ABSTRACT
During Drosophila metamorphosis, nascent testis myotubes migrate from the prospective seminal vesicle of the genital disc onto pupal testes and then further to cover the testes with multinucleated smooth-like muscles. Here we show that DWnt2 is likely required for determination of testis-relevant myoblasts on the genital disc. Knock down of fibroblast growth factor receptor (FGFR) heartless by RNAi and a dominant-negative version revealed multiple functions of Heartless, namely regulation of the amount of myoblasts on the genital disc, connection of seminal vesicles and testes, and migration of muscles along the testes. Live imaging indicated that the downstream effector Stumps is required for migration of testis myotubes on the testis towards the apical tip. After myoblast fusion, myosin II is needed for migration of nascent testis myotubes, in which Thisbe-dependent fibroblast growth factor (FGF) signaling is activated. Cadherin-N is essential for connecting these single myofibers and for creating a firm testis muscle sheath that shapes and stabilizes the testis tubule. Based on these results, we propose a model for the migration of testis myotubes in which nascent testis myotubes migrate as a collective onto and along the testis, dependent on FGF-regulated expression of myosin II.
KEY WORDS: DWnt2, Thisbe, Stumps, FGF, Testes tubules, Muscles
Summary: Drosophila testes and mammalian seminiferous tubules are surrounded by a muscle layer. Drosophila myotubes migrate towards testes in dependence of the FGF receptor Heartless, myosin II and Cadherin-N.
INTRODUCTION
Cell migration is essential for many developmental and physiological processes throughout the animal kingdom, and is also implicated in diseases, e.g. cancer metastasis (Roca-Cusachs et al., 2013). In Drosophila, various single as well as collective cell migration processes have been described, such as border cell migration in the ovary and embryonal mesoderm migration (Pocha and Montell, 2014).
In the current study, we focus on the migration of muscle cells during the development of the male reproductive tract of Drosophila. The five different organs of the inner male reproductive system of Drosophila develop from two different tissues. The testes are of gonadal origin located in segment A5, whereas the somatic parts arise from a single genital imaginal disc (hereafter called genital disc) in segments A8/A9/A10 (Estrada et al., 2003; Greig and Akam, 1995; Stern, 1941). During metamorphosis, the genital disc and pupal testes grow towards each other, and the developing seminal vesicles fuse with the terminal epithelium of the testes (Kozopas et al., 1998; Nanda et al., 2009; Stern, 1941). Nascent myotubes migrate over the developing seminal vesicles onto the pupal testes and build the muscle sheath surrounding the adult testis (Kozopas et al., 1998; Kuckwa et al., 2016). This musculature is composed of multinucleated, smooth-like myofibers (Susic-Jung et al., 2012).
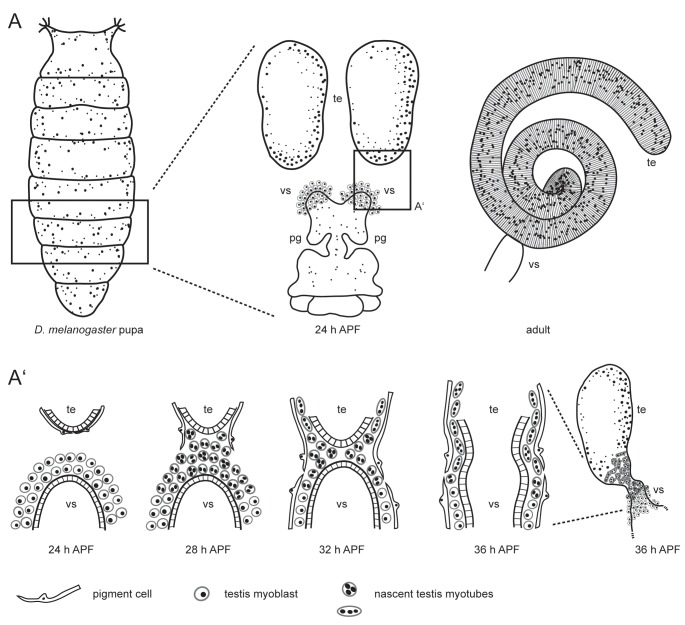
Myoblasts of the genital disc build muscle sheaths for all parts of the male reproductive system (Susic-Jung et al., 2012). The myoblasts that form the testis muscle sheath originate from a common pool and accumulate during the first day of metamorphosis on the prospective seminal vesicles of the genital disc (Fig. 1A). Founder-cell-like (FC-like) myoblasts and fusion-competent-myoblast-like (FCM-like) cells start to fuse around 28 h after puparium formation (APF) to build multinucleated myotubes (Kuckwa et al., 2016). Around 30 h APF, the multinucleated nascent myotubes begin to migrate from the genital disc towards the testis, contact the gonad at the distal end, and migrate further to cover the entire testis (Fig. 1A′) (Kozopas et al., 1998; Kuckwa et al., 2016). Migration of testis myotubes is independent of successful fusion of testis-relevant myoblasts (Kuckwa et al., 2016). Early evidence indicated that this migration process might be dependent on the presence of the Wnt ligand DWnt2 in addition to, or as a consequence of, the failure of pigment cell migration, since in DWnt2 mutant males smooth-like muscles do not accumulate on the testis (Kozopas et al., 1998).
