ABSTRACT
Microtubules are essential for neuronal structure and function. Axonal and dendritic microtubules are enriched in post-translational modifications that impact microtubule dynamics, transport and microtubule-associated proteins. Acetylation of α-tubulin lysine 40 (K40) is a prominent and conserved modification of neuronal microtubules. However, the cellular role of microtubule acetylation remains controversial. To resolve how microtubule acetylation might affect neuronal morphogenesis, we mutated endogenous α-tubulin in vivo using a new Drosophila strain that facilitates the rapid knock-in of designer αTub84B alleles (the predominant α-tubulin-encoding gene in flies). Leveraging our new strain, we found that microtubule acetylation, as well as polyglutamylation and (de)tyrosination, is not essential for survival. However, we found that dendrite branch refinement in sensory neurons relies on α-tubulin K40. Mutagenesis of K40 reveals moderate yet significant changes in dendritic lysosome transport, microtubule polymerization and Futsch protein distribution in dendrites but not in axons. Our studies point to an unappreciated role for α-tubulin K40 and acetylation in dendrite morphogenesis. While our results are consistent with the idea that acetylation tunes microtubule function within neurons, they also suggest there may be an acetylation-independent requirement for α-tubulin K40.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Microtubule, Acetylation, Neuron, Dendrite, Drosophila
Highlighted Article: Neurons are enriched in post-translationally modified microtubules. Targeted mutagenesis of endogenous α-tubulin in flies reveals that dendrite branch refinement is altered by acetylation-blocking mutations.
INTRODUCTION
Microtubules provide the basis for neuronal architecture. The ability of neurons to transmit and receive signals depends on the proper morphogenesis of axons and dendrites. Axons and dendrites differ in structure as well as function. Microtubules in each compartment are uniquely organized and enriched in post-translational modifications (PTMs), including acetylation, detyrosination and polyglutamylation (Chakraborti et al., 2016; Song and Brady, 2015). The patterns of microtubule PTMs between and within axons and dendrites are thought to be critical for functional compartmentalization, acting by locally regulating microtubule dynamics and/or transport. However, the role that microtubule PTMs, in particular acetylation, may play in neuronal morphogenesis has been controversial.
Several conserved lysine residues in α- and β-tubulin are acetylated, and acetylation of the α-tubulin luminal residue lysine 40 (K40) has been the most well-studied since its discovery over 30 years ago (Choudhary et al., 2009; Chu et al., 2011; Howes et al., 2014; L'Hernault and Rosenbaum, 1983, 1985; Soppina et al., 2012). Acetylation of α-tubulin K40 was initially characterized as a marker of microtubules resistant to depolymerizing drugs (Piperno et al., 1987). Although acetylation typically correlates with stable, long-lived microtubules in cells, acetylation itself does not confer stability, but rather may make microtubules more resilient to mechanical forces as microtubules age (Coombes et al., 2016; Howes et al., 2014; Ly et al., 2016; Palazzo et al., 2003; Portran et al., 2017; Szyk et al., 2014; Webster and Borisy, 1989; Wilson and Forer, 1997; Xu et al., 2017). However, despite years of study, the effects of acetylation on microtubules and microtubule function in cells are still debated.
In cultured mammalian neurons, young axons are enriched in acetylated microtubules in comparison to dendrites. This difference initially led to the idea that acetylation might label microtubule tracks for selective transport to one compartment or the other (Song and Brady, 2015). Consistent with this idea, acetylation has been shown to distinguish the microtubule tracks that are preferentially bound by kinesin-1 motors, which transport cargo from the cell body to axon terminal (Dompierre et al., 2007; Guardia et al., 2016). The neuron-wide expression of α-tubulin K40Q, which mimics acetylation, has also been reported to redirect kinesin-1 to dendrites (Farías et al., 2015). Similarly, in immature unpolarized neurons, increasing microtubule acetylation redirects kinesin-1 to multiple neurites (Hammond et al., 2010; Reed et al., 2006). However, in mature polarized neurons, microtubule acetylation by itself is not sufficient to alter kinesin-1 localization (Cai et al., 2009; Hammond et al., 2010). In addition, microtubule acetylation does not affect kinesin-1 motility in purified in vitro systems (Kaul et al., 2014; Walter et al., 2012). Thus, there are conflicting reports about whether microtubule acetylation is necessary and/or sufficient to affect motor activity and localization in neurons.
There is also conflicting evidence regarding the role of microtubule acetylation in neuronal development. The function of microtubule acetylation in the developing nervous system has been investigated mainly through the loss or overexpression of the primary α-tubulin acetyltransferase and deacetylase enzymes αTAT1 and HDAC6, respectively (Akella et al., 2010; Hubbert et al., 2002; Shida et al., 2010). On one hand, there are reports that inhibiting HDAC6 disrupts axon initial segment formation in cultured neurons (Tapia et al., 2010; Tsushima et al., 2015), and that cortical neuron migration is impeded by either the knockdown of αTAT1 or the overexpression of α-tubulin K40A, which cannot be acetylated (Creppe et al., 2009; Li et al., 2012). On the other hand, Hdac6 and Atat1 knockout mice are homozygous viable. Neither knockout results in any gross neurological defect, such as a disruption in cortical layering, which is typically associated with abnormal neuronal polarity (Kalebic et al., 2013; Kim et al., 2013; Zhang et al., 2008). Worms lacking αTAT1 (known as Mec-17) activity are viable, but touch insensitive (Akella et al., 2010; Cueva et al., 2012; Shida et al., 2010; Topalidou et al., 2012; Zhang et al., 2002). A recent study has shown that Atat1 knockout mice are also insensitive to mechanical touch and pain (Morley et al., 2016), indicating that the functional effects of microtubule acetylation are likely conserved between invertebrates and vertebrates. These functional studies raise the question of whether and how microtubule acetylation might sculpt neuronal architecture. Here again, there is conflicting evidence arguing both for and against the importance of acetylated microtubules to axonal morphology (Morley et al., 2016; Neumann and Hilliard, 2014). It is not known whether only axons rely on acetylated microtubules; indeed, a potential role for microtubule acetylation in dendrite morphogenesis has not been explored.
We sought to resolve the role of microtubule acetylation in neuronal transport and morphogenesis through targeted mutagenesis of endogenous αTub84B (the predominant α-tubulin-encoding gene) in Drosophila. A key advantage of mutating endogenous α-tubulin is that we can directly and specifically assess the involvement of α-tubulin residues in the development of axons and dendrites as well as microtubule growth and microtubule-dependent activities. Our approach leverages a new fruit fly strain that we created to enable the rapid knock-in of designer α-tubulin alleles. By directly targeting the α-tubulin residues that are modified, we avoid complications often associated with targeting the modifying enzymes. For example, several modifying enzymes have cellular targets in addition to α-tubulin. While this is not the case for αTAT1, which acetylates itself and α-tubulin K40, HDAC6 deacetylates multiple proteins in addition to α-tubulin (Valenzuela-Fernández et al., 2008). Some enzymes, such as glutamylases, can modify several tubulin residues on both α- and β-tubulin, and some modifying enzymes, such as the carboxypeptidase that removes the terminal tyrosine on α-tubulin, remain unidentified (Janke, 2014; Natarajan et al., 2017; Song and Brady, 2015; van Dijk et al., 2007). This presents challenges to using an enzyme-based approach to dissect the role of microtubule PTMs in cells.
Through live imaging of sensory neurons in developing fruit flies, we found that targeted mutagenesis of endogenous α-tubulin K40 does not disrupt selective transport to axons or dendrites, or neuronal polarity, but does affect the refinement of dendrite branches. Acetylation-blocking mutations increase branch number with a correlative increase in terminal branch growth. Both α-tubulin K40A and K40R mutations block acetylation. However, only the arginine substitution conserves the length and charge of the lysine sidechain; alanine does not and thus may alter α-tubulin structure. We found that the K40R mutation does not phenocopy the effects of the K40A mutation on dendrite dynamics, suggesting that K40 may be essential for α-tubulin and/or microtubule structure. In the α-tubulin K40A mutant dendrites, we observed modest, yet significant, changes in lysosome transport, microtubule growth and the distribution of the protein Futsch that might underlie an increase in branch number. Combined, our data point to a previously unappreciated role for K40 and acetylation in fine-tuning dendrite patterning.
RESULTS
Characterization of α-tubulin mutations that disrupt microtubule PTMs
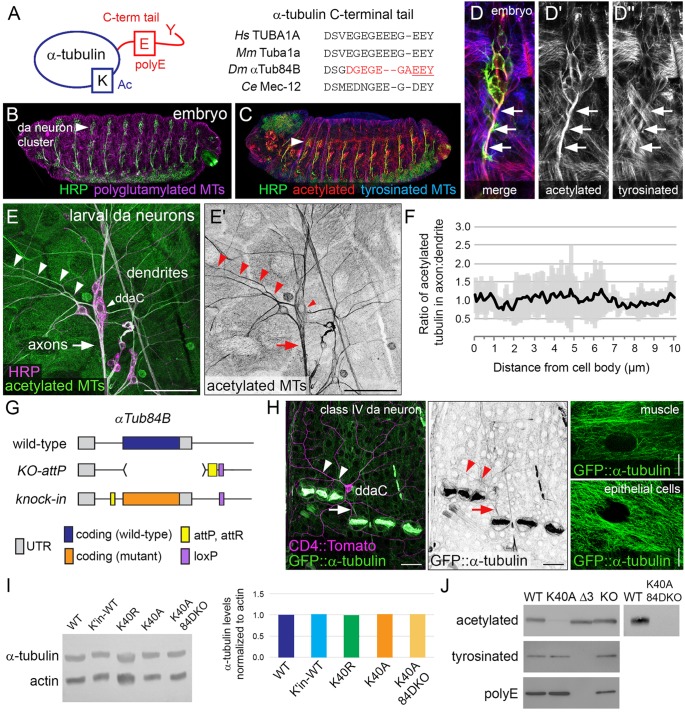
To determine the function of microtubule PTMs in neurons in vivo, we undertook targeted mutagenesis of α-tubulin in fruit flies. Like other organisms, the Drosophila melanogaster genome has several distinct α-tubulin genes that encode unique protein isotypes, which assemble into microtubules that are modified. The four Drosophila α-tubulin genes have been named based on their cytological location: αTub84B, αTub84D, αTub85E and αTub67C (Raff, 1984). αTub84B is likely the predominant α-tubulin in flies and is 97% identical to human TUBA1A, with only five non-conservative amino acid differences, four of which are within the C-terminal tail (Fig. 1A). Like α-tubulin in other organisms, αTub84B is modified at multiple residues (Bobinnec et al., 1999; Piperno and Fuller, 1985; Warn et al., 1990; Wolf et al., 1988). In the sensory class IV dendritic arborization (da) neurons that we use as a model, microtubules are acetylated, polyglutamylated and tyrosinated (Fig. 1B–F and data not shown). In embryos, axonal microtubules are heavily acetylated (Fig. 1C,D), consistent with findings that young axons of mammalian neurons in culture are also enriched in acetylated microtubules (Witte et al., 2008). In mature larval da neurons, microtubule acetylation levels are equivalent between axons and dendrites (Fig. 1E,F).
