Abstract
Background
Glaucoma is a serious eye disease that can lead to loss of vision. Unfortunately, effective treatments are limited by poor bioavailability of antiglaucoma medicine due to short residence time on the preocular surface.
Materials and methods
To solve this, we successfully prepared novel controlled-release ion-exchange microparticles to deliver betaxolol hydrochloride (BH). Montmorillonite/BH complex (Mt-BH) was prepared by acidification-intercalation, and this complex was encapsulated in microspheres (Mt-BH encapsulated microspheres [BMEMs]) by oil-in-oil emulsion–solvent evaporation method. The BH loaded into ion-exchange Mt was 47.45%±0.54%. After the encapsulation of Mt-BH into Eudragit microspheres, the encapsulation efficiency of BH into Eudragit microspheres was 94.35%±1.01% and BH loaded into Eudragit microspheres was 14.31%±0.47%.
Results
Both Fourier transform infrared spectra and X-ray diffraction patterns indicated that BH was successfully intercalated into acid-Mt to form Mt-BH and then Mt-BH was encapsulated into Eudragit microspheres to obtain BMEMs. Interestingly, in vitro release duration of the prepared BMEMs was extended to 12 hours, which is longer than both of the BH solution (2.5 hours) and the conventional BH microspheres (5 hours). Moreover, BMEM exhibited lower toxicity than that of BH solution as shown by the results of cytotoxicity tests, chorioallantoic membrane-trypan blue staining, and Draize rabbit eye test. In addition, both in vivo and in vitro preocular retention capacity study of BMEMs showed a prolonged retention time. The pharmacodynamics showed that BMEMs could extend the drug duration of action.
Conclusion
The developed BMEMs have the potential to be further applied as ocular drug delivery systems for the treatment of glaucoma.
Keywords: glaucoma, montmorillonite, controlled release, betaxolol hydrochloride, preocular retention, microspheres
Introduction
Glaucoma is a slowly progressive atrophy of the optic nerve, characterized by loss of peripheral visual function and an excavated appearance of the optic disc by ophthalmoscopy.1 It is commonly considered as the second leading cause of blindness and about 60.5 million individuals have been affected worldwide.2 High intraocular pressure (IOP) is the main risk factor, which leads to the degeneration of axons from the retinal ganglion cells. Therefore, many kinds of IOP lowering medications are used to manage the disease symptoms, such as timolol, betaxolol, epinephrine, pilocarpine, and dorzolamide.3 However, effective treatments are often limited owing to poor bioavailability of topically administered ocular drugs caused by a number of factors, including rapid tear turnover, transient residence time in the cul-de-sac, and washout of tear.4,5 These factors lead to rapid elimination of the drugs from the precorneal area. Thus, glaucoma therapy is still a significant challenge for clinical applications due to the absence of adequate drug concentration and short residence duration in the ocular tissues. In order to solve the problem, many significant efforts toward advanced ocular drug delivery systems have been made over the last few decades. Thermosensitive hydrogels, implants, and particulate carrier systems have been investigated to sustain the release of drug, prolong retention time on the ocular surface, and improve bioavailability.6,7 Among these strategies, particulate carrier systems have gained considerable attention for ocular applications due to their convenient application of liquid form, prolonged residence time on the cornea, and low irritability.8
Microspheres, as particulate carrier systems, are promising drug delivery systems for eye application because of their properties of easy preparation, wide compatibility, and sustained release.9–14 There are several methods that can be used to form microspheres from polymers, such as spray drying method, emulsion–solvent evaporation method, and phase separation method. One of the most common approaches for the preparation of microparticles for site-specific drug delivery is based on emulsification solvent evaporation, including oil-in-water, oil-in-oil (O/O), and water-in-oil-in-water. For water-soluble drugs and expensive protein drugs, O/O is the most popular method due to its flexibility (easy to operate), high efficiency (high entrapment rate and drug loading rate [DL%]), and reduced burst release.15–17 Eudragit RS and RL polymers are commonly used for the preparation of controlled-release drug forms due to their positive charge, which can allow a longer residence time on the corneal surface.18 They are two copolymers synthesized from acrylic and metacrylic acid esters, containing an amount of quaternary ammonium groups between 4.5%–6.8% and 8.8%–12% for RS and RL, respectively.19 Previous studies have demonstrated that Eudragit has been used as inert carriers to formulate controlled-release delivery systems of nonsteroidal anti-inflammatory drugs, such as ibuprofen and flurbiprofen.19,20
More recently, many considerable attempts have been made to improve the preparation method of microspheres. For example, hybrid materials combining polymers with inorganic solids were synthesized to obtain a functional delivery system. Montmorillonite (Mt), as an effective inorganic solid, has been proposed for modulating drug release properties as in our previous studies, due to its good biocompatibility, high adsorption ability, and high cation exchange capacity (CEC). Previous results indicated that the release duration and burst release could be effectively improved by integrating Mt with polymers to form nanocomposites or microspheres.21–25 Meanwhile, Mt is a 2:1 type swelling phyllosilicate with fine grain and large interlayer-planar spacing, which can allow cationic drug molecules intercalating into this space through ion-exchange method. It was reported that Mt intercalated with 5-fluorouracial and ibuprofen effectively improved the sustained release and loading capacity of drug molecules.26–28 Though this improvement is encouraging, there are a few reports that focus on the anomalous diffusion mechanism of drug release from these nanocomposites and/or microspheres.29 Therefore, a detailed release mechanism is required to interpret the release process of drug molecules for guiding future research of microspheres.
Inspired by the advantages of Mt, microspheres, and Eudragit RS/RL polymers, in this paper, we have made an effort to integrate Mt with Eudragit microspheres as an ocular drug carrier. To the best of our knowledge, there is no related report about Mt/Eudragit microspheres yet, as controlled drug delivery systems for glaucoma therapy. Herein, betaxolol hydrochloride (BH), a β1 antagonist for glaucoma therapy, was investigated as a model drug. Acidification-intercalation and O/O emulsion–solvent evaporation technique were used for the preparation of the Mt/BH complex (Mt-BH) and Mt-BH encapsulated microspheres (BMEMs), respectively. The structural characteristics of Mt-BH and BMEMs were measured by Fourier transform infrared spectra (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM). The release mechanism of BH from BMEMs was investigated. The thiazolyl blue method (MTT), chorioallantoic membrane-trypan blue staining (CAM-TBS) assay, and Draize test were used for determining the irritation of BMEMs. Moreover, the preocular retention capacity was investigated by in vivo and in vitro experiments. Finally, pharmacodynamic observations were conducted to investigate the capacity of reducing intraocular pressure (IOP).
Materials and methods
Materials
Mt with a CEC of 90 mmol⋅g−1 was bought from Zhejiang Sanding Technology Co, Ltd (Shaoxing, China). BH was purchased from Hao Industrial (Shandong, China). Eudragit RS100 and RL100 were purchased from Evonik Degussa (Germany). 1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) and all components of buffer solutions were from Sigma-Aldrich Co (St Louis, MO, USA). All other chemical reagents used in the study were of high performance liquid chromatography (HPLC) or analytical grade. Human immortalized cornea epithelial cells (iHCECs) were kindly provided by Shandong Eye Institute (Qingdao, China) and the study was approved by the Ethics Committee of Shandong Eye Institute.
Sample preparation
Preparation of Mt-BH
Mt was activated by 5% H2SO4 for 0.5 hours at 70°C.24 After the exchange of interlayer metal ions with H+, Mt was ultrasonically treated for 5 minutes to obtain ultrafine montmorillonite treated with acid (acid-Mt) particles. Then, the acid-Mt was centrifuged and washed with deionized water until the pH was equal to 7.0. The solids were dried at 100°C and crushed.
One gram acid-Mt and 3 g BH were added to 1,000 mL deionized water, and the adsorption process was carried out in a water bath (50°C) for 6 hours. After washing, centrifuging, and drying, solid products of Mt-BH were obtained. DL% (Q) was determined with HPLC (Agilent 1200; Agilent Technologies, Santa Clara, CA, USA) using Equation 1:
| (1) |
where C0 and C (mg·mL−1) are the BH concentrations before and after DL into acid-Mt, respectively, Macid-Mt (mg) is the mass of acid-Mt, and VBH (mL) is the volume of the BH solution.
