Abstract
After the Fontan procedure, patients with univentricular hearts can experience long-term complications due to chronic low-shear non-pulsatile pulmonary blood flow. We sought to evaluate hemorheology and its relationship to hemodynamics in children with univentricular hearts. We hypothesized that low-shear blood viscosity and red blood cell (RBC) aggregation would be associated with increased pulmonary vascular resistance (PVR) and decreased pulmonary blood flow (PBF). We performed a cross-sectional analysis of 62 children undergoing cardiac catheterization—20 with isolated atrial septal defect (ASD), 22 status post Glenn procedure (Glenn), and 20 status post Fontan procedure (Fontan). Shear-dependent blood viscosity, RBC aggregation and deformability, complete blood count, coagulation panel, metabolic panel, fibrinogen, and erythrocyte sedimentation rate were measured. PVR and PBF were calculated using the Fick equation. Group differences were analyzed by ANOVA and correlations by linear regression. Blood viscosity at all shear rates was higher in Glenn and Fontan, partially due to normocytic anemia in ASD. RBC aggregation and deformability were similar between all groups. Low-shear viscosity negatively correlated with PBF in Glenn and Fontan only (R2 = 0.27, p < 0.001); it also negatively correlated with pulmonary artery pressure in Glenn (R2 = 0.15, p = 0.01), and positively correlated with PVR in Fontan (R2 = 0.28, p = 0.02). Our data demonstrate that elevated low-shear blood viscosity is associated with negative hemodynamic perturbations in a passive univentricular pulmonary circulation, but not in a pulsatile biventricular pulmonary circulation.
Keywords: Congenital heart disease, Univentricular heart, Fontan, Pulmonary vascular resistance, Rheology
Introduction
Collectively, univentricular heart defects are among the most severe forms of congenital heart disease. Children with these defects require staged surgical palliation over the first several years of life, concluding with the Fontan procedure. This creates a unique circulation where the systemic venous return from the superior and inferior vena cavae bypasses the heart and passively fills the pulmonary arteries. Although the Fontan procedure has significantly improved early survival, transplant-free 30-year survival remains at only 60 % [7]. These patients can also experience significant long-term complications including heart failure, protein-losing enteropathy, and plastic bronchitis [8]. The pathophysiology of these complications is poorly understood, but may be linked to limited pulmonary artery growth, low-shear non-pulsatile pulmonary blood flow (PBF), and thrombus formation leading to increased pulmonary vascular resistance (PVR) [18]. Even among asymptomatic patients, exercise tolerance is universally decreased, presumably due to power loss in the Fontan circuit and inability to augment ventricular preload through the pulmonary vasculature [14].
In general, abnormally low shear in blood vessels can lead to intimal hyperplasia and red blood cell (RBC) aggregation [19]; in the Fontan circulation, low-shear flow may also alter the responsiveness of endothelial nitric oxide synthase [13]. Indeed, multiple studies suggest that chronic non-pulsatile pulmonary blood flow leads to endothelial dysfunction in these patients [10, 13]. In a normal biventricular circulation, RBC aggregates formed in the venous circulation are disaggregated as they are pumped through the right heart. However, the lack of a subpulmonary ventricle in the Glenn and Fontan circulations leads to low-shear flow and RBC aggregation in the pulmonary arterial system. The rheologic behavior of blood and its formed elements (i.e., hemorheology), including shear-dependent blood viscosity and RBC aggregation, and its relationship to hemodynamics in children with the Fontan circulation have not been studied previously. Characterization of hemorheology and correlation with PBF and PVR are expected to provide insight into the pathophysiology of pulmonary vascular disease in these patients, creating the basis for novel therapeutic strategies.
We hypothesized that elevated low-shear blood viscosity and RBC aggregation would be associated with elevated PVR in univentricular circulations, but not in a normal biventricular circulation. In order to address this hypothesis, we aimed to: (1) characterize differences in hemorheological characteristics between children with univentricular heart defects undergoing staged surgical palliation with the Glenn and Fontan procedures and those with a biventricular circulation; and (2) determine how hemorheological parameters influence pulmonary hemodynamics, particularly pulmonary artery pressure, PBF, and PVR in univentricular circulations compared to a biventricular circulation.
Materials and Methods
Study Design and Patient Population
We performed a single-center cross-sectional study comparing children with surgically palliated univentricular congenital heart disease (Glenn and Fontan) to children with biventricular hearts and isolated atrial septal defect (ASD). Inclusion criteria were as follows: (1) diagnosis of either univentricular congenital heart disease or isolated atrial septal defect; (2) age 0–18 years; and (3) referral by the patient’s cardiologist for a clinically indicated cardiac catheterization. ASD was chosen as a convenience control population since children with anatomically normal hearts do not routinely undergo cardiac catheterization. The only exclusion criterion was a concomitant hematologic disorder that would alter hemorheology (e.g., sickle cell disease). The study protocol was approved by the Institutional Review Board of the University of Southern California/ Children’s Hospital Los Angeles.
Patient demographic information including age, anthropometrics, cardiac anatomy, medical and surgical history, and medications were obtained from the most recent outpatient cardiology clinic note in the electronic medical record.
Cardiac Catheterization and Clinical Laboratories
A venous blood sample of 15 mL or no more than 2 mL/kg was drawn from each patient after placement of a femoral venous sheath; EDTA (1.5 mg/mL) was used as an anticoagulant. Blood was analyzed for blood viscosity, RBC deformability, and RBC aggregation as described below. A subset of patients additionally had blood sent to the hospital’s clinical laboratory to measure complete blood count with differential, basic coagulation panel, comprehensive metabolic panel, erythrocyte sedimentation rate (ESR), fibrinogen, and plasma-free hemoglobin.
Hemodynamic data were obtained in the routine manner as indicated by the clinical purpose of the catheterization. Systemic blood flow (SBF), PBF, and indexed pulmonary vascular resistance (PVRi) were calculated using the Fick equation: Blood flow = VO2/(CaO2–CvO2), where CO = cardiac output, VO2 = oxygen consumption assumed based on Lafarge estimates [15], and CaO2–CvO2 = arteriovenous oxygen content difference across the systemic or pulmonary vasculature; and PVRi = TPG/PBF, where TPG = transpulmonary gradient, the difference between mean pulmonary artery and left atrial pressures. For the subset of Glenn patients in whom oxygen saturation was measured in the inferior vena cava, we also calculated superior and inferior vena cava blood flow using the method described by Salim et al. [23]. In patients with the Fontan circulation, where direct left atrial pressures could not be obtained, either a pulmonary capillary wedge pressure or ventricular end-diastolic pressure was used as a surrogate for the left atrial pressure. None of the patients had pulmonary vein stenosis or other anatomic abnormalities that would cause a discrepancy between the pulmonary capillary wedge and left atrial pressures.
