ABSTRACT
For hundreds of years, biologists have studied accessible organisms such as garden peas, sea urchins collected at low tide, newt eggs, and flies circling rotten fruit. These organisms help us to understand the world around us, attracting and inspiring each new generation of biologists with the promise of mystery and discovery. Time and time again, what we learn from such simple organisms has emphasized our common biological origins by proving to be applicable to more complex organisms, including humans. Yet, biologists are increasingly being tasked with developing applications from the known, rather than being allowed to follow a path to discovery of the as yet unknown. Here, we provide examples of important lessons learned from research using selected non-vertebrate organisms. We argue that, for the purpose of understanding human disease, simple organisms cannot and should not be replaced solely by human cell-based culture systems. Rather, these organisms serve as powerful discovery tools for new knowledge that could subsequently be tested for conservation in human cell-based culture systems. In this way, curiosity-driven biological research in simple organisms has and will continue to pay huge dividends in both the short and long run for improving the human condition.
KEY WORDS: C. elegans, Drosophila, Invertebrates, Sea urchin, Yeast
Summary: Curiosity-driven research using simple organisms has and will continue to lead to fundamental discoveries about biology that are directly applicable to improving the human condition.
Introduction
The emphases and directions of medical practice are influenced not only by scientific evidence but also by factors such as financial interests and societal trends. For example, interventions such as cupping, the application of suction cups to the skin, have gained popularity not due to scientific evidence (Lee et al., 2011), but based on popular figures promoting the practice. In contrast, impactful progress in medicine invariably follows major advances in biological understanding due to scientific discovery. Examples extend from Pasteur's recognition of the infectious basis of many diseases to the recent discovery of CRISPR/Cas9-based genome editing (see Box 1 for a glossary of terms) (McNutt, 2015; Porter, 1961). Such advances in our understanding of biology have driven revolutionary advances in medicine, in addition to the incremental and unsteady progress that medicine typically makes. Despite this history of success, science is increasingly being tasked to focus on application rather than discovery (Fang and Casadevall, 2010; Hand et al., 2013; Minogue and Wolinsky, 2010). It is therefore with a sense of urgency that we write this article to defend the winning strategy of ‘discovery first’.
Box 1. Glossary.
Centrosomes: multi-protein structures that determine spindle polarity in mitosis through their function as primary microtubule-organizing centers of the cell.
Chromatin: DNA complexed with associated proteins, particularly histones.
Cleavage furrow: the constriction that cleaves the cell between the separated sister chromosomes at the end of mitosis.
Conditional mutants: mutants that show the phenotype of interest only under specific conditions.
CRISPR/Cas9: clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9. This term is used to refer to a genome editing tool.
Differential interference contrast (DIC): a type of microscopy that uses polarized light to distinguish subcellular features in transparent objects.
Epigenetic regulation: alteration in gene transcription due to changes in chemical modification of DNA or associated proteins.
Epistasis: a genetic analysis method in which the phenotype of a mutant defective in two genes of interest is compared to the phenotype of single mutants in each gene. The results can be used to infer the order in which the products of the two genes act in a biological pathway.
Genetic screen: an experimental technique in which individuals are selected from a mutagenized population based on a specific phenotype.
Mechanical fragmentation: method used to cut sea urchin zygotes into viable fragments containing different nuclear makeups to, for example, dissect the contribution of nucleus vs cytoplasm.
Mendelian ratio: the ratio of genotypes or phenotypes in the progeny when a trait is inherited according to the law of Mendel.
Mendelism: inheritance of traits according to the laws of Mendel.
Microinjection: injection of foreign substances into cells. Microinjection of sea urchin zygotes can be used, for example, to knock down gene expression with antisense morpholino oligonucleotides.
Model organism: experimentally tractable organism used to understand fundamental biological mechanisms that also apply to other organisms of interest.
Organoid: an in vitro 3D cellular cluster derived exclusively from primary tissue, embryonic stem cells or induced pluripotent stem cells (iPSCs) that is capable of self-renewal and self-organization, and that exhibits similar organ functionality as the tissue of origin. Definition accredited to Fatehullah et al., 2016.
Polyspermy: fertilization with more than one sperm per egg.
Position effect variegation (PEV): a phenotype whereby cells with the same genotype sometimes exhibit different phenotypes because of transcriptional inactivation of a gene that is abnormally juxtaposed to heterochromatin.
Our understanding of human diseases and the ability to treat them hinges on a foundation of knowledge about basic biology. This knowledge has been gained largely by research using organisms that are experimentally tractable in ways that humans or human cells can never be. Those who do not know the history of yeast, flies and other non-vertebrate organisms and their contributions to biomedicine may consider such studies as unworthy of participation or of funding. Given the growing excitement about human stem-cell-based cell culture systems, particularly organoids (Box 1), some may argue that organisms that do not recapitulate all of the complexity of humans will cease to be useful in the near future. This line of thought is not only incorrect but could hamper scientific progress.
In this article, we highlight the continuing value of curiosity-driven research, with a focus on widely used non-vertebrate experimental organisms. These are often referred to as ‘model organisms’ (Box 1) to highlight their utility in discovering and understanding fundamental biological principles that also apply to other organisms, particularly humans, and to mechanisms of disease. Although we use this term for the remainder of the article, we wish to emphasize that research using such organisms need not be motivated solely for the purpose of modeling human biology in order to significantly enhance our understanding of human disease.
We will illustrate the utility of each model organism with seminal historical examples, rather than providing a comprehensive overview. We hope to motivate the reader to look into additional contributions. Indeed, it is hard to think of an aspect of biology that has not benefited from studies in model organisms, be it behavior (e.g. Kravitz and Fernandez, 2015), aging (e.g. Kenyon, 2011) or memory (e.g. Dubnau and Tully, 1998). Highlighting past achievements will not necessarily encourage future work. Therefore, we also discuss potential future breakthroughs that might come from curiosity-driven research in model organisms. This is not to say that we disagree with the well-supported contention that no one can predict at the time of discovery how applicable findings from basic research will turn out to be. For example, John S. Dexter, investigating mutant notched wings of fruit flies (Dexter, 1914), could not have predicted the prominent role of the Notch cell surface receptor in cancer (Nowell and Radtke, 2017; Ranganathan et al., 2011). We do not have such foresight either. Nonetheless, it is worth pondering what more we can learn from model organisms, particularly in the era of human stem cells, organoids and facile genome editing using CRISPR/Cas9-based technologies.
The historical impact of model organism research
Biological research was historically driven by curiosity about the natural world. The four model organisms discussed below – yeast, fruit fly, worm and sea urchin – came into use for practical reasons, such as the ease of rearing or their natural abundance. We describe examples of their contributions and discuss their attributes that have enabled investigators to ask questions that would be impractical or impossible to address using mammalian experimental systems.
Yeast
We owe a great deal of our understanding of eukaryotic biology to two species of yeast: Saccharomyces cerevisiae (budding yeast) and Schizosaccharomyces pombe (fission yeast). They have been standout model organisms largely because of their simple life cycles and suitability for large-scale genetic analyses as described below. The relevance of such simple cells to the biology of complex organisms might not be obvious, but, as we discover time and time again, fundamental biological mechanisms are largely the same in simple and more complex organisms, as nicely illustrated by two influential studies (Table 1).
