Abstract
Vascular endothelial growth factor (VEGF) and the pigment epithelium-derived factor (PEDF) serve an important role in prostate cancer (PCa). The aim of the present study was to evaluate whether the levels of VEGF and PEDF in serum are associated with the severity of PCa, and whether they can differentiate from patients with benign prostatic hyperplasia (BPH). Two groups of patients were recruited, patients with PCa or BPH that were newly diagnosed without other comorbidities, and were compared with healthy individuals. The levels of VEGF and PEDF were measured by ELISA in serum, and by immunohistochemistry in biopsies. A correlation analysis was performed for the values in biopsies and serum, comparing the VEGF/PEDF ratio, total-prostate-specific antigen (t-PSA) levels and the status of each sample as acinar Ad (Gleason score) or as benign hyperplasia. The results demonstrated that serum levels of VEGF, PEDF, and t-PSA between PCa and BPH were similar to each other, but different to healthy individuals (P<0.05). The VEGF/PEDF ratio in serum had a significant difference between acinar Ad with Gleason score 8–10 and BPH groups (P<0.05). The VEGF and PEDF immunostaining intensities were correlated with its circulating levels in all cases of PCa, but not in BPH. These preliminary results suggest that VEGF and PEDF levels by themselves or in combination with t-PSA did not differentiate between malignant, and benign prostate diseases. However, there was a significant difference observed in the VEGF/PEDF ratio in serum between the groups, suggesting that it may be used as an index for diagnosis and prognosis in a personalized manner, although more studies are necessary.
Keywords: prostate cancer, benign prostatic hyperplasia, vascular endothelial growth factor, pigment epithelium-derived factor, VEGF/PEDF ratio
Introduction
Prostate cancer (PCa) is the second most frequent malignant neoplastic disease among men, with 241,740 cases in America, and 28,170 deaths from PCa in 2012; in the Mexican population, the incidence of PCa is underrated, but there is a high occurrence of high-grade lesions (1,2). Due to its impact, and to promptly treat this illness, several programs for prevention and early diagnosis are currently active. The primary diagnosis tools for PCa are the level measurement of total prostate specific antigen (t-PSA) in serum along with clinical and digital rectal examination; nevertheless, none of these method is specific enough to differentiate cases of adenocarcinoma (Ad) from benign prostatic hyperplasia (BPH) (3,4). To distinguish between prostatic pathologies and to determine progressiveness of PCa, is useful to perform a histological inspection of biopsies with Gleason score (GS) grading since it allows the physicians to distinguish benign and malignant neoplasias. However, the specificity of histology interpretation could decrease depending on the number of analyzed biopsies, the captured area and the expertise of the pathologist. There is sufficient evidence suggesting that angiogenesis plays an important role in PCa. PCa cells secrete proteic factors such as the vascular endothelial growth factor (VEGF), which is extensively studied and known as the major angiogenic marker. VEGF acts as a direct mediator in endothelial cell proliferation, vascular permeation, tumor growth promotion, and metastasis. Several authors report that there are higher levels of VEGF in biopsies and serum of PCa patients as compared to healthy individuals (5–8). Although there is a correlation between levels of VEGF in serum and the stages of the disease, its validity as a prognosis marker is still controversial because VEGF is also augmented in BPH and its plasma concentration does not concur with the clinical classification as benign or malignant forms (9–13).
Other protein related to angiogenesis is the pigment epithelium-derived factor (PEDF), an antiangiogenic factor with antitumoral properties (14). In PCa and other solid tumors, low levels of PEDF are associated with higher vascular density and to a metastatic phenotype, indicating a decrease of its expression along with tumor progression (15,16). Likewise, tumor growth in PCa diminishes when treated with recombinant PEDF or with diverse epitopes of this protein (14,17,18). Also, the levels of PEDF are lower in serum and biopsies of PCa, suggesting that it as a prediction marker of the disease (19,20). However, there are not studies about the levels of PEDF in PCa and BPH as a diagnosis marker.
Angiogenesis depends on the critical equilibrium between pro- and anti-angiogenic factors (VEGF/PEDF). Several studies in vivo and in vitro show an association between an increase in the VEGF/PEDF ratio and a bad prognosis in nasopharyngeal carcinoma and ophthalmic neovascular illnesses, suggesting that the VEGF/PEDF ratio in serum could be useful as a prognostic value for other diseases (21–25). Though, there are no reports of the differences in the measurements of VEGF/PEDF ratio between PCa and BPH. In here, we aim to describe the serum levels of VEGF, PEDF and the VEGF/PEDF ratio among patients recently diagnosed with PCa or BPH and whether these measurements are related to the detection of both proteins in prostate biopsies. The combination of these data might allow the discrimination of the grade of angiogenesis associated with the disease, and it could become a valuable theranostic tool.
Materials and methods
The present study was performed under the approval of the ethics and research local committees. All participants gave their informed consent through a written format, under the 1975 Helsinki's Declaration and the nationally approved guidelines (26). Patients with PCa (n=40) and BPH (n=57) were recently diagnosed by digital rectal exam, serum t-PSA measurement (t-PSA>4.0 ng/ml) and by detection of diffuse growth at the prostatic transition zone, near the bladder base, with nodular and heterogeneous echo. Acinar Ad and BPH diagnosis were confirmed by histological examination, using a biopsy extracted with a guided transrectal ultrasound (TRUS). None of the PCa and BPH patients had history of other malignancies, previous surgery or any other PCa treatments (deprivation therapy, chemotherapy or androgen radiotherapy), neither presented active infections at the time of their blood test.
Healthy adult volunteers (n=35) were recruited from the blood bank under the criteria established by the Mexican Official Standard NOM-253-SSA1-2012 (27), showing no complaints or signs of malignancies or inflammatory diseases and with t-PSA<4 ng/ml. Diabetes mellitus, cardiovascular disease, and other systemic diseases were excluded both in ill and healthy individuals.