Rheology
Blood samples were kept at room temperature for no more than 4 h before being analyzed for rheology. Whole blood kept at room temperature for this duration has been shown to have minimal effect on blood rheology [1]. Blood viscosity was measured with a Rheolog automated tube viscometer (Rheovector Inc., Wilmington, DE) at 37 °C [1]; a temperature of 37 °C is the standard used for physiologic estimates of viscosity. For each patient, 2 mL of whole blood was introduced into a disposable tube assembly, and an initial pressure gradient across the tube was established. Blood then flowed automatically through the tube, continuously decreasing this gradient. The instrument continuously detects both volumetric blood flow rate and pressure gradient, with these two parameters used to calculate and tabulate viscosity-shear rate relations over a range of shear rates from 1 to 1000 s−1.
RBC deformability was measured at room temperature with a LORCA (Laser-assisted Optical Rotational Cell Analyzer) ektacytometer (Mechatronics, Hoorn, The Netherlands). A 25-μL aliquot of blood was suspended in 5 mL of a viscous isotonic solution of 360 kDa polyvinylpyrrolidone (Sigma Chemical Co., St. Louis, MO). An RBC elongation index (EI) was measured at 37 °C at nine shear-stress levels between 0.5 and 50 Pascal, with increased EI at any given shear stress indicating greater RBC deformability. Further details of the instrument have been described elsewhere [2].
RBC aggregation was measured at room temperature with a Myrenne aggregometer (Myrenne GmbH, Roetgen, Germany). Full details of this device have also been described elsewhere [3]. Aggregation was assessed in autologous plasma and in a defined isotonic pro-aggregating polymer solution. Aggregation in plasma reflects the combined effects of cellular and suspending phase properties, whereas aggregation in the polymer solution reflects only RBC properties (i.e., ‘‘RBC aggregability’’). For the native plasma sample, RBC and plasma were combined to obtain a standardized hematocrit of 40 %. For the non-plasma sample, plasma was removed and discarded, the remaining RBC washed twice with isotonic phosphate-buffered saline (PBS, 0.01 M phosphate, pH 7.4, 290 ± 5 mOsm/kg) and then re-suspended in 3 % solution of 70 kDa dextran in PBS with 0.1 % BSA (Sigma Chemical Co., St. Louis, MO) to a hematocrit of 40 %. Both the plasma and dextran samples were run in duplicate on the aggregometer. Two unitless indices are provided by the Myrenne device: M, representing aggregation at stasis, and M1, representing aggregation at a low-shear rate of 3 s−1.
Statistical Analysis
The degree of normality of each variable was assessed by Shapiro–Wilk test. Comparison between patient groups of each laboratory, rheology, and hemodynamic parameter was performed with ANOVA for variables fitting a normal distribution, and Kruskal–Wallis test for those not fitting a normal distribution. Univariate linear regression was performed to determine the relationships between hemodynamic parameters and laboratory parameters. Statistics were performed in JMP statistical software (SAS Institute Inc., Cary, NC). p < 0.05 was considered statistically significant.
Since this was a novel area of investigation, there were no existing estimates of effect size or sample variance in the patient groups. The coefficient of variation in the rheologic assays was approximately 3–5 %, while that for estimates of PVR was about 10–15 %. Using data from control subjects over various hematocrits, 24 patients were needed to achieve 80 % power for α = 0.05 and an expected effect size of 0.75.
Results
Patient Characteristics
Sixty-two patients were included in the study—20 ASD, 22 Glenn, and 20 Fontan. Table 1 summarizes the patient demographic information. Since the Glenn procedure is done prior to the Fontan, the Glenn cohort was expectedly younger and smaller than the Fontan cohort. The majority (30/42, 71.4 %) of the univentricular cohort had a systemic right ventricle with hypoplastic left heart syndrome being the most common anatomy (15/42, 35.7 %). Significant comorbidities in the univentricular cohort included decreased ventricular function by echocardiography in 10 (23.8 %), pulmonary hypertension in 2 (4.8 %), arteriovenous malformations in 2 (4.8 %), protein-losing enteropathy in 2 (4.8 %), arrhythmia in 4 (9.6 %), and pacemaker in 2 (4.8 %). Eleven (26.2 %) also had a history of thrombus not related to catheter placement.
Table 1.
Patient demographics
| ASD (n = 20) | Glenn (n = 22) | Fontan (n = 20) | p | |
|---|---|---|---|---|
| Male | 5 (25 %) | 13 (59 %) | 16 (80 %) | <0.01*† |
| Age, years | 5.41 ± 1.99 | 3.2 ± 0.63 | 10.79 ± 3.88 | <0.0001*†‡ |
| Weight, kg | 20.4 ± 8.8 | 13.7 ± 2.1 | 37.8 ± 18.8 | <0.0001*†‡ |
| Height, cm | 111.1 ± 14 | 92.7 ± 5.4 | 137.2 ± 24.6 | <0.0001*†‡ |
| BSA, m2 | 0.78 ± 0.2 | 0.58 ± 0.06 | 1.18 ± 0.4 | <0.0001*†‡ |
| Race | ||||
| Asian | 2 (10 %) | 3 (13.6 %) | 2 (10 %) | |
| Black | 2 (10 %) | 2 (9.1 %) | 0 (0 %) | |
| Hispanic | 11 (55 %) | 12 (54.5 %) | 11 (55 %) | |
| White | 4 (20 %) | 4 (18.1 %) | 6 (30 %) | |
| Other | 1 (5 %) | 1 (4.5 %) | 1 (5 %) | |
| Cardiac diagnosis | ||||
| HLHS | 9 (40.9 %) | 6 (30 %) | ||
| UAVC | 4 (18.1 %) | 4 (20 %) | ||
| DORV | 2 (9.1 %) | 3 (15 %) | ||
| DILV | 1 (4.5 %) | 3 (15 %) | ||
| TA | 1 (4.5 %) | 1 (5 %) | ||
| PA/IVS | 1 (4.5 %) | 1 (5 %) | ||
| CCTGA | 1 (4.5 %) | 1 (5 %) | ||
| Other | 3 (13.5 %) | 1 (5 %) | ||
| Systemic ventricle | ||||
| Right | 17 (77.3 %) | 13 (65 %) | ||
| Left | 5 (22.7 %) | 7 (35 %) |
Categorical variables expressed as n (%), continuous variables as mean ± SD
BSA body surface area, HLHS hypoplastic left heart syndrome, UAVC unbalanced atrioventricular canal, DORV double outlet right ventricle, DILV double inlet left ventricle, TA tricuspid atresia, PA/IVS pulmonary atresia/intact ventricular septum, CCTGA congenitally corrected transposition of the great arteries
p < 0.05 for ASD versus Glenn
p < 0.05 for ASD versus Fontan
p < 0.05 for Glenn versus Fontan
Clinical Laboratories
A subset of relevant clinical laboratory values is summarized in Table 2. Overall, the ASD patients were unexpectedly anemic with a mean hemoglobin of 11.3 ± 0.7 g/dL. ESR was also unexpectedly higher (although still in normal range) in ASD compared to the Glenn and Fontan groups; this difference remained significant when adjusted for hematocrit. Elevation in this inflammatory marker in conjunction with normal red blood cell size (average mean corpuscular volume 85.8 fL) was suggestive of anemia of chronic disease. Glenn patients had expectedly higher hemoglobin and hematocrit due to chronic cyanosis. Prothrombin time and international normalized ratio were slightly higher in Fontan patients even when excluding patients taking warfarin. Alanine aminotransferase was also slightly higher in the Fontan group. Creatinine differed between the three groups and was likely reflective of the age differences between the groups. Of note, several plasma components that could influence RBC aggregation including fibrinogen, protein, albumin, and plasma-free hemoglobin were not different between ASD and the univentricular patients.
