Abstract
Vernal keratoconjunctivitis (VKC) is a chronic allergic conjunctivitis that is most often seen in young, males. Although most types of allergic conjunctivitis do not affect vision, VKC is unusual in that damage to the cornea from the condition can result in vision loss. Although it is typically seasonal, year-round symptoms can be seen, which can lead to uncertain diagnoses being made. Although the pathophysiology of VKC is better understood in recent years, allowing more targeted therapies, management of these patients can still be very challenging, and complications can occur. As such, aggressive management of VKC is necessary, especially since vision loss in the amblyogenic age range can be permanent.
Keywords: vernal keratoconjunctivitis, allergic conjunctivitis, inflammation
Introduction
Vernal keratoconjunctivitis (VKC) is a unique disorder among a spectrum of allergic eye diseases. It is a chronic, bilateral, inflammatory condition most commonly involving the upper tarsal conjunctiva. It more commonly affects young, male patients, but it is frequently observed in tropical regions where it may affect both sexes equally.1 VKC has also often been associated with higher socioeconomic status. The incidence, as well as the type, of VKC varies depending on geographic region: limbal VKC is the predominate form in central and southern African countries,2 while the palpebral form is most frequent in Europe and the Americas.3 Seasonal exacerbations may occur based on the time of year, most often in Europe and Asia, but a significant number of patients may develop chronic perennial disease.4 Although the disease is most often self-limiting, and will often resolve after puberty, some patients can develop sight-threatening complications. Immunosuppressive therapy is the mainstay of treatment, but surgical intervention may be required to manage the disease and its complications.
Pathophysiology
VKC is characterized by infiltration of the conjunctiva by a variety of inflammatory cell types, especially eosinophils. Although VKC has previously been thought of as an IgE-mediated disease,5 several other immunologic pathways have also been implicated. Patients with VKC have been shown to have an increased number of activated CD4+ T-lymphocytes, predominantly Th2, indicating that there is a hypersensitivity reaction to an unknown pathogen.6 Increased levels of inflammatory cytokines IL-3, IL-4, and IL-5 have also been demonstrated.7 Conjunctival papillae formation is related to fibroblast activation and production, whereas limbal conjunctival nodules are related to infiltration of inflammatory cells.6
Studies using in vivo confocal microscopy have shown cellular irregularities in patients with VKC. Patients have been shown to have not only injury to the superficial corneal epithelial layer but also involvement of the basal epithelium and anterior stroma. Corneal nerves may be affected in VKC, and they have been shown to have decreased density as well as increased concentration of adjacent inflammatory cells.8
It is also thought that aberrations in the normal ocular surface microbiome may play a role in VKC. In a recent study, Staphylococcus aureus was more frequently isolated from the conjunctival specimens from patients with VKC, and may be a significant cause of exacerbations, while S. epidermidis was more frequently found in normal control patients.9
Clinical findings
Patients with VKC often present with symptoms of intense itching, redness, and watering eyes.4 They also may complain of photophobia and foreign body sensation. Clinical signs of VKC include a papillary reaction of the upper tarsal conjunctiva, and throughout the limbus. VKC is classified as tarsal, limbal, or mixed3 based on the location, and the papillae may range in size from 1 mm to giant cobblestone papillae. The eyelid margins are not involved, in contrast to other types of allergic keratoconjunctivitis. Other typical signs of VKC include bulbar conjunctival hyperemia, a thick mucus discharge, and corneal involvement, including superficial punctate keratitis, epithelial erosions, shield ulcers, or plaques (Figure 1). A classic sign of VKC is Tranta’s dots which represent lymphocytic infiltration of the limbal conjunctiva.
Figure 1.
