ABSTRACT
Mutations in MECP2 cause Rett syndrome, a severe neurological disorder with autism-like features. Duplication of MECP2 also causes severe neuropathology. Both diseases display immunological abnormalities that suggest a role for MECP2 in controlling immune and inflammatory responses. Here, we used mecp2-null zebrafish to study the potential function of Mecp2 as an immunological regulator. Mecp2 deficiency resulted in an increase in neutrophil infiltration and upregulated expression of the pro- and anti-inflammatory cytokines Il1b and Il10 as a secondary response to disturbances in tissue homeostasis. By contrast, expression of the proinflammatory cytokine tumor necrosis factor alpha (Tnfa) was consistently downregulated in mecp2-null animals during development, representing the earliest developmental phenotype described for MECP2 deficiency to date. Expression of tnfa was unresponsive to inflammatory stimulation, and was partially restored by re-expression of functional mecp2. Thus, Mecp2 is required for tnfa expression during zebrafish development and inflammation. Finally, RNA sequencing of mecp2-null embryos revealed dysregulated processes predictive for Rett syndrome phenotypes.
KEY WORDS: Inflammation, mecp2, tnfa, Zebrafish, Rett syndrome
Summary: As shown by evaluating the levels of pro- and anti-inflammatory cytokines in mecp2-null zebrafish, Mecp2 is required for tnfa expression during zebrafish development and inflammation.
INTRODUCTION
The human X-chromosomal gene methyl-CpG-binding protein 2 (MECP2) was identified as an epigenetic factor capable of binding to methylated DNA (Lewis et al., 1992). Mutations in human MECP2 lead to Rett syndrome (RTT) (Amir et al., 1999), a severe neurological disorder associated with autistic features and motor skill regression after an apparently normal early development (Lyst and Bird, 2015). RTT patients often also display growth retardation (Tarquinio et al., 2012), gastrointestinal (GI) and biliary tract disorders (Motil et al., 2012) and oxidative stress (Filosa et al., 2015). Conversely, overexpression of human MECP2 caused by duplication of its genetic locus (Xq28) results in severe mental retardation and progressive neurological symptoms (Van Esch et al., 2005). Although neurological defects are the most striking clinical presentation of RTT and MECP2-duplication syndrome, both diseases display immunological abnormalities that point towards a role for MECP2 in regulating immune and inflammatory responses.
Disturbances in tissue homeostasis are detected by pattern recognition receptors (PRRs), such as the family of Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) (Beg, 2002; Matzinger, 2002; Medzhitov and Janeway, 2000). Activation of TLRs by infection or cellular damage initiates a signaling cascade that leads to the production of proinflammatory cytokines and chemokines (Akira and Takeda, 2004). The primary function of proinflammatory cytokines, including tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL1B), is to initiate an appropriate cellular or humoral immune response to neutralize the disturbance. Anti-inflammatory cytokines, including interleukin 10 (IL10) and transforming growth factor beta (TGFB), balance the activity of proinflammatory cytokines by stimulating resolution of inflammation and tissue repair. Alterations in the balance between pro- and anti-inflammatory cytokines are potentially harmful, as prolonged inflammation can be damaging to tissues, while inadequate immune responses leave the body vulnerable to infections.
RTT patients showed a dysregulated cytokine and chemokine profile and displayed subclinical inflammation (Cortelazzo et al., 2014; Pecorelli et al., 2016). Data obtained using a mouse model of RTT demonstrated that MECP2 regulates microglia and macrophage responsiveness to inflammatory stimulation, hypoxia and glucocorticoids (Cronk et al., 2015). Transplantation of wild-type microglia has even been suggested as a therapeutic strategy for RTT patients based on findings obtained using RTT mice (Derecki et al., 2012), but these findings have since been disputed by others in the field (Wang et al., 2015). Although investigations concerning the role of the immune system in the onset of RTT are ongoing, MECP2 duplication syndrome is linked to immunodeficiency with increased susceptibility to infections for reasons that remain to be uncovered (Bauer et al., 2015). An emerging theme is that MECP2 normally regulates the immune response towards inflammatory stimuli and other stress factors.
The zebrafish was originally employed as a model organism to study vertebrate embryogenesis because of its external fertilization and development, genetic tractability, and optical transparency allowing noninvasive intravital imaging (Kimmel et al., 1988). These characteristics have also helped to develop the zebrafish as a useful model for the study of vertebrate immunity (Renshaw and Trede, 2011; van der Vaart et al., 2012). A recently described mecp2-null zebrafish mutant showed altered motor behaviors (Pietri et al., 2013), and mecp2 was found to be required for normal zebrafish brain development (Gao et al., 2015). Zebrafish mecp2 was broadly expressed early in embryonic development, after which it became enriched in the brains of zebrafish larvae (Gao et al., 2015). This is similar to the distribution of MECP2 in mice, where it is highly expressed in neurons, but also ubiquitously found at lower levels in other cell types (Song et al., 2014).
Here, we studied the potential function of zebrafish Mecp2 as an immunological regulator during development and inflammation. We found that mecp2-null zebrafish display several previously unappreciated phenotypes also present in RTT patients, including growth retardation, GI tract phenotypes and dysregulated expression of cytokines. The gene expression levels of the pro- and anti-inflammatory cytokines il1b and il10 showed a peak during development, but were not hyper-responsive to inflammatory stimulation in mecp2-null larvae. We therefore suggest that the increased expression levels of these inflammatory cytokines during development were a response to a disruption of tissue homeostasis in the absence of Mecp2. Remarkably, we found that the expression levels of zebrafish tnfa were profoundly downregulated during the first hours of development in mecp2-null embryos, preceding the first noticeable disease phenotypes. To the best of our knowledge, this finding represents the earliest developmental phenotype associated with MECP2-deficieny. The lower tnfa expression levels persisted throughout larval development, and tnfa was unresponsive to inflammatory stimulation in mecp2-null larvae. Finally, the expression of tnfa in mecp2-null embryos could be partially restored by enforced expression of wild-type mecp2. However, re-expression of tnfa in mecp2-null embryos was not sufficient to rescue the observed RTT phenotypes. Based on these findings, we conclude that zebrafish Mecp2 is required for tnfa expression during development and inflammation. To assess the earliest changes attributable to loss of Mecp2 function, we utilized RNA sequencing to analyze the transcriptome of mecp2-null embryos shortly after initiation of embryonic transcription (Kane and Kimmel, 1993). Strikingly, this revealed disrupted biological processes that are highly predictive of RTT phenotypes that develop much later in human patients. Further exploration of this transcriptome data and its changes over time might generate novel insights into additional developmental functions of MECP2.
RESULTS
mecp2-null zebrafish display growth retardation, GI tract phenotypes and systemic inflammation
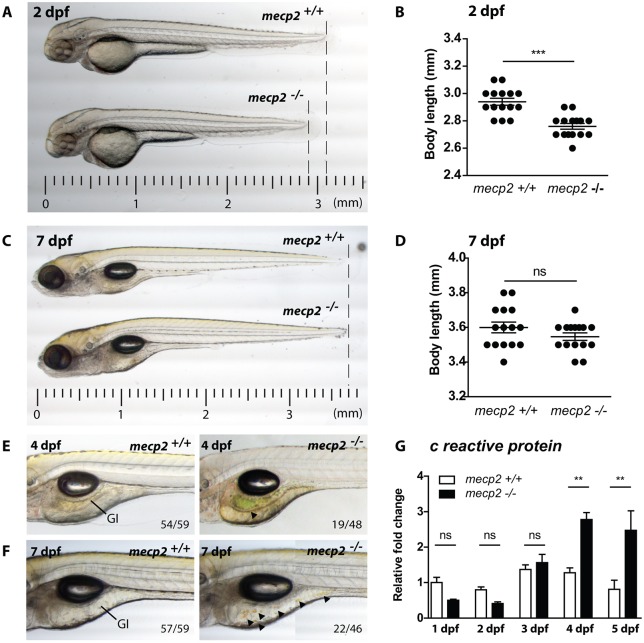
To study the function of Mecp2 during zebrafish development, we used a mutant line containing a premature stop codon in the mecp2 gene (mecp2Q63*) that truncates the protein before the methyl binding domain (MBD) and transcriptional repression domain (TRD), both of which are vital to its function (Lyst and Bird, 2015; Pietri et al., 2013). Although adult mecp2-null zebrafish are viable and fertile with no overt phenotypes, these animals display behavioral alterations during their larval development (Pietri et al., 2013). Upon further characterization, we found that developing mecp2-null embryos displayed growth retardation at 2 days postfertilization (dpf) (Fig. 1A,B). However, no significant difference in total body length was discernible between mecp2-null and wild-type embryos at 7 dpf (Fig. 1C,D). At ∼4 dpf, green/yellow discoloration was observed in the GI tracts of mecp2-null larvae (Fig. 1E), indicative of an accumulation of, or disruption in, flow of bile (Delous et al., 2012). At 7 dpf, dark yellow droplets were regularly observed in the GI tracts of mecp2-null larvae (Fig. 1F), consistent with bile overproduction. To investigate whether these phenotypes are preceded or accompanied by systemic inflammation, we analyzed gene expression of the inflammation marker C reactive protein (crp) by quantitative real-time PCR (qPCR) (Okamura et al., 1990). In the first 3 days of zebrafish development, we found no difference in crp expression between wild-type and mecp2-null larvae, but crp levels were significantly elevated in mecp2-null larvae by 4 dpf and 5 dpf (Fig. 1G). This demonstrates that mecp2-null larvae mount an inflammatory response at 4 dpf and 5 dpf that is detectable at a whole-organism level, after an early developmental period with no overt signs of systemic inflammation. Together, these results show that mecp2-null zebrafish display several RTT features during their development, including growth retardation, GI tract phenotypes and systemic inflammation.