Serum sample collection and measurement of VEGF and PEDF
Venous blood samples were collected after an overnight fast, serum was separated and stored at −80°C. VEGF serum levels were quantified using the Quantikine assay kit (R&D Systems) according to the manufacturer's instructions. PEDF was measured using an enzyme-linked immunosorbent assay (ELISA) kit (ChemiKine™; Chemicon International; Millipore Inc., Billerica, MA, USA). To prevent PEDF from associating with other circulating proteins that may interfere with its total serum quantification, samples were pre-treated with urea (8 M final concentration) for 60 min on ice and diluted in dilution buffer before being applied in duplicate to ELISA plates, as recommended by the manufacturer. All plates VEGF and PEDF were read at 450 nm using a microplate reader (Eon™Microplate Spectrophotometer; bioTek, Winooski, VT, USA).
Immunohistochemical staining
A range of 9–12 cores was taken at the initial prostate biopsy, which were divided into three biopsies per paraffin block. Tissue specimens were processed using conventional procedures for paraffin embedding. Three-micron sections were serially cut. The pathologist analyzed hematoxylin/eosin-stained slides for classification. Subsequently, the highest score Gleason representative paraffin block (containing at least 2 cores positive) was sectioned, dewaxed and rehydrated up to wash buffer (Dako wash; North America, Inc.) and loaded onto Shandon sequenza chamber (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Labeled polymer-based immunodetection system (Mouse/Rabbit PolyVue™ HRP/DAB Detection System; Diagnostic BioSystems, Pleasanton, CA, USA) was used as recommended by the manufacturer's protocol. Monoclonal mouse anti-VEGF antibody (1:50; SC-7269; Santa Cruz Biotech, Inc., Santa Cruz, CA, USA) or polyclonal goat PEDF antibody (1:200; AF1177; Millipore, R&D Systems, Minneapolis, MN, USA) were applied. Then enhancer Polyvue Plus and HRP were added, and incubated with DAB plus/chromogen substrate. Histological observation and image capture were performed using an Axio Imager.A2 (ZEISS, Oberkochen, Germany). To prevent artifactual formation, VEGF or PEDF staining were processed the same day. The criterion of analysis was applied to the regions where VEGF staining showed a higher intensity.
The intensity of VEGF-A and PEDF expression in the biopsies selected above were evaluated in the entire tissue, subsequently three to five fields by cylinder were captured (magnification, ×40) and then processed by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA), which detected the brown spots of the image. According to the data of detected area, samples were classified as four grades: none (0–50), mild (51–166), moderate (167–283), and strong (284–400), and the percentage was plotted as total intensity. Two blinded observers independently performed analysis and immunostaining interpretation. A third observer was required in the discording cases.
Statistical analysis
All data were analyzed by SPSS v.20 (IBM SPSS Inc., Armonk, NY, USA) using descriptive statistics. Data were presented as the mean ± SD. One-way analysis of variance (ANOVA) with Tukey post hoc test was used to compare groups of normally distributed data of the variables studied; Pearson's correlation for serum levels or Spearman's correlation coefficients for serum vs. immunostained intensity percentage in biopsies were used to test associations between variables. Student's t-test was used to compare median for t-PSA with the AdGS. P<0.05 was considered to indicate a statistically significant difference.
Results
Group description
Table I describes the clinical parameters of the enrolled patients and healthy participants. The means of age between patients and healthy participants were similar (P=0.109). BMI means were similar in BPH and PCa (P=0.170) but higher as compared to healthy individuals (P=0.001). The t-PSA values in the serum of PCa and BPH patients were higher than 4 ng/ml, and there was not a significant difference between them, but both were different from the healthy group (P=0.001). According to the clinical and histological evaluation, from the 40 cases diagnosed as PCa (PCa total), nine were classified as Ad with GS 6 (AdGS6); sixteen were AdGS7, thirteen were AdGS8, and two were AdGS10. PCa cases were grouped into two subsets, AdGS6-7 (well and moderately differentiated), and AdGS8-10 (poorly differentiated or undifferentiated) to further analysis. There was a difference statistically significant between the t-PSA mean values of AdGS6-7 and AdGS8-10 (P=0.045).
Table I.
Data comparison of Age, BMI and t-PSA of patients with PCa, AdGS6-7, AdGS8-10, BPH and healthy participants.
| Characteristic | PCa(n=40) | BPH(n=57) | Healthy(n=35) |
|---|---|---|---|
| Age | 65.32±4,28 | 64.35±5,56 | 62.80±5,41 |
| BMI | 26.05±2,88a | 27.20±3,59a | 23.18±1,59 |
| t-PSA | 12.81±1.76a | 14.88±2.83a | 1.08±0.14 |
| 9.41±3.73b,c | – | – | |
| 17.91±9.80d | – | – |
Mean ± SD.
P≤0.05 vs. Healthy in a one-way analysis of variance
P≤0.05 vs. AdGS8-10 in a Student's t-test
AdGS6-7 (n=25)
AdGS8-10 (n=15). pCA, prostate cancer; BPH, benign prostatic hyperplasia; BMI, body mass index; t-PSA, total-prostate-specific antigen; AdGSC, adenocarcinoma with Gleason score.
VEGF and PEDF measurements in serum
The VEGF levels (pg/ml) were increased in PCa compared to healthy individuals (360.55±292.10 vs. 157.1±49.73; P=0.039), but there was not a difference with BPH cases (298.17±178.6; P=0.274). Nonetheless, the stratification of PCa in GS showed that only AdGS8-10 (475.7±405.7) had a significant increase compared to the control group (P=0.009) (Fig. 1A).
Figure 1.
