ABSTRACT
The small GTPase Arf4 and the Arf GTPase-activating protein (GAP) ASAP1 cooperatively sequester sensory receptor cargo into transport carriers targeted to primary cilia, but the input that drives Arf4 activation in this process remains unknown. Here, we show, by using frog retinas and recombinant human proteins, that during the carrier biogenesis from the photoreceptor Golgi/trans-Golgi network (TGN) a functional complex is formed between Arf4, the Arf guanine nucleotide exchange factor (GEF) GBF1 and the light-sensing receptor, rhodopsin. Rhodopsin and Arf4 bind the regulatory N-terminal dimerization and cyclophillin-binding (DCB)-homology upstream of Sec7 (HUS) domain of GBF1. The complex is sensitive to Golgicide A (GCA), a selective inhibitor of GBF1 that accordingly blocks rhodopsin delivery to the cilia, without disrupting the photoreceptor Golgi. The emergence of newly synthesized rhodopsin in the endomembrane system is essential for GBF1-Arf4 complex formation in vivo. Notably, GBF1 interacts with the Arf GAP ASAP1 in a GCA-resistant manner. Our findings indicate that converging signals on GBF1 from the influx of cargo into the Golgi/TGN and the feedback from Arf4, combined with input from ASAP1, control Arf4 activation during sensory membrane trafficking to primary cilia.
KEY WORDS: Cilium, Arf GTPases, Sensory receptors, Rhodopsin
Highlighted Article: Here, we show that sensory receptor cargo promotes its intracellular progression by providing input to a specific Arf GEF to activate a cognate Arf directing transport to the cilia.
INTRODUCTION
The Arf family of small G-proteins constitutes a crucial component of the intracellular membrane trafficking machinery. Through the control of lipid metabolism and the recruitment of canonical coat complexes and protein adaptors that recognize and sequester the appropriate membrane cargo, Arf GTPases play a central role in key processes such as the maintenance of the Golgi architecture, progression of cargo through the Golgi complex, as well as the Golgi-to-plasma-membrane targeting that is responsible for the delivery of sensory receptors and their associated complexes to primary cilia (Deretic, 2013; Donaldson and Jackson, 2011; Ezratty et al., 2016; Hilgendorf et al., 2016; Humbert et al., 2012; Schou et al., 2015; Schwarz et al., 2012; Wang and Deretic, 2014; Wright et al., 2011). The distal ciliary membrane of vertebrate retinal rod photoreceptor cells elaborates a unique sensory organelle, the rod outer segment (ROS), which is filled with several thousand membranous disks containing as many as a billion copies of the light receptor rhodopsin (Besharse, 1986). Rhodopsin is directed to cilia through the ciliary targeting signal (CTS) VxPx, which directly binds activated Arf4 at the Golgi/TGN (Deretic et al., 2005; Mazelova et al., 2009; Wang et al., 2012). The importance of this trafficking pathway is underscored by autosomal dominant retinitis pigmentosa (ADRP), a group of blinding diseases that result from mutations in >25 genes. Mutations affecting the rhodopsin CTS VxPx are among the most severe forms of ADRP (Berson et al., 2002). On the other hand, different targeting signals and trafficking mechanisms direct other ROS membrane components to cilia. Cyclic nucleotide-gated (CNG) channel transport relies on the cytoskeletal adaptor ankyrin-G (Kizhatil et al., 2009). Guanylyl cyclase 1 (GC1) and the progressive rod-cone degeneration (PRCD) protein appear to require rhodopsin for their ciliary trafficking (Pearring et al., 2015; Spencer et al., 2016), whereas targeting of the ROS disk rim protein peripherin-2/rds (P/rds, also known as Prph2) requires its C terminus and interactions with SNARE proteins (Salinas et al., 2013; Tam et al., 2004; Zulliger et al., 2015). Defects in P/rds cause retinitis pigmentosa and macular dystrophies (Goldberg et al., 2016), while defects in trafficking of prenylated ROS proteins cause retinitis pigmentosa 2 (Zhang et al., 2014).
Broad dysfunction of ciliary trafficking causes human genetic diseases and syndromic disorders collectively known as ciliopathies (Reiter and Leroux, 2017). The transition zone and basal body multiprotein complexes NPHP-JBTS-MKS and BBS participate in ciliary morphogenesis and gating. These processes are affected by mutations causing nephronophthisis, as well as Joubert, Meckel and Bardet Biedel syndrome, which affect multiple organs, including the eyes (Craige et al., 2010; Datta et al., 2015; Garcia-Gonzalo et al., 2011; Nachury et al., 2010; Sang et al., 2011; Shimada et al., 2017; van Reeuwijk et al., 2011). Intraflagellar transport regulates the entrance and exit of regulatory components and the progression of ciliary cargo, including rhodopsin, through the transition zone (Bhowmick et al., 2009; Eguether et al., 2014; Keady et al., 2011; Krock et al., 2009; Liew et al., 2014; Zhao and Malicki, 2011). These ciliary networks are directly linked to the small GTPase Rab8 and its GEF Rabin8 (also known as Rab3ip) (Bachmann-Gagescu et al., 2011; Chiba et al., 2013; Nachury et al., 2007; Omori et al., 2008), which are crucial regulators of ciliary membrane trafficking (Deretic et al., 1995; Feng et al., 2012; Moritz et al., 2001; Wang and Deretic, 2015; Westlake et al., 2011). Dysfunction of Arf GTPases and their regulators is also a known cause of ciliopathies (Seixas et al., 2013; Wiens et al., 2010; Zhang et al., 2013).
Arf GTPases exert their regulatory function through the cycles of GTP binding and hydrolysis that are regulated by Arf guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), which control their membrane association and signaling pathways through activation cascades and positive-feedback loops (Bui et al., 2009; Casanova, 2007; Jackson and Casanova, 2000; Lowery et al., 2013; Stalder and Antonny, 2013). One of the outstanding questions in the regulation of Arf GTPases is the role of protein cargo in their activation. It has been proposed that the cargo acts upstream of Arf activation, in a manner analogous to the activation of heterotrimeric G-proteins by G-protein-coupled receptors (GPCRs) that serve as their GEFs, upon light or ligand stimulation (Caster et al., 2013). Although multiple Arf GEFs activate Arfs in spatiotemporally restricted manners, it is not clear what signals Arf GEFs recognize in order to activate Arfs. The specific cargo has the capacity to regulate the Arf-dependent recruitment of the protein adaptors (Caster et al., 2013), which suggests that a functional complex between the cargo, the cognate Arf and an Arf GEF likely exists during membrane trafficking. However, currently the evidence for such a complex is absent.
The BIG/GBF family of Golgi-localized large Arf GEFs contains the highly conserved Sec7 domain involved in the nucleotide-exchange activity, surrounded by several conserved domains involved in functional interactions that regulate their activity and membrane association (Bui et al., 2009; Casanova, 2007; Wright et al., 2014). Golgi-localized large Arf GEFs are autoinhibited in solution. Their catalytic activity and membrane association are controlled by cooperative allosteric regulation via coincidence detection by dimerization and cyclophillin-binding (DCB) and homology downstream of Sec7 (HDS) domains, which integrate direct inputs from membranes and multiple activated Arfs and Rabs (Alvarez et al., 2003; Bouvet et al., 2013; McDonold and Fromme, 2014; Monetta et al., 2007; Nawrotek et al., 2016; Richardson and Fromme, 2012; Richardson et al., 2012; Stalder and Antonny, 2013). GBF1 and Arf4 function within the early Golgi, and at the TGN (Ben-Tekaya et al., 2010; Chun et al., 2008; Garcia-Mata et al., 2003; Kawamoto et al., 2002; Mazelova et al., 2009; Nakai et al., 2013; Szul et al., 2005, 2007; Wang et al., 2012; Zhao et al., 2006). At the TGN, GBF1 initiates an Arf activation cascade through direct interactions of Arf4 with the DCB domains of BIG1 (also known as Arfgef1) and BIG2 (also known as Arfgef2) (Lowery et al., 2013). It is thus plausible to hypothesize that GBF1 might function as the Arf4 GEF that activates Arf4 in ciliary receptor targeting.
Although the directed cargo delivery is tightly regulated in all cells, the limited quantity of a specific ciliary cargo often necessitates its overexpression to analyze ciliary transport, thus retinal rod photoreceptors provide a clear advantage for these studies (Pearring et al., 2013; Wang and Deretic, 2014; Wensel et al., 2016). Because of their extensive ROS membrane turnover, amphibian rods have consistently offered a unique model in which biochemical and morphological data can be correlated in a single experimental system for the study of otherwise basic mechanisms underlying ciliary and photoreceptor membrane biogenesis (Besharse, 1986; Hall et al., 1969; Papermaster et al., 1975, 1985; Young, 1967, 1976). Although photoreceptors are highly specialized cells, a recombinant rhodopsin–GFP fusion protein expressed in epithelial cells maintains the restricted ciliary localization, indicating that certain aspects of ciliary transport are highly conserved (Mazelova et al., 2009; Trivedi et al., 2012; Wang et al., 2012; Ward et al., 2011). In this study, we take advantage of the photoreceptor paradigm because of the abundance of rhodopsin transport carriers (RTCs) that mediate Golgi-to-cilia transport in photoreceptors (Deretic and Mazelova, 2009; Wang and Deretic, 2014), and examine the role of the ciliary cargo, rhodopsin, in the recruitment of the Arf GEF GBF1 and the activation of Arf4. We find that GBF1 interacts with rhodopsin at the Golgi/TGN, and that the activity of GBF1 is essential for their interaction and for communication with Arf4, which, in turn, regulates the formation of RTCs and the delivery of rhodopsin to sensory cilia.
