Abstract
Background
Interest in comparative effectiveness research and the rising number of negative or “small effect” trials have stimulated research into differential response to treatment among subgroups of patients.
Objective
To develop and test the Potential for Benefit Scale (PBS), a composite measure to identify subgroups of patients with differential potential for response to treatment, using diabetes as a model.
Design
Cross-sectional and longitudinal cohort study.
Subjects and Setting
Type 2 diabetes patients (n = 1361) were identified from 7 outpatient clinics serving a diverse population. Of these, 611 completed a 1-year follow-up.
Measures
To represent patients’ health status, we used the Total Illness Burden Index, the Physical Function Index of the SF-36, the Center for Epidemiologic Studies Depression Scale, and the Diabetes Burden Scale. To represent personality characteristics related to health, we used the Provider-Dependent Health Care Orientation scale. We assessed the contribution of these measures to a composite scale of patients’ potential for treatment response in terms of self-reported medication adherence and glycemic control.
Results
Principal components analysis confirmed associations among these measures. The internal consistency reliability of the PBS was adequate (Cronbach α = 0.65). Patients in the lowest versus highest quartile of the PBS reported poorer adherence (18% vs. 55%, P < 0.001) and poorer glycemic control at baseline (mean hemoglobin A1c values: 7.75 vs. 7.39, P < 0.001). Those in the highest quartile of the PBS also were more likely to reach target values for glycemic control (HbA1c <7%) at 1-year follow-up, (adjusted OR = 1.61, P < 0.05).
Conclusions
The PBS, a composite scale, may be helpful in identifying patients with differential potential for response to treatment.
Keywords: heterogeneity of treatment effects, comorbidity, diabetes
■ If it were not for the great variability among individuals, medicine might as well be a science and not an art.
—Sir William Osler, The Principles and Practice of Medicine 1892.
Called “heterogeneity of treatment effects,” the recognition that patients vary in response to treatment is not a new concept, as illustrated by the quote from Sir William Osler from 1892. However, the recent re-emergence of this concept in the clinical and statistical literature is a reflection of its sustained importance for clinical practice, clinical guidelines, and most importantly, for the design and conduct of clinical trials.1–6 The need to understand and respond to patient variation in treatment response has been fueled by the convergence of 2 phenomena: (1) the rise in the proportion of negative or small effect trials, as entry criteria were broadened to enhance generalizability of results7–12; and (2) the importance in understanding the differential effects of treatment for specific subgroups of patients for comparative effectiveness research and potential health reform initiatives.7,13–19
Heterogeneity of treatment effects has a number of potential sources, including: variation due to methodologic issues (quality and rigor of design and conduct of clinical research); variations due to clinical settings in which studies were conducted; and variations due to individual patient characteristics.2–4 The latter can include variation in: initial severity of disease (risk of a poor outcome independent of treatment)4–6,20; responsiveness to treatment (for physiologic, behavioral or genetic reasons); vulnerability to side effects or adverse events; adherence; patient preferences and risk perceptions; and competing risk, or the presence of multiple comorbid conditions and their treatments.1,21–24 For practicing physicians attempting to tailor treatment to maximize benefit and minimize risk for individual patients and for those developing new approaches to the design and conduct of clinical trials or other clinical research designs, new approaches to the quantification of risk/benefit potential are needed to identify the subgroups of patients for whom treatment would be most effective. Methods and measures that could be used prior to or early in the conduct of clinical studies, in the case of research applications,13 and prior to the delivery of a treatment, for practicing clinicians, could improve the efficiency and effectiveness of clinical research and clinical practice.
Although, in the design and posthoc adjustment of studies, individual measures have been used as stratifying variables to identify important subgroups of patients who might differentially benefit from treatment, the use of multiple measures for these purposes could improve the classification of such subgroups. Creation of a composite of such measures could improve precision and efficiency of the design and analysis of clinical studies, and provide valuable information to practicing clinicians on the potential for benefit from treatment for selected subgroups of patients.
This article describes the development of a new composite measure of the potential of individuals to respond to treatment. Using data from multiple clinics in a cross-sectional and longitudinal cohort study among a diverse population of patients with diabetes, we present evidence for the creation of such a composite measure, the Potential for Benefit Scale (PBS), based on existing patient-reported measures of the severity of multiple comorbid conditions, disease-specific perceived burden, general health status, depression, and passive orientation. We present evidence for the reliability and construct validity of this composite measure, along with its relationship to adherence to treatment at baseline and to glycemic control at baseline and at 1-year follow-up.
METHODS
Setting and Study Sample
Data were collected at seven clinics affiliated with an academic medical center in Southern California. The clinics serve patients from diverse racial and ethnic groups. Patients with at least one visit for type 2 diabetes within the 12 months prior to study onset were identified from a Diabetes Registry maintained by the medical center. This registry included all adult patients seen by a family medicine, internal medicine, or endocrinology physicians within the study period (n = 3894). Patients were excluded if they had been hospitalized for ketoacidosis in the year prior to the study period, were age ≥80, had dementia or other serious mental health problems, or could not speak English, Spanish, or Vietnamese. Patients were sampled to reflect their ethnic/racial distribution in the Registry. Of the 1791 eligible patients approached, 75% (n = 1361) consented to complete the baseline study survey and agreed to allow access to their medical record information, laboratory and administrative data.