The HPLC conditions were as follows: the BH concentration was determined by HPLC. Ultimate® XB-C18 column (Welch, Austin, TX, USA; 4.60×250 mm, 5 μm) was used. The mobile phase was acetonitrile/trimethylamine (30/70, v/v) with pH 3.0. The detector wavelength, flow rate, column temperature, and injection volume were 275 nm, 1 mL·min−1, 25°C, and 20 μL, respectively.
Preparation of BMEMs
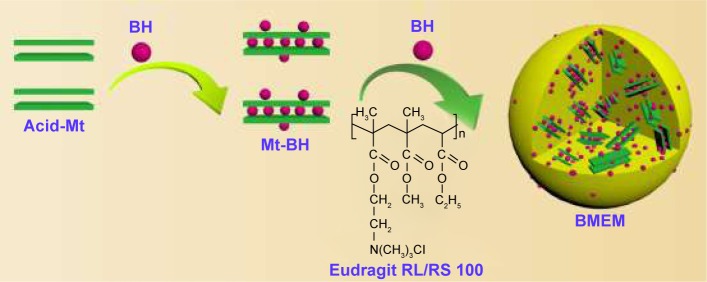
BMEMs were prepared by O/O emulsion–solvent evaporation method.30 Briefly, Eudragit RL/RS 100, triethyl citrate, glycerinum, Tween 80, BH, and Mt-BH were dispersed in a mixed organic solvent (acetonitrile and dichloromethane [DCM] with a volume ratio of 4:1) as an internal oil phase. Span 80 was dispersed in light liquid paraffin as an external oil phase. The internal oil phase was homogenized at a rate of 10,000 r·min−1 for 5 minutes to obtain an ultrafine dispersed suspension. Afterward, the internal oil phase was added into the external oil phase drop-by-drop to form a mixed emulsion (O/O). At room temperature (RT), the emulsion was stirred at a rate of 800 r·min−1 for 4–8 hours until the organic solvent completely evaporated. After washing with n-hexane 7–9 times, BMEMs were dried with pumping filtration. The preparation process and construction of BMEMs are shown in Figure 1.
Figure 1.
A schematic of the preparation process of BMEMs. BMEMs represent Eudragit microspheres incorporated Mt-BH.
Abbreviations: Acid-Mt, montmorillonite treated with acid; BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere; Mt, montmorillonite; Mt-BH, betaxolol hydrochloride loaded into montmorillonite.
Physicochemical characteristics of BMEMs
Entrapment efficiency (EE%) and DL%
BMEMs (30 mg) were dissolved in 1 mL of DCM with the help of ultrasonic treatment. BH was extracted by 30 mL of deionized water through a vortex process. After centrifuging at 3,000 r·min−1 for 10 minutes, the diluted supernatant was measured by HPLC at 275 nm. EE% and DL% of BMEMs were calculated using Equations 2 and 3, respectively:
| (2) |
| (3) |
where m′BH is the weight (mg) of BH encapsulated in BMEMs, mBH is the weight (mg) of initially added BH, and mBMEM is the weight (mg) of BMEMs.
Characterization
FTIR was measured by a Vertex-70 FTIR spectrometer (Bruker Optik GmbH, Ettlingen, Germany) at RT in the range of 400–4,000 cm−1 with a resolution of 4 cm−1 and using 64 scans. XRD patterns were obtained using a Bruker D8 advance diffractometer (Bruker Optik GmbH) from 2° to 15° with a scanning rate of 2°·min−1, by using CuKα radiation with a generator voltage of 40 kV and a generator current of 40 mA. The morphology of all samples was determined by using Hitachi S-3400N SEM (Hitachi Ltd, Tokyo, Japan) at an accelerating voltage of 20 kV. All samples were dried and coated with gold before scanning.
In vitro release studies
In vitro drug release experiments were conducted by using dialysis bag in dialysis medium at 34°C. Exactly 6.7 g NaCl, 2.0 g NaHCO3, 0.06 g CaCl2, and 1.38 g KCl were dissolved in 1,000 mL deionized water to prepare the dialysis medium (artificial tears). Briefly, BMEMs (2 mL, 2.8 mg·mL−1), conventional BH microspheres (2 mL, 2.8 mg·mL−1, absence of Mt), and BH solution (2 mL, 2.8 mg·mL−1) were enclosed in the dialysis bag and then put into 35 mL artificial tears. At designated time intervals, 5 mL of the sample was withdrawn from the bag and immediately replaced with the same volume of fresh artificial tears. The amount of drug released was determined by HPLC. The release behavior and mechanism of drug from BMEMs were analyzed.
Cytotoxicity test
To assess the cytotoxicity of BMEMs, iHCECs were used in the present study. The cells were grown in DMEM/F-12 along with 10% (v/v) fetal bovine serum, 0.1 mg·mL−1 streptomycin, and 1,000 IU·mL−1 penicillin under 5% CO2 at 37°C (series ∏ water jacket).31 When cells achieved >80% confluence, they were exposed to formulations along with media. Different concentrations and durations of the formulations were evaluated by MTT assay. The experiment was performed in four groups: G1= control group, G2= BH solution group, G3= blank-BMEM group, and G4= BMEM group. Each test group had six different concentrations (0.28, 0.56, 0.84, 1.40, 2.24, and 2.8 mg·mL−1). After exposure to formulations for 120 minutes, the medium was removed and the cell was carefully rinsed with phosphate-buffered saline. Then, 5 mg·mL−1 MTT was added to each well and further incubated for 4 hours. Finally, the resulting formazan crystals were dissolved by dimethyl sulfoxide and measured by a spectrophotometer at 490 nm. Cell viability was calculated using Equation 4:
| (4) |
where A′ and A0 are the absorbance of the test and control groups, respectively.
CAM-TBS test
An alternative for the Draize rabbit eye test is the CAM-TBS test, which is an inexpensive and sensitive assay established for ophthalmic irritancy; it has shown good correlation to in vivo irritation studies.32 Briefly, fertilized hen’s eggs were incubated in the horizontal position to ensure correct positioning of the embryo (away from the CAM) at 37°C±0.5°C and 40%±5% relative humidity for 9 days.33 On the 10th day, the section of egg shell above the airspace was removed and several drops of saline solution were added onto the shell membrane with a pipette. Then, the inner membrane was carefully removed to expose the CAM without damaging the underlying CAM. BMEMs (300 μL, 2.8 mg·mL−1), blank-BMEMs (300 μL, BMEMs without BH loaded), and BH solution (300 μL, 2.8 mg·mL−1) were gently pipetted onto the CAM for 5 minutes, respectively. The samples were immediately washed by normal saline and 0.5 mL trypan blue solution (1 mg·mL−1) was added on the CAM to stain for 1 minute. Finally, the dyed CAM was excised and the adsorbed trypan blue was extracted with 1 mL of formamide for 24 hours; the extract was measured spectrophotometrically at 611 nm in quintuplicate. NaOH (0.1 M) was used as a positive control and normal saline as a negative control. Any irritant reaction during the course of the experiments should be carefully observed, such as hyperemia, hemorrhage, clotting, and/or coagulation.
In vitro preocular retention
In this study, iHCECs were used to investigate the precorneal retention of BMEMs. As shown in Figure 2, after iHCECs became confluent, inert permeable polycarbonate inserts were lifted to the air–liquid interface to have a varying period of submerged cultivation (so-called reconstituted multilayer human corneal epithelia). The construct then appeared as a multilayered epithelium, which was histologically similar to that of the normal human corneal epithelium.34 Then, the insert was placed in a thermostat bath at 34°C (the temperature of the human tear film). The experiment was performed by transferring 30 μL of sample (2.8 mg·mL−1) in the turnover chamber prefilled with 270 μL of simulated tear fluid, and the inflow and outflow of tears in the chamber were controlled by a peristaltic pump at the same speed (20 μL·min−1). The above in vitro tear turnover model was used to simulate the drug concentration dynamics in the tear film. Finally, 200 μL of solution was collected every 10 minutes, and the precorneal retention time was determined by HPLC-fluorescence detection.
Figure 2.
