Abstract
Background
Fructose-mediated protein glycation (fructation) has been linked to an increase in diabetic and cardiovascular complications due to over consumption of high-fructose containing diets in recent times. The objective of the present study is to evaluate the protective effect of (R)-α-lipoic acid (ALA) against fructose-induced myoglobin fructation and the formation of advanced glycation end products (AGEs) in vitro.
Methods
The anti-glycation activity of ALA was determined using the formation of AGEs fluorescence intensity, iron released from the heme moiety of myoglobin and the level of fructosamine. The fructation-induced myoglobin oxidation was examined using the level of protein carbonyl content and thiol group estimation.
Results
The results showed that co-incubation of myoglobin (1 mg/mL), fructose (1 M) and ALA (1, 2 and 4 mM) significantly inhibited the formation of AGEs during the 30 day study period. ALA markedly decreased the levels of fructosamine, which is directly associated with the reduction of AGEs formation. Furthermore, ALA significantly reduced free iron release from myoglobin which is attributed to the protection of myoglobin from fructose-induced glycation. The results also demonstrated a significant protective effect of ALA on myoglobin oxidative damages, as seen from decreased protein carbonyl content and increased protein thiols.
Conclusion
These findings provide new insights into the anti-glycation properties of ALA and emphasize that ALA supplementation is beneficial in the prevention of AGEs-mediated diabetic and cardiovascular complications.
Keywords: (R)-α-Lipoic acid, Fructose, Fructation, Myoglobin
Background
Protein glycation is a chemical reaction process between a free amino group of a protein and a carbonyl group of a reducing sugar to form a freely reversible Schiff’s base that further rearranges into a more stable intermediary structure called Amadori product [1]. The Amadori product then undergoes oxidative cleavage, generating dicarbonyl compounds to form cross-linked structures termed advanced glycation end products (AGEs). The gradual formation of AGEs in various body tissues play a vital role in further cross-linking or modifying other intracellular and extracellular proteins and in generating reactive oxygen species (ROS) [2]. Hence, the excessive formation of AGEs and their accumulation in various tissues is responsible for age-related diseases [3] and the development of long-term diabetic complications [4].
Myoglobin, a heme-protein expressed in striated muscles (cardiac myocytes and skeletal muscle fibres) plays an important role in the storage and transport of molecular oxygen for cellular respiration [5–9]. Furthermore, myoglobin acts as an intracellular scavenger of nitric oxide (NO), regulating its level in the cardiac muscle and thereby protecting mitochondrial respiration [10–12]. In a high glucose and/or fructose environment, the amino group of myoglobin readily undergoes a non-enzymatic reaction which results in its structural and functional modification in vitro [13, 14]. Furthermore, prolonged incubation of myoglobin with glucose or fructose produces fructosamine followed by AGEs formation [15, 16]. Glucose and fructose-mediated glycation also induces oxidative modification of myoglobin by generating protein carbonyl compounds which may be associated with oxidative stress [13, 17]. During vigorous repeated physical exercise or muscle injury- related disorder such as rhabdomyolysis produce muscle damage releasing myoglobin into circulation, where myoglobin comes in contact with circulatory glucose and/or fructose [18]. Free myoglobin in circulation can undergo glycation reactions specifically in the poor glycaemic situation and may be a source of various pathophysiological complications such as diabetic ketoacidosis and renal failure [19, 20]. However, intracellular myoglobin glycation in muscles cell, particularly in hyperglycaemic condition and its implications in the development of complications, is still not known.
Although many synthetic and natural anti-glycation compounds have been evaluated in vivo and in vitro, no single compound effectively suppresses protein glycation in a clinical setting. Aminoguanidine has been shown to be a potent inhibitor of the protein glycation process and fluorescent AGEs formation in animals and in humans [21]. However, its clinical use is limited due to severe adverse effects such as flu-like symptoms, gastrointestinal problems and anaemia [22–24]. Therefore, there is an urgent need to evaluate new compounds that inhibit protein glycation and thus may be beneficial in preventing diseases mediated by AGEs.
(R)-α-Lipoic acid (ALA; 1,2-dithiolane-3-pentanoic acid), also known as thioctic acid, is traditionally recognized as an essential cofactor in mitochondrial respiratory enzymes that catalyse the oxidative decarboxylation reactions [25]. Chemically, ALA is a short-chain fatty acid with a disulfide group in its dithiolane ring and a chiral carbon resulting in R and S enantiomers. Although the majority of the commercially produced ALA consists of a racemic admixture, the R form is the biologically active form that is endogenously produced by the body, while the S form is produced from chemical manufacture and is not biologically active [26]. At the cellular level, ALA is reduced to dihydrolipoic acid (DHLA), which has a number of cellular actions including free radical scavenging and modulating oxidative stress and inflammatory pathways [26]. ALA, when exogenously administered, is readily absorbed from the gut and has been clinically used in Europe for the treatment of diabetic polyneuropathy [27].
ALA has been shown to possess various pharmacological properties including anti-oxidant, anti-inflammatory, detoxifying, antidiabetic, cardiovascular, anti-ageing, anticancer, cognitive and neuroprotective [28, 29]. In laboratory experiments, the effect of ALA on protein glycation and AGEs formation has been investigated both in vitro and in vivo. Dietary supplementation of ALA in rats fed chronically with glucose significantly decreased mitochondrial superoxide in the heart and AGEs formation in the aorta [30]. Furthermore, chronic supplementation of ALA in fructose-fed-rats significantly attenuated AGEs-mediated skin-collagen cross-linking and other physicochemical abnormalities [31]. In addition, chronic treatment of ALA in high-fructose fed rats markedly lowered glucose, glycated protein, glycated hemoglobin and fructosamine in circulation; enhanced in vitro glucose utilization with prevention of glycation and accumulation of AGEs in isolated rat diaphragm [32]. In obese Zucker rats, chronic treatment of ALA significantly inhibited protein carbonyls content and improved insulin sensitivity in skeletal muscle [33]. ALA also inhibited AGEs production and down-regulated the receptor for advanced glycation end products (RAGE) expression in streptozotocin-induced diabetic rats [34] and in human embryonic kidney cells and in rat sensory neurons [35, 36]. In addition, topical treatment of ALA nanoparticles significantly down-regulated the expression of RAGE and enhanced cutaneous wound healing in streptozotocin-induced diabetic mice [37].
In an in vitro glycation model containing bovine serum albumin and glucose, ALA markedly inhibited fructosamine, protein carbonyls and fluorescent AGEs production [38, 39]. Moreover, ALA markedly suppressed AGEs-induced activation of NF-kB in cultured vascular endothelial cells [40] and in retinal endothelial cells [41]. In another independent study, exogenous administration of ALA diminished AGEs-induced endothelial expression of vascular cell adhesion molecule-1 (VCAM-1) and monocyte binding to endothelium [42]. Furthermore, ALA prevented the up-regulation of AGEs-induced inducible nitric oxide synthase (iNOS) expression and nitric oxide (NO) production in murine microglial cells [43]. ALA also reduced the AGEs-mediated formation of lipid peroxidation products in human neuronal cells [44, 45] and in rat cortical neurones [46]. However, ALA has not been examined in myoglobin glycation induced by fructose. Hence, in the present study, we investigated the effects of ALA on fructose-induced myoglobin fructation and AGEs formation.