Fig. 1.
Scheme of Drosophila testis myotube migration. (A) The male reproductive tract develops during metamorphosis. At 24 h APF, the single genital disc and paired testes (te) are separate organs. The seminal vesicles (vs) and the paragonia (pg) already start to grow. In the adult, the tubular testis is connected to the seminal vesicle. (A’) During metamorphosis, the prospective seminal vesicles and testes grow towards each other and fuse. On genital discs 24 h APF, testis-relevant myoblasts accumulate on the prospective seminal vesicle. Pigment cells cover the pupal testis. At 28 h APF, myoblasts fuse to build multinucleated testis myotubes. These nascent testis myotubes migrate beneath the pigment cells onto the pupal testis, while pigment cells migrate from the testis onto the developing seminal vesicle. By 36 h APF, the epithelia of seminal vesicles and the terminal epithelium of the testes have fused. Modified after Bodenstein (1950), Kozopas et al. (1998), Kuckwa et al. (2016).
Another relevant pathway for the development of the male reproductive organs of Drosophila is fibroblast growth factor (FGF) signaling. The FGF receptor (FGFR) Breathless (Btl) and its ligand Branchless recruit larval mesodermal cells, which become epithelial and give rise to paragonia and seminal vesicles (Ahmad and Baker, 2002). Btl is also essential for cell migration during embryonal tracheal development (Glazer and Shilo, 1991) and for directed cell migration of midline glial cells (Klämbt et al., 1992). The second FGFR in Drosophila, Heartless (Htl), becomes activated by its ligands Thisbe (Ths) and Pyramus (Pyr) (Gryzik and Müller, 2004; Stathopoulos et al., 2004) and is implicated in various migration processes. Htl is expressed in the mesoderm during gastrulation (Shishido et al., 1993), where it is necessary for the epithelial-mesenchymal transition, i.e. the dorsolateral migration of individual mesodermal cells along the ectoderm (Gisselbrecht et al., 1996). Htl is also needed during ovarian muscle tissue development (Irizarry and Stathopoulos, 2015). Htl-dependent FGF signaling also guides the migration of founder cells of the longitudinal midgut muscles during Drosophila embryogenesis (Kadam et al., 2012; Reim et al., 2012). During migration, these longitudinal founder cells fuse with fusion-competent myoblasts to build syncytia. Rudolf et al. (2014) have shown that this migration and fusion process is dependent on cytoskeletal rearrangements, particularly Arp2/3-induced actin polymerization. The function of cytoskeleton components and their regulators is also implicated in other cell migration processes. In vertebrate cells, Arp2/3 is needed for actin nucleation in lamellipodia-dependent cell migration (Campellone and Welch, 2010), while non-muscle myosin II plays a fundamental role in promoting directional cell migration (Vicente-Manzanares et al., 2009).
In Drosophila, cadherins such as Shotgun (Shg, the Drosophila homologue of E-Cadherin) and Cadherin-N (Cad-N) can mediate adhesion between neighboring cells. Catenins, such as beta-catenin, mediate the link between cadherins and the cytoskeleton (Bulgakova et al., 2012). Exemplarily, during the epithelial-mesenchymal transition in the Drosophila embryo, a series of changes in cell polarity, cell adhesion, and motility occur. Cells undergo a switch from an adhesive state towards a migrating state (Lim and Thiery, 2012). Thereby, the transcription factor Snail down-regulates epithelial genes, e.g. Shg, while Twist induces the transcription of Cad-N in the mesoderm (Leptin and Grunewald, 1990).
Here, we report first insights into the migration process of nascent myotubes from the prospective seminal vesicle onto the pupal testis and further towards the apical tip of the testis. Based on our results, we propose a model that links Ths- and Htl-dependent FGF signaling to myosin II-dependent processes during the migration of nascent myotubes from the genital disc onto the testes.