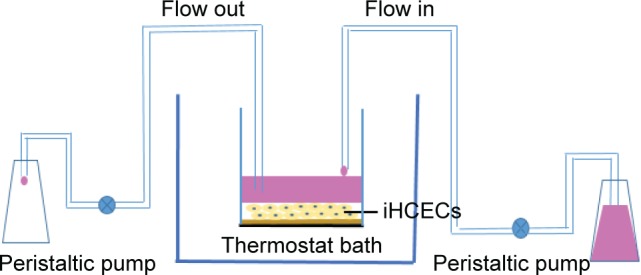
Schematic diagram of the in vitro tear turnover apparatus. The model incorporates an insert containing iHCECs as turnover chamber; the external basal side of the insert is sealed to avoid down diffusion of material. The temperature of the system is 34°C (the human tear film temperature). Two peristaltic pumps were used to control the inflow and outflow of simulated tears in the chamber.
Abbreviation: iHCECs, human immortalized cornea epithelial cells.
In vivo test
Draize test
Rabbits were provided by the animal center of Guangdong Pharmaceutical University with a weight of 2.5–3.0 kg and without eye disease. They were housed in standard cages in a light-controlled room at 19°C±1°C, with food and water. All the animals were treated according to the Association for Research in Vision and Ophthalmology resolution for the use of animals in research and were approved for use by the Central Animal Ethical Committee of Guangdong Pharmaceutical University (approval number of the animal experiment protocols is gdpulac2017014). Both eyes were carefully checked before the experiments to ensure that they were without any defects.
The Draize test was used to evaluate the ocular irritation of normal saline, BH solution (2.8 mg·mL−1), blank-BMEMs, and BMEMs (2.8 mg·mL−1) by observing any signs of opacity, redness, inflammation, or increased tear production after application to the eyes of Albino rabbits. The single high-dosage ocular irritation test was conducted as follows. The left eyes of every group were treated with saline solution (1 mL) as control and the right eyes were subjected to a high dose of sample (1 mL) as the test group. The ocular tissue (cornea, iris, and conjunctiva) was examined at 1, 2, 4, 24, 48, and 72 hours after administration. The irritation was evaluated by “Draize eye test guideline.”35 In the multiple dosing ocular irritation tests, the left eyes of every group were treated with saline solution as control and the right eyes were subjected to 50 μL of the sample (2.8 mg·mL−1) twice a day for 7 days. After the irritation test, these rabbits were sacrificed by intravenous injection of air. Then, the eyeballs were excised for observation of cornea tissues’ morphology: cornea tissue treated with 10% formalin for 24 hours, embedded in paraffin, making a pathological section, stained with hematoxylin and eosin, and observed by a light microscope.36
In vivo preocular retention
The ability of precorneal retention was evaluated by tears elimination. The animals used for the experiments were New Zealand White rabbits. Rabbits, in spite of having a nictitating membrane and very low blinking frequency, are generally considered as the reference animal for ocular experiments due to several similarities between the anatomy of the rabbit and the human eye.31 Briefly, the rabbits were given an instillation of 100 μL of formulations (2.8 mg·mL−1) in the lower conjunctival sac of each cornea and kept the eyelids closed for 5 seconds to prevent loss of the instilled solution. After drug instillation, tear samples were collected by using 8×8 mm filter paper at 10, 30, 60, 90, 120, 180, and 240 minutes. The filter paper with tear was stored in microcentrifuge tubes and the weight of the collected tear was recorded. Then, the collected sample was dried by nitrogen flow and 200 μL of methanol solvent was added to dissolve it. Finally, the samples were centrifuged for 30 minutes. Quantitation was performed by HPLC assays.
Pharmacodynamics
After 1 week of adaptation in the facilities, the eyes of all animals were examined with a slit lamp to exclude any disease that could interfere with the experimental results. IOP (mmHg) was measured using an indentation tonometer (YZ7A; Suzhou Visual Technology Co, Ltd, Suzhou, China). The high-IOP model rabbits were induced by gavage with high dose of saline.37 The right eye conjunctival sac of the high-IOP model rabbit was administered 100 μL of sample (2.8 mg·mL−1) and the left eye was treated with physiological saline as the control group. After the application of samples, IOP determination was carried out at 0, 15, 30, 45, 60, 90, 120, 180, 240, 300, and 360 minutes. All the measurements were done six times at each interval by the same operator under the same environmental conditions.
Statistical analysis
Statistical analysis of the results was performed using one-way analysis of variances, referring to a level of p<0.05. The analysis was computed using Origin 8 software (OriginLab company, Hampton, MA, USA).
Results and discussion
Preparation
Acidification and DL of Mt
After acid treatment, the interlayer cation of Mt was replaced by H+. This process resulted in an increased layer spacing and CEC of the products. In this study, the effect of concentration of sulfuric acid (V/V), solid-to-liquid ratio (mg/mL), treatment temperature, and time were investigated on the structural properties and on the BH intercalation feature of Mt. Optimal acid treatment of Mt was done by using a solid-to-liquid ratio of 1:10 (Mt:5% sulfuric acid, mg/V) at 70°C for 0.5 hours. The optimal intercalation of acid-Mt with BH was as follows: a mass ratio of 1:3 (acid-Mt:BH) was dissolved in deionized water and heated at 50°C for intercalation of BH into acid-Mt. The result showed that maximum loading rates of Mt increased from 153.6±7.6 to 474.52±5.4 mg·g−1 (n=5) after acid treatment. This increase in DL after acid treatment can be attributed to both the increased basal spacing and increased ion-exchange reaction.38 This is in good agreement with the result of XRD and FTIR (Figures 3 and 4).
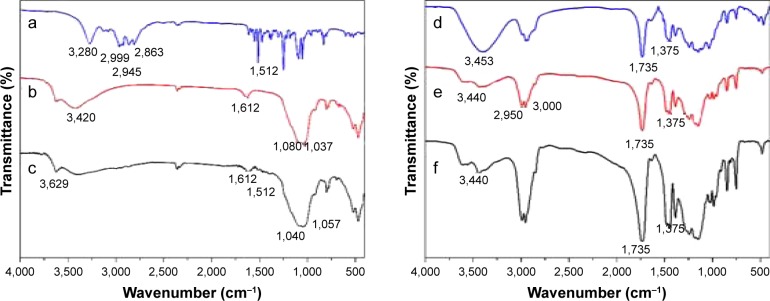
Figure 3.
The FTIR spectrum of (a) BH, (b) acid-Mt, (c) Mt-BH, (d) BMEM, (e) Eudragit RL100, and (f) Eudragit RS100.
Abbreviations: Acid-Mt, montmorillonite treated with acid; BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere; FTIR, Fourier transform infrared; Mt, montmorillonite; Mt-BH, betaxolol hydrochloride loaded into montmorillonite.
Figure 4.
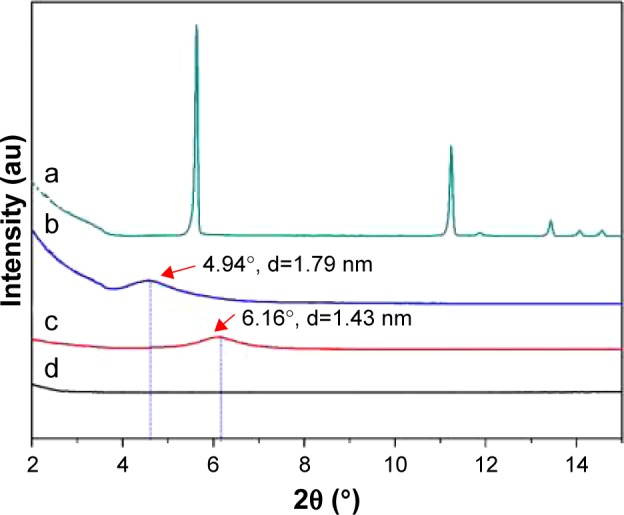
The XRD patterns of (a) BH, (b) Mt-BH, (c) acid-Mt, and (d) BMEM.
Abbreviations: Acid-Mt, montmorillonite treated with acid; Mt, montmorillonite; BH, betaxolol hydrochloride; Mt-BH, betaxolol hydrochloride loaded into montmorillonite; BMEM, betaxolol hydrochloride encapsulated microsphere; XRD, X-ray diffraction.
