Abstract
Although the MELDNa score is now used for liver transplant allocation in the United States, mortality prediction may be underestimated by the score. Using aggregated electronic health record data from 7,834 adult patients with cirrhosis, we determined whether the cause of cirrhosis/cirrhosis complications is associated with an increased risk of death among patients with a MELDNa ≤15 and whether patients with the greatest risk of death could benefit from liver transplantation (LT). Over a median follow-up of 2.3 years, 3,715 patients had a maximum MELDNa score ≤15. 3.4% were wait-listed for LT. Severe hypoalbuminemia, hepatorenal syndrome and hepatic hydrothorax conferred the greatest risk of death independent of MELDNa score with a 1 year predicted mortality >14%. Approximately 10% possessed these risk factors. Of these high risk patients, only 4% were wait-listed for liver transplantation despite no difference in non-liver comorbidities between patients wait-listed and those not listed. Also, risk factors for death among patients wait-listed were the same as those not wait-listed although the effect of malnutrition was significantly greater for wait-listed patients (HR 8.65 CI 2.57–29.11 versus HR 1.47 CI 1.08–1.98). Using the MELDNa score for allocation may continue to limit access to liver transplantation.
Introduction
Model for End-stage Liver Disease (MELD)-based liver allocation, which was implemented in the United States (US) in 2002, prioritizes access to liver transplantation based on medical urgency.(1) As an objective measure, MELD was a significant improvement from the prior allocation policy which used the Child-Turcote-Pugh score and waiting time.(2) However, despite present MELD based allocation policy, the annual mortality on the waiting list remains substantial with approximately 2,000 patients dying and another 1,000 patients removed because they are too sick for transplant.(3) While many of these deaths are due to the shortage of organs, some are attributable to imperfect mortality risk prediction.
Previous studies have identified subgroups of patients at higher risk of mortality than predicted by their MELD score thereby limiting their access to liver transplantation.(4) Hyponatremia, for example, has been recognized as a prominent risk factor for mortality in end-stage liver disease (ESLD) independent of the MELD score. (5) Therefore, since January 2016, the MELD sodium (MELDNa) score has been used for liver allocation instead of MELD.(6) ESLD patients with a low MELD score are the predominant beneficiaries of this new liver allocation system because the greatest improvement in mortality risk prediction using MELDNa is seen in patients with a low MELD score and low serum sodium levels.(5)
Other predictors of mortality, besides serum sodium, which confer significant risk of death independent of the MELD score have been previously reported.(7, 8) For instance, ascites (7) and hepatic encephalopathy (8), albeit subjective, are complications of ESLD, which portend a poor outcome despite a low MELD score. However, it remains unknown whether mortality risk continues to be underestimated in specific subgroups of patients with cirrhosis and low MELDNa given the improvement in risk prediction using the MELDNa score instead of MELD.
A large epidemiological study of all patients with cirrhosis is limited by the absence of a national registry. To date cirrhosis patient data have largely been provided by single institutions and the national liver transplant registry (4, 5, 8) that only captures patients listed for transplantation. This data source predominantly includes patients with MELDNa scores above 15. Novel data sources that consist of a wide-ranging population of patients with liver cirrhosis can improve on the limitations of current data sources.(9) Therefore, in the absence of a national cirrhosis database, this study used aggregated electronic health record (EHR) data (HealthLNK)(10) from multiple healthcare networks encompassing the greater Chicago metropolitan area, to determine whether subgroups of patients with cirrhosis and MELDNa ≤ 15 continue to be disadvantaged by the current MELDNa-based liver allocation system, thereby limiting their access to life-saving liver transplantation.
Materials and Methods
This retrospective cohort study of patients with liver cirrhosis was conducted between January 1, 2006 and December 31, 2012 and was approved by the Northwestern University Institutional Review Board (IRB number STU00104145)
Data Sources
Three sources of data were used: HealthLNK,(10) United Network for Organ Sharing (UNOS), (11) and the Social Security death master file.(12)
The HealthLNK Data Repository is an assembly of electronic health records (de-identified) from six health care institutions: five large Academic Medical Centers (Northwestern Medicine, University of Chicago Hospitals and Clinic, Rush University Medical Center, University of Illinois at Chicago Medical Center, and Loyola University Medical Center); and one large academic safety net health care system (Cook County Health and Hospitals System). (10) HealthLNK Data Repository currently contains records for encounters between January 1, 2006 and Dec 31, 2012 for patients residing in the city of Chicago and surrounding suburbs. Data provided by the various institutions included demographics, diagnoses, medications, laboratory values and procedures.(10)
UNOS provided data about liver transplantation including waiting list status and transplant date. (11) Death dates were obtained from the Social Security death master file for Illinois. (12)
Study Population
Patients with liver cirrhosis and 18 years of age or older were identified using the Ninth Revision of International Classification of Diseases and Health Related Problems (ICD-9) codes 571.2 or 571.5 or 571.6, which have previously been validated. (13–15)
Among patients identified with liver cirrhosis, MELD and MELDNa scores were calculated from multiple laboratory test results in accordance with recent Organ Procurement and Transplantation Network (OPTN) rules.(16) Median international normalized ratio (INR), creatinine, bilirubin and sodium laboratory values were determined for each month. Patients on warfarin, those with hepatocellular carcinoma, and those for whom a MELD and MELDNa score could not be calculated were excluded.