Methods
Chemicals and reagents
(R)-α-Lipoic acid, myoglobin, nitro blue tetrazolium (NBT), hydroxylamine hydrochloride, ferrozine, dinitrophenylhydrazine (DNPH), guanidine hydrochloride, ethyl acetate, ethanol, trichloroacetic acid (TCA), dimethyl sulfoxide (DMSO), fructose, 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB), L-cysteine and aminoguanidine (AG) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fructosamine and iron standards were obtained from PM Separations (Capalaba DC, QLD, Australia). All other chemicals and reagents used were of analytical grade.
Evaluation of myoglobin fructation inhibitory effect of α-lipoic acid under fructose overload in vitro
Myoglobin fructation was performed according to the methods previously described by Roy and colleagues with minor modification [13, 16]. Briefly, 500 μL of myoglobin (final concentration: 1 mg/mL) was incubated with 400 μL of fructose (final concentration: 1 M) solution at 37 °C in the dark for up to 30 days in the presence or absence of ALA (100 μL; dissolved in DMSO) at a final concentration of 1, 2 and 4 mM. Aminoguanidine (100 μL; dissolved in DMSO), at a final concentration of 5 mM was used as a positive control. After the specified incubation period (10, 20 or 30 days), aliquots of the glycated reaction mixtures were assayed for fluorescent AGEs, free iron, fructosamine (glycated protein), protein carbonyls and protein thiols.
Determination of fluorescent AGEs formation
The formation of fluorescent AGEs in the reaction mixture after 30 days of incubation was measured according to the method of Wrobel et al. [46]. Briefly, to 1 mL of the reaction mixture, 250 μL of TCA (100%) was added. The resulting mixture was vortexed for 60 s and centrifuged in a refrigerated centrifuge (Biofuge Stratos, Thermo Scientific) at 14,000 rpm for 4 min. The supernatant was collected in a disposable polystyrene cuvette, and fluorescence intensity was read at an excitation wavelength 355 nm and emission wavelength 460 nm using a spectrofluorometer (Wallac 1420 Victor 3 V, Perkin Elmer). The percentage inhibition of fluorescent AGEs formation was calculated as follows:
Estimation of free iron in fructated myoglobin (ferrozine test)
The free iron, as a measure of fructation-induced iron release in the reaction mixture after 10, 20 and 30 days of incubation, was estimated according to the method of Panter [47]. Briefly, to 250 μL of the reaction mixture, 250 μL of ice cold TCA (20%) was added and centrifuged in a refrigerated centrifuge at 15,000 rpm for 4 min. To 250 μL of the supernatant, 2.5 mL of iron buffer (1.5% hydroxylamine hydrochloride in acetate buffer, pH 4.5) and 50 μL iron colour reagent (0.85% ferrozine in iron buffer) were added. The resultant mixture was incubated at 37 °C for 30 min and the absorbance was measured at 560 nm using a UV-visible spectrophotometer (Ultrospec 2100 Pro, Biochrom). The concentration of liberated free iron was calculated as follows:
Estimation of fructosamine (fructated myoglobin)
The concentration of fructosamine, as a measure of glycated protein in the reaction mixture after 10, 20 and 30 days of incubation was measured according to the method of Ohkawara et al. [48]. Briefly, to 250 μL of the reaction mixture, 1 mL of 0.5 mM NBT reagent (in carbonate buffer; pH 10.8) was added in a disposable polystyrene cuvette inside a UV-visible spectrophotometer. The absorbance difference at 540 nm after 10 min and 15 min was used to calculate the formation of fructosamine using the following formula:
Estimation of protein carbonyls content
The level of protein carbonyls, as a measurement of fructation-induced protein oxidation in the reaction mixture after 10, 20 and 30 days of incubation, was estimated according to the method of Levine et al. [49]. Briefly, to 200 μL of the reaction mixture, 200 μL of 10 mM DNPH (in 2.5 M hydrochloric acid) was added. After thorough mixing, 250 μL of TCA (30%) was added and centrifuged in a refrigerated centrifuge at 15,000 rpm for 4 min. The pellet was collected and washed three times with 1 mL ethanol: ethyl acetate (1:1) mixture to remove any unreacted DNPH. The pellet was then dissolved in 1 mL of 6 M guanidine hydrochloride (in 20 mM phosphate buffer, pH 6.6), incubated at 37 °C for 15 min and centrifuged in a refrigerated centrifuge at 15,000 rpm for 4 min. The absorbance of the supernatant was then measured at 375 nm using a UV-visible spectrophotometer. The concentration of protein carbonyls was expressed as nanomoles of carbonyls per milligram of protein using the molar absorption coefficient of DNPH (22,000 M−1 cm−1).
Estimation of free protein thiols
The concentration of free protein thiols, as a measure of fructation-induced antioxidant defence in the reaction mixture after 10, 20 and 30 days of incubation, was measured by Ellman’s assay [50] with minor modifications. Briefly, 70 μL of the reaction mixture was incubated with 130 μL of 5 mM DTNB (in 0.1 M phosphate buffered saline) at room temperature for 15 min and the absorbance was measured at 412 nm using a UV-visible spectrophotometer. The concentration of protein thiols was calculated using a standard curve of L-cysteine and expressed as nanomoles of L-cysteine per milligram of protein.
Statistical analysis
The results were expressed as a means ± SD (n = 6). To examine the quantitative differences among the experimental groups, the respective data were subjected to one-way analysis of variance (ANOVA) using GraphPad Prism-5.0 (GraphPad Software Inc., California, USA) statistical programme. Post hoc comparisons were made using Dunnett’s multiple comparison test. Statistical differences in individual groups at different time points were detected using Student’s paired t-test. In all tests, p < 0.05 was used as the criterion for statistical significance.