Preparation of BMEMs
There are many factors that influenced the structure and property of BMEMs, including starting materials, reactants ratio, and temperature. For example, Eudragit RS100, as a microsphere skeleton material, possesses a typical low burst effect, while the hypertonicity of Eudragit RL100 contributes to the complete release of drug. Therefore, the combination of Eudragit RS100 with Eudragit RL100 has generated a great deal of interest to obtain microspheres, possessing a structure with low burst effect and complete release feature.39–41 In this study, the Mt-BH:Eudragit RL/RS 100 ratios in the range of 1:4 to 1:8 contributed to the spherical morphology of the particles with fine size and high encapsulation efficiency (>80%). Temperature was another important parameter, which affected mainly the emulsification and evaporation process. Emulsification with an ice bath led to well-distributed droplets which were beneficial to form uniform microspheres with a significantly lower burst effect and improved sustained release. Furthermore, the evaporation temperature also showed a great effect on the EE% and DL% of microspheres. The EE% and DL% values of microspheres evaporated at the ice bath, RT and 30°C–40°C were 66.26%, 90.1%, 74.5%, and 7.62%, 10.82%, 8.94%, respectively. Hence, the ice bath and RT were chosen for the emulsification reaction and evaporation of organic solvent, respectively.
The EE% and DL% of BMEMs were 94.35%±1.01% and 14.31%±0.47% (n=5), respectively, which indicated that the method was reproducible and stable. Mt-BH could serve as a co-emulsifier in the preparation process, which increased the stability of the emulsions and showed little effect on EE% and DL%.42
FTIR, XRD, and SEM studies of samples
The FTIR spectrum of BH (Figure 3a) showed characteristic absorptions at 3,280, 3,000–2,800, and 1,512 cm−1, corresponding to the −OH stretching, alkyl chain C−H stretching, and aromatic ring C−H deformation vibrations, respectively. The infrared spectrum of acid-Mt (Figure 3b) showed absorptions at 3,420, 1,080, and 1,037 cm−1 due to the −OH stretching of adsorbed water, Si−O out-of-plane, and in-plane stretching vibrations of Mt, respectively.29 After the loading of BH, the products showed a group of new absorptions at 3,000–2,800 and 1,512 cm−1 (Figure 3c), which were attributed to the alkyl chain C−H stretching and aromatic ring C−H deformation vibrations, respectively. Compared with the absorption bands of BH, the absorption bands of BH from Mt-BH showed small band shifts and intensity reductions (or disappeared) in the wavenumber region of 3,280–2,800 cm−1. These changes suggested that intercalation of BH into Mt was successful and both physical and chemical interactions existed between loaded BH and acid-Mt. The FTIR spectra of Eudragit RL/RS 100 are showed in Figure 3e and f. The peak at 2,950–3,000, 1,375, 1,735, and 3,440 cm−1 can be assigned to O−H, −CH3, C=O, and free OH stretching, respectively. This indicates a typical vibration mode of Eudragit RL/RS 100. For the FTIR of BMEMs, all the characteristic bands of acid-Mt cannot be distinguished in the spectrum of BMEMs, including the surface–OH stretching of acid-Mt at 3,629 cm−1, water bending at 1,612 cm−1, and Si−O related stretching at 1,100 cm−1. Meanwhile, some peaks appeared at 3,440 cm−1 (free OH), 2,950–3,000 cm−1 (O−H), 1,375 cm−1 (−CH3), and 1,735 cm−1 (C=O), which confirmed the formation of BMEMs from Eudragit RL/RS 100.
As shown in the XRD patterns (Figure 4), the XRD peaks for the BH pure powder are relatively sharp, which showed that BH was in its perfect crystalline form. Reflections for the (001) plane of acid-Mt and Mt-BH were observed at 2θ=6.2° and 4.9°, respectively. The calculated d001 values of acid-Mt and Mt-BH were 1.43 and 1.79 nm, respectively. The increase in d value resulted from the intercalation of BH into the interlayer space of Mt. Furthermore, the XRD results (Figure 4) showed that the characteristic reflections of acid-Mt and Mt-BH disappeared as shown in the BMEM pattern. All the above-mentioned changes implied that Mt-BH was successfully encapsulated into the microspheres and BMEM was successfully formed.
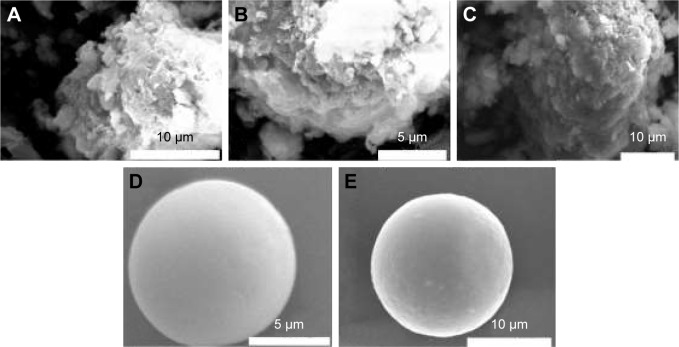
Both SEM images of Mt and acid-Mt showed irregular layer particles and randomly orientated aggregations (Figure 5A and B), which are characteristic morphologies of natural clays and acid-treated clays.43 Intercalation of BH apparently led to a smoother surface of the particles as observed from Mt-BH (Figure 5C). Both conventional BH microspheres (absence of Mt) and BMEMs showed spherical morphologies (Figure 5D and E), and the latter was relatively rough in surface, due to the encapsulation of Mt-BH.
Figure 5.
The SEM images of (A) raw Mt, (B) acid-Mt, (C) Mt-BH, (D) conventional microsphere, and (E) BMEM.
Abbreviations: Acid-Mt, montmorillonite treated with acid; BMEM, betaxolol hydrochloride encapsulated microsphere; Mt, montmorillonite; Mt-BH, betaxolol hydrochloride loaded into montmorillonite; SEM, scanning electron microscopy.