The study population was then divided into 3 groups based on their highest MELD and MELDNa achieved during the study period: (1) Low-MELDNa patients with a maximum MELD and MELDNa ≤ 15 (i.e. MELD/MELDNa was never above 15); (2) Mixed-MELD patients with a maximum MELD ≤ 15 but a MELDNa >15; and (3) High-MELD patients with a MELD and MELDNa >15. This study focuses on the Low-MELDNa group because this group of patients is less likely to be listed for liver transplantation because of their low MELDNa score. Factors associated with an increased risk of mortality independent of MELDNa score were therefore assessed in this group of patients only. The Mixed-MELD group was used to assess the effect of a low serum sodium on mortality among patients with a MELD ≤ 15. Mortality rates between the 3 groups were compared to confirm the effect of an increasing MELDNa score on mortality.
Complications of cirrhosis were defined using various combinations of ICD-9 codes, current procedural terminology (CPT) codes, medications, and laboratory values, using previously validated methods.(17) The definitions used to define the study population and complications of cirrhosis are provided in the Appendix. To ascertain non-liver related morbidity, the Elixhauser comorbidity index (18) was adapted and used.
Data Analysis
A summary of demographic and clinical characteristics are presented as mean and standard deviation or median and interquartile range for continuous variables. Categorical variables are presented as number and percent.
Data were retrieved for patients during the study period (January 1, 2006 – December 31, 2012), beginning with their first ICD-9 code for cirrhosis (defined above) until death, liver transplantation, or end of the study period. The primary endpoint of the study was defined as all-cause mortality with liver transplantation treated as a competing risk for death.
The predicted cumulative incidence function of death over time among the three groups was plotted and subdistribution hazards compared using the Fine and Gray Methods.(19)
A multivariate Fine and Gray subdistribution hazards model (20) was fitted to determine factors associated with mortality in the Low-MELDNa group independent of MELDNa score. Covariates were chosen for inclusion in the multivariate model if the observed univariate association was significant with p-value <0.05, as well as, if the covariate was deemed by author consensus to be potentially clinically significant. The multivariate model was adjusted for age, gender, race, insurance status, Elixhauser comorbidity index, (18) and maximum MELDNa score. A p-value of < 0.05 was considered significant in the final model.
Subgroup and further analysis
First, in order to assess whether low-MELDNa patients on the transplant waitlist had similar risk factors for death compared to non-waitlisted patients, a Fine and Gray subdistribution hazards model using interaction terms was used. We adopted this approach to assess whether risk factors are the same in the 2 populations as well as to determine whether the relative effects of each risk factor differ depending on whether a patient is on the transplant wait-list or not.
Second, in order to assess whether patients who possess risk factors that confer an increased risk of death independent of MELDNa score could potentially benefit from liver transplantation in terms of survival, the predicted probability of death at 1 year was calculated (using cumulative incidence functions) for each complication of cirrhosis with a hazard ratio >1.1 obtained from the multivariate subdistribution hazards model. Recent data demonstrate that living donor liver transplantation in the US has a 1 year survival probability of 90.32 %. (21) Thus, a subgroup of patients with cirrhosis complication who had a 1 year predicted mortality risk ≥14% was selected. (The cutoff of 14% was decided upon following author consensus as a mortality risk reasonably greater than approximately 10% seen following living donor liver transplantation). This subgroup was stratified by liver transplant waitlist status and compared by age, gender, insurance, etiology of cirrhosis, Elixhauser score, and MELDNa score, using 2 sample t-tests, Chi-square test, Fishers Exact test, and the Wilcoxon Rank Sum test as appropriate.
Analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC)
Results
Study Population
A total of 10,302 adult patients with liver cirrhosis and at least 1 MELD/MELDNa score were identified. Of these 782 (8%) patients were on warfarin, 1,668 (16%) patients had hepatocellular carcinoma, and 18 (0.2%) patients had an abnormal time-censoring variable (i.e. an event occurred before the appearance of the ICD-9 code for cirrhosis) and therefore, were excluded resulting in a study population of 7,834 patients. Of these, 3,715 (47%) were in the Low-MELDNa group; 484 (6%) were in the Mixed-MELD group, and 3,635 (46%) patients were in the High-MELD group. A comparison of demographic and clinical characteristics between the 3 groups is provided in Table 1.
TABLE 1.
Characteristics of Patients with Liver Cirrhosis* (N=7,834)
| Variable | Low-MELDNa group N=3715 |
Mixed- MELD group N=484 |
High-MELD group N=3635 |
P Value | |
|---|---|---|---|---|---|
| Age-years | 55±11 | 56±11 | 55±11 | 0.2168a | |
| Male-no. (%) | 1955 (53) | 627 (65) | 2292 (63) | <.0001c | |
| Race-no. (%) | |||||
| White | 1458 (39) | 191 (39) | 1499 (41) | 0.0115c | |
| Black | 801 (22) | 97 (20) | 813 (22) | ||
| Hispanic | 799 (22) | 129 (27) | 747 (21) | ||
| Asian | 102 (3) | 6 (1) | 75 (2) | ||
| Other | 555 (15) | 61 (13) | 501 (14) | ||
| Etiology of Cirrhosis†-no. (%) | |||||
| Alcohol | 1149 (31) | 269 (56) | 2125 (58) | <.0001c | |
| Hepatitis C | 1532 (41) | 186 (38) | 1291 (36) | <.0001c | |
| NASH | 695 (19) | 73 (15) | 670 (18) | 0.1510c | |
| Hepatitis B | 291 (8) | 30 (6) | 313 (9) | 0.1365c | |
| Cholestatic Disease | 416 (11) | 24 (5) | 349 (10) | <.0001c | |
| Median Elixhauser Score-(IQR) | 2 (1–4) | 4 (1–6) | 4 (2–7) | <.0001b | |
| Insurance-no. (%) | |||||
| Medicare/Medicaid | 1501 (40) | 213 (44) | 1610 (44) | <.0001c | |
| Private Insurance | 1277 (34) | 128 (26) | 1072 (30) | ||
| Other | 937 (25) | 143 (30) | 953 (26) | ||
| Number of MELDNa Scores | 5±5 | 6±6 | 7±7 | <.0001a | |
| Median MELDNa (IQR) | 9(8–11) | 17 (16–20) | 24(20–30) | <.0001b | |
Plus-minus values are means+/− SD.