The racial/ethnic characteristics of the final cross-sectional study sample reflected the sampling parameters: Vietnamese (19.0%), Hispanic (54.5%), Non-Hispanic white (26.5%) (Table 1). This cross-sectional sample had a mean age of 59.0 (SD, 11.6), mean years of education of 9.6 (SD, 5.1); 40% were male and the median annual household income was $23,800. Of the patients in the cross-sectional sample, 611 (44.9%) participated in a 1-year longitudinal cohort study. Characteristics for the longitudinal cohort sample were comparable to those in the cross-sectional sample.
TABLE 1.
Sample Characteristics (n = 1361)*
| Characteristics | Cross-sectional Sample | Cohort Sample |
|---|---|---|
| N | 1361 | 611 |
| Mean age (yr) | 59.0 (11.6) | 59.0 (11.6) |
| Mean education (yr) | 9.6 (5.1) | 9.6 (5.1) |
| Percent male | 40.0% | 42.9% |
| Race/ethnicity (%) | ||
| Non-Hispanic white | 26.5 (44.1) | 29.1 (45.5) |
| Hispanic | 54.5 (49.8) | 58.9 (49.2) |
| Vietnamese | 19.0 (39.2) | 12.0 (32.5) |
| Median annual household income ($) | $23,800 | $26,700 |
Table entries based on responses to patient questionnaire; entries are means with standard deviations in parentheses or percents as noted.
Design
The study was a cross-sectional observational study, with a longitudinal cohort followed for 1-year.
Data Collection
Data were derived from patient surveys, medical record abstraction, and laboratory and administrative databases maintained by the medical center. The survey questions require, on average, 20 minutes to complete.
Conceptual Framework
The framework underpinning the development of the composite measure of potential for treatment response integrates elements derived from a number of theoretical approaches to health behavior and illness management. These approaches include the Common Sense Model of illness,25 the “ecological approach” to disease management,26 the biopsychosocial model of health and illness,27 and models linking socioeconomic position, cultural influences, and personal influences to health outcomes.28–31 The framework we postulate (Fig. 1), asserts that “immutable” characteristics of patients (eg, demographic characteristics, genetic profiles), along with more mutable and variable characteristics that collectively constitute a “health profile” (eg, comorbidity, perceived disease burden, mental/physical function, biomarkers of disease, etc), a “personality profile” (eg, passive orientation to healthcare, organizational skills, a “behavioral profile” (eg, disease management skills, positive or negative health habits, coping skills, etc), a “medical or treatment context” (eg, doctor-patient relationships, continuity of care, access to care, etc), and a “life context” (eg, stressful life events, social support) will contribute to the specification of subgroups with greater and lesser potential for the expected response to treatment. Measures within each of the constructs have previously been linked with outcomes of treatment and may, therefore, have a direct relationship with the expected treatment response from a specific intervention. For example, prostate cancer patients with high levels of comorbid disease have been shown to benefit less from treatment than those with lower levels of comorbidity.22
FIGURE 1.
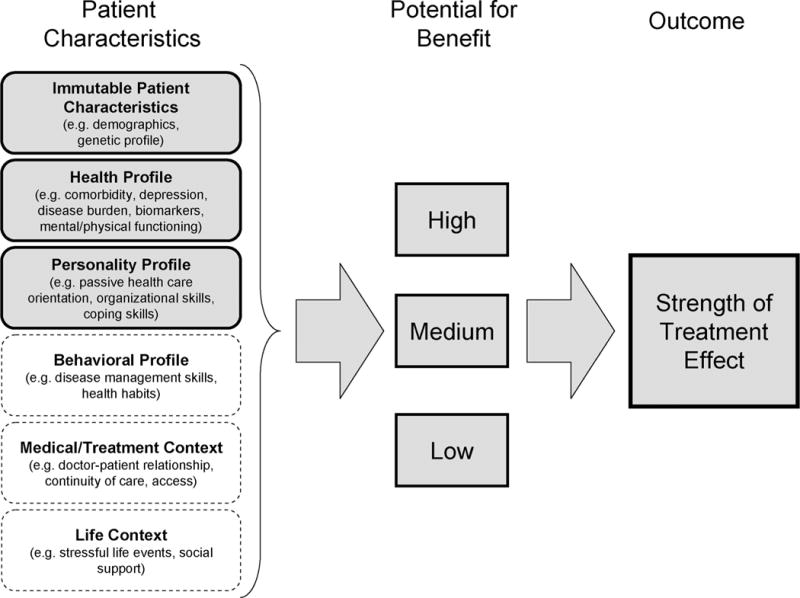
Conceptual model for the development of the Potential for Benefit Scale (PBS).