Results
Effect of α-lipoic acid on fluorescent AGEs formation
The effect of ALA on the formation of fluorescent AGEs in myoglobin-fructose glycation system was observed on day-30 of incubation. As shown in Fig. 1a, incubation of myoglobin with fructose (fructated control) significantly (p < 0.001) increased the formation of fluorescent AGEs by 17-fold as shown by increased fluorescence intensity (9226.7 ± 398.4 vs 539.7 ± 4.8) compared with myoglobin incubation alone (non-fructated control). On the other hand, ALA co-treatment in the myoglobin-fructose glycation system at 1, 2 and 4 mM concentrations elicited significant (p < 0.01) concentration-dependent inhibition of fluorescent AGEs formation with a maximum reduction of 49.7% (4641.7 ± 590.2 vs 9226.7 ± 398.4) at 4 mM concentration compared with the fructated control. The concentration of ALA required to inhibit 50% (IC50) of fluorescent AGEs as determined from linear regression analysis was found to be 3.97 mM (Fig. 1b). In comparison, co-treatment of aminoguanidine (a known inhibitor of glycation process; positive control) at a concentration of 5 mM in myoglobin-fructose glycation system produced a significant (p < 0.01) 78.6% inhibition of fluorescent AGEs formation (1969.8 ± 276.0 vs 9226.7 ± 398.4) compared with the fructated control.
Fig. 1.
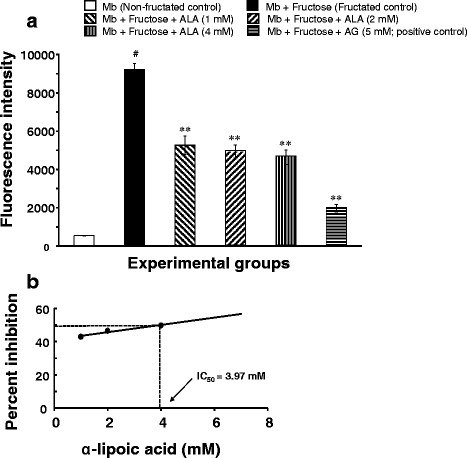
Effect of α-lipoic acid on the formation of fluorescent AGEs in fructose-induced myoglobin fructation. Each bar represents the mean ± SEM in six separate experiments (n = 6). Significant difference from fructated control: **p < 0.01. Significant difference from non-fructated control: #p < 0.001. ALA = (R)-α-lipoic acid; AG = Aminoguanidine; Mb = Myoglobin
Effect of α-lipoic acid on free iron release
Figure 2a shows the effect of ALA on fructation-induced iron release in myoglobin-fructose glycation system as observed on day-10, 20 and 30 of incubation. A significant (p < 0.001) 13-fold increase in free iron (121.8 ± 5.3 vs 9.4 ± 2.2) was observed on day-10 when myoglobin was co-incubated with fructose (fructated control) compared with myoglobin incubation alone (non-fructated control) and moreover, this difference was consistent throughout the study period. Nevertheless, no significant time-dependent change in free iron level was observed in the myoglobin-fructose co-incubation on day-20 or day-30 compared with day-10 value. However, ALA co-treatment in myoglobin-fructose glycation system at 1, 2 and 4 mM concentrations significantly (p < 0.01) displayed a concentration-dependent reduction in free iron levels on day-10 compared with the fructated control and moreover, this difference was consistent throughout the study period. On day-30 of co-treatment, ALA significantly (p < 0.01) decreased the free iron levels with a maximum reduction of 44.5% (69.5 ± 2.9 vs 125.3 ± 1.1) at 4 mM concentration compared with the fructated control. Nevertheless, at all the studied ALA concentrations, no significant time-dependent change in free iron levels was observed within groups on day-20 or day-30 compared with day-10 values. The concentration of ALA required to inhibit 50% (IC50) of free iron release as determined from linear regression analysis was found to be 4.4 mM (Fig. 2b). In comparison, co-treatment of aminoguanidine (a known inhibitor of glycation process; positive control) at a concentration of 5 mM in myoglobin-fructose glycation system produced a significant (p < 0.01) reduction in free iron release on day-10 compared with the fructated control which was consistent throughout the study period, achieving a maximum 48.8% reduction (64.2 ± 2.4 vs 125.3 ± 1.1) on day-30.
Fig. 2.
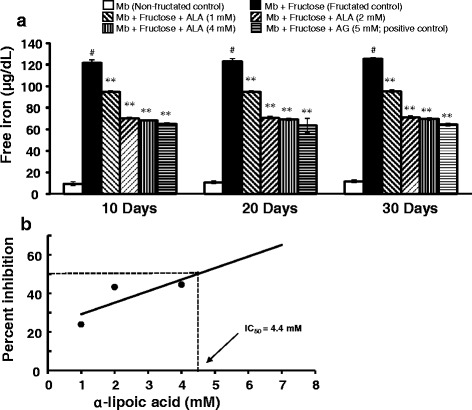
Effects of α-lipoic acid on free iron release in fructose-induced myoglobin fructation. Each bar represents the mean ± SEM in six separate experiments (n = 6). Significant difference compared to fructated control at identical times: **p < 0.01. Significant difference from non-fructated control: #p < 0.001. ALA = α-lipoic acid; AG = Aminoguanidine; Mb = Myoglobin
Effect of α-lipoic acid on fructosamine formation
The effect of ALA on fructosamine formation in myoglobin-fructose glycation system as observed on day-10, 20 and 30 of incubation is shown in Fig. 3a. A significant 3-fold increase in fructosamine (1300.2 ± 72.5 vs 486.4 ± 39.4; p < 0.001) was observed on day-10 when myoglobin was co-incubated with fructose (fructated control) compared with myoglobin incubation alone (non-fructated control) and moreover, this difference was more pronounced after day-20 (1608.8 ± 84.1 vs 557.4 ± 23.3; p < 0.001) and day-30 (1754.8 ± 83.2 vs 564.9 ± 51.8; p < 0.001) of the study. Moreover, a marked time-dependent increase in fructosamine was observed in myoglobin-fructose co-incubation that gained significance by day-20 (1608.8 ± 84.1 vs 1300.2 ± 72.5; p < 0.05) and day-30 (1754.8 ± 83.2 vs 1300.2 ± 72.5; p < 0.01) compared with day-10. On the other hand, ALA co-treatment in the myoglobin-fructose glycation system at 1, 2 and 4 mM concentrations significantly (p < 0.05 to p < 0.01) displayed a concentration-dependent reduction in fructosamine levels on day-10 compared with the fructated control and moreover, this difference was consistent throughout the study period. On day-30 of co-treatment, ALA showed a significant (p < 0.01) decrease in fructosamine levels with a maximum reduction of 34.5% (1275.9 ± 66.4 vs 1754.8 ± 83.2) at 4 mM concentration compared with the fructated control. Nevertheless, at all the studied ALA concentrations, no significant time-dependent change in fructosamine levels was observed on day-20 or day-30 compared with day-10 values. The concentration of ALA required to inhibit 50% (IC50) of fructosamine as determined from linear regression analysis was found to be 8.9 mM (Fig. 3b). In comparison, co-treatment of aminoguanidine (a known inhibitor of glycation process; positive control) at a concentration of 5 mM in myoglobin-fructose glycation system produced a significant (p < 0.01) reduction in fructosamine on day-10 compared with the fructated control, which was consistent throughout the study period, achieving a maximum 40.6% reduction (1088.8 ± 69.7 vs 1754.8 ± 83.2) on day-30.