RESULTS
The Arf GEF GBF1 is localized at the trans-Golgi, where it interacts with Arf4, the Arf GAP ASAP1 and the ciliary cargo, rhodopsin
To determine which Arf GEF is responsible for the activation of Arf4 in retinal photoreceptor cells, we examined the distribution of GBF1, a candidate Arf GEF that is reported to activate Arf4 in HeLa cells (Lowery et al., 2013). By confocal microscopy, GBF1 exhibited a distribution that differed from that of the cis-Golgi marker GM130 (UniProt Q08379) and closely resembled that of the trans-Golgi marker Rab6 (also known as Rab6a) (Fig. 1A–D, arrows). The pixel colocalization of Rab6 with GBF1 was significantly higher than with GM130 (P=3.42×10−5, n=5 cells) (Fig. 1D). In photoreceptors, Golgi is localized within the myoid region (M) of the rod inner segment (RIS), as schematically presented in Fig. 1E. To pinpoint the localization of GBF1 within the Golgi, we performed in situ proximity ligation assay (PLA), a molecular technique suitable for proteomic analysis, because a positive signal is possible only when the fluorescent PLA probes are <40 nm apart (Raykova et al., 2016; Söderberg et al., 2006). We employed PLA modified for studies of brain and retinal tissue (Blasic et al., 2012; Trifilieff et al., 2011; Wang and Deretic, 2015; Wang et al., 2012; Zulliger et al., 2015). GBF1-Rab6 interaction sites (Fig. 1F, red dots), aligned well with the trans-Golgi, which was identified post-PLA by staining with anti-Rab6 conjugated to Alexa Fluor 488 (Fig. 1F, green). No interaction sites were detected between GBF1 and the cis-Golgi markers GM130 (Fig. 1G) and p115 (UniProt O60763) (Fig. 1H), despite the robust Golgi labeling with the antibody to p115 (Fig. 1I). Thus, in photoreceptor cells, GBF1 does not associate with the cis-Golgi but is specifically localized at the trans-Golgi.
Fig. 1.
The Arf GEF GBF1 is localized at the trans-Golgi and interacts with Arf4, the Arf GAP ASAP1 and the ciliary cargo, rhodopsin. (A–D) Retinas were labeled with rabbit anti-GBF1 and mouse anti-GM130, followed by Cy3- and Cy5-conjugated secondary antibodies, followed by rabbit anti-Rab6 conjugated to Alexa Fluor 488. An individual optical section is shown. G, Golgi; N, nucleus. Scale bar: 3 µm. GBF1 (red) overlaps with the trans-Golgi marker Rab6 (green) (arrows), significantly better than with the cis-Golgi marker GM130 (blue), as per pixel colocalization analysis performed within the Golgi and expressed as the Pearson's coefficient (***P=3.42×10−5) (n=5 cells). (E) Schematic of a photoreceptor cell. The ROS is an elaborate primary cilium. Golgi and the TGN are localized in the myoid region (M) of the RIS. RTCs bud from the TGN and travel to the cilium (arrow), through the ellipsoid region (E) packed with mitochondria. Adherens junctions (AJ) form the outer limiting membrane (OLM) throughout the retina. (F) GBF1+Rab6 interaction sites (red dots) detected by PLA using mouse (m) anti-GBF1 and rabbit (r) anti-Rab6. Following the detection of interaction sites by PLA (arrows), sections were subsequently stained with an antibody against Rab6 conjugated to Alexa Fluor 488 (green). Nuclei were stained with TO-PRO-3 (blue). (G,H) PLA for GM130(m)+GBF1(r) (G) and P115(m)+GBF1(r) (H). (I) Golgi staining with Rab6(Alexa Fluor 488), p115(r) and GM130(m). (J–M) PLA for GBF1(m)+Arf4(r) (J), rhodopsin(m)+GBF1(r) (K), rhodopsin(m)+Arf4(r) (L) and ASAP1(m)+GBF1(r) (M). Scale bar: 5 µm. (N) Red dots were counted for the PLA pairs shown in panels J–M (30 cells each) in three separate experiments. The data from a representative experiment were expressed as a percentage of total interaction sites within the RIS, analyzed using Student's t-test (n=30) and presented as mean±s.e.m. (O) PNS (0.1 retina), or T/G/E, RTC and cytosolic fractions (0.25 retina each), were analyzed by immunoblotting (IB), as indicated. All antibodies were tested on a single blot.
Next, we determined that, in the RIS, GBF1 and Arf4 interact in close proximity to the trans-Golgi (Fig. 1J, red dots), identified by Rab6, as above (Fig. 1J, green). Notably, within the same area, GBF1 also interacted with rhodopsin (Fig. 1K). The distribution of these interaction sites was comparable to the distribution of rhodopsin-Arf4 interaction sites (Fig. 1L), as noted previously (Wang et al., 2012). Unexpectedly, GBF1 also interacted with the Arf GAP ASAP1, which is known to form a complex with rhodopsin and Arf4 (Wang et al., 2012). As shown in Fig. 1M, GBF1-ASAP1 interactions were not restricted to the Golgi area, but were distributed throughout the RIS. To quantify the number of protein-protein interaction sites in these experiments, the red fluorescent signals detected by PLA in the Golgi area of the myoid region were assigned to the Golgi/TGN and those in the ellipsoid region (E) to RTCs, as described (Wang et al., 2012). The quantitative analysis revealed that interactions of rhodopsin with GBF1 and Arf4 occur nearly exclusively at the Golgi/TGN, whereas GBF1-ASAP1 interactions occur at the Golgi/TGN and on RTCs (Fig. 1N).
To further characterize the subcellular localization of GBF1, we performed retinal subcellular fractionation by a standard procedure (Deretic and Mazelova, 2009), generating ROS and retinal postnuclear supernatant (PNS), highly enriched in photoreceptor biosynthetic membranes (Deretic and Papermaster, 1991; Papermaster et al., 1975). PNS was separated into three fractions designated as the Golgi/TGN/ER-enriched, RTC-enriched (T/G/E and RTCs hereafter) and cytosol, as previously reported (Deretic, 2000; Deretic et al., 1995, 1996; Mazelova et al., 2009; Morel et al., 2000; Wang et al., 2012). In agreement with microscopy data, GBF1 was present in the T/G/E fraction, on RTCs and in the cytosol (Fig. 1O), paralleling the known distribution of the Arf GAP ASAP1 (Mazelova et al., 2009) (Fig. 1O). Arf4 was detected only in the T/G/E fraction and in the cytosol, as previously determined (Mazelova et al., 2009; Wang et al., 2012) (Fig. 1O). Subcellular fractionation corroborated the PLA data and revealed that despite the lack of Arfs (Mazelova et al., 2009; Wang et al., 2012), both the Arf GEF GBF1 and the Arf GAP ASAP1 also associate with RTCs.
Golgicide A, a selective inhibitor of GBF1, significantly disrupts rhodopsin-GBF1-Arf4-ASAP1 interactions
To determine whether the activity of GBF1 affects its interactions with the ciliary cargo, Arf4 and ASAP1, we inactivated GBF1 in cultured eyecups with Golgicide A (GCA) for 3 h. GCA is a selective inhibitor of GBF1 that has no effect on other Arf GEFs, owing to the unique conformation of the nucleotide-binding pocket of GBF1 (Sáenz et al., 2009). In control retinas, GBF1 interactions were detected around the Golgi/TGN (Fig. 2A,C), as before. Rhodopsin-Arf4 interactions were detected at the Golgi (Fig. 2B, arrows). ASAP1 interactions with rhodopsin and GBF1 were detected at the Golgi and on RTCs (Fig. 2D,E, arrows). GCA treatment greatly diminished rhodopsin-GBF1, rhodopsin-Arf4, Arf4-GBF1 and rhodopsin-ASAP1 interactions (Fig. 2F–I), but had minimal effect on GBF1-ASAP1 interactions (Fig. 2J). In GCA-treated retinas, rhodopsin-ASAP1 interactions were diminished both at the Golgi and on RTCs, and the remaining interaction sites were observed along the myoid-ellipsoid border (Fig. 2I, arrows), at an unusual location not seen in the controls. These data suggest that GCA affected both the formation of nascent RTCs at the Golgi/TGN, and the cilia-directed trafficking of RTCs formed before the addition of GCA. The activity of GBF1 was essential for rhodopsin-GBF1-Arf4-ASAP1 communications, as GCA caused a significant decrease in their interaction sites (P<2.2×10−6) (Fig. 2K–N). By contrast, GBF1-ASAP1 interactions were unaffected (Fig. 2O). From the comparable number and distribution of rhodopsin interaction sites with GBF1 and Arf4 detected by PLA at the Golgi/TGN, we conclude that the specific complex between the cargo, the cognate Arf and the Arf GEF may be formed there.
Fig. 2.
GCA, a selective inhibitor of GBF1, significantly disrupts rhodopsin-GBF1-Arf4-ASAP1 interactions. (A) Rhodopsin(m)+GBF1(r) interaction sites (red dots) detected by PLA in control retinas. Retinal sections were visualized by DIC. (B–E) The same was repeated for rhodopsin(m)+Arf4(r) (B), GBF1(m)+Arf4(r) (C), rhodopsin(m)+ASAP1(r) (D) and ASAP1(m)+GBF1(r) (E). (F–J) PLA of GCA-treated retinas for rhodopsin(m)+GBF1(r) (F), rhodopsin(m)+Arf4(r) (G), GBF1(m)+Arf4(r) (H), rhodopsin(m)+ASAP1(r) (I) and ASAP1(m)+GBF1(r) (J). Scale bar: 5 µm. (K–O) Experiments were repeated three times and >10 Z-stacks containing ≥10 confocal optical sections were generated for each PLA pair. From these Z-stacks, two representative confocal sections encompassing at least five photoreceptors with clearly visible RIS demarcations were selected from each experiment. Interaction sites were counted (10 photoreceptors×3 experiments) for each PLA pair, analyzed using Student's t-test (n=30) and presented as in Fig. 1 (***P<2.2×10−6).
GCA minimally affects the morphology of photoreceptor Golgi
Given the reported disassembly of the Golgi by GCA (Lowery et al., 2013; Sáenz et al., 2009), we examined the state of the Golgi complex in GCA-treated retinas. Surprisingly, in photoreceptor cells, GCA had minimal effect on the Golgi morphology. Both cis-Golgi, identified by GM130 staining, and trans-Golgi, identified by Rab6, were largely unchanged in GCA-treated retinas (Fig. 3A). By contrast, Brefeldin A (BFA), a noncompetitive inhibitor of Golgi Arf GEFs (Peyroche et al., 1999), caused substantial swelling and perturbation of the photoreceptor Golgi, as previously described (Deretic and Papermaster, 1991; Mazelova et al., 2009) (Fig. 3A). Thus, in all probability, the Golgi organization in photoreceptors is not controlled by GBF1, but by another BFA-sensitive Arf GEF, very likely to be BIG1 (Boal and Stephens, 2010), which is detected in the mouse retinal transcriptome at a similar level to GBF1 (Brooks et al., 2011).