Fig. 1.
mecp2-deficient zebrafish display inflammation during larval development. (A,C) Representative stereo microscopy images of 2 dpf and 7 dpf wild-type and mecp2-null zebrafish larvae. (B,D) Total body lengths of the 2 dpf and 7 dpf wild-type and mecp2-null larvae, as measured in millimeters (n=15 per condition; Student’s t-test; ***P<0.001; data are representative of three individual experiments). (E,F) Stereo microscopy images of 4 dpf and 7 dpf wild-type and mecp2-null zebrafish illustrating the GI tract phenotypes regularly observed (indicated by arrowheads). The frequency of these phenotypes is shown in relation to the total number of examined animals (19 of 48 mecp2-null animals at 3 dpf; 22 of 46 mecp2-null animals at 7 dpf). (G) qPCR was performed to determine the whole-organism gene expression level of the inflammation marker crp relative to the expression of the housekeeping gene tbp. Wild-type and mecp2-null samples (n=3 with 20 embryos or larvae pooled per sample) were taken every day for the first 5 days of development. The relative fold change versus gene expression in a 1 dpf wild type is shown (one-way ANOVA with Tukey's post hoc test; **P<0.01; ns, not significant; data are representative of two individual experiments).
Neutrophil numbers and mobilization confirm the presence of inflammation in mecp2-null larvae
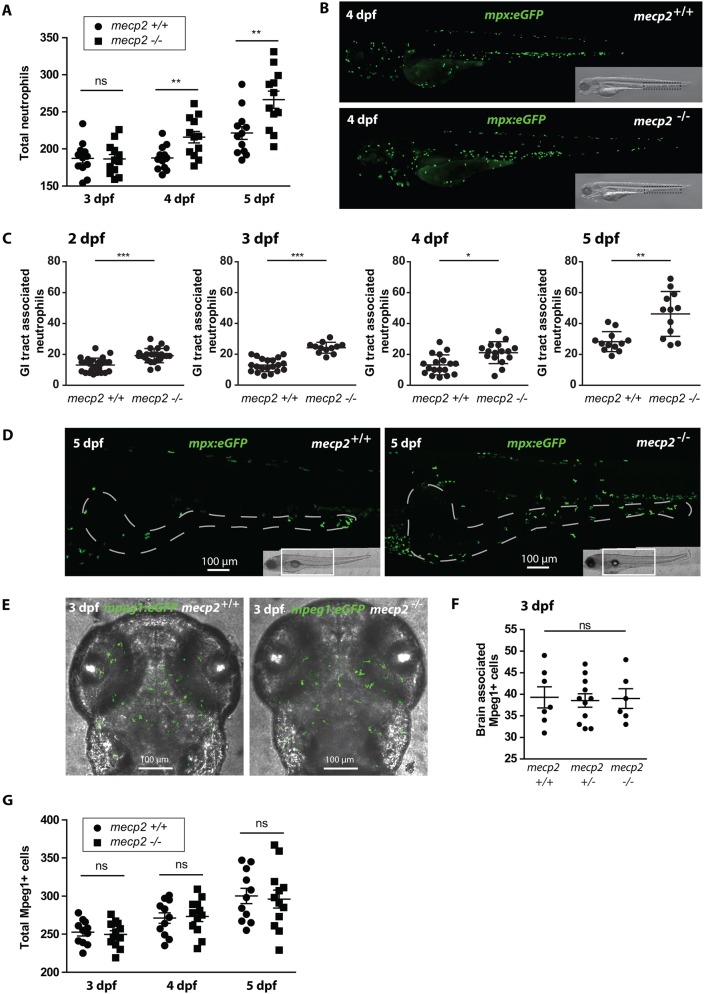
To further investigate and characterize the possible inflammatory response in mecp2-null larvae suggested by increased crp levels, we first analyzed neutrophil numbers. Neutrophils are among the first innate immune cells that respond to disturbances in tissue homeostasis; increased tissue infiltration has previously been used to mark inflammation in zebrafish models of wounding, infection and inflammatory bowel disease (Brudal et al., 2014; Brugman et al., 2009; Oehlers et al., 2011; Renshaw et al., 2006). We used Tg(mpx:eGFP) animals (Renshaw et al., 2006), in which neutrophils are fluorescently labeled, to assess the number and distribution of neutrophils in the mecp2-null background over several developmental time points. Correlating with our crp results, we did not find any difference in neutrophil number between wild-type and mecp2-null larvae at 3 dpf, but total neutrophil numbers were significantly increased in mecp2-null larvae at 4 dpf and 5 dpf (Fig. 2A,B). These findings reproduce the neutrophilia observed in Mecp2-null mice displaying RTT phenotypes, and underscore the conserved function of Mecp2 in lower vertebrates (Cronk et al., 2015).
Fig. 2.
Neutrophil number and distribution confirm the presence of inflammation in mecp2-null larvae. (A) Total numbers of Tg(mpx:eGFP)-positive neutrophils were counted in 3, 4 and 5 dpf wild-type and mecp2-null larvae using stereo fluorescent microscopy (n=12 larvae per condition pooled from two individual experiments; larvae were scored for three consecutive days). (B) Representative stereo microscopy images of 4 dpf Tg(mpx:eGFP) wild-type and mecp2-null larvae. (C) Numbers of Tg(mpx:eGFP)-positive neutrophils associated with the GI tract of 2, 3, 4 and 5 dpf wild-type and mecp2-null larvae were counted (n≥12 embryos per condition; data are representative of three individual experiments). (D) Representative confocal micrographs (maximum projection) of the GI tracts of 5 dpf Tg(mpx:eGFP) wild-type and mecp2-null larvae in which the GI tract has been delineated with a white dashed line based on the transmitted light images. (E) Representative confocal micrographs (maximum projection) of the brain region of 3 dpf Tg(mpeg1:eGFP) wild-type and mecp2-null larvae. (F) Brain-associated Tg(mpeg1:eGFP)-positive cells were counted for 3 dpf wild-type, heterozygous and mecp2-null larvae (n=7, n=11, n=6, respectively; one-way ANOVA with Tukey's post hoc test; ns, not significant; data are representative of two individual experiments). (G) Total numbers of Tg(mpeg1:eGFP)-positive cells were counted in 3, 4 and 5 dpf wild-type and mecp2-null larvae using stereo fluorescent microscopy (n=11 and n=12 embryos per condition, respectively; data are representative of two individual experiments). A Student’s t-test was used for all statistical analyses, except for the data analyzed in F, by comparing wild-type and mecp2-null numbers per day (***P<0.001; **P<0.01; *P<0.05; ns, not significant).
Neutrophilic granulocytes begin to accumulate in the caudal hematopoietic tissue (CHT) of developing zebrafish embryos following initiation of circulation at 26 hpf (Bertrand et al., 2007; Le Guyader et al., 2008; Stachura and Traver, 2011). A large number of neutrophils continue to reside in the CHT in uninflamed larvae, from which they can be mobilized to migrate towards inflamed tissues when needed (Yoo and Huttenlocher, 2011). We therefore aimed to approximate the source of inflammation in mecp2-null larvae by determining which tissues displayed increased neutrophil infiltration. Although the head region of mecp2-null larvae contained a slightly increased number of neutrophils at 5 dpf, we did not observe any significant infiltration of neutrophils into the brains of mecp2-null animals (Fig. S1A-C). Starting at 2 dpf, we observed increases in neutrophil numbers associated with the GI tract of mecp2-null larvae (Fig. 2C,D), indicating this tissue as a potential source of inflammation. We reproduced this observation by using a previously characterized anti-sense morpholino oligonucleotide approach designed to block initiation of zebrafish Mecp2 protein translation (Fig. S1D) (Gao et al., 2015). These findings are in agreement with our previous observation of GI tract phenotypes during mecp2-null larval development.
Because microglia and macrophages have previously been implicated in RTT-like etiology in mice, and become depleted with disease progression (Cronk et al., 2015), we also assessed their number and localization by using Tg(mpeg1:eGFP) animals with fluorescently labeled microglia and macrophages (Ellett et al., 2011). At 3 dpf, mpeg1-expressing microglia have colonized the brain and are capable of mounting a functional immune response (Herbomel et al., 2001; Svahn et al., 2013). However, we found no distinguishable difference in microglia or macrophage numbers or localization between mecp2-null and wild-type larvae from 3 to 5 dpf (Fig. 2E-G). In summary, our results indicate that disrupting Mecp2 function during zebrafish development leads to a systemic immune response that appears to originate from the GI tract, based on observed GI tract phenotypes and neutrophil influx into this tissue.