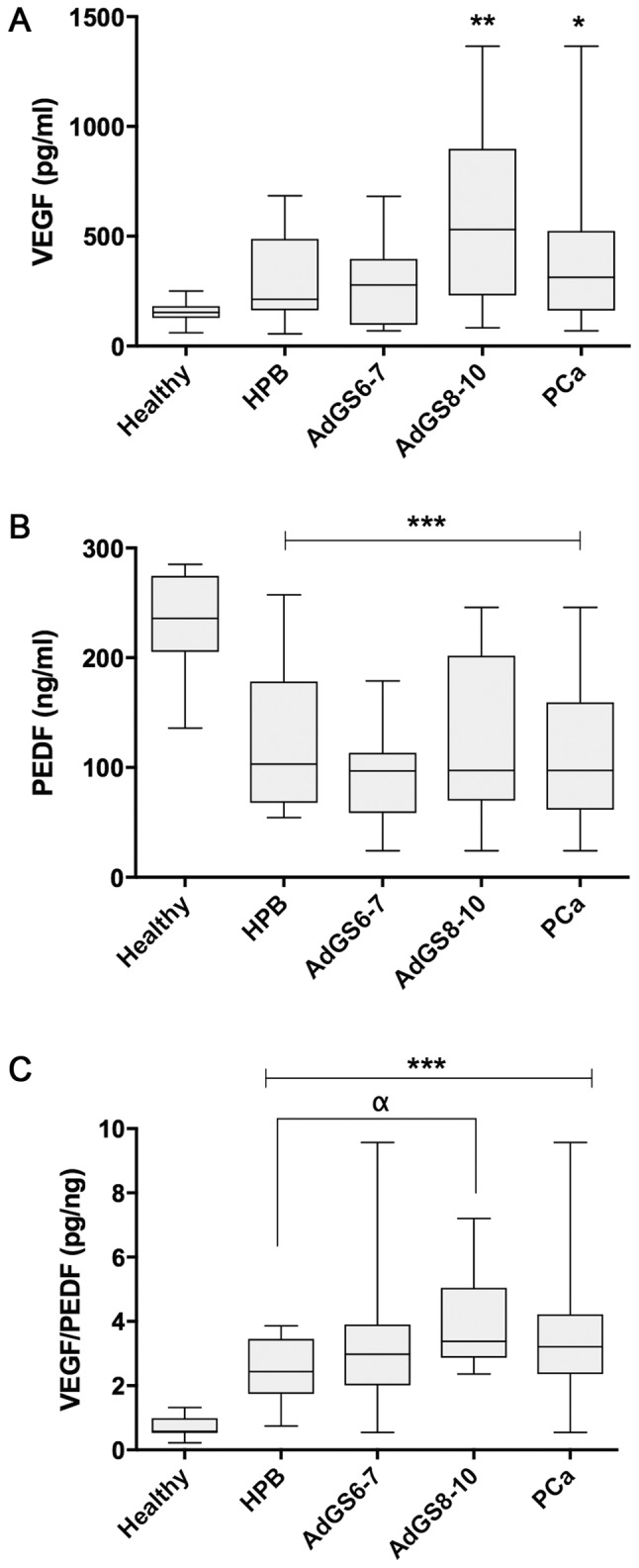
Serum values of VEGF (A), PEDF (B), VEGF/PEDF ratio (C) from healthy (n=35) and prostatic diseases groups (BPH n=57; PCa n=40; AdGS6-7 n=25 and AdGS8-10 n=15). Values are mean ± SD. *P≤0.05, **P≤0.01 and ***P≤0.001 vs. healthy; αP≤0.05 by ANOVA test. VEGF, vascular endothelial growth factor; PEDF, pigment epithelium-derived factor BPH, benign prostatic hyperplasia; ANOVA, one-way analysis of variance.
Fig. 1B indicates that the means of PEDF values in ng/ml were significantly inferior in BPH (122.15±58.84), AdGS6-7 (98.93±38.02), AdGS8-10 (121.58±84.13) and PCa (107.99±59.79) when weighed against the healthy group (233.6±9.25; P=0.001), although there was no significant difference between the prostatic diseases (P>0.05).
When analyzing the VEGF/PEDF ratio (pg/ng) among the samples, its mean was 2.47±0.94 in BPH, 3.18±1.11 in PCa and 0.71±0.3 in the healthy group. There are statistically significant differences between all groups compared to the healthy group (P=0.001); meanwhile, there was no difference between PCa or AdG6-7 vs. BPH. Nevertheless, AdGS8-10 was significant compared to BPH (3.71±1.25; P=0.015) (Fig. 1C).
Table II illustrates the correlation coefficient analyses between serum levels of t-PSA, VEGF, PEDF and VEGF/PEDF ratio from healthy, PCa and BPH groups. In PCa, VEGF presented a positive correlation with t-PSA (P=0.042), PEDF (P=0.001), and the ratio (P=0.004); in AdGS6-7 and AdGS8-10 groups a positive correlation was also found between VEGF and PEDF (P=0.003 and P=0.001, respectively). For BPH there was a positive correlation between VEGF and PEDF (P=0.001), and it was negative when the t-PSA vs. ratio analysis was performed (P=0.008). As it is expected, the correlation of VEGF with VEGF/PEDF ratio was positive and significant between PCa and BPH (P=0.004 and P=0.003). In healthy individuals, a strong negative correlation was observed between VEGF and PEDF with the ratio (P=0.001 and P=0.001).
Table II.