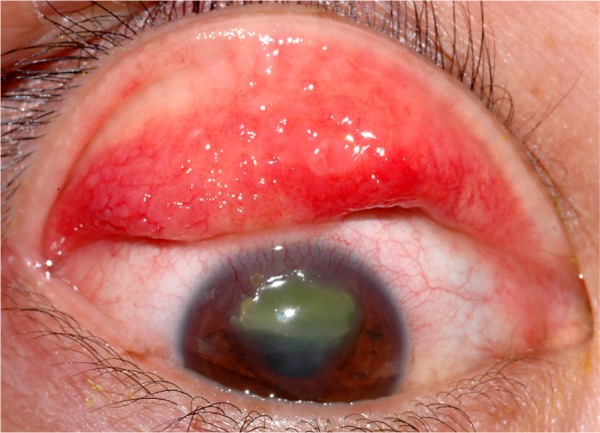
Ten-year-old boy with vernal keratoconjunctivitis demonstrating superior palpebral conjunctiva with giant papillae within an intensely inflamed conjunctiva and shield ulcer in the cornea with fluorescein staining. (Courtesy of Todd P. Margolis, MD, PhD).
A clinical grading system based on clinical symptoms and signs has been proposed to help guide treatment and identify patients at high risk for recurrences, corneal complications, and vision loss. In a recent study, patients were stratified by symptoms: grade 0 being no symptoms and no therapy; grade 1 being presence of symptoms without photophobia and occasional allergic eye drop use; grade 2 being the presence of symptoms including photophobia; grade 3 representing daily anti-allergic treatment and occasional topical steroid use; and grade 4 being diffuse punctate keratitis or corneal ulcer, and use of pulsed high-dose topical steroid. This grading system identifies those patients with more severe disease and at higher risk of recurrence and complications associated with VKC, leading to worse visual outcomes.10
Differential diagnoses
Differential diagnosis of VKC includes any of the chronic allergic conjunctivitis’s including seasonal allergic conjunctivitis, perennial allergic conjunctivitis, atopic keratoconjunctivitis, or giant papillary conjunctivitis, as well as chlamydial infection, especially in the early stages on the disease.11 VKC may be differentiated from the above disorders by hypertrophic papillae in the tarsal form of VKC or Horner Trantas dots in the limbal form, or a combination of the two, along with no involvement of the eyelids.12
Currently there is limited utility in testing for the diagnosis of VKC. Skin tests and IgE levels are rarely useful and may be negative in 50% of patients with VKC. In cases where the diagnosis is unclear, conjunctival scrapings showing eosinophilic infiltration may be beneficial in aiding the diagnosis.5
Treatment
There is currently no defined gold-standard treatment algorithm for VKC, but there are many options available, and treatment should be tailored to the individual.
Whatever treatment regimen is prescribed, it should be initiated promptly and the patient should be monitored closely for the development of any corneal complications.
A Cochrane review by Mantelli et al included 27 randomized controlled trials encompassing 2,184 eyes in an effort to evaluate topical therapies for the treatment of VKC. They found that all common antiallergic eye drops are effective in reducing signs and symptoms of disease, and that some patients even respond to placebo.13 A critical initial component of all allergic disease involves avoidance of inciting factors. Patients should be encouraged to wash hands frequently and avoid touching or rubbing their eyes. Cold compresses and artificial tears may help to alleviate symptoms in mild cases or as adjunctive therapy to pharmacologic treatment. First-line pharmacologic therapy for VKC is topical treatment, and there is considerable therapeutic overlap with other forms of allergic conjunctivitis. Mast cell stabilizers are a mainstay for prophylaxis. In milder cases, antihistamines may be of benefit, but more severe cases may require corticosteroid treatment. Long-term steroid use is associated with many complications, so other immunomodulatory treatments have been studied extensively.
Mast cell stabilizers
Mast cell stabilizers are thought to prevent mast cell degranulation via inhibition of calcium channels, but their mechanism is not completely understood. There is increasing evidence that mast cell stabilizers act more broadly on inflammatory cell chemotaxis.14 Commonly used mast cell stabilizers include cromolyn sodium and lodoxamide, which are frequently used as first-line therapy. Lodoxamide has been shown to be superior to cromolyn sodium in relieving symptoms and clinical signs of VKC.15 The medications should be used 4–6 times daily and may take up to 2 weeks to show response in mild cases of VKC.
Antihistamines
Antihistamines are sometimes used in the treatment of VKC: they are most often used in alleviating the symptoms of mild disease, but they have limited utility in severe cases.