Table 2.
Subset of clinical laboratory values
| ASD (n = 15) | Glenn (n = 11) | Fontan (n = 14) | p | |
|---|---|---|---|---|
| Hemoglobin, g/dL | 11.3 ± 0.7 | 15.1 ± 1.1 | 13.8 ± 1 | <0.0001*†‡ |
| Hematocrit, % | 32.8 ± 1.8 % | 43.3 ± 3.2 % | 40.3 ± 2.9 % | <0.0001*†‡ |
| ESR, mm/h | 8.3 ± 6.1 | 1.9 ± 1.2 | 2.5 ± 1 | <0.0001*† |
| ESR/Hct | 6.1 ± 4.4 | 1.8 ± 1.1 | 2.3 ± 0.8 | 0.003*† |
| PT, seconds | 12.1 ± 0.7 | 13.1 ± 1.6 | 15.4 ± 4.2 | 0.0005† |
| INR | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.4 ± 0.4 | 0.02*† |
| Creatinine, mg/dL | 0.36 ± 0.08 | 0.29 ± 0.06 | 0.47 ± 0.15 | 0.0008*†‡ |
| Fibrinogen, mg % | 203 ± 28 | 211 ± 54 | 219 ± 29 | 0.74 |
| Protein, g/dL | 6.4 ± 0.3 | 5.9 ± 0.6 | 6.5 ± 0.8 | 0.02*‡ |
| Albumin, g/dL | 3.7 ± 0.3 | 3.4 ± 0.4 | 3.7 ± 0.5 | 0.2 |
| Bilirubin, mg/dL | 0.5 ± 0.1 | 0.7 ± 0.4 | 0.9 ± 0.5 | 0.04† |
| AST, U/L | 37 ± 6 | 39 ± 9 | 39 ± 9 | 0.74 |
| ALT, U/L | 26 ± 5 | 31 ± 7 | 37 ± 11 | 0.009*† |
| Plasma Hb, mg/dL | 15 ± 10 | 16 ± 9 | 16 ± 12 | 0.99 |
Data expressed as mean ± SD
PT prothrombin time, INR international normalized ratio, AST aspartate aminotransferase, ALT alanine aminotransferase
p < 0.05 for ASD versus Glenn
p < 0.05 for ASD versus Fontan
p < 0.05 for Glenn versus Fontan
Rheology
Figure 1a presents mean blood viscosity versus shear rate for the three cohorts. Viscosity was lowest in ASD and highest in Glenn at all shear rates. These differences were statistically significant at all shear rates except for at 1000 s−1, where ASD and Fontan were not significantly different. The hematocrit/viscosity ratio (HVR), a marker of RBC oxygen transport efficiency [5], was also calculated over the same range of shear rates; these curves are illustrated in Fig. 1b. HVR was lowest in Glenn and highest in ASD at low shear rates. Interestingly, this relationship changed at higher shear rates, with Fontan having the highest HVR and ASD having the lowest.
Fig. 1.
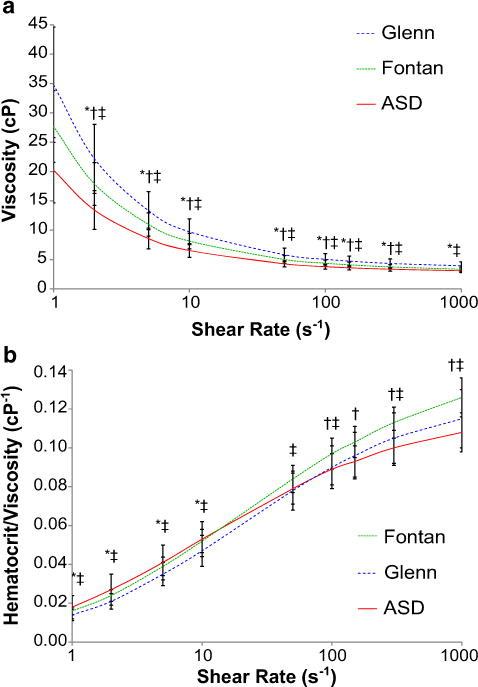
a Comparison of mean viscosity between groups across range of shear rates. b Comparison of mean HVR between groups across range of shear rates. Error bars are ± 1 SD. *p < 0.05 for ASD versus Glenn, †p < 0.05 for ASD versus Fontan, ‡p < 0.05 for Glenn versus Fontan
The results of RBC deformability and aggregation analyses are summarized in Fig. 2. In terms of RBC deformability (Fig. 2a), elongation indices were similar at all shear rates between all three groups. Pediatric normative data for RBC deformability have not been published previously, so the expected standard deviation in this population is unknown. Our cohort’s RBC deformability, however, was similar to normal adult values [2]. RBC aggregation (Fig. 2b), both in plasma and dextran, was also similar between groups. There are also nonormative dataonRBC aggregation in the pediatric population; however, the aggregation values we obtained were about one-third of normal adult values [3].
Fig. 2.
