Abstract
The aim of the present study was to investigate the effect of a hypoxic environment on the biological behavior of breast cancer MCF-7 cells, using CoCl2 to mimic the hypoxia model in breast cancer cells. Using 50, 100, 150 and 200 µM CoCl2 as a hypoxic inducer, a hypoxic model was established in MCF-7 cells in vitro. MTT, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and western blotting assays were performed to detect MCF-7 cell proliferation under hypoxic conditions and the expression of the hypoxic markers hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF) and C-X-C motif chemokine receptor 4 (CXCR4) mRNA and that of the associated proteins. The RT-qPCR results revealed that there were no obvious changes in the expression of HIF-1α mRNA; however, the expression of CXCR4 and VEGF mRNA increased significantly following treatment with different CoCl2 concentrations (P<0.05). The results of western blotting identified that CoCl2 significantly induced the expression of HIF-1α, CXCR4 and VEGF proteins (P<0.05). The MTT assay revealed that different concentrations of CoCl2 inhibited the proliferation of MCF-7 cells. The TUNEL assay demonstrated that CoCl2 was able to trigger apoptosis of MCF-7 cells. Therefore, the results of the present study identified that CoCl2 is able to control MCF-7 cell proliferation and apoptosis, also increasing the expression of HIF-1α, CXCR4 and VEGF. The present study may aid the discovery of a novel method to prevent cell damage and decrease cell proliferation in order to prevent the occurrence and development of breast cancer.
Keywords: breast cancer, hypoxia model, MCF-7 cell, cell proliferation, CoCl2, hypoxia-inducible factor-1α, vascular endothelial growth factor, C-X-C motif chemokine receptor 4
Introduction
Hypoxia refers to a decrease in the concentration of oxygen available and the decrease in the pressure of oxygen below normal range. Hypoxia is able to limit and even halt the physiological function of organs, organisms and cells (1). Tumor cells commonly induce hypoxic conditions, owing to the rapid growth of tumor cells and the relatively limited blood supply in tumors (2,3). Clinical d experimental research indicates that the hypoxic tumor environment may be associated with the development and metastasis of solid tumors (4,5).
Breast cancer is the most common type of cancer in women (6). The pathological grade and prognosis of breast cancer are directly associated with tumor hypoxia (7). Therefore, the study of hypoxic microenvironments may have a crucial function in targeted therapy adopted by clinics in the future. Hypoxia-inducible factor-1α (HIF-1α) is an essential transcriptional regulatory factor in hypoxia microenvironments and has 100 types of downstream gene, including cell proliferation, angiogenesis and energy metabolism (8,9). A previous study indicated that the expression of HIF-1α, C-X-C motif chemokine receptor 4 (CXCR4) and vascular endothelial growth factor (VEGF) is involved in tumor progression, angiogenesis, metastasis and survival, and their expression may be induced by hypoxia (10).
Understanding how to mimic a precise hypoxic environment in vitro and establish a reliable easy-to-operate model of hypoxia is the first step in studying the hypoxic tumor microenvironment. Cobalt ions are substrates of the iron-chelating enzymes; they can substitute for the iron ions of the oxygen sensor hemoglobin and combine with oxygen at high concentrations, leading to molecules entering the deoxidization phase (11). As has been documented in previous studies, treatment with CoCl2 is able to mimic hypoxia (12). In the present study, different concentrations of CoCl2 and MCR-7 breast cancer cells were cultured together in vitro to find the optimal hypoxia model. The changes in the biological behavior of breast cancer cells in a hypoxic microenvironment were examined and the effects of CoCl2 on the MCR-7 cell proliferation of breast cancer and the tumor angiogenesis factor investigated.
Materials and methods
Cell culture
The breast cancer cell line MCF-7 was purchased from Shanghai Bo Valley Biological Technology Co., Ltd. (Shanghai, China), and incubated in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum, 100 U/ml penicillin and 0.1 mg/ml streptomycin at 37°C with 5% CO2. CoCl2 was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). MCF-7 cells in the exponential phase were used for further detection. Different CoCl2 concentrations were added to DMEM. Cells were incubated with 50, 100, 150 and 200 µM CoCl2 for different periods of time [0, 24, 48 and 72 h for MTT assay; 24 h for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay], with an equivalent volume of PBS added to the control group. The morphological changes of MCF-7 cells were observed using an inverted phase-contrast microscope following treatment with CoCl2.