Fig. 1.
In vivo analysis of α-tubulin modifications. (A) Cartoon showing α-tubulin modifications (left) and sequence alignment of the C-terminal tails of human, mouse, fly, and worm TUBA1A orthologs (right). Red, ten amino acids that were deleted in αTub84BΔC. Underline, three residues (EEY) deleted in αTub84BΔ3. (B–D) Microtubules in developing fly embryos are modified, and da neuron axons are enriched in acetylated microtubules (D–D″). Embryos were stained for HRP (green), a neuronal membrane marker, as well as polyglutamylated (magenta, B), acetylated (red, C,D) and tyrosinated (blue, C,D) microtubules. Arrowheads, da neuron cluster; arrows, axons. (E,F) In larval da neurons, microtubules in axons and dendrites are acetylated at equivalent levels. The acetylated microtubule signals in proximal axonal and dendritic segments (E) were compared as a ratio (mean±s.d.), n=7 class IV ddaC neurons (F). Green, acetylated microtubules; magenta, HRP. Arrowheads, dendrite and ddaC cell body marker; arrow, axons. Images are from fixed tissue. Scale bars: 50 µm. (G) Cartoon of the αTub84BKO-attP allele that enables rapid knock-in of designer alleles to interrogate α-tubulin function in vivo. Top, wild-type αTub84B (blue); middle, the major coding exon of αTub84B was deleted (brackets) and replaced by an attP site (yellow); bottom, knock-in of a mutant αTub84B (orange). (H) GFP-tagged αTub84B is broadly expressed in developing larvae. In muscles and epithelial cells, a filamentous pattern indicates GFP::αTub84B is likely incorporated into microtubules. Arrowheads, dendrites; arrow, axon. Green, GFP; magenta, CD4::Tomato. Scale bars: 25 µm (left), 10 µm (muscle and epithelial cell images, right). Images from live third-instar larvae. (I) Western blot analysis (left) and quantification (right) of α-tubulin levels (normalized to actin) in wild-type (WT) or mutant strains as indicated. All strains are homozygous. K40A 84DKO refers to the αTub84BK40A chromosome with αTub84D deleted (αTub84BK40A, αTub84DKO); K′in-WT is a knock-in for wild-type αTub84B. (J) Western blot of lysates from wild-type and αTub84B mutant fly heads probed for acetylated, tyrosinated and polyglutamylated α-tubulin, as indicated. KO, single αTub84D knockout. The two-lane blot (right) was probed for acetylated α-tubulin and includes lysate from double-mutant αTub84BK40A αTub84DKO fly heads. The αTub84B Δ3 mutation eliminates both the anti-tyrosinated and anti-polyglutamylated α-tubulin signals.
By using a genome-engineering approach, we created a new fly strain that enables us to readily knock-in designer αTub84B alleles via site-directed recombination (Fig. 1G). We used an ends-out gene-targeting strategy (Huang et al., 2009) to replace αTub84B with an attP ‘landing’ site. Consistent with previous reports, deleting αTub84B resulted in lethality (Table 1), indicating that αTub84B is essential for survival and that the other α-tubulin genes could not compensate for its loss (Matthews and Kaufman, 1987). This includes αTub84D, which has a similar expression pattern and encodes a nearly identical protein that differs from αTub84B by only two amino acids (Matthews et al., 1989; Raff, 1984). The knockout strain was rescued by knocking-in wild-type αTub84B (αTub84BK'in-WT), indicating that the attP replacement strategy did not disrupt the function of the αTub84B locus (Table 1). To confirm that αTub84B is indeed broadly expressed, including in the nervous system (Raff, 1984), we generated flies that express GFP-tagged αTub84B (GFP::αTub84B). As expected, GFP::αTub84B was expressed in most cell types, including neurons (Fig. 1H). In muscles and epithelial cells, GFP::αTub84B appeared to be filamentous, suggesting that GFP-tagged tubulin was incorporated into microtubules (Fig. 1H). However, it should be noted that the GFP::αTub84B allele is dominant male sterile and does not survive in trans to a deletion that removes αTub84B. This suggests that GFP::αTub84B does not function equivalently to the wild-type untagged protein. Thus, we have created a unique and powerful tool to manipulate and visualize endogenous α-tubulin in vivo.
Table 1.
Effects of α-tubulin mutations on survival
We targeted K40 acetylation as well as two additional α-tubulin modifications that have also been implicated in neuronal development and transport, namely polyglutamylation and detyrosination of the C-terminal tail. Much of what is known about microtubule polyglutamylation and (de)tyrosination is based on studies from vertebrate models and manipulating the modifying enzymes. Not all the modifying enzymes have clear fly homologs; for example, only a minor fraction of fly microtubules are detyrosinated and there is no known α-tubulin tyrosine ligase in flies (Warn et al., 1990). The fly and mammalian C-terminal tails also differ in several amino acids (Fig. 1A). We tested whether the function of the α-tubulin C-terminal tails from flies and mammals might be conserved despite these differences. Replacing the αTub84B C-terminal tail with that of the mammalian TUBA1A did not affect viability (Table 1), indicating the mammalian TUBA1A C-terminal tail can functionally substitute for the fly αTub84B C-terminal tail (Fig. 1A). Deletion of the C-terminal tail resulted in lethality (Table 1), indicating that the C-terminal tail is essential for proper α-tubulin function in vivo. Blocking two different modifications of the C-terminal tail, polyglutamylation (αTub84BAAAA) and detyrosination (αTub84BΔ3), did not affect animal survival (Table 1). Since the glutamate residues in the C-terminal tail are thought to mediate interactions with essential motors and other microtubule-binding proteins, it was particularly surprising that eliminating virtually all the negatively charged residues in αTub84BAAAA did not affect viability (Bonnet et al., 2001; Boucher et al., 1994; Lacroix et al., 2010; Larcher et al., 1996; Roll-Mecak, 2015; Sirajuddin et al., 2014; Valenstein and Roll-Mecak, 2016; Wang and Sheetz, 2000). Combined, our results suggest that the C-terminal tail has a conserved role in α-tubulin function in vivo, yet polyglutamylation and modification of the terminal residues of the C-terminal tail are dispensable for survival.
To test the role of αTub84B K40 acetylation in survival and neuronal morphogenesis, we introduced K40A and K40R mutations to eliminate acetylation. Both αTub84B alleles were viable and fertile in trans to the αTub84B knockout (Table 1), consistent with reports that loss of K40 acetylation does not affect survival (Akella et al., 2010; Cueva et al., 2012; Kalebic et al., 2013; Kim et al., 2013; Mao et al., 2017; Shida et al., 2010; Topalidou et al., 2012; Zhang et al., 2008). Our viability and fertility results agree with a recent study that used CRISPR-Cas9 to introduce the K40R mutation into αTub84B (Mao et al., 2017). Western blot analysis of adult fly head lysate revealed that the amounts of the mutant αTub84B proteins were equivalent to those in wild-type and that α-tubulin K40 acetylation was virtually abolished in the αTub84BK40A flies (Fig. 1I,J). The residual signal in the western blot may reflect acetylation of another α-tubulin isotype, most likely αTub84D, which is also broadly expressed (Raff, 1984). We used CRISPR-Cas9 genome editing to delete the entire αTub84D gene in the αTub84BK40A strain. The αTub84BK40A αTub84DKO double mutant eliminated the residual acetylated tubulin signal in western blots (Fig. 1J) and was viable in trans to a large deletion that removes both these α-tubulin genes (Table 1). Genetic complementation tests also unexpectedly revealed that αTub84D is a non-essential gene (Table 1). Combined, these data indicate that α-tubulin K40 acetylation is not essential for survival.
αTub84B K40A does not affect selective transport to axons, but has a compartment-specific effect on retrograde lysosome transport in dendrites
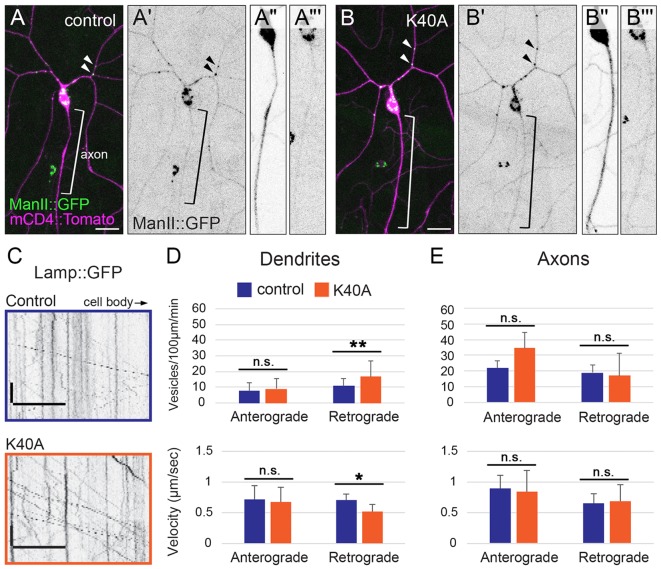
Microtubule acetylation has been shown to affect microtubule-based transport in cultured cells, including in neurons (Dompierre et al., 2007; Reed et al., 2006). One recent model suggests that acetylated microtubules are part of an exclusion zone that prevents dendritic cargos from entering axons (Farías et al., 2015). However, there is also evidence that microtubule acetylation alone is not sufficient to direct motors to a specific compartment (Atherton et al., 2013). The class IV da neurons that we use as a model reside just below the transparent larval cuticle, allowing for live imaging of transport in neurons in intact animals. First, we examined the distribution of a polarized organelle population, Golgi outposts, which localize to dendrites and regulate dendrite patterning in flies and mammals (Horton and Ehlers, 2003; Horton et al., 2005; Ye et al., 2007). We found that the polarized dendritic localization of Golgi outposts was not altered in αTub84BK40A neurons (Fig. 2A–B‴). Thus, microtubule acetylation is not an essential part of the mechanism that prevents Golgi outposts from entering axons.
Fig. 2.