Categories are not mutually exclusive
P-Values determined by ANOVA,
P-Values determined by Kruskal Wallis test,
P-Values determined by Chi-squared test
Table Legend: Demographics of three liver cirrhosis cohorts are shown. For each patient the maximum MELD and MELDNa was calculated. If both the highest MELD and MELDNa score was ≤15 patients were stratified into a Low-MELDNa group; if MELD was ≤15 and MELDNa >15, into a Mixed-MELD group and; if both the highest MELD and MELDNa >15 into a High-MELD group.
Abbrev: MELDNa: MELD Sodium, NASH: Nonalcoholic steatohepatitis
Predicted Mortality
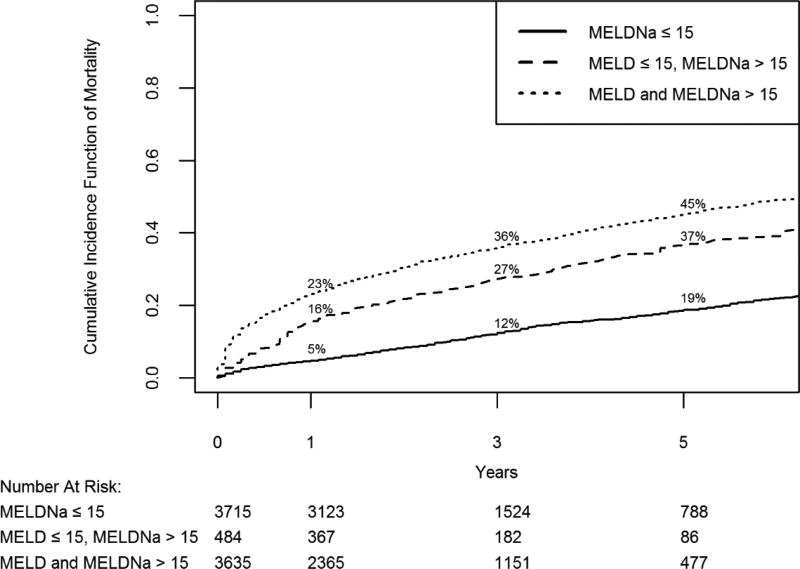
Figure 1 shows the cumulative incidence of death having accounted for liver transplantation as a competing risk. The maximum length of follow-up was 7 years with a median follow-up time of 2.3 years. Cumulative incidence of death in the Low-MELDNa group was 4.7% (95% CI 4.1–5.5%) at 1 year, 12.1% (95% CI 10.9–13.3 %) at 3 years, and 18.6% (95% CI 16.9–20.4%) at 5 years. Improved mortality prediction, using MELDNa instead of MELD is demonstrated in the Mixed- MELD group, as patients with MELD ≤15 but MELDNa >15, because of low serum sodium, have an increased cumulative incidence of death.
Figure 1. Predicted Mortality of Patients with Liver Cirrhosis.
Cumulative incidence function of mortality when treating liver transplantation as a competing risk for 3 groups of patients with liver cirrhosis. Predicted 1, 3 and 5 year mortality for each group is shown. If both the highest MELD and MELDNa score was ≤15, patients were stratified into a Low-MELDNa cohort; if the highest MELD was ≤15 but highest MELDNa >15, into a Mixed-MELD cohort and ; if both the highest MELD and highest MELDNa was > 15 into a High-MELD cohort
Gray’s test for equality of cumulative incidence function shows a statistical difference between the 3 groups (p<0.0001)
Abbrev: MELD/MELDNa: Model for end-stage liver disease sodium
Risk Factors Associated with Mortality among the Low-MELDNa Group
Results from univariate subdistribution hazards models are presented in Supplementary Table 1(Appendix). A serum albumin consistently below 2.8 gram per deciliter (g/dL) (severe hypoalbuminemia) or between 2.8 and 3.5 g/dL (moderate hypoalbuminemia), hepatorenal syndrome, hepatic hydrothorax, transjugular intrahepatic portosystemic shunt (TIPS), spontaneous bacterial peritonitis, ascites, malnutrition, hepatic encephalopathy, maximum platelet count ≤ 50 ×109 /L or between 50–100 ×109 /L, alcoholic cirrhosis, portal hypertension, MELDNa score, and Elixhauser comorbidity index (16) were all associated with a statistically significant increase in mortality risk. Nonalcoholic steatohepatitis (NASH) cirrhosis, and cholestatic cirrhosis were associated with a statistically significant reduced mortality risk.
Jaundice, the presence of esophageal varices without bleeding, variceal bleeding, hepatitis B and hepatitis C were not statistically significant. Hepatopulmonary syndrome was excluded from analysis as only 4 (0.1%) patients in the Low-MELDNa group had this risk factor and imprecise estimates were obtained from the subdistribution hazards model.