Table 1.
Studies on non-vertebrate eukaryotic model organisms that were recognized by Nobel prizes in Physiology or Medicine in the last 25 years

The first study asked a simple but fundamental question: which genes control how one cell becomes two? Leland Hartwell and his colleagues recognized that the way budding yeast grew and divided would allow the identification of mutants that are defective in the cell division cycle by simply observing the cells (Hartwell et al., 1970, 1973). The researchers took advantage of a useful feature of yeast. Because yeast can grow at different temperatures, one can isolate conditional mutants (Box 1) in which a gene product is active at a low temperature but becomes inactive at a high temperature. These temperature-sensitive conditional mutants could be grown and propagated at the low temperature and their defects examined after a shift to the high temperature. Because yeast cells are small and easy to grow, Hartwell and his colleagues were able to isolate many temperature-sensitive mutants (Fig. 1A). By simply observing cells at the high temperature, these researchers identified mutants that were blocked at different stages of the cell cycle (Fig. 1B). This genetic screen, combined with genetic tools such as epistasis analysis (Box 1), allowed Hartwell and colleagues to identify virtually all of the genes involved in controlling the cell division cycle, or the ‘CDC genes’ (Hartwell et al., 1970, 1973). A parallel analysis led by Paul Nurse and colleagues using similar methods identified the CDC genes in fission yeast (Lee and Nurse, 1987; Nurse, 1975).
Fig. 1.
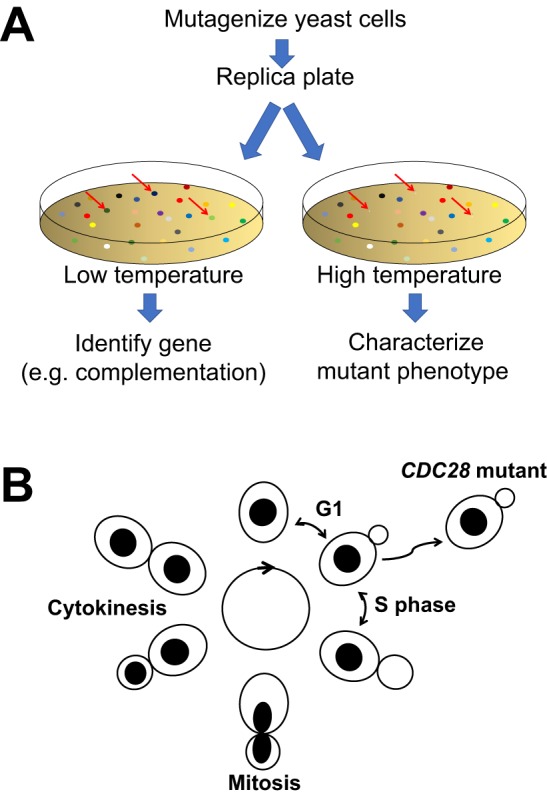
The basis for a genetic screen for budding-yeast cell-cycle mutants. (A) A genetic screen in yeast. Mutagenized yeast cells are cultured on replica plates. One plate is incubated at the low temperature to allow growth, whereas the other is incubated at a high temperature. Conditional mutants that fail to grow at the high temperature (arrows) are thus selected against when the plates are incubated at the high temperature. Microscopic analysis of the high-temperature plate identifies conditional mutants that are blocked in the cell cycle. These mutants, which retain the ability to grow at low temperature, are then isolated from the low-temperature plate for subsequent analysis, which includes complementation tests with wild-type genes to identify the gene responsible for the phenotype (Forsburg, 2001). (B) A budding-yeast cell cycle, modified from Hartwell et al., 1970, 1973. A mother cell produces a daughter by growing a bud that enlarges and eventually separates from the mother. Different stages of the cell cycle can be scored by the shape and size of the cells. For instance, the onset of DNA replication corresponds to the emergence of a bud. Mutants in CDC28, encoding the major cyclin-dependent kinase, arrest with a ‘small bud’, unable to enter a new cell cycle.
The identification of the CDC genes defined a new problem: how do they work in concert to guide cell proliferation, perhaps the most basic of life's processes? Combining molecular biology and genetics in yeast, and with contributions from other model organisms (e.g. sea urchins; see below), the roles of the different CDC gene products were discovered. These advances came with the realization that the genes that control the cell cycle are largely conserved from yeast to human (e.g. Hartwell et al., 1970, 1973; Nurse, 1975). An exceptionally good example of this evolutionary conservation is the gene encoding cyclin-dependent kinase 1 (CDK1); the human CDK1 gene was first identified by its ability to substitute for the fission yeast version and allow yeast that were mutant for CDC2 (CDK1 homolog) to continue dividing (Lee and Nurse, 1987). The regulation of CDK1 by inputs from many of the CDC genes governs the progress of the cell cycle. Moreover, the impact of yeast studies extended in many directions, raising questions such as: how is cell proliferation regulated during development; how do disruptions in this regulation derail proliferation control in cancer; and how can drugs modulating the function of CDC genes be used to treat cancer (Table 1)?
In another example, a simple observation in budding yeast ignited research in the field of protein secretion, which is of central importance to cellular function (Novick et al., 1980; Novick and Schekman, 1979). The isolation of the first secretory (SEC) mutants, in the laboratory of Randy Schekman, was made possible because of the realization that protein secretion is required to build a cell wall outside the cell membrane (Novick and Schekman, 1979). Subsequently, secretion-defective yeast mutants were recognized to be denser than their wild-type counterparts. This trait was used to isolate mutants in additional secretory pathway components using gradient sedimentation (Novick et al., 1980). Again, the genes identified provided the foundation for the mechanistic dissection of protein secretion. The resulting understanding of how an intricate system of membranous vesicles traffics proteins from the inside to the outside of cells also led to our understanding of other processes, such as how nerves signal to one another through the release of neurotransmitters. Yeast studies thus provided an entrée into a new area of cell biology (Table 1).
These genetic screens in yeasts (Fig. 1A) have changed the face of modern science and stimulated subsequent genetic analyses that allowed researchers to place genes in functional pathways even when insight into the biochemical properties of the gene products was lacking (e.g. Garvik et al., 1995). The resulting deep understanding of cell cycle regulation and membrane biology underpins many branches of modern biology and has guided medical research and biotechnology. For example, our understanding of protein secretion from yeast allowed us to manufacture recombinant human insulin for therapeutic purposes (Nielsen, 2013) and small-molecule inhibitors of WEE1, a regulator of CDK1, are being assessed in clinical trials for cancer (Matheson et al., 2016).