The αTub84B K40A mutation does not affect the polarized distribution of Golgi outposts, but does affect lysosome motility in dendrites. (A–B‴) Golgi outposts, marked by ManII::GFP (green), localize to dendrites in both control (A–A‴) and αTub84BK40A neurons (B–B‴). ManII::GFP (green in A,B; black in A′,A‴,B′,B‴) and CD4::Tomato (magenta in A,B, black in A″,B″) are expressed in class IV da neurons under the control of the ppk enhancer. Bracket, axon; arrowheads, Golgi outposts in dendrites. Scale bars: 25 µm. (C) Representative kymographs of lysosome dynamics in the dendrites of control (top) and αTub84BK40A (bottom) neurons. Scale bar x-axis: 10 µm; scale bar y-axis: 10 s. Lysosomes are marked by Lamp1::GFP. The cell body is to the right. (D,E) In dendrites (D), lysosomes traveling in the retrograde direction in αTub84BK40A neurons display increased flux (top) and reduced velocity (bottom). Lysosome motility in axons (E) is unaffected by the αTub84B K40A mutation. Dendrites (D, flux): 30 wild-type control dendrite segments and 29 αTub84BK40A dendrite segments were analyzed (mean±s.d.); P=0.008. Dendrites (D, velocity): 32 wild-type control dendrite segments and 29 αTub84BK40A dendrite segments were analyzed (mean±s.d.); P=0.012. Axons (E, flux): 7 wild-type control axons and 5 αTub84BK40A axons were analyzed (mean±s.d.). Axons (E, velocity): 7 wild-type control axons and 5 αTub84BK40A axons were analyzed (mean±s.d.). *P=0.01–0.05; **P=0.001–0.01; n.s., not significant (two-tailed Student's t-test).
Although selective transport to dendrites or axons is not perturbed, it is possible that microtubule acetylation affects other aspects of microtubule-based transport. The trafficking of lysosomes, an organelle component of the autophagy pathway, is sensitive to microtubule acetylation in cultured cells (Chauhan et al., 2015; Guardia et al., 2016; Xie et al., 2010). Notably, a recent study revealed that microtubule acetylation distinguishes a set of tracks that are preferentially used by kinesin-1 to transport lysosomes in the perinuclear region in HeLa cells (Guardia et al., 2016). We expressed the lysosome marker Lamp1::GFP in the da neurons, and analyzed its dynamic localization in the axons and dendrites of control and αTub84BK40A neurons. In αTub84BK40A dendrites, the velocity of lysosomes traveling in a retrograde direction was significantly reduced while their flux nearly doubled (Fig. 2C,D). Lysosomes moving in an anterograde direction in αTub84BK40A dendrites were not affected (Fig. 2D). Since microtubules in da neuron dendrites are oriented predominantly with the minus-end distally (Rolls et al., 2007), lysosomes moving in an anterograde direction are likely transported by dynein whereas those that move in a retrograde direction are likely transported by kinesin. We found that lysosome transport in axons was unchanged in the αTub84BK40A neurons (Fig. 2E). Our data indicate that the αTub84B K40A mutation selectively alters the retrograde, likely kinesin-mediated, transport of lysosomes in dendrites, but does not affect either the retrograde or anterograde transport of lysosomes in axons. While the αTub84B K40A mutation does not affect selective transport to axons or dendrites, it does have a dendrite-specific effect on lysosome transport.
αTub84B K40A and K40R mutations increase dendrite branch number
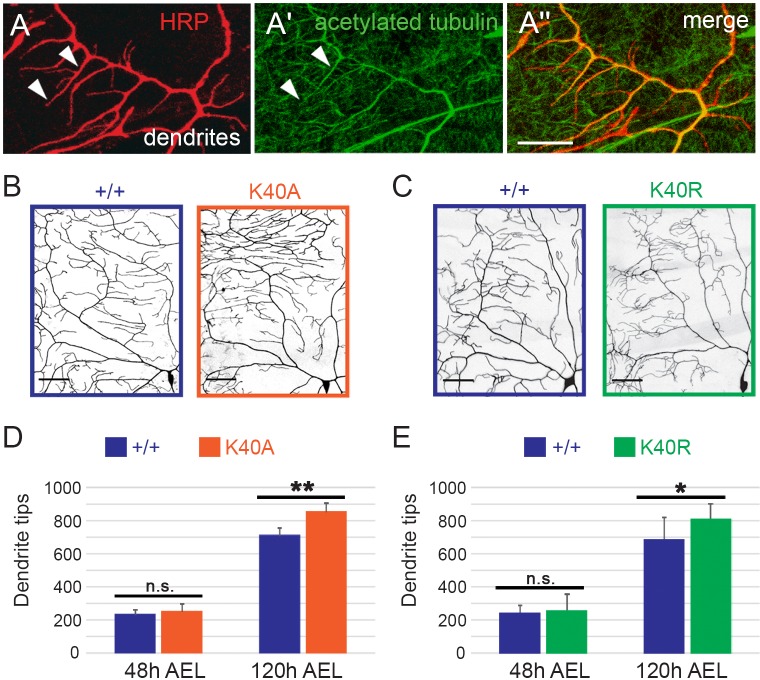
The axons of young neurons in culture are enriched in acetylated microtubules relative to dendrites (Song and Brady, 2015), which has led to a focus on the role of microtubule acetylation in axons. However, our analyses of lysosome transport in αTub84BK40A neurons suggest that dendritic, not axonal, microtubules are sensitive to α-tubulin K40 mutagenesis. We next tested whether dendrite morphogenesis is affected by K40 mutations. In developing larvae, the class IV da neurons extend an expansive dendritic arbor over a shallow depth, making them ideal for analyzing dendrite growth and patterning (Grueber et al., 2002). Acetylated microtubules are present in the main dendrite branches and a subset of terminal branches of class IV da neurons (Fig. 3A). To visualize dendrite arbors and quantify dendrite branching in control and αTub84B K40 mutants, we used the transgene ppk-CD4::GFP, which expresses a GFP-tagged transmembrane protein (human CD4) under the control of the class IV-specific pickpocket (ppk) enhancer. The overall dendritic coverage of the αTub84BK40A and αTub84BK40R neurons appears normal in that the mutant arbors extended to the segment boundaries normally and tiled properly with their neighbors. However, our quantification of the number of dendrite tips revealed that both the K40A and K40R mutations resulted in an increased number of terminal branches compared to that in age-matched wild-type controls at 120 h after egg laying (AEL) (Fig. 3B–E). We did not detect any defects in axon termination in the ventral nerve cord (data not shown).
Fig. 3.
Sensory dendrite tip number is increased in αTub84B K40 mutant neurons. (A) In class IV da neuron dendrites, acetylated microtubules are present in main dendrite branches and some terminal dendrites (arrowheads). Red, HRP, a neuronal membrane marker; green, anti-acetylated α-tubulin. Scale bar: 10 µm. (B–E) Mutations that prevent αTub84B K40 acetylation (K40A, K40R) increase the number of dendrite tips at 120 h AEL. Terminal branch number is not significantly affected at 48 h AEL. (B,C) Quadrant of class IV da neuron dendrite arbor at 120 h AEL illuminated with ppk-CD4::GFP. Scale bars: 50 µm. Experiments to analyze the morphogenesis of each mutant included age-matched controls that were imaged and analyzed in parallel. (D,E) The numbers of dendrite tips in control neurons do not significantly differ between each other. αTub84BK40A dendrite analysis (D): 6 wild-type control and 6 αTub84BK40A neurons were analyzed (mean±s.d.), P=0.001. αTub84BK40R dendrite analysis (E): 8 wild-type control and 14 αTub84BK40R neurons were analyzed (mean±s.d.), P=0.04. *P=0.01–0.05; **P=0.001–0.01; n.s., not significant (one-way ANOVA with post hoc two-tailed Student's t-tests between experimentally matched control and mutant neurons).
Dendrite branch growth is increased in αTub84B K40A mutant neurons
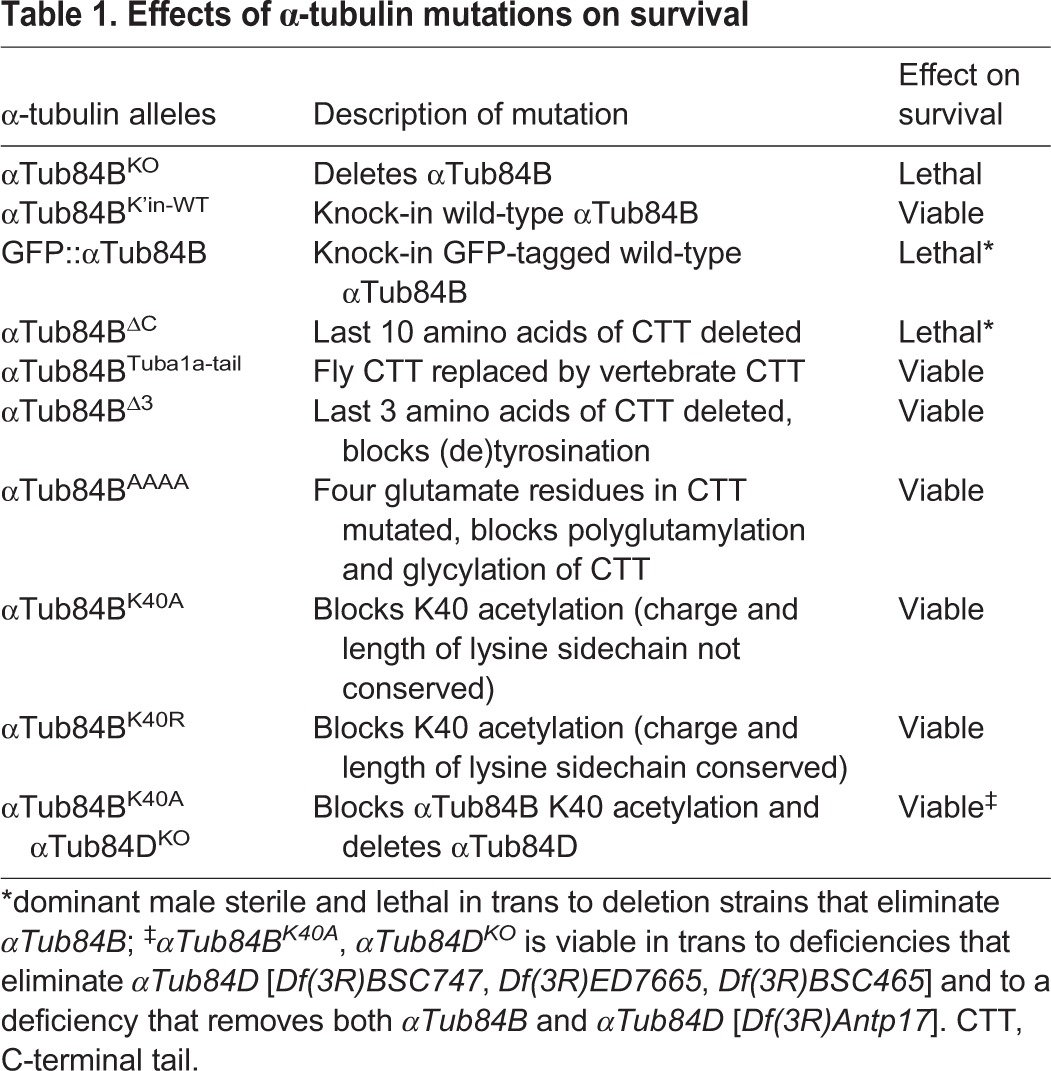
The class IV da neuron dendrites initially extend during late embryonic stages and continue to grow throughout larval stages. Dendrite branches undergo remodeling and refinement through bouts of de novo growth, extension and retraction as larvae grow in size (Parrish et al., 2009; Stewart et al., 2012). During early larval stages (48 h AEL; at the beginning of the second larval instar), terminal branches are dynamic and new branches are added to the arbor whereas during third instar larval stages (120 h AEL), terminal branches are less dynamic and fewer new branches appear. At 48 h AEL, neurons with both the K40A and K40R mutations had the same number of dendrite tips as did the control neurons (Fig. 3B–E), indicating that the increase in dendrite tip number was likely not due to a defect in the initial stages of dendrite extension during the embryonic stages. Rather, we reasoned that the increase in terminal dendrites might have resulted from changes in dendrite dynamics during early larval stages. To test whether dendrite tip number increased due to increased branch growth and/or decreased branch retraction, we used time-lapse imaging to record dendrite dynamics in larvae at 48 h AEL. We then quantified the number of dendrite tips that formed de novo, or extended or retracted over a 15-min interval (Fig. 4A). In the αTub84BK40A neurons, significantly more branches extended compared to the number in controls, although de novo branch growth did not significantly increase over this time interval (Fig. 4B–D). While αTub84BK40R mutant neurons had an increased number of dendrite tips, as with αTub84BK40A, neither dendrite growth nor retraction was significantly altered (Fig. 4B–D). This suggests the increase in dendrite tips in the αTub84BK40A arbors is likely due to an increase in dendrite growth. Moreover, the difference between dendrite dynamics for the αTub84B K40A versus K40R mutant neurons suggests that K40 may be structurally important for α-tubulin and/or microtubule function in neurons.