More recent medications that combine antihistamine properties with mast cell stabilization include ketotifen and olopatadine. Both have been shown to improve the signs and symptoms of VKC, and there is evidence that ketotifen may be more effective.16 Some antihistamines may have a drying effect, which could exacerbate symptoms.
Nonsteroidal anti-inflammatory drugs (NSAIDs)
NSAIDs may also be a useful therapeutic option in VKC. Topical diclofenac or ketorolac have been shown to be effective in treating symptoms.17,18 NSAIDs may be used in conjunction with other topical anti-inflammatory medications to provide rapid relief of symptoms; however, they have limited utility in those patients with corneal involvement.19
While NSAIDs are a frequently prescribed ocular medication, there have been reports of severe corneal toxicity, with even ulceration and corneal perforation in otherwise healthy eyes. As such, their use should be limited to short-term treatment.12
Topical corticosteroids
Topical corticosteroids are a critical component of VKC management, particularly in VKC exacerbations, but they have extensive side effect profiles including cataract development and glaucoma. Initial treatment in an acute exacerbation should begin with steroids with low intraocular penetration such as loteprednol or fluorometholone to avoid complications associated with steroid use. More resistant cases should be treated with more potent steroids such as prednisolone acetate or dexamethasone.20
Cyclosporine
Cyclosporine is a calcineurin inhibitor that acts by inhibiting cytokine production by T-lymphocytes. It also has inhibitory effects on eosinophil and mast cell activation.21 Low-dose cyclosporine has emerged as an alternative to corticosteroids in some cases of VKC. It has an excellent side effect profile, limited to only a burning sensation and few systemic effects at the doses being used.22 Commercially available topical 0.05% cyclosporine A has been shown to improve clinical signs and symptoms of VKC, as well as decreasing tear cytokine concentration when used 4–6 times per day.23 Higher concentrations of 1%–2% have been studied and shown to be safe and effective for even severe VKC, but the disadvantage is that they need to be extemporaneously compounded.24,25 More recent studies have shown even a dose of cyclosporine 0.05% 4 times per day may be effective in drug-resistant VKC in conjunction with topical corticosteroids.26
Tacrolimus
Tacrolimus is another calcineurin inhibitor that acts to inhibit T-lymphocyte activation and it causes the release of inflammatory cytokines.27 In recent studies tacrolimus has proven to be very effective in the treatment of VKC at various concentrations. Chatterjee et al studied response to 0.03% tacrolimus ointment in steroid-refractory patients with VKC. Out of 23 patients, only 4 required additional corticosteroid therapy after 4 weeks of tacrolimus treatment.28
Tacrolimus may also be effective in patients with corneal involvement. Miyazaki et al treated patients with epitheliopathy, shield ulcers, or corneal plaques with 0.1% topical tacrolimus, and showed similar improvement in epitheliopathy scores when compared to those using adjuvant topical corticosteroids.29
A recent randomized control trial compared treatment of VKC with 0.005% tacrolimus eye drops versus IFN alpha-2b who had failed topical antihistamines and corticosteroids. The main outcome measure was improvement of subjective symptoms, but objective improvement of exam findings was also measured. All of the patients with tacrolimus showed improvement of subjective symptoms while one patient treated with interferon had persistent symptoms.30
Other therapies
