Abstract
We investigated whether stratifying by the interval between first-line (T1) and second-line (T2) treatments could identify a subgroup of older patients with relapsed CLL/SLL with an expectation of normal overall survival. Longer time-to-T2 was associated with a modestly improved prognosis; however, even among those retreated ≥ 3 years after T1, survival was poor compared with the general population (5-year relative survival = 50%).
Background
Novel targeted therapies offer excellent short-term outcomes in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma (CLL/SLL). However, there is disagreement over how widely these therapies should be used in place of standard chemo-immunotherapy (CIT). We investigated whether stratification on the length of the interval between first-line (T1) and second-line (T2) treatments could identify a subgroup of older patients with relapsed CLL/SLL with an expectation of normal overall survival, and for whom CIT could be an acceptable treatment choice.
Patients and Methods
Patients with relapsed CLL/SLL who received T2 were identified from the SEER-Medicare Linked Database. Five-year relative survival (RS5; ie, the ratio of observed survival to expected survival based on population life tables) was assessed after stratifying patients on the interval between T1 and T2. We then validated our findings in the Mayo Clinic CLL Database.
Results
Among 1974 SEER-Medicare patients (median age = 77 years) who received T2 for relapsed CLL/SLL, longer time-to-retreatment was associated with a modestly improved prognosis (P = .01). However, even among those retreated ≥ 3 years after T1, survival was poor compared with the general population (RS5 = 0.50 or lower in SEER-Medicare). Similar patterns were observed in the younger Mayo validation cohort, although prognosis was better overall among the Mayo patients, and patients with favorable fluorescence in situ hybridization retreated ≥ 3 years after T1 had close to normal expected survival (RS5 = 0.87).
Conclusion
Further research is needed to quantify the degree to which targeted therapies provide meaningful improvements over CIT in long-term outcomes for older patients with relapsed CLL/SLL.
Keywords: Mortality, Prognosis, SEER-Medicare
Introduction
Novel therapies available targeting Bruton’s tyrosine kinase, PI-3 kinase, and bcl-2 have demonstrated excellent short-term outcomes in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma (CLL/SLL).1–3 Particularly for patients with an otherwise poor prognosis, these treatments are likely to be transformative. However, these agents must be taken indefinitely, are costly ($100,000–$150,000 annually),4,5 and carry risks of significant side effects (eg, atrial fibrillation, colitis, infection, and tumor lysis syndrome).6 For these reasons, it is important to identify the CLL/SLL patients for whom these new agents are most likely to provide a meaningful benefit compared with standard chemo-immunotherapy (CIT) regimens. Additionally, it would be useful to identify subgroups of patients with relapsed or refractory CLL/SLL who are at low risk for premature mortality with standard CIT options, and thus would have a suitable alternative to long-term use of newer agents.
In patients with relapsed CLL/SLL, the time interval from initial therapy until relapse is recognized as an important prognostic marker. Patients who relapse sooner are less responsive to subsequent treatments and have shorter overall survival (OS).7–9 This association has been characterized in a younger patient cohort (median age < 60 years) that received first-line treatment with fludarabine, cyclophosphamide, and rituximab (FCR). Median OS following relapse was 13 months among patients who relapsed in less than 3 years, as compared with 63 months in those who relapsed in ≥ 3 years.10 However, because the median age at diagnosis for CLL/SLL is 71 years,11 these results may not be generalizable to most patients with CLL/SLL. Older patients typically receive less intensive antineoplastic therapies, and OS patterns following relapse are likely to be different in older adults due to competing risks of mortality. A more precise characterization of this relationship in older adults will inform clinicians’ efforts to risk-stratify and appropriately treat older patients with relapsed CLL/SLL.
In this study we assessed the prognostic value of time-until-retreatment in predicting subsequent OS among older CLL/SLL cases identified from the Surveillance, Epidemiology, and End Results (SEER)-Medicare Linked Database (SEER-Medicare) and an independent validation cohort identified from the Mayo Clinic CLL Database. We hypothesized that, before the era of novel targeted therapies, patients retreated ≥ 3 years after their first course of antineoplastic therapy would have OS close to that expected in the US general population after controlling for age, sex, and calendar year.