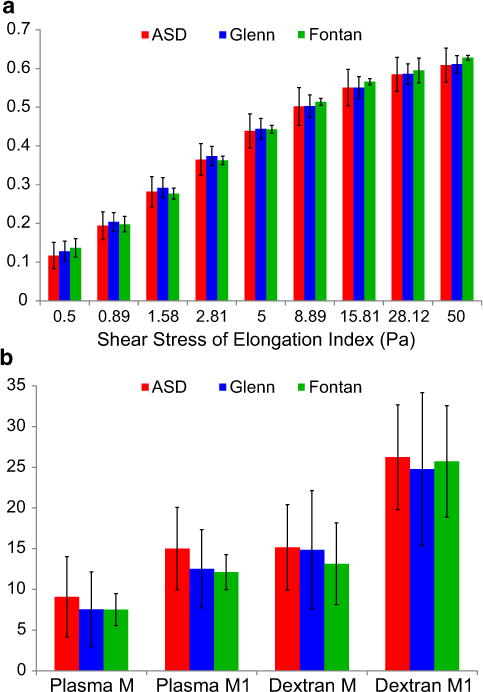
RBC deformability and aggregation. a RBC deformability was similar among groups across the entire range of shear stresses. b RBC aggregation in both plasma and dextran was also similar among groups. Error bars are ±1 SD. p > 0.05 for all comparisons
Cardiac Catheterization
Hemodynamic data from the cardiac catheterizations is summarized in Table 3. One patient with ASD had unexpected pulmonary hypertension (PVRi > 4 W.U. × m2), so this patient was excluded from further analyses.
Table 3.
Cardiac catheterization data
| ASD (n = 20) | Glenn (n = 22) | Fontan (n = 20) | p | |
|---|---|---|---|---|
| Arterial oxygen saturation, % | 99 ± 2 | 85 ± 4 | 92 ± 7 | <0.0001*†‡ |
| Mixed venous (SVC) oxygen saturation, % | 76 ± 5 | 65 ± 6 | 70 ± 7 | <0.0001*†‡ |
| Mean pulmonary artery pressure, mm Hg | 18 ± 5 | 11 ± 2 | 13 ± 2 | <0.0001*† |
| Pulmonary blood flow, L/min/m2 | 8.6 ± 3.7 | 2.7 ± 0.7 | 3.2 ± 0.7 | <0.0001*† |
| Systemic blood flow, L/min/m2 | 4.4 ± 0.8 | 4.2 ± 1.2 | 3.4 ± 0.8 | 0.003*† |
| Pulmonary/systemic blood flow ratio | 1.9 ± 0.6 | 0.7 ± 0.1 | 0.9 ± 0.1 | <0.0001*†‡ |
| Pulmonary vascular resistance, W.U. × m2 | 1.1 ± 0.4 | 1.6 ± 0.4 | 1.8 ± 0.9 | 0.0007*† |
Data expressed as mean ± SD
SVC superior vena cava
p < 0.05 for ASD versus Glenn
p < 0.05 for ASD versus Fontan
p < 0.05 for Glenn versus Fontan
Figures 3, 4, 5, and 6 demonstrate correlations between viscosity and hemodynamic variables obtained by cardiac catheterization. In each figure, viscosity at five different shear rates is illustrated. For reference, the estimated mean shear rate in the vena cavae is on the order of 10–50 s−1 [6], although it is important to note that a range of shear rates is seen in any given blood vessel. Interestingly, there were significant differences in these relationships between the three cohorts. Both Glenn and Fontan patients demonstrated a moderate negative correlation between viscosity and PBF (Fig. 3). This relationship was constant at all shear rates in the Glenn cohort, but was only significant at lower shear rates (≤50 s−1) in the Fontan cohort. Comparing viscosity and pulmonary artery pressure, only the Glenn cohort showed a significant negative correlation at the majority of shear rates (Fig. 4). At 1000 s−1, however, there was a moderate negative correlation in the ASD group and a trend toward significant negative correlation in the Fontan group. Only the Fontan cohort demonstrated a moderate positive correlation between viscosity and PVRi (Fig. 5). Importantly, hematocrit alone was not associated with PBF or PVRi in Glenn or Fontan patients, whereas HVR, a correction for the interdependence of hematocrit and viscosity, at low shear rates was associated with both PBF and PVRi in the Fontan patients (data not shown).
Fig. 3.
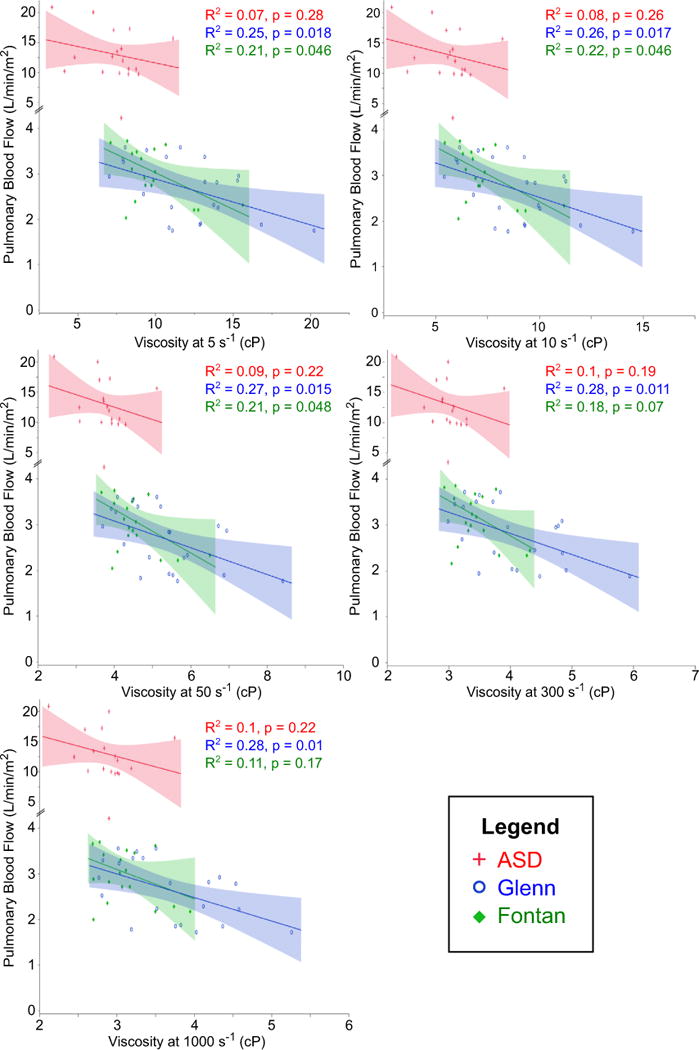
Correlation between viscosity and PBF. Moderate negative correlation was seen at all shear rates in Glenn and at lower shear rates in Fontan. No correlation was seen in ASD
Fig. 4.