Fig. 4.
Terminal-dendrite extension is increased in αTub84BK40A neurons. (A) Overlaid photographs of a dendrite imaged at two time points 15 min apart in control (left) and αTub84BK40A (right) larvae at 48 h AEL. The initial dendrite image (t=0) is pseudo-colored red and the second image (t=15 min) is shown in green. Dendrites that have retracted will appear red and those that have extended will appear green. Scale bar: 10 µm. (B,C) Percentage of the total number of terminal branches that extended (B) or retracted (C) during the 15 min imaging interval in wild-type control, αTub84BK40A, and αTub84BK40R neurons. Boxes represent first and third quartiles (median indicated by line) and whiskers indicate minimum and maximum values. (D) Percentage of the dynamic terminal branches (mean±s.d.) that either extended (dark bar) or retracted (light bar). Experiments included age-matched controls that were imaged and analyzed in parallel; all dendrites in the entire arbor of each neuron were analyzed. Dendrite dynamics between control neurons are not significantly different. αTub84BK40A dendrite analysis (B–D): 12 wild-type control and 8 αTub84BK40A neurons were analyzed, P=0.002 (% total dendrites that extended), P=0.014 (% dynamic dendrites that extended or retracted). αTub84BK40R dendrite analysis (B–D): 11 wild-type control and 8 αTub84BK40R neurons were analyzed. *P=0.01–0.05; **P=0.001–0.01; n.s., not significant (one-way ANOVA with post hoc two-tailed Student's t-tests between experimentally matched control and mutant neurons).
Dendritic microtubule polymerization frequency is reduced by αTub84B K40A
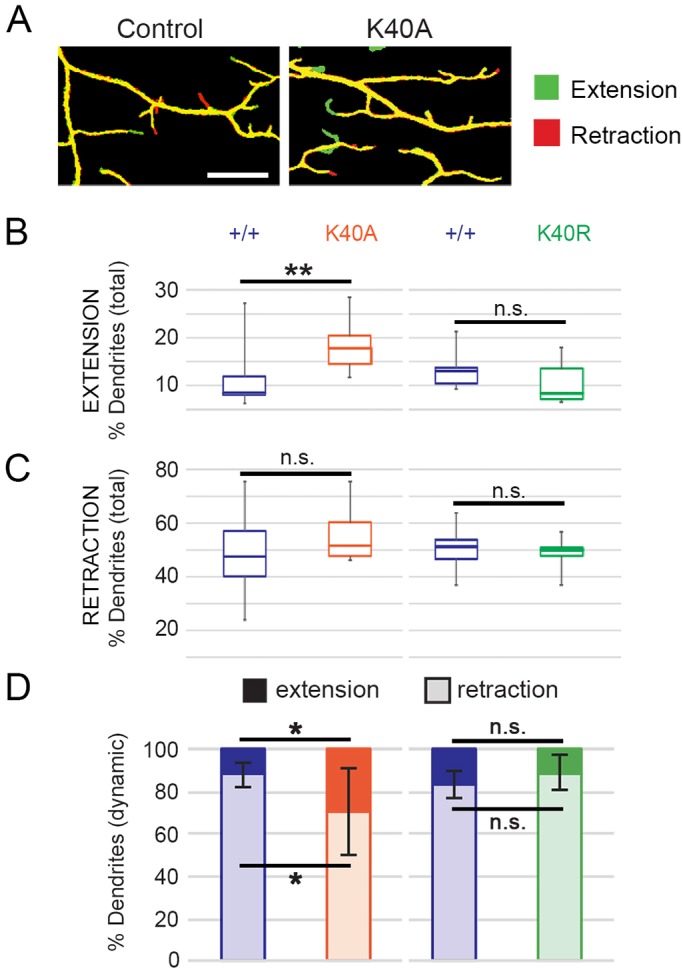
Next, we analyzed whether the increase in dendrite growth in the αTub84B K40A mutants might reflect a change in the growth of the microtubules themselves. We focused on the αTub84B K40A mutant as it resulted in significantly altered dynamic dendrite growth. Although acetylation does not affect microtubule polymerization in vitro (Dompierre et al., 2007; Howes et al., 2014; Maruta et al., 1986), we nonetheless tested this possibility since microtubule growth has been previously correlated with terminal branch growth in da neurons (Ori-McKenney et al., 2012; Sears and Broihier, 2016; Yalgin et al., 2015). Based on these reports, we predicted that the increase in terminal branch growth in the αTub84B K40 mutants might correlate with an increase in microtubule growth. To monitor microtubule growth, we used EB1::GFP, which associates with the plus-ends of growing microtubules in neurites (Fig. 5A). Our data reveal that the αTub84B K40A mutation resulted in a reduced number of EB1::GFP comets specifically in dendrites (Fig. 5B,C) demonstrating that blocking K40 acetylation affected the polymerization of dendritic, but not axonal, microtubules. The rate at which microtubules polymerized was not affected by the K40A mutation (control dendrites: 0.123±0.028 µm min−1, n=25, and αTub84BK40A dendrites: 0.113±0.029 µm min−1, n=30, P=0.19; mean±s.d.). Thus, similar to its effect on lysosomes, the αTub84B K40A mutation had a compartment-specific effect on microtubule polymerization.
Fig. 5.
Reduced microtubule growth frequency in dendrites of αTub84BK40A neurons. (A) In a terminal dendrite (box, left panel), EB1::GFP (arrowhead, right panels) marks a microtubule growing towards the dendrite tip in a αTub84BK40A neuron. Scale bars: 10 µm (left panel); 2 µm (right panel). (B,C) Microtubule polymerization frequency, quantified as the number of EB1::GFP comets per µm per minute, is significantly decreased in dendrites (B), but not axons (C), of αTub84BK40A neurons. Boxes represent first and third quartiles (median indicated by line) and whiskers indicate minimum and maximum values. Experiments included age-matched controls that were imaged and analyzed in parallel. EB1::GFP analysis: 27 wild-type control and 30 αTub84BK40A dendrites were analyzed (B), P=0.003; 6 wild-type control and 6 αTub84BK40A axons were analyzed (C). **P=0.001–0.01; n.s., not significant (two-tailed Student's t-test).
The proximal-distal gradient of Futsch in dendrites is disrupted by αTub84B K40A
We next considered whether mutating K40 might affect dendrite branch growth via an effect on microtubule-associated proteins (MAPs), which are also known to regulate dendrite branch growth (Conde and Cáceres, 2009). Microtubule acetylation might impact the activity and/or distribution of a MAP that is important for proper dendrite branching. We initially tested whether loss of microtubule acetylation disrupts microtubule severing by katanin (the catalytic subunit of katanin is Katanin 60). Katanin has been previously shown to be sensitive to microtubule acetylation levels in dendrites (Sudo and Baas, 2010). However, we found that the loss of αTub84B K40 acetylation neither blocks nor enhances katanin-induced changes in dendrite morphogenesis (Fig. S1). This is consistent with a recent report that found that modulating HDAC6 levels does not affect katanin-induced dendrite growth defects (Mao et al., 2014).
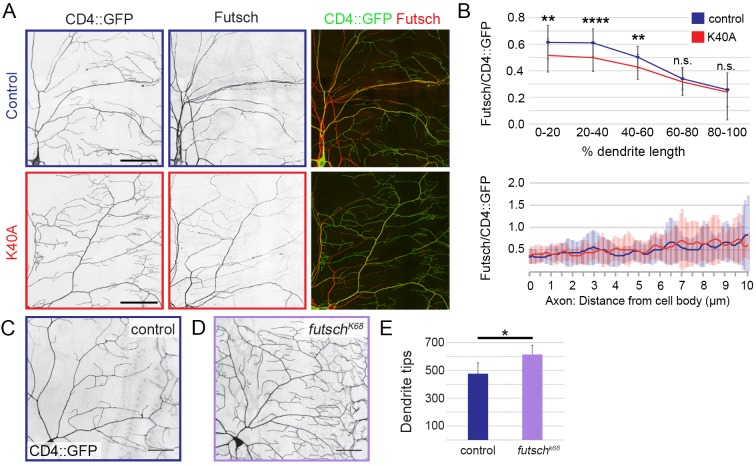
We next turned to Futsch, the fly homolog of MAP1B, which has been shown to regulate dendrite branching (Sears and Broihier, 2016; Yalgin et al., 2015) and has a similar distribution pattern to that of acetylated microtubules in dendrites (Fig. 6A, Fig. 1E,E′ and Grueber et al., 2002; Jinushi-Nakao et al., 2007). We asked whether the distribution of Futsch might be affected by the acetylation-blocking K40A mutation. Consistent with previous reports (Grueber et al., 2002; Jinushi-Nakao et al., 2007), in wild-type control neurons, we found that Futsch was enriched in the main dendrite branches and decreased towards the dendrite tips, where it was detected in some, but not all, terminal branches (Fig. 6A). In control dendrites, Futsch levels decayed ∼70% from the cell body to distal dendrite tip (Fig. 6B). In αTub84BK40A neurons, the proximal-medial dendrite segments showed a significant decrease in Futsch, but Futsch levels were comparable to those in control dendrites in the medial-distal segments (Fig. 6B). Futsch levels in control and αTub84BK40A axons were equivalent (Fig. 6B). While decreased levels of Futsch have been shown to increase dendrite branch number in one study (Yalgin et al., 2015), another study has found the opposite (Sears and Broihier, 2016). In agreement with the first study, we found that the reduction of Futsch in a futsch hypomorph (futschK68) increased dendrite branch number (Fig. 6C–E). Combined, our results suggest a model in which a change in Futsch distribution in the dendrite arbor may contribute to the increase in dendrite tips in the αTub84BK40A neurons.