Fig. 3.
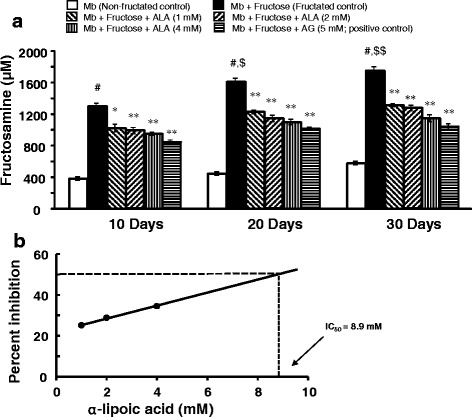
Effects of α-lipoic acid on fructosamine formation in fructose-induced myoglobin fructation. Each bar represents the mean ± SEM in six separate experiments (n = 6). Significant difference compared to fructated control at identical times: * p < 0.05; **p < 0.01. Significant difference from non-fructated control: #p < 0.001. Significant difference from the respective Day-10 value: $p < 0.05; $$p < 0.01. ALA = α-lipoic acid; AG = Aminoguanidine; Mb = Myoglobin
Effect of α-lipoic acid on protein carbonyls formation
Figure 4a depicts the effect of ALA on protein carbonyls formation in myoglobin-fructose glycation system as observed on day-10, 20 and 30 of incubation. A significant 3.6-fold increase in protein carbonyls (7.1 ± 0.4 vs 2.0 ± 0.2; p < 0.001) was observed on day-10 when myoglobin was co-incubated with fructose (fructated control) compared with myoglobin incubation alone (non-fructated control) and moreover, this difference was more pronounced after day-20 (8.01 ± 0.2 vs 2.3 ± 0.2; p < 0.001) and day-30 (9.1 ± 0.2 vs 2.3 ± 0.2; p < 0.001) of the study. Furthermore, a marked time-dependent increase in protein carbonyls was observed in myoglobin-fructose co-incubation that gained significance by day-20 (8.0 ± 0.2 vs 7.1 ± 0.4; p < 0.05) and day-30 (9.1 ± 0.2 vs 7.1 ± 0.4; p < 0.01) compared with day-10. On the other hand, ALA co-treatment in the myoglobin-fructose glycation system at 1, 2 and 4 mM concentrations significantly (p < 0.01) displayed a concentration-dependent reduction in protein carbonyls levels on day-10 compared with the fructated control and moreover, this difference was consistent throughout the study period. On day-30 of co-treatment, ALA showed a significant (p < 0.01) decrease in protein carbonyls levels with a maximum reduction of 41.9% (5.7 ± 0.4 vs 9.1 ± 0.2) at 4 mM concentration compared with the fructated control. Nevertheless, at all the studied ALA concentrations, no significant time-dependent change in protein carbonyls levels was observed on day-20 or day-30 compared with day-10 values. The concentration of ALA required to inhibit 50% (IC50) of protein carbonyls as determined from linear regression analysis was found to be 7.09 mM (Fig. 4b). In comparison, co-treatment of aminoguanidine (a known inhibitor of glycation process; positive control) at a concentration of 5 mM in myoglobin-fructose glycation system produced a significant (p < 0.01) reduction in protein carbonyls on day-10 compared with the fructated control which was consistent throughout the study period achieving a maximum 48.7% reduction (4.7 ± 0.4 vs 9.1 ± 0.2) on day-30.
Fig. 4.
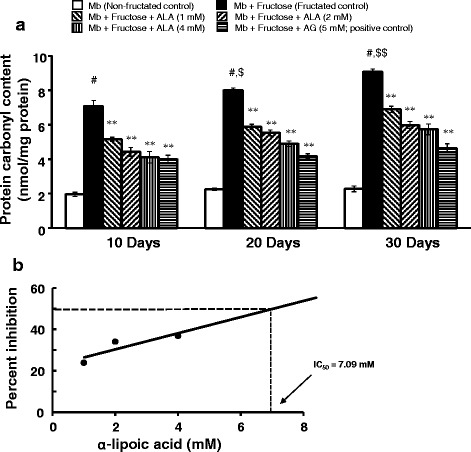
Effect of α-lipoic acid on protein carbonyl content in fructose-induced myoglobin fructation. Each bar represents the mean ± SEM in six separate experiments (n = 6). Significant difference compared to fructated control at identical times: * p < 0.05; **p < 0.01. Significant difference from non-fructated control: #p < 0.001. Significant difference from the respective Day-10 value: $p < 0.05, $$p < 0.01. ALA = α-lipoic acid; AG = Aminoguanidine; Mb = Myoglobin
Effect of α-lipoic acid on protein thiols oxidation
The effect of ALA on free protein thiols in myoglobin-fructose glycation system as observed on day-10, 20 and 30 of incubation is depicted in Fig. 5a. A significant 2.8-fold decrease in free protein thiols (2.6 ± 0.2 vs 7.2 ± 0.8; p < 0.001) was observed on day-10 when myoglobin was co-incubated with fructose (fructated control) compared with myoglobin incubation alone (non-fructated control) and moreover, this difference was consistent throughout the study period. Nevertheless, no significant time-dependent change in free protein thiols was observed in the myoglobin-fructose co-incubation on day-20 or day-30 compared with day-10 value. On the other hand, ALA co-treatment in the myoglobin-fructose glycation system at 1, 2 and 4 mM concentrations significantly (p < 0.05 to p < 0.01) displayed a concentration-dependent increase in free protein thiols levels on day-10 compared with the fructated control and moreover, this difference was consistent throughout the study period. On day-30 of co-treatment, ALA showed a significant (p < 0.01) increase in free protein thiols levels with a maximum increment of 68.1% (3.7 ± 0.4 vs 2.2 ± 0.7) at 4 mM concentration compared with the fructated control. Nevertheless, at all the studied ALA concentrations, no significant time-dependent change in free protein thiols levels was observed on day-20 or day-30 compared with day-10 values. The concentration of ALA required to increase 50% (EC50) of free protein thiols as determined from linear regression analysis was found to be 0.65 mM (Fig. 4b). In comparison, co-treatment of aminoguanidine (a known inhibitor of glycation process; positive control) at a concentration of 5 mM in myoglobin-fructose glycation system produced a significant (p < 0.01) increase in free protein thiols on day-10 compared with the fructated control which was consistent throughout the study period achieving a maximum 36.3% increase (3.0 ± 1.9 vs 2.2 ± 0.7) on day-30.
Fig. 5.