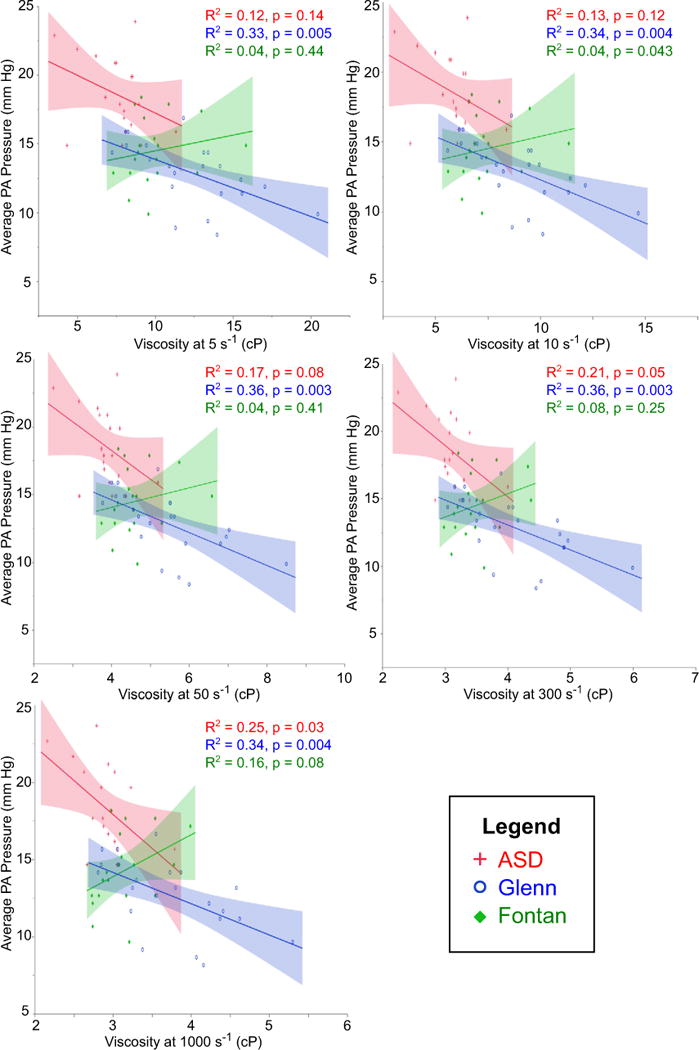
Correlation between viscosity and pulmonary artery pressure. Moderate negative correlation was seen in Glenn at all shear rates, and in ASD only at 1000 s−1
Fig. 5.
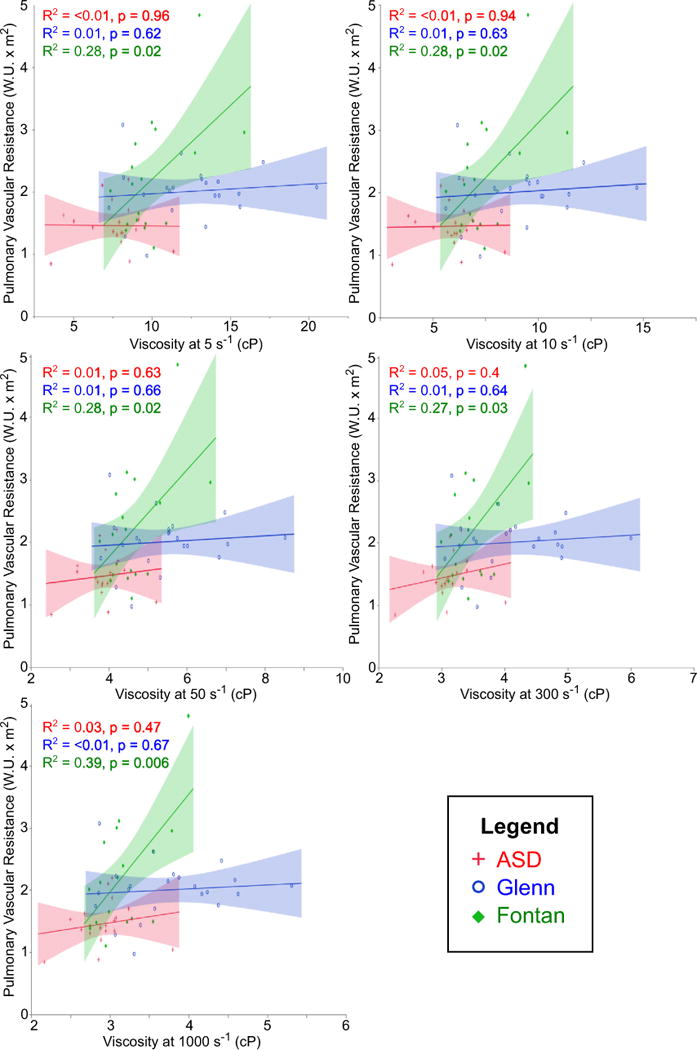
Correlation between viscosity and pulmonary vascular resistance. Moderate positive correlation was seen only in Fontan patients
Fig. 6.
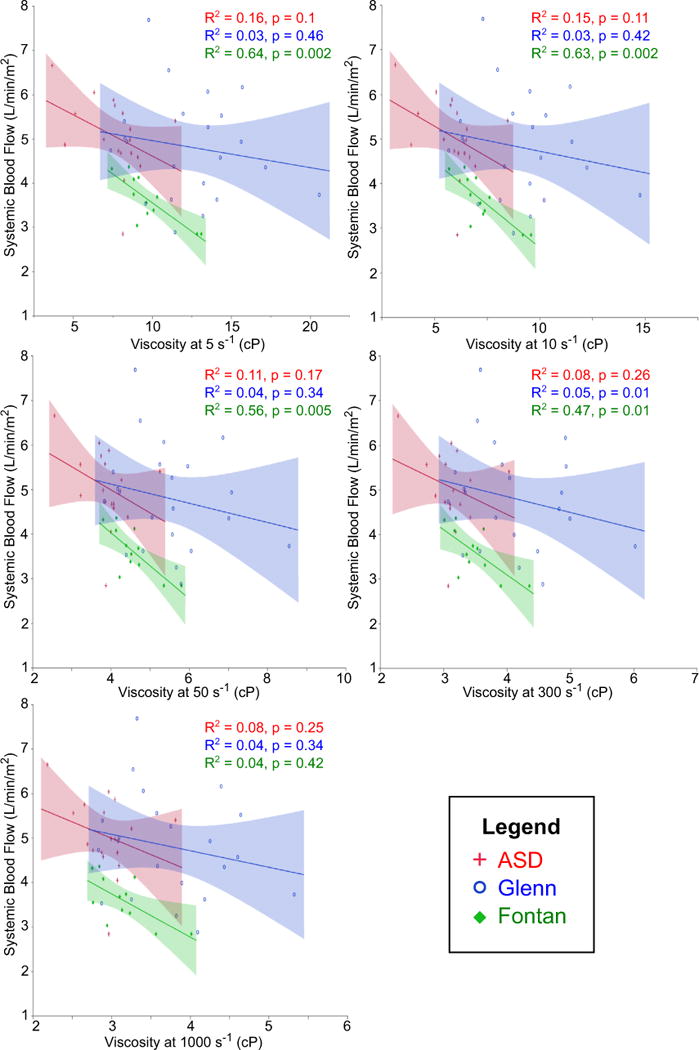
Correlation between viscosity and systemic blood flow. Strong negative correlation was seen only in Fontan patients. The correlation weakened at higher shear rates
Including the entire cohort, viscosity did not correlate with SBF in any group. However, after excluding patients with anatomy that allowed blood to bypass the pulmonary capillary bed (Fontan fenestration, venovenous collateral vessels, and pulmonary arteriovenous malformations), there was a strong negative correlation between viscosity and SBF in the Fontan group as shown in Fig. 6. This correlation was significant at all shear rates ≤300 s−1. The lack of correlation between viscosity and SBF in the Glenn group appeared to be related to concomitant counteractive changes in superior and inferior vena cava blood flow. As shown in Fig. 7, with increasing viscosity there was a simultaneous decrease in superior vena cava blood flow and trend toward increasing inferior vena cava blood flow; the net result was relatively constant SBF.