Fig. 6.
Futsch levels are decreased in αTub84B K40A mutant dendrites. (A) Representative images of a quadrant of da neuron dendrite arbors immunostained for CD4::GFP (left panel, green) and Futsch (middle panel, red). Top row, wild-type control; bottom row, αTub84BK40A. (B) Quantification of Futsch levels in wild-type control and αTub84BK40A neurons (mean±s.d.). Futsch levels measured along a dendrite (0–100% length, top) and proximal axon (bottom) were normalized to CD4::GFP levels. (C–E) In hemizygous Futsch mutant animals (D), the number of dendrite tips are increased relative to in wild-type control neurons (C,E). Experiments included age-matched controls that were imaged and analyzed in parallel. αTub84BK40A Futsch analysis (B, upper panel): 27 wild-type control and 45 αTub84BK40A dendrites were analyzed, P=0.002 (0–20% length), P=0.00004 (20–40% length), P=0.001 (40–60% length); 12 wild-type control and 18 αTub84BK40A axons were analyzed (B, lower panel), no significant difference was detected at any 0.1 µm interval. Futsch loss-of-function dendrite tip analysis (mean±s.d., E): 4 wild-type control and 8 futschK68 neurons were analyzed in third-instar male larvae, P=0.01. *P=0.01–0.05; **P=0.001–0.01; ****P<0.0001; n.s., not significant (two-tailed Student's t-test). Scale bars: 50 µm.
DISCUSSION
Acetylation of α-tubulin K40 is a highly conserved and well-studied microtubule modification. While acetylated microtubules have been shown to mediate touch sensation in invertebrates and vertebrates (Akella et al., 2010; Cueva et al., 2012; Morley et al., 2016; Shida et al., 2010; Topalidou et al., 2012; Zhang et al., 2002), a role, if any, for acetylated microtubules in neuronal morphogenesis has remained elusive. To investigate how acetylation of α-tubulin K40 might affect neuronal development we leveraged our new fly strain, which facilitates the rapid knock-in of designer αTub84B alleles and thus is a versatile tool for interrogating α-tubulin function in vivo. Our targeted mutagenesis of endogenous αTub84B points to a role for α-tubulin K40 acetylation and K40 in refining the terminal dendrite branches of developing sensory neurons. Although microtubules in young axons of cultured mammalian neurons are enriched in acetylated microtubules (Song and Brady, 2015), we found that microtubule acetylation levels are equivalent between axons and dendrites in mature sensory neurons in vivo. Mutating αTub84B K40 does not affect selective transport to axons or dendrites in these neurons, consistent with previous reports showing that microtubule acetylation alone is not sufficient to direct transport to either compartment (Hammond et al., 2010; Kaul et al., 2014; Witte et al., 2008). Instead, our results show that mutating αTub84B K40 alters microtubule growth, lysosome transport and Futsch levels in dendrites but not in axons. Our findings are consistent with the idea that α-tubulin K40 may be important for locally and dynamically modulating microtubule function in neurons.
Our data suggest that the increase in the number of dendrite tips in the αTub84B K40 mutants likely reflects a change in the refinement of terminal branches that occurs during larval stages rather than an effect on dendrite outgrowth during embryogenesis. The class IV da neurons have both microtubule- and actin-rich dendrite branches (Grueber et al., 2002; Jinushi-Nakao et al., 2007), and our analyses indicate that only a subset of terminal dendrites contain acetylated microtubules (although dendrites with just a few microtubules might be below our level of detection). It is possible that the effect of mutating K40 on dendrite branching is modest since only a fraction of terminal dendrites contains microtubules. In contrast, mutations that disrupt the actin cytoskeleton typically produce striking changes in terminal branching (Ferreira et al., 2014; Jinushi-Nakao et al., 2007; Lee et al., 2003, 2015; Soba et al., 2015). One model consistent with our results and the findings of others is that microtubule acetylation fine-tunes the dynamic remodeling of microtubule-based dendrite branches. Another possibility is that mutating K40 alters the structure of α-tubulin in a way that disrupts dendrite branching.
Our data show that the αTub84B K40A mutation has modest yet significant effects on lysosome transport, microtubule growth and Futsch distribution in dendrites. While these changes might all independently contribute to an increase in dendrite branch number, it is also possible that they are mechanistically linked. We found that retrograde lysosome flux nearly doubles in αTub84BK40A dendrites while anterograde lysosome transport is normal. Studies in fly and mammalian neurons indicate that MAPs, including Futsch and MAP6, can selectively disrupt anterograde or retrograde transport (Schwenk et al., 2014; Stephan et al., 2015). Notably, MAP6-regulated retrograde lysosome transport affects dendrite growth in cultured hippocampal neurons (Schwenk et al., 2014), which suggests that dendrite branching may be sculpted by the flux of lysosomes moving to and away from the cell body. It is unclear why lysosome transport, microtubule growth and Futsch distribution are not significantly altered in the αTub84BK40A axons, although compartment-specific differences in microtubule regulators and/or MAPs might make dendrites more sensitive to the αTub84B K40 mutations than axons. It is possible that several microtubule-based activities that impinge on dendrite branching are affected by mutating α-tubulin K40.
Recent studies suggest that acetylation increases the resiliency of microtubules and protects them against mechanical breakage (Portran et al., 2017; Xu et al., 2017). The da neuron dendrites, which are sandwiched between the larval cuticle and muscles, are potentially exposed to repeated external and internal mechanical forces. Unacetylated microtubules in αTub84BK40A dendrites may be less resilient to mechanical stresses and thus may contain a higher proportion of damaged and broken microtubules than wild-type neurons. The breakage of unacetylated microtubules in worm neurons lacking αTAT1 is suppressed by paralyzing the worms (Topalidou et al., 2012). These broken microtubules are postulated to stimulate neurite branching by promoting microtubule growth from the broken microtubule ends. A similar mechanism may increase terminal branching in the αTub84BK40A dendrites; however, we observe a decrease, not increase, in microtubule growth frequency. A change in microtubule flexibility and/or lattice integrity may also affect the binding of MAPs. For example, the MAP doublecortin preferentially binds to curved microtubule segments, which may be prevalent in neurons with flexible microtubules (Bechstedt et al., 2014). It would be interesting to determine whether wild-type and αTub84BK40A dendrites are differentially sensitive to mechanical force, and whether changes in mechanical stress would modify any of the αTub84BK40A phenotypes.
An alternative interpretation of our data is that the increase in branch number is not due specifically to the loss of microtubule acetylation. For example, other modifications of α-tubulin K40 have been reported, including methylation by SetD2 (Park et al., 2016). However, it is not known whether α-tubulin K40 is methylated or otherwise modified in neurons. Lysine-to-arginine or -alanine mutations are often used interchangeably to block acetylation, although some of our results suggest that these mutations may not be entirely equivalent. This raises the possibility that intact K40 may be important to the structure of α-tubulin and/or microtubules in neurons. Consistent with this idea, work in plants has revealed that plant growth is disrupted by the expression of α-tubulin with a K40A, but not a K40R, mutation (Xiong et al., 2013).
Neuronal microtubules are enriched in other α-tubulin modifications, including (de)tyrosination and polyglutamylation, whose roles in neuronal development and function are still being unraveled. We found that targeted mutagenesis of residues that are modified in the α-tubulin C-terminal tail (αTub84B Δ3 and AAAA) has no effect on animal survival. This was somewhat unexpected, given the findings, for example, showing that the detyrosination–tyrosination cycle affects kinesin activity (Sirajuddin et al., 2014) as well as the loading of dynein onto microtubules (McKenney et al., 2016; Nirschl et al., 2016), and that the loss of glutamylase activity alters synaptic function (Ikegami et al., 2007). However, it is important to consider that fly and mammalian microtubules may be differentially enriched in these modifications. An early report suggests that fly microtubules are only weakly detyrosinated (Warn et al., 1990). Moreover, differences in the repertoire of modifying enzymes between flies and mammals suggest that PTM dynamics may differ as well. For example, although fly microtubules are tyrosinated and detyrosinated, the lack of a known α-tubulin tyrosine ligase makes it unclear whether microtubules cycle between these two states in flies.
Our results are consistent with the possibility that PTMs may function synergistically rather than independently to regulate microtubule function (Atherton et al., 2013; Hammond et al., 2010; Kaul et al., 2014). In addition, these modifications may be important to preserving microtubule-based functions in aging neurons given that changes in acetylation, detyrosination/tyrosination and polyglutamylation have been implicated in neurodegeneration (Song and Brady, 2015). In support of this idea, we have found that adult αTub84BK40A flies display an age-related deficit in righting behavior (H.L.R., B.V.J., J.W., unpublished data). Our current studies are not an exhaustive analysis of all known modifications of α-tubulin and microtubules. It will be of great interest to determine whether combinations of known modifications or currently uncharacterized modifications contribute to creating a polarized neuron. Proteomic studies have identified additional α-tubulin lysine residues that are acetylated (Choudhary et al., 2009; Liu et al., 2015a,b), raising the possibility that the acetylation of other lysine residues might play an essential role in neuronal development.