The most resistant cases of VKC may require treatment with systemic medications, including oral corticosteroids or other immunomodulatory agents. Recent case reports have shown treatment of VKC with omalizumab: 4 patients who had failed topical therapy of both cyclosporine and tacrolimus showed improvement of ocular symptoms of itching and tearing, as well as ocular signs of hyperemia, and papillae.31 There may also be a role for allergen-specific immunotherapy which avoids the side effects often seen with topical therapies. Patients with VKC often have hypersensitivity to pollens and house dust and have been shown to have improvement in signs and symptoms with subcutaneous allergen-specific immunotherapy (SCIT).32 In this study, treatment by SCIT was more effective in improving the clinical symptoms and reducing the serum IgE than topical treatment because there was a greater reduction in symptoms in group 1 of immunotherapy (72%) than in group 2 of medical treatment (59%) (P<0.05). Also, there was a significant reduction in total serum IgE (P<0.05) in group 1 (62%) compared to group 2 (42%).32
Surgical intervention
Persistent corneal complications such as non-healing shield ulcers or corneal plaques may require surgical treatment. These interventions may range from scraping to superficial keratectomy. In rare cases of refractory giant papillae that do not respond to medical treatment, surgical interventions such as cryoablation may be employed. Therapies in conjunction with surgical excision to prevent recurrence include mitomycin-C application, autologous conjunctival graft, or mucous membrane transplantation. Amniotic membrane transplantation in cases of refractory giant papillae has been shown to aid healing of corneal complications associated with giant papillae such as epitheliopathy and ulcers.33
Complications
Severe vernal keratoconjunctivitis is most frequently a self-limiting disease; however, in some cases sight-threatening complications may develop. The corneal epithelium acts as a barrier to circulating pathogens, but may become damaged in severe disease both due to trauma from upper tarsal papillae and a complex array of inflammatory molecules.34 This combination of repeated trauma and inflammatory milieu may then lead to shield ulcers and plaques. Shield ulcers usually form on the upper third of the cornea and can lead to sight-threatening complications in up to 6% of patients.35 They begin as punctate epithelial erosions which coalesce to form macroerosions which then develop into shield ulcers which can be self-limiting or develop further consequences such as bacterial keratitis.36 Plaques form when inflammatory debris accumulates at the base of a shield ulcer. They are particularly resistant to topical therapy and may require surgical intervention.
Patients with long-standing vernal keratoconjunctivitis may also develop limbal stem cell deficiency due to longstanding inflammation. The prevalence of limbal stem cell deficiency in patients with VKC may be as high as 1.2% and occurs in older patients with VKC. Treatment may include amniotic membrane transplantation or allo-limbal stem cell transplantation.37 Other commonly associated complications from vernal keratoconjunctivitis include keratoconus and irregular astigmatism due to frequent eye rubbing in the atopic pediatric population38 and steroid-induced glaucoma from frequent use of topical corticosteroids.3,38
Prognosis
The prognosis for VKC patients is generally good and the disease is generally self-limiting with appropriate treatment. Despite an overall good prognosis, up to 6% of patients will develop vision loss due to complications associated with VKC. In patients studied, over half will continue to have symptoms after 5 years and the presence of giant papilla may indicate a worse prognosis.3
Future research
The incidence of allergic disease is continuing to climb39 and personalized therapeutic approaches are currently being studied. Further developments in allergic sensitization may prove to be the next innovation of VKC treatment.
Conclusion
VKC is a bilateral inflammatory disease that can cause visual loss. Although little has changed in the diagnosis of VKC, which continues to be largely clinical, based on symptoms and clinical signs, newer therapies have been developed. This armamentarium of therapies continues to expand with increasing use of immunomodulators. Unfortunately, severe, protracted cases of VKC remain a challenge to treat. As such, continued research is necessary to better understand the complex nature of VKC and to develop more effective therapies.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chenge B, Makumyamviri AM, Kaimbo WA, Kaimbo D. La limbo-conjunctivite endemique des tropiques a Lubumbashi, Republique Democratique du Congo. [Tropical endemic limbo-conjunctivitis in Lúbumbashi, Democratic Republic of the Congo] Bull Soc Belge Ophtalmol. 2003;(290):9–16. French. [PubMed] [Google Scholar]