Drosophila
How does one choose examples to adequately illustrate the impact of a model organism that is responsible for the confirmation of Mendelism (Box 1), the discovery of the first mutation and the demonstration that genetic traits are carried on chromosomes? The fruit fly Drosophila melanogaster has taught us many fundamental biological mechanisms, thanks to powerful genetic tools, which include phenotypic tractability, special ‘balancer’ chromosomes that allow long-term maintenance of lethal mutations, high fecundity and short life cycle, and the dedication of early fly geneticists, who learned to recognize every bristle on the fly or to detect the subtlest of deviations in wing shape or eye color. Many regulatory pathways studied in modern biology were either discovered in Drosophila or organized into regulatory circuits as a result of studies in Drosophila. For instance, although studies in mammals identified the Ras oncogenes (and others), we owe our understanding of their function to genetic studies of eye development in Drosophila, which revealed that Ras transmits signals from receptor tyrosine kinases (Simon et al., 1991; similar insights from worms are discussed below). Indeed, many signaling pathways of central interest to both normal biology and disease research bear the names of the genes discovered in Drosophila by scientists such as Christiane Nüsslein-Volhard, Eric F. Wieschaus and colleagues, including hedgehog (Nüsslein-Volhard and Wieschaus, 1980), Notch (Dexter, 1914; Fig. 2A,B) and Toll (Table 1). Toll was identified as a gene that functions in the establishment of dorsal/ventral polarity in the Drosophila embryo (Anderson et al., 1985a,b). The elegant genetic dissection of Toll function helped outline an elaborate pathway, which extends from extracellular signals to the activation of key conserved transcription factors, including NF-κB (called Dorsal in flies) and its relatives (Roth et al., 1989; Sen and Baltimore, 1986; Steward, 1989). Subsequently, it was shown that the same signaling system triggers an innate immune response in Drosophila through the work of Jules A. Hoffmann and his colleagues, and that this signaling system is the core component of innate immunity in mammals (Lemaitre et al., 1996; Poltorak et al., 1998). This pivotal discovery promoted a dramatic shift in the study of immunology from an emphasis on adaptive immunity to the more conserved innate system; the ratio of PubMed search results for ‘innate immunity’ vs ‘adaptive immunity’ was 1285:2860 in 1980 and 4423:2658 in 2017. Moreover, we now appreciate that NF-κB family transcription factors regulate almost all aspects of cell biology, from proliferation to inflammation and cell death (Zhang et al., 2017).
Fig. 2.
Drosophila mutants to illustrate landmark studies. (A,B) Wild-type (A) and Notch mutant (B) wings showing notched (arrow) wing blades. Figure reproduced from Casso et al., 2011, with copyright permission from the publisher. (C,D) Sex combs on the front legs of a male D. melanogaster (arrows in C) are magnified in D. In polycomb mutants, anterior/posterior patterning is disrupted, resulting in sex combs appearing also on the middle and hind legs. Reproduced under a Creative Commons license from Wikicommons and with permission from http://flymove.uni-muenster.de. See also Weigmann et al., 2003. (E,F) Suppression of eye color variegation, from Qi et al., 2006. Variegation of eye color (F; juxtaposition of patches of white and red) is suppressed in heterozygotes of a mutation in Su(var)3-9, a gene that encodes an enzyme that methylates lysine 9 of histone H3 (E; uniformly red). TM3 is a balancer chromosome and serves as a ‘wild type’ control. Reproduced with copyright permission from the publisher.
Unusual as it may seem, bristles on the leg of a fly helped us understand how the activity of genes is epigenetically regulated (Box 1). The regions that looked different within the chromosomes of moss and Drosophila were recognized in the early 1900s: densely stained heterochromatin and less densely stained euchromatin (Passarge, 1979). Continuing investigations showed that genes in heterochromatin were transcriptionally repressed and that this repression could be passed on to the next generation of cells (Brown and Nur, 1964). Surprisingly, the discovery of the underlying mechanisms of epigenetic inheritance involved studies of flies' bristles. The accurate formation of a set of leg bristles on male Drosophila, called the ‘sex comb’ for their role in mating (Fig. 2C,D), depends on the mechanisms that form heterochromatin. Mutations that result in the formation of additional sex combs identified a number of genes, with names like Polycomb and extra sex combs (Hannah-Alava, 1958; Reute and Spierer, 1992). Subsequent analyses demonstrated that these genes encode proteins that work together to regulate the addition or removal of chemical modifications to and from histones, the proteins that bind to and determine how DNA is packaged (Piunti and Shilatifard, 2016). Another assay in Drosophila allowed the isolation of additional chromatin-modulating genes. The red color of the fly eye requires the expression of the gene white, which encodes a pigment transporter (O'Hare et al., 1984; Sullivan et al., 1974). Translocation of the white gene near heterochromatin (for example by a chromosome rearrangement) can produce flies with eyes that show ‘variegation’, whereby white patches appear next to normally pigmented red patches (Fig. 2E,F; e.g. Qi et al., 2006). This process is known as position effect variegation (Box 1), and it happens because of changes in chromatin state, so that the white gene locus is in the heterochromatin state and thus transcriptionally inactive in some cells but not others, whereas the DNA sequence of the gene remains unchanged (Ebert et al., 2004). This phenotype illustrates how epigenetic regulation determines gene expression profiles, not on the basis of DNA sequence changes, but on the basis of chromatin state. Founder cells with an epigenetically inactive white gene produce daughter populations that form a white patch in the eye, whereas neighboring cells with a transcriptionally active white gene are red. Mutations that suppress the formation of these white patches are called suppressors of variegation, or Su(var) mutations, and their discovery identified additional chromatin-modulating genes and gene regulatory networks (Ebert et al., 2004; Reute and Spierer, 1992; Sinclair et al., 1983). For example, Su(var)3-9 encodes a histone methyltransferase and Su(var)2-5 encodes a protein, now called heterochromatin protein 1 (HP1), that binds to histones methylated by Su(var)3-9 (Aagaard et al., 1999; Ebert et al., 2004; Eissenberg and Elgin, 2014; Eissenberg et al., 1990; James and Elgin, 1986; Rea et al., 2000; Tschiersch et al., 1994). Polycomb, Su(var)3-9 and HP1 are just three of many genes with crucial roles in maintaining cell identity through epigenetic regulation of gene expression that were discovered in Drosophila. Their initial identification in Drosophila subsequently led to the characterization of their highly conserved counterparts in humans, where they also regulate transcription and influence many aspects of cell identity, physiology and pathogenesis (Allis and Jenuwein, 2016; Lanzuolo and Orlando, 2012; Piunti and Shilatifard, 2016; Schuettengruber et al., 2007; Timms et al., 2016).