Individual patients have different profiles of characteristics within this set of constructs, which may alter the expected response to treatment. Creation of a composite measure of these constructs would summarize the collective effect of variations in these individual characteristics to provide a single “potential for benefit” score to assess the likely appropriateness of any additional treatment. By refining the categorization of patients into a priori subgroups, such a composite measure could be useful in the future design of clinical studies and, eventually, in guiding ‘tailored treatment’ decisions in clinical settings.
Study Measures
Our dataset included survey-based measures representing three of these constructs, immutable patient characteristics, health profile and personality profile. We did not include measures of behavioral profile, medical/treatment context and life context in this version of the PBS. The following section describes candidate measures for each of the represented constructs.
Health Profile Measures
In considering overall potential for benefit from treatment, probably the most clinically credible and conceptually plausible construct is patient’s health status at baseline.1,5 Since health is a complex, multidimensional construct, we chose to represent patient’s “health profile” with both global and disease-specific health status in the creation of the composite PBS.
Comorbidity was measured using the Total Illness Burden Index (TIBI),1,22–24 a 63-item summary measure of the presence and severity of the patient’s diseases and symptoms that has been previously modified to reflect different index conditions.1,23 The TIBI has been demonstrated to predict new cardiovascular events and all-cause mortality in a recently published five-year follow-up study among type 2 diabetic patients.1 Concurrent validity of the TIBI has been previously demonstrated using the SF-36.22,24 Those with more severe total comorbidity had poorer health-related quality of life.
To measure physical function, we used the 10-item physical function scale (PFI-10) of the Short Form 36.32 To represent mental health, specifically depressive symptomatology, we used a modified version of the Center for Epidemiologic Studies Depression Scale (CES-D).33 The latter was shortened to 11 items to reduce respondent burden, with scale scores ranging from 0 to 3. To evaluate the disease-specific burden of diabetes, we used the 8-item Diabetes Burden Scale from the Type 2 Diabetes Patient Outcomes Research Team.34,35
Personality Profile Measures
Although numerous personality characteristics have been linked with health outcomes36–40 and treatment response,41–43 we chose to represent at least one of the dimensions of this multidimensional construct using a measure of a passive orientation to health and health care, the Provider Dependent Health Care Orientation (PDHCO).44 Based on the mastery model of health and illness,45–49 the 13-item PDHCO scale measures a dependent or passive approach to healthcare and disease management, and has been linked with poor transitions in physical functioning over time.44
“Immutable” Patient Characteristics
We used age, gender, education, and race/ethnicity to represent patient demographic characteristics.
Dependent Variables
To represent treatment response, in this case for diabetes, we assessed the level of patients’ glycemic control using glycated hemoglobin (HbA1c) based on laboratory data measured at baseline, and 12 months after completing the patient questionnaire.
In addition, we measured adherence to treatment, using a 13-item measure of patient adherence to provider recommendations for medication regimens in the face of specific barriers.50,51
Statistical Analysis
All data were analyzed using SPSS release 17.0.0.52 All derived multi-item measures were tested for reliability using Cronbach alpha. Univariate and distributional analyses included measures of central tendency, kurtosis, and skew. The standard error of measurement was computed for each multi-item derived variable. Associations among independent variables were computed used Pearson product-moment correlation coefficients. Construct validity for derived multi-item scales was assessed using confirmatory principal components and varimax rotated factor analysis. Higher-order confirmatory principal components analysis and varimax rotated factor analyses were also performed on the scales representing the composite “potential for benefit” latent construct, with all measures transformed to a 0–100 scale to assure comparable contribution to covariance structure. The composite PBS was computed as the average of the scores of the standardized candidate scales, weighted by the factor loading of each score on the latent variable.
Separate analyses of covariance were used to estimate the contribution of the composite PBS to baseline adherence to treatment and hemoglobin A1c, adjusted for patient demographic characteristics. Logistic regression was used to assess the relationship of the Potential for Benefit Scale to hemoglobin A1c at the 1-year follow-up period, adjusted for baseline HbA1c, and patient demographic characteristics.
RESULTS
Patients in the study sample had relatively well controlled diabetes, with a mean HbA1c of 7.6, although there was substantial variation (SD, 1.7; Table 2). Only 32.8% reported no problems in adhering to treatment regimens related to their diabetes.
TABLE 2.
Description of Study Measures (n = 1361)
| Study Variables | No. of tems | Range | Mean | SD | Cronbach Alpha |
|---|---|---|---|---|---|
| Dependent variables | |||||
| Optimal adherence to treatment (%)*† | 13 | 0–100 | 32.8 | 47.0 | 0.80 |
| Glycated hemoglobin (HbA1c)‡ | 1 | — | 7.6 | 1.7 | N/A |
| Independent variables | |||||
| Total Illness Burden Index (TIBI)*§ | 9 | 0–25 | 5.1 | 3.5 | 0.69 |
| Passivity (PDHCO)*¶ | 13 | 0–100 | 52.9 | 18.0 | 0.75 |
| Functional status (PFI-10)*‖ | 10 | 0–100 | 64.4 | 29.7 | 0.93 |
| Depressive symptomatology (CES-D)*** | 11 | 0–33 | 12.5 | 8.0 | 0.90 |
| Perceived diabetes burden (Diabetes Burden Scale)*†† | 8 | 0–100 | 39.7 | 28.9 | 0.94 |
From patient questionnaire.