RESULTS
The adhesion molecule Cadherin-N is essential for building a continuous testis sheath
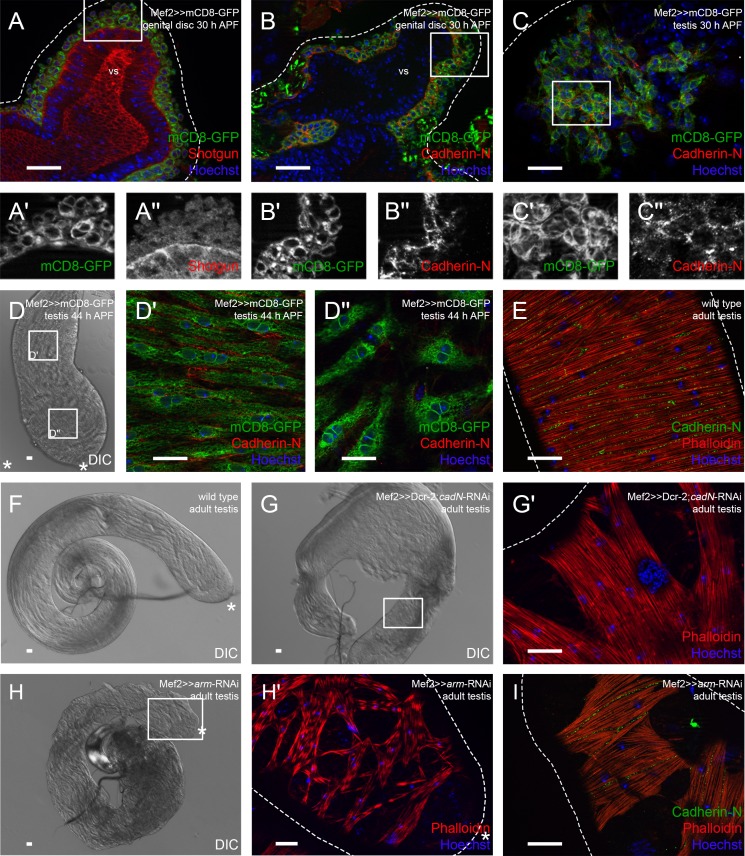
Since the Drosophila adult testis is encircled by a tight muscle sheath, we asked whether cadherins mediate the adhesion between myotubes in the testis muscle sheath before, during, and after migration. In expression analyses, we used Mef2-Gal4-driven UAS-mCD8-GFP flies to visualize myoblasts on genital discs and pupal testes at distinct time points. We observed that on genital discs 30 h APF, Shg (Cad-E) was expressed in the epithelium of the prospective seminal vesicle but was hardly detectable in myoblasts (Fig. 2A–A″).
Fig. 2.
Knock-down of Cadherin-N or Armadillo strongly reduces the adhesion between testis myotubes. Immunofluorescence analyses of genital discs and testes. (A) Seminal vesicles 30 h APF stained or marked with anti-Shotgun (red), GFP (green; myoblasts and myotubes on genital discs and pupal testes marked with Mef2≫mCD8-GFP), and Hoechst (blue; nuclei). (A′,A″) Enlargement of boxed area in A, stained or marked as indicated. (B) Genital discs 30 h APF and (C) testis 30 h APF stained or marked with anti-Cad-N (red), GFP (green), and Hoechst (blue), magnification of prospective seminal vesicle is shown. (B′,B″,C′,C″) Enlargement of boxed area in B and C stained or marked as indicated. (D–D″) Testis 44 h APF. (D) Differential interference contrast (DIC) micrograph of testis 44 h APF, (D′,D″) enlargement of boxed area in D stained or marked with anti-Cad-N (red), GFP (green), and Hoechst (blue). (E) Adult testis stained with Hoechst (blue), Phalloidin to visualize F-actin (red), and anti-Cad-N (green). (F) DIC micrograph of wild-type testis. (G) DIC micrograph of cad-N knock-down testis; (G′) enlargement of boxed area in G showing Phalloidin (red) and Hoechst (blue) staining of testis muscle sheath. (H) DIC micrograph of arm knock-down testis; (H′) enlargement of boxed area in H showing Phalloidin (red) and Hoechst (blue) staining of testis muscle sheath. (I) Adult arm knock-down testis stained with Hoechst (blue), Phalloidin to visualize F-actin (red), and anti-Cad-N (green). Dotted lines reflect approximate shape of the organ. Asterisk, hub region; vs, seminal vesicle. Scale bars: 20 µm.
By contrast, Cad-N was detected in the membranes of nascent myotubes on genital discs 30 h APF (Fig. 2B–B″) as well as on testes at 30 h APF (Fig. 2C–C″). Nascent myotubes of testes 44 h APF expressed Cad-N in correlation to their stage of migration. Specifically, myotubes at the basal testis end already started to elongate and to build a sheath, and expressed Cad-N (Fig. 2D,D′), whereas Cad-N was barely detected in nascent myotubes near the apical tip in mCD8-GFP-labeled myotubes in an optical section (Fig. 2D,D″). In the testis sheath of adult males, Cad-N was distinctly localized in the adjacent membranes of multinucleated myotubes (Fig. 2E).
Hence, we then knocked down cad-N by RNA interference (RNAi) with the driver line UAS-Dcr-2;;Mef2-Gal4 specifically in myoblasts. This resulted in a disturbed morphology of the testis. In the wild type, the adult testis is a long, thin tubule of 2.5 coils (Fig. 2F). In the RNAi-mediated knock-down of cad-N, the adult testis was partly irregular in shape and had roughly only one coil (Fig. 2G). The testis muscle sheath exhibited holes, which indicated that the myotubes were not properly attached to one another (Fig. 2G′). Genital discs 24 h APF exhibit Duf-expressing FC-like and sticks and stones (SNS)-expressing FCM-like myoblasts on prospective seminal vesicles (Kuckwa et al., 2016). In differential interference contrast (DIC) images, FC-like and FCM-like cells are visible (Fig. S2A), Cad-N expression is largely restricted to FC-like myoblasts (Fig. S2A′). mefGAL4-driven knock-down of Cad-N was efficient in FC-like myoblasts, whereas expression was hardly affected in myoblasts lying over the paragonia (compare Fig. S2B′ to wild type Fig. S2A′). At 44 h APF, cad-N knock-down testes displayed nascent myotubes, which were distributed all over the testes (Fig. S2C′). In contrast to wild-type testes (Fig. S2C), the nascent myotubes in these cad-N knock-down testes are less elongated and their number seemed to be reduced (Fig. S2C). Nevertheless, adult males with this phenotype were able to produce offspring (89%, Fig. S1).