Pearson's Correlation Coefficient of PSA, VEGF, PEDF and Ratio VEGF/PEDF serum in PCa, BPH and healthy groups.
| Serum | ||||
|---|---|---|---|---|
| Group | VEGF | PEDF | Ratio | |
| PCa | t-PSA | 0.458a | 0.389 | 0.316 |
| VEGF | – | 0.900c | 0.613b | |
| PEDF | – | – | 0.259 | |
| AdGS6-7 | t-PSA | −0.129 | 0.037 | −0.251 |
| VEGF | – | 0.775b | 0.695 | |
| PEDF | – | – | 0.112 | |
| AdGS8-10 | t-PSA | 0.460 | 0.426 | 0.314 |
| VEGF | – | 0.927b | 0.556 | |
| PEDF | – | – | 0.262 | |
| BPH | t-PSA | −0.300 | 0.077 | −0.578b |
| VEGF | – | 0.690b | 0.620b | |
| PEDF | – | – | −0.079 | |
| Healthy | t-PSA | −0.046 | −0.051 | −0.008 |
| VEGF | – | −.347 | −0.883c | |
| PEDF | – | – | −0.730c | |
P≤0.05
P<0.01
P<0.001. pCA, prostate cancer; BPH, benign prostatic hyperplasia; AdGSC, adenocarcinoma with Gleason score; VEGF, vascular endothelial growth factor; PEDF, pigment epithelium-derived factor; t-PSA, total-prostate-specific antigen.
Immunohistochemical analysis
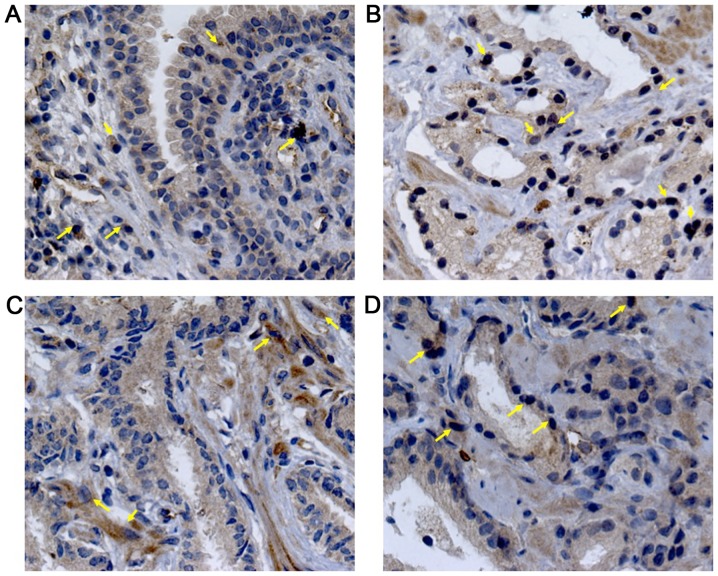
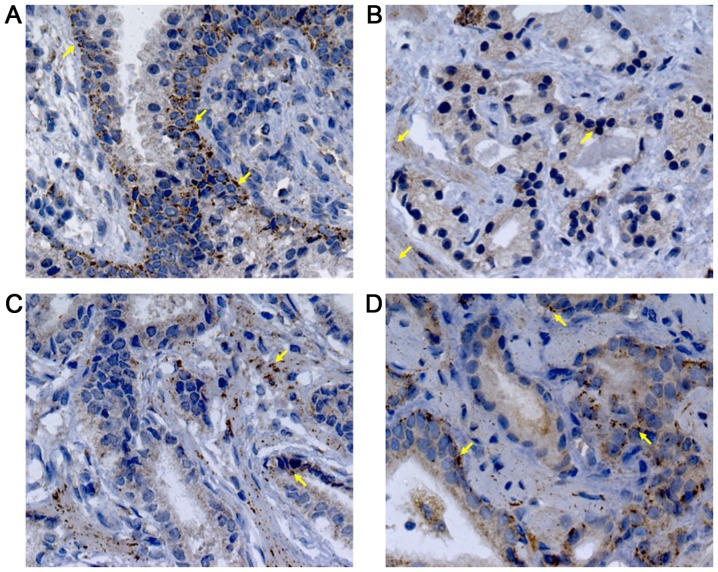
Immunohistochemical analysis was performed to demonstrate if the serum levels of these proteins were related to its expression intensity in prostatic tissues. VEGF staining presented a diffuse pattern; meanwhile, PEDF showed a granular staining. Representative photomicrographs and the analysis of the staining intensity in prostatic tissues are illustrated in Figs. 2–4. In BPH tissues, there was a moderate VEGF staining (33.75±12.86, 4A), confined mainly into the cytoplasm of glandular epithelial cells, endothelial cells and stromal fibroblasts (Fig. 2A). In contrast, PEDF staining showed a mild intensity (24.73±12.85, 4B) and it was limited to the perinuclear region of basal cells (Fig. 3A). The percentage of VEGF staining in PCa (49.90±18.31) was different to BPH (P=0.003). Particularly, AdGS6-7 mean staining was 45±13.31 with a mild to moderate intensity (Fig. 2B and C) but was no different from BPH. However, we found intensity from moderate to high in AdGS8-10 (Fig. 2D) with mean staining values of 57.25±23 (Fig. 4A), which was statistically significant compared to BPH (P=0.002). On the other hand, PEDF staining intensity for PCa was 31±13.72; with a mild staining for AdGS6-7 (Fig. 3B and C) with a mean intensity 29.58±9.4 (Fig. 4B) and mild to moderate in the AdGS8-10 (Fig. 3D) with 33.13±19.07 mean intensity (Fig. 4B). However, on the microscopic examination, most tissue samples of BPH and PCa showed superior staining areas for VEGF over PEDF (P<0.05).
Figure 2.
Immunostaining for VEGF in BPH (A); AdGS6 (B), AdGS7 (C) and AdGS8 (D) biopsies. VEGF-A nuclear expression in acinar and peri-acinar stromal areas (yellow arrows) was observed. Nuclei were counterstained with hematoxylin; magnification, ×400. BPH, benign prostatic hyperplasia; VEGF, vascular endothelial growth factor; AdGSC, adenocarcinoma with Gleason score.
Figure 4.