The proportion of patients reporting optimal adherence a to medication regimens, based on 13 items on scored on a 5-point Likert scale.
Glycated hemoglobin (HbA1c) collected at baseline study enrollment.
Total Illness Burden Index, a measure of the presence and severity of comorbid conditions; 9 disease-specific scale scores computed from 63 individual questionnaire items; scores range from 0 to 25; higher TIBI scores indicate greater comorbidity.
Provider-Dependent Health Care Orientation scale, measuring patient passivity; scores range from 0 to 100; higher scores indicate greater passivity.
Physical Function scale of the Medical Outcomes Study Short-Form 36; scores range from 0 to 100; higher scores indicate better physical functioning.
Center for Epidemiology Studies Depression Scale, 11-item version; scores range from 0 to 33; higher scores indicating the presence of more depressive symptoms.
Diabetes Burden scale measures impact of diabetes on health, social activities, lifestyle and finances; scores range from 0 to 100; higher scores indicate a greater degree of diabetes burden.
Patients reported moderate levels of comorbidity (TIBI mean = 5.1, SD = 3.5; Table 2). Scores on the PFI-10 (mean = 64.4, SD = 29.7) were comparable to our previous studies among patients with type 2 diabetes,53 and suggest relatively poor functioning compared with the general population.32 Compared with patients with diabetes in our prior studies,54 patients in this study were very passive (mean PDHCO = 52.9, SD = 18.0). Scores in this range have been associated with declines of ≥0.5 standard deviations in the SF-36 PFI-10 over a 1-year period.23 Study patients also reported relatively high levels of depressive symptoms (modified CES-D mean = 12.5, SD = 8.0), exceeding the level observed in other studies of patients with type 2 diabetes.53 Study patients showed substantial variation in the amount of perceived diabetes burden (mean diabetes burden = 39.7, SD = 28.9).
There were statistically significant correlations among all of the candidate measures of the higher-order construct of “potential for benefit,” lending support for the creation of a composite measure (Table 3). Factor loadings resulting from a principal components analysis confirm that all candidate variables had primary loadings on the first factor, which explained 44.0% of the variance in the covariance matrix. This same pattern of findings was observed in the longitudinal cohort subsample.
TABLE 3.
Correlation Among Candidate Measures for Inclusion in Composite Potential for Benefit Scale (n = 1361)
| Measures | Pearson Product Moment Correlation Coefficients
|
Factor Loading on Latent Valuable* | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| TIBI† | — | 0.74 | ||||
| SF-36 PFI-10‡ | −0.46§ | — | 0.72 | |||
| PDHCO¶ | 0.14§ | −0.19§ | — | 0.46 | ||
| CES-D11 | 0.46§ | −0.41§ | 0.21§ | — | 0.77 | |
| Diabetes Burden Scale** | 0.24§ | −0.21§ | 0.26§ | 0.34§ | — | 0.58 |
Factor loadings on the primary factor in a principal components analysis. This factor explains 44.0% of the total variation in the latent variable.
Total Illness Burden Index, a measure of the presence and severity of comorbid conditions; 9 disease-specific scale scores computed from 63 individual questionnaire items; scores range from 0 to 25; higher TIBI scores indicate greater comorbidity.
Physical Function scale of the Medical Outcomes Study Short-Form 36; scores range from 0–100; higher scores indicate better physical functioning.
P < 0.001.
Provider-Dependent Health Care Orientation scale, measuring patient passivity; scores range from 0 to 100; higher scores indicate greater passivity.
Center for Epidemiology Studies Depression Scale, 11-item version; scores range from 0 to 33; higher scores indicating the presence of more depressive symptoms.
Diabetes burden scale measures impact of diabetes on health, social activities, lifestyle and finances; scores range from 0 to 100; higher scores indicate a greater degree of diabetes burden.
We created the composite Potential for Benefit Scale (PBS), using a weighted average of the scores of the candidate measures (mean PBS = 25.3, SD = 11.3; data not shown). The composite PBS was significantly correlated with baseline adherence to treatment (Pearson r = −0.31, P < 001), and mean HbA1c (Pearson r = 0.12, P < 0.001; data not shown).
Results of analyses of covariance adjusted for age, gender, education, and race/ethnicity showed that patients in the highest quartile of the PBS had the highest proportion of those reporting the optimal adherence to treatment regimens (55%). Those in the lowest quartile reported the poorest adherence (18% reporting optimal adherence, P < 0.001; Table 4). Proportions of patients adhering to treatment decreased for each quartile from highest to lowest; however, the lowest 2 quartiles were not statistically significantly different from each other. These findings parallel what was observed in the longitudinal cohort subsample at baseline.
TABLE 4.