MATERIALS AND METHODS
Fly strains
The αTub84BattP-KO strain was created using an ends-out recombination approach (Huang et al., 2009). All αTub84B knock-in strains were made by using standard molecular biology methods to modify αTub84B in a plasmid containing an attB site; the plasmid with the modified αTub84B was then injected into αTub84BattP-KO embryos expressing ΦC31 by BestGene Inc. (Chino Hills, CA). A fly strain with wild-type αTub84B knocked into the locus (αTub84BK'in-WT) rescued the lethality of the αTub84B knockout. α-tubulin protein levels and dendrite branch number were equivalent between αTub84BK'in-WT and wild-type flies. Thus, wild-type flies were used as controls in the experiments. The following alleles and transgenes were used in this study: ppk-CD4::tdGFP, ppk-CD4::tdTomato, ppk-Gal4, UAS-Lamp1::GFP and FutschK68 (Hummel et al., 2000) were obtained from the Bloomington Stock Center (Bloomington, IN); UAS-EB1::GFP (Rolls et al., 2007) from Melissa Rolls (Penn State University, University Park, PA), and UAS-katanin-60 (Mao et al., 2014) from Shan Jin and Yong Q. Zhang (Hubei University, Wuhan, Hubei and Chinese Academy of Sciences, Beijing, China, respectively). ppk-ManII::GFP was created by cloning ManII::GFP (Ye et al., 2007) downstream of the ppk enhancer in the pACUH vector (plasmid 58374, Addgene, Cambridge, MA); ppk-ManII::GFP was integrated at attP VK00037 by BestGene Inc.
Imaging and analysis
Images were acquired on a Leica SP5 laser-scanning confocal microscope, equipped with two standard PMTs and a HyD GaAsP detector, using a 20×0.7 NA oil immersion HC PL APO objective or a 40×1.3 NA oil immersion HCX PL APO objective. Fruit fly larvae were imaged live in a drop of 50% glycerol (catalog number G153-1, Fisher Scientific, Hampton, NH) in phosphate-buffered saline (PBS). The ratio of anti-acetylated microtubules in axons and dendrites was obtained from class IV ddaC neurons in fixed larval fillets stained with anti-acetylated tubulin (6-11-B1, see the Immunohistochemistry section for full details) and horseradish peroxidase (HRP). Acetylated α-tubulin signal in axons and dendrites was traced and captured via line scan analysis in ImageJ/FIJI (NIH) and exported to Excel (Microsoft). For live neuron imaging, larvae were immobilized during imaging by pressure from a coverglass secured by two lines of vacuum grease flanking the animal. EB1::GFP and Lamp1::GFP movies were collected at rates of 1.25 frames per second (f s−1) and 0.5 f s−1 (EB1::GFP), or 1.51 f s−1 (Lamp1::GFP). Kymographs were generated and traced in Metamorph software (Molecular Devices, Sunnyvale, CA), and the data were analyzed in Excel. Velocity (for EB1::GFP and Lamp1) was calculated from the slope of the trajectory traced in Metamorph. The trajectories of lysosomes that changed speed or direction were segmented, and the segments were included in the total tallies. Lamp1 flux describes the number of lysosomes moving within an axon or dendrite segment within an ∼1-min-long movie segment. For analyzing Futsch levels in dendrites and axons, images of neurons in fixed tissue were acquired and then dendrite segments were traced in ImageJ/FIJI using the CD4::GFP signal as a guide. Data from line scans of the CD4::GFP and Futsch signals in the proximal axon and dendrite segments were imported into Excel. We normalized the anti-Futsch (see the Immunohistochemistry section for full details) signal intensity by generating a ratio of Futsch to CD4::GFP. Since the dendrites included for analysis varied somewhat in length, we normalized dendrite length by dividing each dendrite into five segments that represented a percentage of the total dendrite length (e.g. the most proximal segment represented 0–20% of the total length). Dendrite tips were counted by using either Imaris (automated dendrite tip counting following manual adjustments) or Metamorph (manual tip marking) software. Dendrite extension, retraction, and de novo growth were analyzed as previously described (Soba et al., 2015). Briefly, z-stack images of neurons expressing CD4::GFP in larvae at 48 h AEL were acquired 15 min apart. Maximum projections of images taken at both time points were aligned in ImageJ by using the bUnwarpJ plugin and then overlaid in Metamorph. The first image (t=0) was pseudo-colored red and the second image (t=15 min) was pseudo-colored green. Overlaid images were manually scored for red and green tips using Metamorph and Photoshop (Adobe, San Jose, CA), and data were analyzed in Excel (Microsoft). All data were double-blinded before analysis and a portion of data sets were analyzed independently by two people to ensure samples were scored equivalently. Experiments were replicated at least twice.
Immunohistochemistry
Larvae were dissected in PHEM buffer (80 mM PIPES pH6.9, 25 mM HEPES pH 7.0, 7 mM MgCl2 and 1 mM EGTA) and fixed in 4% paraformaldehyde (PFA) in 1× PBS with 3.2% sucrose for 45–60 min. The fixed fillets were then permeabilized in PBS with 0.3% Triton X-100, quenched with 50 mM NH4Cl, and blocked in blocking buffer composed of 2.5% bovine serum albumin (BSA; Sigma catalog number A9647), 0.25% fish-skin gelatin (FSG; Sigma catalog number G7765), 10 mM glycine, 50 mM NH4Cl and 0.05% Triton X-100. The fillets were incubated with primary antibodies overnight at 4°C in blocking buffer. Samples were washed extensively in PBS with 0.1% Triton X-100 and then incubated with secondary antibodies in blocking buffer overnight at 4°C in the dark. After washing, samples were mounted on slides using elvanol with antifade (polyvinyl alcohol, Tris-HCl pH 8.5, glycerol and DABCO, catalog number 11247100, Fisher Scientific, Hampton, NH). Embryos were dechorionated in bleach for 1–2 min, fixed in 4% formaldehyde overlaid with heptane for 20 min, and devitellinized by rapid passage of embryos through a heptane–methanol interface. Embryos were incubated with primary antibodies diluted in PBS with 0.1% Triton X-100 overnight at 4°C and with secondary antibodies for 2.5 h at room temperature (following each antibody incubation step, embryos were washed three times for 20 min each time with PBS with 0.1% Triton X-100 at room temperature). Antibodies used were: anti-acetylated α-tubulin 6-11B-1 antibody (1:1000 or 1 µg ml−1, catalog number T6793, Sigma-Aldrich, St. Louis, MO), anti-polyglutamylated α-tubulin GT335 antibody (1:1000, gift of Carsten Janke, Institut Curie, Paris Sciences et Lettres Research University, Orsay, France), anti-tyrosinated α-tubulin YL1/2 antibody (1:250, or 4 µg ml−1, AbD Serotec MCA77G, Bio-Rad, Hercules, CA), anti-Futsch 22C10 antibody (1:50, Developmental Studies Hybridoma Bank, Iowa City, IA), anti-HRP antibody conjugated to Alexa Fluor 647 (1:1000, or 1.4 µg ml−1, catalog number 123-605-021, Jackson ImmunoResearch, West Grove, PA), Dylight 550-conjugated anti-mouse-IgG antibody (1:1000, or 0.5 µg ml−1, catalog number SA5-10167, ThermoFisher, Waltham, MA).
Immunoblotting
For western blot analysis of tubulin expression, 10 fly heads were homogenized in 30 µl of 1× SDS loading buffer. Lysate from the equivalent of one fly head (3 µl) was loaded into each lane. Proteins were transferred to PVDF membranes (catalog number 162-0177, Bio-Rad, Hercules, CA) overnight and the membranes were stained with Ponceau S (catalog number BP 103-10, Fisher Scientific, Hampton, NH) to check for efficient transfer. Membranes were blocked (5% milk, in TBS with 0.1% Tween-20) for 1–2 h at room temperature and incubated with primary antibody overnight. After washing, membranes were incubated with secondary antibody for 2–4 h at room temperature. The membranes were then imaged with either chemiluminescence (SuperSignal West Pico, catalog number 34077, ThermoFisher, Waltham, MA) or fluorescent imaging by using an Odyssey Imaging System (Li-Cor Biosciences, Lincoln, NE). Fluorescence intensity ratios obtained from the Odyssey were analyzed using FIJI and Excel. Antibodies used were: anti-α-tubulin DM1A (1:1000, or 1 µg ml−1, catalog number T6199, Sigma-Aldrich, St. Louis, MO), anti-acetylated α-tubulin 6-11B-1 (1:10,000, or 0.1 µg ml−1, catalog number T6793, Sigma-Aldrich, St. Louis, MO), anti-tyrosinated α-tubulin (1:1000, 1 µg ml−1, AbD Serotec MCA77G, Bio-Rad, Hercules, CA), anti-polyglutamylated α-tubulin (1:4000, catalog number T9822 Sigma-Aldrich, St. Louis, MO), and anti-actin (1:5000, Chemicon MAB 1501, EMD-Millipore, Billerica, MA).
Statistical analysis
Multiple comparisons were performed using one-way ANOVA with post-hoc two-tailed Student's t-tests between experimentally matched control and mutant samples. Two-tailed Student's t-tests were used to compare two conditions. *P=0.05–0.01; **P=0.01–0.001; ***P=0.0001–0.001; ****P<0.0001; n.s., not significant. Error bars indicate standard deviation.
Supplementary Material
Acknowledgements
We thank Yuh Nung Jan (University of California, San Francisco, CA) for generously supporting the initial stages of this research. We thank Drs Yongqing Zhang and Shan Jin for katanin-60 reagents, the Developmental Studies Hybridoma Bank (created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242) for anti-Futsch 22C10 antibody, the Bloomington Drosophila Stock Center (NIH P40 OD018537) for stocks, Kevin Eliceiri and the Laboratory for Optical and Computational Instrumentation for image analysis support, and the Wickens lab for sharing reagents. We thank Jay Parrish (University of Washington), Melissa Gardner (University of Minnesota), and Wildonger laboratory members for discussion and comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: B.V.J., J.W.; Methodology: B.V.J., J.W.; Validation: B.V.J., H.A.J.S., H.L.R., D.M.J.-S., J.W.; Formal analysis: B.V.J., H.A.J.S., J.W.; Investigation: B.V.J., H.A.J.S., H.L.R., D.M.J.-S., J.W.; Resources: B.V.J., H.A.J.S., H.L.R., D.M.J.-S., J.W.; Data curation: B.V.J., H.A.J.S., H.L.R., J.W.; Writing - original draft: B.V.J., J.W.; Writing - review & editing: B.V.J., H.A.J.S., H.L.R., D.M.J.-S., J.W.; Visualization: B.V.J., H.A.J.S., H.L.R., J.W.; Supervision: B.V.J., J.W.; Project administration: J.W.; Funding acquisition: J.W.