Methods
Data Sources
The study data for the SEER-Medicare patient cohort were obtained from the 2014 SEER-Medicare linkage, which included SEER cancer cases diagnosed from 1973 to 2011 and their Medicare claims from 1992 to 2013. Within SEER registry catchment areas, 93% of patients aged 65+ years diagnosed with cancer have been linked to their Medicare claims data.12 Participating registries collect data for all patients with cancer diagnosed within their defined geographic area. Registry data include month and year of diagnosis, age at diagnosis, race, tumor stage, and histology. Medicare files from the Centers for Medicare and Medicaid Services (CMS) include demographic and enrollment information, date of death, and all bills submitted for inpatient hospital care, outpatient hospital care, physician services, and prescription fills.
Normative mortality rates based on the US general population were obtained from The Human Mortality Database,13 which assembles historical US life tables based on data published by the US Census Bureau14 and National Center for Health Statistics.15 As described in the statistical methods section, these data were used for the relative survival estimates used to describe CLL/SLL patients’ prognosis compared with age- and sex-matched controls.
Institutional Review Board Review and Research Ethics
This research project was approved by SEER-Medicare Program staff and the University of Iowa Institutional Review Board (IRB). The University of Iowa IRB granted a waiver of informed consent because the project consisted of a secondary analysis of existing data. SEER-Medicare Program staff reviewed the manuscript to ensure that it met reporting requirements to protect patient confidentiality. The validation study in the Mayo Clinic CLL Database, described at the end of the methods section, was performed with the approval of the Mayo Clinic IRB.
Cohort Definition
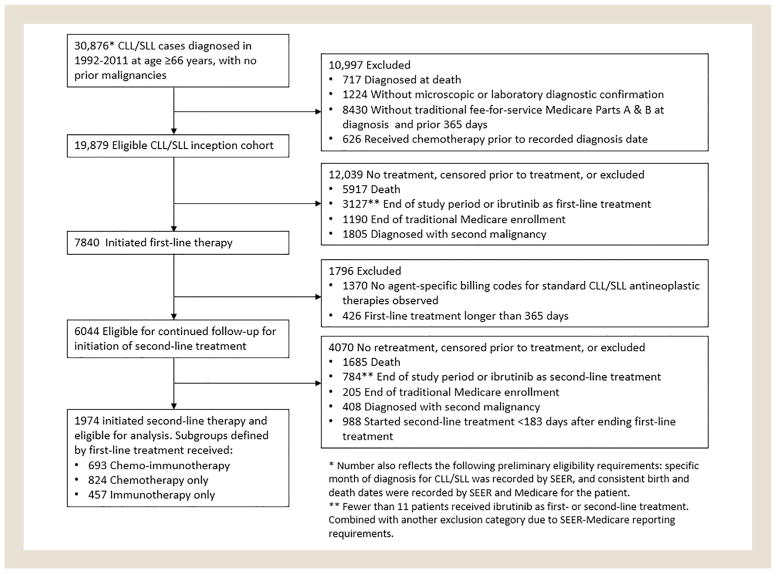
Our study population consisted of patients who at age ≥ 66 years were diagnosed with CLL/SLL from 1992 to 2011. Cases were excluded if they had no specific diagnosis month recorded by SEER, had a prior malignancy, had inconsistent birth or death dates recorded by SEER and CMS, were diagnosed at death, did not have microscopic or laboratory diagnostic confirmation, were not enrolled in traditional fee-for-service Medicare Parts A and B at diagnosis and the prior 365 days, or had claims for antineoplastic therapy before the diagnosis date recorded by SEER (Figure 1). The age and Medicare eligibility restrictions ensured that we could evaluate patients’ receipt of antineoplastic therapy, comorbidity burden, and proxies for CLL/SLL progression using Medicare claims data.
Figure 1.
Flow Diagram Showing Identification of Eligible Surveillance, Epidemiology, and End Results (SEER)-Medicare Patients With Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma (CLL/SLL)
Patients in this CLL/SLL inception cohort became eligible for inclusion in the present study on receiving first- and second-line courses of antineoplastic therapy. Follow-up began on the date that second-line therapy was initiated. Patients were excluded if prior to second-line treatment any of the following censoring events occurred: death, the end of the study period (December 31, 2013), loss of traditional Medicare enrollment, diagnosis with a second primary malignancy, or initiation of ibrutinib (a novel Bruton tyrosine kinase inhibitor initially approved in 2013). Cohort identification steps are shown in Figure 1.