Caenorhabditis elegans
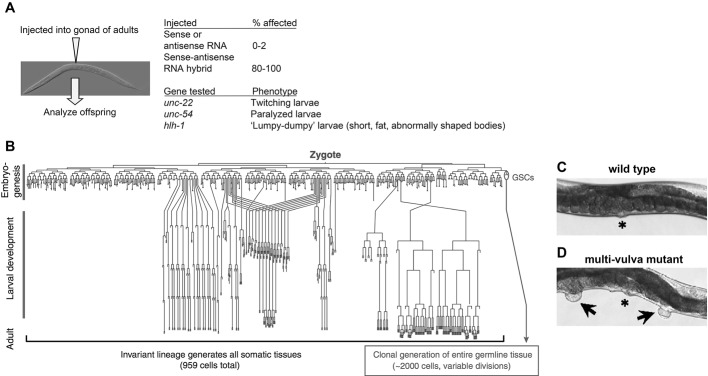
Studies of C. elegans development, like those in Drosophila, led to breakthroughs in our understanding of cellular signaling. Early researchers of C. elegans took advantage of the transparent bodies of these small nematodes and used simple polarization or differential interference contrast (DIC) optics (Box 1) to track the fate of cells as an embryo developed (Brenner, 1974; Sulston and Horvitz, 1977). What they learned was the remarkable conservation of cell fate decisions from worm to worm. In fact, cell behavior is so rigid in this organism that Sydney Brenner, John Soulston, Bob Horvitz and colleagues were able to construct an accurate lineage map of all post-embryonic cell divisions and cell deaths (Fig. 3; Sulston and Horvitz, 1977). This was a gold mine for geneticists looking for mutants that deviated from the wild-type pattern of cell division and cell death. C. elegans are hermaphrodites and can be mated to themselves (much like Mendel's garden peas) to produce progeny, typically in large numbers and in the expected Mendelian ratios (Box 1) (Brenner, 1974). These and the other benefits of C. elegans have led to many fundamental insights into biology, including the identification of the regulators of Ras signaling that arose from the first cloning of the Ras gene (Beitel et al., 1990; Han and Sternberg, 1990; Sternberg and Han, 1998), the discovery of RNA interference (RNAi; Fire et al., 1998; Table 1) and of the first microRNA (Lee et al., 1993; Wightman et al., 1993). The discovery of RNAi by Craig Mello, Andrew Fire and colleagues came on the heels of prior recognition that experimental addition of exogenous RNA could interfere with the expression of the corresponding endogenous genes in plants and worms (Sen and Blau, 2006). Some of the results, such as the finding that both sense and anti-sense strands had the same repressive effect, however, could not be explained by the prevailing model that exogenous RNA interfered with gene function simply by hybridization with the endogenous RNA. Instead, C. elegans studies showed that double-stranded RNA acted catalytically to suppress gene expression, thereby spearheading a field that now has wide applications in basic research, biomedicine and pest control, among others (Perrimon et al., 2010).
Fig. 3.
C. elegans cell-lineage map and multi-vulva mutants. (A) Experiment and data that led to the discovery of RNAi, summarized from Fire et al., 1998. (B) Cell-lineage map of C. elegans. Reproduced under a Creative Commons license from Kimble and Seidel, 2013. (C,D) Wild-type and multi-vulva (Muv) mutant worms. * indicates the vulva; arrows point to ectopic vulvae. Figures modified and reproduced under a Creative Commons license from de la Cova and Greenwald, 2012. Specification of the vulval fate occurs through Ras/MAPK signaling (Kornfeld, 1997; Sundaram and Han, 1996). Mutants in which this pathway is misregulated can show the Muv phenotype (e.g. Gu et al., 1998), which led to the discovery of regulators of Ras/MAPK signaling.
A key disease-relevant field that C. elegans genetics research pioneered is programmed cell death. Apoptosis, a form of programmed cell death, was first recognized in mammalian cells (Kerr et al., 1972) and it was known that enzymes called caspases were important for executing this cellular process. But it was not until Bob Horvitz and colleagues took a genetic approach to analyzing programmed cell death during worm development that our understanding of apoptosis truly advanced. These researchers exploited the invariant sequence of cell division and cell death during C. elegans development to screen for mutants in which too many or too few cells died (e.g. Ellis and Horvitz, 1986). Further studies led to the isolation of the gene that encodes the key caspase, ced-3 (for cell death mutant number 3) (Yuan et al., 1993). C. elegans studies thus identified the genes that match mammalian caspase genes. Using genetic analysis in C. elegans, the enzymes that trigger and execute apoptosis were placed in an ordered pathway and their activators and repressors were identified (Metzstein et al., 1998). These efforts laid the foundation for a deeper understanding of apoptosis in mammalian systems (Table 1).
Sea urchins
Classical forward genetics is not the only tool in the biologist's toolkit. Research into the embryonic development of marine invertebrates, such as the sea urchin, provides an excellent example of how combining biochemistry and cell biology results in a powerful discovery strategy. The practical advantages of using sea urchins attracted many researchers: low cost and relative ease of maintenance (Sluder, 2016); availability of a large quantity of sperm and eggs to enable biochemical studies; rapid, nearly synchronous cell cycles (40-90 min depending upon the temperature) that allow one to study multiple divisions in a short time; and optical transparency of the eggs of some species, useful for imaging. Sea urchin zygotes are remarkably robust and tolerate, for example, mechanical fragmentation or microinjection (Box 1) (Heasman, 2002). The publicly available genome sequence of some species provides access to genes of interest (Cameron et al., 2015; Sea Urchin Genome Sequencing Consortium et al., 2006) and genome editing in zygotes with CRISPR/Cas9 has been reported (Lin and Su, 2016).
Sea urchin gametes and zygotes have been studied since the late 1800s. Theodore Boveri used polyspermy (Box 1) to force a fertilized egg to divide its contents unequally into daughter cells. He analyzed the resulting abnormal zygotes and, with crisp deductive logic, Boveri considered and then discarded each cellular component as the bearer of genetic information until he identified the structures that we now call chromosomes (Boveri, 1974). Boveri's chromosome theory of inheritance was beautifully complemented by Mendel's work as re-discovered by Sutton (Sutton, 1903) and was amply supported by Morgan's experimental data from Drosophila (Morgan, 1911). Since then, many fundamental insights into cell biology have been gained using sea urchin gametes and zygotes. These include the identification of bindin, a protein that facilitates egg-sperm interactions for fertilization (Glabe and Vacquier, 1977; Vacquier and Moy, 1977); an understanding of how complex changes to the cytoskeleton and cell membranes are orchestrated to form a cleavage furrow (Box 1) (Pollard, 2004); the identification of gene regulatory networks that control early zygotic development (Davidson et al., 2002); understanding how centrosomes (Box 1) are assembled and duplicated (reviewed in Sluder, 2014); and the discovery of the cyclin proteins that drive the cell cycle in sea urchin zygotes and also in clam embryo extracts (Evans et al., 1983; Hunt et al., 1992; Standart et al., 1990; Standart and Hunt, 1990). We, the authors, still remember the excitement at the recognition that the cyclins that drive cleavage in sea urchin zygotes are homologous to the CDC proteins that drive the cell cycle in yeast (Booher and Beach, 1988; Hagan et al., 1988). These and many other findings from sea urchin models paved the way for subsequent discoveries in mammalian systems.
Concluding remarks
As useful as model organisms are, they are not human, and have their limitations. Three-dimensional (3D) cell-culture model systems, such as organoids derived from human stem cells, have been much-heralded because they are the only human-cell-based model system to recapitulate the cellular complexity of tissues (Huch et al., 2017). Challenges exist, such as high cost and the phenotypic variability in traits, including organoid size, shape, cellular composition and 3D architecture, even when the organoids are produced under identical conditions (Huch et al., 2017), but these may be overcome as the technology matures. Lab-grown organoids should be useful for toxicity testing and for the generation of tissues for biobanks, but experts doubt that they will replace animal models as discovery tools (Bredenoord et al., 2017). We would go further to argue that organoids will not and should not replace non-vertebrate model organisms as discovery tools. We, the authors, speculate that the best use of organoids may be to transfer knowledge acquired in model organisms to humans. Given this position, what do we think are future discoveries that could be expected from curiosity-driven research in model organisms?