MTT assay
MCF-7 cells (1×103) were seeded in 96-well plates and cultured overnight. Subsequently, the culture medium was removed and fresh DMEM containing the aforementioned concentrations of CoCl2 was added. Cells were then incubated for different periods of time, and the culture medium was removed and replaced with fresh DMEM with different concentrations of CoCl2 as previously used. MTT solution (5 mg/ml, 10 µl) was added to each well prior to incubation for 4 h. Next, culture medium was removed and 100 µl dimethylsulfoxide was added to dissolve the formazan crystals. The absorbance value was determined at 490 nm.
RT-qPCR analysis
RT-qPCR was performed to quantitatively estimate the changes in the expression of HIF-1α, CXCR4 and VEGF mRNA in MCF-7 cells treated with the aforementioned CoCl2 concentrations for 24 h. Total RNA was isolated from cells using an RNeasy kit (Sigma-Aldrich; Merck KGaA). The RNA was reverse-transcribed into cDNA using the PrimeScript® First Strand cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan) at 42°C for 15 min, then at 85°C for 5 min. The Maxima® SYBR Green qPCR Master Mix (2X) kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to perform qPCR. Primer sequences for β-actin, HIF-1α, CXCR4 and VEGF are presented in Table I (13,14). The following PCR conditions were used: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec (annealing and extension, respectively). The experiment was performed in triplicate and independently repeated at least twice. RT-qPCR data were normalized and quantified using the 2−ΔΔCq method (15). The relative expression level of HIF-1α, CXCR4 and VEGF mRNA was calculated by determining the ratio between the amount of the gene and β-actin.
Table I.
Primer sequences used in reverse transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence | Product size, bp |
|---|---|---|
| β-actin (13) | 162 | |
| Forward | 5′-GACTTAGTTGCGTTACACCCTTTCT-3′ | |
| Reverse | 5′-GAACGGTGAAGGTGACAGCAGT-3′ | |
| HIF-1α (14) | 150 | |
| Forward | 5′-TCTGGGTTGAAACTCAAGCAACTG-3′ | |
| Reverse | 5′-CAACCGGTTTAAGGACACATTCTG-3′ | |
| CXCR4 (14) | 184 | |
| Forward | 5′-TCTGTGACCGCTTCTACC-3′ | |
| Reverse | 5′- AGGATGAGGATGACTGTGG-3′ | |
| VEGF (14) | 176 | |
| Forward | 5′-TGCTTCTGAGTTGCCCAGGA-3′ | |
| Reverse | 5′-TGGTTTCAATGGTGTGAGGACATAG-3′ |
HIF-1α, hypoxia-inducible factor-1α; CXCR4, C-X-C motif chemokine receptor 4; VEGF, vascular endothelial growth factor.