Fig. 7.
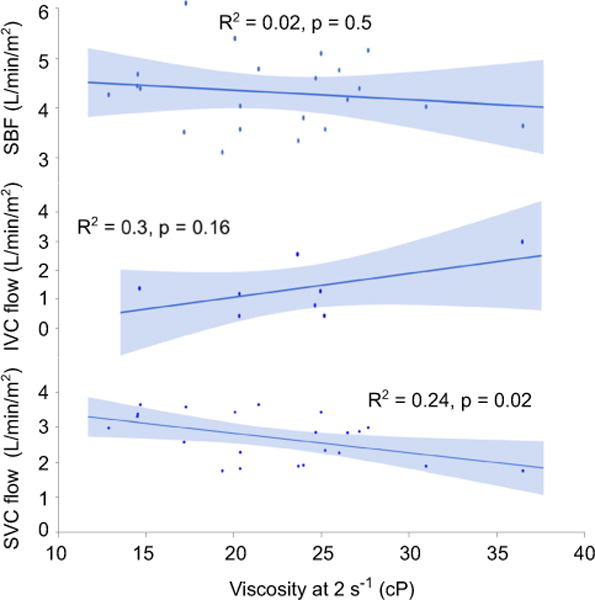
Correlation between viscosity, systemic blood flow, and caval blood flows in Glenn patients. SVC, superior vena cava; IVC, inferior vena cava
Discussion
We found that blood viscosity has a strong and significant association with PBF and PVR in univentricular circulations where low-shear non-pulsatile blood flow is present in the pulmonary arterial tree. These associations were not present in patients with a biventricular circulation. The linear relationship between PVR and blood viscosity has been well described in the normal biventricular circulation, where both the pulmonary and systemic arterial beds are exposed to high-shear pulsatile flow [11]. Yet, this relationship has not been re-examined in patients with univentricular circulation previously. Under high-shear conditions, the relationship between viscosity and shear rate is linear and constant. However, this relationship becomes nonlinear over the low shear rate range observed in the systemic venous system, particularly in the vena cavae [6]. In this range, small decreases in shear rate have a large effect on blood viscosity and thus PVR, as demonstrated in the steep slope of the viscosity-shear rate curve in our data.
In addition to the acute effects of changes in shear rate, ample evidence in the literature demonstrates chronic changes in pulmonary and systemic arteries where low-shear or turbulent flow predominates. Elevated whole blood viscosity has been implicated as a significant independent risk factor of atherosclerosis and resultant cardiovascular disease in adults [12]. Hyperviscosity has also been shown to decrease cerebral blood flow both in neonatal and adult patients with polycythemia; interestingly, this effect has been observed in hyperviscous but normocythemic animal models as well [9]. In the pulmonary circulation, hyperviscosity can have similarly detrimental effects. In a murine model of chronic mountain sickness, a disorder characterized by hypoxia-induced polycythemia and arterial desaturation, chronic hypoxia led to an increase in PVR which was both preventable and reversible via reduction in blood viscosity with acetazolamide [20]. Small studies in humans also suggest that the adverse hemodynamic effects of hyperviscosity are reversible, at least in the short term. In a cohort of adults with chronic obstructive pulmonary disease and secondary pulmonary hypertension, serial reduction in blood viscosity with phlebotomy and substitution with hydroxyethyl starch resulted in decreased PVR and improvement in cardiac output and exercise tolerance [4]. A similar intervention of intraoperative reduction in blood viscosity with hydroxyethyl starch in children with cyanotic congenital heart disease undergoing Blalock-Taussig shunt demonstrated a protective effect on shunt patency in the immediate postoperative period [22]. Therefore, in patients with the Glenn circulation, where PBF is dependent on upper body/cerebral blood flow, elevated blood viscosity may have dual adverse effects by simultaneously decreasing cerebral blood flow and increasing PVR.
A complex interdependence exists between blood viscosity, hematocrit, RBC aggregation, and RBC deformability. In our study population, Glenn and Fontan patients demonstrated similar RBC deformability compared to ASD patients as well as published adult norms. RBC aggregation was also similar between the three groups. Since there were not any significant abnormalities in plasma elements that could affect RBC aggregation in our patient population, this result is not necessarily surprising. Interestingly and unexpectedly, there was a significant normocytic anemia seen in the ASD patients, in the presence of slightly elevated ESR. While this is intriguing, our study design was not suitable to address the etiology of this finding. Future studies directly testing the individual effects of hematocrit, aggregation, and deformability on both blood viscosity and pulmonary hemodynamics are warranted.
In the normal systemic circulation, elevated blood viscosity increases systemic vascular resistance, which in turn increases the workload of the left ventricle. The increased energy expended by the heart is then transmitted to the systemic arteries. According to the protective adaptation theory, the resultant chronic stretch injury to the endothelium promotes a self-preserving fibroproliferative response, which over time leads to decreased arterial compliance and further increases systemic vascular resistance [12]. The focal nature of atherosclerotic plaques suggests that certain areas in the vasculature are particularly susceptible to mechanical injury from local flow abnormalities. Indeed, areas exposed to chronic low shear, such as the outer walls of vessel bifurcation points, demonstrate an abnormal morphology and diminished vasodilatory capacity compared to areas of chronic high shear [16].