Expression of central pro- and anti-inflammatory cytokines is dysregulated in mecp2-null larvae
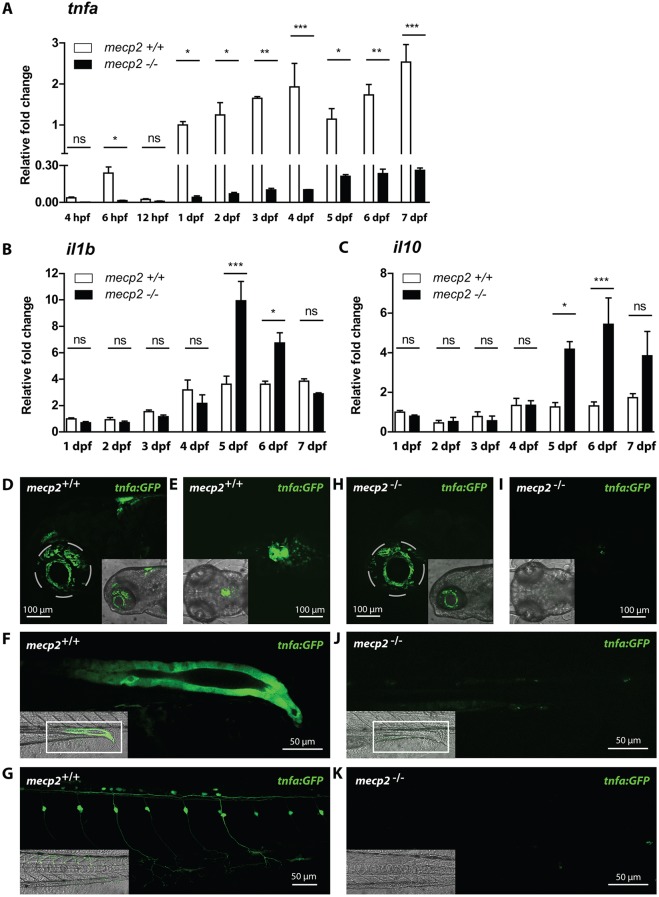
Because inflammation is mainly controlled by the expression and activity of pro- and anti-inflammatory cytokines and chemokines, we queried whether the expression of these regulatory molecules is affected by Mecp2 deficiency. We used qPCR to analyze the gene expression levels of a panel of zebrafish inflammatory cytokines and chemokines in mecp2-null and wild-type larvae during the first 7 days of development. The panel consisted of the proinflammatory cytokines il1b, interleukin 6 (il6) and tnfa; the proinflammatory chemokine interleukin 8 (cxcl8a); and the anti-inflammatory cytokines il10 and tgfb1. At each time point analyzed over the 7-day time course, tnfa was expressed at dramatically lower levels in mecp2-null embryos and larvae compared with wild-type embryos and larvae of the same age (Fig. 3A). Even at 6 hpf, the earliest time point with clearly detectable tnfa expression in wild-type embryos, its expression was significantly reduced in mecp2-null animals (Fig. 3A). By contrast, we found no significant difference in expression levels of il6, cxcl8a and tgfb1 between mecp2-null and wild-type animals over the developmental time course of 7 days (Fig. S2A-C). We did detect a significant increase in whole-organism il1b and il10 expression in mecp2-null larvae at 5 dpf, after being expressed at wild-type levels for the first 4 days of development (Fig. 3B,C). While il1b reverted back to wild-type levels over the next 2 days (Fig. 3B), the significantly increased expression of il10 peaked at 6 dpf, after which it also trended downwards (Fig. 3C). The expression levels of il1b and il10 indicate a temporal increase in inflammatory signaling, followed by resolution of inflammation.
Fig. 3.
Expression of central inflammatory cytokines is dysregulated in mecp2-null larvae. (A,B,C) qPCR was performed to determine the whole-organism expression of tnfa from 4 hpf to 7 dpf (A), and il1b and il10 from 1 dpf to 7 dpf (B,C), in wild-type or mecp2-null zebrafish. Gene expression is related to the expression of the housekeeping gene tbp, where the fold change relative to gene expression in 1 dpf wild-type embryos is shown (n=3 with 20 embryos or larvae pooled per sample for 1-7 dpf; 30 embryos were pooled per sample for the 4-12 hpf time points; data are representative of two individual experiments). One-way ANOVA with Tukey's post hoc test was used for all statistical analyses (***P<0.001; **P<0.01; *P<0.05; ns, not significant). (D-K) Representative confocal micrographs of 3 dpf Tg(tnfa:eGFP) wild-type and mecp2-null larvae showing the eGFP expression pattern in brain regions in a lateral view (D,H), brain regions in a dorsal view (E,I), posterior gut epithelium in a lateral view (F,J) and dorsal root ganglion neurons in a lateral view (G,K).
We sought to confirm the specific downregulation of tnfa by confocal microscopy imaging of wild-type and mecp2-null larvae carrying a Tg(tnfa:eGFP) reporter that expresses eGFP under control of tnfa regulatory sequences (Fig. S3) (Marjoram et al., 2015). Wild-type larvae of 3 dpf expressed eGFP in brain regions (Fig. 3D,E), posterior gut epithelium (Fig. 3F) and dorsal root ganglion neurons (Fig. 3G). By contrast, 3 dpf mecp2-null larvae had no detectable expression of GFP in any of these tissues (Fig. 3H-K). Because the Tg(tnfa:eGFP) reporter construct introduced an additional tnfa promoter region at a random location in the zebrafish genome (Marjoram et al., 2015), the lack of eGFP expression caused by Mecp2 deficiency appears to be linked to the DNA sequence of the tnfa promoter, rather than its chromosomal location. The decreased expression of tnfa precedes any observable phenotype, suggesting that this is not part of a secondary inflammatory response, but rather caused by genetic dysregulation.
mecp2-null larvae are unable to activate tnfa expression during an acute inflammatory response
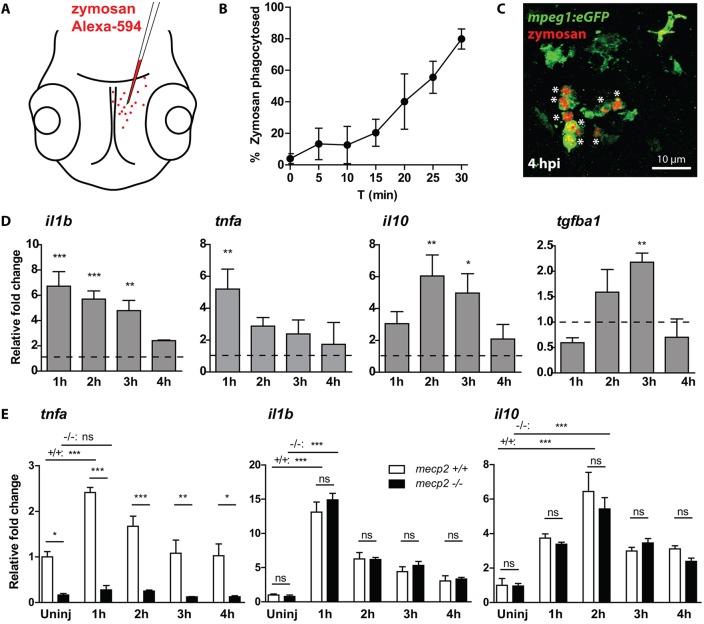
Our finding that tnfa is downregulated in mecp2-null as early as 6 hpf is highly suggestive of a direct effect of Mecp2 on tnfa expression. To test whether the tnfa gene has lost its responsiveness to inflammatory stress signals, we designed an acute inflammation assay by injecting the yeast cell wall particle zymosan, a TLR2 ligand (Underhill et al., 1999), into the brains of 3 dpf zebrafish larvae (Fig. 4A). Fluorescently labeled zymosan injected into the brains of wild-type larvae was rapidly phagocytosed by Tg(mpeg1:eGFP)-positive microglia (Fig. 4B), and all zymosan particles were cleared from the brain tissue at 4 h postinjection (hpi) (Fig. 4C). The clearance of zymosan is accompanied by an acute inflammatory response characterized by an initial upregulation of the proinflammatory cytokine genes il1b and tnfa, followed by an upregulation of the anti-inflammatory cytokine genes il10 and tgfb1 (Fig. 4D). The gene expression levels of these inflammatory cytokines had returned to baseline levels at 4 hpi of zymosan (Fig. 4D). The inflammatory response to zymosan injected into the brain is strongest in dissected heads of wild-type larvae, but its effects on il1b gene expression can also be detected in whole animal preparations of injected zebrafish larvae (Fig. S4A).
Fig. 4.
mecp2-null larvae are unable to increase tnfa expression during an acute inflammatory response. (A) Schematic of the injection of Alexa Fluor 594-labeled zymosan into the brains of 3 dpf zebrafish larvae. (B) The percentage of zymosan particles phagocytosed by Tg(mpeg1:eGFP)-positive cells in wild-type larvae was determined using confocal microscopy of samples fixed every 5 min after injection (n=5 larvae per time point). (C) Representative confocal micrograph of a wild-type Tg(mpeg1:eGFP) larva at 4 hpi. Asterisks indicate zymosan particles phagocytosed by Tg(mpeg1:eGFP)-positive cells. (D) qPCR was performed to determine the whole-organism gene expression level of il1b, tnfa, il10 and tgfb1 relative to the expression of the housekeeping gene tbp. Samples (n=3 with 10 embryos per sample) were taken at 1, 2, 3 and 4 hpi of zymosan or PBS as a control. The relative fold change of zymosan- versus PBS-injected samples is shown for each time point to account for a possible wounding effect by the injection itself. (E) qPCR was performed to determine the whole-organism expression level of il1b, tnfa, il10 and tgfb1 relative to the expression of the housekeeping gene tbp. Wild-type or mecp2-null samples (n=3 with 10 embryos per sample) were taken at 1, 2, 3 and 4 hpi of zymosan. The relative fold change of zymosan-injected larvae versus uninjected wild-type controls is shown for each time point to not exclude a potential different response in mecp2-null samples towards the wound caused by the injection. One-way ANOVA with Tukey's post hoc test was used for all statistical analyses (***P<0.001; **P<0.01; *P<0.05; ns, not significant; data are representative of at least two individual experiments).