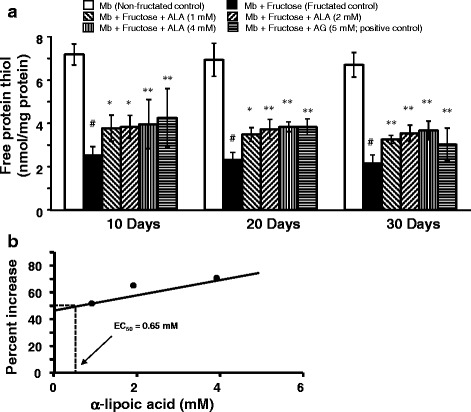
Effect of α-lipoic acid on protein thiol oxidation group in fructose-induced myoglobin fructation. Each bar represents the mean ± SEM in six separate experiments (n = 6). Significant difference compared to fructated control at identical times: * p < 0.05; **p < 0.01. Significant difference from non-fructated control: #p < 0.001. ALA = α-lipoic acid; AG = Aminoguanidine; Mb = Myoglobin
Discussion
Protein glycation, also known as the Maillard reaction, is a complex biochemical reaction beginning with the non-enzymatic interaction of a reducing sugar with an amino group of protein [51]. Molecular rearrangements (Schiff base formation and Amadori rearrangements) lead to the formation of AGEs that trigger pathogenic signalling pathways and cross-link extracellular matrix proteins [52]. In recent decades, over consumption of high-fructose diets has dramatically increased and has been linked to an increase in obesity and diabetic complications [53]. Furthermore, in the hyperglycaemic condition, the polyol pathway becomes active and facilitates the conversion of intracellular glucose to fructose. In the context of intracellular protein glycation, the rate of fructose-induced glycation is faster than glucose-induced glycation and thus, fructose and its metabolites are considered as important precursors in the intracellular formation of AGEs [54–56].
Myoglobin is an oxygen-binding heme-protein localized in oxidative muscle (i.e. cardiac myocardium and skeletal muscle fibres) and functions to transport and store oxygen, scavenge nitric oxide and reactive oxygen species [57]. Recently, it has been demonstrated that myoglobin non-enzymatically reacts with reducing sugars and generates various AGEs [13, 14]. Furthermore, it has also been shown that fructose has a greater rate of myoglobin glycation and AGEs formation than glucose [56, 58, 59]. Recently, Banerjee and Chakraborti [60, 61] demonstrated that myoglobin, when incubated with methylglyoxal, a known AGEs precursor, induced structural modifications of myoglobin and generated various AGEs. Studies have shown that many antioxidant-rich natural products have the ability to prevent reducing sugar-mediated protein glycation [62, 63]. In this study, we examined the preventive effects of ALA in fructose-induced myoglobin glycation (fructation) and AGEs formation using an in vitro glycation model. The clinical pharmacokinetic characteristics of ALA (200 mg/day to 600 mg/day) have been widely reported with varied pharmacokinetic profiles in multiple studies as reviewed by Shay and colleagues [26]. The disparity in the observed oral bioavailability and peak plasma concentration has been attributed to whether R-enantiomer, its salt form, or a racemic mixture of ALA has been used in the studies, plus any associated formulation and/or biopharmaceutical factors. Hence, has yet to be established a clear relationship between the oral dosage, bioavailability and peak plasma concentration of ALA. Therefore, the selected concentrations of ALA used in this study were rationalised based on our previous experience and on the published literature [64–66]. To our knowledge, this is the first study which investigates the protective effects of α-lipoic acid in fructose-induced myoglobin glycation.
Based on fluorescence property, we examined the influence of ALA on the formation of total AGEs. Our results demonstrated that ALA efficiently inhibited fructose-induced AGEs formation which supports a previous report on the inhibitory effects of ALA on fluorescent AGEs formation in vivo [34, 35, 67]. The possible mechanism of action of ALA in inhibiting the formation of fluorescent AGEs include: (i) blocking the amino groups of protein, thus preventing its glycation with free sugar, (ii) blocking the carbonyl groups of reducing sugars, (iii) preventing the formation of Amadori products by blocking the Schiff’s base to Amadori products conversion, (iv) blocking the Amadori products and dicarbonyl intermediates which may reduce glycation, as well as AGEs formation, and/or (v) preventing autoxidation of fructose and glycoxidation of Amadori products. The protein glycation reaction generates various fluorescent AGEs such as pentosidine and crossline which are implicated in the development of diabetic cardiovascular complications [1, 68]. It has been demonstrated that serum fluorescent AGEs (such as pentosidine) were significantly higher in diabetic patients and was associated with an increased incidence of cardiovascular disease [69, 70]. Moreover, AGEs can cross-link with extracellular matrix proteins such as collagen, thereby increasing arterial wall and myocardial stiffness. This leads to systolic and diastolic dysfunction of the heart and potentiates heart failure in diabetic patients [71]. Furthermore, binding AGEs to its receptor (RAGE) cause modifications to LDL (i.e. oxidation of LDL) and subsequently, generate foam cells, which are hallmarks of atherosclerosis [72]. Therefore, preventing the formation of AGEs or removing cross-linked AGEs is an efficient way of interrupting the glycation cascade and preventing the potential pathological consequences of AGEs.
In our study, ALA also displayed a significant, time-dependent inhibition of fructosamine formation. A possible mechanism for inhibiting the formation of Amadori products could be either by competing with sugar molecules or protecting the protein amino group from the nucleophilic addition of the carbonyl group of the sugar. In the early stages of protein glycation, the reaction between the carbonyl group of sugar and the amino group of protein form freely reversible Schiff’s bases which further rearrange to form more stable ketoamine or Amadori products such as fructosamine [73]. At this stage, the reducing sugar itself undergoes autoxidation in the presence of transition metals and generates various highly reactive superoxide radical and hydroxyl radical. The harmful radicals further accelerate the glycation process to form AGEs. In addition, the Amadori products also react with free protein and generate AGEs [72]. Thus, the reduction of fructosamine would be beneficial in the suppression of AGEs formation and therapeutically delay the occurrence of AGEs-mediated complications.
Furthermore, in this study, myoglobin-fructose glycation effectively released free iron from the heme moiety of myoglobin in a time-dependent manner which is effectively suppressed by ALA. During myoglobin glycation, iron is liberated from the heme and most likely ligated to distal histidine in the heme pocket of myoglobin. This iron termed as “mobile reactive iron” can catalyse the Haber-Weiss reaction producing free radicals (particularly hydroxyl (OH) radicals), which increase cellular oxidative stress and damage different cellular constituents [15, 16, 74]. Roy and co-workers demonstrated that the in vitro, non-enzymatic glycation of myoglobin induces the release of free iron from the heme pocket of myoglobin, and the iron release was found to be proportional to the extent of myoglobin glycation [13].