One obvious answer concerns the inner workings deep within the cell. For example, all cells, regardless of origin, need to make proteins, but also must get rid of proteins that are no longer needed. Genetic studies in budding yeast, complemented by biochemical and structural analyses, are providing us with exquisite details on how the protein machines responsible for destroying other proteins are assembled (e.g. Li et al., 2017; Sokolova et al., 2015). Likewise, we anticipate that studies in yeast and other model organisms may provide a deeper understanding of how proteins are synthesized, how they are modified with different chemical moieties, and how they are transported across and out of the cell. For such dissection of fundamental cellular processes, stem cells or organoids are neither required nor cost effective. Additionally, as with examples described in this article, we expect fundamental insights gleaned from model organisms to lead to better understanding of biology in all organisms, including humans.
Moving beyond cell biology to whole-animal biology, two examples illustrate the kind of new knowledge that can be gained from studying the biology of model organisms. An interesting question that has intrigued biologists for many years is how organisms, and the organs within them, attain their characteristic size. The Drosophila Hippo kinase pathway acts as a growth rheostat that fine-tunes the balance of proliferation and cell death during development, thereby helping organs attain their correct size and shape. It does so by regulating the transcription factor Yorkie (Yki; YAP in human), the targets of which include genes that regulate cell proliferation and apoptosis (Hariharan, 2015; Irvine and Harvey, 2015). The latest twist in the Hippo signaling tale involves Drosophila insulin-like peptide 8 (Dilp8), a peptide hormone secreted by injured tissues (Colombani et al., 2012; Garelli et al., 2012). Secreted Dilp8 accumulates in the circulatory system of the fly, from where it finds its way to the brain and stimulates neurons that subsequently repress the production of the molting hormone, thereby postponing the next stage in fly development. This signaling throughout the animal allows injured organs more time to grow and catch up in size relative to their undamaged counterparts. Strikingly, dilp8 mutant flies lose coordinated organ growth, such that wings are mismatched in size (Garelli et al., 2012). dilp8 was recently found to be a transcriptional target of the Hippo pathway (Boone et al., 2016). The current thinking is that Hippo not only fine-tunes growth within a tissue, but also acts via Dilp8 to delay development until organs at distant sites in a body are matched in size. When the pathway is functional, wing growth on opposite sides of the body is coordinated. The Hippo pathway also has emerged as an important regulator of the growth needed to repair injured tissues in a variety of organisms, including vertebrates (Hong et al., 2016; Patel et al., 2017; Zhang and Del Re, 2017). Given these roles, it is not surprising that the Hippo pathway – first discovered in Drosophila (Harvey et al., 2003; Justice et al., 1995; Pantalacci et al., 2003; Udan et al., 2003; Wu et al., 2003) – is also increasingly implicated in growth control in human cells and in the development of many human cancers (Hong et al., 2016; Patel et al., 2017). This example highlights how a strategy of ‘discovery first’ can be of tremendous benefit to biomedicine.
C. elegans provides another example of a model in which new insights about biology remain to be made. C. elegans hermaphrodites can produce sperm or egg, but not both simultaneously. A recently published study found that fatty acids (FAs) in the gut can alter the germline of hermaphrodites to stimulate production of either oocytes or sperm (Tang and Han, 2017). FAs exert their effect by altering the level of myristic acid (a form of FA) in the germline, which in turn affects the level of myristoylation, a process in which myristic acid is covalently attached to proteins. One protein controlled by myristoylation turns out to be the MAP kinase MPK-1, which determines germ-cell fate (Tang and Han, 2017). This biological phenomenon is conserved in Caenorhabditis remanei, a worm species that lives as male or female and not as a hermaphrodite, where the knocking down of the myristic acid-processing enzyme ACS-4 can masculinize genetically female worms into producing sperm.
What do these examples tell us about what the future might hold? They illustrate the complex interconnections between the germline and the soma through nutrients and signaling in worms, and a complex system of growth control and tissue repair that involves multiple organs and many secreted hormones in flies. They exemplify how model organisms can be used to understand how multiple organ systems interact inside an intact body, and to identify molecules that mediate these interactions. Such a deep understanding of biology and its complexities, we argue, will provide a basis for future breakthroughs in medicine. It will be a long time, if ever, before we can model complex interactions in organoids or cultured cells. Until then, it is curiosity-driven research in model organisms that will continue to satiate our appetite for understanding the wonderful and mysterious natural world that we live in.
Acknowledgements
We thank our colleagues Min Han and Corrie Detweiler for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
National Institutes of Health (NIH) grants R01 DA036897 and R01 GM058921 to R.J.D., R37 GM037193 to P.H.O., R01 GM30758 to G.S., and R01 GM106317 to T.T.S. support work in the Duronio, O'Farrell, Sluder and Su labs, respectively.
References
- Aagaard L., Laible G., Selenko P., Schmid M., Dorn R., Schotta G., Kuhfittig S., Wolf A., Lebersorger A., Singh P. B. et al. (1999). Functional mammalian homologues of the Drosophila PEV-modifier Su(var)3-9 encode centromere-associated proteins which complex with the heterochromatin component M31. EMBO J. 18, 1923-1938. 10.1093/emboj/18.7.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis C. D. and Jenuwein T. (2016). The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487-500. 10.1038/nrg.2016.59 [DOI] [PubMed] [Google Scholar]
- Anderson K. V., Bokla L. and Nüsslein-Volhard C. (1985a). Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42, 791-798. 10.1016/0092-8674(85)90275-2 [DOI] [PubMed] [Google Scholar]
- Anderson K. V., Jürgens G. and Nüsslein-Volhard C. (1985b). Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42, 779-789. 10.1016/0092-8674(85)90274-0 [DOI] [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A. and Rothman J. E. (1984). Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39, 405-416. 10.1016/0092-8674(84)90019-9 [DOI] [PubMed] [Google Scholar]
- Bargiello T. A., Jackson F. R. and Young M. W. (1984). Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature 312, 752-764. 10.1038/312752a0 [DOI] [PubMed] [Google Scholar]
- Beitel G. J., Clark S. G. and Horvitz H. R. (1990). Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348, 503-509. 10.1038/348503a0 [DOI] [PubMed] [Google Scholar]