We confirmed that Tg(mpeg1:eGFP)-positive cells present in the brain of wild-type and mecp2-null larvae phagocytosed zymosan at comparable rates (Fig. S4B,C). We then used qPCR to compare the gene expression levels of il1b, il10 and tnfa between mecp2-null and wild-type larvae over a 4-h time course after injection of zymosan into the brain. Strikingly, mecp2-null larvae were unable to increase the gene expression level of tnfa in response to zymosan injection, unlike the wild-type control group (Fig. 4E). By comparison, we found no difference in gene expression levels of il1b and il10 between mecp2-null and wild-type larvae during this inflammatory response (Fig. 4E). This demonstrates that the genetic regulation of il1b and il10 in response to a danger signal is not disturbed by Mecp2 deficiency, and suggests that their upregulation during mecp2-null larval development is part of an inflammatory response to disturbances in tissue homeostasis. We conclude that zebrafish tnfa was unresponsive to inflammatory stimulation in the absence of functional Mecp2. These results show that even during an acute stress event, mecp2-null larvae cannot activate tnfa expression, and further suggests that Mecp2 is required for proper expression of tnfa.
Re-expression of mecp2 in mecp2-null zebrafish embryos partially rescues tnfa gene expression
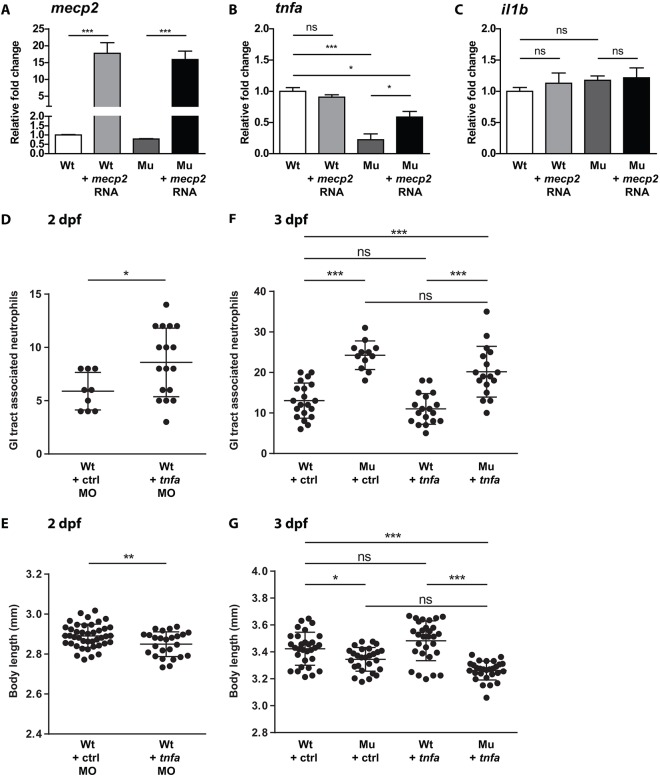
Because mecp2-null larvae were unable to express tnfa at wild-type levels during development or during an acute inflammatory response, we asked whether re-expression of wild-type mecp2 was sufficient to restore tnfa gene expression levels in mecp2 mutants. For this purpose, we injected full-length mecp2 mRNA into mecp2-null or wild-type zygotes. Injection of mecp2 mRNA resulted in a 15- to 20-fold overexpression of mecp2 at 1 dpf (Fig. 5A), or ∼50-fold overexpression at twice the dose (Fig. S5). Even at the highest dose tested, we were unable to detect increased mecp2 expression levels at 3 dpf (Fig. S5), suggesting a rapid decay of the injected mecp2 mRNA. We analyzed the effect of mecp2 overexpression on tnfa and il1b gene expression levels by qPCR. We found that overexpression of wild-type mecp2 in mecp2-null could partially rescue tnfa gene expression levels at 1 dpf, but did not affect tnfa gene expression in wild-type embryos (Fig. 5B). Overexpression of wild-type mecp2 mRNA had no noticeable effect on gene expression levels of il1b in either mecp2-null or wild-type embryos (Fig. 5C). The lower tnfa expression throughout embryonic and larval development, combined with the unresponsiveness of tnfa to inflammatory stimulation in mecp2-null larvae, suggested a direct effect of Mecp2 deficiency on tnfa gene expression. Based on the mecp2 mRNA re-expression experiments, we conclude that Mecp2 is required to allow normal expression of tnfa in zebrafish embryos and larvae. However, overexpression of mecp2 mRNA in wild-type embryos did not alter tnfa expression, indicating that Mecp2 alone is not sufficient to induce tnfa expression. This suggests a mechanism in which Mecp2 allows additional transcriptional regulators to be recruited to modulate tnfa gene expression.
Fig. 5.
Re-expression of mecp2 in mecp2-null zebrafish embryos partially rescues tnfa gene expression, while enforced expression of tnfa does not alleviate phenotypes caused by Mecp2 deficiency. (A,B,C) Wild-type and mecp2-null one-cell stage embryos were injected with 50 pg of full-length mecp2 mRNA. qPCR was performed to determine the whole-organism gene expression level of mecp2 (A), tnfa (B) and il1b (C), relative to the expression of the housekeeping gene tbp. Wild-type and mecp2-null samples (n=3 with 30 embryos pooled per sample) were taken at 24 hpf. The relative fold change of each condition versus uninjected wild-type controls is shown. (D,E) Oligonucleotide morpholino targeting tnfa expression was injected as previously described by Candel et al. (2014). Numbers of Tg(mpx:eGFP)-positive neutrophils associated with the GI tract of 2 dpf control and tnfa morpholino-injected larvae were counted (n≥9 embryos per condition) (D), and the total body lengths of 2 dpf control and tnfa morpholino-injected larvae (n≥25 per condition) were measured (E). (F,G) Wild-type and mecp2-null one-cell stage embryos were injected with tnfa cDNA-containing plasmids as previously described by López-Muñoz et al. (2011). Numbers of Tg(mpx:eGFP)-positive neutrophils associated with the GI tract of 3 dpf wild-type or mecp2-null larvae (injected with control or tnfa cDNA-containing plasmids) were counted (n≥12 embryos per condition) (F), and the total body lengths of the larvae were measured (n≥26 embryos per condition) (G). One-way ANOVA with Tukey's post hoc test was used for all statistical analyses involving more than two groups. Student's t-test was used for all statistical analyses comparing two groups (***P<0.001; **P<0.01; *P<0.05; ns, not significant; data are representative of at least two individual experiments).
Re-expression of tnfa in mecp2-null zebrafish embryos does not rescue RTT phenotypes
We observed that tnfa expression is significantly reduced in mecp2-null zebrafish during embryonic and larval development. The posterior gut epithelium is a prominent source of tnfa expression in wild-type larvae (Fig. 3F), whereas mecp2-null animals had no detectable tnfa expression in this tissue (Fig. 3J). Because dysregulated tnfa expression has previously been implicated in the onset of inflammatory bowel disease in zebrafish larvae (Marjoram et al., 2015), we hypothesized that the lack of tnfa expression might contribute to the development of inflammatory phenotypes in the GI tract of mecp2-null zebrafish. To test for the potential involvement of reduced tnfa expression in the development of RTT phenotypes, we injected a previously described morpholino oligonucleotide targeting tnfa expression into wild-type zygotes (López-Muñoz et al., 2011). We found that knockdown of tnfa resulted in a significant increase in the number of GI tract-associated neutrophils compared to control-injected individuals (Fig. 5D), as well as a significant decrease in total body length (Fig. 5E). Both these phenotypes are also observed in mecp2-null larvae. Next, we attempted to rescue the GI tract neutrophil infiltration and growth reduction observed in mecp2-null larvae by re-expressing tnfa. For this purpose, we injected plasmid encoding full-length tnfa mRNA into mecp2-null or wild-type zygotes (Fig. S6). Enforced expression of tnfa did not reduce the number of neutrophils associated with the GI tract in mecp2-null larvae (Fig. 5F), nor did it restore the reduced body length of mecp2-null larvae (Fig. 5G). In summary, although knockdown of tnfa mimicked the phenotypes observed in mecp2-null larvae, restoring tnfa expression was not sufficient to rescue the growth retardation and GI tract inflammatory phenotypes observed in mecp2-null animals.