Furthermore, we down-regulated Armadillo (Arm), the beta-catenin homologue in Drosophila (Peifer et al., 1992), specifically in myoblasts. Adult testes with down-regulated arm had about two coils (Fig. 2H). The testis muscle sheath exhibited numerous small holes, which indicated that the adhesion between single myotubes was disturbed (Fig. 2H′). These males also had a reduced fertility (60%, Fig. S1). Cad-N expression between adjacent myotubes was preserved upon arm knock-down, whereas no Cad-N expression was detected when testis myotubes did not adhere to each other (Fig. 2I).
We conclude that Cad-N in cooperation with Arm is involved in testis myotube migration and is necessary both to stabilize the testis muscle sheath and to shape the testis.
Non-muscle myosin II regulates the migration of nascent myotubes onto the testes
Many migratory processes depend on the actin-myosin network (Campellone and Welch, 2010; Vicente-Manzanares et al., 2009). Using myoblast-specific RNAi experiments, we therefore investigated the relevant myosins of nascent myotubes as they populated the testis. Down-regulation of the light or heavy chain of non-muscle myosin II led to drastic defects.
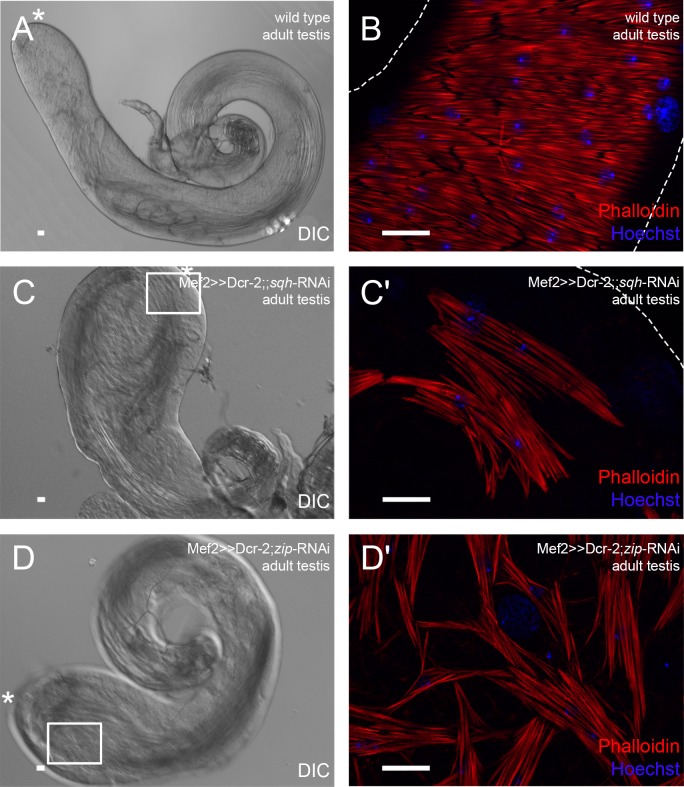
When we myoblast-specifically knocked down spaghetti squash (sqh), the regulatory light chain of non-muscle myosin II (Karess et al., 1991). The testes were smaller than in the wild type (Fig. 3A), and had bulky tips and 1.5 coils in adult males (Fig. 3C). The muscle sheath did not cover the entire testes and had numerous holes; single myofibers appeared shorter (Fig. 3C′) than in the wild type (Fig. 3B). These males had reduced fertility (43%, Fig. S1).
Fig. 3.
Myoblast-specific down-regulation of non-muscle myosin II leads to inefficient population of the testis with muscles. Analysis of adult (A,B) wild-type testes, (C,C′) sqh knock-down testes, and (D,D′) zip knock-down testes. (A,C,D) DIC micrograph; asterisk, hub region. (B,C′,D′) Phalloidin staining to visualize F-actin (red), and Hoechst staining of nuclei (blue). C′ and D′ are enlargements of areas boxed in C and D, respectively. Dotted lines reflect approximate shape of the organ. Scale bars: 20 µm.
Testes morphology and musculature were also disturbed when zipper (zip), the non-muscle myosin II heavy chain (Mansfield et al., 1996), was down-regulated. Adult testes had 1.5 coils and a bulky head (Fig. 3D), comparable to the phenotype observed in the sqh knock-down, and the muscles resembled a broad-meshed net rather than a sheath (Fig. 3D′). In contrast to sqh knock-down males, the fertility of zip knock-down males was preserved (100%, Fig. S1). This was surprising because the sqh and zip knock-down phenotypes resembled each other and are components of myosin II. The observed differences in male fertility might be due to an unpredicted off-target in the sqh RNAi line.