- 2.Dahan E, Appel R. Vernal keratoconjunctivitis in the black child and its response to therapy. Br J Ophthalmol. 1983;67(10):688–692. doi: 10.1136/bjo.67.10.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000;107(6):1157–1163. doi: 10.1016/s0161-6420(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 4.Saboo US, Jain M, Reddy JC, Sangwan VS. Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J Ophthalmol. 2013;61(9):486–489. doi: 10.4103/0301-4738.119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. 2004;18(4):345–351. doi: 10.1038/sj.eye.6700675. [DOI] [PubMed] [Google Scholar]
- 6.Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002;21(3):319–339. doi: 10.1016/s1350-9462(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 7.Metz DP, Hingorani M, Calder VL, Buckley RJ, Lightman SL. T-cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol. 1997;100(6 Pt 1):817–824. doi: 10.1016/s0091-6749(97)70279-3. [DOI] [PubMed] [Google Scholar]
- 8.Leonardi A, Lazzarini D, Bortolotti M, Piliego F, Midena E, Fregona I. Corneal confocal microscopy in patients with vernal keratoconjunctivitis. Ophthalmology. 2012;119(3):509–515. doi: 10.1016/j.ophtha.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hakami AM, Al-Amri A, Abdulrahim I, Hamid ME. Is there is an association between the presence of Staphylococcus species and occurrence of vernal keratoconjunctivitis? Saudi J Ophthalmol. 2015;29(4):255–258. doi: 10.1016/j.sjopt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacchetti M, Lambiase A, Mantelli F, Deligianni V, Leonardi A, Bonini S. Tailored approach to the treatment of vernal keratoconjunctivitis. Ophthalmology. 2010;117(7):1294–1299. doi: 10.1016/j.ophtha.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Mannis MJ, Holland EJ. Cornea. 2nd ed. Vol. 1. Edinburgh: Elsevier; 2017. [Google Scholar]
- 12.Leonardi A, Bogacka E, Fauquert JL, et al. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67(11):1327–1337. doi: 10.1111/all.12009. [DOI] [PubMed] [Google Scholar]
- 13.Mantelli F, Santos MS, Petitti T, et al. Systematic review and meta-analysis of randomised clinical trials on topical treatments for vernal keratoconjunctivitis. Br J Ophthalmol. 2007;91(12):1656–1661. doi: 10.1136/bjo.2007.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruijnzeel PL, Warringa RA, Kok PT, Kreukniet J. Inhibition of neutrophil and eosinophil induced chemotaxis by nedocromil sodium and sodium cromoglycate. Br J Pharmacol. 1990;99(4):798–802. doi: 10.1111/j.1476-5381.1990.tb13009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caldwell DR, Verin P, Hartwich-Young R, Meyer SM, Drake MM. Efficacy and safety of lodoxamide 0.1% vs cromolyn sodium 4% in patients with vernal keratoconjunctivitis. Am J Ophthalmol. 1992;113(6):632–637. doi: 10.1016/s0002-9394(14)74786-5. [DOI] [PubMed] [Google Scholar]
- 16.Hida WT, Nogueira DC, Schaefer A, Dantas PEC, Dantas MCN. Comparação entre o uso tópico do fumarato de cetotifeno 0,025% e do cloridrato de olopatadina 0,1% no tratamento da ceratoconjuntivite primaveril. [Comparative study between 0.025% ketotifen fumarate and 0.1% olopatadine hydrochloride in the treatment of vernal keratoconjunctivitis] Arq Bras Oftalmol. 2006;69(6):851–856. doi: 10.1590/s0004-27492006000600013. Portuguese. [DOI] [PubMed] [Google Scholar]
- 17.D’Angelo G, Lambiase A, Cortes M, et al. Preservative-free diclofenac sodium 0.1% for vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2003;241:192–195. doi: 10.1007/s00417-002-0612-6. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Gupta R, Ram J, Gupta A. Topical ketorolac 0.5% solution for the treatment of vernal keratoconjunctivitis. Indian J Ophthalmol. 1997;45(3):177–180. [PubMed] [Google Scholar]
- 19.Kosrirukvongs P, Luengchaichawange C. Topical cyclosporine 0.5 per cent and preservative-free ketorolac tromethamine 0.5 per cent in vernal keratoconjunctivitis. J Med Assoc Thai. 2004;87(2):190–197. [PubMed] [Google Scholar]