RNA sequencing reveals early developmental effects of mecp2 deficiency and predicts RTT phenotypes
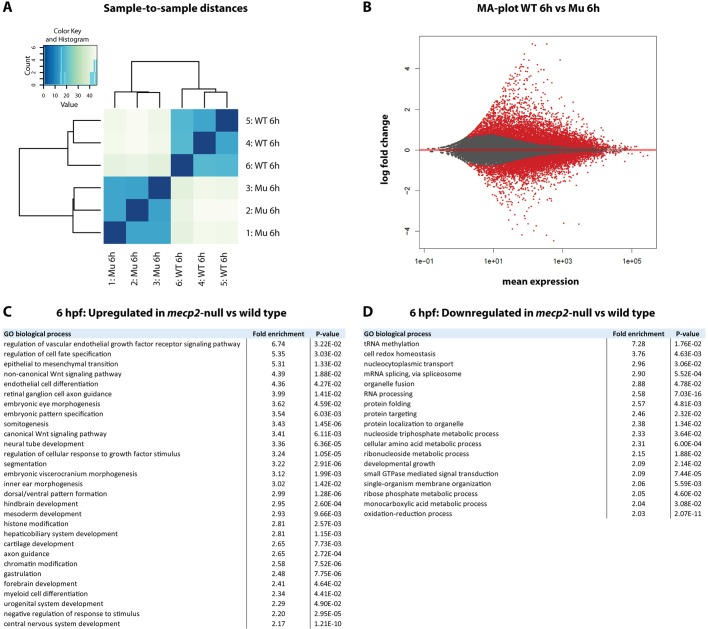
In this study, we have demonstrated that Mecp2 regulates tnfa gene expression levels during early zebrafish embryonic development. Even at 6 hpf, when low levels of tnfa expression can first be detected in wild-type embryos, mecp2-null embryos express significantly reduced levels of tnfa. At this time point of development, zebrafish embryos are undergoing epiboly and gastrulation, which initiate formation of the basic body plan. Although we were able to detect the effect of mecp2 deficiency at this early developmental stage in zebrafish embryos, RTT patients do not display phenotypes until at least 6 months after birth. Therefore, we reasoned that the zebrafish embryo could be highly informative regarding the earliest effects of disrupted MECP2-function that ultimately result in disease phenotypes. To assess the genes that are disrupted during early development of mecp2-null, we performed RNA sequencing to reveal whole-transcriptome differences between 6 hpf mecp2-null and wild-type embryos. For the mecp2-null group, embryos were derived from homozygous mecp2-null parents to avoid the confounding influence of maternally deposited wild-type mecp2 RNA. The three biological replicates of each condition clustered closely together after DESEq2 analysis (Fig. 6A). At 6 hpf, 3837 transcripts were significantly upregulated in mecp2-null versus wild-type embryos, whereas 4217 transcripts were significantly downregulated (Fig. 6B). Although the raw counts for tnfa were lower in mecp2-null compared to wild-type embryos, the average number of raw counts for tnfa in wild-type embryos was too low to demonstrate significance (data not shown).
Fig. 6.
RNA sequencing reveals early developmental effects of mecp2 deficiency relevant to RTT. RNA sequencing was performed on RNA isolated from groups of 6 hpf wild-type and mecp2-null embryos (n=3 biological replicates per condition with 30 embryos pooled per replicate). DESeq2 analysis was performed using http://usegalaxy.org/. (A) A sample-to-sample distances plot for the three biological replicates per condition was used to detect potential outliers. (B) An MA-plot of differential expression caused by Mecp2 deficiency is shown. The log2 fold change is plotted on the y-axis and the average of the counts normalized by size factor is shown on the x-axis. Each gene is represented by a dot. Genes with an adjusted P value <0.05 are shown in red. (C,D) Enriched GO processes for significantly up- or downregulated genes in mecp2-null versus wild-type embryos are listed in the tables. Only GO terms with at least twofold enrichment are shown. For hierarchically clustered GO terms, only the most specific term is included in the list.
For an unbiased assessment of potentially disrupted biological processes in mecp2-null embryos at 6 hpf, we submitted the subsets of differentially up- or downregulated genes to gene ontology (GO) analysis. GO analysis revealed that genes associated with a large range of biological processes were significantly enriched in the differentially expressed subsets, illustrating that mecp2 deficiency has a broad effect on transcription. We limited our further analysis to GO terms with at least twofold enrichment, and only included the most specific GO term for groups of hierarchically clustered terms (Fig. 6C,D). This strict GO term analysis revealed significantly enriched biological processes that are linked to known MECP2-functions, such as epigenetic regulation of transcription and mRNA splicing (Lyst and Bird, 2015). Importantly, GO analysis also identified enriched processes at this early developmental stage, which become relevant to RTT phenotypes at later stages, including neurological development, craniofacial development, vascular dysfunction, redox homeostasis, developmental growth, myeloid cell differentiation and hepatobiliary system development. Finally, GO analysis identified enriched biological processes that, to the best of our knowledge, have not been previously linked to MECP2-function or RTT, including dorsal/ventral pattern formation; protein folding; and intracellular protein targeting. The analysis of the RNA sequencing data underscores the relevance of the zebrafish model for the study of MECP2 function and RTT, while potentially identifying new biological processes of interest.
At the same time, the unbiased analysis of enriched GO terms appears predictive for the growth retardation, myeloid cell number disruption (neutrophilia) and hepatobiliary dysfunction that occurs later during development. For the GO term ‘developmental growth’, 44 out of a total of 187 genes linked to this biological process were significantly downregulated in 6 hpf mecp2-null versus wild-type embryos. For the GO terms ‘myeloid cell differentiation’ and ‘hepaticobiliary system development’, 32 out of a total of 133 or 111 genes linked to this process, respectively, were significantly upregulated in mecp2-null embryos. To investigate the extent of individual gene dysregulation in the absence of Mecp2, we plotted the normalized fold change in all differentially expressed genes linked to these three GO terms in a heatmap (Fig. 7A). Mecp2 deficiency affected the expression levels of a large number of genes related to these biological processes; ∼75% of these genes were up- or downregulated, at a twofold change or less (Fig. 7B). The same observation was made on a genome-wide scale for all significantly upregulated genes in mecp2-null embryos, or to a lesser degree for all significantly downregulated genes (Fig. 7B). Notable exceptions to this general tendency for small differences in gene expression levels are bbs4 and nos1 (GO term ‘developmental growth’); sptb, smad9 and casp3b (GO term ‘myeloid cell differentiation’); and sfrp5 and a2ml (GO term ‘hepaticobiliary system development’). While the neuron-expressed Nitric oxide synthase 1 (Nos1) protein is well known for its role in neurotransmission, mutations in genes from the Bardet-Biedl syndrome (BBS) family, such as bbs4, result in an autosomal recessive disorder characterized by mental retardation and other severe symptoms. The anti-inflammatory adipocytokine Sfrp5 modulates metabolic dysfunction during obesity in mice (Ouchi et al., 2010), and a2ml (Alpha2 macroglobulin-like) was shown to be essential for liver development in zebrafish (Hong and Dawid, 2008). A potential role for Sptb, Smad9 and Casp3b in neutrophilia is not directly clear. For all genes with relatively high differential expression between mecp2-null and wild-type embryos at 6 hpf, it will be interesting to investigate whether their dysregulation extends into developmental phases when RTT phenotypes first arise.
Fig. 7.

Heatmap of differentially expressed genes in mecp2-null embryos. (A) Heatmap displaying the extent of differential gene expression between 6 hpf mecp2-null versus wild-type embryos. The genes incorporated in the heatmap represent all differentially expressed genes that belong to the GO terms ‘developmental growth’ (downregulated genes), ‘myeloid cell differentiation’ (upregulated genes) and ‘hepaticobiliary system development’ (upregulated genes). For all genes, the positive or negative normalized fold change (nFC) for mecp2-null embryos versus wild-type embryos is shown. (B) The graph displays the percentage of significantly differentially expressed genes in mecp2-null versus wild-type embryos with a fold change ≤2 or >2. The following groups are shown: significantly differentially expressed genes belonging to the GO terms ‘developmental growth’, ‘myeloid cell differentiation’ and ‘hepaticobiliary system development’; genome-wide significantly upregulated genes; genome-wide significantly downregulated genes; and all significantly differentially expressed genes.
DISCUSSION
The large body of literature on MECP2 and RTT contains evidence that mutations in MECP2, as well as its overexpression caused by duplication of its genetic locus, result in abnormal functioning of the immune system (Bauer et al., 2015; Cortelazzo et al., 2014; Cronk et al., 2015; Derecki et al., 2012; Leoncini et al., 2015; Pecorelli et al., 2016). Furthermore, because RTT patients acquire disease symptoms after an apparently normal early development, we hypothesized that misregulated responses to external or internal inflammatory stimuli encountered during development could play a key role in the onset of RTT. We therefore set out to test the potential function of zebrafish Mecp2 as an epigenetic regulator of immune and inflammatory responses during development.
Indeed, gene expression levels of the inflammation marker crp and mobilization of neutrophils provided evidence for the presence of inflammation in mecp2-null larvae after an inflammation-free early development. Increased gene expression levels of il1b and il10 measured at a whole-organism level were found to be involved in this inflammatory response. We hypothesized that, when lacking the epigenetic regulator Mecp2, the zebrafish genes encoding Il1b and Il10 were hyper-responsive to inflammatory stimulation. By submitting both wild-type and mecp2-null larvae to an acute inflammation assay, we were able to disprove this hypothesis. The expression levels of il1b and il10 were regulated in a similar fashion in response to inflammatory stimulation in wild-type and mecp2-null larvae. Combined with the fact that il1b and il10 were expressed at wild-type levels in mecp2-null embryos during early development, we suggest that the peak in expression of these pro- and anti-inflammatory cytokines was a response to a disturbance in tissue homeostasis in the absence of Mecp2.