Western blotting
The protein expression of HIF-1α, CXCR4 and VEGF was assessed by western blotting. MCF-7 cells were treated with the aforementioned CoCl2 concentrations for 24 h and cells were lysed with ice-cold radioimmunoprecipitation assay buffer (150 mM NaCl, 1.0% NP-40, 0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris-HCl, pH 8.0) and protease inhibitors (AEBSF at 2 mM, Aprotinin at 0.3 µM, Bestatin at 116 µM, E-64 at 14 µM, Leupeptin at 1 µM and EDTA at 1 mM; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Protein concentrations were quantified using a Bicinchoninic Acid Protein assay kit. Proteins (30 µg/lane) were separated by SDS-PAGE (10% gels) and transferred onto nitrocellulose membranes. Membranes were blocked for 1 h with 5% non-fat dried milk in Tris-buffered saline containing 20% Tween-20 (TBST) and incubated overnight at 4°C with the primary antibody Following washing with TBST, membranes were incubated for 1 h with secondary antibodies. Labeled protein bands were detected using the enhanced chemiluminescence method (ProteinTech Group, Inc., Chicago, IL, USA). Western blotting was performed three times. The relative expression level of HIF-1α, CXCR4 and VEGF proteins was quantified by densitometry analysis using ImageJ (version 1.6.0; National Institutes of Health, Bethesda, MD, USA) relative to β-actin. The antibodies were as follows: Anti-HIF-1α (ab69836; 1:600), anti-CXCR4 (ab124824; 1:1,000) and anti-β-actin (ab8226; 1:1,000) were purchased from Abcam (Cambridge, MA, USA); anti-VEGF (AF5131, 1:1,000) was purchased from Affinity Biosciences (Cincinnati, OH, USA), and the secondary antibodies were purchased from ProteinTech Group, Inc.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
In situ detection of apoptosis was performed on sides using the TUNEL technique using an In Situ Cell Death Detection kit (Roche Applied Science, Penzberg, Germany). MCF-7 cells were seeded onto sterile glass cover slips in a 6-well plate, and incubated with 150 µM CoCl2 for 48 h. Cells were then assessed by a TUNEL assay, according to the manufacturer's protocol. Steps were as follows: Cells were fixed with 4% paraformaldehyde (pH 7.4, Beijing Solarbio Science& Technology Co., Ltd.) at room temperature for 1 h and washed with PBS for 5 min; cells were incubated in Blocking solution (3% H2O2 in methanol) for 10 min at room temperature and washed with PBS for 5 min. Cells were then incubated with penetrating fluid (0.1% Triton X-100 in 0.1% sodium citrate solution) on ice for 2 min and then 50 µl TUNEL reaction mixture (1:9) was added prior to incubation in a damp box at 37°C for 1 h. Following washing with PBS for 5 min, a drop of PBS was added to the slide. For staining of the nuclei, cells were washed with PBS and incubated with 1.0 µg/ml DAPI (Sigma-Aldrich; Merck KGaA). The samples were analyzed using a fluorescence microscope (magnification, ×100). A total of 10 fields of view were analyzed.
Statistical analysis
SPSS statistical software (version 19.0; IBM Corp., Armonk, NY, USA) to analyze the experimental data. Quantitative data are presented as the mean ± standard deviation. One-way analysis of variance with Least Significant Difference post-hoc test was used to compare between groups. Student's t-test was used to determine the significance for all pairwise comparisons of interest. P<0.05 was considered to indicate a statistically significant difference.
Results
CoCl2 induces the expression of CXCR4 and VEGF mRNA, but not HIF-1α mRNA
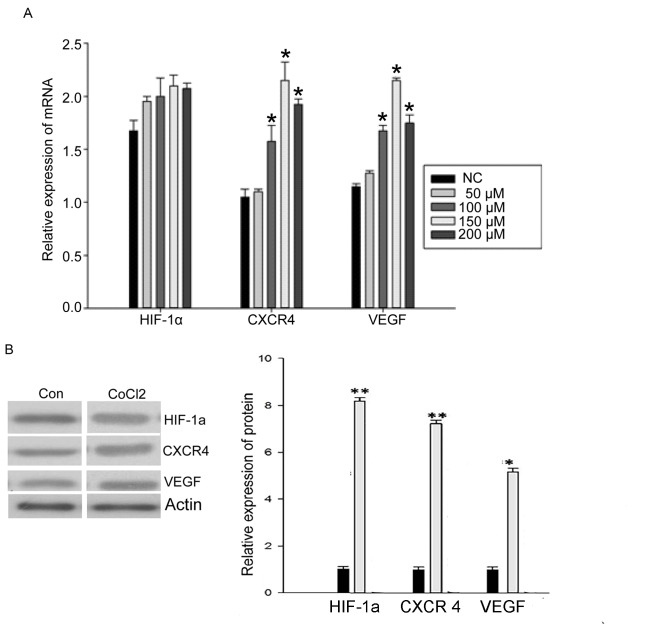
To examine whether hypoxia affected the mRNA expression level of HIF-1α, CXCR4 and VEGF, RT-qPCR was used to quantify mRNA levels in MCF-7 cells cultured for 48 h with different CoCl2 concentrations. There was no significant change in the expression of HIF-1α mRNA following treatment with different CoCl2 concentrations (Fig. 1A). However, the expression of CXCR4 mRNA and VEGF mRNA was significantly increased by treatment with CoCl2 (50, 100, 150 and 200 µM). The 150 µM concentration of CoCl2 induced the maximum effect (P<0.05; Fig. 1A), which indicated that hypoxia is able to promote the expression of CXCR4 and VEGF mRNA.