In vitro release studies
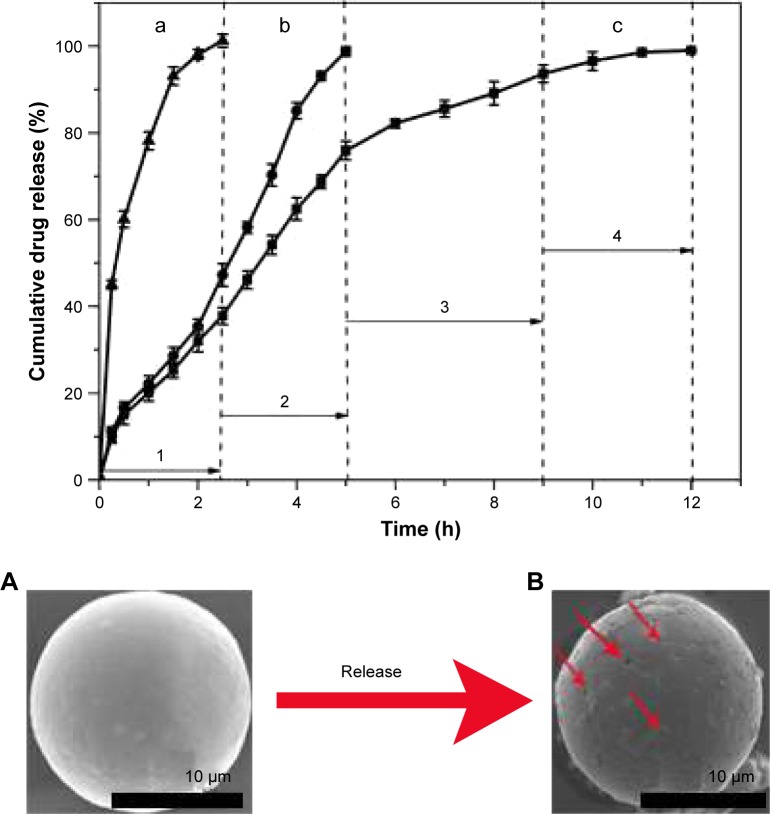
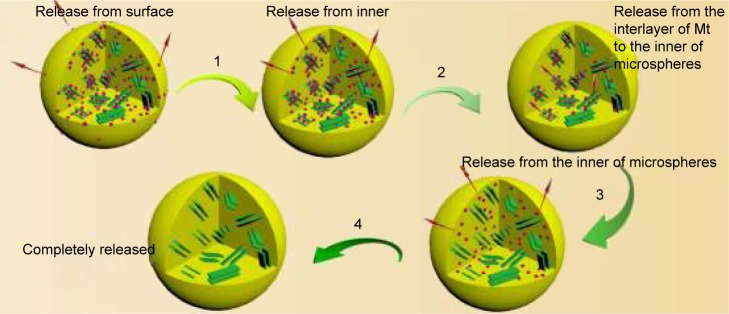
The burst release would cause many adverse reactions due to the sharp increase of drug concentration. As shown in Figure 6a, the BH solution exhibited a rapid release in artificial tears (over 90% in the initial 1 hour and almost 100% within 2.5 hours). Conventional BH microspheres (absence of Mt) could reduce such a burst at limited extent and prolong the release duration to 5 hours (Figure 6b). As for BMEMs, the release duration can be extended to 12 hours. And the whole release process included four stages (Figure 6c). For the first stage, BH absorbed on the surface of microspheres was released (Figures 6c-1 and 7). Second, the BH encapsulated in the microsphere matrix but not intercalated into the Mt layer was released (Figures 6c-2 and 7). Third, the BH intercalated into the Mt layer was released into the inner region of the microsphere matrix (Figures 6c-3 and 7). Fourth, BH from the microspheres was released into the medium (Figures 6c-4 and 7). At the first burst process, the drug released sharply from the surface of BMEMs with an initial release rate of 37.58% (2.5 hours) and from conventional microspheres at a rate of 46.14% at 2.5 hours, which is potentially beneficial to producing a therapeutic concentration.44 The significant induction of the initial release rate can be attributed to relatively more drug molecules being encapsulated into the microsphere than conventional microspheres. Also, only a few drugs were adsorbed to the surface of the microsphere, so the proportion of the release of the drug at the first burst process was low. For conventional microspheres (Figure 6b), the drug was completely released from the conventional microspheres within 5 hours, while that of BMEMs was nearly 76% at 5 hours. Besides, BMEMs also exhibited a unique drug release process in the duration of 5–9 hours with a cumulative release of 98.8% at 12 hours, due to the characteristics of high ion exchange of Mt in the drug release process. Therefore, BH release from BMEMs was significantly prolonged after encapsulation of Mt-BH. The drug release process showed that the in vitro drug release profile of BMEMs was consistent with first-order kinetics (Log (100−Y) =2.071−0.134t, r=0.9915). This indicated that the BH release from BMEMs was controlled by diffusion.
Figure 6.
In vitro dialysis release study of BH from formulations: (a) BH solution, (b) conventional BH microsphere, and (c) BMEM. The cumulative drug release (%) is plotted against time (h). Values are presented as the mean ± SD (n=5). SEM micrographs of BMEM: (A) microsphere before release process and (B) microsphere after release process.
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere; SEM, scanning electron microscopy.
Figure 7.
In vitro release processes of BMEM. The whole release of BMEM could be divided into 4 stages, including the release of BH (1) absorbed on the surface of the microsphere, (2) encapsulated in the microsphere matrix but not intercalated into the Mt layer, (3) absorbed on the surface of Mt, and (4) intercalated in Mt.
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere; Mt, montmorillonite.
In addition, Figure 6A and B shows the morphology of BMEMs after release duration. It is clearly seen that the general morphology of BMEMs after release duration had no significant difference with that of before release duration. Interestingly, some of the holes on the surface of BMEMs were clearly seen in Figure 6B. It could be predicted that the medicine molecules released slowly from BMEMs through diffusion from those holes without dissolution and/or degradation of microspheres.
Cytotoxicity study
It is well known that a promising microparticle system used for ocular purposes must demonstrate ocular tolerability. In this study, ocular irritation of the developed formulation was directly measured using the MTT test to evaluate the cytotoxicity on iHCECs. MTT can be oxidized by the mitochondrial dehydrogenase in living cells to obtain a dark blue formazan product. The cell survival rate was <50%, which indicates that mitochondrial activity was reduced.40 iHCECs were exposed to BH solution, blank-BMEMs, and BMEMs at different concentrations for 120 minutes (Figure 8). In three formulations, the cell viability decreased with increase of drug concentration. For BH solution, the cell viability was above 60% when the drug concentration was <0.84 mg·mL−1. However, when the drug concentration was >1.4 mg·mL−1, the cell survival rate fell to <5%, which showed that BH had a toxic effect on iHCECs in higher concentration levels. In comparison with the BH solution, for the cell viability of iHCECs treated with blank-BMEMs and BMEMs at low level (<0.84 mg·mL−1), the cell survival rate was very high, but at high values (>1.4 mg·mL−1), the cell survival rates of blank-BMEMs and BMEMs were significantly higher than that of the BH solution. Meanwhile, it could be found that the cell viability of iHCEC cells treated with blank-BMEMs was higher than BMEMs at the same amounts. This may be explained by the fact that microspheres could protect the cells from damage by BH (raw drug). Each sample of cytotoxicity was in such a sequence: BH solution > BMEMs > blank-BMEMs. From these results, it is clearly obvious that BMEMs could effectively maintain superior cell viability within an appropriate range.
Figure 8.
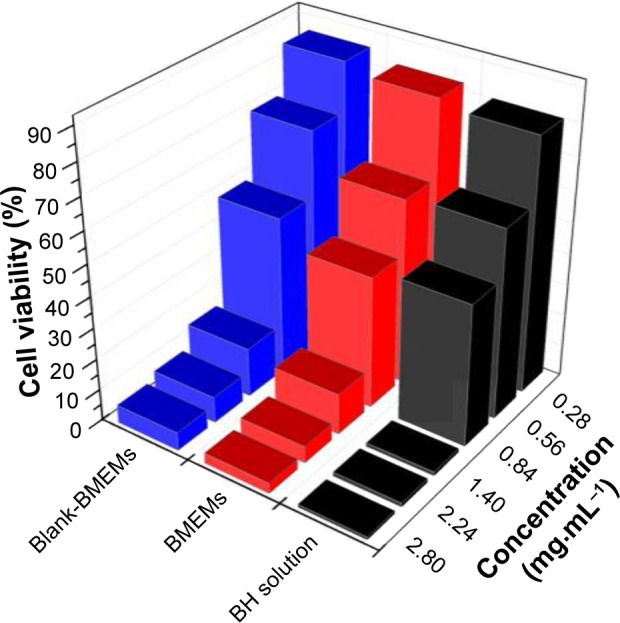
Cell viability (%) of iHCECs determined by MTT assay after exposing them to different concentrations (0.28, 0.56, 0.84, 1.40, 2.24, and 2.8 mg·mL−1) of BH solution, blank-BMEM, and BMEM for 120 minutes.
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere; iHCECs, human immortalized cornea epithelial cells.
CAM-TBS
CAM-TBS test is an alternative toxicological method widely used to determine ocular irritation potential.41 The CAM is a highly vascular embryonic membrane and stratified tissue, which can respond to injury in a similar manner as mucosal and subcutaneous tissue. It might provide very useful information for predicting the potential eye irritation caused by a substance. The irritant effect of these substances is determined by the amount of trypan blue absorption of CAM. It is very simple and reproducible, as well as quantitative.45 Figure 9 shows the trypan blue absorption onto the CAM after exposure to BMEMs, BH solution, blank-BMEMs, saline solution, and NaOH. It is clear that each sample followed this sequence for cytotoxicity: NaOH > BH solution > BMEMs > blank-BMEMs > saline solution. These results revealed that BMEMs had less irritation for ocular drug delivery compared with BH solution.
Figure 9.