We observed an increased infiltration of neutrophils into the GI tract of mecp2-null larvae, combined with GI tract phenotypes and a potential disturbance of bile production or flow. These observations are relevant, because RTT patients frequently display GI tract phenotypes, including GI dismotility (Baikie et al., 2014). Additionally, cholesterol metabolism is altered in RTT patients (Segatto et al., 2014), and limiting cholesterol biosynthesis alleviated RTT symptoms and increased the survival of mecp2-null mice (Buchovecky et al., 2013). While bile acids, a major component of cholesterol, have immunomodulatory effects (Brestoff and Artis, 2013), inflammation can also suppress the expression of bile transporters and thereby reduce the flow of bile (Kosters and Karpen, 2010). With the proven contribution of zebrafish larval and embryonic models to the study of liver diseases and inflammatory bowel diseases (Goessling and Sadler, 2015; Love et al., 2007), the zebrafish mecp2-null mutant might be ideally suited to illuminating the role of inflammation in the GI tract of RTT patients.
The most striking result obtained during this study was that zebrafish tnfa was not expressed at normal levels in the absence of functional Mecp2 during embryonic and larval development, or during an acute inflammatory response. Combined with our finding that re-expression of wild-type Mecp2 can partially rescue tnfa expression in mecp2-null embryos, we conclude that zebrafish Mecp2 influences the transcriptional potential of tnfa. Importantly, the dysregulated expression levels of tnfa at 6 hpf precede any of the developmental phenotypes observed in the absence of functional Mecp2, and could potentially be a causative factor for RTT features displayed later during development. Indeed, knockdown of tnfa gene expression induced neutrophilic infiltration into the GI tract of zebrafish larvae, a phenotype resembling that observed in mecp2-null individuals. In this light, it is interesting to note that genetic inhibition of Tnfa and Tnfr2 in zebrafish previously resulted in the mobilization of neutrophils to the skin, revealing a crucial role for the TNFα/TNFR2 axis in the protection against Duox1-mediated oxidative stress (Candel et al., 2014). RTT patients often display oxidative stress, and we identified the GO term ‘redox homeostasis’ as one of the biological pathways altered in mecp2-null embryos. The potential link between reduced tnfa expression in the GI tract and inflammation caused by increased oxidative stress is therefore an interesting topic for further study in mecp2-null zebrafish embryos and larvae.
However, we found that re-expression of tnfa did not alleviate the phenotypes observed in mecp2-null zebrafish. Transcriptome analysis revealed that a total of 8054 genes are differentially expressed between mecp2-null embryos and wild types at 6 hpf. Even if the enforced expression of tnfa could be titrated to match wild-type endogenous levels, which differ according to tissue and circumstance, it indeed seems unlikely that re-expression of only one dysregulated gene would be sufficient to alleviate the observed RTT features.
The observation that overexpression of mecp2 in wild-type embryos did not raise tnfa gene expression levels indicates that the presence of Mecp2 alone is not sufficient to increase transcription of tnfa. The experiments performed in this study also provide clues into which aspect of the diverse Mecp2 functions might be involved in the regulation of tnfa (Lyst and Bird, 2015). The Tg(tnfa:eGFP) construct (Marjoram et al., 2015), introducing an additional copy of the tnfa promoter in the genome, did not drive expression of eGFP in the absence of Mecp2, indicating that the regulatory sequences of the tnfa transgene are critically important for its regulation by Mecp2. It is possible that sequence-specific DNA-binding of Mecp2 results in chromatin remodeling that increases the transcriptional potential of the zebrafish tnfa gene (Ballestar et al., 2000; Baubec et al., 2013; Yusufzai and Wolffe, 2000). Another plausible explanation is that Mecp2 is involved in the transcriptional activation of tnfa by recruiting the co-activator CREB1, since the CREB-binding protein (CBP)/p300 was shown to play a stimulus-dependent role in T cell receptor-activated TNFα gene expression (Falvo et al., 2000).
Several in vivo and in vitro models exist for the study of RTT and MECP2 function, including Mecp2-null mutant mice (Chen et al., 2001; Guy et al., 2001); Xenopus laevis with truncated Mecp2 (Stancheva et al., 2003); induced pluripotent stem cells (iPSCs) from RTT patients' fibroblasts (Marchetto et al., 2010); mecp2-null mutant zebrafish (Pietri et al., 2013); and most recently transgenic monkeys overexpressing MECP2 (Liu et al., 2016). The results obtained using these different models are sometimes conflicting and Mecp2 function varies between different tissues or cells of the same organism. For instance, the NFκB-pathway component Irak1 was specifically upregulated in cortical callosal projection neurons in Mecp2-null mice, but not in distinct organs such as the lungs, heart, spleen or kidney (Kishi et al., 2016). Even when the same model organism and experimental conditions are used, results can still differ fundamentally (Derecki et al., 2012; Wang et al., 2015). In this regard, while we consistently found zebrafish tnfa to be downregulated in mecp2-null animals, Cronk et al. (2015) found an increase in Tnfa-induced transcriptional signature genes specifically in isolated Mecp2-null microglia. The different cell source utilized in these experiments might explain the conflicting results, making it worthwhile to analyze tnfa transcript levels in isolated zebrafish mecp2-null microglia and other immune cells.
With the sometimes conflicting findings on the effect of Mecp2 deficiency under differing conditions and from various model systems, it is challenging to reach a unified and evolutionary conserved conclusion on Mecp2 function. Nonetheless, we believe that contributions from each individual model system will ultimately help to understand the function of MECP2 in health and disease. We have used the zebrafish embryonic and larval system to demonstrate that Mecp2 is required for tnfa expression during zebrafish development and inflammation. Besides this, our RNA sequencing results provide insights into the earliest genetic alterations that occur in the absence of MECP2 function, which ultimately could result in RTT phenotypes. Furthermore, zebrafish embryos are amenable to high-throughput screening for drugs with the potential to remedy these phenotypes (Tan and Zon, 2011). We believe that these findings have the potential to instruct future studies in zebrafish and other model systems to increase our understanding of MECP2 function and its role in RTT pathogenesis.
MATERIALS AND METHODS
Zebrafish husbandry and maintenance
Zebrafish (Danio rerio) were maintained and used for experiments according to the guidelines of the UCSD Institutional Animal Care and Use Committee. The following zebrafish lines were used: AB (wild-type strain); mecp2Q63* mutants (Pietri et al., 2013); Tg(mpx:eGFP)i114 (Renshaw et al., 2006); Tg(mpeg1:eGFP)gl22 (Ellett et al., 2011); Tg(tnfa:eGFP) (Marjoram et al., 2015). Genotyping of mecp2Q63* mutants occurred as previously described (Pietri et al., 2013). When needed for experimental purposes, zebrafish were anesthetized using Tricaine (200 µg/ml).
Microscopy
For stereomicroscopy, embryos and larvae were mounted in E3 medium containing 3% methyl cellulose (Sigma-Aldrich). Brightfield images were acquired using a Leica MZ16 stereomicroscope with a Leica DFC295 camera (Leica Microsystems). Epifluorescence images were acquired using an AxioZoom.V16 stereomicroscope (Zeiss). For confocal microscopy, larvae were mounted in E3 medium containing 0.5% low melting point agarose (Sigma-Aldrich). Confocal micrographs were acquired using a Leica SP5 confocal system (Leica Microsystems). Images were created using Imaris (Bitplane) and ImageJ (https://imagej.nih.gov/ij/) software.
qPCR
mRNA was isolated using the RNeasy mini kit according to the manufacturer's instructions (Qiagen). cDNA was synthesized using the iScript cDNA synthesis kit according to the manufacturer's instructions (BioRad). qPCR was performed using iQ SYBR Green Supermix (BioRad) and the BioRad CFX96 real-time system according to the manufacturer's instructions. Gene expression levels were calculated relative to the expression of the housekeeping gene TATA box binding protein according to the 2−ΔΔCt method. Primers used for qPCR analysis of gene expression are listed in Table S1.
Microinjection of zymosan particles
Zebrafish larvae of 3 dpf were positioned with the dorsal side up to allow injection of 1 nl PBS containing 100-150 Alexa Fluor 594-labeled Zymosan A (S. cerevisiae) BioParticles (Molecular Probes) into the brain. As a control for a potential wounding effect, 1 nl of sterile PBS was injected in a similar manner. The percentage of zymosan particles phagocytosed by Tg(mpeg1:eGFP)-positive cells was determined based on confocal micrographs of the brain.
Microinjection of mRNA, plasmids and antisense oligonucleotide morpholinos
A gBlock (Integrated DNA Technology) containing full-length zebrafish mecp2 cDNA (ENSDART00000040672) was cloned into a Zero Blunt TOPO PCR vector according to the manufacturer's instructions (Life Sciences). Zebrafish mecp2 mRNA was synthesized using the mMessage mMachine SP6 Transcription Kit according to the manufacturer's instructions (Invitrogen). Then, 50 pg or 100 pg mecp2 mRNA was injected into the yolk of one-cell stage zebrafish embryos. The antisense oligonucleotide morpholino targeting mecp2 expression was injected as described by Gao et al. (2015), while the antisense oligonucleotide morpholino targeting tnfa expression was injected as described by López-Muñoz et al. (2011). Control plasmid (pCS2+) and Tnfa plasmid (Roca et al., 2008) (20 pg) were injected into the yolk sac of one-cell stage embryos.