Relationship of Composite Potential for Benefit Scale to Adherence to Treatment, Glycemic Control at Baseline (n = 1361)
| Levels of Potential for Benefit Scale* | Adherence to Treatment† Mean (SE) | HbA1c <7%‡ % (SE) | HbA1c§ Mean (SE) |
|---|---|---|---|
| Quartile 1 (highest) | 0.55 (0.03)¶ | 50.2 (2.9)¶ | 7.39 (0.09)¶ |
| Quartile 2 | 0.36 (0.03)‖ | 44.6 (2.8)‖ | 7.57 (0.09)‖ |
| Quartile 3 | 0.24 (0.02)** | 44.1 (2.9)‖ | 7.69 (0.09)‖ |
| Quartile 4 (lowest) | 0.18 (0.03)** | 38.0 (2.9)‖ | 7.75 (0.09)‖ |
| R2 | 0.16 | 0.12 | 0.19 |
| F(3,1358) | 37.89†† | 2.87†† | 2.81‡‡ |
Adherence to treatment compared across quartile groups for the Potential for Benefit Scale (PBS) using analysis of covariance models adjusting for age, gender, education and ethnicity. Adjusted means for each outcome are reported.
Proportion of patients in each quartile group reporting optimal adherence to treatment regimens.
The proportion of patients with an average glycated hemoglobin (HbA1c) less than 7% at baseline.
Mean glycated hemoglobin (HbA1c) collected at baseline.
Significant differences between quartiles in contrast analyses (P < 0.05).
P < 0.001.
P < 0.05.
Similarly, after adjustment, those in the highest quartile of the PBS had a mean HbA1c of 7.39% (SE, 0.09) with 50.2% having HbA1c values less than 7%, while those in the lowest PBS quartile had a mean HbA1c of 7.75% (SE, 0.09), with only 38.0% having HbA1c values less than 7% (Table 4). In all 3 models, PBS was statistically significantly associated with the dependent variable, accounting for 16%, 12%, and 19% of the explained variance in adherence to treatment, proportion of patients with HbA1c values less than 7% and mean HbA1c, respectively. Replicating these analyses in the longitudinal cohort subsample produced results that parallel the findings reported here.
To assess the relative contribution of the composite PBS to glycemic control at 1-year follow-up, we conducted a logistic regression with HbA1c less than 7% as the dependent variable, using baseline PBS, baseline HbA1c values, and patient’s age, gender, education, and race/ethnicity as independent variables and covariates. The composite PBS was statistically significantly related to the target HbA1c value (less than 7%), after adjustment (adjusted OR = 1.61, P < 0.05) (Table 5).
TABLE 5.
Relationship of Composite Potential for Benefit Scale to One Year Postenrollment Glycemic Control (HbA1c <7%) in the Longitudinal Cohort Subsample, Results From a Logistic Regression Analysis (n = 611)*
| Independent Variables | HbA1c <7% at One-Year Follow-up
|
|
|---|---|---|
| Odds Ratio (95% CI) | P | |
| Potential for Benefit | 1.62 (1.004, 2.62) | 0.048 |
| Scale†‡ (score in highest quartile) | ||
| Baseline Hemoglobin A1c§ | 0.28 (0.21, 0.35) | <0.001 |
| Age† | 1.01 (0.99, 1.04) | 0.162 |
| Gender (male)† | 0.91 (0.59, 1.41) | 0.663 |
| Hispanic ethnicity† | 0.57 (0.29, 1.11) | 0.097 |
| Vietnamese ethnicity† | 1.14 (0.57, 2.31) | 0.709 |
| Years of education† | 1.00 (0.95, 1.06) | 0.911 |
| Constant | 4132.13 | — |
Subset of patients from the cross-sectional sample participating in 1-year longitudinal cohort study; logistic regression analysis modeling attaining a glycated hemoglobin (HbA1c) value less than 7% at 1-year follow-up was used to derive table entries.
Reported from patient questionnaire at baseline.
A composite measure scored as the weighted mean of scores on the Total Illness Burden Index (TIBI), PFI-10, PDHCO scale, CES-D, and the Diabetes Burden Scale; scores ranged from 0 to 100; weighted derived from factor loadings from a principal components analysis.
The mean glycated hemoglobin (HbA1c) value collected at baseline.
DISCUSSION
Recent negative or “small effect” clinical trials have directed attention to the differential response of important and often large subgroups of patients.8–12 In addition to the severity or stage of the specific disease being studied, other patient characteristics such as comorbidity or passivity may change the potential of some patients to respond to treatment, particularly in trials where the effectiveness of treatment is contingent on successful patient behaviors. As has frequently been observed,13,15,17 the a priori risk-stratification by subgroups of patients with differential potential for benefit from treatment, could both improve the efficiency and effectiveness of clinical studies and provide useful information for ‘tailoring’ treatment for specific subgroups to practicing clinicians.
This article focuses on selected patient characteristics, each representing key constructs from our proposed conceptual framework, to help identify groups of patients with greater or lesser potential to respond to treatment. The intent was to identify candidates for a composite measure that, with further development and testing, could be used as a design variable for clinical studies, and to provide useful information for eventual “tailoring” of treatment to improve the effectiveness of care.