Figure 1.
mRNA and protein expression levels of HIF-1α, CXCR4 and VEGF following treatment with CoCl2. (A) Reverse transcription-quantitative polymerase chain reaction analysis of HIF-1α, CXCR4 and VEGF mRNA levels following treatment with CoCl2 (n=3). (B) Changes in HIF-1α, CXCR4 and VEGF protein expression following treatment with 150 µM CoCl2 (n=3). *P<0.05, **P<0.01 compared with NC. HIF-1α, hypoxia-inducible factor-1α; CXCR4, C-X-C motif chemokine receptor 4; VEGF, vascular endothelial growth factor; NC, negative control; Con, control.
CoCl2 induces the protein expression of HIF-1α, CXCR4 and VEGF
To examine further whether hypoxia affects the protein expression of HIF-1α, CXCR4 and VEGF, levels were examined by western blotting in MCF-7 cells cultured for 48 h with different CoCl2 concentrations. The expression of HIF-1α protein significantly increased following treatment with CoCl2 (150 µM; P<0.05; Fig. 1B). Expression of CXCR4 and VEGF protein was also significantly increased by CoCl2 treatment (P<0.05; Fig. 1B). These results indicated that hypoxia also promotes the expression of HIF-1α, CXCR4 and VEGF protein. Owing to these results, a concentration of 150 µM CoCl2 was used for further experiments.
Effects of CoCl2 on MCF-7 cell apoptosis
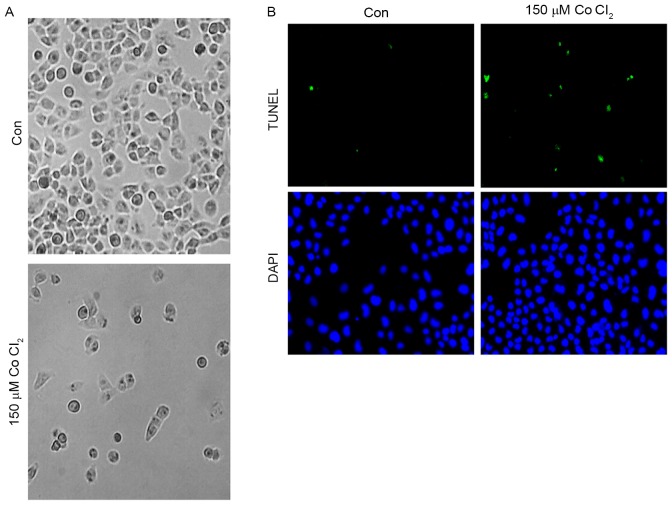
First, in order to determine the effect of CoCl2 on MCF-7 cell morphology, 150 µM CoCl2 was added to MCF-7 cells for 48 h. As shown, the MCF-7 cell morphology did not change significantly after treatment for 24 h. However, 48 h later, an accumulation of cell metabolites was observed in the culture dish, and a number of the cells exhibited plasmatorrhexis (Fig. 2A). These results indicated that the high intensity of the hypoxia microenvironment may have an effect on cell morphology. A TUNEL assay was performed to investigate whether CoCl2 was able to trigger apoptosis of MCF-7 cells. As presented in Fig. 2B, the results of the TUNEL assay demonstrated that incubation with 150 µM CoCl2 for 48 h induced MCF-7 cells to exhibit a significant increase (5±2% TUNEL-positive cells in the control group vs. 30±5% of TUNEL-positive cells in the CoCl2-treatment group; P<0.05) in TUNEL-positive cells, which indicated that CoCl2 is involved in the regulation of apoptosis in MCF-7 cells.