Funding
This work was supported by start-up funds provided by the University of Wisconsin-Madison and grants from the National Institutes of Neurological Disorders and Stroke, National Institutes of Health [grant R00NS072252 and R21NS101553] to J.W. Deposited in PMC for immediate release.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.210203.supplemental
References
- Akella J. S., Wloga D., Kim J., Starostina N. G., Lyons-Abbott S., Morrissette N. S., Dougan S. T., Kipreos E. T. and Gaertig J. (2010). MEC-17 is an alpha-tubulin acetyltransferase. Nature 467, 218-222. 10.1038/nature09324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton J., Houdusse A. and Moores C. (2013). MAPping out distribution routes for kinesin couriers. Biol. Cell 105, 465-487. 10.1111/boc.201300012 [DOI] [PubMed] [Google Scholar]
- Bechstedt S., Lu K. and Brouhard G. J. (2014). Doublecortin recognizes the longitudinal curvature of the microtubule end and lattice. Curr. Biol. 24, 2366-2375. 10.1016/j.cub.2014.08.039 [DOI] [PubMed] [Google Scholar]
- Bobinnec Y., Marcaillou C. and Debec A. (1999). Microtubule polyglutamylation in Drosophila melanogaster brain and testis. Eur. J. Cell Biol. 78, 671-674. 10.1016/S0171-9335(99)80053-3 [DOI] [PubMed] [Google Scholar]
- Bonnet C., Boucher D., Lazereg S., Pedrotti B., Islam K., Denoulet P. and Larcher J. C. (2001). Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J. Biol. Chem. 276, 12839-12848. 10.1074/jbc.M011380200 [DOI] [PubMed] [Google Scholar]
- Boucher D., Larcher J.-C., Gros F. and Denoulet P. (1994). Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein Tau and tubulin. Biochemistry 33, 12471-12477. 10.1021/bi00207a014 [DOI] [PubMed] [Google Scholar]
- Cai D., McEwen D. P., Martens J. R., Meyhofer E. and Verhey K. J. (2009). Single molecule imaging reveals differences in microtubule track selection between Kinesin motors. PLoS Biol. 7, e1000216 10.1371/journal.pbio.1000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti S., Natarajan K., Curiel J., Janke C. and Liu J. (2016). The emerging role of the tubulin code: From the tubulin molecule to neuronal function and disease. Cytoskeleton (Hoboken) 73, 521-550. 10.1002/cm.21290 [DOI] [PubMed] [Google Scholar]
- Chauhan S., Ahmed Z., Bradfute S. B., Arko-Mensah J., Mandell M. A., Won Choi S., Kimura T., Blanchet F., Waller A., Mudd M. H. et al. (2015). Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat. Commun. 6, 8620 10.1038/ncomms9620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V. and Mann M. (2009). Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834-840. 10.1126/science.1175371 [DOI] [PubMed] [Google Scholar]
- Chu C.-W., Hou F., Zhang J., Phu L., Loktev A. V., Kirkpatrick D. S., Jackson P. K., Zhao Y. and Zou H. (2011). A novel acetylation of beta-tubulin by San modulates microtubule polymerization via down-regulating tubulin incorporation. Mol. Biol. Cell 22, 448-456. 10.1091/mbc.E10-03-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C. and Cáceres A. (2009). Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319-332. 10.1038/nrn2631 [DOI] [PubMed] [Google Scholar]
- Coombes C., Yamamoto A., McClellan M., Reid T. A., Plooster M., Luxton G. W. G., Alper J., Howard J. and Gardner M. K. (2016). Mechanism of microtubule lumen entry for the alpha-tubulin acetyltransferase enzyme alphaTAT1. Proc. Natl. Acad. Sci. USA 113, E7176-E7184. 10.1073/pnas.1605397113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creppe C., Malinouskaya L., Volvert M.-L., Gillard M., Close P., Malaise O., Laguesse S., Cornez I., Rahmouni S., Ormenese S. et al. (2009). Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 136, 551-564. 10.1016/j.cell.2008.11.043 [DOI] [PubMed] [Google Scholar]
- Cueva J. G., Hsin J., Huang K. C. and Goodman M. B. (2012). Posttranslational acetylation of alpha-tubulin constrains protofilament number in native microtubules. Curr. Biol. 22, 1066-1074. 10.1016/j.cub.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dompierre J. P., Godin J. D., Charrin B. C., Cordelieres F. P., King S. J., Humbert S. and Saudou F. (2007). Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 27, 3571-3583. 10.1523/JNEUROSCI.0037-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías G. G., Guardia C. M., Britt D. J., Guo X. and Bonifacino J. S. (2015). Sorting of Dendritic and Axonal Vesicles at the Pre-axonal Exclusion Zone. Cell Rep. 13, 1221-1232. 10.1016/j.celrep.2015.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira T., Ou Y., Li S., Giniger E. and van Meyel D. J. (2014). Dendrite architecture organized by transcriptional control of the F-actin nucleator Spire. Development 141, 650-660. 10.1242/dev.099655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber W. B., Jan L. Y. and Jan Y. N. (2002). Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129, 2867-2878. [DOI] [PubMed] [Google Scholar]
- Guardia C. M., Farías G. G., Jia R., Pu J. and Bonifacino J. S. (2016). BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep 17, 1950-1961. 10.1016/j.celrep.2016.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J. W., Huang C.-F., Kaech S., Jacobson C., Banker G. and Verhey K. J. (2010). Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol. Biol. Cell 21, 572-583. 10.1091/mbc.E09-01-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. C. and Ehlers M. D. (2003). Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J. Neurosci. 23, 6188-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. C., Rácz B., Monson E. E., Lin A. L., Weinberg R. J. and Ehlers M. D. (2005). Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48, 757-771. 10.1016/j.neuron.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Howes S. C., Alushin G. M., Shida T., Nachury M. V. and Nogales E. (2014). Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. Mol. Biol. Cell 25, 257-266. 10.1091/mbc.E13-07-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhou W., Dong W., Watson A. M. and Hong Y. (2009). Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl. Acad. Sci. USA 106, 8284-8289. 10.1073/pnas.0900641106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.-F. and Yao T.-P. (2002). HDAC6 is a microtubule-associated deacetylase. Nature 417, 455-458. 10.1038/417455a [DOI] [PubMed] [Google Scholar]
- Hummel T., Krukkert K., Roos J., Davis G. and Klämbt C. (2000). Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron 26, 357-370. 10.1016/S0896-6273(00)81169-1 [DOI] [PubMed] [Google Scholar]
- Ikegami K., Heier R. L., Taruishi M., Takagi H., Mukai M., Shimma S., Taira S., Hatanaka K., Morone N., Yao I. et al. (2007). Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. USA 104, 3213-3218. 10.1073/pnas.0611547104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C. (2014). The tubulin code: molecular components, readout mechanisms, and functions. J. Cell Biol. 206, 461-472. 10.1083/jcb.201406055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinushi-Nakao S., Arvind R., Amikura R., Kinameri E., Liu A. W. and Moore A. W. (2007). Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron 56, 963-978. 10.1016/j.neuron.2007.10.031 [DOI] [PubMed] [Google Scholar]
- Kalebic N., Sorrentino S., Perlas E., Bolasco G., Martinez C. and Heppenstall P. A. (2013). alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat. Commun. 4, 1962 10.1038/ncomms2962 [DOI] [PubMed] [Google Scholar]
- Kaul N., Soppina V. and Verhey K. J. (2014). Effects of alpha-tubulin K40 acetylation and detyrosination on kinesin-1 motility in a purified system. Biophys. J. 106, 2636-2643. 10.1016/j.bpj.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.-W., Li L., Gorbani M., You L. and Yang X.-J. (2013). Mice lacking alpha-tubulin acetyltransferase 1 are viable but display alpha-tubulin acetylation deficiency and dentate gyrus distortion. J. Biol. Chem. 288, 20334-20350. 10.1074/jbc.M113.464792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix B., van Dijk J., Gold N. D., Guizetti J., Aldrian-Herrada G., Rogowski K., Gerlich D. W. and Janke C. (2010). Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 189, 945-954. 10.1083/jcb.201001024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher J. C., Boucher D., Lazereg S., Gros F. and Denoulet P. (1996). Interaction of kinesin motor domains with alpha- and beta-tubulin subunits at a tau-independent binding site. Regulation by polyglutamylation. J. Biol. Chem. 271, 22117-22124. [DOI] [PubMed] [Google Scholar]
- Lee A., Li W., Xu K., Bogert B. A., Su K. and Gao F. B. (2003). Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130, 5543-5552. 10.1242/dev.00792 [DOI] [PubMed] [Google Scholar]
- Lee J., Peng Y., Lin W.-Y. and Parrish J. Z. (2015). Coordinate control of terminal dendrite patterning and dynamics by the membrane protein Raw. Development 142, 162-173. 10.1242/dev.113423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W. and Rosenbaum J. L. (1983). Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J. Cell Biol. 97, 258-263. 10.1083/jcb.97.1.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W. and Rosenbaum J. L. (1985). Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 24, 473-478. 10.1021/bi00323a034 [DOI] [PubMed] [Google Scholar]
- Li L., Wei D., Wang Q., Pan J., Liu R., Zhang X. and Bao L. (2012). MEC-17 deficiency leads to reduced alpha-tubulin acetylation and impaired migration of cortical neurons. J. Neurosci. 32, 12673-12683. 10.1523/JNEUROSCI.0016-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Xiong Y., Li S., Ren Y., He Q., Gao S., Zhou J. and Shui W. (2015a). New HDAC6-mediated deacetylation sites of tubulin in the mouse brain identified by quantitative mass spectrometry. Sci. Rep. 5, 16869 10.1038/srep16869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Xiong Y., Ren Y., Zhang L., He X., Wang X., Liu M., Li D., Shui W. and Zhou J. (2015b). Proteomic profiling and functional characterization of multiple post-translational modifications of tubulin. J. Proteome Res. 14, 3292-3304. 10.1021/acs.jproteome.5b00308 [DOI] [PubMed] [Google Scholar]
- Ly N., Elkhatib N., Bresteau E., Piétrement O., Khaled M., Magiera M. M., Janke C., Le Cam E., Rutenberg A. D. and Montagnac G. (2016). alphaTAT1 controls longitudinal spreading of acetylation marks from open microtubules extremities. Sci. Rep. 6, 35624 10.1038/srep35624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C.-X., Xiong Y., Xiong Z., Wang Q., Zhang Y. Q. and Jin S. (2014). Microtubule-severing protein Katanin regulates neuromuscular junction development and dendritic elaboration in Drosophila. Development 141, 1064-1074. 10.1242/dev.097774 [DOI] [PubMed] [Google Scholar]
- Mao C.-X., Wen X., Jin S. and Zhang Y. Q. (2017). Increased acetylation of microtubules rescues human tau-induced microtubule defects and neuromuscular junction abnormalities in Drosophila. Dis. Model. Mech. 10, 1245-1252. 10.1242/dmm.028316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta H., Greer K. and Rosenbaum J. L. (1986). The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J. Cell Biol. 103, 571-579. 10.1083/jcb.103.2.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K. A. and Kaufman T. C. (1987). Developmental consequences of mutations in the 84B alpha-tubulin gene of Drosophila melanogaster. Dev. Biol. 119, 100-114. 10.1016/0012-1606(87)90211-9 [DOI] [PubMed] [Google Scholar]