Transposing this pathophysiology to the Fontan circulation, where the vena cavae and pulmonary arteries are exposed to chronic low-shear flow, one can easily envision how chronic low-shear-related endothelial dysregulation and subsequent maladaptive vascular responses could similarly develop. There is ample clinical and experimental evidence to support that adverse pulmonary remodeling and vascular dysfunction are common with the Fontan circulation. While children normally have significant pulmonary artery growth until around 8 years of age, those with univentricular hearts have almost complete arrest of growth after the Glenn [24] and Fontan procedures [18]. Histologic studies of autopsy specimens have shown that these patients have adverse pulmonary vascular remodeling, including intimal thickening from fibrosis and collagen deposition as well as time-dependent alterations in medial smooth muscle [21]. A porcine model of a unilateral cavopulmonary anastomosis suggests that chronic non-pulsatile flow to the lungs resulting in endothelial dysfunction may be the underlying mechanism of this pathology [10]. Clinical evidence to support preservation of some degree of pulsatile antegrade pulmonary blood flow to improve pulmonary artery growth after the Glenn procedure, however, has been mixed [24].
Elevated blood viscosity, especially at low shear, could significantly impede flow through the Fontan circulation by increasing energy losses via wall shear stress [17]. Consistent with this, our data demonstrated that elevated low-shear blood viscosity was associated with negative hemodynamic perturbations in Glenn and Fontan patients, but not in ASD patients. Most notably, elevated viscosity was associated with decreased PBF. While this increased viscous drag could theoretically stimulate nitric oxide production in the pulmonary arteries, the absence of pulmonary artery growth in this population again highlights their inherent endothelial dysfunction.
The association between low-shear viscosity and pulmonary artery pressure, PBF, and PVR was different between our Glenn and Fontan cohorts. This is likely due to fundamental differences between the two circulations. In the Glenn circulation, PBF stems from systemic venous flow from the upper body. Upper body blood flow during the period when patients usually have the Glenn circulation is primarily cerebral blood flow. Hyperviscosity from polycythemia results in greater oxygen content, resulting in a compensatory increase in cerebral vascular tone in order to decrease cerebral blood flow and maintain constant cerebral oxygen delivery. This mechanism is supported by several animal models as well as both pediatric and adult studies of cerebral blood flow in patients with polycythemia; in these studies, despite decreased cerebral blood flow, oxygen delivery is maintained due to increased cerebral oxygen extraction [9]. Similarly, in the Glenn circulation, hyper-viscosity could cause a decrease in cerebral blood flow, in turn causing a proportional decrease in PBF. In this case, concomitant decreases in PBF and pulmonary artery pressure would result in unchanged calculated PVR, which may explain the lack of association between PVRi and viscosity in our Glenn cohort. In the Fontan circulation, by comparison, all systemic venous flow must traverse the pulmonary vasculature to return to the heart. Increased impedance to blood flow in this scenario would result in decreased PBF, increased pulmonary artery pressure, and increased calculated PVR. In those without an alternative pathway to bypass the pulmonary vasculature, such as a Fontan fenestration, this would also cause decreased SBF. These differences between the Glenn and Fontan circulations are highlighted in Figs. 6 and 7.
As mentioned previously, even among asymptomatic Fontan patients, exercise tolerance is universally decreased, presumably due to inability to augment ventricular preload through the pulmonary vasculature [14]. Although many agents including pulmonary vasodilators, beta-blockers, and angiotensin-converting enzyme inhibitors have been tried, no medical therapies have been consistently proven to improve pulmonary blood flow and therefore cardiac output in this population [8]. Given that our data suggest that increased blood viscosity limits PBF, medications that instead lower blood viscosity may be beneficial therapy. This is a crucial first step establishing controlled hemorheologic manipulation as a therapeutic option in patients with univentricular physiology.
Limitations of this study include the small size of the study population and low prevalence of comorbidities, such as protein-losing enteropathy, that could affect rheology. In this study population, there were no notable abnormalities in any of the clinical laboratories measured. Potentially, fibrinogen and plasma-free hemoglobin could have been elevated as markers of chronic, low-level vascular inflammation. Protein and albumin could have also been decreased in Fontan patients due to chronic hepatic congestion and fibrosis. With a larger and older cohort of Fontan patients, these abnormalities may have become apparent. As normative rheology data do not exist for pediatric patients, children with completely normal biventricular circulations would have been the ideal control group. However, we chose ASD as a convenience population since children with completely normal cardiac anatomy do not routinely undergo cardiac catheterization. Although their anemia resulted in low viscosity, this did not correlate with any of the hemodynamic parameters as it did in the Glenn and Fontan patients. Lastly, determining the mechanism of how viscosity alters pulmonary hemodynamics is beyond the scope of this study, although there is a reasonable amount of evidence to suggest that it is mediated by pulmonary endothelial dysfunction.
Due to the cross-sectional nature of this study, we did not evaluate blood rheology in relation to clinical outcomes. However, based on our data, future studies examining pulmonary vascular disease in this population should include blood viscosity as a possible determinant of PBF. Evaluating plasma viscosity separately from whole blood viscosity may also be warranted.
In conclusion, our data demonstrate that elevated low-shear blood viscosity is associated with negative hemodynamic perturbations in a passive univentricular pulmonary circulation, but not in a pulsatile biventricular pulmonary circulation. Most notably, elevated viscosity is associated with decreased PBF. The differences in pulmonary artery pressure and PVR responses between Glenn and Fontan patients were likely due to fundamental differences between the two circulations. Further prospective data are warranted to investigate how this may relate to clinical outcomes.
Acknowledgments
This work was supported by a Grant-in-Aid (Section on Cardiology and Cardiac Surgery Research Fellowship Award) from the American Academy of Pediatrics (A.L.C.), a University of Southern California Provost’s Postdoctoral Scholar Research Grant (A.L.C.), a K12 award (2 K12 HD 52954-6 A1) and K23 award (1 K23 HL 119627-01 A1) from the National Institutes of Health (J.A.D.), and an institutional grant (NIH #RR00043-43) from the Children’s Hospital Los Angeles General Clinical Research Center (J.A.D.). The authors acknowledge Hesham Mahmoud for assistance with obtaining informed consents, as well as Honglei Liu, Iris Xu, and Israel Orta for assistance with the rheology experiments.