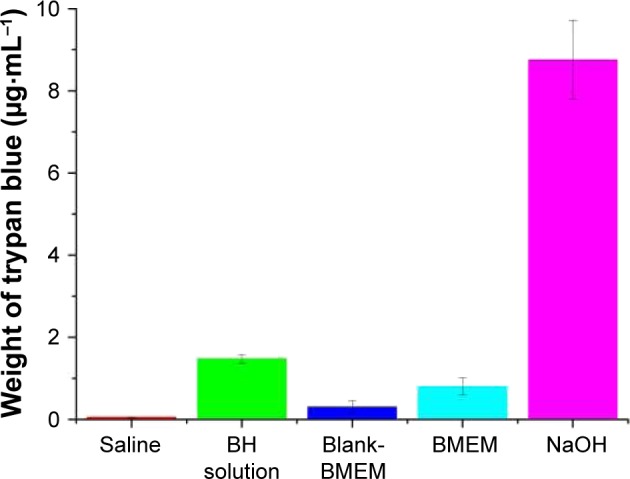
Eye irritancy induced by different concentrations of BH solution, blank-BMEM, and BMEM determined by the in vitro CAM-TBS method. NaOH was used as a positive control and normal saline as a negative control. Values are presented as the mean ± SD (n=5).
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere; CAM, chorioallantoic membrane; TBS, trypan blue staining.
In vitro preocular retention
Cell culture models of ocular barriers offer the advantage of highly defined, compliant systems to investigate drug behavior, bio-adhesion characteristics, and pharmacokinetic properties of medicine preparation. The experimental parameters and conditions can be easily adjusted according to our requirements, and cultured human cells have good correlations with in vivo behavior to some extent, thereby avoiding species difference and the use of animal tissue. Histologically, cell culture models of ocular barriers resemble the normal human corneal epithelium, which can provide powerful systems to investigate the residence of a drug in the precorneal area. The concentration of BH in cornea/tear film compartment as a function of time was monitored after administration of BH solution and BMEMs (Figure 10). For the BH solution, the BH concentration in tears rapidly fell from about 65.45 down to 6.33 μg·mL−1 only within 40 minutes. Although the Cmax of BH solution was significantly higher than BMEMs, it could remain in the precorneal position for a short time, only just 80 minutes. BMEMs decreased from about 50.25 down to 9.33 μg·mL−1 during the period of 50 minutes (which is 1.5-fold compared to the BH solution at 50 minutes). Meanwhile, BMEMs could still be detected at 120 minutes, which may explain the sustained release of BMEMs. These results revealed that BH could release from BMEMs slowly over time and maintained a certain BH concentration in tear fluid for a prolonged period of time.
Figure 10.
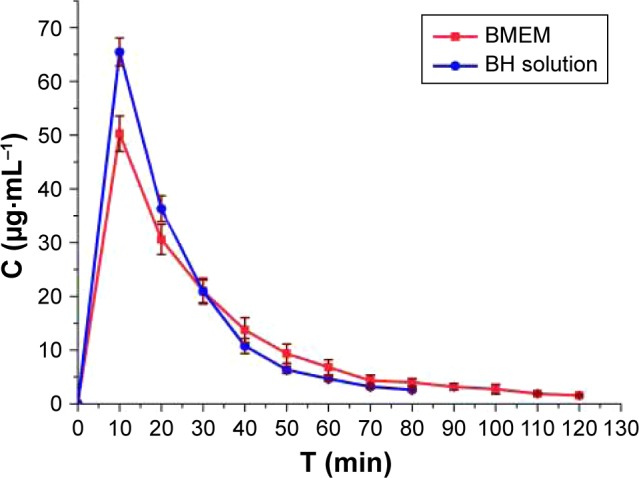
Concentration–time curve in the cornea/tear film compartment after the instillation of BH solution (2.8 mg·mL−1) and BMEM (2.8 mg·mL−1). Values are presented as the mean ± SD (n=5).
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere.
In vivo test
Draize test
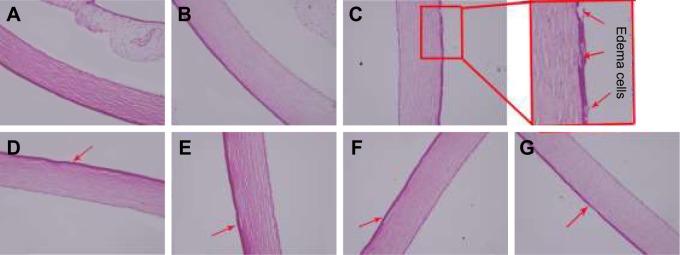
Eye safety is of crucial importance to be evaluated to avoid damage to the ocular tissues due to the sensitive nature of the eye. The experiment was implemented by using rabbits as a model due to their large eyes with well-described anatomy and physiology. However, the eyes of rabbits are generally more sensitive to irritating materials than the humans’ eyes.43 From the results of Draize study (Table 1), it was found that all groups appeared to have a slight congestion status in conjunctiva including the normal saline group (control group), which might be attributed to the high sensitivity to external structure materials. However, the Draize test revealed that the total score for all the rabbits were less than all three scores, which indicated that all samples had no significant damage to ocular tissues. Furthermore, cornea histological experiment was carried out for a more accurate evaluation of the safety of BMEMs on the eye. The histological analysis of corneal sections after the high-dose irritation test are shown in Figure 11. The normal cornea tissue showed a smooth surface and a clear organizational structure of layers (Figure 11A). Corneal tissue had no obvious pathological changes after administration of normal saline (Figure 11B). However, after treatment with BH solution, the corneal epithelial cells showed mild edema with some abnormal cells (Figure 11C). As shown in Figure 11D and E, the corneal epithelial cells exhibited a handful of edema cells after the multiple dosing of BMEMs and blank-BMEMs. Figure 11F and G showed no significant pathological changes after administration of a single dose of BMEMs and blank-BMEMs. The histological analysis demonstrated that BMEMs had very little irritation for the cornea, which was consistent with the results of the cytotoxicity study and CAM-TBS. Based on these findings, it was confirmed that BMEMs were safer and less toxic to the eye than BH solution for ophthalmic administration.
Table 1.
Draize test scores after single and multiple dose formulations: saline, BH solution, blank-BMEMs, and BMEMs (n=5)
| Symptom | Normal saline
|
Blank-BMEM
|
BMEM
|
BH solution
|
Normal | ||||
|---|---|---|---|---|---|---|---|---|---|
| Single | Multiple | Single | Multiple | Single | Multiple | Single | Multiple | ||
| Cornea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Iris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 |
| Conjunctiva congestion | 1 | 0.8 | 0 | 0.2 | 1 | 1 | 1 | 1 | 0.2 |
| Conjunctive edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 |
| Secretions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 | 0 |
| Score | 1 | 0.8 | 0 | 0.2 | 1 | 1 | 1 | 2 | 0.2 |
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere.
Figure 11.
Cornea histopathology analysis after the high-dose irritation test by microscopy. (A) Normal cornea, (B) saline, (C) BH solution, (D) a single high dose of blank-BMEM, (E) a single high dose of BMEM, (F) multiple dose of blank-BMEM, and (G) multiple dose of BMEM.
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere.
In vivo preocular retention
It was estimated that about 80% of the topically administered eye drops were drained through the nasolacrimal duct, with high tear fluid turnover, and systemically absorbed.46 Only <5% of the applied drug could penetrate the cornea and reach the intraocular tissues, resulting in low ocular bioavailability. The precorneal retention time is shown after the application of BH solution and BMEMs (Figure 12). It is obvious that the BH solution was quickly removed by tear fluid turnover, and the concentration of the BH solution was only 478.44 μg·mL−1 after the administration of 10 minutes, which can explain the low viscosity of the aqueous solution and strong stimulation. It is worth noting that the drug concentration of BH solution cannot be observed after 90 minutes, since it was below the detection limit of HPLC. BMEMs could still maintain drug concentration after >240 minutes. BMEM was composed of polyacrylic acid, which was a mucoadhesive polymer. These mucoadhesive polymers could interact with the precorneal mucin layer via noncovalent bonds. Thus, it could increase the residence time of the drug on the ocular surface, decrease drug loss, and eventually improve bioavailability. These results illustrated that BMEMs could be of value as a vehicle for ocular applications due to the reduction of precorneal loss caused by drainage and high tear fluid turnover.
Figure 12.