Figure 2.
Morphological changes and apoptosis of MCF-7 cells following 48 h of treatment with CoCl2. (A) The morphological changes of MCF-7 cells following 48 h of treatment with 150 µM CoCl2. (B) Apoptosis was determined by TUNEL staining (green dots) and doubly stained with DAPI (blue dots). Magnification, ×100. Con, control; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling.
CoCl2 inhibits MCF-7 cell proliferation
An MTT assay was performed to determine whether CoCl2 affects the proliferation of MCF-7 cells. The MTT assay demonstrated that MCF-7 cell proliferation significantly decreased following treatment with CoCl2 compared with the control group (P<0.05; Fig. 3). The effects of different CoCl2 concentrations on MCF-7 cell proliferation were as follows: The higher the concentration of CoCl2, the higher the inhibition efficiency and the longer the treatment time, the higher the inhibition efficiency (Fig. 3). The inhibitory effect of CoCl2 on MCF-7 cell proliferation was therefore dependent on the treatment time periods and on CoCl2 concentration, which indicated that the time and intensity of hypoxia was able to inhibit the proliferation of cells to various extents.
Figure 3.
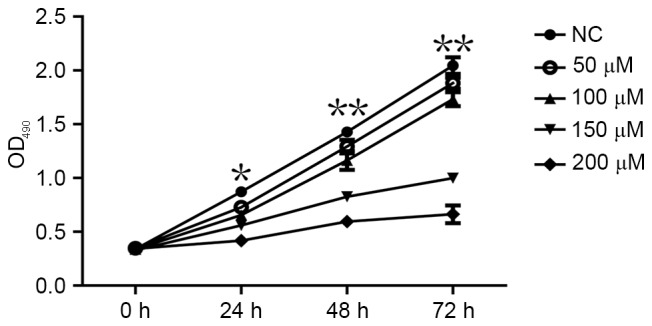
Effect of CoCl2 on MCF-7 cell proliferation assessed using an MTT assay. *P<0.05, **P<0.01 compared with NC; n=3. OD, optical density; NC, negative control.
Discussion
A hypoxic tumor microenvironment is a key feature in a number of solid tumor tissues (16). This is due to the rapid proliferation of tumor cells and the presence of tumor vascular structure and functional abnormalities (17,18). The presence of hypoxia may lead to a series of biological behaviors in solid tumors, and these changes may possibly become the primary reason for the development of resistance to radiotherapy and chemotherapy (19). On the basis of previous studies, tissue hypoxia is known to alter the oxygen balance in tumor microenvironment (16). All of the aforementioned features may elicit adverse effects in patients with breast cancer.
Breast cancer is the most frequently diagnosed cancer in women and a major cause of mortality in women worldwide, with high relapse rates (20–22). Breast cancer is sensitive to hypoxia; 25–40% of invasive breast cancer exhibits hypoxic microenvironments (23,24). Hypoxia may promote the stemming of breast cancer cells and epithelial-mesenchymal transition-mediated breast cancer cell migration, with this intratumoral hypoxia having a negative impact on the survival rate of patients with breast cancer (25).
Hypoxia causes extensive responses in cells and tissues; the expression of the transcription factor 1 HIF-1 is key to allowing cells to adapt to the hypoxic environment (26,27). HIF-1 regulates the expression of a series of hypoxia-inducible genes, resulting in a series of hypoxia adaptations. It has been demonstrated that the expression of HIF-1α and its target genes are increased in breast cancer (27). HIF-1α expression may have a notable function early in breast cancer progression (27). High levels of HIF-1α expression at diagnosis may be used to predict early recurrence and metastasis, and are also associated with poor clinical outcomes in patients with breast cancer (28,29).