Fig. 3.
GBF1 regulates Golgi-to-cilia transport of rhodopsin. (A) Control, GCA- and BFA-treated retinas were labeled with antibody to Rab6(r) (green) and GM130(m) (red). Scale bars: 5 µm (1 µm in inset). (B) Isolated retinas were incubated in the presence or absence of GCA during the pulse-chase experiment. Following treatment, T/G/E, RTC and ROS fractions were analyzed by SDS-PAGE and autoradiography. (C) Autoradiogram of the gel shown in B. (D) Radiolabeled rhodopsin was quantified in three separate experiments. The data were analyzed using Student's t-test (n=3) and presented as mean±s.e.m. (*P=0.01). (E) Control and GCA-treated retinas were labeled with anti-Rab6 (green), GBF1 (red) and GM130 (blue), as in Fig. 1. Rab6 and GBF1 colocalize (arrows) in control and GCA-treated retinas. Labeling with anti-GBF1(m) and anti-ASAP1(r) in control and GCA-treated retinas shows Golgi and RTC colocalization (arrows), whereas colocalization detected with anti-GBF1(m) and anti-Arf4(r) in controls is lost upon GCA treatment (arrows). Scale bar: 5 µm. (F) Following the pulse-chase experiment, subcellular fractions of control or GCA-treated retinas were separated by SDS-PAGE and immunoblotted as indicated. All antibodies were tested on a single blot. ASAP1 detected in the crude ROS fraction originates from a minor contamination with RIS proteins. (G) The distribution of GBF1, Arf4 and ASAP1 in the T/G/E fraction, RTCs and the cytosol was quantified in three separate experiments and presented as mean±s.e.m.
The activity of GBF1 is necessary for the Golgi export of ciliary cargo
Because the activity of GBF1 is important for its interactions with Arf4 and the ciliary cargo at the Golgi, we asked whether it is also necessary for ciliary trafficking. We performed pulse-chase experiments in isolated retinas using established methodology: following a 1 h pulse and a 2 h chase, photoreceptor ER membranes are cleared of newly synthesized proteins and radiolabeled rhodopsin localizes in the Golgi/TGN, RTCs and the ROS, with the kinetics paralleling its trafficking in vivo (Deretic and Papermaster, 1991; Mazelova et al., 2009). We followed the progression of radiolabeled proteins through the biosynthetic membranes separated on sucrose density gradients in control retinas, or in the continuous presence of GCA. At the GCA concentration tested, the majority of the newly synthesized rhodopsin was arrested in the T/G/E fraction and its delivery to the ROS was significantly inhibited (P<0.01) (Fig. 3B–D). Based on its uniform molecular weight in all fractions, we determined that, in the presence of GCA, newly synthesized rhodopsin had left the ER and reached the Golgi, where it was terminally glycosylated. This is in contrast to BFA-induced trafficking disruption, which also affects oligosaccharide trimming (Deretic and Papermaster, 1991). Furthermore, GCA did not alter the Golgi localization of GBF1 (Fig. 3E, arrows), in line with the report that in GCA-treated cells, GBF1 remains on the Golgi membranes (Lowery et al., 2013). Consistent with the preservation of interactions between GBF1 and ASAP1 detected in Fig. 2, their colocalization was unaltered by GCA (Fig. 3E, arrows). By contrast, colocalization between Arf4 and GBF1 was disrupted by GCA treatment (Fig. 3E, arrows), in accord with the absence of a PLA signal observed in Fig. 2. Finally, GCA treatment had minimal effect on the distribution of GBF1, Arf4 and ASAP1 among retinal subcellular fractions (Fig. 3F,G). Because GCA significantly slowed down the exit of rhodopsin while maintaining the Golgi structure and localization of key associated proteins, we conclude that in retinal photoreceptors the activity of GBF1 is necessary for the Golgi export of ciliary cargo.
GBF1 directly interacts with Arf4 and the ciliary cargo, rhodopsin
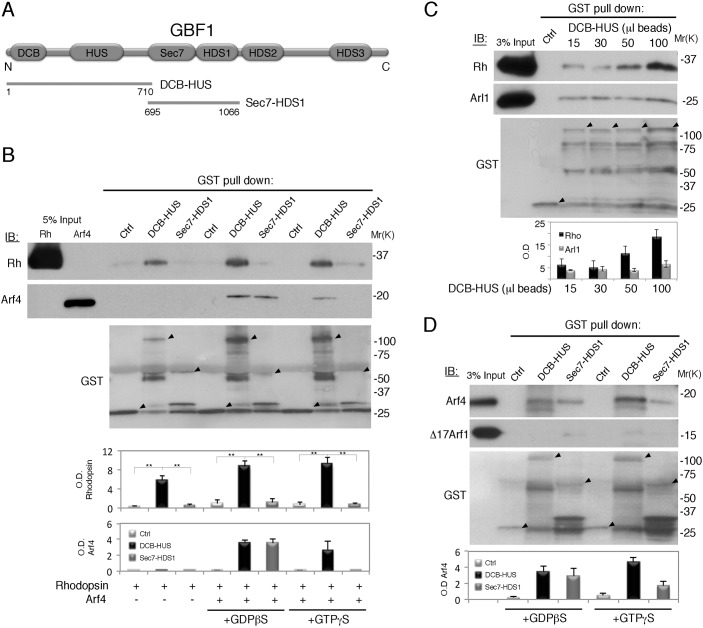
In addition to the catalytic Sec7 domain, GBF1 contains a DCB domain, a homology upstream of Sec7 (HUS) domain and three HDS domains (Bui et al., 2009; Mouratou et al., 2005). To determine whether the binding of GBF1 to rhodopsin is direct, we employed human DCB-HUS [amino acids (AA) 1–710] and Sec7-HDS1 (AA 695–1066), fused to glutathione S-transferase (GST) (Bouvet et al., 2013) (Fig. 4A). GST fusion proteins were incubated with purified bovine rhodopsin, with or without recombinant human Arf4 preloaded with GTPγS or GDPβS. GST DCB-HUS pulled down rhodopsin significantly better than GST Sec7-HDS1 or GST alone (P<0.005), both in the presence and absence of Arf4, demonstrating a direct cargo-GBF1 interaction (Fig. 4B). GST DCB-HUS pulled down Arf4, bound to GTPγS or GDPβS, whereas Sec7-HDS1 preferably interacted with GDPβS-bound Arf4. To ascertain that GBF1 DCB-HUS is properly folded and binds rhodopsin specifically, we used Arl1 as a negative control, as its binding to the DCB domain is conserved in BIG1 and BIG2, but not in GBF1 (Christis and Munro, 2012; Galindo et al., 2016). We incubated purified bovine rhodopsin, or Arl1Q71L, with increasing amounts of GBF1 DCB-HUS bound to glutathione beads. Rhodopsin binding robustly increased with the increase of DCB-HUS beads, in contrast to the barely detectable increase in nonspecific Arl1 binding (Fig. 4C). To examine the specificity of GBF1 interaction with Arf GTPases, we compared the full-length Arf4 to Δ17Arf1, a truncated construct in which the N-terminal α-helix of Arf1 was removed to facilitate nucleotide loading in the absence of membranes (Randazzo et al., 1995). Unlike Arf1, Arf4 does not require myristoylation and membranes for activation (Chun et al., 2008; Duijsings et al., 2009); therefore, full-length Arf4 was preloaded with GTPγS or GDPβS. GBF1 is known to co-precipitate and activate both Arf1 and Arf4 in vivo (Szul et al., 2007). However, GST DCB-HUS pulled down only Arf4 (Fig. 4D), whereas Δ17Arf1 did not show above background binding (Fig. 4D). Although these data point to the specificity of Arf4 binding to the DCB-HUS domain of GBF1, they could be attributed to the absence of the N-terminal helix of Arf1, which, although different from Arf4 (Duijsings et al., 2009), might still have the ability to interact with the DCB-HUS of GBF1. Lack of strong discrimination between the nucleotide-bound states of Arf4 by GST DCB-HUS indicates that the contact surface on Arf4 does not undergo conformational changes upon nucleotide binding.
Fig. 4.
GBF1 directly interacts with Arf4 and the ciliary cargo, rhodopsin. (A) Schematic of GBF1. DCB-HUS (AA 1–710) and Sec7-HDS1 (AA 695–1066) are indicated. (B) GST-DCB-HUS, GST-Sec7-HDS1 or GST were incubated with purified bovine rhodopsin, with or without recombinant human Arf4 bound to GDPβS or GTPγS. Rhodopsin and Arf4 were detected by immunoblotting. The GST fusion proteins were detected with anti-GST antibody. Arrowheads point to the GST fusion proteins used in pulldowns. Breakdown products of GBF1 DCB-HUS were also observed by Galindo et al. (2016). Sec7-HDS1 is partially obscured by the BSA present in all samples. Rhodopsin and Arf4 were quantified in three separate experiments. The data were analyzed using Student's t-test (n=3) and presented as mean±s.e.m. (**P<0.005). (C) Comparable amounts of bovine rhodopsin and human Arl1Q71L were subjected to pulldowns by GST-DCB-HUS. Bound proteins and GST fusion proteins were detected by specific antibodies. Rhodopsin and Arl1 were quantified in two separate experiments and presented as mean±range (for two experiments). (D) GST pulldown of human Arf4, or Δ17Arf1, bound to GDPβS or GTPγS. Bound Arfs and GST fusion proteins were detected by immunoblotting, and Arf4 was quantified in three separate experiments and presented as mean±s.e.m.