- 20.Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2(2):73–88. doi: 10.1007/s40123-013-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54(3):321–338. doi: 10.1016/j.survophthal.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Pucci N, Caputo R, Mori F, et al. Long-term safety and efficacy of topical cyclosporine in 156 children with vernal keratoconjunctivitis. Int J Immunopathol Pharmacol. 2010;23(3):865–871. doi: 10.1177/039463201002300322. [DOI] [PubMed] [Google Scholar]
- 23.Oray M, Toker E. Tear cytokine levels in vernal keratoconjunctivitis: the effect of topical 0.05% cyclosporine a therapy. Cornea. 2013;32(8):1149–1154. doi: 10.1097/ICO.0b013e31828ffdf8. [DOI] [PubMed] [Google Scholar]
- 24.Tesse R, Spadavecchia L, Fanelli P, et al. Treatment of severe vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatr Allergy Immunol. 2010;21(2 pt 1):330–335. doi: 10.1111/j.1399-3038.2009.00948.x. [DOI] [PubMed] [Google Scholar]
- 25.Kiliç A, Gürler B. Topical 2% cyclosporine A in preservative-free artificial tears for the treatment of vernal keratoconjunctivitis. Can J Ophthalmol. 2006;41(6):693–698. doi: 10.3129/i06-061. [DOI] [PubMed] [Google Scholar]
- 26.Yücel OE, Ulus ND. Efficacy and safety of topical cyclosporine A 0.05% in vernal keratoconjunctivitis. Singapore Med J. 2016;57(9):507–510. doi: 10.11622/smedj.2015161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai J, Gu J, Yuan J, Chen J. Tacrolimus in the treatment of ocular diseases. BioDrugs. 2011;25:89–103. doi: 10.2165/11587010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee S, Agrawal D. Tacrolimus in corticosteroid-refractory vernal keratoconjunctivitis. Cornea. 2016;35(1):1444–1448. doi: 10.1097/ICO.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki D, Fukushima A, Ohashi Y, et al. Steroid-sparing effect of 0.1% Tacrolimus eye drop for treatment of shield ulcer and corneal epitheliopathy in refractory allergic ocular diseases. Ophthalmology. 2017;124(3):287–294. doi: 10.1016/j.ophtha.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Zanjani H, Aminifard MN, Ghafourian A, et al. Comparative evaluation of tacrolimus versus interferon alpha-2b eye drops in the treatment of vernal keratoconjunctivitis: a randomized, double-masked study. Cornea. 2017;36(6):675–678. doi: 10.1097/ICO.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 31.Occasi F, Duse M, Nebbioso M, et al. Vernal keratoconjunctivitis treated with omalizumab: a case series. Pediatr Allergy Immunol. 2017;28(5):503–505. doi: 10.1111/pai.12737. [DOI] [PubMed] [Google Scholar]
- 32.Mahdy RAR, Nada WM, Marei AA. Subcutaneous allergen-specific immunotherapy versus topical treatment in vernal keratoconjunctivitis. Cornea. 2012;31(5):525–528. doi: 10.1097/ICO.0b013e3181eae270. [DOI] [PubMed] [Google Scholar]
- 33.Guo P, Kheirkhah A, Zhou W-W, Qin L, Shen X-L. Surgical resection and amniotic membrane transplantation for treatment of refractory giant papillae in vernal keratoconjunctivitis. Cornea. 2013;32(6):816–820. doi: 10.1097/ICO.0b013e31826a1e53. [DOI] [PubMed] [Google Scholar]
- 34.Fukuda K, Nishida T. Ocular allergic inflammation: interaction between the cornea and conjunctiva. Cornea. 2010;29:S62–S67. doi: 10.1097/ICO.0b013e3181ea9b2b. [DOI] [PubMed] [Google Scholar]
- 35.Neumann E, Gutmann MJ, Blumenkrantz N, Michaelson IC. A review of four hundred cases of vernal conjunctivitis. Am J Ophthalmol. 1959;47(2):166–172. doi: 10.1016/s0002-9394(14)76417-7. [DOI] [PubMed] [Google Scholar]
- 36.Cameron JA. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. 1995;102(6):985–993. doi: 10.1016/s0161-6420(95)30925-6. [DOI] [PubMed] [Google Scholar]
- 37.Sangwan VS, Jain V, Vemuganti GK, Murthy SI. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea. 2011;30(5):491–496. doi: 10.1097/ico.0b013e3181cbf9d3. [DOI] [PubMed] [Google Scholar]
- 38.Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. 1999;34(2):88–92. [PubMed] [Google Scholar]
- 39.Backman H, Räisänen P, Hedman L, et al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016 – results from three population surveys. Clin Exp Allergy. 2017;47(11):1426–1435. doi: 10.1111/cea.12963. [DOI] [PubMed] [Google Scholar]