When attempting to create subgroups of patients with differing potential for benefit from treatment, the construct with the greatest clinical credibility and the strongest empirical link with treatment outcomes is patients’ health profile. We represented patients’ health profile with both general and disease-specific measures. In this study and elsewhere,22,24 the TIBI has also been shown to have a significant relationship to functional status, as measured by the SF-36. Although conceptually distinct, the modest association between the severity of multiple comorbidities, as measured by the TIBI, and the performance of routine daily activities, as measured by the SF-36, suggested that both could contribute independently to the categorization of patients’ potential for response to treatment. When considered together, our analyses showed that both were significantly associated with compliance and HbA1c, and explained more variation in both dependent variables that when each was considered alone.
Depression has been linked with poor health outcomes, including higher HbA1c among patients with diabetes.1 The association of depressive symptoms and lack of engagement in health enhancing behaviors,55–56 along with the interrelationship between depression, functional status and multiple comorbidities led us to consider a measure of depressive symptoms as a candidate for inclusion in the PBS.
Finally, the perceived burden of the disease under study, in this case diabetes, could also be an important and independent contributor to the patient’s potential for response to treatment. As the management of any specific chronic disease becomes more complex, and is perceived to be more difficult or arduous by the patient, the potential for response to any additional treatment may be reduced. Therefore, we also considered this variable as a candidate contributor to the PBS.
To represent the many personality characteristics with established relationships to health outcomes that could contribute to a composite potential for benefit measure, we focused on a passive approach to health and healthcare. Since the effectiveness of a treatment often requires the active engagement of patients in sometimes complex regimens, a passive approach to disease management could reduce the potential for treatment response.45–46,49 Data from the Type II Diabetes Patient Outcomes Research Team have shown that passive patients have substantial and statistically significant declines in health status, as measured by the PFI-10, over a 1-year period.44 Data from the current study also show that passivity is associated with poorer physical functioning, more depressive symptoms, greater total illness burden and perceived diabetes-specific burden, and with poorer adherence to treatment and poorer glycemic control. This variable was therefore considered as a candidate for reducing patients’ potential for treatment response.
Empirical testing of this group of candidate measures supported the creation of a composite measure, the PBS, that could be used to categorize overall potential for a differential response to treatment. The significant and substantial relationship of the PBS to adherence at baseline, as well as to glycemic control at baseline and at 1-year follow-up, supports the validity of this higher-order construct to reflect a differential responsiveness to treatment in this study.
Clearly this research is preliminary and represents a first step in the development of a composite that could classify patients by potential for treatment response. Further research is needed to assess the relative contribution of other dimensions of potential for benefit, eg, elements of a “behavioral profile,” “life context,” and “medical context,” as well as other measures of the “health” and “personality” profiles, in a more refined categorization of subgroups of patients with greater and lesser likelihood to respond to treatment. Longitudinal studies, in other diseases, other patient populations, and other clinical settings are needed to assess generalizability of the findings of this study.
If successful, a more fully developed composite measure could be used to identify a priori treatment groups with differential potential for treatment response, with varying effect sizes, for more interpretable and efficient design of randomized controlled clinical trials. Such redesign of trials could potentially obviate the need for posthoc analyses of subgroups. Although each of the contributors to the composite PBS could be used independently as an a priori design variable, composite measures simplify the creation of subgroups, increase the precision of the identification of such groups, and offer efficiencies in adjustment of trial results for baseline states. Data on such subgroups could aid practicing physicians in the tailoring of treatments to maximize treatment effectiveness.
Limitations
This study has a number of limitations. First, we used a single disease, type 2 diabetes, to develop the composite potential for treatment response variable. Study findings will need to be replicated in other chronic conditions before such a composite measure should be used widely in the design of clinical trials. However, because diabetes is a complex, multisystem disease, it includes a broader spectrum of chronic diseases and the findings from this study are more likely to generalize to at least those diseases. Second, we used data from a poor and ethnically diverse population seen at multiple clinics in a single academic medical center. Results from this study may not replicate among other patient populations and in other treatment settings. Third, we used a selected group of variables to represent potential for treatment response. The inclusion of other categories of variables, such as those described in the conceptual framework, may enhance the prediction of potential for response. Finally, although we followed a cohort for a 1-year period, the results of this study should be replicated with longer term transitions in health outcomes to specify the baseline potential for treatment response for use in the design of clinical trials.
In summary, we conclude that the measures we tested representing patients’ health status and passive orientation toward their healthcare can form a composite that reflects their potential for responsiveness to treatment. This composite measure, the PBS, was associated with adherence to treatment and to baseline and follow-up glycemic control. Such a measure could be useful in the interpretation of comparative effectiveness research and may be useful in the redesign of clinical trials.
Acknowledgments
This work was supported by The Robert Wood Johnson Foundation (Grants #1051084 and #59758), Princeton, New Jersey, The NovoNordisk Foundation, Corporate Diabetes Programmes, Novo Nordisk, Bagsvaerd, Denmark, and the National Institute of Diabetes, Digestive and Kidney Diseases (R18DK69846 and K01DK078939), Building 31. Rm 9A06, 31 Center Drive, MSC 2560 Bethesda, MD 20892-2560, USA.