Rhodopsin transiting the RIS provides a signal crucial to Arf4 interaction with GBF1
A key step in the assembly of the ciliary-targeting complex is the binding of rhodopsin to activated Arf4 at the TGN (Mazelova et al., 2009). We thus wanted to test whether the influx of rhodopsin plays a role in the activation of Arf4. For this purpose, we treated retinas with cycloheximide, which essentially abolished rhodopsin-GBF1, rhodopsin-Arf4 and Arf4-GBF1 interactions (Fig. 5A–C,E–G), but, like GCA, had minimal effect on GBF1-ASAP1 interactions (Fig. 5D,H). A significant decrease in interaction sites detected by PLA in the RIS (P<2.0×10−13) indicates that not only rhodopsin interactions, but also the Arf4-GBF1 interaction, were contingent upon the presence of ciliary-targeted cargo in biosynthetic membranes (Fig. 5I). A parallel pulse-chase experiment confirmed a near complete inhibition of protein synthesis by a 3 h treatment with cycloheximide (Fig. 5J,L). Subcellular fractionation showed that cycloheximide minimally affected the intracellular distribution of Arf4 and ASAP1 (Fig. 5K). Cycloheximide did not alter the localization of GBF1 and Arf4 (Fig. 5M), but completely depleted rhodopsin from the RIS endomembrane system (Fig. 5N,O). Although it is formally possible that the depletion of proteins other than rhodopsin might have contributed to the observed effects of cycloheximide on Arf4-GBF1 interactions, this is highly unlikely considering that rhodopsin represents as much as 90% of the ROS membrane protein, has by far the fastest turnover in the retina, and is the major protein synthesized and transported to the cilia of photoreceptor cells (Brooks et al., 2011; Deretic and Papermaster, 1991; Hall et al., 1969; Papermaster et al., 1975, 1985; Papermaster and Dreyer, 1974). The dominance of rhodopsin is also evident in Fig. 5L, where only a couple of other radiolabeled proteins are clearly detected in the autoradiogram. They most likely correspond to the subunits of the next most abundant photoreceptor protein, the heterotrimeric G-protein transducin, which is activated by rhodopsin in the ROS upon light stimulation. Fig. 5P schematically represents molecular interactions between the cargo, rhodopsin, the cognate Arf, Arf4, and the Arf GEF GBF1 during the formation of nascent RTCs from the TGN, consistent with the results of our study.
Fig. 5.
Influx of rhodopsin provides a signal crucial to Arf4 interaction with GBF1. (A–H) PLA was performed to detect various interaction sites in control (A,B,C,D) and cycloheximide-treated (E,F,G,H) retinas: rhodopsin(m)+GBF1(r) (A,E), rhodopsin(m)+Arf4(r) (B,F), GBF1(m)+Arf4(r) (C,G) and ASAP1(m)+GBF1(r) (D,H). Scale bar: 5 µm. (I) Interaction sites were analyzed and presented as in Fig. 2 (***P<2.0×10−13). (J) Isolated retinas were incubated in the presence or absence of cycloheximide. Following a pulse-chase experiment, T/G/E, RTCs, ROS and cytosol were analyzed by SDS-PAGE. (K) Distribution of Arf4 and ASAP1 among subcellular fractions in control and cycloheximide-treated cells was determined by immunoblotting, as indicated. (L) Autoradiogram of the gel shown in J. (M) Golgi localization (arrows) of GBF1 (red) and Arf4 (green) in control and cycloheximide-treated retinas. (N,O) Retinas were labeled with antibody to Rab6(r) (green) and rhodopsin(m) (red). Arrows indicate Golgi-localized rhodopsin in the control (N), but not in cycloheximide-treated retinas (O). Scale bars: represents 8 µm for M–O; 5 µm for insets in N and O. (P) Schematic of the apparent sequence of events in ciliary trafficking leading to the formation of the complex consisting of the cargo, the cognate Arf and an Arf GEF. Cytosolic Arf4 becomes membrane associated and activated, either through random activation events, or by an Arf-GEF. Through interactions with Rab6, GBF1 is positioned at the trans-Golgi membranes, where it associates with rhodopsin and Arf4. This process is inhibited by cycloheximide, which chiefly blocks the influx of rhodopsin into the endomembrane system. GBF1, stimulated by the cargo and Arf4 binding to the regulatory DCB-HUS domain, quickly builds up levels of active Arf4, a process inhibited by GCA. Activated Arf4 recognizes and directly binds to the VxPx C-terminal signal of incoming rhodopsin, which leads to the assembly of the ciliary targeting complex, starting with the Arf GAP ASAP1. GTP hydrolysis on Arf4, catalyzed by ASAP1, releases inactive Arf4 into the cytosol and directs rhodopsin into the nascent RTCs that contain both ASAP1 and GBF1.
DISCUSSION
In this study, we provide evidence for the role of protein cargo in the regulation of Arf GTPases in vertebrate rod photoreceptors by establishing the existence of a functional complex between the ciliary cargo, rhodopsin, the cognate Arf, Arf4, and the Arf GEF GBF1. Although ciliary transport is highly conserved, our present study reveals a particular adaptation of photoreceptor cells that are synthesizing and transporting considerable amounts of rhodopsin-containing membranes, through apparent concentration of the Arf GEF GBF1 at the Golgi exit where it senses emergence of the cargo essential for ciliary biogenesis. Our study also broadly implicates the protein cargo in promoting its progression through the endomembrane system by providing input to a specific Arf GEF to activate a cognate Arf directing cargo transport to its correct subcellular location.
The Arf GEF GBF1 activates Arf4, which was initially identified as an essential factor for the generation of ciliary-targeted post-Golgi carriers (RTCs) via interaction with the VxPx CTS of rhodopsin (Deretic et al., 2005). Recent in vivo studies of trafficking of rhodopsin fused to the photoconvertible fluorescent protein Dendra2 in Xenopus photoreceptors showed that the VxPx motif enhances ciliary targeting ≥10-fold and accelerates trafficking of post-Golgi vesicular structures (Lodowski et al., 2013), most likely acting through Arf4. In further support of Arf4 function in ciliary trafficking, the reduction in Arf4 also caused a delay in delivery of ciliary sensory receptor fibrocystin from the Golgi to the cilium (Follit et al., 2014). In photoreceptors, the Arf4-based complex forms in sequential order at the TGN and includes the Arf GAP ASAP1, the Rab8 GEF Rabin8, Rab11 (also known as Rab11a), and the Arf/Rab11 effector FIP3 (also known as Rab11fip3) (Mazelova et al., 2009; Wang and Deretic, 2015; Wang et al., 2012). The notion that membrane-targeting modules assemble through multiple weak interactions that create high-avidity complexes was recently reinforced by crystallization and analysis of the Rab11–FIP3–Rabin8 dual effector complex (Vetter et al., 2015).
A partially redundant role for Arf1 and Arf4 in intracellular trafficking was proposed based on double knockouts, which caused tubulation and vesiculation of the Golgi and defects in HeLa cells (Volpicelli-Daley et al., 2005). However, a recent study revealed not only similarities but also many differences between Arf1 and Arf4 (Christis and Munro, 2012). Arf1 and Arf4 showed similar interactions with COP I coat proteins and GM130 from insect cell extracts, but Arf4 preferentially bound Rab6 and Rab11, the two Rabs known to be involved in trafficking of both Drosophila and vertebrate rhodopsin (Deretic and Papermaster, 1993; Mazelova et al., 2009; Satoh et al., 2005; Shetty et al., 1998). A more specific role for Arf4 in directing transport out of the Golgi complex is further substantiated by its binding to GMAP210, which is implicated in ciliary trafficking in photoreceptors (Follit et al., 2008; Keady et al., 2011), and a ninefold higher interaction with Src, a kinase that regulates Golgi exit (Pulvirenti et al., 2008) and phosphorylates the Arf4 GAP ASAP1 (Brown et al., 1998).
Our earlier study in transgenic frogs showed that the Arf4I46D mutant, deficient in GTP hydrolysis by ASAP1, caused dysfunctional rhodopsin trafficking and rapid retinal degeneration (Mazelova et al., 2009). Nevertheless, the role of Arf4 in rhodopsin trafficking in mouse retinas has been brought into question by monitoring morphology of photoreceptors in a conditional knockout mouse (Pearring et al., 2017). Using a mouse model system with low demands on membrane trafficking volumes, the authors reported that the absence of Arf4 caused no mislocalization of rhodopsin, as evidenced by the morphological appearance of the published data. However the data are difficult to interpret as no quantification of rhodopsin localization was performed, although mild mislocalization was evident. Notably, in the same mouse, in cells with high volumes of cargo and membrane transiting through the secretory pathway, such as the exocrine pancreas, the absence of Arf4 caused a major phenotype. The most likely explanation for the data showing that mouse photoreceptors lacking Arf4 appear to deliver rhodopsin to the ROS is that the compensatory mechanisms, probably involving Arf1, which interacts with many of the same proteins (Christis and Munro, 2012), allow the process to proceed, perhaps at a suboptimal level. Over time, an Arf4 knockout retina might prove to be more susceptible to light damage and other stress leading to slow retinal degeneration, as Arf4 is also implicated in the signaling pathway mediating Golgi stress response (Reiling et al., 2013).
There are four issues of significance when comparing the data from the two published Arf4 in vivo models. (1) The absence of a gene versus a dominant-negative action often has different effects ex vivo (in cellular models) and in vivo e.g. even retinas of a rhodopsin hypomorph mouse develop normally, rods elaborate ROS of normal size, and retinas look identical to controls at P41, whereas comparable expression of a dominant negative mutant affecting the VxPx CTS of rhodopsin causes retinal degeneration modeling ADRP (Concepcion and Chen, 2010; Concepcion et al., 2002; Humphries et al., 1997; Lem et al., 1999; Li et al., 1996). (2) The volume of membrane trafficking in the frog eye exceeds by an order of magnitude that of the rodent rods. Xenopus and Rana photoreceptors synthesize and transport ∼3 µm2 and ∼1.5 µm2 of membrane per minute, respectively, versus 0.1 µm2 synthesized by rodent photoreceptors (Besharse, 1986). Additionally, owing to their larger size, light-sensing membranes in amphibians contain 6×104 molecules of rhodopsin versus 2000 molecules of rhodopsin in rats. (3) Mouse models do not always recapture retinal membrane trafficking disease phenotype e.g. despite a relatively faithful manifestation of the hearing and balance disorders found in Usher syndrome, none of the Usher 1 mouse models undergo retinal degeneration (Williams, 2008). (4) Neither the frog nor mouse models are faithful representations of the human eye, but they are useful when dissecting disease-related processes. The frog, by magnifying the role of trafficking through its high volumes of membrane and cargo synthesis and vectorial transport, allows us to dissect the stages and molecular machineries involved. The mouse has its own advantages, and it would be of interest to follow up on the absence of, or only very mild morphological change as discussed above, by generating a knock-in mouse with a dominant-negative mutant to assess the role of Arf4 in this particular model.