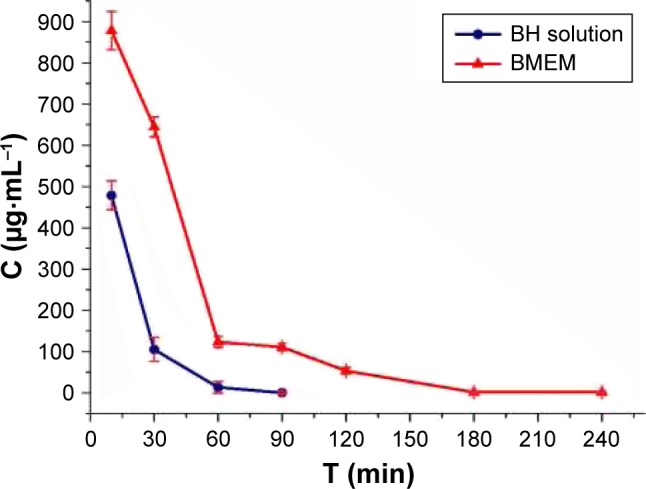
The tear fluid concentration–time curve after topical application of BH solution (2.8 mg·mL−1) and BMEM (2.8 mg·mL−1) in rabbit eyes. Values are presented as the mean ± SD (n=5).
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere.
Pharmacodynamics
As shown in Figure 13, two formulations could significantly reduce the high IOP to some extent. BH solution attained the maximum effect (5.04 mmHg) of lowering IOP after administration for 30 minutes and the IOP significantly declined after administration for 60 minutes. BMEMs have a sustained IOP-lowering effect and attained the maximum effect (4.89 mmHg) of lowering IOP after 120-minute administration. In comparison with the BH solution, BMEMs could maintain a longer effect of lowering IOP than that of the BH solution. This could be because BMEMs could stay on the preocular surface to some extent and simultaneously release the drug in a sustained manner. Therefore, the IOP-lowering effect was continuous, steady, and would avoid many side effects caused the sharp reduction of IOP.47–49 These results suggested that BMEMs had a sustained IOP-lowering effect.
Figure 13.
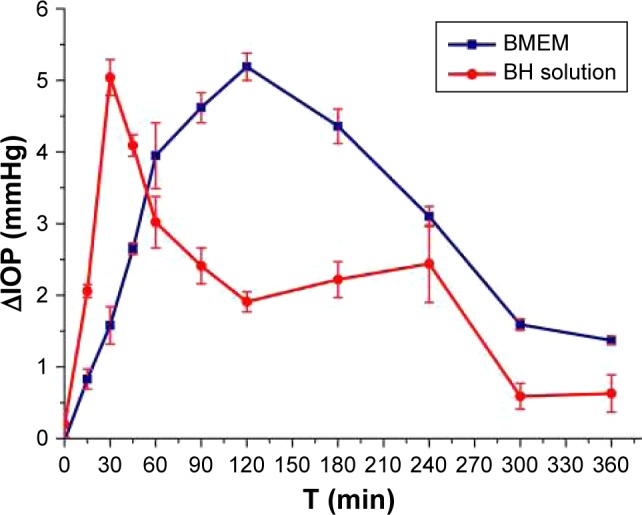
Effect on IOP changes induced by a single topical instillation of BMEM (2.8 mg·mL−1) in rabbit eyes in comparison with BH solution (2.8 mg·mL−1). Values are presented as the mean ± SD (n=6).
Abbreviations: BH, betaxolol hydrochloride; BMEM, betaxolol hydrochloride encapsulated microsphere; IOP, intraocular pressure.
Conclusion
A new controlled drug delivery carrier (BMEM) for glaucoma therapy was successfully prepared by a combination of acidification-intercalation and O/O emulsion–solvent evaporation method. This carrier was composed of bio-adhesive materials and demonstrated the ion-exchange ability of Mt. With the acid treatment, the Q of Mt was significantly increased from 153.6±7.6 to 474.52±5.4 mg·g−1. Both FTIR and XRD measurements proved that BH was successfully intercalated into acid-Mt. SEM images showed that BMEMs were rougher and slightly larger than conventional BH microspheres, due to the encapsulation of Mt-BH. BMEMs exhibited the longest sustained release duration (12 hours) in comparison with the BH solution (2.5 hours) and conventional BH microspheres (5 hours), since the incorporation of Mt-BH has prolonged the release time of BH by ion exchange. We hypothesized that the in vitro release process may have four stages (last for 12 hours) that caused the BH to be slowly released. The MTT test, CAM-TBS, and Draize test have shown higher security than BH solution. In vitro and in vivo precorneal retention have also shown that BMEMs have a prolonged retention time because BMEMs have intimate contact with the epithelial mucosal surface of the eye to prevent tear washout. It is noteworthy that BMEMs present a strong effect on reducing the high IOP. Taken together, BMEMs presented extended release, prolonged precorneal retention time, and better tolerability at the corneal site, which would have potential applications in glaucoma treatment.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant no 51102052), Medical Scientific Research Foundation of Guangdong Province (grant no A2016275), and a collaborative project from Science and Technology Planning Project of Guangdong Province, China (2017B030314175). iHCEC was kindly provided by Shandong Eye Institute (Qingdao, China).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natarajan JV, Ang M, Darwitan A, Chattopadhyay S, Wong TT, Venkatraman SS. Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int J Nanomedicine. 2012;7:123–131. doi: 10.2147/IJN.S25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho IM, Marques CS, Oliveira RS, Coelho PB, Costa PC, Ferreira DC. Sustained drug release by contact lenses for glaucoma treatment – a review. J Control Release. 2015;202:76–82. doi: 10.1016/j.jconrel.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Dai Y, Zhou R, Liu L, Lu Y, Qi J, Wu W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine. 2013;8:1921–1933. doi: 10.2147/IJN.S44487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Zhang Z, Li J, Sun S, Weng Y, Chen H. Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale. 2012;4(15):4667–4673. doi: 10.1039/c2nr30924f. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliv Rev. 1995;16(1):61–73. [Google Scholar]
- 7.Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269(1):1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59(6):478–490. doi: 10.1016/j.addr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Guan Q, Chen W, Hu X. Development of lovastatin-loaded poly(lactic acid) microspheres for sustained oral delivery: in vitro and ex vivo evaluation. Drug Des Devel Ther. 2015;9:791–798. doi: 10.2147/DDDT.S76676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He P, Liu H, Tang Z, et al. Poly(ester amide) blend microspheres for oral insulin delivery. Int J Pharm. 2013;455(1–2):259–266. doi: 10.1016/j.ijpharm.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Uemoto S, Tabata Y. Prevention of trinitrobenzene sulfonic acid-induced experimental colitis by oral administration of a poly(lactic-coglycolic acid) microsphere containing prostaglandin E(2) receptor subtype 4 agonist. J Pharmacol Exp Ther. 2012;341(2):340–349. doi: 10.1124/jpet.111.190447. [DOI] [PubMed] [Google Scholar]
- 12.Gavini E, Rassu G, Haukvik T, Lanni C, Racchi M, Giunchedi P. Mucoadhesive microspheres for nasal administration of cyclodextrins. J Drug Target. 2009;17(2):168–179. doi: 10.1080/10611860802556842. [DOI] [PubMed] [Google Scholar]
- 13.Huh Y, Cho HJ, Yoon IS, et al. Preparation and evaluation of spray-dried hyaluronic acid microspheres for intranasal delivery of fexofenadine hydrochloride. Eur J Pharm Sci. 2010;40(1):9–15. doi: 10.1016/j.ejps.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Andres-Guerrero V, Zong M, Ramsay E, et al. Novel biodegradable polyesteramide microspheres for controlled drug delivery in ophthalmology. J Control Release. 2015;211:105–117. doi: 10.1016/j.jconrel.2015.05.279. [DOI] [PubMed] [Google Scholar]
- 15.Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin Drug Deliv. 2008;5(6):615–628. doi: 10.1517/17425247.5.6.615. [DOI] [PubMed] [Google Scholar]
- 16.Nilkumhang S, Basit AW. The robustness and flexibility of an emulsion solvent evaporation method to prepare pH-responsive microparticles. Int J Pharm. 2009;377(1–2):135–141. doi: 10.1016/j.ijpharm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res. 2004;27(1):1–12. doi: 10.1007/BF02980037. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Li X, Ye T, et al. Nanostructured lipid carrier surface modified with Eudragit RS 100 and its potential ophthalmic functions. Int J Nanomedicine. 2014;9:4305–4315. doi: 10.2147/IJN.S63414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignatelloa R, Bucolob C, Spedalieria G, Malteseb A, Puglisia G. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;16(1–2):53–61. doi: 10.1016/s0928-0987(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 20.Pignatelloa R, Bucolob C, Spedalieria G, Malteseb A, Puglisia G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23(15):3247–3255. doi: 10.1016/s0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 21.Ennajih H, Bouhfid R, Essassi EM, Bousmina M, El Kadib A. Chitosan–montmorillonite bio-based aerogel hybrid microspheres. Microporous Mesoporous Mater. 2012;152:208–213. [Google Scholar]