We restricted our study sample to patients whose first course of antineoplastic therapy was no more than 365 days. For most CLL/ SLL chemotherapy regimens, the treatment plan would consist of 6 cycles of treatment at 4-week intervals. Some patients may discontinue treatment early or take breaks between treatment cycles, and treatment with some agents (eg, chlorambucil or rituximab monotherapy) may continue for 12 months. We further restricted to patients in whom the start of the second treatment course was at least 183 days after the end of the first treatment course. This requirement was motivated by concerns that parsing shorter gaps between treatment episodes into distinct treatment courses would be difficult based solely on claims data. In addition, patients who relapse fewer than 6 months after their last antineoplastic treatment are considered to have refractory CLL/SLL, and are known to have a poor prognosis.9,16
Exposure Definition
The main prognostic factor of interest was time-until-retreatment, defined as the difference in years between the end of the first course of antineoplastic therapy and the beginning of the second course of antineoplastic therapy. Receipt of antineoplastic treatment was assessed using procedure, diagnosis, and drug codes recorded in Medicare inpatient, outpatient, and prescription claims. See Supplemental Tables 1 and 2 (in the online version) for details on the codes and code ranges that were used. Corticosteroids were not included in our study definition of antineoplastic therapy because they are frequently used for other indications, and rarely used as monotherapy for CLL/SLL. Drug-specific Healthcare Common Procedure Coding System (HCPCS) and National Drug Codes (NDCs) were used to identify specific antineoplastic agents administered. Patients were classified as having received chemo-immunotherapy (CIT), chemotherapy, or immunotherapy. More details on the specific treatment regimens that patients received are provided in Supplemental Table 3 (in the online version).
A course of treatment was defined as beginning on the start date associated with the first claim where antineoplastic therapy was recorded, continuing for as long as additional claims were observed (allowing for gaps of no more than 90 days), and ending on the end date of the last claim in the series. A prior chart validation study performed in patients with lymphoma found that Medicare claims data generally provide reliable information on whether and when patients receive chemotherapy.17
Study Endpoint
The study endpoint was all-cause mortality. The SEER-Medicare dataset includes each patient’s date of death as recorded in the Social Security Death Master File. In the files used in the present study from the 2014 SEER-Medicare linkage, mortality data were available through the end of 2013. Patients who were alive on December 31, 2013, were right-censored in our survival analyses.
Statistical Methods
Five-year relative survival (RS5) was used to characterize the prognosis of patients with CLL/SLL. RS5, the ratio of 5-year observed OS relative to OS in the general population after conditioning on age, sex, and calendar year, was estimated with the Ederer II method.18–20 RS measures are frequently used in cancer epidemiology as indirect estimates of the burden of cancer-specific mortality in defined patient populations. The statistical significance of differences and trends in RS across patient subgroups was assessed using the additive hazards model endorsed by Dickman et al19 for analyses of relative survival data within a generalized linear models framework.
Covariates
A number of demographic and clinical characteristics were assessed to characterize our study sample (see Table 1), and to explore possible modification of the relationship between time-to-retreatment and relative survival. As a summary measure of comorbidity burden, we report a count of the following 12 major comorbidities included in the National Cancer Institute and Charlson comorbidity indices21–23: cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes, hemiplegia/paraplegia, liver disease, myocardial infarction, peptic ulcer disease, renal disease, rheumatic disease, and human immunodeficiency virus infection. In addition, we provide data on the frequency of several health conditions associated with advanced CLL/SLL disease (eg, anemia and infection).
Table 1.