The cognate-activating Arf GEFs principally control the membrane association of Arfs (Bui et al., 2009; Casanova, 2007; Nawrotek et al., 2016; Stalder and Antonny, 2013), but their membrane recruitment also involves protein interactions that include SNAREs and the ciliary cargo (Honda et al., 2005; Mazelova et al., 2009). Initially, Arf1 weakly associates with membranes through the N-terminal myristoyl group, but GEF activation and GTP binding cause a conformational transition, termed the ‘myristoyl switch’, which tightly couples Arf1 activation with stable membrane association (Antonny et al., 1997; Franco et al., 1996; Goldberg, 1998; Pasqualato et al., 2002; Randazzo et al., 1995). Activation of Arf1 by Arf GEF Sec7 is amplified by the DCB and HUS regulatory domains that form a single compact helical structural unit, which facilitates membrane insertion of the Arf1 amphipathic N-terminal helix (Richardson et al., 2016). By contrast, GDP-bound Arf4 and Arf5 stably associate with membranes independently of GBF1 (Chun et al., 2008). The membrane-binding properties of Arf4 and Arf5 that differ from those of Arf1 and Arf3 are mediated by the N-terminal amphipathic helix and a class-specific residue in the conserved interswitch domain (Duijsings et al., 2009). Our study suggests that unique properties of Arf4 and its N-terminal amphipathic helix could be responsible for GBF1 DCB-HUS interactions that involve both GDP and GTP-bound Arf4. If activation and membrane association are uncoupled in class II Arfs, then multiple, random activation events at the Golgi/TGN can create a small number of active Arf4 clusters. Through the operation of an autocatalytic amplification mechanism (positive feedback) (Jackson, 2014), these may serve to quickly build up levels of active Arf4 that recognizes and directly binds to the VxPx C-terminal signal of the incoming ciliary cargo, such as rhodopsin, leading to the assembly of the ciliary-targeting complex (Mazelova et al., 2009). Similar molecular interactions might be involved in epidermal differentiation, as Arf4 recognizes the VxPx motif of presenilin-2 and regulates its localization to basal bodies/cilia to modulate Notch signaling (Ezratty et al., 2016).
Further studies will be necessary to determine whether the GBF1-ASAP1 interaction results in reciprocal changes in their catalytic activity to modulate the location and the duration of Arf4 signaling. However, they will require a new paradigm: the comprehensive analysis of full-length Arf GEFs to reveal aspects of their regulation and functions that cannot be identified by using isolated domains and truncated proteins as employed thus far. Nevertheless, given the remarkably high conservation of the GEF and GAP cascades that regulate the ordered recruitment and activation of small GTPases (Deretic, 2013; Mizuno-Yamasaki et al., 2012; Stalder and Antonny, 2013), our finding that the prototypical ciliary membrane receptor rhodopsin might promote its transport from the Golgi to the primary cilium through Arf GEF-cognate-Arf interaction implies that other membrane cargo may also promote its progression through the endomembrane system through hierarchical interactions with the highly conserved functional GTPase networks.
MATERIALS AND METHODS
Materials
GST DCB-HUS (AA 1–710) and GST Sec7-HDS1 (AA 695–1066) in the pGEX-4T1 vector (Bouvet et al., 2013), as well as the purified human Δ17Arf1, were kind gifts from Cathy Jackson (Institut Jacques Monod, CNRS, Paris, France). Purified bovine rhodopsin was a gift from Kris Palczewski (Case Western Reserve University, Cleveland, OH) (Palczewski et al., 2000). Purified Arl1 and anti-Arl1 were kind gifts from Antonio Galindo (MRC LMB, Cambridge, UK) (Galindo et al., 2016). Recombinant human Arf4 was expressed and purified as described previously (Wang et al., 2012). GCA and cycloheximide were from Sigma-Aldrich. Antibodies used in this study were as follows: rabbit polyclonal anti-Arf4 (Mazelova et al., 2009); anti-rhodopsin C-terminus mAb 11D5 (Deretic and Papermaster, 1991) and mAb 1D4 (ab5417, Abcam); rabbit anti-GBF1 (ab105111, Abcam); mouse monoclonal anti-GBF1 (612116, BD Biosciences), anti-ASAP1 (612073, BD Biosciences) and anti-GM130 (610823, BD Biosciences); rabbit polyclonal anti-Rab6 (sc-310, Santa Cruz Biotechnology); rabbit anti-p115 (13509-1-AP, Proteintech); rabbit anti-ASAP1 [a kind gift from Paul Randazzo (National Cancer Institute, Bethesda, MD) (Randazzo et al., 2000)]; mouse anti-Arf1 (ThermoFisher Scientific), mouse anti-GST (SAB4200237, Sigma-Aldrich); Cy3- and Cy5-conjugated secondary antibodies (Jackson ImmunoResearch) and To-Pro3 (Life Technologies). The Duolink II Rabbit/Mouse Red Kit (excitation, 598 nm; emission, 634 nm) was from Sigma-Aldrich (DUO92101). For some experiments, rabbit anti-Rab6 antibody was directly conjugated to Alexa Fluor 488 using an Antibody Labeling Kit (Invitrogen), according to the manufacturer's instructions.
Pulse-chase labeling, preparation of the photoreceptor-enriched PNS and retinal subcellular fractionation
These experiments were performed according to established procedures (Deretic, 2000; Deretic and Papermaster, 1991; Deretic et al., 1996; Mazelova et al., 2009; Morel et al., 2000; Wang et al., 2012). Our animal protocol is approved by the Office of Animal Care Compliance at The University of New Mexico. Briefly, following 1 h pulse labeling of seven isolated frog retinas (from Rana berlandierii; Carolina Biological Supply Company, Burlington, NC) and 2 h chase, ROSs were removed and pelleted without further purification (crude ROS), and retinal pellets were homogenized and centrifuged to generate the PNS. The PNS was centrifuged at 17,500 g for 10 min to obtain a pellet enriched in Golgi/TGN/ER membranes. The supernatant was centrifuged at 336,000 g for 30 min to separate the RTCs from the cytosol. In some experiments, retinas were incubated with 10 µm GCA or 30 µg/ml cycloheximide for 3 h, during pulse-chase labeling.
Confocal microscopy and PLA
Confocal microscopy was performed on dark-adapted frog retinas as described (Mazelova et al., 2009). In some experiments isolated eyecups were incubated for 3 h with 10 µm GCA or 30 µg/ml cycloheximide. Eyecups were fixed with 4% paraformaldehyde overnight and embedded in 5% agarose. Then, 100 μm sections were cut, permeabilized in 0.3% Triton X-100, and labeled with specific antibodies as described in the figure legends. Antibodies used for confocal microscopy were as follows: rabbit polyclonal anti-Arf4 (1:400); anti-rhodopsin C-terminus mAb 11D5 (1:400); rabbit anti-GBF1 (1:200); mouse anti-GBF1 (1:200), mouse anti-ASAP1 (1:200); mouse anti-GM130 (1:200); rabbit polyclonal anti-Rab6 (1:200); rabbit anti-p115 (1:200); rabbit anti-ASAP1 (1:200). Incubation with primary antibodies was followed by incubation with Cy3- and Cy5-conjugated secondary antibodies (1:200). Nuclei were stained with To-Pro3 (1:1000). PLA was performed on fixed retinal sections using a Duolink II Rabbit/Mouse Red Kit, as described previously (Wang et al., 2012). In some experiments, retinal sections labeled with Duolink were stained overnight at 4°C with anti-Rab6 antibody conjugated to Alexa Fluor 488 (1:100) to highlight Golgi localization. Confocal optical sections were generated on a Zeiss 800 LSM (Carl Zeiss). Digital images were prepared using Adobe Photoshop CS4 (Adobe Systems). Colocalization analysis (Pearson's coefficient) was calculated using SlideBook Image Analysis software (Intelligent Imaging Innovations). To quantify interaction sites detected by the PLA in control, GCA- or cycloheximide-treated retinas, three separate experiments were conducted, each including the rhodopsin-Arf4 pair as a positive control (Wang et al., 2012), and >10 Z-stacks containing ≥10 confocal optical sections were generated for each PLA pair. From these Z-stacks, two representative confocal sections, generally from the middle of the stack, encompassing at least five photoreceptors with clearly visible RIS demarcations were selected from each experiment. Interaction sites were counted (10 photoreceptors×3 experiments) for each PLA pair. In control retinas, numerous interaction sites were detected in nearly every photoreceptor, whereas in GCA- and cycloheximide-treated retinas, occasional interaction sites were detected in <50% of the cells counted, except in the GBF1-ASAP1 pairs.
GST fusion protein pulldown assay
To analyze direct protein interactions, purified human proteins, Arf4 (Wang et al., 2012), Δ17Arf1 or Arl1 (5 µg each), were preincubated with 100 µM GDPβS or GTPγS in 100 µl nucleotide loading buffer (25 mM HEPES, pH 7.4, 100 mM NaCl, 0.5 mM MgCl2, 1 mM EDTA, 1 mM ATP and 1 mM DTT) at 30°C for 1 h. Purified bovine rhodopsin [functionally equivalent to frog rhodopsin (Deretic et al., 1998)], was in a 1.6 mg/ml solution in a buffer containing 0.5 mM n-dodecyl-β-maltoside (DDM). GST and GST fusion proteins were expressed in Rosetta 2 E.coli cells and direct protein interactions were analyzed as described previously (Wang et al., 2012). Briefly, GST fusion proteins on glutathione-Sepharose 4B beads (50 µl per sample) were washed 2× with PBS. Immobilized GST fusion proteins were incubated at RT for 2 h in 500 µl reaction buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM MgCl2 6H2O, 0.1% Triton X-100, 0.1% BSA and 1 mM PMSF) with 5 µg each of Arf4, Δ17Arf1, Arl1 and/or rhodopsin, as indicated. Glutathione-Sepharose 4B beads were then washed eight times with the reaction buffer. Bound proteins were eluted by 20 µl of 2× SDS-PAGE sample buffer. Protein-protein interactions were analyzed by SDS-PAGE and western blotting.