- Block M. R., Glick B. S., Wilcox C. A., Wieland F. T. and Rothman J. E. (1988). Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl. Acad. Sci. U S A 85, 7852-7856. 10.1073/pnas.85.21.7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R. and Beach D. (1988). Involvement of cdc13+ in mitotic control in Schizosaccharomyces pombe: possible interaction of the gene product with microtubules. EMBO J. 7, 2321-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone E., Colombani J., Andersen D. S. and Léopold P. (2016). The Hippo signalling pathway coordinates organ growth and limits developmental variability by controlling dilp8 expression. Nat. Commun. 7, 13505 10.1038/ncomms13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. (1974). On multipolar mitosis as a means of analysis of the cell nucleus. In Foundations of Experimental Embryology (ed. Willier B. H. and Oppenheimer J. M.), pp. 74-97. New York: Hafner Press. [Google Scholar]
- Bredenoord A. L., Clevers H. and Knoblich J. A. (2017). Human tissues in a dish: the research and ethical implications of organoid technology. Science 355, eaaf9414 10.1126/science.aaf9414 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. W. and Nur U. (1964). Heterochromatic chromosomes in the coccids. Science 145, 130-136. 10.1126/science.145.3628.130 [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Kudtarkar P., Gordon S. M., Worley K. C. and Gibbs R. A. (2015). Do echinoderm genomes measure up? Mar. Genomics 22, 1-9. 10.1016/j.margen.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casso D. J., Biehs B. and Kornberg T. B. (2011). A novel interaction between hedgehog and Notch promotes proliferation at the anterior-posterior organizer of the Drosophila wing. Genetics 187, 485-499. 10.1534/genetics.110.125138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J., Andersen D. S. and Leopold P. (2012). Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336, 582-585. 10.1126/science.1216689 [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Rast J. P., Oliveri P., Ransick A., Calestani C., Yuh C. H., Minokawa T., Amore G., Hinman V., Arenas-Mena C. et al. (2002). A genomic regulatory network for development. Science 295, 1669-1678. 10.1126/science.1069883 [DOI] [PubMed] [Google Scholar]
- de la Cova C. and Greenwald I. (2012). SEL-10/Fbw7-dependent negative feedback regulation of LIN-45/Braf signaling in C. elegans via a conserved phosphodegron. Genes Dev. 26, 2524-2535. 10.1101/gad.203703.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter J. S. (1914). The analysis of a case of continuous variation in Drosophila by a study of its linkage relations. Am. Nat. 48, 712-758. 10.1086/279446 [DOI] [Google Scholar]
- Dubnau J. and Tully T. (1998). Gene discovery in Drosophila: new insights for learning and memory. Annu. Rev. Neurosci. 21, 407-444. 10.1146/annurev.neuro.21.1.407 [DOI] [PubMed] [Google Scholar]
- Ebert A., Schotta G., Lein S., Kubicek S., Krauss V., Jenuwein T. and Reuter G. (2004). Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18, 2973-2983. 10.1101/gad.323004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C. and Elgin S. C. R. (2014). HP1a: a structural chromosomal protein regulating transcription. Trends Genet. 30, 103-110. 10.1016/j.tig.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., James T. C., Foster-Hartnett D. M., Hartnett T., Ngan V. and Elgin S. C. (1990). Mutation in a heterochromatin-specific chromosomal protein is associated with suppression of position-effect variegation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 87, 9923-9927. 10.1073/pnas.87.24.9923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. M. and Horvitz H. R. (1986). Genetic control of programmed cell death in the nematode C. elegans. Cell 44, 817-829. 10.1016/0092-8674(86)90004-8 [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D. and Hunt T. (1983). Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33, 389-396. 10.1016/0092-8674(83)90420-8 [DOI] [PubMed] [Google Scholar]
- Fang F. C. and Casadevall A. (2010). Lost in translation--basic science in the era of translational research. Infect. Immun. 78, 563-566. 10.1128/IAI.01318-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehullah A., Tan S. H. and Barker N. (2016). Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246-254. 10.1038/ncb3312 [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S. Q., Montgomery M. K., Kostas S. A., Driver S. E. and Mello C. C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806-811. 10.1038/35888 [DOI] [PubMed] [Google Scholar]
- Forsburg S. L. (2001). The art and design of genetic screens: yeast. Nat. Rev. Genet. 2, 659-668. 10.1038/35088500 [DOI] [PubMed] [Google Scholar]
- Garelli A., Gontijo A. M., Miguela V., Caparros E. and Dominguez M. (2012). Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579-582. 10.1126/science.1216735 [DOI] [PubMed] [Google Scholar]
- Garvik B., Carson M. and Hartwell L. (1995). Single-stranded DNA arising at telomeres in cdc13 mutants may constitute a specific signal for the RAD9 checkpoint. Mol. Cell. Biol. 15, 6128-6138. 10.1128/MCB.15.11.6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C. G. and Vacquier V. D. (1977). Species specific agglutination of eggs by bindin isolated from sea urchin sperm. Nature 267, 836-838. 10.1038/267836a0 [DOI] [PubMed] [Google Scholar]
- Greider C. W. and Blackburn E. H. (1985). Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405-413. 10.1016/0092-8674(85)90170-9 [DOI] [PubMed] [Google Scholar]
- Gu T., Orita S. and Han M. (1998). Caenorhabditis elegans SUR-5, a novel but conserved protein, negatively regulates LET-60 Ras activity during vulval induction. Mol. Cell. Biol. 18, 4556-4564. 10.1128/MCB.18.8.4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I., Hayles J. and Nurse P. (1988). Cloning and sequencing of the cyclin-related cdc13+ gene and a cytological study of its role in fission yeast mitosis. J. Cell Sci. 91, 587-595. [DOI] [PubMed] [Google Scholar]
- Han M. and Sternberg P. W. (1990). let-60, a gene that specifies cell fates during C. elegans vulval induction, encodes a ras protein. Cell 63, 921-931. 10.1016/0092-8674(90)90495-Z [DOI] [PubMed] [Google Scholar]
- Hand E., Mole B., Morello L., Tollefson J., Wadman M. and Witze A. (2013). A back seat for basic science. Nature 496, 277-279. 10.1038/496277a [DOI] [PubMed] [Google Scholar]
- Hannah-Alava A. (1958). Developmental genetics of the posterior legs in Drosophila Melanogaster. Genetics 43, 878-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C. and Rosbash M. (1990). Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536-540. 10.1038/343536a0 [DOI] [PubMed] [Google Scholar]
- Hariharan I. K. (2015). Organ size control: lessons from Drosophila. Dev. Cell 34, 255-265. 10.1016/j.devcel.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J. and Reid B. (1970). Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc. Natl. Acad. Sci. USA 66, 352-359. 10.1073/pnas.66.2.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J. and Culotti M. (1973). Genetic control of the cell division cycle in yeast: V. Genetic analysis of cdc mutants. Genetics 74, 267-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K. F., Pfleger C. M. and Hariharan I. K. (2003). The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457-467. 10.1016/S0092-8674(03)00557-9 [DOI] [PubMed] [Google Scholar]
- Heasman J. (2002). Morpholino oligos: making sense of antisense? Dev. Biol. 243, 209-214. 10.1006/dbio.2001.0565 [DOI] [PubMed] [Google Scholar]