We also examined the influence of ALA against fructose-mediated non-enzymatic glycation and oxidation-dependent damage to myoglobin. ALA suppressed the formation of protein carbonyls content and oxidation of thiols in this study. ALA is a potent biological antioxidant and is capable of scavenging many free radicals such as hydroxyl groups [75, 76]. During protein glycation, reactive di-carbonyl intermediates and protein carbonyl derivatives generate AGEs formation and also modify protein structure which is prone to oxidative reaction with amino acids such as cysteine, particularly the thiol side chain. The reactive oxygen species and reactive nitrogen species are also generated during glycation and glycoxidation. In the meantime, they also can oxidize side chains of amino acid residues of the protein to form a carbonyl derivative and diminish the oxidative defense of protein by eliminating the thiol groups [77, 78]. These alterations are reflective of oxidative protein damage, with oxidative stress and the formation of AGEs. Therefore, a possible mechanism of ALA in suppressing the formation of protein dicarbonyls is through scavenging the highly reactive free radicals generated during chronic glycation.
Conclusion
In conclusion, these findings demonstrate that ALA protects against fructose-mediated myoglobin glycation in vitro by inhibiting the early and intermediate glycation reactions involved in the formation of AGEs. Thus, the present findings emphasize that ALA supplementation is beneficial in the prevention of AGEs-mediated diabetic and cardiovascular complications. Further studies are warranted to investigate the ability of ALA on late-stage glycation events that lead to AGEs production and on AGEs-mediated protein cross-linking and cellular signaling pathways.
Acknowledgments
Funding
This research study was supported under the Higher Degree Research Training Scheme funds provided to HG by the Western Sydney University, Australia.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to Research Data Management and Intellectual Property Policies of Western Sydney University that prevent research data sharing in public repositories, but are available from the corresponding author on reasonable request.
Abbreviations
- AG
Aminoguanidine
- AGEs
Advanced glycation end products
- ALA
(R)-α-Lipoic acid
- DNHP
Dinitrophenylhydrazine
- LDL
Low-density lipoproteins
- Mb
Myoglobin
- NBT
Nitro-blue tetrazolium
- RAGE
Receptor for advanced glycation end products
- ROS
Reactive oxygen species
- TCA
Trichloro acetic acid
Authors’ contributions
HG, SN, VRN and RRP made substantial contributions to conception design and conduction of research. HG performed all of the experiments in the laboratory. Data collection, analysis, graphical representation and interpretation were done by HG and SN. Article was written by HG. Critical revision of the article was done by SN, VRN and RRP. Critical statistical analysis was done by HG and SN. SN, VRN and RRP made the necessary corrections in the write up. All authors read and approved the final manuscripts.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hardik Ghelani, Email: h.ghelani@westernsydney.edu.au.
Valentina Razmovski-Naumovski, Email: v.naumovski@westernsydney.edu.au.
Rajeswara Rao Pragada, Email: profprrau@gmail.com.
Srinivas Nammi, Phone: +61 2 4620 3038, Email: s.nammi@westernsydney.edu.au.
References
- 1.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Monnier VM, Taniguchi N. Advanced glycation in diabetes, aging and age-related diseases: editorial and dedication. Glycoconj J. 2016;33(4):483–486. doi: 10.1007/s10719-016-9704-0. [DOI] [PubMed] [Google Scholar]
- 4.Rondeau P, Bourdon E. The glycation of albumin: structural and functional impacts. Biochimie. 2011;93(4):645–658. doi: 10.1016/j.biochi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Ordway GA, Garry DJ. Myoglobin: an essential hemoprotein in striated muscle. J Exp Biol. 2004;207:3441–3446. doi: 10.1242/jeb.01172. [DOI] [PubMed] [Google Scholar]
- 6.Chung Y, Huang S-J, Glabe A, Jue T. Implication of CO inactivation on myoglobin function. Am J Physiol Cell Physiol. 2006;290:C1616–C1624. doi: 10.1152/ajpcell.00360.2005. [DOI] [PubMed] [Google Scholar]
- 7.Jürgens KD, Papadopoulos S, Peters T, Gros G. Myoglobin: just an oxygen otore or also an oxygen transporter. News Physiol Sci. 2000;15:269–274. doi: 10.1152/physiologyonline.2000.15.5.269. [DOI] [PubMed] [Google Scholar]
- 8.Lin PC, Kreutzer U, Jue T. Myoglobin translational diffusion in rat myocardium and its implication on intracellular oxygen transport. J Physiol. 2007;578:595–603. doi: 10.1113/jphysiol.2006.116061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merx MW, Flögel U, Stumpe T, Gödecke A, Decking UK, Schrader J. Myoglobin facilitates oxygen diffusion. FASEB J. 2001;15(6):1077–1079. doi: 10.1096/fj.00-0497fje. [DOI] [PubMed] [Google Scholar]
- 10.Flogel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: a scavenger of bioactive NO. Proc Natl Acad Sci U S A. 2001;98(2):735–740. doi: 10.1073/pnas.98.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunori M. Nitric oxide moves myoglobin centre stage. Trends Biochem Sci. 2001;26(4):209–210. doi: 10.1016/S0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- 12.Wunderlich C, Flogel U, Godecke A, Heger J, Schrader J. Acute inhibition of myoglobin impairs contractility and energy state of iNOS-overexpressing hearts. Circ Res. 2003;92(12):1352–1358. doi: 10.1161/01.RES.0000079026.70629.E5. [DOI] [PubMed] [Google Scholar]
- 13.Roy A, Sen S, Chakraborti AS. In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress. Free Radic Res. 2004;38(2):139–146. doi: 10.1080/10715160310001638038. [DOI] [PubMed] [Google Scholar]
- 14.Hendgen-Cotta UB, Kelm M, Rassaf T. Myoglobin functions in the heart. Free Radic Biol Med. 2014;73:252–259. doi: 10.1016/j.freeradbiomed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S, Maity S, Chakraborti AS. Methylglyoxal-induced modification causes aggregation of myoglobin. Spectrochim Acta A Mol Biomol Spectrosc. 2016;155:1–10. doi: 10.1016/j.saa.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Roy A, Sil R, Chakraborti AS. Non-enzymatic glycation induces structural modifications of myoglobin. Mol Cell Biochem. 2010;338(1-2):105–114. doi: 10.1007/s11010-009-0343-7. [DOI] [PubMed] [Google Scholar]