Footnotes
Compliance with Ethical Standards
Conflicts of interest The authors declare that they have no conflicts of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Alexy T, Wenby RB, Pais E, Goldstein LJ, Hogenauer W, Meiselman HJ. An automated tube-type blood viscometer: validation studies. Biorheology. 2005;42:237–247. [PubMed] [Google Scholar]
- 2.Baskurt OK, Hardeman MR, Uyuklu M, Ulker P, Cengiz M, Nemeth N, Shin S, Alexy T, Meiselman HJ. Parameterization of red blood cell elongation index–shear stress curves obtained by ektacytometry. Scand J Clin Lab Invest. 2009;69:777–788. doi: 10.3109/00365510903266069. [DOI] [PubMed] [Google Scholar]
- 3.Baskurt OK, Uyuklu M, Ulker P, Cengiz M, Nemeth N, Alexy T, Shin S, Hardeman MR, Meiselman HJ. Comparison of three instruments for measuring red blood cell aggregation. Clin Hemorheol Microcirc. 2009;43:283–298. doi: 10.3233/CH-2009-1240. [DOI] [PubMed] [Google Scholar]
- 4.Borst MM, Leschke M, König U, Worth H. Repetitive hemodilution in chronic obstructive pulmonary disease and pulmonary hypertension: effects on pulmonary hemodynamics, gas exchange, and exercise capacity. Respiration. 1999;66:225–232. doi: 10.1159/000029382. [DOI] [PubMed] [Google Scholar]
- 5.Connes P, Lamarre Y, Hardy-Dessources MD, Lemonne N, Waltz X, Mougenel D, Mukisi-Mukaza M, Lalanne-Mistrih ML, Tarer V, Tressières B, Etienne-Julan M, Romana M. Decreased hematocrit-to-viscosity ratio and increased lactate dehydrogenase level in patients with sickle cell anemia and recurrent leg ulcers. PLoS One. 2013;8:e79680. doi: 10.1371/journal.pone.0079680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connes P, Alexy T, Detterich J, Romana M, Hardy-Dessources MD, Ballas SK. The role of blood rheology in sickle cell disease. Blood Rev. 2015 doi: 10.1016/j.blre.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elder RW, McCabe NM, Veledar E, Kogon BE, Jokhadar M, Rodriguez FH, McConnell ME, Book WM. Risk factors for major adverse events late after Fontan palliation. Congenit Heart Dis. 2015;10:159–168. doi: 10.1111/chd.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, et al. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol. 2012;59:S1–S42. doi: 10.1016/j.jacc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein M, Stonestreet BS, Brann BS, Oh W. Cerebral cortical blood flow and oxygen metabolism in normocythemic hyperviscous newborn piglets. Pediatr Res. 1988;24:486–489. doi: 10.1203/00006450-198810000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Henaine R, Vergnat M, Bacha EA, Baudet B, Lambert V, Belli E, Serraf A. Effects of lack of pulsatility on pulmonary endothelial function in the Fontan circulation. J Thorac Cardiovasc Surg. 2013;146:522–529. doi: 10.1016/j.jtcvs.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman JI. Pulmonary vascular resistance and viscosity: the forgotten factor. Pediatr Cardiol. 2011;32:557–561. doi: 10.1007/s00246-011-9954-3. [DOI] [PubMed] [Google Scholar]
- 12.Kensey KR. The mechanistic relationships between hemorheological characteristics and cardiovascular disease. Curr Med Res Opin. 2003;19:587–596. doi: 10.1185/030079903125002289. [DOI] [PubMed] [Google Scholar]
- 13.Khambadkone S, Li J, de Leval MR, Cullen S, Deanfield JE, Redington AN. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation. 2003;107:3204–3208. doi: 10.1161/01.CIR.0000074210.49434.40. [DOI] [PubMed] [Google Scholar]
- 14.Khiabani RH, Whitehead KK, Han D, Restrepo M, Tang E, Bethel J, Paridon SM, Fogel MA, Yoganathan AP. Exercise capacity in single-ventricle patients after Fontan correlates with haemodynamic energy loss in TCPC. Heart. 2015;101:139–143. doi: 10.1136/heartjnl-2014-306337. [DOI] [PubMed] [Google Scholar]
- 15.LaFarge CG, Miettinen OS. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 17.Moyle KR, Mallinson GD, Occleshaw CJ, Cowan BR, Gentles TL. Wall shear stress is the primary mechanism of energy loss in the Fontan connection. Pediatr Cardiol. 2006;27:309–315. doi: 10.1007/s00246-005-0918-3. [DOI] [PubMed] [Google Scholar]
- 18.Ovroutski S, Ewert P, Alexi-Meskishvili V, Hölscher K, Miera O, Peters B, Hetzer R, Berger F. Absence of pulmonary artery growth after fontan operation and its possible impact on late outcome. Ann Thorac Surg. 2009;87:826–831. doi: 10.1016/j.athoracsur.2008.10.075. [DOI] [PubMed] [Google Scholar]
- 19.Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hell J Cardiol. 2005;46:9–15. [PubMed] [Google Scholar]
- 20.Pichon A, Connes P, Quidu P, Marchant D, Brunet J, Levy BI, Vilar J, Safeukui I, Cymbalista F, Maignan M, Richalet JP, Favret F. Acetazolamide and chronic hypoxia: effects on haemorheology and pulmonary haemodynamics. Eur Respir J. 2012;40:1401–1409. doi: 10.1183/09031936.00216011. [DOI] [PubMed] [Google Scholar]
- 21.Ridderbos FJ, Wolff D, Timmer A, van Melle JP, Ebels T, Dickinson MG, Timens W, Berger RM. Adverse pulmonary vascular remodeling in the Fontan circulation. J Heart Lung Transpl. 2015;34:404–413. doi: 10.1016/j.healun.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Sahoo TK, Chauhan S, Sahu M, Bisoi A, Kiran U. Effects of hemodilution on outcome after modified Blalock-Taussig shunt operation in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth. 2007;21:179–183. doi: 10.1053/j.jvca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Salim MA, Case CL, Sade RM, Watson DC, Alpert BS, DiSessa TG. Pulmonary/systemic flow ratio in children after cavopulmonary anastomosis. J Am Coll Cardiol. 1995;25:735–738. doi: 10.1016/0735-1097(94)00441-R. [DOI] [PubMed] [Google Scholar]
- 24.Turner ME, Richmond ME, Quaegebeur JM, Shah A, Chen JM, Bacha EA, Vincent JA. Intact right ventricle-pulmonary artery shunt after stage 2 palliation in hypoplastic left heart syndrome improves pulmonary artery growth. Pediatr Cardiol. 2013;34:924–930. doi: 10.1007/s00246-012-0576-1. [DOI] [PubMed] [Google Scholar]