As an example, we first analyzed adult sqh knock-down testes in more detail. Cad-N was expressed between the few remaining adjacent myotubes (Fig. S2D), which suggested that the muscle sheath defect was not due to faults in Cad-N-mediated attachment of single myotubes. Instead, it was likely caused by insufficient population of the testis with muscles. We further analyzed the status of the extracellular matrix by monitoring Terribly reduced optic lobes (Trol) (Voigt et al., 2002), which is expressed in the testis muscle sheath (Susic-Jung et al., 2012). Expression of Trol revealed no changes in adult sqh knock-down testes (Fig. S2E,F), which indicated extracellular matrix integrity. At 44 h APF, pupal testes with myoblast-specific sqh knock-down were populated with fewer nascent myotubes than wild-type testes (Fig. S2H, compare to G). However, the testis shape was not affected at this time of development.
Taken together, these data led us to conclude that non-muscle myosin II is required for testis myotube migration and that correct shaping of the testis depends on the presence of an intact tight muscle sheath.
DWnt2 signaling controls determination of testis-relevant myoblasts
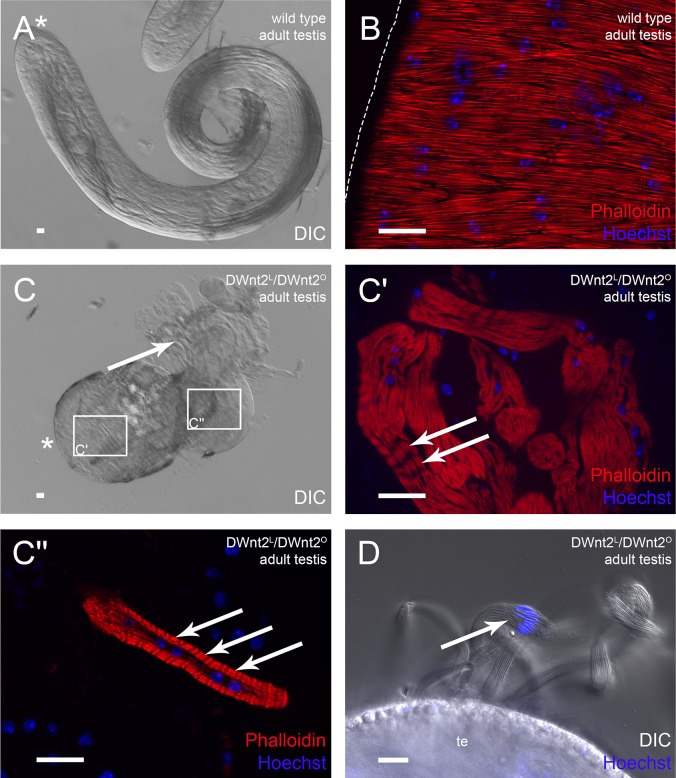
It has been previously suggested that DWnt2 plays a role in the migration of myoblasts from the prospective seminal vesicle onto the testes (Kozopas et al., 1998). Therefore, we analyzed the testis shape and muscle sheath of two amorphic DWnt2 alleles in trans (DWnt2L, DWnt2O; Kozopas et al., 1998). The resulting flies were not able to hatch, and pharate lethal. The development and morphology of testes was variable in these flies, as has also been recently observed by Linnemannstöns et al. (2014). The most abundant phenotypes were deformed and not elongated adult testes that did not resemble the wild-type adult testis shape (Fig. 4A). Instead, the shapes were comparable to those of pupal testes (Fig. 4C). Testis stability was decreased, and germ cells leaked (Fig. 4C, arrow). Unlike the wild-type musculature (Fig. 4B), smooth-like muscles were not detected on these testes, but some multinucleated actin-rich structures that contained repetitive actin filaments resembling striated muscles were visible (Fig. 4C′,C″, arrows). The leaking cysts with elongated spermatids that appeared shortly before individualization (Fig. 4D, arrow) indicated that germ cell maturation proceeded normally. This is in agreement with our earlier observations that spermatogenesis in vitro in cultured isolated cysts proceeds until shortly before individualization, independently of the presence of the testis muscle sheath (Awe and Renkawitz-Pohl, 2010; Gärtner et al., 2014). Other organs of the reproductive system showed no obvious defects (data not shown), which suggested a selective effect on testis-relevant myoblasts.
Fig. 4.
DWnt2 affects testis muscle determination. Analysis of adult (A,B) wild-type testes and (C-D) DWnt2L/DWnt2O testes. (A,C) DIC micrographs; arrow, leaking sperm; asterisk, hub region. (C′,C″) Enlargement of the respective boxed areas in C stained with Phalloidin to visualize F-actin (red) and Hoechst to visualize nuclei (blue). Arrows, thin actin filaments. (D) DIC micrograph and Hoechst staining (blue) of adult DWnt2L/DWnt2O testis. Arrow, nuclei of leaking spermatid bundles. Scale bars: 20 µm.
From these data, we concluded that DWnt2L/DWnt2O mutant flies fail to determine testis-specific myoblasts from the common pool of myoblasts. However, this does not exclude an additional later role of DWnt2 during migration of muscles onto the testes.