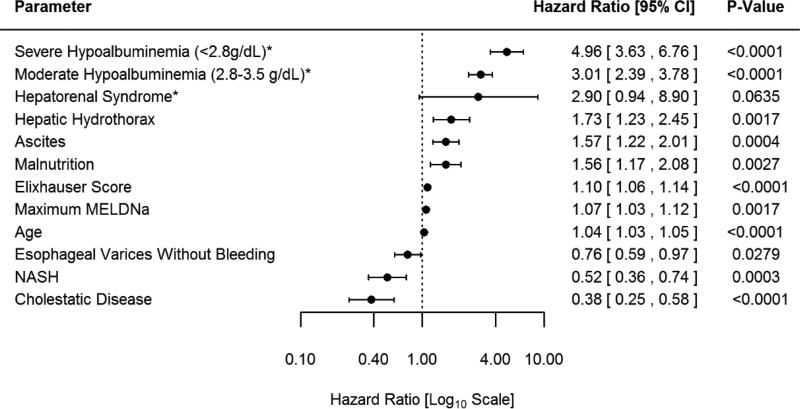
The multivariate subdistribution hazards model is shown in Figure 2. All significant univariate predictors and the non-significant predictors listed above (determined to be clinically significant by the authors) were included in the model. After adjusting for age, gender, race, insurance, MELDNa, and Elixhauser comorbidity index, the most significant predictors of mortality were severe hypoalbuminemia (HR 5.0; p<0.0001) and moderate hypoalbuminemia (HR 3.0; p<0.0001). Other important predictors of mortality include hepatic hydrothorax, ascites and malnutrition. In this model, hepatorenal syndrome was not statistically significant (p=0.06) however, in the subsequent model where interaction terms are introduced a statistically significant p value (p=0.04) for hepatorenal syndrome is observed. Thus, we considered hepatorenal syndrome a statistically significant predictor of mortality. A diagnosis of NASH (p=0.003), cholestatic cirrhosis (p<0.0001) and the presence of esophageal varices without bleeding (p=0.03) were associated with a reduced risk of death. Predictors that were not statistically significant are provided in Supplementary Table 2 (Appendix). TIPS was noted to convey an increased risk of death in the univariate model (HR 2.8 p=0.03). A non-statistically significant reduced risk of death for TIPS was observed in the multivariate model (HR 0.4 p=0.38).
Figure 2. Multivariate Subdistribution Hazards Model.
*Reference >3.5g/dL
The Figure demonstrates the Hazard Ratio for risk factors for mortality determined by the multivariate subdistribution hazards model among patients with MELDNa ≤ 15 (Low-MELDNa). The model is adjusted for age, gender, race, insurance, Elixhauser score and maximum MELDNa score. Severe hypoalbuminemia is defined as maximum serum albumin <2.8g/dl during the study period. Moderate hypoalbuminemia is defined as a maximum serum albumin 2.8–3.5g/dl during the study period. In this model hepatorenal syndrome is not statistically significant, however in a subsequent model (figure 3) where interaction terms for transplant waitlist are introduced the main effect of hepatorenal syndrome is observed to be statistically significant p=0.0414 (HR 3.27 95% CI 1.048–10.23)
Abbrev: CI: confidence interval, g/dl: gram per deciliter, NASH: Nonalcoholic steatohepatitis MELDNa: Model for end-stage liver disease sodium
Assessment of whether risk factors differ depending on transplant waitlist status
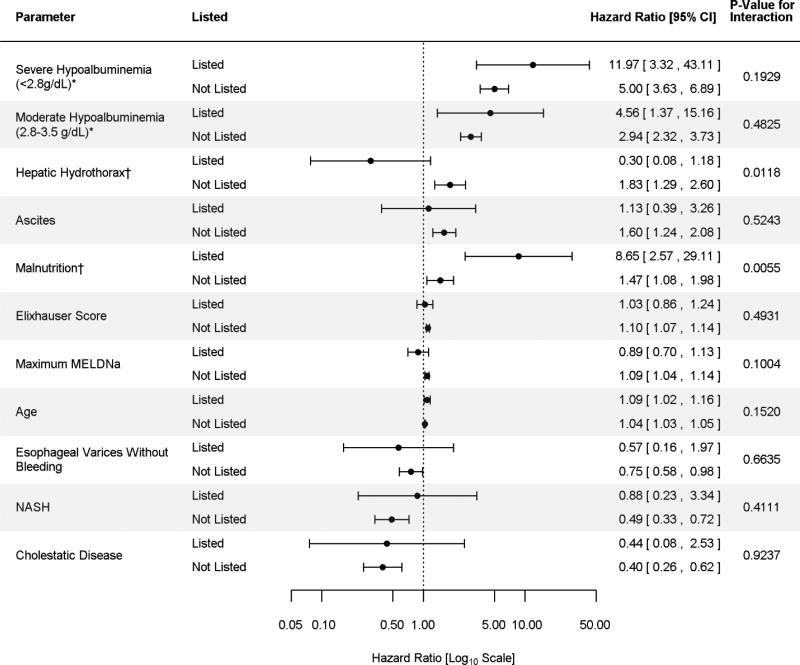
Among Low-MELDNa patients, 3.4% (n=125) were wait-listed for liver transplantation. Risk factors for death among patients on the transplant waitlist were the same as the risk factors for death among those not on the transplant waitlist Figure 3. However, the effect of hepatic hydrothorax and malnutrition differed by waitlist status as evidenced by statistically significant interaction terms Figure 3. Hepatic hydrothorax is associated with an increased risk of death for those not wait-listed for liver transplantation. A non-statistically significant association is however observed for those wait-listed. Malnutrition on the other hand is associated with significantly greater risk of death among waitlisted patients than among non-waitlisted patients.