RNA sequencing
mRNA was isolated using the RNeasy mini kit according to the manufacturer's instructions (Qiagen). Library preparation and sequencing was performed by the Institute for Genomic Medicine Center at the University of California, San Diego. RNA sequencing was performed on an Illumina HiSeq4000 platform using single reads of 50 bases in length. RNA sequencing data were mapped to the zebrafish genome (version Zv9) using TopHat 2.1.1 (http://ccb.jhu.edu/software/tophat/index.shtml). Raw counts were submitted to DESeq2 analysis using the Galaxy website (http://usegalaxy.org/). GO analysis was performed using the Gene Ontology website (http://geneontology.org/). The heatmap displaying differential gene expression was created using Gene-E software (Broad Institute; https://software.broadinstitute.org/GENE-E/). RNA sequencing data are accessible under Gene Expression Omnibus accession number GSE80348.
Statistical analysis
All data (mean±s.e.m.), except for the RNA sequencing data, were analyzed (Prism 5.0, GraphPad Software) using unpaired, two-tailed Student's t-tests for comparisons between two groups, or one-way ANOVA with Tukey's Multiple Comparison method as a post hoc test for other data (***P<0.001; **P<0.01; *P<0.05; ns, not significant).
Supplementary Material
Acknowledgements
We thank Karen Ong and Jingjing Kobayashi-Sun for laboratory support; Roger Rainville for animal care; and Pankaj Sahai and Kanako Lewis for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.T., M.V., B.G.W., A.R.M.; Methodology: D.T., M.V., V.S., A.R.M.; Validation: O.S., B.G.W., R.E.-P., V.S.; Formal analysis: M.V.; Investigation: M.V., O.S., B.G.W., R.E.-P., V.S.; Resources: T.P., M.B.; Writing - original draft: D.T., M.V.; Writing - review & editing: D.T., O.S., B.G.W., R.E.-P., A.R.M.; Supervision: D.T., A.R.M.; Project administration: D.T.; Funding acquisition: D.T., M.V.
Funding
This work was supported by a Simons Foundation Autism Research Initiative Pilot Award (346154).
Data availability
RNA sequencing data are available at Gene Expression Omnibus under accession number GSE80348.
Supplementary information
Supplementary information available online at http://dmm.biologists.org/lookup/doi/10.1242/dmm.026922.supplemental
References
- Akira S. and Takeda K. (2004). Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499-511. 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- Amir R. E., Veyver I. B., Wan M., Tran C. Q., Francke U. and Zoghbi H. Y. (1999). Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185-188. 10.1038/13810 [DOI] [PubMed] [Google Scholar]
- Baikie G., Ravikumara M., Downs J., Naseem N., Wong K., Percy A., Lane J., Weiss B., Ellaway C., Bathgate K. et al. (2014). Gastrointestinal Dysmotility in Rett Syndrome. J. Pediatr. Gastr. Nutr. 58, 237 10.1097/MPG.0000000000000200 [DOI] [PubMed] [Google Scholar]
- Ballestar E., Yusufzai T. M. and Wolffe A. P. (2000). Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry 39, 7100-7106. 10.1021/bi0001271 [DOI] [PubMed] [Google Scholar]
- Baubec T., Ivánek R., Lienert F. and Schübeler D. (2013). Methylation-Dependent and -Independent Genomic Targeting Principles of the MBD Protein Family. Cell 153, 480-492. 10.1016/j.cell.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Bauer M., Kölsch U., Krüger R., Unterwalder N., Hameister K., Kaiser F. M., Vignoli A., Rossi R., Botella M. P., Budisteanu M. et al. (2015). Infectious and immunologic phenotype of MECP2 duplication syndrome. J. Clin. Immunol. 35, 168-181. 10.1007/s10875-015-0129-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A. A. (2002). Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23, 509-512. 10.1016/S1471-4906(02)02317-7 [DOI] [PubMed] [Google Scholar]
- Bertrand J. Y., Kim A. D., Violette E. P., Stachura D. L., Cisson J. L. and Traver D. (2007). Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 134, 4147-4156. 10.1242/dev.012385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff J. R. and Artis D. (2013). Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 14, 676-684. 10.1038/ni.2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudal E., Ulanova L. S., Lampe E. O., Rishovd A.-L., Griffiths G. and Winther-Larsen H. C. (2014). Establishment of Three Francisella Infections in Zebrafish Embryos at Different Temperatures. Infect. Immun. 82, 2180-2194. 10.1128/IAI.00077-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S., Liu K. Y., Lindenbergh–Kortleve D., Samsom J. N., Furuta G. T., Renshaw S. A., Willemsen R. and Nieuwenhuis E. E. S. (2009). Oxazolone-Induced Enterocolitis in Zebrafish Depends on the Composition of the Intestinal Microbiota. Gastroenterology 137, 1757-1767.e1. 10.1053/j.gastro.2009.07.069 [DOI] [PubMed] [Google Scholar]
- Buchovecky C. M., Turley S. D., Brown H., Kyle S. M., McDonald J. G., Liu B., Pieper A. A., Huang W., Katz D. M., Russell D. W. et al. (2013). A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat. Genet. 45, 1013-1020. 10.1038/ng.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candel S., de Oliveira S., López-Muñoz A., García-Moreno D., Espín-Palazón R., Tyrkalska S. D., Cayuela M. L. L., Renshaw S. A., Corbalán-Vélez R., Vidal-Abarca I. et al. (2014). Tnfa signaling through tnfr2 protects skin against oxidative stress-induced inflammation. PLoS Biol. 12, e1001855 10.1371/journal.pbio.1001855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Z., Akbarian S., Tudor M. and Jaenisch R. (2001). Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 27, 327-331. 10.1038/85906 [DOI] [PubMed] [Google Scholar]
- Cortelazzo A., De Felice C., Guerranti R., Signorini C., Leoncini S., Pecorelli A., Zollo G., Landi C., Valacchi G., Ciccoli L. et al. (2014). Subclinical inflammatory status in Rett syndrome. Mediators Inflamm. 2014, 480980 10.1155/2014/480980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk J. C., Derecki N. C., Ji E., Xu Y., Lampano A. E., Smirnov I., Baker W., Norris G. T., Marin I., Coddington N. et al. (2015). Methyl-CpG Binding Protein 2 Regulates Microglia and Macrophage Gene Expression in Response to Inflammatory Stimuli. Immunity 42, 679-691. 10.1016/j.immuni.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M., Yin C., Shin D., Ninov N., Debrito Carten J., Pan L., Ma T. P., Farber S. A., Moens C. B. and Stainier D. Y. R. (2012). sox9b Is a Key Regulator of Pancreaticobiliary Ductal System Development. PLoS Genet. 8, e1002754 10.1371/journal.pgen.1002754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki N. C., Cronk J. C., Lu Z., Xu E., Abbott S. B. G., Guyenet P. G. and Kipnis J. (2012). Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484, 105-109. 10.1038/nature10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F., Pase L., Hayman J. W., Andrianopoulos A. and Lieschke G. J. (2011). mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49-e56. 10.1182/blood-2010-10-314120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo J., Brinkman B. M. N., Tsytsykova A. V., Tsai E. Y., Yao T.-P., Kung A. L. and Goldfeld A. E. (2000). A stimulus-specific role for CREB-binding protein (CBP) in T cell receptor-activated tumor necrosis factor alpha gene expression. Proc. Natl. Acad. Sci. USA 97, 3925-3929. 10.1073/pnas.97.8.3925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa S., Pecorelli A., D'Esposito M., Valacchi G. and Hajek J. (2015). Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome. Free Radic. Biol. Med. 88, 81-90. 10.1016/j.freeradbiomed.2015.04.019 [DOI] [PubMed] [Google Scholar]
- Gao H., Bu Y., Wu Q., Wang X., Chang N., Lei L., Chen S., Liu D., Zhu X., Hu K. et al. (2015). Mecp2 regulates neural cell differentiation by suppressing the Id1 to Her2 axis in zebrafish. J. Cell Sci. 128, 2340-2350. 10.1242/jcs.167874 [DOI] [PubMed] [Google Scholar]
- Goessling W. and Sadler K. C. (2015). Zebrafish: An Important Tool for Liver Disease Research. Gastroenterology 149, 1361-1377. 10.1053/j.gastro.2015.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J., Hendrich B., Holmes M., Martin J. and Bird A. (2001). A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 27, 322-326. 10.1038/85899 [DOI] [PubMed] [Google Scholar]
- Herbomel P., Thisse B. and Thisse C. (2001). Zebrafish Early Macrophages Colonize Cephalic Mesenchyme and Developing Brain, Retina, and Epidermis through a M-CSF Receptor-Dependent Invasive Process. Dev. Biol. 238, 274-288. 10.1006/dbio.2001.0393 [DOI] [PubMed] [Google Scholar]
- Hong S.-K. and Dawid I. B. (2008). Alpha2 macroglobulin-like is essential for liver development in zebrafish. PLoS ONE 3, e3736 10.1371/journal.pone.0003736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane D. A. and Kimmel C. B. (1993). The zebrafish midblastula transition. Development 119, 447-456. [DOI] [PubMed] [Google Scholar]