Heartless-dependent FGF signaling is essential for the migration of nascent myotubes onto the testis
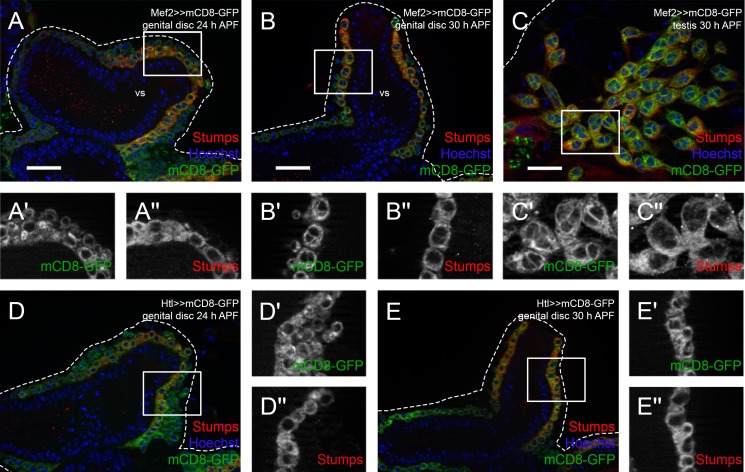
FGF signaling is involved in various migration processes throughout the animal kingdom (Itoh and Ornitz, 2011; Ornitz and Itoh, 2015; Shilo, 2016). Therefore, we examined the role of FGF signaling in the migration of nascent testis myotubes. We analyzed the expression of Stumps, the intracellular adaptor protein specific for FGFRs, which is also known as Heartbroken (Hbr) and Downstream of FGF (Dof) (Imam et al., 1999; Michelson et al., 1998a; Vincent et al., 1998), using a specific antibody (Vincent et al., 1998) on genital discs. At 24 h APF, Stumps was detected in the cytoplasm of myoblasts in the inner layer, the so-called FC-like myoblasts (Fig. 5A–A″). Nascent myotubes on genital discs 30 h APF (Fig. 5B–B″) and testes 30 h APF (Fig. 5C–C″) also expressed Stumps in the cytoplasm. We investigated the potential expression pattern of Htl on male genital discs in an Htl-Gal4 driver line that has been shown to drive expression in the epithelial sheath of the ovary (Irizarry and Stathopoulos, 2015). We observed Htl-driven mCD8-GFP expression in myoblasts on the prospective seminal vesicles at 24 h APF (Fig. 5D) and 30 h APF (Fig. 5E). Notably, Stumps was expressed in FC-like Htl-positive myoblasts on developing seminal vesicles at 24 h APF (Fig. 5D–D″) and in all nascent myotubes at 30 h APF (Fig. 5E–E″), which suggested that FGF signaling might be activated during migration.
Fig. 5.
FGF signaling components are expressed during migration of testis myotubes. Immunofluorescence analysis of Stumps. Myoblasts and nascent myotubes on (A-A″) wild-type genital discs 24 h APF, (B-B″) wild-type genital discs 30 h APF (magnifications of prospective seminal vesicles are shown), and (C-C″) pupal testis 30 h APF stained or marked with anti-Stumps (red), Mef2-driven mCD8-GFP (green), and Hoechst (blue; nuclei). (A′,A″,B′,B″,C′,C″) Enlargement of boxed areas in A, B, and C, respectively, marked with GFP or stained with anti-Stumps. (D-E″) Myoblasts and myotubes on Htl-Gal4≫mCD8-GFP genital discs at (D-D″) 24 h APF (magnification of prospective seminal vesicle is shown) and (E-E″) 30 h APF, stained or marked with anti-Stumps (red), Htl-driven mCD8-GFP (green), and Hoechst (blue). (D′,D″,E′,E″) Enlargement of boxed area in D and E, respectively, marked with Htl-driven mCD8-GFP or stained with anti-Stumps. Dotted lines reflect approximate shape of the organ. vs, seminal vesicle. Scale bars: 20 µm.