Characteristics of Eligible Patients Who Received Second-Line Treatment Stratified by First-Line Therapy
| Characteristic | All Treatments (n = 1974) | Chemo-immuno-therapy (n = 693) | Chemotherapy Alone (n = 824) | Immunotherapy Alone (n = 457) |
|---|---|---|---|---|
| Age at second-line treatment in years | ||||
| 66–74 | 653 (33) | 277 (40) | 268 (33) | 108 (24) |
| 75–79 | 577 (29) | 202 (29) | 261 (32) | 114 (25) |
| 80+ | 744 (38) | 214 (31) | 295 (36) | 235 (51) |
| Sex | ||||
| Female | 874 (44) | 266 (38) | 388 (47) | 220 (48) |
| Male | 1100 (56) | 427 (62) | 436 (53) | 237 (52) |
| Number of treatment cycles included in first-line treatment course | ||||
| 1–2 | 650 (33) | 109 (16) | 231 (28) | 310 (68) |
| 3+ | 1324 (67) | 584 (84) | 593 (72) | 147 (32) |
| Interval between first- and second-line antineoplastic treatments | ||||
| 6 mo to <2 y | 1325 (67) | 434 (63) | 531 (64) | 360 (79) |
| 2 y to <3 y | 355 (18) | 155 (22) | 142 (17) | 58 (13) |
| 3+ y | 294 (15) | 104 (15) | 151 (18) | 39 (9) |
| Type of second-line antineoplastic treatment | ||||
| Chemo-immunotherapy | 576 (29) | 298 (43) | 183 (22) | 95 (21) |
| Chemotherapy alone | 486 (25) | 80 (12) | 375 (46) | 31 (7) |
| Immunotherapy alone | 609 (31) | 207 (30) | 122 (15) | 280 (61) |
| Unknown (nonspecific billing codes) | 303 (15) | 108 (16) | 144 (17) | 51 (11) |
| Indicators of CLL/SLL progression | ||||
| Anemia | 916 (46) | 331 (48) | 345 (42) | 240 (53) |
| Thrombocytopenia | 358 (18) | 145 (21) | 135 (16) | 78 (17) |
| Immune deficiency | 282 (14) | 147 (21) | 93 (11) | 42 (9) |
| Count of major comorbiditiesa | ||||
| None | 964 (49) | 330 (48) | 420 (51) | 214 (47) |
| 1 | 566 (29) | 197 (28) | 236 (29) | 133 (29) |
| 2 or more | 444 (22) | 166 (24) | 168 (20) | 110 (24) |
| Hospitalized in prior year | 777 (39) | 269 (39) | 335 (41) | 173 (38) |
Values are n (%).
Abbreviation: CLL/SLL = chronic lymphocytic leukemia or small lymphocytic lymphoma.
The major comorbidities covariate, adapted from the National Cancer Institute and Charlson comorbidity indices, is a count of the following 12 comorbidities: cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes, hemiplegia/paraplegia, liver disease, myocardial infarction, peptic ulcer disease, renal disease, rheumatic disease, and human immunodeficiency virus infection.
Health conditions and health care utilization were assessed based on administrative diagnosis codes recorded during the year before retreatment. Following the approach of Klabunde et al,23 a condition was considered present if a corresponding inpatient diagnosis code or 2 outpatient diagnosis codes 30+ days apart were observed. A higher standard was required for outpatient diagnosis codes, because they can sometimes reflect diagnoses that were ruled out or merely considered as part of a differential diagnosis.
Validation Study in Mayo Clinic CLL Database
Data for the validation cohort were obtained from the Mayo Clinic CLL Database with the approval of the Mayo Clinic IRB. The database includes patients with a diagnosis of CLL/SLL seen in the Division of Hematology at the Mayo Clinic since 1995. Clinical information regarding date of diagnosis, baseline evaluation, prognostic parameters, treatment history, and disease-related complications was abstracted from clinical records on all patients and maintained in the database on an ongoing prospective basis. Additional details about the Mayo Clinic CLL Database may be found in prior publications.24,25
The validation study cohort included patients diagnosed with CLL/SLL from 1995 to 2013, and longitudinal follow-up data through 2016. All patients with CLL/SLL who received second-line therapy during this period were identified, classified based on the time-to-retreatment, and followed until death or censoring. RS5 from the initiation of second-line therapy was estimated with the relsurv package for R.26,27 RS5 was estimated for patient subgroups defined by time-to-retreatment, type of first-line antineoplastic therapy received (CIT, chemotherapy, or immunotherapy), IGHV mutation status, and whether 11q or 17p chromosome deletions were detected through fluorescence in situ hybridization (FISH).
Results
During 1992 to 2011, there were 6044 CLL/SLL cases identified from SEER-Medicare who initiated first-line therapy and were eligible for continued follow-up. Of this group, 1974 initiated second-line therapy before censoring and were included in our analyses (Figure 1). As first-line treatment, 693 patients had received CIT (35%), 824 chemotherapy alone (42%), and 457 immunotherapy alone (23%). Frequencies of specific treatment regimens are provided in Supplemental Table 3 (in the online version). At retreatment, the patients had a median age of 77 years (interquartile range: 73–82); 44% were women. Additional patient characteristics are shown in Table 1.