References
- 1.Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151:854–860. doi: 10.7326/0003-4819-151-12-200912150-00005. [DOI] [PubMed] [Google Scholar]
- 2.Greenfield S, Kravitz RL, Duan N, et al. Heterogeneity of treatment effects: implications for guidelines, payment, and quality assessment. Am J Med. 2007;120:S3–S9. doi: 10.1016/j.amjmed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield S, Kravitz RL. Engaging the implications of subgroup heterogeneity: the increasing inadequacy of randomized trials for guidelines, payment and quality. Washington, DC: Institute of Medicine Report on the Learning Healthcare Environment: National Academy of Sciences; 2007. [Google Scholar]
- 4.Kravitz RL, Duan N, Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q. 2004;82:661–687. doi: 10.1111/j.0887-378X.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kent D, Hayward R. When Averages hide individual differences in clinical trials: analyzing the results of clinical trials to expose individual patients’ risks might help doctors make better treatment decisions. Am Sci. 2007;95:60–68. [Google Scholar]
- 6.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 7.Sox HC, Greenfield S. Comparative effectiveness research: a report from the institute of medicine. Ann Intern Med. 2009;151:203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]
- 8.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Advance Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 10.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt DL, Fox AA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 13.Luce BR, Kramer JM, Goodman SN, et al. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009;151:206–210. doi: 10.7326/0003-4819-151-3-200908040-00126. [DOI] [PubMed] [Google Scholar]
- 14.Peterson ED. Innovation and comparative-effectiveness research in cardiac surgery. N Engl J Med. 2009;361:1897–1899. doi: 10.1056/NEJMe0907887. [DOI] [PubMed] [Google Scholar]
- 15.Hayward RA, Kent DM, Vijan S, et al. Reporting clinical trial results to inform providers, payers, and consumers. Health Affairs. 2005;24:1571–1581. doi: 10.1377/hlthaff.24.6.1571. [DOI] [PubMed] [Google Scholar]
- 16.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 18.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. Br Med J. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karanicolas PJ, Montori VM, Devereaux PJ, et al. A new “mechanistic-practical” framework for designing and interpreting randomized trials. J Clin Epidemiol. 2009;62:479–484. doi: 10.1016/j.jclinepi.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Kent DM, Ruthazer R, Selker HP. Are some patients likely to benefit from recombinant tissue-type plasminogen activator for acute ischemic stroke even beyond 3 hours from symptom onset? Stroke. 2003;34:464–467. doi: 10.1161/01.str.0000051506.43212.8b. [DOI] [PubMed] [Google Scholar]
- 21.Turner BJ, Hollenbeak CS, Weiner M, et al. Effect of unrelated comorbid conditions on hypertension management. Ann Intern Med. 2008;148:578–586. doi: 10.7326/0003-4819-148-8-200804150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Litwin M, Greenfield S, Elkin E, et al. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109:1777–1783. doi: 10.1002/cncr.22615. [DOI] [PubMed] [Google Scholar]
- 23.Stier DM, Greenfield S, Lubeck DP, et al. Quantifying comorbidity in a disease-specific cohort: adaptation of the total illness burden index to prostate cancer. Urology. 1999;54:424–429. doi: 10.1016/s0090-4295(99)00203-4. [DOI] [PubMed] [Google Scholar]
- 24.Greenfield S, Sullivan L, Dukes KA, et al. Development and testing of a new measure of case mix for use in office practice. Med Care. 1995;33:AS47–AS55. [PubMed] [Google Scholar]
- 25.Hagger MS, Orbell S. A meta-analytic review of the common-sense model Of illness representations. Psychol Health. 2003;18:141–184. [Google Scholar]
- 26.Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot. 1996;10:282–298. doi: 10.4278/0890-1171-10.4.282. [DOI] [PubMed] [Google Scholar]
- 27.Suls J, Rothman A. Evolution of the biopsychosocial model: prospects and challenges for health psychology. Health Psychol. 2004;23:119–125. doi: 10.1037/0278-6133.23.2.119. [DOI] [PubMed] [Google Scholar]
- 28.Brown AF, Ettner SL, Piette J, et al. Socioeconomic position and health among persons with diabetes mellitus: a conceptual framework and review of the literature. Epidemiol Rev. 2004;26:63–77. doi: 10.1093/epirev/mxh002. [DOI] [PubMed] [Google Scholar]
- 29.Franzini L, Fernandez-Esquer ME. Socioeconomic, cultural, and personal influences on health outcomes in low income Mexican-origin individuals in Texas. Soc Sci Med. 2004;59:1629–1646. doi: 10.1016/j.socscimed.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 31.Kogan SM, Brody GH, Chen Y. Depressive symptomatology mediates the effect of socioeconomic disadvantage on HbA1c among rural African Americans with type 2 diabetes. J Psychosom Res. 2009;67:289–296. doi: 10.1016/j.jpsychores.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 34.Silliman RA, Bhatti S, Knan A, et al. The care of older persons with diabetes mellitus: families and primary care physicians. J Am Geriatr Soc. 1996;44:1314–1321. doi: 10.1111/j.1532-5415.1996.tb01401.x. [DOI] [PubMed] [Google Scholar]