- Hong A. W., Meng Z. and Guan K.-L. (2016). The Hippo pathway in intestinal regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 13, 324-337. 10.1038/nrgastro.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Knoblich J. A., Lutolf M. P. and Martinez-Arias A. (2017). The hope and the hype of organoid research. Development 144, 938-941. 10.1242/dev.150201 [DOI] [PubMed] [Google Scholar]
- Hunt T., Luca F. C. and Ruderman J. V. (1992). The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J. Cell Biol. 116, 707-724. 10.1083/jcb.116.3.707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K. D. and Harvey K. F. (2015). Control of organ growth by patterning and hippo signaling in Drosophila. Cold Spring Harb. Perspect. Biol. 7, a019224 10.1101/cshperspect.a019224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T. C. and Elgin S. C. (1986). Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 6, 3862-3872. 10.1128/MCB.6.11.3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice R. W., Zilian O., Woods D. F., Noll M. and Bryant P. J. (1995). The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534-546. 10.1101/gad.9.5.534 [DOI] [PubMed] [Google Scholar]
- Kenyon C. (2011). The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 9-16. 10.1098/rstb.2010.0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F. R., Wyllie A. H. and Currie A. R. (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239-257. 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J. and Seidel H. C. (2013). C. elegans germline stem cells and their niche. In StemBook [Internet] (ed. L. Girard). Cambridge, MA: Harvard Stem Cell Institute. [PubMed] [Google Scholar]
- Konopka R. J. and Benzer S. (1971). Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. U S A 68, 2112-2116. 10.1073/pnas.68.9.2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld K. (1997). Vulval development in Caenorhabditis elegans. Trends Genet. 13, 55-61. 10.1016/S0168-9525(97)01005-6 [DOI] [PubMed] [Google Scholar]
- Kravitz E. A. and Fernandez M. P. (2015). Aggression in Drosophila. Behav. Neurosci. 129, 549-563. 10.1037/bne0000089 [DOI] [PubMed] [Google Scholar]
- Lanzuolo C. and Orlando V. (2012). Memories from the polycomb group proteins. Annu. Rev. Genet. 46, 561-589. 10.1146/annurev-genet-110711-155603 [DOI] [PubMed] [Google Scholar]
- Lee M. G. and Nurse P. (1987). Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature 327, 31-35. 10.1038/327031a0 [DOI] [PubMed] [Google Scholar]
- Lee R. C., Feinbaum R. L. and Ambros V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843-854. 10.1016/0092-8674(93)90529-Y [DOI] [PubMed] [Google Scholar]
- Lee M. S., Kim J. I. and Ernst E. (2011). Is cupping an effective treatment? An overview of systematic reviews. J. Acupunct. Meridian Stud. 4, 1-4. 10.1016/S2005-2901(11)60001-0 [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Nicolas E., Michaut L., Reichhart J.-M. and Hoffmann J. A. (1996). The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973-983. 10.1016/S0092-8674(00)80172-5 [DOI] [PubMed] [Google Scholar]
- Lewis E. B. (1978). A gene complex controlling segmentation in Drosophila. Nature 276, 565-570. 10.1038/276565a0 [DOI] [PubMed] [Google Scholar]
- Li F., Tian G., Langager D., Sokolova V., Finley D. and Park S. (2017). Nucleotide-dependent switch in proteasome assembly mediated by the Nas6 chaperone. Proc. Natl. Acad. Sci. USA 114, 1548-1553. 10.1073/pnas.1612922114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-Y. and Su Y.-H. (2016). Genome editing in sea urchin embryos by using a CRISPR/Cas9 system. Dev. Biol. 409, 420-428. 10.1016/j.ydbio.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Matheson C. J., Backos D. S. and Reigan P. (2016). Targeting WEE1 Kinase in Cancer. Trends Pharmacol. Sci. 37, 872-881. 10.1016/j.tips.2016.06.006 [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Missler M., Li C. and Sudhof T. C. (1995). Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83, 111-119. 10.1016/0092-8674(95)90239-2 [DOI] [PubMed] [Google Scholar]
- McNutt M. (2015). Breakthrough to genome editing. Science 350, 1445 10.1126/science.aae0479 [DOI] [PubMed] [Google Scholar]
- Metzstein M. M., Stanfield G. M. and Horvitz H. R. (1998). Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 14, 410-416. 10.1016/S0168-9525(98)01573-X [DOI] [PubMed] [Google Scholar]
- Minogue K. and Wolinsky H. (2010). Lost in translation. EMBO Rep. 11, 93-96. 10.1038/embor.2009.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Noda T., Yoshimori T., Tanaka Y., Ishii T., George M. D., Klionsky D. J., Ohsumi M. and Ohsumi Y. (1998). A protein conjugation system essential for autophagy. Nature 395, 395-398. 10.1038/26506 [DOI] [PubMed] [Google Scholar]
- Morgan T. H. (1911). The origin of five mutations in eye color in Drosophila and their modes of inheritance. Science 33, 534-537. 10.1126/science.33.849.534-a [DOI] [PubMed] [Google Scholar]
- Nielsen J. (2013). Production of biopharmaceutical proteins by yeast: advances through metabolic engineering. Bioengineered 4, 207-211. 10.4161/bioe.22856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P. and Schekman R. (1979). Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 76, 1858-1862. 10.1073/pnas.76.4.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C. and Schekman R. (1980). Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205-215. 10.1016/0092-8674(80)90128-2 [DOI] [PubMed] [Google Scholar]
- Nowell C. S. and Radtke F. (2017). Notch as a tumour suppressor. Nat. Rev. Cancer 17, 145-159. 10.1038/nrc.2016.145 [DOI] [PubMed] [Google Scholar]
- Nurse P. (1975). Genetic control of cell size at cell division in yeast. Nature 256, 547-551. 10.1038/256547a0 [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C. and Wieschaus E. (1980). Mutations affecting segment number and polarity in Drosophila. Nature 287, 795-801. 10.1038/287795a0 [DOI] [PubMed] [Google Scholar]
- O'Hare K., Murphy C., Levis R. and Rubin G. M. (1984). DNA sequence of the white locus of Drosophila melanogaster. J. Mol. Biol. 180, 437-455. 10.1016/0022-2836(84)90021-4 [DOI] [PubMed] [Google Scholar]
- Pantalacci S., Tapon N. and Leopold P. (2003). The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 5, 921-917 10.1038/ncb1051 [DOI] [PubMed] [Google Scholar]
- Passarge E. (1979). Emil Heitz and the concept of heterochromatin: longitudinal chromosome differentiation was recognized fifty years ago. Am. J. Hum. Genet. 31, 106-115. [PMC free article] [PubMed] [Google Scholar]
- Patel S. H., Camargo F. D. and Yimlamai D. (2017). Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology 152, 533-545. 10.1053/j.gastro.2016.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon N., Ni J.-Q. and Perkins L. (2010). In vivo RNAi: today and tomorrow. Cold Spring Harb. Perspect. Biol. 2, a003640 10.1101/cshperspect.a003640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piunti A. and Shilatifard A. (2016). Epigenetic balance of gene expression by Polycomb and COMPASS families. Science 352, aad9780 10.1126/science.aad9780 [DOI] [PubMed] [Google Scholar]
- Pollard T. D. (2004). Ray Rappaport chronology: twenty-five years of seminal papers on cytokinesis in the Journal of Experimental Zoology. J. Exp. Zool. A Comp. Exp. Biol. 301A, 9-14. 10.1002/jez.a.20000 [DOI] [PubMed] [Google Scholar]
- Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C. et al. (1998). Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085-2088. 10.1126/science.282.5396.2085 [DOI] [PubMed] [Google Scholar]