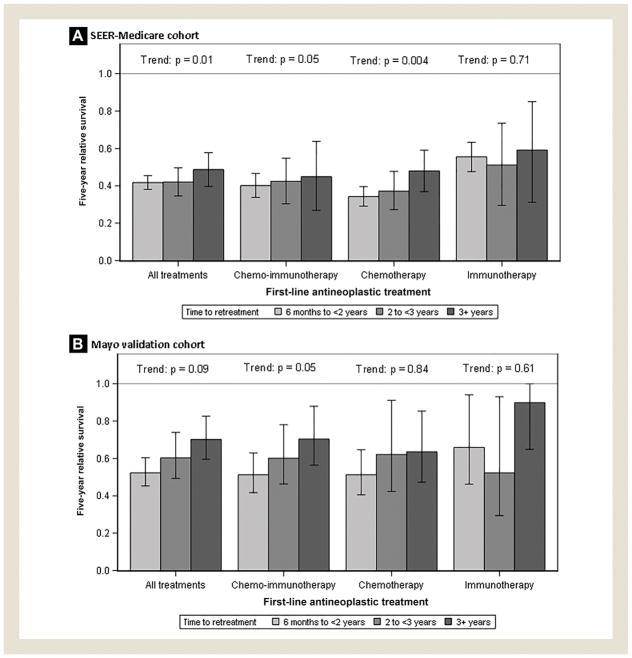
RS5 following second-line treatment was modestly higher in patients retreated ≥ 3 years after first-line treatment (0.49; 95% confidence interval, 0.40–0.58) compared with patients retreated in 6 months to < 2 years (0.42; 95% CI, 0.38–0.45) or 2 to < 3 years (0.42; 95% CI, 0.35–0.50; test for trend: P = .01). The relationship between time-until-retreatment and subsequent RS5 varied by the type of first-line treatment received (Figure 2A). A protective association between longer time-until-retreatment and RS5 was seen among patients who received chemotherapy alone (P = .004) or CIT (P = .054). No clear pattern was observed in patients who received immunotherapy alone (P = .71) as first-line treatment.
Figure 2.
Estimated 5-year Relative Survival Following Initiation of Second-Line Treatment in the Surveillance, Epidemiology, and End Results (SEER)-Medicare Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Patient Cohort (A) and Mayo Validation Cohort (B)
In additional subgroup analyses, we explored whether the association between time-until-retreatment and subsequent RS5 varied by number of treatment cycles received during first-line therapy, patient age, or comorbidity burden. The relationship between longer time-until-retreatment and improved subsequent RS5 was most pronounced among those whose first-line treatment course included 3+ treatment cycles (Supplemental Figure 1 in the online version), who were 80+ years or older (Supplemental Figure 2 in the online version), and who had no major comorbidities (Supplemental Figure 3 in the online version). However, across all subgroups of retreated patients, survival was meaningfully reduced compared with appropriate age- and gender-matched cohorts from the US general population.