- 17.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a congress series sponsored by UNESCO-MCBN, the American Diabetes Association and the German diabetes society. Diabetes Metab Res Rev. 2001;17(3):189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 18.Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rumpf KW, Kaiser H, Grone HJ, Trapp VE, Meinck HM, Goebel HH, Kunze E, Kreuzer H, Scheler F. Myoglobinuric renal failure in hyperosmolar diabetic coma. Dtsch Med Wochenschr. 1981;106(22):708–711. doi: 10.1055/s-2008-1070386. [DOI] [PubMed] [Google Scholar]
- 20.Nakano S, Mugikura M, Endoh M, Ogami Y, Otsuki M. Acute pancreatitis with diabetic ketoacidosis associated with hypermyoglobinemia, acute renal failure, and DIC. J Gastroenterol. 1996;31(4):623–626. doi: 10.1007/BF02355070. [DOI] [PubMed] [Google Scholar]
- 21.Khalifah RG, Baynes JW, Hudson BG. Amadorins: novel post-Amadori inhibitors of advanced glycation reactions. Biochem Biophys Res Commun. 1999;257(2):251–258. doi: 10.1006/bbrc.1999.0371. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson BO. Biological effects of aminoguanidine: an update. Inflamm Res. 1999;48(10):509–515. doi: 10.1007/s000110050495. [DOI] [PubMed] [Google Scholar]
- 23.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M. Lilly lecture 1993. Glycation and diabetic complications. Diabetes. 1994;43(6):836–841. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 25.Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19(2):227–250. doi: 10.1016/0891-5849(95)00017-R. [DOI] [PubMed] [Google Scholar]
- 26.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790(10):1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29(3):315–331. doi: 10.1016/S0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 28.Goraca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol Rep. 2011;63(4):849–858. doi: 10.1016/S1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- 29.Ghelani H, Razmovski-Naumovski V, Nammi S. Chronic treatment of (R)-α-lipoic acid reduces blood glucose and lipid levels in high-fat diet and low-dose streptozotocin-induced metabolic syndrome and type 2 diabetes in Sprague-Dawley rats. Pharmacol Res Perspect. 2017;5(3):e00306. doi: 10.1002/prp2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Midaoui AE, Elimadi A, Wu L, Haddad PS, de Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens. 2003;16(3):173–179. doi: 10.1016/S0895-7061(02)03253-3. [DOI] [PubMed] [Google Scholar]
- 31.Thirunavukkarasu V, Nandhini AT, Anuradha CV. Fructose diet-induced skin collagen abnormalities are prevented by lipoic acid. Exp Diabesity Res. 2004;5(4):237–244. doi: 10.1080/154386090506148. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Thirunavukkarasu V, Anitha Nandhini AT, Anuradha CV. Lipoic acid improves glucose utilisation and prevents protein glycation and AGE formation. Pharmazie. 2005;60(10):772–775. [PubMed] [Google Scholar]
- 33.Muellenbach EM, Diehl CJ, Teachey MK, Lindborg KA, Hasselwander O, Matuschek M, et al. Metabolic interactions of AGE inhibitor pyridoxamine and antioxidant alpha-lipoic acid following 22 weeks of treatment in obese Zucker rats. Life Sci. 2009;84(15-16):563–568. doi: 10.1016/j.lfs.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XZ, Yan HD, Wang J. Extract of Ginkgo Biloba and alpha-lipoic acid attenuate advanced glycation end products accumulation and RAGE expression in diabetic nephropathy rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31(4):525–531. [PubMed] [Google Scholar]
- 35.Leu JG, Lin CY, Jian JH, Shih CY, Liang YJ. Epigallocatechin-3-gallate combined with alpha lipoic acid attenuates high glucose-induced receptor for advanced glycation end products (RAGE) expression in human embryonic kidney cells. An Acad Bras Cienc. 2013;85(2):745–752. doi: 10.1590/S0001-37652013005000023. [DOI] [PubMed] [Google Scholar]
- 36.Vincent AM, Perrone L, Sullivan KA, Backus C, Sastry AM, Lastoskie C, Feldman EL. Receptor for advanced glycation end products activation injures primary sensory neurons via oxidative stress. Endocrinology. 2007;148(2):548–558. doi: 10.1210/en.2006-0073. [DOI] [PubMed] [Google Scholar]
- 37.Chen SA, Chen HM, Yao YD, Hung CF, Tu CS, Liang YJ. Topical treatment with anti-oxidants and au nanoparticles promote healing of diabetic wound through receptor for advance glycation end-products. Eur J Pharm Sci. 2012;47(5):875–883. doi: 10.1016/j.ejps.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Akihiko SMY, Satoshi H, Takayuki N, Masafumi I, Seigo K, Yoshikazu Y. Anti-Glycation activity of alpha-Lipoic acid derivatives and vitamin E derivatives. Anti-Aging Med. 2013;10:42–54. [Google Scholar]
- 39.Suzuki YJ, Tsuchiya M, Packer L. Lipoate prevents glucose-induced protein modifications. Free Radic Res Commun. 1992;17(3):211–217. doi: 10.3109/10715769209068167. [DOI] [PubMed] [Google Scholar]
- 40.Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T, et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes. 1997;46(9):1481–1490. doi: 10.2337/diab.46.9.1481. [DOI] [PubMed] [Google Scholar]
- 41.Kowluru RA. Effect of advanced glycation end products on accelerated apoptosis of retinal capillary cells under in vitro conditions. Life Sci. 2005;76(9):1051–1060. doi: 10.1016/j.lfs.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Kunt T, Forst T, Wilhelm A, Tritschler H, Pfuetzner A, Harzer O, et al. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin Sci (Lond) 1999;96(1):75–82. doi: 10.1042/cs0960075. [DOI] [PubMed] [Google Scholar]
- 43.Wong A, Dukic-Stefanovic S, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer P, et al. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur J Neurosci. 2001;14(12):1961–1967. doi: 10.1046/j.0953-816x.2001.01820.x. [DOI] [PubMed] [Google Scholar]
- 44.Gasic-Milenkovic J, Loske C, Munch G. Advanced glycation endproducts cause lipid peroxidation in the human neuronal cell line SH-SY5Y. J Alzheimers Dis. 2003;5(1):25–30. doi: 10.3233/JAD-2003-5104. [DOI] [PubMed] [Google Scholar]
- 45.Yin QQ, Dong CF, Dong SQ, Dong XL, Hong Y, Hou XY, et al. AGEs induce cell death via oxidative and endoplasmic reticulum stresses in both human SH-SY5Y neuroblastoma cells and rat cortical neurons. Cell Mol Neurobiol. 2012;32(8):1299–1309. doi: 10.1007/s10571-012-9856-9. [DOI] [PubMed] [Google Scholar]
- 46.Wrobel K, Wrobel K, Garay-Sevilla ME, Nava LE, Malacara JM. Novel analytical approach to monitoring advanced glycosylation end products in human serum with on-line spectrophotometric and spectrofluorometric detection in a flow system. Clin Chem. 1997;43(9):1563–1569. [PubMed] [Google Scholar]
- 47.Panter SS. Release of iron from hemoglobin. Methods Enzymol. 1994;231:502–514. doi: 10.1016/0076-6879(94)31034-3. [DOI] [PubMed] [Google Scholar]