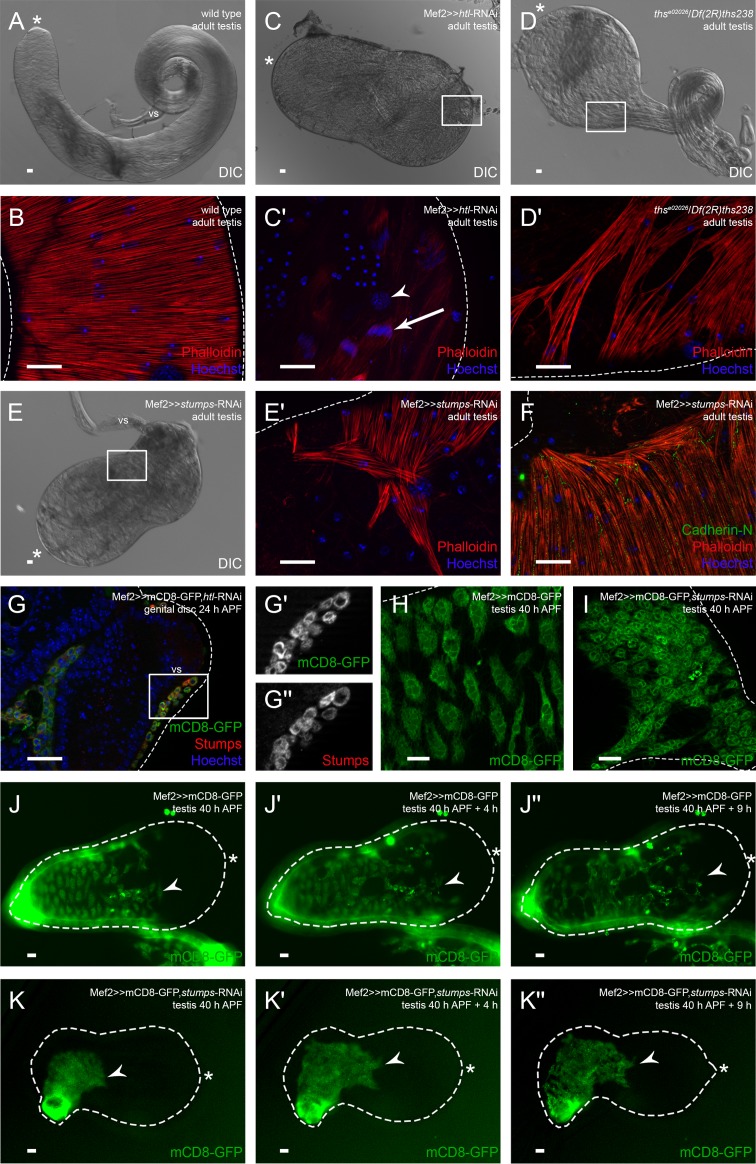
In Drosophila, Stumps is a unique adaptor protein in FGF signaling that can be activated by two FGFRs (Vincent et al., 1998). Myoblast-specific knock-down of the FGFR Btl did not result in any defects in testis musculature or shape, but resembled the wild-type situation (Fig. S3A). By contrast, we observed very drastic defects when the FGFR Htl was knocked down. Specifically, all flies were pharate lethal, and most testes were not attached to the seminal vesicles and did not elongate (Fig. 6C). The testes did not contain muscles, and the sheath consisted solely of pigment cells (Fig. 6C′, arrowhead). Nevertheless, spermatogenesis appeared to be normal in these testes since we observed bundles of spermatids shortly before or during individualization (Fig. 6C′, arrow). This indicates that the presence of testis muscles is not essential for germ cell maturation, as we also observed in hypomorph DWnt2 alleles (Fig. 4). In the htl knock-down, the other organs of the male reproductive system were malformed and partly degraded, and muscles were mainly absent (data not shown). This might be due to myoblast determination defects, since htl knock-down genital discs 24 h APF contained fewer myoblasts than wild-type genital discs 24 h APF. This was especially the case in the posterior part of the genital discs, where myoblasts for the muscle sheaths of the ejaculatory duct and sperm pump are localized (Fig. S3B,C, arrows). At 30 h APF, prospective seminal vesicles of htl knock-down genital discs contained fewer nascent myotubes than the wild type (compare Fig. S3F-F″ to Fig. 5B). This is in agreement with the observed Htl-driven expression of mCD8-GFP in most myoblasts on genital discs 24 h APF (Fig. S3D). Myoblast-specific expression of a dominant-negative (DN) version of Htl lacking the kinase domain (Michelson et al., 1998b) resulted in a milder phenotype than the htl knock-down, comparable to the other htl-RNAi line (Table S1). The adult testes were elongated and coiled, but had bulky tips as observed with the DN-version of Htl (Fig. S3E). The testis muscles covered the organs except for the tips (Fig. S3E′). To gain further insight into which FGF ligand activates Htl in nascent myotubes, we analyzed the testis shape and musculature of hypomorph thse02026/Df(2R)ths238 mutants. Adult testes had approximately two coils and a very bulky tip (Fig. 6D). The smooth-like testis muscle sheath had numerous holes, and the testis tip was free of muscles (Fig. 6D′) as previously observed for DN-version of Htl and knock down by the RNAi line BL35024. Unfortunately, we could not analyze the other FGF ligand Pyr because no hypomorph alleles were available.
Fig. 6.