CXCR4 is a member of the C-X-C motif chemokine receptor family associated with aggressive, proliferative and motile breast cancer phenotypes (30–32). CXCR4 may represent a novel independent prognostic marker for patients with lymph-node-positive breast cancer (33). Previous studies have demonstrated that HIF-1α can markedly induce and regulate the expression of CXCR4 and its ligand stromal cell-derived factor 1 (SDF-1) in breast cancer tissues and cells, providing it with a vital function in the migration of tumor cells (34–36). The CXCR4-SDF-1 interaction potentially mediates the trafficking of circulating tumor cells in primary breast cancer (36).
VEGF is able to regulate a number of cell functions, including mitosis, permeability and vasoconstrictor tension (37). VEGF expression is closely associated with tumor angiogenesis and lymphatic formation in breast cancer (38). VEGF is a target gene of HIF-1 (39). The HIF-1 transcription complex is able to induce the expression of VEGF and induce the corresponding biological effects (40,41).
HIF-1, CXCR4 and VEGF represent important targets in the prevention and treatment of breast cancer under hypoxic conditions. In recent years, HIFs have become the focus of a great deal of research (42,43); however, only certain studies concern HIF-1α, CXCR4 and VEGF and their association with the oxygen homeostasis of microenvironments in breast tumors (44). For this reason, it is important to investigate the association between the expression of these three factors in breast cancer. Therefore, the present study established an in vitro model to simulate the hypoxic microenvironment present in human breast cancer cells. The present study revealed that CoCl2 inhibited MCF-7 cell proliferation, and this inhibitory effect was dependent on the length of time and CoCl2 concentration. The results of RT-qPCR and western blot analysis revealed that the expression of HIF-1α mRNA was not significantly induced by CoCl2 (P>0.05); however, the expression of CXCR4 and VEGF mRNA increased significantly upon treatment with a range of different CoCl2 concentrations (100, 150 and 200 µM). The results of western blotting revealed that CoCl2 significantly induced the protein expression of HIF-1α, CXCR4 and VEGF. Additionally, the CoCl2-simulated hypoxic conditions generated cytotoxicity and apoptosis in MCF-7 cells. The expression of HIF-1α, CXCR4 and VEGF was associated with the CoCl2 treatment length and concentration. Thus, CoCl2 treatment was identified to induce the proliferation and metastasis of tumors.
Further efforts to develop a suitable model of hypoxia, or to discover an anticancer antioxidant to prevent damage to cells may help to decrease tumor cell proliferation and decrease the expression and transferal ability of HIF-1α, CXCR4 and VEGF. This may provide a novel method for the prevention and treatment of breast cancer.
Acknowledgements
The present study was supported by the Natural Science Funds of Shandong Province Project: Mutation and Expression of Parathyroid Carcinoma Susceptibility Gene HRPT2, MEN1, CyclinD1 and RET (grant no. ZR2012HL04); and the Science and Technology Development Plan of Shandong Province Project: Clinical Application of Selective ALND of CN+ Breast Cancer Patients (grant no. 2012YD18062).