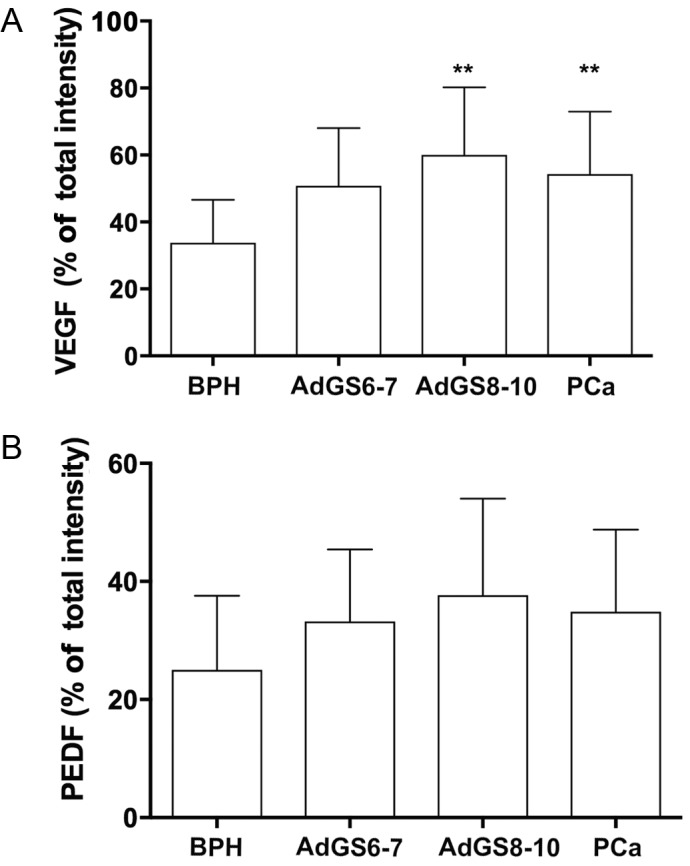
Analysis of tissue immunostaining (magnification, ×40). Total intensity percentage of VEGF (A) and PEDF (B) for BPH (n=57); PCa (n=40); AdGS6-7 (n=25) and AdGS8-10 (n=15), bars indicate mean ± SD. **P≤0.01 vs. BPH, one-way ANOVA. BPH, benign prostatic hyperplasia; VEGF, vascular endothelial growth factor; PEDF, pigment epithelium-derived factor; ANOVA, one-way analysis of variance; AdGSC, adenocarcinoma with Gleason score.
Figure 3.
Immunohistochemistry for PEDF in (A) BPH; (B) AdGS6, (C) AdGS7, and (D) AdGS8 biopsies. PEDF cytoplasmic expression in acinar and peri-acinar stromal areas was observed. PEDF staining was localized at basal cells (yellow arrows). Nuclei were counterstained with hematoxylin; magnification, ×400. PEDF, pigment epithelium-derived factor; BPH, benign prostatic hyperplasia; AdGSC, adenocarcinoma with Gleason score.
A correlation analysis of serum VEGF and PEDF levels with staining intensity in tissues was additionally performed (Table III). In BPH we found no association in both measurements. In PCa a positive correlation was shown for VEGF and PEDF (P=0.002 and 0.001, respectively), nonetheless, in AdGS6-7 a correlation was seen only with VEGF values (P=0.048). AdGS8-10 displayed a correlation with PEDF values (P=0.004) and a positive tendency with VEGF; showing that the heterogeneity found in serum corresponds to the observations in biopsies.
Table III.
Spearman's Correlation Coefficient of serum values VEGF and PEDF with immunostaining intensity percentage in prostatic diseases.
| Serum | ||
|---|---|---|
| Biopsy (%) | VEGF | PEDF |
| BPH | ||
| VEGF | 0.166 | – |
| PEDF | – | −0.198 |
| PCa | ||
| VEGF | 0.661b | – |
| PEDF | – | 0.661b |
| AdGS6-7 | ||
| VEGF | 0.580a | – |
| PEDF | – | 0.344 |
| AdGS8-10 | ||
| VEGF | 0.611 | – |
| PEDF | – | 0.881b |
P≤0.05
P<0.01. pCA, prostate cancer; BPH, benign prostatic hyperplasia; AdGSC, adenocarcinoma with Gleason score; VEGF, vascular endothelial growth factor; PEDF, pigment epithelium-derived factor.
Discussion
Increased levels of t-PSA determine possible anomalies in the prostate, so it has been proposed as a prognostic biomarker in PCa. However, it remains contradictory since its positive predictive value is ~30% (4). Other biomarkers have been proposed to improve this value such as a factors related to angiogenesis (VEGF and MMP9), and to cell processes like PCA3, ANXA3 and TERT (28).
We describe for the first time the behavior simultaneously of VEGF and PEDF in benign and malignant prostate environments, both serum and tissue in individuals without comorbidities related to chronic inflammation (9,29).
VEGF is narrowly related to the malignancy grade and metastasis of PCa, suggesting that it has a diagnostic and prognostic value of this illness. Our results and other studies reveal that serum expression of VEGF is not correlated, neither can discriminate a benign form (30–33). We have shown that levels of VEGF in the serum of PCa and BPH are not significantly different. Probably the inflammatory response in BPH causes an increase in the VEGF expression leading to stromal hypervascularization, endothelial vessel permeability (34–36), or it might occur through a decrease in the androgen receptors and inhibition of apoptosis in epithelial cells (10).
On the other hand, PEDF is a glycoprotein with antitumor properties, because it diminishes the tumor volume and metastases, by acting directly on migration and differentiation into type I tumor-associated macrophages (TAM-1) (37–40), suggesting that it could be used as a predictor of the disease and with therapeutic utility (19,20). Nonetheless, little is known about the levels of PEDF in serum. Ide H et al reported that there are lower levels in BPH in comparison with PCa patients (41). However, we found that PEDF levels were not different in both pathologies but were lower compared to healthy individuals. There is a high expression of PEDF in our PCa group, particularly on AdGS8-10, probably due to the aforementioned (15).