Figure 3. Multivariate Subdistribution Hazards Model by Liver Transplant Waitlist Status.
*Reference >3.5g/dL
† Statistically significant interaction term
The Figure demonstrates the Hazard Ratio for risk factors for mortality determined by the subdistribution hazards model among patients with MELDNa ≤ 15 according to transplant waitlist status. Overall risk factors for patients on the waitlist are the same as risk factors for those not waitlisted. However, the effect of hepatic hydrothorax and malnutrition differ whether an individual is the liver transplant waitlist or not. The model is adjusted for age, gender, race, insurance, Elixhauser score and maximum MELDNa score. Severe hypoalbuminemia is defined as maximum serum albumin <2.8g/dl during the study period. Moderate hypoalbuminemia is defined as a maximum serum albumin 2.8–3.5g/dl during the study period.
Abbrev: CI: confidence interval, g/dl: gram per deciliter, NASH: Nonalcoholic steatohepatitis MELDNa: Model for end-stage liver disease sodium
Potential for liver transplantation
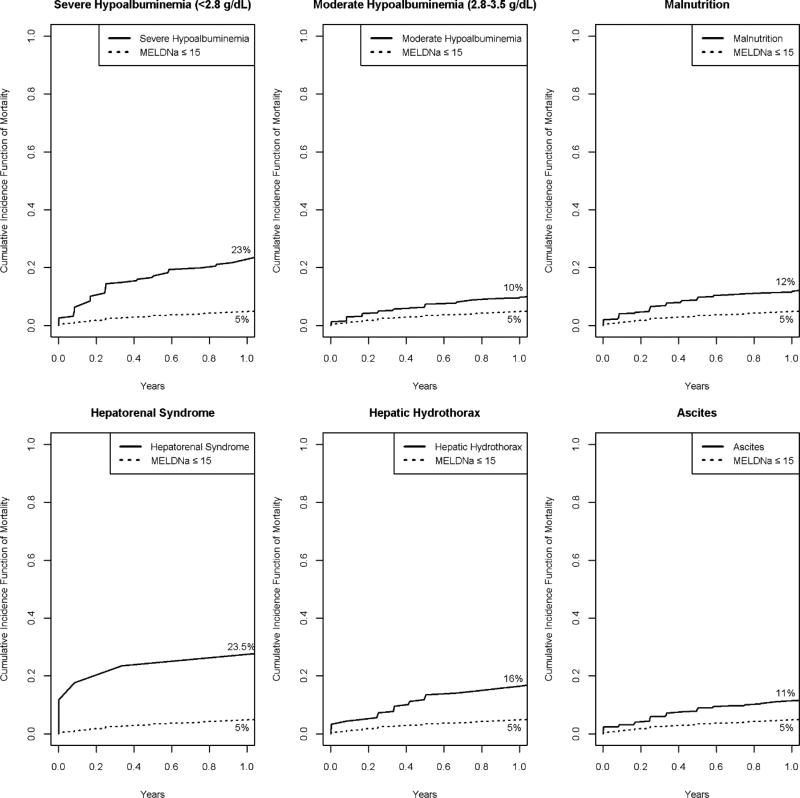
Cumulative incidence functions for death up to 1 year for risk factors obtained from the multivariate subdistribution hazards model are shown in Figure 4. One-year mortality following living donor liver transplantation in the US is approximately 10%.(21)
Figure 4. *. Cumulative Incidence Function of Mortality plots showing 1 year predicted mortality among patients in Low-MELDNa cohort according to specific risk factor.
*Risk factors included are those obtained from the multivariate subdistribution hazard model figure 2.
The figure demonstrates 1 year predicted mortality accounting for liver transplantation as a competing risk among patients with Low-MELDNa according to specific risk factor. The dotted line represents the curve for all patients with MELDNa≤15. Solid line represent the curve for patients with MELDNa≤15 and specific risk factor of cirrhosis.
Abbrev: g/dl: gram per deciliter, MELDNa: Model for end-stage liver disease sodium
A predicted 1-year mortality of 4.7% was seen in the Low-MELDNa group overall as previously stated above. However, patients with severe hypoalbuminemia had a 1-year predicted mortality of 22.9% (95% CI 17–29%) while those with moderate hypoalbuminemia had a 1 year predicted mortality of 9.6% (95% CI 7.3–12.1%). Patients with hepatorenal syndrome had a predicted 1 year mortality of 23.5% (95% CI 6.9–45.6), hepatic hydrothorax 16.5% (95% CI 11.4–22.3%), malnutrition11.5% (95%CI 8.4–15.1%) and ascites 11.4% (95%CI 8.4–14.2%).
Patients with severe hypoalbuminemia, hepatorenal syndrome, or hepatic hydrothorax comprised 9.7% of the Low-MELDNa group and there were considered at high risk for death (1-year mortality ≥ 14%). Among this high risk subgroup, only 4.2% (n=15) were waitlisted for liver transplantation. When the high risk subgroup was further stratified by transplant waitlist status, no statistically significant differences were seen between those listed and those not listed for liver transplantation in terms of age, gender, insurance status, or Elixhauser comorbidity index. Those listed for liver transplantation had a higher MELDNa score and were more likely to have cholestatic disease as shown in Table 2.