References
- 1.Hales CA. Hypoxic Pulmonary Vasoconstriction. Springer US; 2004. Physiological Function of Hypoxic Pulmonary Vasoconstriction; pp. 3–14. [DOI] [Google Scholar]
- 2.K L Eales, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5:e190. doi: 10.1038/oncsis.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaupel P, Harrison L. Tumor hypoxia: Causative factors, compensatory mechanisms and cellular response. Oncologist. 2004;9(Suppl 5):S4–S9. doi: 10.1634/theoncologist.9-90005-4. [DOI] [PubMed] [Google Scholar]
- 4.Yamada D, Kobayashi S, Yamamoto H, Tomimaru Y, Noda T, Uemura M, Wada H, Marubashi S, Eguchi H, Tanemura M, et al. Role of the hypoxia-related gene, JMJD1A, in hepatocellular carcinoma: Clinical impact on recurrence after hepatic resection. Ann Surg Oncol. 2011;19(Suppl 3):S355. doi: 10.1245/s10434-011-1797-x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen S, Donnem T, Al-Saad S, Al-Shibli K, Stenvold H, Busund LT, Bremnes RM. Correlation and coexpression of HIFs and NOTCH markers in NSCLC. Anticancer Res. 2011;31:1603–1606. [PubMed] [Google Scholar]
- 6.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–318. doi: 10.1016/j.canep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti J, Turley H, Campo L, Han C, Harris AL, Gatter KC, Fox SB. The transcription factor DEC1 (stra13, SHARP2) is associated with the hypoxic response and high tumour grade in human breast cancers. Br J cancer. 2004;91:954–958. doi: 10.1038/sj.bjc.6602059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hänze J, Eul BG, Savai R, Krick S, Goyal P, Grimminger F, Seeger W, Rose F. RNA interference for HIF-1alpha inhibits its downstream signalling and affects cellular proliferation. Biochem Biophys Res Commun. 2003;312:571–577. doi: 10.1016/j.bbrc.2003.10.153. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Chen J, Liu F, Gao C, Wang X, Zhao T, Liu J, Gao S, Zhao X, Ren H, Hao J. CypA, a gene downstream of HIF-1α, promotes the development of PDAC. PLoS One. 2014;9:e92824. doi: 10.1371/journal.pone.0092824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Du KM, Xue ZH, Yan H, Li D, Liu W, Chen Z, Zhao Q, Tong JH, Zhu YS, Chen GQ. Cobalt chloride and low oxygen tension trigger differentiation of acute myeloid leukemic cells: possible mediation of hypoxia-inducible factor-1alpha. Leukemia. 2003;17:2065–2073. doi: 10.1038/sj.leu.2403141. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Zhang J, Yang H, Wu C, Dang X, Liu Y. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci Rep. 2015;5:12410. doi: 10.1038/srep12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen B, Zheng MQ, Lu JW, Jiang Q, Wang TH, Huang XE. CXCL12-CXCR4 promotes proliferation and invasion of pancreatic cancer cells. Asian Pac J Cancer Prev. 2013;14:5403–5408. doi: 10.7314/APJCP.2013.14.9.5403. [DOI] [PubMed] [Google Scholar]
- 14.Luo HQ, Xu M, Zhong WT, Cui ZY, Liu FM, Zhou KY, Li XY. EGCG decreases the expression of HIF-1α and VEGF and cell growth in MCF-7 breast cancer cells. J BUON. 2014;19:435–439. [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data usingreal-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863:382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsutsui S, Matsuyama A, Yamamoto M, Takeuchi H, Oshiro Y, Ishida T, Maehara Y. The Akt expression correlates with the VEGF-A and -C expression as well as the microvessel and lymphatic vessel density in breast cancer. Oncol Rep. 2010;23:621–630. doi: 10.3892/or_00000677. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Wu J, Liang B, Huangfu CS. Correlationbetween hypoxia inducible factor (HIF-1α) and epithelium-mesenchyma transform of ductal carcinoma with invasive breast cancer. Shandong Med J. 2012;52:90–92. [Google Scholar]
- 19.Moser C, Lang SA, Mori A, Hellerbrand C, Schlitt HJ, Geissler EK, Fogler WE, Stoeltzing O. ENMD-1198, a novel tubulin binding agent rdduces HIF-lalpha and STAT3 activity in human hepatocellular carcinoma (HCC) cells and inhibits growth and vascularization in vivo. BMC Cancer. 2008;8:206. doi: 10.1186/1471-2407-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: An independent review. Br J Cancer. 2013;108:2205–2240. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 22.Lech R, Przemyslaw O. Epidemiological models for breast cancer risk estimation. Ginekol Pol. 2011;82:451–454. [PubMed] [Google Scholar]