SDS-PAGE and immunoblotting
Proteins were separated by SDS-PAGE on 4–15% TGX gels (BioRad). Gels were either dried and exposed to autoradiography, or blotted onto Immobilon-P membranes (BioRad) and probed with specific antibodies, as indicated. The antibodies used for western blotting were as follows: rabbit polyclonal anti-Arf4 (1:1000); anti-rhodopsin C-terminus mAb 11D5 (1:1000), mAb 1D4 (1:500); anti-Arl1 (1:500); rabbit anti-GBF1 (1:1000); mouse monoclonal anti-GBF1 (1:1000); mouse anti-ASAP1 (1:1000); rabbit anti-ASAP1; mouse monoclonal anti-GST (1:1000) and anti-Arf1 (1:100). Bound antibodies were detected using a chemiluminescent Western Lightning immunodetection system (Perkin Elmer Life Sciences). Because of high retinal tissue requirements, and for more accurate quantification obviating the need for additional loading controls, immunoblots were cut into strips and multiple antibodies were tested on the same blot. Before its use on the strips, each antibody was tested on an entire western blot of the frog retinal PNS to ascertain its specificity. The distribution of radiolabeled rhodopsin, or antigens detected by immunoblotting, was quantified using Quantity One 1-D analysis software (BioRad) and expressed in arbitrary OD units.
Acknowledgements
We thank Drs Antonio Galindo, Cathy Jackson, Kris Palczewski and Paul Randazzo for their generous gifts of reagents.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.D.; Methodology: J.W., T.F., V.K.; Formal analysis: J.W., T.F., V.K.; Data curation: J.W.; Writing - original draft: D.D.; Writing - review & editing: D.D.; Funding acquisition: D.D.
Funding
This work was supported by the National Eye Institute [EY-12421]. Deposited in PMC for release after 12 months.
References
- Alvarez C., Garcia-Mata R., Brandon E. and Sztul E. (2003). COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol. Biol. Cell 14, 2116-2127. 10.1091/mbc.E02-09-0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B., Beraud-Dufour S., Chardin P. and Chabre M. (1997). N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 36, 4675-4684. 10.1021/bi962252b [DOI] [PubMed] [Google Scholar]
- Bachmann-Gagescu R., Phelps I. G., Stearns G., Link B. A., Brockerhoff S. E., Moens C. B. and Doherty D. (2011). The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum. Mol. Genet. 20, 4041-4055. 10.1093/hmg/ddr332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tekaya H., Kahn R. A. and Hauri H.-P. (2010). ADP ribosylation factors 1 and 4 and group VIA phospholipase A(2) regulate morphology and intraorganellar traffic in the endoplasmic reticulum-Golgi intermediate compartment. Mol. Biol. Cell 21, 4130-4140. 10.1091/mbc.E10-01-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson E. L., Rosner B., Weigel-DiFranco C., Dryja T. P. and Sandberg M. A. (2002). Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest. Ophthalmol. Vis. Sci. 43, 3027-3036. [PubMed] [Google Scholar]
- Besharse J. C. (1986). Photosensitive membrane turnover: differentiated membrane domains and cell-cell interaction. In The Retina: A Model for Cell Biological Studies (ed. Adler R. and Farber D.), pp. 297-352. New York: Academic Press. [Google Scholar]
- Bhowmick R., Li M., Sun J., Baker S. A., Insinna C. and Besharse J. C. (2009). Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic 10, 648-663. 10.1111/j.1600-0854.2009.00896.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasic J. R. Jr, Lane Brown R. and Robinson P. R. (2012). Light-dependent phosphorylation of the carboxy tail of mouse melanopsin. Cell. Mol. Life Sci. 69, 1551-1562. 10.1007/s00018-011-0891-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boal F. and Stephens D. J. (2010). Specific functions of BIG1 and BIG2 in endomembrane organization. PLoS ONE 5, e9898 10.1371/journal.pone.0009898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet S., Golinelli-Cohen M.-P., Contremoulins V. and Jackson C. L. (2013). Targeting of the Arf-GEF GBF1 to lipid droplets and Golgi membranes. J. Cell Sci. 126, 4794-4805. 10.1242/jcs.134254 [DOI] [PubMed] [Google Scholar]
- Brooks M. J., Rajasimha H. K., Roger J. E. and Swaroop A. (2011). Next-generation sequencing facilitates quantitative analysis of wild-type and Nrl(-/-) retinal transcriptomes. Mol. Vis. 17, 3034-3054. [PMC free article] [PubMed] [Google Scholar]
- Brown M. T., Andrade J., Radhakrishna H., Donaldson J. G., Cooper J. A. and Randazzo P. A. (1998). ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol. Cell. Biol. 18, 7038-7051. 10.1128/MCB.18.12.7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui Q. T., Golinelli-Cohen M.-P. and Jackson C. L. (2009). Large Arf1 guanine nucleotide exchange factors: evolution, domain structure, and roles in membrane trafficking and human disease. Mol. Genet. Genomics 282, 329-350. 10.1007/s00438-009-0473-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E. (2007). Regulation of Arf Activation: the Sec7 family of guanine nucleotide exchange factors. Traffic 8, 1476-1485. 10.1111/j.1600-0854.2007.00634.x [DOI] [PubMed] [Google Scholar]
- Caster A. H., Sztul E. and Kahn R. A. (2013). A role for cargo in Arf-dependent adaptor recruitment. J. Biol. Chem. 288, 14788-14804. 10.1074/jbc.M113.453621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Amagai Y., Homma Y., Fukuda M. and Mizuno K. (2013). NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J. 32, 874-885. 10.1038/emboj.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christis C. and Munro S. (2012). The small G protein Arl1 directs the trans-Golgi-specific targeting of the Arf1 exchange factors BIG1 and BIG2. J. Cell Biol. 196, 327-335. 10.1083/jcb.201107115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Shapovalova Z., Dejgaard S. Y., Presley J. F. and Melancon P. (2008). Characterization of class I and II ADP-ribosylation factors (Arfs) in live cells: GDP-bound class II Arfs associate with the ER-Golgi intermediate compartment independently of GBF1. Mol. Biol. Cell 19, 3488-3500. 10.1091/mbc.E08-04-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion F. and Chen J. (2010). Q344ter mutation causes mislocalization of rhodopsin molecules that are catalytically active: a mouse model of Q344ter-induced retinal degeneration. PLoS ONE 5, e10904 10.1371/journal.pone.0010904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concepcion F., Mendez A. and Chen J. (2002). The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision Res. 42, 417-426. 10.1016/S0042-6989(01)00195-X [DOI] [PubMed] [Google Scholar]
- Craige B., Tsao C.-C., Diener D. R., Hou Y., Lechtreck K.-F., Rosenbaum J. L. and Witman G. B. (2010). CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927-940. 10.1083/jcb.201006105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Allamargot C., Hudson J. S., Andersen E. K., Bhattarai S., Drack A. V., Sheffield V. C. and Seo S. (2015). Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet-Biedl syndrome. Proc. Natl. Acad. Sci. USA 112, E4400-E4409. 10.1073/pnas.1510111112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D. (2000). Rhodopsin trafficking in photoreceptors using retinal cell-free system. Methods Enzymol. 315, 77-88. 10.1016/S0076-6879(00)15836-7 [DOI] [PubMed] [Google Scholar]
- Deretic D. (2013). Crosstalk of Arf and Rab GTPases en route to cilia. Small GTPases 4, 70-77. 10.4161/sgtp.24396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D. and Mazelova J. (2009). Assay for in vitro budding of ciliary-targeted rhodopsin transport carriers. Methods Cell Biol. 94, 241-257. 10.1016/S0091-679X(08)94012-7 [DOI] [PubMed] [Google Scholar]
- Deretic D. and Papermaster D. S. (1991). Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J. Cell Biol. 113, 1281-1293. 10.1083/jcb.113.6.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D. and Papermaster D. S. (1993). Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J. Cell Sci. 106, 803-813. [DOI] [PubMed] [Google Scholar]
- Deretic D., Huber L. A., Ransom N., Mancini M., Simons K. and Papermaster D. S. (1995). rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J. Cell Sci. 108, 215-224. [DOI] [PubMed] [Google Scholar]
- Deretic D., Puleo-Scheppke B. and Trippe C. (1996). Cytoplasmic domain of rhodopsin is essential for post-Golgi vesicle formation in a retinal cell-free system. J. Biol. Chem. 271, 2279-2286. 10.1074/jbc.271.4.2279 [DOI] [PubMed] [Google Scholar]
- Deretic D., Schmerl S., Hargrave P. A., Arendt A. and McDowell J. H. (1998). Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc. Natl. Acad. Sci. USA 95, 10620-10625. 10.1073/pnas.95.18.10620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic D., Williams A. H., Ransom N., Morel V., Hargrave P. A. and Arendt A. (2005). Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc. Natl. Acad. Sci. USA 102, 3301-3306. 10.1073/pnas.0500095102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G. and Jackson C. L. (2011). ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 12, 362-375. 10.1038/nrm3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijsings D., Lanke K. H. W., van Dooren S. H. J., van Dommelen M. M. T., Wetzels R., de Mattia F., Wessels E. and van Kuppeveld F. J. M. (2009). Differential membrane association properties and regulation of class I and class II Arfs. Traffic 10, 316-323. 10.1111/j.1600-0854.2008.00868.x [DOI] [PubMed] [Google Scholar]
- Eguether T., San Agustin J. T., Keady B. T., Jonassen J. A., Liang Y., Francis R., Tobita K., Johnson C. A., Abdelhamed Z. A., Lo C. W. et al. (2014). IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev. Cell 31, 279-290. 10.1016/j.devcel.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezratty E. J., Pasolli H. A. and Fuchs E. (2016). A Presenilin-2-ARF4 trafficking axis modulates Notch signaling during epidermal differentiation. J. Cell Biol. 214, 89-101. 10.1083/jcb.201508082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Knödler A., Ren J., Zhang J., Zhang X., Hong Y., Huang S., Peränen J. and Guo W. (2012). A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J. Biol. Chem. 287, 15602-15609. 10.1074/jbc.M111.333245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J. A., San Agustin J. T., Xu F., Jonassen J. A., Samtani R., Lo C. W. and Pazour G. J. (2008). The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 4, e1000315 10.1371/journal.pgen.1000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J. A., San Agustin J. T., Jonassen J. A., Huang T., Rivera-Perez J. A., Tremblay K. D. and Pazour G. J. (2014). Arf4 is required for Mammalian development but dispensable for ciliary assembly. PLoS Genet. 10, e1004170 10.1371/journal.pgen.1004170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M., Chardin P., Chabre M. and Paris S. (1996). Myristoylation-facilitated binding of the G protein ARF1 to membrane phospholipids is required for its activation by a soluble nucleotide exchange factor. J. Biol. Chem. 271, 1573-1578. 10.1074/jbc.271.3.1573 [DOI] [PubMed] [Google Scholar]