- 35.Greenfield S, Kaplan SH, Silliman RA, et al. The uses of outcomes research for medical effectiveness, quality of care, and reimbursement in type II diabetes. Diabetes Care. 1994;17(suppl 1):32–39. [PubMed] [Google Scholar]
- 36.Brickman AL, Yount SE, Blaney NT, et al. Personality traits and long-term health status: the influence of neuroticism and conscientiousness on renal deterioration in type-1 diabetes. Psychosomatics. 1996;37:459–468. doi: 10.1016/S0033-3182(96)71534-7. [DOI] [PubMed] [Google Scholar]
- 37.Hemingway H, Marmot M. Psychosocial factors in the aetiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. Br Med J. 1999;318:1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steptoe A, Molloy GJ. Personality and heart disease. Heart. 2007;93:783–784. doi: 10.1136/hrt.2006.109355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mols F, Denollet J. Type D personality in the general population: a systematic review of health status, mechanisms of disease, and work-related problems. Health Qual Life Outcomes. 2010;8:9. doi: 10.1186/1477-7525-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousfi S, Matthews G, Amelang M, et al. Personality and disease: correlations of multiple trait scores with various illnesses. J Health Psychol. 2004;9:627–647. doi: 10.1177/1359105304045339. [DOI] [PubMed] [Google Scholar]
- 41.Bagby RM, Quilty LC, Segal ZV, et al. Personality and differential treatment response in major depression: a randomized controlled trial comparing cognitive-behavioural therapy and pharmacotherapy. Can J Psychiatry. 2008;53:361–370. doi: 10.1177/070674370805300605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kool S, Schoevers R, de Maat S, et al. Efficacy of pharmacotherapy in depressed patients with and without personality disorders: a systematic review and meta-analysis. J Affect Disord. 2005;88:269–278. doi: 10.1016/j.jad.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Denollet J, Vaes J, Brutsaert DL. Inadequate response to treatment in coronary heart disease: adverse effects of type D personality and younger age on 5-year prognosis and quality of life. Circulation. 2000;102:630–635. doi: 10.1161/01.cir.102.6.630. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan SH, Dukes KA, Sullivan LM, et al. Is passivity a risk factor for poor health outcomes? J Gen Intern Med. 1996;11:76. [Google Scholar]
- 45.Seeman TE. Personal control and coronary artery disease: how generalized expectancies about control may influence disease risk. J Psychosom Res. 1991;35:661–669. doi: 10.1016/0022-3999(91)90116-6. [DOI] [PubMed] [Google Scholar]
- 46.Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. J Pers Soc Psychol. 1998;74:763–773. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- 47.Pudrovska T, Schieman S, Pearlin LI, et al. The sense of mastery as a mediator and moderator in the association between economic hardship and health in late life. J Aging Health. 2005;17:643–660. doi: 10.1177/0898264305279874. [DOI] [PubMed] [Google Scholar]
- 48.Schieman S, Meersman SC. Neighborhood problems and health among older adults: received and donated social support and the sense of mastery as effect modifiers. J Gerontol B Psychol Sci Soc Sci. 2004;59:S89–S97. doi: 10.1093/geronb/59.2.s89. [DOI] [PubMed] [Google Scholar]
- 49.Street RL, Jr, Krupat E, Bell RA, et al. Beliefs about control in the physician-patient relationship: effect on communication in medical encounters. J Gen Intern Med. 2003;18:609–616. doi: 10.1046/j.1525-1497.2003.20749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherbourne CD, Hays RD, Ordway L, et al. Antecedents of adherence to medical recommendations: results from the Medical Outcomes Study. J Behav Med. 1992;15:447–468. doi: 10.1007/BF00844941. [DOI] [PubMed] [Google Scholar]
- 51.Toobert DJ, Glasgow RE. Assessing diabetes self-management: the summary of diabetes self-care activities questionnaire. In: Bradley C, editor. Handbook of Psychology and Diabetes. Newark, NJ: Harwood Academic Publishers; 1994. pp. 355–356. [Google Scholar]
- 52.SPSS Statistical Software [computer program] Version 17.0 for Windows. Chicago, IL: SPSS; 2008. [Google Scholar]
- 53.Carusoa LB, Sillimana RA, Demissieb S, et al. What can we do to improve physical function in older persons with Type 2 diabetes? J Gerontol A Biol Sci Med Sci. 2000;55:M372–M377. doi: 10.1093/gerona/55.7.m372. [DOI] [PubMed] [Google Scholar]
- 54.Kaplan SH, Dukes KA, Sullivan LM, et al. Is passivity a risk factor for poor health outcomes? J Gen Intern Med. 1996;11:76. [Google Scholar]
- 55.Dimatteo RM, Lepper HD, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 56.Ciechanowski PS, Katon WJ, Russo JO. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–3285. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]