- Porter J. R. (1961). Louis PASTEUR; achievements and disappointments, 1861. Bacteriol. Rev. 25, 389-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D., Jin H., Lilja T. and Mannervik M. (2006). Drosophila Reptin and other TIP60 complex components promote generation of silent chromatin. Genetics 174, 241-251. 10.1534/genetics.106.059980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P., Weaver K. L. and Capobianco A. J. (2011). Notch signalling in solid tumours: a little bit of everything but not all the time. Nat. Rev. Cancer 11, 338-351. 10.1038/nrc3035 [DOI] [PubMed] [Google Scholar]
- Rea S., Eisenhaber F., O'Carroll D., Strahl B. D., Sun Z.-W., Schmid M., Opravil S., Mechtler K., Ponting C. P., Allis C. D. et al. (2000). Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593-599. 10.1038/35020506 [DOI] [PubMed] [Google Scholar]
- Reute G. and Spierer P. (1992). Position effect variegation and chromatin proteins. BioEssays 14, 605-612. 10.1002/bies.950140907 [DOI] [PubMed] [Google Scholar]
- Roth S., Stein D. and Nüsslein-Volhard C. (1989). A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59, 1189-1202. 10.1016/0092-8674(89)90774-5 [DOI] [PubMed] [Google Scholar]
- Schuettengruber B., Chourrout D., Vervoort M., Leblanc B. and Cavalli G. (2007). Genome regulation by polycomb and trithorax proteins. Cell 128, 735-745. 10.1016/j.cell.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing Consortium, Sodergren E., Weinstock G. M., Davidson E. H., Cameron R. A., Gibbs R. A., Angerer R. C., Angerer L. M., Arnone M. I., Burgess D. R., et al. (2006). The genome of the sea urchin Strongylocentrotus purpuratus. Science 314, 941-952. 10.1126/science.1133609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R. and Baltimore D. (1986). Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46, 705-716. 10.1016/0092-8674(86)90346-6 [DOI] [PubMed] [Google Scholar]
- Sen G. L. and Blau H. M. (2006). A brief history of RNAi: the silence of the genes. FASEB J. 20, 1293-1299. 10.1096/fj.06-6014rev [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D. L., Dodson G. S., Laverty T. R. and Rubin G. M. (1991). Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67, 701-716. 10.1016/0092-8674(91)90065-7 [DOI] [PubMed] [Google Scholar]
- Sinclair D. A. R., Mottus R. C. and Grigliatti T. A. (1983). Genes which suppress position-effect variegation in Drosophila melanogaster are clustered. Mol. Gen. Genet. 191, 326-333. 10.1007/BF00334834 [DOI] [Google Scholar]
- Sluder G. (2014). One to only two: a short history of the centrosome and its duplication. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369 10.1098/rstb.2013.0455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G. (2016). Using sea urchin gametes and zygotes to investigate centrosome duplication. Cilia 5, 20 10.1186/s13630-016-0043-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova V., Li F., Polovin G. and Park S. (2015). Proteasome activation is mediated via a functional switch of the Rpt6 C-terminal tail following chaperone-dependent assembly. Sci. Rep. 5, 14909 10.1038/srep14909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standart N. and Hunt T. (1990). Control of translation of masked mRNAs in clam oocytes. Enzyme 44, 106-119. 10.1159/000468751 [DOI] [PubMed] [Google Scholar]
- Standart N., Dale M., Stewart E. and Hunt T. (1990). Maternal mRNA from clam oocytes can be specifically unmasked in vitro by antisense RNA complementary to the 3′-untranslated region. Genes Dev. 4, 2157-2168. 10.1101/gad.4.12a.2157 [DOI] [PubMed] [Google Scholar]
- Sternberg P. W. and Han M. (1998). Genetics of RAS signaling in C. elegans. Trends Genet. 14, 466-472. 10.1016/S0168-9525(98)01592-3 [DOI] [PubMed] [Google Scholar]
- Steward R. (1989). Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell 59, 1179-1188. 10.1016/0092-8674(89)90773-3 [DOI] [PubMed] [Google Scholar]
- Sullivan D. T., Grillo S. L. and Kitos R. J. (1974). Subcellular localization of the first three enzymes of the ommochrome synthetic pathway in Drosophila melanogaster. J. Exp. Zool. 188, 225-233. 10.1002/jez.1401880210 [DOI] [PubMed] [Google Scholar]
- Sulston J. E. and Horvitz H. R. (1977). Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56, 110-156. 10.1016/0012-1606(77)90158-0 [DOI] [PubMed] [Google Scholar]
- Sundaram M. and Han M. (1996). Control and integration of cell signaling pathways during C. elegans vulval development. BioEssays 18, 473-480. 10.1002/bies.950180609 [DOI] [PubMed] [Google Scholar]
- Sutton W. S. (1903). The chromosomes in heredity. Biol. Bull. 4, 231-250. 10.2307/1535741 [DOI] [Google Scholar]
- Szostak J. W. and Blackburn E. H. (1982). Cloning yeast telomeres on linear plasmid vectors. Cell 29, 245-255. 10.1016/0092-8674(82)90109-X [DOI] [PubMed] [Google Scholar]
- Takeshige K., Baba M., Tsuboi S., Noda T. and Ohsumi Y. (1992). Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119, 301-311. 10.1083/jcb.119.2.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. and Han M. (2017). Fatty acids regulate germline sex determination through ACS-4-dependent myristoylation. Cell 169, 457-469.e13. 10.1016/j.cell.2017.03.049 [DOI] [PubMed] [Google Scholar]
- Timms R. T., Tchasovnikarova I. A. and Lehner P. J. (2016). Position-effect variegation revisited: HUSHing up heterochromatin in human cells. BioEssays 38, 333-343. 10.1002/bies.201500184 [DOI] [PubMed] [Google Scholar]
- Tschiersch B., Hofmann A., Krauss V., Dorn R., Korge G. and Reuter G. (1994). The protein encoded by the Drosophila position-effect variegation suppressor gene Su(var)3-9 combines domains of antagonistic regulators of homeotic gene complexes. EMBO J. 13, 3822-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan R. S., Kango-Singh M., Nolo R., Tao C. and Halder G. (2003). Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 5, 914-920. 10.1038/ncb1050 [DOI] [PubMed] [Google Scholar]
- Vacquier V. D. and Moy G. W. (1977). Isolation of bindin: the protein responsible for adhesion of sperm to sea urchin eggs. Proc. Natl. Acad. Sci. USA 74, 2456-2460. 10.1073/pnas.74.6.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann K., Klapper R., Strasser T., Rickert C., Technau G., Jäckle H., Janning W. and Klämbt C. (2003). FlyMove--a new way to look at development of Drosophila. Trends Genet. 19, 310-311. 10.1016/S0168-9525(03)00050-7 [DOI] [PubMed] [Google Scholar]
- Wightman B., Ha I. and Ruvkun G. (1993). Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855-862. 10.1016/0092-8674(93)90530-4 [DOI] [PubMed] [Google Scholar]
- Wu S., Huang J., Dong J. and Pan D. (2003). hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445-456. 10.1016/S0092-8674(03)00549-X [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M. and Horvitz H. R. (1993). The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell 75, 641-652. 10.1016/0092-8674(93)90485-9 [DOI] [PubMed] [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M. and Hall J. C. (1984). P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell 39, 369-376. 10.1016/0092-8674(84)90015-1 [DOI] [PubMed] [Google Scholar]
- Zhang Y. and Del Re D. P. (2017). A growing role for the Hippo signaling pathway in the heart. J. Mol. Med. (Berl.) 95, 465-472. 10.1007/s00109-017-1525-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Lenardo M. J. and Baltimore D. (2017). 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell 168, 37-57. 10.1016/j.cell.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]