- Galindo A., Soler N., McLaughlin S. H., Yu M., Williams R. L. and Munro S. (2016). Structural insights into Arl1-mediated targeting of the Arf-GEF BIG1 to the trans-Golgi. Cell Rep 16, 839-850. 10.1016/j.celrep.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo F. R., Corbit K. C., Sirerol-Piquer M. S., Ramaswami G., Otto E. A., Noriega T. R., Seol A. D., Robinson J. F., Bennett C. L., Josifova D. J. et al. (2011). A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43, 776-784. 10.1038/ng.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R., Szul T., Alvarez C. and Sztul E. (2003). ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol. Biol. Cell 14, 2250-2261. 10.1091/mbc.E02-11-0730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. (1998). Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 95, 237-248. 10.1016/S0092-8674(00)81754-7 [DOI] [PubMed] [Google Scholar]
- Goldberg A. F. X., Moritz O. L. and Williams D. S. (2016). Molecular basis for photoreceptor outer segment architecture. Prog. Retin. Eye Res. 55, 52-81. 10.1016/j.preteyeres.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. O., Bok D. and Bacharach A. D. (1969). Biosynthesis and assembly of the rod outer segment membrane system. Formation and fate of visual pigment in the frog retina. J. Mol. Biol. 45, 397-406. 10.1016/0022-2836(69)90114-4 [DOI] [PubMed] [Google Scholar]
- Hilgendorf K. I., Johnson C. T. and Jackson P. K. (2016). The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr. Opin. Cell Biol. 39, 84-92. 10.1016/j.ceb.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A., Al-Awar O. S., Hay J. C. and Donaldson J. G. (2005). Targeting of Arf-1 to the early Golgi by membrin, an ER-Golgi SNARE. J. Cell Biol. 168, 1039-1051. 10.1083/jcb.200409138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M. C., Weihbrecht K., Searby C. C., Li Y., Pope R. M., Sheffield V. C. and Seo S. (2012). ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc. Natl. Acad. Sci. USA 109, 19691-19696. 10.1073/pnas.1210916109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. M., Rancourt D., Farrar G. J., Kenna P., Hazel M., Bush R. A., Sieving P. A., Sheils D. M., McNally N., Creighton P. et al. (1997). Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 15, 216-219. 10.1038/ng0297-216 [DOI] [PubMed] [Google Scholar]
- Jackson C. L. (2014). GEF-effector interactions. Cell Logist. 4, e943616 10.4161/21592780.2014.943616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. L. and Casanova J. E. (2000). Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10, 60-67. 10.1016/S0962-8924(99)01699-2 [DOI] [PubMed] [Google Scholar]
- Kawamoto K., Yoshida Y., Tamaki H., Torii S., Shinotsuka C., Yamashina S. and Nakayama K. (2002). GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic 3, 483-495. 10.1034/j.1600-0854.2002.30705.x [DOI] [PubMed] [Google Scholar]
- Keady B. T., Le Y. Z. and Pazour G. J. (2011). IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol. Biol. Cell 22, 921-930. 10.1091/mbc.E10-09-0792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizhatil K., Baker S. A., Arshavsky V. Y. and Bennett V. (2009). Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science 323, 1614-1617. 10.1126/science.1169789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock B. L., Mills-Henry I. and Perkins B. D. (2009). Retrograde intraflagellar transport by cytoplasmic dynein-2 is required for outer segment extension in vertebrate photoreceptors but not arrestin translocation. Invest. Ophthalmol. Vis. Sci. 50, 5463-5471. 10.1167/iovs.09-3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J., Krasnoperova N. V., Calvert P. D., Kosaras B., Cameron D. A., Nicolo M., Makino C. L. and Sidman R. L. (1999). Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc. Natl. Acad. Sci. USA 96, 736-741. 10.1073/pnas.96.2.736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Snyder W. K., Olsson J. E. and Dryja T. P. (1996). Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc. Natl. Acad. Sci. USA 93, 14176-14181. 10.1073/pnas.93.24.14176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew G. M., Ye F., Nager A. R., Murphy J. P., Lee J. S., Aguiar M., Breslow D. K., Gygi S. P. and Nachury M. V. (2014). The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev. Cell 31, 265-278. 10.1016/j.devcel.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodowski K. H., Lee R., Ropelewski P., Nemet I., Tian G. and Imanishi Y. (2013). Signals governing the trafficking and mistrafficking of a ciliary GPCR, rhodopsin. J. Neurosci. 33, 13621-13638. 10.1523/JNEUROSCI.1520-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery J., Szul T., Styers M., Holloway Z., Oorschot V., Klumperman J. and Sztul E. (2013). The Sec7 guanine nucleotide exchange factor GBF1 regulates membrane recruitment of BIG1 and BIG2 guanine nucleotide exchange factors to the trans-Golgi network (TGN). J. Biol. Chem. 288, 11532-11545. 10.1074/jbc.M112.438481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J., Astuto-Gribble L., Inoue H., Tam B. M., Schonteich E., Prekeris R., Moritz O. L., Randazzo P. A. and Deretic D. (2009). Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 28, 183-192. 10.1038/emboj.2008.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonold C. M. and Fromme J. C. (2014). Four GTPases differentially regulate the Sec7 Arf-GEF to direct traffic at the trans-golgi network. Dev. Cell 30, 759-767. 10.1016/j.devcel.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno-Yamasaki E., Rivera-Molina F. and Novick P. (2012). GTPase networks in membrane traffic. Annu. Rev. Biochem. 81, 637-659. 10.1146/annurev-biochem-052810-093700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monetta P., Slavin I., Romero N. and Alvarez C. (2007). Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol. Biol. Cell 18, 2400-2410. 10.1091/mbc.E06-11-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel V., Poschet R., Traverso V. and Deretic D. (2000). Towards the proteome of the rhodopsin-bearing post-Golgi compartment of retinal photoreceptor cells. Electrophoresis 21, 3460-3469. [DOI] [PubMed] [Google Scholar]
- Moritz O. L., Tam B. M., Hurd L. L., Peranen J., Deretic D. and Papermaster D. S. (2001). Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol. Biol. Cell 12, 2341-2351. 10.1091/mbc.12.8.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouratou B., Biou V., Joubert A., Cohen J., Shields D. J., Geldner N., Jürgens G., Melançon P. and Cherfils J. (2005). The domain architecture of large guanine nucleotide exchange factors for the small GTP-binding protein Arf. BMC Genomics 6, 20 10.1186/1471-2164-6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury M. V., Loktev A. V., Zhang Q., Westlake C. J., Peränen J., Merdes A., Slusarski D. C., Scheller R. H., Bazan J. F., Sheffield V. C. et al. (2007). A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201-1213. 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- Nachury M. V., Seeley E. S. and Jin H. (2010). Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26, 59-87. 10.1146/annurev.cellbio.042308.113337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai W., Kondo Y., Saitoh A., Naito T., Nakayama K. and Shin H.-W. (2013). ARF1 and ARF4 regulate recycling endosomal morphology and retrograde transport from endosomes to the Golgi apparatus. Mol. Biol. Cell 24, 2570-2581. 10.1091/mbc.E13-04-0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrotek A., Zeghouf M. and Cherfils J. (2016). Allosteric regulation of Arf GTPases and their GEFs at the membrane interface. Small GTPases 7, 283-296. 10.1080/21541248.2016.1215778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y., Zhao C., Saras A., Mukhopadhyay S., Kim W., Furukawa T., Sengupta P., Veraksa A. and Malicki J. (2008). Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 10, 437-444. 10.1038/ncb1706 [DOI] [PubMed] [Google Scholar]
- Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E. et al. (2000). Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739-745. 10.1126/science.289.5480.739 [DOI] [PubMed] [Google Scholar]
- Papermaster D. S. and Dreyer W. J. (1974). Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry 13, 2438-2444. 10.1021/bi00708a031 [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Converse C. A. and Siu J. (1975). Membrane biosynthesis in the frog retina: opsin transport in the photoreceptor cell. Biochemistry 14, 1343-1352. 10.1021/bi00678a001 [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Schneider B. G. and Besharse J. C. (1985). Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest. Ophthalmol. Vis. Sci. 26, 1386-1404. [PubMed] [Google Scholar]
- Pasqualato S., Renault L. and Cherfils J. (2002). Arf, Arl, Arp and Sar proteins: a family of GTP-binding proteins with a structural device for ‘front-back’ communication. EMBO Rep. 3, 1035-1041. 10.1093/embo-reports/kvf221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring J. N., Salinas R. Y., Baker S. A. and Arshavsky V. Y. (2013). Protein sorting, targeting and trafficking in photoreceptor cells. Prog. Retin. Eye Res. 36, 24-51. 10.1016/j.preteyeres.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring J. N., Spencer W. J., Lieu E. C. and Arshavsky V. Y. (2015). Guanylate cyclase 1 relies on rhodopsin for intracellular stability and ciliary trafficking. Elife 4, e12058 10.7554/eLife.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearring J. N., San Agustin J. T., Lobanova E. S., Gabriel C. J., Lieu E. C., Monis W. J., Stuck M. W., Strittmatter L., Jaber S. M., Arshavsky V. Y. et al. (2017). Loss of Arf4 causes severe degeneration of the exocrine pancreas but not cystic kidney disease or retinal degeneration. PLoS Genet. 13, e1006740 10.1371/journal.pgen.1006740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche A., Antonny B., Robineau S., Acker J., Cherfils J. and Jackson C. L. (1999). Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol. Cell 3, 275-285. 10.1016/S1097-2765(00)80455-4 [DOI] [PubMed] [Google Scholar]
- Pulvirenti T., Giannotta M., Capestrano M., Capitani M., Pisanu A., Polishchuk R. S., San Pietro E., Beznoussenko G. V., Mironov A. A., Turacchio G. et al. (2008). A traffic-activated Golgi-based signalling circuit coordinates the secretory pathway. Nat. Cell Biol. 10, 912-922. 10.1038/ncb1751 [DOI] [PubMed] [Google Scholar]