Mayo Clinic Validation Study
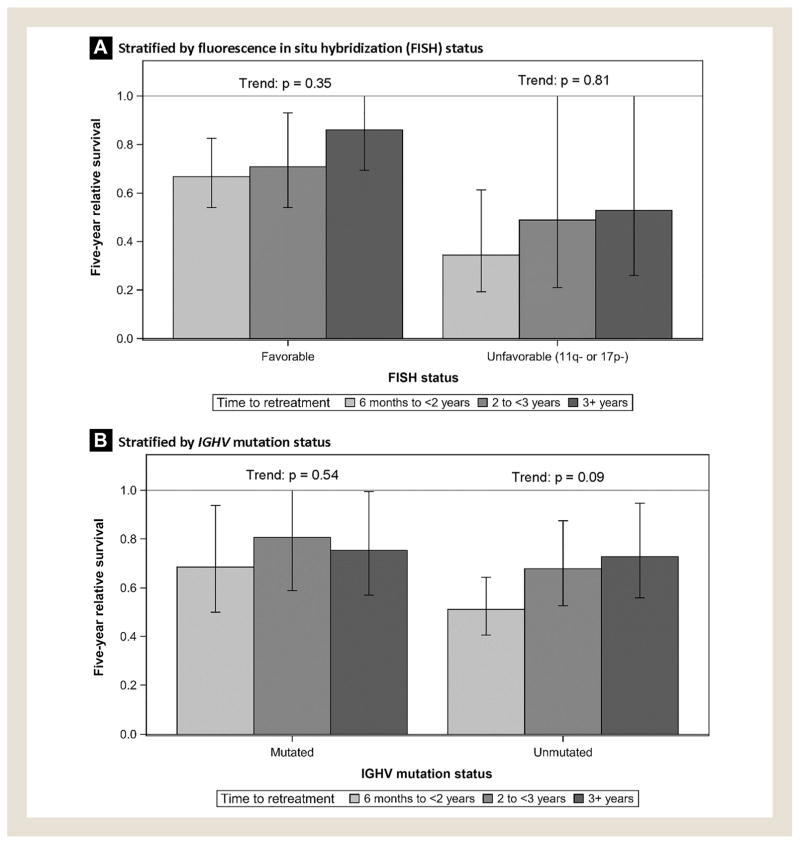
The validation cohort consisted of 508 patients identified from the Mayo Clinic CLL Database who received second-line antineo-plastic therapy. The median patient age was 66 years (interquartile range: 59–74); 28% were women; and 58%, 29%, and 12% had received CIT, chemotherapy alone, and immunotherapy alone as first-line treatment, respectively (see Table 2). RS5 estimates were 0.52, 0.60, and 0.70 for patients retreated in 6 months to < 2 years, 2 to < 3 years, and 3+ years, respectively (test for trend: P = .09). Similar patterns were observed when patients were stratified by type of first-line antineoplastic therapy received (Figure 2A). In subgroup analyses, we stratified patients by IGHV mutation and FISH status. Time-to-retreatment was more strongly predictive of survival in patients with favorable FISH status (ie, no 11q or 17p deletions; Figure 3A) and in patients with unmutated IGHV (Figure 3B), although these trends did not reach statistical significance.
Table 2.
Baseline Characteristics of Eligible Patients in Mayo Validation Cohort Who Received Second-Line Treatment
| Characteristic | Patient Count (n = 508), n (%) |
|---|---|
| Age at second-line treatment in years | |
| ≤65 | 245 (48) |
| 66–74 | 148 (29) |
| 75–79 | 77 (15) |
| 80+ | 38 (7) |
| Sex | |
| Female | 144 (28) |
| Male | 364 (72) |
| Type of first-line treatment received | |
| Chemo-immunotherapy (CIT) | 295 (58) |
| Chemotherapy alone | 148 (29) |
| Immunotherapy alone | 59 (12) |
| Missing | 6 (1) |
| Interval between end of first-line therapy and start of second-line therapy | |
| 6 mo to <2 y | 275 (54) |
| 2 y to <3 y | 105 (21) |
| 3+ y | 128 (25) |
| Fluorescence in situ hybridization status | |
| High risk (11q- or 17p-) | 52 (10) |
| Other/favorable | 187 (37) |
| Missing | 269 (53) |
| IGHV mutation status | |
| Unmutated | 217 (43) |
| Mutated | 84 (17) |
| Missing | 207 (41) |
Figure 3.
Estimated 5-year Relative Survival Following Initiation of Second-Line Treatment in the Mayo Validation Cohort, Stratified by Fluorescence In Situ Hybridization and IGHV Mutation Status
Discussion
In our primary population-based SEER-Medicare study of mortality among older patients with relapsed CLL/SLL who received second-line therapy, we found that longer time-until-retreatment was associated with moderately improved subsequent RS5. Contrary to our initial study hypothesis, and in contradistinction to previous findings in follicular lymphoma (FL), another chronic B-cell malignancy, stratification on time-to-retreatment did not allow us to identify a patient subgroup with normal longevity. Even among patients retreated 3+ years after the end of first-line therapy, mortality was high among the SEER-Medicare patients relative to the general population (RS5 = 0.50). These results indicate that a significant number of older patients with relapsed CLL/SLL retreated with rituximabera CIT regimens are likely to have an unmet therapeutic need.