Some studies have linked an increase in the angiogenic balance VEGF/PEDF as a prognostic marker in neovascular diseases (21–25). On prostatic diseases, the measurement of VEGF/PEDF ratio in serum has been unexplored; this is the first study that shows data of their expression in PCa. We observed that the VEGF/PEDF ratio in AdGS8-10 patients was higher as compared to BPH and, even more, to healthy individuals (Fig. 1C). We suggest that the VEGF/PEDF ratio is a kind of normalization of the individually measured data, denoting that the simultaneous measurement of VEGF and PEDF, not the isolated observation of their levels, could help to determine the disease status in an individualized manner. We interpret this idea through the correlation between VEGF, PEDF, and t-PSA (Table III). VEGF was associated to t-PSA only in AdGS8-10 meanwhile this association was negative in BPH. Conversely, PEDF did not present association with t-PSA in any pathology. These results show that levels of t-PSA are not related to VEGF and PEDF in benign hyperplasia and lower grades of PCa (AdGS6-7).
On the other hand, the relationship of VEGF with PEDF shows a positive significance in both GSs and BPH; indicating that both are increased independently of the pathology. Suggesting that the individual analysis of VEGF or PEDF does not differentiate between benign and malignant forms; except for healthy individuals where a negative tendency was shown. Regarding the VEGF/PEDF ratio, there is a significant relation with the decrease of t-PSA in BPH. While in healthy individuals it is maintained in balance.
Additionally, PEDF and VEGF were detected by immunostaining in biopsies. It was noticeable that the intensity of PEDF was lower compared to VEGF in most samples. Doll et al reported a downregulation of PEDF expression in PCa and high levels in BPH (42). Perhaps our divergence is due to the origin of the samples (patients vs. animal model, respectively) (43). Furthermore, we found marked differences in the localization of PEDF among glandular regions. For instance, in BPH, PEDF is located in the cytoplasmic region of basal epithelium, meanwhile, in malignant glands, it was found in the acinar cytoplasm. The intensity of VEGF in the PCa glands was higher compared to hyperplastic glands. We observed that AdGS8-10 significantly contributed to the higher staining intensity. As it has been previously found, VEGF is increased according to the severity of PCa; nonetheless, our data were unable to discriminate between early stages (AdG6-7) and the benign hyperplastic disease. These particularities could allow the histological discrimination between malignant and benign regions constituting relevant information for the pathological analysis. Nonetheless, these results should be verified using ELISA to quantitatively assess VEGF and PEDF expression in tissues, specially with those from prostatectomies where the volume of biological material is abundant.
To study if the serum values of VEGF and PEDF in PCa and BPH were similar to its staining intensity in biopsies, we perform a correlation analysis. Interestingly, we found that in PCa had a significant difference and a positive trend with its levels in biopsies, this is to say, the phenomenon in the tumor is reflected by the circulating levels of both proteins. Similarly, PEDF had a higher correlation between the levels in serum and biopsy, contrary to the common pre-conception, we found a simultaneous increment of both pro-angiogenic (VEGF), and anti-angiogenic (PEDF) factors.
Our results seem to reveal a fine-tuning performed by the balance of VEGF and PEDF levels. Several anti-angiogenic mechanisms, where PEDF acts upon VEGF in a direct or indirect manner, have been proposed in physiological conditions. First, an interference of the VEGFR1 signaling through transmembranal excision activated by the PEDF-induced gamma-secretase (44). Second, antagonist activity of PEDF upon VEGFR1 and VEGFR2 to promote its internalization and degradation inside endothelial cells (45). Finally, stimulation by PEDFR/PPARγ signaling that leads to apoptosis of endothelial cells via FAS-L (17,39,46). On the contrary, the growth of malignant cells is caused by alterations in the balance between VEGF and PEDF, releasing in consequence matrix metalloproteases (MMPs) that influence migration and proliferation of endothelial cells and extracellular PEDF degradation (14).
Prospective and simultaneous measurements of serum levels of VEGF, PEDF or the use of their ratio, along with other diagnostic methods, including t-PSA could be clinically relevant for determining the progression of the disease in a personalized manner, and allow the physicians to make better decisions in doubtful cases. However, these are only preliminary descriptive data and further research is required to determine the role VEGF and PEDF in PCa.
Acknowledgements
The authors would like to thank Mr. Alejandro Herrera-Mundo for his support and valuable consultation on the histological procedures and immunohistochemistry techniques.