Table 2.
*. Comparison of Patients with Severe hypoalbuminemia <2.8g/dL or Hepatorenal Syndrome or Hepatic Hydrothorax by liver transplant waitlist status Total N=360
| Variable | Listed for transplant n=15 |
Not Listed n=345 |
P Value | |
|---|---|---|---|---|
| Age-years | 54±12 | 58±12 | 0.1799a | |
| Male-no. (%) | 8 (53) | 196 (57) | 0.7901b | |
| Insurance-no. (%) | ||||
| Medicare/Medicaid | 8 (53) | 174 (50) | 0.1294c | |
| Private Insurance | 6 (40) | 79 (23) | ||
| Other | 1 (7) | 92 (27) | ||
| Etiology of Cirrhosis†-no. (%) | ||||
| Alcohol | 4 (27) | 120 (35) | 0.5173b | |
| Hepatitis C | 8 (53) | 121 (35) | 0.1488b | |
| NASH | 4 (27) | 53 (15) | 0.2713c | |
| Hepatitis B | 0 (0) | 14 (4) | 0.9999c | |
| Cholestatic Disease | 6 (40) | 28 (8) | 0.0012c | |
| Median Elixhauser Score-(IQR) | 4 (1–6) | 4 (2–6) | 0.6223d | |
| MELDNa Median(IQR) | 13(11–14) | 11 (9–13) | 0.0452d | |
No statistically significant difference is seen between those listed for liver transplant and those not listed except for MELDNa Score and Cholestatic Disease
P-Values determined by 2 sample t test,
P-Values determined by Chi-squared test t,
P-Value determined by Fishers Exact test
P-Values determined by Wilcoxon Rank Sum test
Table Legend: The table shows a comparison, by liver transplant waitlist status, of patients with MELDNa ≤15 and severe hypoalbuminemia or hepatorenal syndrome or hepatic hydrothorax. These complications have a predicted 1 year risk of death ≥ 14%.
Abbreviations: NASH non-alcoholic steatohepatitis
Discussion
Although an improvement from MELD, mortality prediction is still underestimated for important subgroups of patients with liver cirrhosis by using the MELDNa score. These subgroups include patients with hypoalbuminemia, hepatorenal syndrome, hepatic hydrothorax, malnutrition, and ascites. The recent adoption of the MELDNa score for liver allocation in the US will thus continue to have important consequences for these subgroups of patients. For the broader population of patients with cirrhosis and a low-MELDNa score, access to liver transplantation is the issue of concern. On the other hand, for those wait-listed, underestimation of death on the wait-list will persist.
This study uses population-based data and is distinct from other studies. By using data from a large population of patients with liver cirrhosis, we find that very few Low-MELDNa patients are wait-listed for liver transplantation, even though approximately 10% possess risk factors that confer a 1 year mortality greater than that of living donor liver transplantation. Furthermore, among Low-MELDNa patients at high risk, only approximately 4% were wait-listed. The comparison between those wait-listed with those not wait-listed in this subgroup showed that wait-listed patients were more likely to have a higher MELDNa score and more likely to have the diagnosis of cholestatic disease. However, no differences in age, gender, non-liver comorbidities or insurance status between the 2 groups were observed. Consequently, it is likely that there are many Low-MELDNa patients who are not currently listed for liver transplantation who can benefit from the procedure. In fact, many may not even undergo evaluation for liver transplantation.
The American Association for the Study of Liver Diseases (AASLD) guidelines from 2013 recommend that “the evaluation for liver transplant should be considered once a patient with cirrhosis has experienced an index complication such as ascites, hepatic encephalopathy, or variceal hemorrhage or hepatocellular dysfunction resulting in a MELD score equal or greater than 15.” (22) As a result, this cutoff of 15 has largely been viewed by clinicians as the threshold to waitlist a patient.(23) However, in this study, the highest median MELDNa score achieved among the Low-MELDNa group, after median follow-up of 2.3 years, was 9 with an interquartile range of 8–11. Thus, these patients died without ever having a MELDNa score of at least 15.
Patients with low MELDNa scores, who may be at high risk of death, currently have less access to liver transplantation as evidenced by a decline in the proportions of Low-MELD patients wait-listed nationally between 2004 and 2014. In 2004, 58.5% of the waitlist population were patients with MELD scores < 15 and by 2014, the proportion dropped to 45.6 %. (3) More importantly, in 2004, 11.7% of wait-listed patients who received a liver transplant had a MELD of <15, compared to only 2.7% in 2014.(3) The MELD allocation policy has had a significant effect on selection of patients for liver transplantation that cannot be ignored. This negative effect is likely to persist, despite the current use of the MELDNa score for allocation instead of MELD. Importantly, additional new changes in policy, such as regional sharing of organs for patients with MELDNa >35,(24) will likely further decrease the listing of patients with MELDNa <15. Due to the regional disparity in organ availability in the US, this negative effect will likely impact highly competitive regions to a greater extent. (25)
It is often argued that many Low-MELDNa patients at high risk are not transplant candidates. Thus, in this study we perform a subgroup analysis to examine mortality risk factors among Low-MELDNa patients on the liver transplant waiting-list in the context of the broader population of Low-MELDNa patients with cirrhosis. We find that among Low-MELDNa patients listed for liver transplantation risk factors for mortality are the same as those not listed however, the effect of malnutrition (after adjusting for multiple variables including a low albumin) was significantly greater for patients on the wait-list compared to patients not on the wait-list. For hepatic hydrothorax, the risk of death was increased in those not wait-listed though not statistically significant for those on the waitlist.