Overall, similar patterns were observed among patients in the Mayo Clinic CLL Database. Prognosis was better across the board for retreated patients in the younger Mayo cohort, with RS5 estimates ranging from 0.52 for patients retreated in 6 months to < 2 years to 0.70 for patients retreated in 3+ years. It is possible that the better prognosis observed in the Mayo cohort relative to the SEER-Medicare cohort is attributable to its younger age (median age of 66 years vs. 77 years in SEER-Medicare cohort) and other case-mix differences, as well as differences in the selection of patients for treatment.
Because clinical prognostic markers were available in the Mayo Clinic CLL Database, we were able to stratify the Mayo cohort by IGHV and FISH status. These subgroup analyses suggested that time-to-retreatment had greater prognostic value among patients with unmutated IGHV and favorable FISH. Notably, among patients with time-to-retreatment ≥ 3 years and favorable FISH, subsequent survival was close to normal (RS5 = 0.86). Survival was also close to normal for patients with time-to-retreatment ≥ 3 years who had received immunotherapy as first-line treatment (RS5 = 0.90).
Our results differ from those reported in prior work in FL, another typically chronic and indolent lymphoproliferative disease. In FL, it was demonstrated that early events following initial therapy are strongly predictive of subsequent outcomes, independent of baseline clinical prognostic scores.28 In patients with FL who received CIT as first-line treatment, disease progression within 24 months is predictive of meaningfully reduced OS. However, in patients with FL without an event in the 24 months following CIT (or 12 months following less aggressive treatment), OS is comparable to expected rates based on the age- and sex-matched general population. Our differing results may be explained by differences in the biology of FL and CLL/SLL, and in the typical trajectory of disease progression following first-line treatment. In addition, the research questions in the FL study and ours were slightly different: we focused on survival outcomes among patients who initiated second-line treatment, whereas the FL study sought to characterize subsequent survival trajectories among patients who at 24 months had not been retreated or experienced a relapse.
Our primary study relied on data available from SEER registries and Medicare claims, and thus had several limitations. Treatment dates and the type of antineoplastic therapy received were determined from procedure and diagnosis codes recorded by health care providers for reimbursement and Part D pharmacy claims. A prior validation study of billing codes for antineoplastic treatment in Medicare beneficiaries with lymphoma indicated that claims data generally provide valid information on chemotherapeutic agents and services dates.17 We would expect that modest misclassification of the timing and type of treatment would mean that the observed differences in RS5 across patient subgroups may underestimate the true between-group differences in survival outcomes. In addition, clinical prognostic markers were unavailable for the SEER-Medicare cohort.
Because our RS5 estimates reflect comparisons between observed and expected survival based on mortality rates observed in the US general population, it is important to consider the possibility of treatment selection bias. The patients in our SEER-Medicare and Mayo cohorts were all determined to be fit enough for first- and second-line antineoplastic therapy. For these reasons, our sample is likely to be slightly healthier than the general population in terms of comorbidity burden and functional status. Despite this possible source of bias toward improved survival outcomes in the patients with CLL/SLL who received antineoplastic therapy, survival rates were still meaningfully reduced among most patients receiving second-line therapy.
Our findings have several implications. During the rituximab era, most older patients with relapsed and retreated CLL/SLL had meaningfully reduced survival and a clear unmet therapeutic need. Possible exceptions, based on subgroup analyses in the younger Mayo cohort, were patients with favorable FISH and time-to-retreatment ≥ 3 years, or immunotherapy as first-line treatment and time-to-retreatment ≥ 3 years. Further research is needed to quantify the degree to which targeted therapies provide meaningful improvements over CIT in long-term outcomes for older patients with relapsed CLL/SLL.
Clinical Practice Point
There is disagreement over how widely novel targeted therapies should be used in place of standard chemo-immunotherapy (CIT) for the treatment of CLL/SLL.
We investigated whether stratifying by the interval between first-line (T1) and second-line (T2) treatments could identify a subgroup of older patients with relapsed CLL/SLL with an expectation of normal overall survival.
Longer time-to-T2 was associated with a modestly improved prognosis; however, during the rituximab era, most older patients with relapsed and retreated CLL/SLL had meaningfully reduced survival and a clear unmet therapeutic need.
