240:832–839. doi: 10.1097/01.sla.0000143243.81014.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumor ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–132. doi: 10.1046/j.1365-2559.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 3.Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor ‘budding℉ in patients with colorectal cancer. Dis Colon Rectum. 1993;36:627–635. doi: 10.1007/BF02238588. [DOI] [PubMed] [Google Scholar]
- 4.Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, Sheahan K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer. 2016;115:831–840. doi: 10.1038/bjc.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukazawa S, Shinto E, Tsuda H, Ueno H, Shikina A, Kajiwara Y, Yamamoto J, Hase K. Laminin β3 expression as a prognostic factor and a predictive marker of chemoresistance in colorectal cancer. Jpn J Clin Oncol. 2015;45:533–540. doi: 10.1093/jjco/hyv037. [DOI] [PubMed] [Google Scholar]
- 6.Shinto E, Tsuda H, Ueno H, Hashiguchi Y, Hase K, Tamai S, Mochizuki H, Inazawa J, Matsubara O. Prognostic implication of laminin-5 gamma 2 chain expression in the invasive front of colorectal cancers, disclosed by area-specific four-point tissue microarrays. Lab Invest. 2005;85:257–266. doi: 10.1038/labinvest.3700199. [DOI] [PubMed] [Google Scholar]
- 7.Masaki T, Goto A, Sugiyama M, Matsuoka H, Abe N, Sakamoto A, Atomi Y. Possible contribution of CD44 variant 6 and nuclear beta-catenin expression to the formation of budding tumor cells in patients with T1 colorectal carcinoma. Cancer. 2001;92:2539–2546. doi: 10.1002/1097-0142(20011115)92:10<2539::AID-CNCR1605>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Kajiwara Y, Ueno H, Hashiguchi Y, Shinto E, Shimazaki H, Mochizuki H, Hase K. Expression of l1 cell adhesion molecule and morphologic features at the invasive front of colorectal cancer. Am J Clin Pathol. 2011;136:138–144. doi: 10.1309/AJCP63NRBNGCTXVF. [DOI] [PubMed] [Google Scholar]
- 9.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 10.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akamine S, Nakagoe T, Sawai T, Tsuji T, Tanaka K, Hidaka S, Shibasaki S, Nanashima A, Yamaguchi H, Nagayasu T, Yasutake T. Differences in prognosis of colorectal cancer patients based on the expression of sialyl Lewisa, sialyl Lewisx and sialyl Tn antigens in serum and tumor tissue. Anticancer Res. 2004;24:2541–2546. [PubMed] [Google Scholar]
- 12.Nakagoe T, Fukushima K, Tanaka K, Sawai T, Tsuji T, Jibiki M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Arisawa K. Evaluation of sialyl Lewis(a), sialyl Lewis(x), and sialyl Tn antigens expression levels as predictors of recurrence after curative surgery in node-negative colorectal cancer patients. J Exp Clin Cancer Res. 2002;21:107–113. [PubMed] [Google Scholar]
- 13.Hoff SD, Matsushita Y, Ota DM, Cleary KR, Yamori T, Hakomori S, Irimura T. Increased expression of sialyl-dimeric LeX antigen in liver metastases of human colorectal carcinoma. Cancer Res. 1989;49:6883–6888. [PubMed] [Google Scholar]
- 14.Schiffmann L, Schwarz F, Linnebacher M, Prall F, Pahnke J, Krentz H, Vollmar B, Klar E. A novel sialyl Le(X) expression score as a potential prognostic tool in colorectal cancer. World J Surg Oncol. 2012;10:95. doi: 10.1186/1477-7819-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono M, Sakamoto M, Ino Y, Moriya Y, Sugihara K, Muto T, Hirohashi S. Cancer cell morphology at the invasive front and expression of cell adhesion-related carbohydrate in the primary lesion of patients with colorectal carcinoma with liver metastasis. Cancer. 1996;78:1179–1186. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1179::AID-CNCR3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Japanese Society for Cancer of the Colon and Rectum, corp-author. Japanese Classification of Colorectal Carcinoma. 2nd. Kanehara & Co., Ltd.; Tokyo: 2009. [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg RA. The biology of cancer. 2nd. Garland Science; New York, NY: 2014. pp. 685–689. [Google Scholar]
- 19.Leber MF, Efferth T. Molecular principles of cancer invasion and metastasis (Review) Int J Oncol. 2009;34:881–895. doi: 10.3892/ijo_00000214. [DOI] [PubMed] [Google Scholar]
- 20.Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, Kannagi R. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53:354–361. [PubMed] [Google Scholar]
- 21.Gassmann P, Haier J, Schlüter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B, Müller A. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia. 2009;11:651–661. doi: 10.1593/neo.09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujisaki T, Tanaka Y, Fujii K, Mine S, Saito K, Yamada S, Yamashita U, Irimura T, Eto S. CD44 stimulation induces integrin-mediated adhesion of colon cancer cell lines to endothelial cells by up-regulation of integrins and c-Met and activation of integrins. Cancer Res. 1999;59:4427–4434. [PubMed] [Google Scholar]
- 23.Nakagoe T, Sawai T, Tsuji T, Jibiki M, Ohbatake M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Tagawa Y. Differences in release mechanisms and distributions for sialyl Le(a) and sialyl Le(x) antigens in colorectal cancer. Ann Surg Oncol. 2000;7:289–295. doi: 10.1007/s10434-000-0289-1. [DOI] [PubMed] [Google Scholar]
- 24.Nakagoe T, Sawai T, Tsuji T, Jibiki M, Ohbatake M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H, Arisawa K. Prognostic value of serum sialyl Lewis(a), sialyl Lewis(x) and sialyl Tn antigens in blood from the tumor drainage vein of colorectal cancer patients. Tumour Biol. 2001;22:115–122. doi: 10.1159/000050605. [DOI] [PubMed] [Google Scholar]
- 25.Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal cancer resection. Lancet. 2000;355:395–399. doi: 10.1016/S0140-6736(99)06552-6. [DOI] [PubMed] [Google Scholar]
- 26.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–474. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 27.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D'Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: Trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–755. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- 30.Schmoll HJ, Van Custem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 31.Benson AB, III, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]