- 48.Ohkawara E, Nohara Y, Kanno Y, Suzuki H, Matsumoto G, Kinoshita T, Watanabe M. Fructosamine assay using albumin extracted from serum. Biol Pharm Bull. 2002;25(9):1121–1124. doi: 10.1248/bpb.25.1121. [DOI] [PubMed] [Google Scholar]
- 49.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 50.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 51.Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Increased glycation and oxidative damage to apolipoprotein B100 of LDL cholesterol in patients with type 2 diabetes and effect of metformin. Diabetes. 2010;59(4):1038–1045. doi: 10.2337/db09-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wells-Knecht KJ, Brinkmann E, Wells-Knecht MC, Litchfield JE, Ahmed MU, Reddy S, et al. New biomarkers of Maillard reaction damage to proteins. Nephrol Dial Transplant. 1996;11:41–47. doi: 10.1093/ndt/11.supp5.41. [DOI] [PubMed] [Google Scholar]
- 53.Stanhope KL, Schwarz J-M, Havel PJ. Adverse metabolic effects of dietary fructose: results from recent epidemiological, clinical, and mechanistic studies. Curr Opin Lipidol. 2013;24(3):198–206. doi: 10.1097/MOL.0b013e3283613bca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruderman NB, Williamson JR, Brownlee M. Glucose and diabetic vascular disease. FASEB J. 1992;6(11):2905–2914. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- 55.Sakai M, Oimomi M, Kasuga M. Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci. 2002;48(5-6):125–136. [PubMed] [Google Scholar]
- 56.Suarez G, Rajaram R, Oronsky AL, Gawinowicz MA. Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose. J Biol Chem. 1989;264(7):3674–3679. [PubMed] [Google Scholar]
- 57.Kanatous SB, Mammen PP, Rosenberg PB, Martin CM, White MD, Dimaio JM, et al. Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am J Physiol Cell Physiol. 2009;296(3):C393–C402. doi: 10.1152/ajpcell.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dearlove RP, Greenspan P, Hartle DK, Swanson RB, Hargrove JL. Inhibition of protein glycation by extracts of culinary herbs and spices. J Med Food. 2008;11(2):275–281. doi: 10.1089/jmf.2007.536. [DOI] [PubMed] [Google Scholar]
- 59.Bhattacherjee A, Chakraborti AS. Fructose-induced modifications of myoglobin: change of structure from met (Fe3+) to oxy (Fe2+) form. Int J Biol Macromol. 2011;48(1):202–209. doi: 10.1016/j.ijbiomac.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Banerjee S, Chakraborti AS. Structural alterations of hemoglobin and myoglobin by glyoxal: a comparative study. Int J Biol Macromol. 2014;66(0):311–318. doi: 10.1016/j.ijbiomac.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee S, Chakraborti A. In Vitro study on structural alteration of Myoglobin by Methylglyoxal. Protein J. 2013;32(3):216–222. doi: 10.1007/s10930-013-9480-7. [DOI] [PubMed] [Google Scholar]
- 62.Ramkissoon JS, Mahomoodally MF, Ahmed N, Subratty AH. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med. 2013;6(7):561–569. doi: 10.1016/S1995-7645(13)60097-8. [DOI] [PubMed] [Google Scholar]
- 63.Elosta A, Ghous T, Ahmed N. Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev. 2012;8(2):92–108. doi: 10.2174/157339912799424528. [DOI] [PubMed] [Google Scholar]
- 64.Ziegler D, Gries FA. α-Lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes. 1997;46(2):S62–S66. doi: 10.2337/diab.46.2.S62. [DOI] [PubMed] [Google Scholar]
- 65.Chen WL, Kang CH, Wang SG. Lee HM: alpha-Lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia. 2012;55(6):1824–1835. doi: 10.1007/s00125-012-2530-4. [DOI] [PubMed] [Google Scholar]
- 66.Sun LQ, Chen YY, Wang X, Li XJ, Xue B, Qu L, Zhang TT, Mu YM, Lu JM. The protective effect of alpha lipoic acid on Schwann cells exposed to constant or intermittent high glucose. Biochem Pharmacol. 2012;84(7):961–973. doi: 10.1016/j.bcp.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Muellenbach EA, Diehl CJ, Teachey MK, Lindborg KA, Archuleta TL, Harrell NB, et al. Interactions of the advanced glycation end product inhibitor pyridoxamine and the antioxidant alpha-lipoic acid on insulin resistance in the obese Zucker rat. Metabolism. 2008;57(10):1465–1472. doi: 10.1016/j.metabol.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kerkeni M, Saïdi A, Bouzidi H, Letaief A, Ben Yahia S, Hammami M. Pentosidine as a biomarker for microvascular complications in type 2 diabetic patients. Diab Vasc Dis Res. 2013;10(3):239–245. doi: 10.1177/1479164112460253. [DOI] [PubMed] [Google Scholar]
- 69.Sugiyama S, Miyata T, Ueda Y, Tanaka H, Maeda K, Kawashima S, Van Ypersele de Strihou C, Kurokawa K. Plasma levels of pentosidine in diabetic patients: an advanced glycation end product. J Am Soc Nephrol. 1998;9(9):1681–1688. doi: 10.1681/ASN.V991681. [DOI] [PubMed] [Google Scholar]
- 70.Weiss MF, Rodby RA, Justice AC, Hricik DE. Free pentosidine and neopterin as markers of progression rate in diabetic nephropathy. Collaborative study group. Kidney Int. 1998;54(1):193–202. doi: 10.1046/j.1523-1755.1998.00982.x. [DOI] [PubMed] [Google Scholar]
- 71.Yoshida N, Okumura K, Aso Y. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism. 2005;54(3):345–350. doi: 10.1016/j.metabol.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 73.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sil S, Bose T, Roy D, Chakraborti AS. Protoporphyrin IX-induced structural and functional changes in human red blood cells, hemoglobin and myoglobin. J Biosci. 2004;29(3):281–291. doi: 10.1007/BF02702610. [DOI] [PubMed] [Google Scholar]
- 75.Wollin SD, Jones PJ. Alpha-lipoic acid and cardiovascular disease. J Nutr. 2003;133:3327–3330. doi: 10.1093/jn/133.11.3327. [DOI] [PubMed] [Google Scholar]
- 76.Koriyama Y, Nakayama Y, Matsugo S, Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cellline. Brain Res. 2013;1499:145–157. doi: 10.1016/j.brainres.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 77.Miyata T, Kurokawa K, Van Ypersele De Strihou C. Advanced glycation and lipoxidation end products: role of reactive carbonyl compounds generated during carbohydrate and lipid metabolism. J Am Soc Nephrol. 2000;11(9):1744–1752. doi: 10.1681/ASN.V1191744. [DOI] [PubMed] [Google Scholar]
- 78.Zeng J, Dunlop RA, Rodgers KJ, Davies MJ. Evidence for inactivation of cysteine proteases by reactive carbonyls via glycation of active site thiols. Biochem J. 2006;398(2):197–206. doi: 10.1042/BJ20060019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to Research Data Management and Intellectual Property Policies of Western Sydney University that prevent research data sharing in public repositories, but are available from the corresponding author on reasonable request.