- Matthews K. A., Miller D. F. B. and Kaufman T. C. (1989). Developmental distribution of RNA and protein products of the Drosophila alpha-tubulin gene family. Dev. Biol. 132, 45-61. 10.1016/0012-1606(89)90203-0 [DOI] [PubMed] [Google Scholar]
- McKenney R. J., Huynh W., Vale R. D. and Sirajuddin M. (2016). Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J. 35, 1175-1185. 10.15252/embj.201593071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. J., Qi Y., Iovino L., Andolfi L., Guo D., Kalebic N., Castaldi L., Tischer C., Portulano C., Bolasco G. et al. (2016). Acetylated tubulin is essential for touch sensation in mice. Elife 5, e20813 10.7554/eLife.20813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K., Gadadhar S., Souphron J., Magiera M. M. and Janke C. (2017). Molecular interactions between tubulin tails and glutamylases reveal determinants of glutamylation patterns. EMBO Rep. 18, 1013-1026. 10.15252/embr.201643751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B. and Hilliard M. A. (2014). Loss of MEC-17 leads to microtubule instability and axonal degeneration. Cell Rep. 6, 93-103. 10.1016/j.celrep.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirschl J. J., Magiera M. M., Lazarus J. E., Janke C. and Holzbaur E. L. F. (2016). alpha-tubulin tyrosination and CLIP-170 phosphorylation regulate the initiation of dynein-driven transport in neurons. Cell Rep. 14, 2637-2652. 10.1016/j.celrep.2016.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney K. M., Jan L. Y. and Jan Y.-N. (2012). Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron 76, 921-930. 10.1016/j.neuron.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo A., Ackerman B. and Gundersen G. G. (2003). Cell biology (Communication arising): tubulin acetylation and cell motility. Nature 421, 230 10.1038/421230a [DOI] [PubMed] [Google Scholar]
- Park I. Y., Powell R. T., Tripathi D. N., Dere R., Ho T. H., Blasius T. L., Chiang Y.-C., Davis I. J., Fahey C. C., Hacker K. E. et al. (2016). Dual chromatin and cytoskeletal remodeling by SETD2. Cell 166, 950-962. 10.1016/j.cell.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish J. Z., Xu P., Kim C. C., Jan L. Y. and Jan Y. N. (2009). The microRNA bantam functions in epithelial cells to regulate scaling growth of dendrite arbors in drosophila sensory neurons. Neuron 63, 788-802. 10.1016/j.neuron.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G. and Fuller M. T. (1985). Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 101, 2085-2094. 10.1083/jcb.101.6.2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., LeDizet M. and Chang X. J. (1987). Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 104, 289-302. 10.1083/jcb.104.2.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portran D., Schaedel L., Xu Z., Théry M. and Nachury M. V. (2017). Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 19, 391-398. 10.1038/ncb3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff E. C. (1984). Genetics of microtubule systems. J. Cell Biol. 99, 1-10. 10.1083/jcb.99.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed N. A., Cai D., Blasius T. L., Jih G. T., Meyhofer E., Gaertig J. and Verhey K. J. (2006). Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166-2172. 10.1016/j.cub.2006.09.014 [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A. (2015). Intrinsically disordered tubulin tails: complex tuners of microtubule functions? Semin. Cell Dev. Biol. 37, 11-19. 10.1016/j.semcdb.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls M. M., Satoh D., Clyne P. J., Henner A. L., Uemura T. and Doe C. Q. (2007). Polarity and intracellular compartmentalization of Drosophila neurons. Neural Dev. 2, 7 10.1186/1749-8104-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk B. M., Lang C. M., Hogl S., Tahirovic S., Orozco D., Rentzsch K., Lichtenthaler S. F., Hoogenraad C. C., Capell A., Haass C. et al. (2014). The FTLD risk factor TMEM106B and MAP6 control dendritic trafficking of lysosomes. EMBO J. 33, 450-467. 10.1002/embj.201385857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears J. C. and Broihier H. T. (2016). FoxO regulates microtubule dynamics and polarity to promote dendrite branching in Drosophila sensory neurons. Dev. Biol. 418, 40-54. 10.1016/j.ydbio.2016.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida T., Cueva J. G., Xu Z., Goodman M. B. and Nachury M. V. (2010). The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 107, 21517-21522. 10.1073/pnas.1013728107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M., Rice L. M. and Vale R. D. (2014). Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335-344. 10.1038/ncb2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soba P., Han C., Zheng Y., Perea D., Miguel-Aliaga I., Jan L. Y. and Jan Y. N. (2015). The Ret receptor regulates sensory neuron dendrite growth and integrin mediated adhesion. Elife 4, e05491 10.7554/eLife.05491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. and Brady S. T. (2015). Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 25, 125-136. 10.1016/j.tcb.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppina V., Herbstman J. F., Skiniotis G. and Verhey K. J. (2012). Luminal localization of alpha-tubulin K40 acetylation by cryo-EM analysis of fab-labeled microtubules. PLoS ONE 7, e48204 10.1371/journal.pone.0048204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan R., Goellner B., Moreno E., Frank C. A., Hugenschmidt T., Genoud C., Aberle H. and Pielage J. (2015). Hierarchical microtubule organization controls axon caliber and transport and determines synaptic structure and stability. Dev. Cell 33, 5-21. 10.1016/j.devcel.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A., Tsubouchi A., Rolls M. M., Tracey W. D. and Sherwood N. T. (2012). Katanin p60-like1 promotes microtubule growth and terminal dendrite stability in the larval class IV sensory neurons of Drosophila. J. Neurosci. 32, 11631-11642. 10.1523/JNEUROSCI.0729-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H. and Baas P. W. (2010). Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J. Neurosci. 30, 7215-7226. 10.1523/JNEUROSCI.0048-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyk A., Deaconescu A. M., Spector J., Goodman B., Valenstein M. L., Ziolkowska N. E., Kormendi V., Grigorieff N. and Roll-Mecak A. (2014). Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157, 1405-1415. 10.1016/j.cell.2014.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia M., Wandosell F. and Garrido J. J. (2010). Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS ONE 5, e12908 10.1371/journal.pone.0012908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalidou I., Keller C., Kalebic N., Nguyen K. C. Q., Somhegyi H., Politi K. A., Heppenstall P., Hall D. H. and Chalfie M. (2012). Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr. Biol. 22, 1057-1065. 10.1016/j.cub.2012.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima H., Emanuele M., Polenghi A., Esposito A., Vassalli M., Barberis A., Difato F. and Chieregatti E. (2015). HDAC6 and RhoA are novel players in Abeta-driven disruption of neuronal polarity. Nat. Commun. 6, 7781 10.1038/ncomms8781 [DOI] [PubMed] [Google Scholar]
- Valenstein M. L. and Roll-Mecak A. (2016). Graded Control of Microtubule Severing by Tubulin Glutamylation. Cell 164, 911-921. 10.1016/j.cell.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Fernández A., Cabrero J. R., Serrador J. M. and Sánchez-Madrid F. (2008). HDAC6: a key regulator of cytoskeleton, cell migration and cell-cell interactions. Trends Cell Biol. 18, 291-297. 10.1016/j.tcb.2008.04.003 [DOI] [PubMed] [Google Scholar]
- van Dijk J., Rogowski K., Miro J., Lacroix B., Eddé B. and Janke C. (2007). A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol. Cell 26, 437-448. 10.1016/j.molcel.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Walter W. J., Beránek V., Fischermeier E. and Diez S. (2012). Tubulin acetylation alone does not affect kinesin-1 velocity and run length in vitro. PLoS ONE 7, e42218 10.1371/journal.pone.0042218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. and Sheetz M. P. (2000). The C-terminus of tubulin increases cytoplasmic dynein and kinesin processivity. Biophys. J. 78, 1955-1964. 10.1016/S0006-3495(00)76743-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn R. M., Harrison A., Planques V., Robert-Nicoud N. and Wehland J. (1990). Distribution of microtubules containing post-translationally modified alpha-tubulin during Drosophila embryogenesis. Cell Motil. Cytoskeleton 17, 34-45. 10.1002/cm.970170106 [DOI] [PubMed] [Google Scholar]
- Webster D. R. and Borisy G. G. (1989). Microtubules are acetylated in domains that turn over slowly. J. Cell Sci. 92, 57-65. [DOI] [PubMed] [Google Scholar]
- Wilson P. J. and Forer A. (1997). Effects of nanomolar taxol on crane-fly spermatocyte spindles indicate that acetylation of kinetochore microtubules can be used as a marker of poleward tubulin flux. Cell Motil. Cytoskeleton 37, 20-32. [DOI] [PubMed] [Google Scholar]
- Witte H., Neukirchen D. and Bradke F. (2008). Microtubule stabilization specifies initial neuronal polarization. J. Cell Biol. 180, 619-632. 10.1083/jcb.200707042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N., Regan C. L. and Fuller M. T. (1988). Temporal and spatial pattern of differences in microtubule behaviour during Drosophila embryogenesis revealed by distribution of a tubulin isoform. Development 102, 311-324. [DOI] [PubMed] [Google Scholar]
- Xie R., Nguyen S., McKeehan W. L. and Liu L. (2010). Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol. 11, 89 10.1186/1471-2121-11-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Xu D., Yang Z., Huang H. and Cui X. (2013). A single amino-acid substitution at lysine 40 of an Arabidopsis thaliana alpha-tubulin causes extensive cell proliferation and expansion defects. J. Integr. Plant Biol. 55, 209-220. 10.1111/jipb.12003 [DOI] [PubMed] [Google Scholar]
- Xu Z., Schaedel L., Portran D., Aguilar A., Gaillard J., Marinkovich M. P., Théry M. and Nachury M. V. (2017). Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356, 328-332. 10.1126/science.aai8764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalgin C., Ebrahimi S., Delandre C., Yoong L. F., Akimoto S., Tran H., Amikura R., Spokony R., Torben-Nielsen B., White K. P. et al. (2015). Centrosomin represses dendrite branching by orienting microtubule nucleation. Nat. Neurosci. 18, 1437-1445. 10.1038/nn.4099 [DOI] [PubMed] [Google Scholar]
- Ye B., Zhang Y., Song W., Younger S. H., Jan L. Y. and Jan Y. N. (2007). Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130, 717-729. 10.1016/j.cell.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ma C., Delohery T., Nasipak B., Foat B. C., Bounoutas A., Bussemaker H. J., Kim S. K. and Chalfie M. (2002). Identification of genes expressed in C. elegans touch receptor neurons. Nature 418, 331-335. 10.1038/nature00891 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kwon S., Yamaguchi T., Cubizolles F., Rousseaux S., Kneissel M., Cao C., Li N., Cheng H.-L., Chua K. et al. (2008). Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol. Cell. Biol. 28, 1688-1701. 10.1128/MCB.01154-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.