- Randazzo P. A., Terui T., Sturch S., Fales H. M., Ferrige A. G. and Kahn R. A. (1995). The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J. Biol. Chem. 270, 14809-14815. 10.1074/jbc.270.24.14809 [DOI] [PubMed] [Google Scholar]
- Randazzo P. A., Andrade J., Miura K., Brown M. T., Long Y.-Q., Stauffer S., Roller P. and Cooper J. A. (2000). The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 97, 4011-4016. 10.1073/pnas.070552297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raykova D., Koos B., Asplund A., Gelleri M., Ivarsson Y., Danielson U. H. and Soderberg O. (2016). Let there be light! Proteomes 4, 36 10.3390/proteomes4040036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiling J. H., Olive A. J., Sanyal S., Carette J. E., Brummelkamp T. R., Ploegh H. L., Starnbach M. N. and Sabatini D. M. (2013). A CREB3-ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens. Nat. Cell Biol. 15, 1473-1485. 10.1038/ncb2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter J. F. and Leroux M. R. (2017). Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533-547. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. C. and Fromme J. C. (2012). Autoregulation of Sec7 Arf-GEF activity and localization by positive feedback. Small GTPases 3, 240-243. 10.4161/sgtp.21828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. C., McDonold C. M. and Fromme J. C. (2012). The Sec7 Arf-GEF is recruited to the trans-Golgi network by positive feedback. Dev. Cell 22, 799-810. 10.1016/j.devcel.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. C., Halaby S. L., Gustafson M. A. and Fromme J. C. (2016). The Sec7 N-terminal regulatory domains facilitate membrane-proximal activation of the Arf1 GTPase. Elife 5, e12411 10.7554/eLife.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáenz J. B., Sun W. J., Chang J. W., Li J., Bursulaya B., Gray N. S. and Haslam D. B. (2009). Golgicide A reveals essential roles for GBF1 in Golgi assembly and function. Nat. Chem. Biol. 5, 157-165. 10.1038/nchembio.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas R. Y., Baker S. A., Gospe S. M. III and Arshavsky V. Y. (2013). A single valine residue plays an essential role in peripherin/rds targeting to photoreceptor outer segments. PLoS ONE 8, e54292 10.1371/journal.pone.0054292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L., Miller J. J., Corbit K. C., Giles R. H., Brauer M. J., Otto E. A., Baye L. M., Wen X., Scales S. J., Kwong M. et al. (2011). Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145, 513-528. 10.1016/j.cell.2011.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A. K., O'Tousa J. E., Ozaki K. and Ready D. F. (2005). Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development 132, 1487-1497. 10.1242/dev.01704 [DOI] [PubMed] [Google Scholar]
- Schou K. B., Pedersen L. B. and Christensen S. T. (2015). Ins and outs of GPCR signaling in primary cilia. EMBO Rep. 16, 1099-1113. 10.15252/embr.201540530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N., Hardcastle A. J. and Cheetham M. E. (2012). Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vision Res. 75, 2-4. 10.1016/j.visres.2012.07.016 [DOI] [PubMed] [Google Scholar]
- Seixas E., Barros M., Seabra M. C. and Barral D. C. (2013). Rab and Arf proteins in genetic diseases. Traffic 14, 871-885. 10.1111/tra.12072 [DOI] [PubMed] [Google Scholar]
- Shetty K. M., Kurada P. and O'Tousa J. E. (1998). Rab6 regulation of rhodopsin transport in Drosophila. J. Biol. Chem. 273, 20425-20430. 10.1074/jbc.273.32.20425 [DOI] [PubMed] [Google Scholar]
- Shimada H., Lu Q., Insinna-Kettenhofen C., Nagashima K., English M. A., Semler E. M., Mahgerefteh J., Cideciyan A. V., Li T., Brooks B. P. et al. (2017). In vitro modeling using ciliopathy-patient-derived cells reveals distinct cilia dysfunctions caused by CEP290 mutations. Cell Rep. 20, 384-396. 10.1016/j.celrep.2017.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K.-J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L.-G. et al. (2006). Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995-1000. 10.1038/nmeth947 [DOI] [PubMed] [Google Scholar]
- Spencer W. J., Pearring J. N., Salinas R. Y., Loiselle D. R., Skiba N. P. and Arshavsky V. Y. (2016). Progressive Rod-Cone Degeneration (PRCD) protein requires N-terminal S-acylation and rhodopsin binding for photoreceptor outer segment localization and maintaining intracellular stability. Biochemistry 55, 5028-5037. 10.1021/acs.biochem.6b00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder D. and Antonny B. (2013). Arf GTPase regulation through cascade mechanisms and positive feedback loops. FEBS Lett. 587, 2028-2035. 10.1016/j.febslet.2013.05.015 [DOI] [PubMed] [Google Scholar]
- Szul T., Garcia-Mata R., Brandon E., Shestopal S., Alvarez C. and Sztul E. (2005). Dissection of membrane dynamics of the ARF-guanine nucleotide exchange factor GBF1. Traffic 6, 374-385. 10.1111/j.1600-0854.2005.00282.x [DOI] [PubMed] [Google Scholar]
- Szul T., Grabski R., Lyons S., Morohashi Y., Shestopal S., Lowe M. and Sztul E. (2007). Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking. J. Cell Sci. 120, 3929-3940. 10.1242/jcs.010769 [DOI] [PubMed] [Google Scholar]
- Tam B. M., Moritz O. L. and Papermaster D. S. (2004). The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol. Biol. Cell 15, 2027-2037. 10.1091/mbc.E03-09-0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P., Rives M. L., Urizar E., Piskorowski R. A., Vishwasrao H. D., Castrillon J., Schmauss C., Slattman M., Gullberg M. and Javitch J. A. (2011). Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. BioTechniques 51, 111-118. 10.2144/000113719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi D., Colin E., Louie C. M. and Williams D. S. (2012). Live-cell imaging evidence for the ciliary transport of rod photoreceptor opsin by heterotrimeric kinesin-2. J. Neurosci. 32, 10587-10593. 10.1523/JNEUROSCI.0015-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reeuwijk J., Arts H. H. and Roepman R. (2011). Scrutinizing ciliopathies by unraveling ciliary interaction networks. Hum. Mol. Genet. 20, R149-R157. 10.1093/hmg/ddr354 [DOI] [PubMed] [Google Scholar]
- Vetter M., Stehle R., Basquin C. and Lorentzen E. (2015). Structure of Rab11-FIP3-Rabin8 reveals simultaneous binding of FIP3 and Rabin8 effectors to Rab11. Nat. Struct. Mol. Biol. 22, 695-702. 10.1038/nsmb.3065 [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley L. A., Li Y., Zhang C. J. and Kahn R. A. (2005). Isoform-selective effects of the depletion of ADP-ribosylation factors 1-5 on membrane traffic. Mol. Biol. Cell 16, 4495-4508. 10.1091/mbc.E04-12-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. and Deretic D. (2014). Molecular complexes that direct rhodopsin transport to primary cilia. Prog. Retin. Eye Res. 38, 1-19. 10.1016/j.preteyeres.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. and Deretic D. (2015). The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J. Cell Sci. 128, 1375-1385. 10.1242/jcs.162925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Morita Y., Mazelova J. and Deretic D. (2012). The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 31, 4057-4071. 10.1038/emboj.2012.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. H., Brown-Glaberman U., Wang J., Morita Y., Alper S. L., Bedrick E. J., Gattone V. H. II, Deretic D. and Wandinger-Ness A. (2011). A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol. Biol. Cell 22, 3289-3305. 10.1091/mbc.E11-01-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensel T. G., Zhang Z., Anastassov I. A., Gilliam J. C., He F., Schmid M. F. and Robichaux M. A. (2016). Structural and molecular bases of rod photoreceptor morphogenesis and disease. Prog. Retin. Eye Res. 55, 32-51. 10.1016/j.preteteres.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake C. J., Baye L. M., Nachury M. V., Wright K. J., Ervin K. E., Phu L., Chalouni C., Beck J. S., Kirkpatrick D. S., Slusarski D. C. et al. (2011). Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl. Acad. Sci. USA 108, 2759-2764. 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens C. J., Tong Y., Esmail M. A., Oh E., Gerdes J. M., Wang J., Tempel W., Rattner J. B., Katsanis N., Park H.-W. et al. (2010). The Bardet-Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signaling. J. Biol. Chem. 285, 16218-16230. 10.1074/jbc.M109.070953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. S. (2008). Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 48, 433-441. 10.1016/j.visres.2007.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. J., Baye L. M., Olivier-Mason A., Mukhopadhyay S., Sang L., Kwong M., Wang W., Pretorius P. R., Sheffield V. C., Sengupta P. et al. (2011). An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 25, 2347-2360. 10.1101/gad.173443.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J., Kahn R. A. and Sztul E. (2014). Regulating the large Sec7 ARF guanine nucleotide exchange factors: the when, where and how of activation. Cell. Mol. Life Sci. 71, 3419-3438. 10.1007/s00018-014-1602-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. (1967). The renewal of photoreceptor cell outer segments. J. Cell Biol. 33, 61-72. 10.1083/jcb.33.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. W. (1976). Visual cells and the concept of renewal. Invest. Ophthalmol. Vis. Sci. 15, 700-725. [PubMed] [Google Scholar]
- Zhang Q., Hu J. and Ling K. (2013). Molecular views of Arf-like small GTPases in cilia and ciliopathies. Exp. Cell Res. 319, 2316-2322. 10.1016/j.yexcr.2013.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Hanke-Gogokhia C., Jiang L., Li X., Wang P., Gerstner C. D., Frederick J. M., Yang Z. and Baehr W. (2014). Mistrafficking of prenylated proteins causes retinitis pigmentosa 2. FASEB J. 29, 932-942. 10.1096/fj.14-257915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C. and Malicki J. (2011). Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 30, 2532-2544. 10.1038/emboj.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Claude A., Chun J., Shields D. J., Presley J. F. and Melancon P. (2006). GBF1, a cis-Golgi and VTCs-localized ARF-GEF, is implicated in ER-to-Golgi protein traffic. J. Cell Sci. 119, 3743-3753. 10.1242/jcs.03173 [DOI] [PubMed] [Google Scholar]
- Zulliger R., Conley S. M., Mwoyosvi M. L., Stuck M. W., Azadi S. and Naash M. I. (2015). SNAREs interact with retinal degeneration slow and rod outer segment membrane protein-1 during conventional and unconventional outer segment targeting. PLoS ONE 10, e0138508 10.1371/journal.pone.0138508 [DOI] [PMC free article] [PubMed] [Google Scholar]