- 22.dos Santos BR, Bacalhau FB, Pereira Tdos S, Souza CF, Faez R. Chitosan-montmorillonite microspheres: a sustainable fertilizer delivery system. Carbohydr Polym. 2015;127:340–346. doi: 10.1016/j.carbpol.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 23.Hou D, Gui R, Hu S, Huang Y, Feng Z, Ping Q. Preparation and characterization of novel drug-inserted-montmorillonite chitosan carriers for ocular drug delivery. Adv Nanoparticles. 2015;4(3):70–84. [Google Scholar]
- 24.Hou D, Hu S, Huang Y, et al. Preparation and in vitro study of lipid nanoparticles encapsulating drug loaded montmorillonite for ocular delivery. Appl Clay Sci. 2016;119(2):277–283. [Google Scholar]
- 25.Huang Y, Tao Q, Hou D, et al. A novel ion exchange carrier based upon liposome-incorporated montmorillonite for ophthalmic delivery of betaxolol hydrochloride. Int J Nanomedicine. 2017;12:1–15. doi: 10.2147/IJN.S122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng JP, Luan L, Wang HY, Xi LF, Yao KD. Study on ibuprofen/montmorillonite intercalation composites as drug release system. Appl Clay Sci. 2007;36(4):297–301. [Google Scholar]
- 27.Anirudhan TS, Sandeep S. Synthesis and characterization of a novel pH-controllable composite hydrogel for anticancer drug delivery. New J Chem. 2011;35(12):2869. [Google Scholar]
- 28.Shameli K, Bin Ahmad M, Zargar M, et al. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int J Nanomedicine. 2011;6:271–284. doi: 10.2147/IJN.S16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kevadiya BD, Joshi GV, Bajaj HC. Layered bionanocomposites as carrier for procainamide. Int J Pharm. 2010;388(1–2):280–286. doi: 10.1016/j.ijpharm.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Jelvehgari M, Barar J, Valizadeh H, Shadrou S, Nokhodchi A. Formulation, characterization and in vitro evaluation of theophylline-loaded Eudragit RS 100 microspheres prepared by an emulsion-solvent diffusion/evaporation technique. Pharm Dev Technol. 2011;16(6):637–644. doi: 10.3109/10837450.2010.508075. [DOI] [PubMed] [Google Scholar]
- 31.Casolaro M, Casolaro I, Lamponi S. Stimuli-responsive hydrogels for controlled pilocarpine ocular delivery. Eur J Pharm Biopharm. 2012;80(3):553–561. doi: 10.1016/j.ejpb.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Vinardell MP, Garcia L. The quantitive chlorioallantoic membrance test using trypan blue stain to predict the eye irritancy of liquid scintillation cocktails. Toxicol In Vitro. 2000;14(6):551–555. doi: 10.1016/s0887-2333(00)00050-3. [DOI] [PubMed] [Google Scholar]
- 33.Nascimento DF, Silva AC, Mansur CR, et al. Characterization and evaluation of poly(ε-caprolactone) nanoparticles containing 2-ethylhexyl-p-methoxycinnamate, octocrylene, and benzophenone-3 in anti-solar preparations. J Nanosci Nanotechnol. 2012;12(9):7155–7166. doi: 10.1166/jnn.2012.5832. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen DH, Beuerman RW, Wever BD, Rosdy M. Three-dimensional construct of the human corneal epithelium for in vitro toxicology. In: Salem H, Katz SA, editors. Alternative Toxicological Methods. Boca Rato: CRC Press; 2003. pp. 147–159. [Google Scholar]
- 35.York M, Steiling W. A critical review of the assessment of eye irritation potential using the Draize rabbit eye test. J Appl Toxicol. 1998;18(4):233–240. doi: 10.1002/(sici)1099-1263(199807/08)18:4<233::aid-jat496>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Moosa RM, Choonara YE, du Toit LC, et al. In vivo evaluation and in-depth pharmaceutical characterization of a rapidly dissolving solid ocular matrix for the topical delivery of timolol maleate in the rabbit eye model. Int J Pharm. 2014;466(1–2):296–306. doi: 10.1016/j.ijpharm.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 37.Morrison JC, Moore CG, Deppmeier LMH, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64(1):85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 38.Zaghouane-Boudiaf H, Boutahala M. Kinetic analysis of 2,4,5-trichlorophenol adsorption onto acid-activated montmorillonite from aqueous solution. Int J Miner Process. 2011;100(3–4):72–78. [Google Scholar]
- 39.Momoh MA, Kenechukwu FC, Adedokun MO, Odo CE, Attama AA. Pharmacodynamics of diclofenac from novel Eudragit entrapped microspheres. Drug Deliv. 2014;21(3):193–203. doi: 10.3109/10717544.2013.843608. [DOI] [PubMed] [Google Scholar]
- 40.Mundargi RC, Rangaswamy V, Aminabhavi TM. pH-Sensitive oral insulin delivery systems using Eudragit microspheres. Drug Dev Ind Pharm. 2011;37(8):977–985. doi: 10.3109/03639045.2011.552908. [DOI] [PubMed] [Google Scholar]
- 41.Haznedar S, Dortunç B. Preparation and in vitro evaluation of Eudragit microspheres containing acetazolamide. Int J Pharm. 2004;269(1):131–140. doi: 10.1016/j.ijpharm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Dong Y, Feng SS. Poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(30):6068–6076. doi: 10.1016/j.biomaterials.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Mendioroz S, Pajares JA, Benito I, Pesquera C, Gonzalez F, Blanco C. Texture evolution of montmorillonite under progressive acid treatment: change from H3 to H2 type of hysteresis. Langmuir. 1987;3(5):676–681. [Google Scholar]
- 44.Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine. 2010;6(2):324–333. doi: 10.1016/j.nano.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Hagino S, Itagaki H, Kato S, Kobayashi T, Tanaka M. Quantitative evaluation to predict the eye irritancy of chemicals: modification of chorioallantoic membrane test by using trypan blue. Toxicol In Vitro. 1991;5(4):301–304. doi: 10.1016/0887-2333(91)90006-y. [DOI] [PubMed] [Google Scholar]
- 46.Qin F, Zeng L, Zhu Y, Cao J, Wang X, Liu W. Preparation and evaluation of a timolol maleate drug-resin ophthalmic suspension as a sustained-release formulation in vitro and in vivo. Drug Dev Ind Pharm. 2016;42(4):535–545. doi: 10.3109/03639045.2015.1085872. [DOI] [PubMed] [Google Scholar]
- 47.Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. 2007;125(8):1010–1013. doi: 10.1001/archopht.125.8.1010. [DOI] [PubMed] [Google Scholar]
- 48.Konstas AGP, Mantziris DA, Cate EA, Stewart WC. Effect of timolol on the diurnal intraocular pressure in exfoliation and primary open-angle glaucoma. Arch Ophthalmol. 1997;115(8):975–979. doi: 10.1001/archopht.1997.01100160145002. [DOI] [PubMed] [Google Scholar]
- 49.Medeiros FA, Pinheiro A, Moura FC, Leal BC, Susanna R. Intraocular pressure fluctuations in medical versus surgically treated glaucomatous patients. J Ocul Pharmacol Ther. 2002;18(6):489–498. doi: 10.1089/108076802321021036. [DOI] [PubMed] [Google Scholar]