One possible reason hepatic hydrothorax confers an increased risk for non-waitlisted patients is perhaps the tendency for non-transplant professionals to attempt drainage, thus placing these patients at an increased risk of complications. With regard to malnutrition, the risk of death for wait-listed patients we observed is greater than previously published reports. For example in the FrAILT study, (26) a cohort study evaluating functional decline in patients with cirrhosis awaiting liver transplantation, a Short Physical Performance Battery Score of less than 10 (a measure of malnutrition(27)) was associated with a wait-list mortality hazard ratio of 1.45 (CI 1.15–2.20). The hazard ratio in the FrAILT study is similar to the hazard ratio for non-waitlisted patients in our study.
The effect of malnutrition among Low-MELDNa patients on the wait-list found in this study is of clinical importance. Not only is death on the waitlist significantly underestimated for these patients, malnutrition is independently associated with early post-liver transplant mortality and post-transplant outcomes may be poor if these patients undergo transplantation. (28) Post-transplant outcomes are not the focus of this study however, the significant difference in risk observed between patients on the transplant waitlist compared to patients not wait-listed can be explained in part by wait-listing practices of Low-MELDNa patients in the Chicago Metropolitan Area. This study therefore demonstrates that current wait-listing for patients with a Low-MELDNa may on one hand be limiting access for some patients while on the other hand selecting patients whose risk may be too great.
Given very few patients with a Low-MELDNa are wait-listed for liver transplantation, targeting Low-MELDNa patients who are at high risk for evaluation is important. A lack of available organs is a significant barrier to deceased organ transplantation. This shortage means that often Low-MELDNa patients have to “settle” for marginal organs. Therefore, many transplant programs may not list Low-MELDNa patients due to concern regarding poor outcomes and Centers of Medicare and Medicaid Services flagging. Living donor liver transplantation might offer a lifesaving alternative. Living donor liver transplantation has been shown to provide survival benefit for patients with MELD scores <15 (29) and is an underutilized resource in the US. Greater effort can therefore be made to identify Low-MELDNa patients who may benefit from the procedure.
The analysis in this study cannot account for all the factors that are taken into consideration for transplant wait-listing such as ongoing substance abuse. We also cannot determine whether specific patients were referred or underwent transplant evaluation. We accept that indeed not all high risk low MELDNa patients are actually transplant candidates, and if they are transplant candidates, not all of them may have living liver donors. However, our analysis provides evidence that the use of the MELDNa score for wait-listing may reduce access to liver transplantation for some high risk patients and that Low-MELDNa patients currently wait-listed form a highly select population of patients.
Liver transplantation may not be the only management approach for high risk Low-MELDNa patients. In this study we identified esophageal varices without bleeding as being associated with a reduced risk of death in the multivariate model. We postulate that these are patients for whom portal hypertension is diagnosed very early in the clinical course or patients who are receiving better medical management or both. It is also possible that this group of patients has a unique biological advantage in that they develop varices that do not tend to bleed. We hypothesize that among low MELD patients very early diagnosis of portal hypertension and improved care may prevent death and liver transplantation may not be necessary.
While early diagnosis of portal hypertension may reduce the risk of death, we find that Low-MELDNa patients who underwent TIPS had an increased risk of death in the univariate model. In the multivariate model, TIPS was not statistically significant. A larger sample of low MELDNa patients undergoing TIPS will be needed to fully identify whether TIPS reduces mortality in Low-MELDNa patients with portal hypertension.
This study has some limitations. It is retrospective and observational and prone to selection, information, and confounding biases.(30) Data from the Chicago Metropolitan was used; a highly competitive region with regard to available organs for liver transplantation. Also, our data source cannot account for patients who transfer their care outside the Chicago Metropolitan Area. However, given the data used in the study is obtained from different institutions the population is more diverse. Furthermore, follow-up in this study occurred over a period of 7 years allowing the assessment of long term survival.
Risk factors and complications were defined using various combinations of ICD-9 codes, CPT codes, laboratory values and medications. While these approaches have been validated by previous literature (13–15, 17) they are limited by the quality of the medical records. It is possible that the definitions used in this study to capture risk factors and complications overemphasized some risk factors while underemphasizing others. However, contrary to other population datasets, this study is novel as it captures information directly from the EHR as opposed to administrative datasets. EHR data has been shown to be a better and more accurate source of data for studies.(31)
Lastly, the primary end-point of this study was all-cause mortality as opposed to liver associated mortality. It is possible that some of the deaths observed in the study were unrelated to liver disease. A competing risk model which accounts for different causes of death could not be used in this study due to unavailable cause of death data. To mitigate the concern for liver-unrelated disease severity, the Elixhauser comorbidity index (18) was calculated for each patient. Overall, the study results are consistent with what would be expected clinically.
While the allocation policy using MELDNa represents a step forward, persistent limitation should prompt the development of more comprehensive risk prediction tools in the future. The accurate identification of those patients at highest risk for mortality and their targeted selection for liver transplantation or other management modalities provides an opportunity to save additional lives.
Supplementary Material
Table S1: Univariate Subdistribution Hazards Model showing statistically significant predictors of mortality
Table S2: Multivariate Subdistribution Hazards Model showing non-statistically significant predictors of mortality
Acknowledgments
This study was supported by NIH grant T32DK077662 PI MM ABECASSIS, MD.