- 23.Liu ZJ, Semenza GL, Zhang HF. Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B. 2015;16:32–43. doi: 10.1631/jzus.B1400221. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundgren K, Holm C, Landberg G. Hypoxia and breast cancer: Prognostic and therapeutic implications. Cell Mol Life Sci. 2007;64:3233–3247. doi: 10.1007/s00018-007-7390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SJ, Kim JG, Kim ND, Yang K, Shim JW, Heo K. Estradiol, TGF-β1 and hypoxia promote breast cancer stemness and EMT-mediated breast cancer migration. Oncol Lett. 2016;11:1895–1902. doi: 10.3892/ol.2016.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradbury J. Breathing hard to keep up with HIF-1. Lancet. 2001;358:1704. doi: 10.1016/S0140-6736(01)06774-5. [DOI] [PubMed] [Google Scholar]
- 27.Cancer Genome Atlas Network, corp-author. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, Bersiga A, Allevi G, Milani M, Aguggini S, et al. Hypoxiainducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. 2006;12:4562–4568. doi: 10.1158/1078-0432.CCR-05-2690. [DOI] [PubMed] [Google Scholar]
- 29.Gruber G, Greiner RH, Hlushchuk R, Aebersold DM, Altermatt HJ, Berclaz G, Djonov V. Hypoxia-inducible factor 1alpha in high-risk breast cancer: An independent prognostic parameter. Breast Cancer Res. 2004;6:R191–R198. doi: 10.1186/bcr775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 31.Rahimi M, Toth TA, Tang CK. CXCR4 suppression attenuates EGFRVIII mediated invasion and induces p38 MAPK-dependent protein trafficking and degradation of EGFRvIII in breast cancer cells. Cancer Lett. 2011;306:43–51. doi: 10.1016/j.canlet.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helbig G, Christopherson KW, II, Bhat-Nakshatfi P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 33.Parker CC, Kim RH, Li BD, Chu QD. The chemokine receptor CXCR4 as a novel independent prognosic marker for node-positive breast cancer patients. J Surg Oncol. 2012;106:393–398. doi: 10.1002/jso.23113. [DOI] [PubMed] [Google Scholar]
- 34.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Citti A, Boldrini R, Inserra A, Alisi A, Pessolano R, Mastronuzzi A, Zin A, De Sio L, Rosolen A, Locatelli F, Fruci D. Expression of multidrug resistance-associated proteins in paediatric soft tissue sarcomas before and after chemotherapy. Int J Oncol. 2012;41:117–124. doi: 10.3892/ijo.2012.1433. [DOI] [PubMed] [Google Scholar]
- 36.Mego M, Cholujova D, Minarik G, Sedlackova T, Gronesova P, Karaba M, Benca J, Cingelova S, Cierna Z, Manasova D, et al. CXCR4-SDF-1 interaction potentially mediates trafficking of circulating tumor cells in primary breast cancer. Bmc Cancer. 2016;16:127. doi: 10.1186/s12885-016-2143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 38.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–1163. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680–688. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 40.Semenza GL. Expression of hypoxia-inducible factor 1: Mechanisms and consequences. Biochem Pharmacol. 2000;59:47–53. doi: 10.1016/S0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 41.Li G, He L, Zhang E, Shi J, Zhang Q, Le AD, Zhou K, Tang X. Overexpression of human papillomavirus (HPV) type 16 oncoproteins promotes angiogenesis via enhancing HIF-1α and VEGF expression in non-small cell lung cancer cells. Cancer Lett. 2011;311:160–170. doi: 10.1016/j.canlet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Dong X, Wang YS, Dou GR, Hou HY, Shi YY, Zhang R, Ma K, Wu L, Yao LB, Cai Y, Zhang J. Influence of Dll4 via HIF-1α-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS One. 2011;6:e18481. doi: 10.1371/journal.pone.0018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward C, Langdon SP, Mullen P, Harris AL, Harrison DJ, Supuran CT, Kunkler IH. New strategies for targeting the hypoxic tumour microenvironment in breast cancer. Cancer Treat Rev. 2013;39:171–179. doi: 10.1016/j.ctrv.2012.08.004. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Haber C, Barrio-Real L, Casado-Medrano V, Kazanietz MG. Heregulin/ErbB3 signaling enhances CXCR4-driven rac1 activation and breast cancer cell motility via hypoxia-inducible factor 1α. Mol Cell Biol. 2016;36:2011–2026. doi: 10.1128/MCB.00180-16. [DOI] [PMC free article] [PubMed] [Google Scholar]