References
- 1.Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012:152–156. doi: 10.1093/jncimonographs/lgs035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Guerra LS, Martinez-Fierro ML, Alcantara-Aragon V, Ortiz-Lopez R, Martinez-Villarreal RT, Morales-Rodriguez IB, Garza-Guajardo R, Ponce-Camacho MA, Rojas-Martinez A. Population based prostate cancer screening in north Mexico reveals a high prevalence of aggressive tumors in detected cases. BMC Cancer. 2009;9:91. doi: 10.1186/1471-2407-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, DeKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6,630 men. J Urol. 2017;197:S200–S207. doi: 10.1016/j.juro.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZQ, Fang JM, Xiao YY, Zhao Y, Cui R, Hu F, Xu Q. Prognostic role of vascular endothelial growth factor in prostate cancer: A systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:2289–2298. [PMC free article] [PubMed] [Google Scholar]
- 6.Gyftopoulos K, Vourda K, Sakellaropoulos G, Perimenis P, Athanasopoulos A, Papadaki E. The angiogenic switch for vascular endothelial growth factor-A and cyclooxygenase-2 in prostate carcinoma: Correlation with microvessel density, androgen receptor content and Gleason grade. Urol Int. 2011;87:464–469. doi: 10.1159/000329289. [DOI] [PubMed] [Google Scholar]
- 7.Nordby Y, Andersen S, Richardsen E, Ness N, Al-Saad S, Melbø-Jørgensen C, Patel HR, Dønnem T, Busund LT, Bremnes RM. Stromal expression of VEGF-A and VEGFR-2 in prostate tissue is associated with biochemical and clinical recurrence after radical prostatectomy. Prostate. 2015;75:1682–1693. doi: 10.1002/pros.23048. [DOI] [PubMed] [Google Scholar]
- 8.Duque JL, Loughlin KR, Adam RM, Kantoff P, Mazzucchi E, Freeman MR. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: Relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006;61:401–408. doi: 10.1590/S1807-59322006000500006. [DOI] [PubMed] [Google Scholar]
- 9.Silva SA, Gobbo MG, Pinto-Fochi ME, Rafacho A, Taboga SR, Almeida EA, Góes RM, Ribeiro DL. Prostate hyperplasia caused by long-term obesity is characterized by high deposition of extracellular matrix and increased content of MMP-9 and VEGF. Int J Exp Pathol. 2015;96:21–30. doi: 10.1111/iep.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanou D, Batistatou A, Kamina S, Arkoumani E, Papachristou DJ, Agnantis NJ. Expression of vascular endothelial growth factor (VEGF) and association with microvessel density in benign prostatic hyperplasia and prostate cancer. In vivo. 2004;18:155–160. [PubMed] [Google Scholar]
- 11.Walsh K, Sriprasad S, Hopster D, Codd J, Mulvin D. Distribution of vascular endothelial growth factor (VEGF) in prostate disease. Prostate Cancer Prostatic Dis. 2002;5:119–122. doi: 10.1038/sj.pcan.4500575. [DOI] [PubMed] [Google Scholar]
- 12.Botelho F, Pina F, Lunet N. VEGF and prostatic cancer: A systematic review. Eur J Cancer Prev. 2010;19:385–392. doi: 10.1097/CEJ.0b013e32833b48e1. [DOI] [PubMed] [Google Scholar]
- 13.Peyromaure M, Goulvestre C, Fulla Y, Grabar S, Debré B, Dinh-Xuan AT. Serum levels of vascular endothelial growth factor in patients undergoing prostate biopsy for suspicion of prostate cancer. Urology. 2005;66:687–691. doi: 10.1016/j.urology.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 14.Becerra SP, Notario V. The effects of PEDF on cancer biology: Mechanisms of action and therapeutic potential. Nat Rev Cancer. 2013;13:258–271. doi: 10.1038/nrc3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halin S, Wikström P, Rudolfsson SH, Stattin P, Doll JA, Crawford SE, Bergh A. Decreased pigment epithelium-derived factor is associated with metastatic phenotype in human and rat prostate tumors. Cancer Res. 2004;64:5664–5671. doi: 10.1158/0008-5472.CAN-04-0835. [DOI] [PubMed] [Google Scholar]
- 16.Wu QJ, Gong CY, Luo ST, Zhang DM, Zhang S, Shi HS, Lu L, Yan HX, He SS, Li DD, et al. AAV-mediated human PEDF inhibits tumor growth and metastasis in murine colorectal peritoneal carcinomatosis model. BMC Cancer. 2012;12:129. doi: 10.1186/1471-2407-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong Q, Qiu S, Li S, Ma Y, Chen M, Yao Y, Che D, Feng J, Cai W, Ma J, et al. Proapoptotic PEDF functional peptides inhibit prostate tumor growth-a mechanistic study. Biochem Pharmacol. 2014;92:425–437. doi: 10.1016/j.bcp.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Mirochnik Y, Aurora A, Schulze-Hoepfner FT, Deabes A, Shifrin V, Beckmann R, Polsky C, Volpert OV. Short pigment epithelial-derived factor-derived peptide inhibits angiogenesis and tumor growth. Clin Cancer Res. 2009;15:1655–1663. doi: 10.1158/1078-0432.CCR-08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qingyi Z, Lin Y, Junhong W, Jian S, Weizhou H, Long M, Zeyu S, Xiaojian G. Unfavorable prognostic value of human PEDF decreased in high-grade prostatic intraepithelial neoplasia: A differential proteomics approach. Cancer Invest. 2009;27:794–801. doi: 10.1080/07357900802175617. [DOI] [PubMed] [Google Scholar]
- 20.Byrne JC, Downes MR, O'Donoghue N, O'Keane C, O'Neill A, Fan Y, Fitzpatrick JM, Dunn M, Watson RW. 2D-DIGE as a strategy to identify serum markers for the progression of prostate cancer. J Proteome Res. 2009;8:942–957. doi: 10.1021/pr800570s. [DOI] [PubMed] [Google Scholar]
- 21.Grossniklaus HE, Zhang Q, You S, McCarthy C, Heegaard S, Coupland SE. Metastatic ocular melanoma to the liver exhibits infiltrative and nodular growth patterns. Hum Pathol. 2016;57:165–175. doi: 10.1016/j.humpath.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi D, Xie F, Zhang Y, Tian Y, Chen W, Fu L, Wang J, Guo W, Kang T, Huang W, Deng W. TFAP2A regulates nasopharyngeal carcinoma growth and survival by targeting HIF-1α signaling pathway. Cancer Prev Res (Phila) 2014;7:266–277. doi: 10.1158/1940-6207.CAPR-13-0271. [DOI] [PubMed] [Google Scholar]