Supplementary Material
Acknowledgments
This study used the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s SEER Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award P50CA097274, by the University of Iowa Holden Comprehensive Cancer Center (HCCC) Population Research Core, which is supported in part by P30 CA086862, and by a Cancer & Aging Pilot Project Award from the University of Iowa HCCC and Center on Aging.
Footnotes
Disclosure
E.M. Ammann is now employed in Johnson & Johnson’s Medical Device Epidemiology research division. The analyses for this paper were completed and the manuscript was drafted before the start of that role. B.K. Link has received compensation as a consultant for Gilead and AbbVie. The remaining authors have stated that they have no conflicts of interest.
Supplemental tables and figures accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.clml.2017.07.004.
References
- 1.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–22. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanafelt TD, Borah BJ, Finnes HD, et al. Impact of ibrutinib and idelalisib on the pharmaceutical cost of treating chronic lymphocytic leukemia at the individual and societal levels. J Oncol Pract. 2015;11:252–8. doi: 10.1200/JOP.2014.002469. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LA. FDA approves drug for tough-to-treat type of leukemia. Associated Press. Financial News. 2016 [Google Scholar]
- 6.McMullen JR, Boey EJ, Ooi JY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–30. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 7.Rai KR, Stilgenbauer S. Treatment of Relapsed or Refractory Chronic Lymphocytic Leukemia. Waltham, MA: UpToDate; 2016. [Google Scholar]
- 8.Brown JR. The treatment of relapsed refractory chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:110–8. doi: 10.1182/asheducation-2011.1.110. [DOI] [PubMed] [Google Scholar]
- 9.Hallek M. Chronic lymphocytic leukemia: 2015 Update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90:446–60. doi: 10.1002/ajh.23979. [DOI] [PubMed] [Google Scholar]
- 10.Tam CS, O’Brien S, Plunkett W, et al. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab) Blood. 2014;124:3059–64. doi: 10.1182/blood-2014-06-583765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SEER Stat Fact Sheets: Chronic Lymphocytic Leukemia (CLL) National Cancer Institute: Surveillance, Epidemiology, and End Results Program; 2015. [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Shkolnikov V, Barbieri M, Wilmoth J. Human Mortality Database. Department of Demography at the University of California; Berkeley (USA): Max Planck Institute for Demographic Research; Rostock (Germany): 2016. [Google Scholar]
- 14.US Census Bureau. Population Estimates and Projection, Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States. Washington, DC: U.S. Department of Commerce; 1992–2013. [Google Scholar]
- 15.National Center for Health Statistics. Vital Statistics of the United States, Volume II: Mortality, Part A. Washington, DC: Government Printing Office; 1992–2013. [Google Scholar]
- 16.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen-Hardee S, Chrischilles EA, Voelker MD, et al. Population-based assessment of hospitalizations for neutropenia from chemotherapy in older adults with non-Hodgkin’s lymphoma (United States) Cancer Causes Control. 2006;17:647–54. doi: 10.1007/s10552-005-0502-4. [DOI] [PubMed] [Google Scholar]
- 18.Cho H, Howlader N, Mariotto AB, et al. Technical Report #2011–01: Estimating Relative Survival for Cancer Patients From the SEER Program Using Expected Rates Based on Ederer I Versus Ederer II Method. Bethesda, Maryland: National Cancer Institute Surveillance Research Program; 2011. [Google Scholar]
- 19.Dickman PW, Sloggett A, Hills M, et al. Regression models for relative survival. Stat Med. 2004;23:51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 20.Ederer F, Heise H. Methodological Note No. 10: Instructions to IBM 650 Programmers in Processing Survival Computations. Bethesda, MD: National Cancer Institute End Results Evaluation Section; 1959. [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–90. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 24.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 25.Shanafelt TD, Jenkins G, Call TG, et al. Validation of a new prognostic index for patients with chronic lymphocytic leukemia. Cancer. 2009;115:363–72. doi: 10.1002/cncr.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohar M, Stare J. Relative survival analysis in R. Comput Methods Programs Biomed. 2006;81:272–8. doi: 10.1016/j.cmpb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Pohar M, Stare J. Making relative survival analysis relatively easy. Comput Biol Med. 2007;37:1741–9. doi: 10.1016/j.compbiomed.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National LymphoCare Study. J Clin Oncol. 2015;33:2516–22. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.