List of Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ANOVA
Analysis of variance
- CPT
Common Procedural Terminology
- EHR
Electronic Health Records
- ESLD
End Stage Liver Disease
- g/dL
Grams per deciliter
- ICD-9
Ninth Revision of International Classification of Diseases and Health Related Problems
- INR
International Normalized Ratio
- MELD
model for end-stage liver disease
- MELDNa
Model for end-stage liver disease-Sodium
- NASH
Nonalcoholic steatohepatitis
- OPTN
Organ Procurement and Transplantation Network rules OPTN
- TIPS
Transjugular intrahepatic portosytemic shunt
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–6. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 2.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology (Baltimore, Md) 2001;33(2):464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 3.Kim JRL WR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR Annual Data Report 2014 (LIVER) American Journal of Transplantation. 2016;16(S2):69–98. doi: 10.1111/ajt.12581. [DOI] [PubMed] [Google Scholar]
- 4.Huo TI, Lin HC, Wu JC, Hou MC, Lee FY, Lee PC, et al. Limitation of the model for end-stage liver disease for outcome prediction in patients with cirrhosis-related complications. Clinical transplantation. 2006;20(2):188–94. doi: 10.1111/j.1399-0012.2005.00463.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. The New England journal of medicine. 2008;359(10):1018–26. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elwir S, Lake J. Current Status of Liver Allocation in the United States. Gastroenterology & hepatology. 2016;12(3):166–70. [PMC free article] [PubMed] [Google Scholar]
- 7.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology (Baltimore, Md) 2004;40(4):802–10. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 8.Yoo HY, Edwin D, Thuluvath PJ. Relationship of the model for end-stage liver disease (MELD) scale to hepatic encephalopathy, as defined by electroencephalography and neuropsychometric testing, and ascites. The American journal of gastroenterology. 2003;98(6):1395–9. doi: 10.1111/j.1572-0241.2003.07466.x. [DOI] [PubMed] [Google Scholar]
- 9.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(8):1723–30. doi: 10.1111/ajt.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HealthLNK. http://www.healthlnk.org/
- 11.OPTN/UNOS. Available from: https://optn.transplant.hrsa.gov/converge/data/
- 12.Index SSD. Social Security Death Index. Available from: http://www.ntis.gov/products/ssa-dmf.aspx.
- 13.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology. 2013;47(5):e50–4. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo Re V, 3rd, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiology and drug safety. 2011;20(7):689–99. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Lo Re V., 3rd Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiology and drug safety. 2013;22(1):103–7. doi: 10.1002/pds.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.OPTN. Organ Procurement and Transplantation Network (OPTN) Policies-Allocation of Livers and Liver-Intestines. Available from: tps://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_09.
- 17.He D, Mathews SC, Kalloo AN, Hutfless S. Mining high-dimensional administrative claims data to predict early hospital readmissions. Journal of the American Medical Informatics Association : JAMIA. 2014;21(2):272–9. doi: 10.1136/amiajnl-2013-002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Cheng SC, Fine JP, Wei LJ. Prediction of cumulative incidence function under the proportional hazards model. Biometrics. 1998;54(1):219–28. [PubMed] [Google Scholar]
- 20.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(8):2301–8. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SRTR. TABLE C12L. Adult (18+) 1-year patient survival (living donor graft recipients) Single organ transplants performed between 01/01/2013 and 06/30/2015. Retransplants excluded. Available from: www.srtr.org.
- 22.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology (Baltimore, Md) 2014;59(3):1144–65. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg D, French B, Newcomb C, Liu Q, Sahota G, Wallace AE, et al. Patients With Hepatocellular Carcinoma Have Highest Rates of Wait-listing for Liver Transplantation Among Patients With End-Stage Liver Disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(11):1638–46.e2. doi: 10.1016/j.cgh.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow EK, Massie AB, Luo X, Wickliffe C, Gentry SE, Cameron AM, et al. Waitlist Outcomes of Liver Transplant Candidates Reprioritized Under Share-35. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentry SE, Massie AB, Cheek SW, Lentine KL, Chow EH, Wickliffe CE, et al. Addressing geographic disparities in liver transplantation through redistricting. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(8):2052–8. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: Results from the functional assessment in liver transplantation (FrAILT) study. Hepatology (Baltimore, Md) 2016;63(2):574–80. doi: 10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrader E, Grosch E, Bertsch T, Sieber CC, Volkert D. Nutritional and Functional Status in Geriatric Day Hospital Patients - MNA Short Form Versus Full MNA. The journal of nutrition, health & aging. 2016;20(9):918–26. doi: 10.1007/s12603-016-0691-4. [DOI] [PubMed] [Google Scholar]
- 28.Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. Journal of cachexia, sarcopenia and muscle. 2016 doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg CL, Merion RM, Shearon TH, Olthoff KM, Brown RS, Jr, Baker TB, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology (Baltimore, Md) 2011;54(4):1313–21. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet (London, England) 2002;359(9302):248–52. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 31.Tang PC, Ralston M, Arrigotti MF, Qureshi L, Graham J. Comparison of methodologies for calculating quality measures based on administrative data versus clinical data from an electronic health record system: implications for performance measures. Journal of the American Medical Informatics Association : JAMIA. 2007;14(1):10–5. doi: 10.1197/jamia.M2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Univariate Subdistribution Hazards Model showing statistically significant predictors of mortality
Table S2: Multivariate Subdistribution Hazards Model showing non-statistically significant predictors of mortality