- Kimmel C., Sepich D. and Trevarrow B. (1988). Development of segmentation in zebrafish. Dev. Camb. Engl. 104 Suppl, 197-207. [DOI] [PubMed] [Google Scholar]
- Kishi N., MacDonald J. L., Ye J., Molyneaux B. J., Azim E. and Macklis J. D. (2016). Reduction of aberrant NF-κB signalling ameliorates Rett syndrome phenotypes in Mecp2-null mice. Nat. Commun. 7, 10520 10.1038/ncomms10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosters A. and Karpen S. (2010). The Role of Inflammation in Cholestasis: Clinical and Basic Aspects. Semin. Liver Dis. 30, 186-194. 10.1055/s-0030-1253227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guyader D., Redd M. J., Colucci-Guyon E., Murayama E., Kissa K., Briolat V., Mordelet E., Zapata A., Shinomiya H. and Herbomel P. (2008). Origins and unconventional behavior of neutrophils in developing zebrafish. Blood 111, 132-141. 10.1182/blood-2007-06-095398 [DOI] [PubMed] [Google Scholar]
- Leoncini S., Felice C., Signorini C., Zollo G., Cortelazzo A., Durand T., Galano J.-M., Guerranti R., Rossi M. and Ciccoli L. (2015). Cytokine Dysregulation in MECP2-and CDKL5-Related Rett Syndrome: Relationships with Aberrant Redox Homeostasis, Inflammation, and -3 PUFAs. Oxid. Med. Cell. Longev. 2015, 421624 10.1155/2015/421624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. D., Meehan R. R., Henzel W. J., Maurer-Fogy I., Jeppesen P., Klein F. and Bird A. (1992). Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69, 905-914. 10.1016/0092-8674(92)90610-O [DOI] [PubMed] [Google Scholar]
- Liu Z., Li X., Zhang J.-T., Cai Y.-J., Cheng T.-L., Cheng C., Wang Y., Zhang C.-C., Nie Y.-H., Chen Z.-F. et al. (2016). Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature 530, 98-102. 10.1038/nature16533 [DOI] [PubMed] [Google Scholar]
- López-Muñoz A., Sepulcre M. P., Roca F. J., Figueras A., Meseguer J. and Mulero V. (2011). Evolutionary conserved pro-inflammatory and antigen presentation functions of zebrafish IFNγ revealed by transcriptomic and functional analysis. Mol. Immunol. 48, 1073-1083. 10.1016/j.molimm.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Love D. R., Lan C.-C., Dodd A., Shelling A. N., McNabb W. C. and Ferguson L. R. (2007). Modeling inflammatory bowel disease: the zebrafish as a way forward. Expert Rev. Mol. Diagn. 7, 177-193. 10.1586/14737159.7.2.177 [DOI] [PubMed] [Google Scholar]
- Lyst M. J. and Bird A. (2015). Rett syndrome: a complex disorder with simple roots. Nat. Rev. Genet. 16, 261-275. 10.1038/nrg3897 [DOI] [PubMed] [Google Scholar]
- Marchetto M. C. N., Carromeu C., Acab A., Yu D., Yeo G. W., Mu Y., Chen G., Gage F. H. and Muotri A. R. (2010). A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527-539. 10.1016/j.cell.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoram L., Alvers A., Deerhake M. E., Bagwell J., Mankiewicz J., Cocchiaro J. L., Beerman R. W., Willer J., Sumigray K. D., Katsanis N. et al. (2015). Epigenetic control of intestinal barrier function and inflammation in zebrafish. Proc. Natl. Acad. Sci. 112, 2770-2775. 10.1073/pnas.1424089112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. (2002). The danger model: a renewed sense of self. Science 296, 301-305. 10.1126/science.1071059 [DOI] [PubMed] [Google Scholar]
- Medzhitov R. and Janeway C. Jr (2000). The Toll receptor family and microbial recognition. Trends Microbiol. 8, 452-456. 10.1016/S0966-842X(00)01845-X [DOI] [PubMed] [Google Scholar]
- Motil K. J., Caeg E., Barrish J. O., Geerts S., Lane J. B., Percy A. K., Annese F., McNair L., Skinner S. A., Lee H.-S. et al. (2012). Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 55, 292-298. 10.1097/MPG.0b013e31824b6159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlers S. H., Flores M. V., Okuda K. S., Hall C. J., Crosier K. E. and Crosier P. S. (2011). A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Dev. Dyn. 240, 288-298. 10.1002/dvdy.22519 [DOI] [PubMed] [Google Scholar]
- Okamura J. M., Miyagi J. M., Terada K. and Hokama Y. (1990). Potential clinical applications of c-reactive protein. J. Clin. Lab. Anal. 4, 231-235. 10.1002/jcla.1860040316 [DOI] [PubMed] [Google Scholar]
- Ouchi N., Higuchi A., Ohashi K., Oshima Y., Gokce N., Shibata R., Akasaki Y., Shimono A. and Walsh K. (2010). Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329, 454-457. 10.1126/science.1188280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorelli A., Cervellati F., Belmonte G., Montagner G., Waldon P. A., Hayek J., Gambari R. and Valacchi G. (2016). Cytokines profile and peripheral blood mononuclear cells morphology in Rett and autistic patients. Cytokine 77, 180-188. 10.1016/j.cyto.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Pietri T., Roman A.-C., Guyon N., Romano S. A. A., Washbourne P., Moens C. B., de Polavieja G. G. and Sumbre G. (2013). The first mecp2-null zebrafish model shows altered motor behaviors. Front. Neural Circuits 7, 118 10.3389/fncir.2013.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw S. A. and Trede N. S. (2011). A model 450 million years in the making: zebrafish and vertebrate immunity. Dis. Model. Mech. 5, 38-47. 10.1242/dmm.007138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw S. A., Loynes C. A., Trushell D. M. I., Elworthy S., Ingham P. W. and Whyte M. K. B. (2006). A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976-3978. 10.1182/blood-2006-05-024075 [DOI] [PubMed] [Google Scholar]
- Roca F. J., Mulero I., López-Muñoz A., Sepulcre M. P., Renshaw S. A., Meseguer J. and Mulero V. (2008). Evolution of the inflammatory response in vertebrates: fish TNF-alpha is a powerful activator of endothelial cells but hardly activates phagocytes. J. Immunol. 181, 5071-5081. 10.4049/jimmunol.181.7.5071 [DOI] [PubMed] [Google Scholar]
- Segatto M., Trapani L., Di Tunno I., Sticozzi C., Valacchi G., Hayek J. and Pallottini V. (2014). Cholesterol Metabolism Is Altered in Rett Syndrome: A Study on Plasma and Primary Cultured Fibroblasts Derived from Patients. PLoS ONE 9, e104834 10.1371/journal.pone.0104834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Feodorova Y., Guy J., Peichl L., Jost K., Kimura H., Cardoso M., Bird A., Leonhardt H., Joffe B. et al. (2014). DNA methylation reader MECP2: cell type- and differentiation stage-specific protein distribution. Epigenetics Chromatin 7, 17 10.1186/1756-8935-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachura D. L. and Traver D. (2011). Cellular dissection of zebrafish hematopoiesis. Methods Cell Biol. 101, 75-110. 10.1016/B978-0-12-387036-0.00004-9 [DOI] [PubMed] [Google Scholar]
- Stancheva I., Collins A. L., Van, den Veyver I. B., Zoghbi H. and Meehan R. R. (2003). A mutant form of MeCP2 protein associated with human Rett syndrome cannot be displaced from methylated DNA by notch in Xenopus embryos. Mol. Cell 12, 425-435. 10.1016/S1097-2765(03)00276-4 [DOI] [PubMed] [Google Scholar]
- Svahn A. J., Graeber M. B., Ellett F., Lieschke G. J., Rinkwitz S., Bennett M. R. and Becker T. S. (2013). Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev. Neurobiol. 73, 60-71. 10.1002/dneu.22039 [DOI] [PubMed] [Google Scholar]
- Tan J. and Zon L. (2011). Chapter 21 Chemical Screening in Zebrafish for Novel Biological and Therapeutic Discovery. Boston: ScienceDirect. [DOI] [PubMed] [Google Scholar]
- Tarquinio D. C., Motil K. J., Hou W., Lee H.-S., Glaze D. G., Skinner S. A., Neul J. L., Annese F., McNair L., Barrish J. O. et al. (2012). Growth failure and outcome in Rett syndrome: specific growth references. Neurology 79, 1653-1661. 10.1212/WNL.0b013e31826e9a70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill D. M., Ozinsky A., Hajjar A. M. and Stevens A. (1999). The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811-815. 10.1038/35005543 [DOI] [PubMed] [Google Scholar]
- van der Vaart M., Spaink H. P. and Meijer A. H. (2012). Pathogen recognition and activation of the innate immune response in zebrafish. Adv. Hematol. 2012, 159807 10.1155/2012/159807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H., Bauters M., Ignatius J., Jansen M., Raynaud M., Hollanders K., Lugtenberg D., Bienvenu T., Jensen L. R., Gécz J. et al. (2005). Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am. J. Hum. Genet. 77, 442-453. 10.1086/444549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wegener J., Huang T.-W., Sripathy S., De Jesus-Cortes H., Xu P., Tran S., Knobbe W., Leko V., Britt J. et al. (2015). Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature 521, E1-E4. 10.1038/nature14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. K. and Huttenlocher A. (2011). Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J. Leukoc. Biol. 89, 661-667. 10.1189/jlb.1010567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufzai T. M. and Wolffe A. (2000). Functional consequences of Rett syndrome mutations on human MeCP2. Nucleic Acids Res. 28, 4172-4179. 10.1093/nar/28.21.4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.