- 23.Jeng KS, Sheen IS, Jeng WJ, Su JC. PEDF effectively decreases VEGF to PEDF messenger RNA ratio of the inner edge of rat hepatocellular carcinoma induced by diethyl nitrosamine-an ‘in vivo’ study. Hepatogastroenterology. 2012;59:1484–1490. doi: 10.5754/hge11543. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Xu Z, Iuvone PM, Grossniklaus HE. Angiostatin decreases cell migration and vascular endothelium growth factor (VEGF) to pigment epithelium derived factor (PEDF) RNA ratio in vitro and in a murine ocular melanoma model. Mol Vis. 2006;12:511–517. [PubMed] [Google Scholar]
- 25.Bai YJ, Huang LZ, Zhou AY, Zhao M, Yu WZ, Li XX. Antiangiogenesis effects of endostatin in retinal neovascularization. J Ocul Pharmacol Ther. 2013;29:619–626. doi: 10.1089/jop.2012.0225. [DOI] [PubMed] [Google Scholar]
- 26.Regulation on Research for Health of the General Health Law. http://www.salud.gob.mx/unidades/cdi/nom/compi/rlgsmis.html http://www.salud.gob.mx/unidades/cdi/nom/compi/rlgsmis.html
- 27.Official Mexican Norm number NOM-253-SSA1-2012, for the disposal of human blood and its components for therapeutic purposes. http://www.cnts.salud.gob.mx/descargas/PROY_A_NOM_2-1.pdf http://www.cnts.salud.gob.mx/descargas/PROY_A_NOM_2-1.pdf
- 28.Jamaspishvili T, Kral M, Khomeriki I, Student V, Kolar Z, Bouchal J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:12–19. doi: 10.1038/pcan.2009.31. [DOI] [PubMed] [Google Scholar]
- 29.Kim NH, Oh JH, Seo JA, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kang YS, Han SY, et al. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT-1 in diabetic nephropathy. Kidney Int. 2005;67:167–177. doi: 10.1111/j.1523-1755.2005.00067.x. [DOI] [PubMed] [Google Scholar]
- 30.Shariat SF, Anwuri VA, Lamb DJ, Shah NV, Wheeler TM, Slawin KM. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J Clin Oncol. 2004;22:1655–1663. doi: 10.1200/JCO.2004.09.142. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Kantoff PW, Ma J, Stampfer MJ, George DJ. Prediagnostic plasma vascular endothelial growth factor levels and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1557–1561. doi: 10.1158/1055-9965.EPI-04-0456. [DOI] [PubMed] [Google Scholar]
- 32.Mao K, Badoual C, Camparo P, Delongchamps NB, Vieillefond A, Dinh-Xuan AT, Peyromaure M. The prognostic value of vascular endothelial growth factor (VEGF)-A and its receptor in clinically localized prostate cancer: A prospective evaluation in 100 patients undergoing radical prostatectomy. Can J Urol. 2008;15:4257–4262. [PubMed] [Google Scholar]
- 33.Soulitzis N, Karyotis I, Delakas D, Spandidos DA. Expression analysis of peptide growth factors VEGF FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic hyperplasia. Int J Oncol. 2006;29:305–314. [PubMed] [Google Scholar]
- 34.Lekas A, Lazaris AC, Deliveliotis C, Chrisofos M, Zoubouli C, Lapas D, Papathomas T, Fokitis I, Nakopoulou L. The expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and angiogenesis markers in hyperplastic and malignant prostate tissue. Anticancer Res. 2006;26:2989–2993. [PubMed] [Google Scholar]
- 35.Shih SJ, Dall'Era MA, Westphal JR, Yang J, Sweep CG, Gandour-Edwards R, Evans CP. Elements regulating angiogenesis and correlative microvessel density in benign hyperplastic and malignant prostate tissue. Prostate Cancer Prostatic Dis. 2003;6:131–137. doi: 10.1038/sj.pcan.4500637. [DOI] [PubMed] [Google Scholar]
- 36.Voss M, Trojan L, Steidler A, Weiss C, Grobholz R, Alken P, Michel MS. Serum vascular endothelial growth factor C level in patients with prostate cancer and benign prostatic hyperplasia. Anal Quant Cytol Histol. 2008;30:199–202. [PubMed] [Google Scholar]
- 37.Matsui T, Ojima A, Higashimoto Y, Taira J, Fukami K, Yamagishi SI. Pigment epithelium-derived factor inhibits caveolin-induced interleukin-8 gene expression and proliferation of human prostate cancer cells. Oncol Lett. 2015;10:2644–2648. doi: 10.3892/ol.2015.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelius T, Martinez-Marin D, Hirsch J, Miller B, Rinard K, Lopez J, de Riese W, Filleur S. Pigment epithelium-derived factor expression prolongs survival and enhances the cytotoxicity of low-dose chemotherapy in castration-refractory prostate cancer. Cell Death Dis. 2014;5:e1210. doi: 10.1038/cddis.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch J, Johnson CL, Nelius T, Kennedy R, Riese WD, Filleur S. PEDF inhibits IL8 production in prostate cancer cells through PEDF receptor/phospholipase A2 and regulation of NFκB and PPARγ. Cytokine. 2011;55:202–210. doi: 10.1016/j.cyto.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Nelius T, Samathanam C, Martinez-Marin D, Gaines N, Stevens J, Hickson J, de Riese W, Filleur S. Positive correlation between PEDF expression levels and macrophage density in the human prostate. Prostate. 2013;73:549–561. doi: 10.1002/pros.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ide H, Yamagishi S, Lu Y, Sakamaki K, Nakajima A, Horiuchi A, Kitamura K, Hisasue S, Muto S, Yamaguchi R, Horie S. Circulating pigment epithelium-derived factor (PEDF) is associated with pathological grade of prostate cancer. Anticancer Res. 2015;35:1703–1708. [PubMed] [Google Scholar]
- 42.Doll JA, Stellmach VM, Bouck NP, Bergh AR, Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J, Crawford SE. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 43.Carnagarin R, Dharmarajan AM, Dass CR. PEDF-induced alteration of metabolism leading to insulin resistance. Mol Cell Endocrinol. 2015;401:98–104. doi: 10.1016/j.mce.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Cai J, Chen Z, Ruan Q, Han S, Liu L, Qi X, Boye SL, Hauswirth WW, Grant MB, Boulton ME. γ-Secretase and presenilin mediate cleavage and phosphorylation of vascular endothelial growth factor receptor-1. J Biol Chem. 2011;286:42514–42523. doi: 10.1074/jbc.M111.296590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–2070. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]