Ths-activated Heartless is essential for populating the testis with myotubes. Analysis of htl and stumps knock-down and ths mutant. (A) DIC micrograph of adult wild-type testis. (B) Phalloidin staining to visualize F-actin (red), and Hoechst staining of nuclei (blue). (C) DIC micrograph of adult htl knock-down (v6692) testis. (C′) Enlargement of boxed area in C showing adult htl knock-down testis stained with Phalloidin (red; F-actin) and Hoechst (blue; nuclei); arrowhead, pigment cell nuclei; arrow, spermatids during individualization. (D) DIC micrograph of adult ths mutant testis. (D′) Enlargement of boxed area in D showing adult ths mutant testis stained with Phalloidin (red) and Hoechst (blue). (E) DIC micrograph of adult stumps knock-down testis. (E′) Enlargement of boxed area in E showing adult stumps knock-down testis stained with Phalloidin (red) and Hoechst (blue). (F) Adult stumps knock-down testis stained with anti-Cad-N (green), Phalloidin (red), and Hoechst (blue). (G-G″) htl knock-down genital disc 24 h APF stained or marked with anti-Stumps (red), GFP (green), and Hoechst (blue); magnification of prospective seminal vesicle is shown. (G′,G″) Enlargement of boxed area in G marked with GFP or stained with anti-Stumps. (H) Myoblasts on wild-type testis 40 h APF marked with Mef2-driven mCD8-GFP (green). (I) Myotubes of stumps knock-down testis 40 h APF marked with GFP (green). (J-K″) Live imaging over time of testes 40 h APF expressing Mef2-driven mCD8-GFP to reveal the migration of nascent myotubes in an ex vivo culture of (J-J″) wild-type testis and (K-K″) stumps knock-down testis. Dotted lines reflect the approximate shape of the organ. Arrowheads, the front of migrating nascent myotubes; asterisk, hub region; vs, seminal vesicle. Scale bars: 20 µm.
When we down-regulated stumps specifically in myoblasts, a strong phenotype was generated, comparable to that of the htl knock-down. But unlike the strongest htl knock-down testes, adult testes with reduced Stumps levels were attached to seminal vesicles, and about 30% of the testis sheath contained muscles. All flies were pharate lethal, and most testes attached to the seminal vesicles did not elongate (Fig. 6E). The muscles did not build a complete sheath but remained at the basal end of the testis, and the muscle pattern was disturbed (Fig. 6E′). On the adult stumps knock-down testis, Cad-N was normally expressed in the membranes between adjacent myofibers, where muscles formed an intact sheath (Fig. 6F). However, where myofibers did not attach to one another, no Cad-N was detected (Fig. 6F). No Stumps expression was visible in stumps knock-down genital discs 24 h APF, which indicated a strong down-regulation of stumps (Fig. S3G-G″). After stumps knock-down – in contrast to the htl knock-down (Fig. S3F-F″) – we observed many myoblasts lying over the seminal vesicles (Fig. S3G-G″). Stumps expression on htl knock-down genital discs 24 h APF remained unchanged in testis-relevant myoblasts (Fig. 6G-G″). At 40 h APF, mCD8-GFP-positive nascent myotubes were visible on both wild-type (Fig. 6H) and stumps knock-down testes (Fig. 6I), which indicated that the initial migration process was intact upon stumps knock-down.
These results suggest that Htl-controlled FGF signaling activated by Ths is essential for the correct migration of nascent myotubes on testes.
Stumps is necessary for proper myotube migration on the testis ex vivo
To gain further insights into the migration process on the testis, we established ex vivo live imaging of co-cultures of pupal testes and genital discs. For investigating the migrating nascent myotubes on the testis itself, we used 40 h APF testes expressing myoblast-specific mCD8-GFP. The testes were still attached to part of the developing seminal vesicles, but not to the genital disc. When live imaging started, approximately 60% of the pupal testis surface was already covered with multinucleated nascent myotubes (Fig. 6J, arrowhead). Four hours later, the myotubes had spread out and migrated towards the apical region (Fig. 6J′, arrowhead). After nine hours, myotubes covered about 80% of the pupal testis surface (Fig. 6J″, arrowhead). During live imaging, the testis grew in length.
We then tested the effect of down-regulated stumps on the migration process on pupal testes. We cultured stumps knock-down testes 40 h APF expressing myoblast-specific mCD8-GFP. These testes were also still attached to a part of the developing seminal vesicle. When live imaging started, approximately 30% of the pupal testis was covered with nascent myotubes (Fig. 6K, arrowhead). After four hours, myotubes covered about 40% of the testis (Fig. 6K′, arrowhead). After nine hours, myotubes still covered no more than 40% of the testis (Fig. 6K″, arrowhead). Strikingly, the morphology of the nascent myotubes was affected; the cells seemed smaller than in the control (Fig. 6K, compare to J) and did not spread out on the testis during live imaging. In addition, the testis grew only little in length.
From these results, we conclude that Stumps is essential for the migration of nascent myotubes on pupal testes, and that when Stumps is efficiently reduced, testes can grow and even attach to the developing seminal vesicles, but nascent myotubes fail to fully cover the testes.
Non-muscle myosin II expression depends on Heartless signaling
So far, we gained evidence for Heartless-dependent migration of nascent myotubes along the testes and showed that knock-down of Cad-N (Fig. 2G-G′) and Sqh (Fig. 3) lead to distortion of the coverage of testes with muscles. We now asked whether the expression of Cad-N or Sqh or both depends on Heartless signaling. As the htl-RNAi line with the strongest phenotype after myoblast-specific activation had no muscles on the testis, we applied a second htl-RNAi line (BL35024) in our analysis, which resembles the Htl-DN (Fig. S3E) phenotype and the thisbe hypomorph mutant and thus had muscles on the testes. As RNAi-mediated knock-down of Stumps (Fig. S3G-G′) was very efficient (Fig. S3G-G″), we focused on stumps in our analysis in parallel to the htl-RNAi line BL35024.
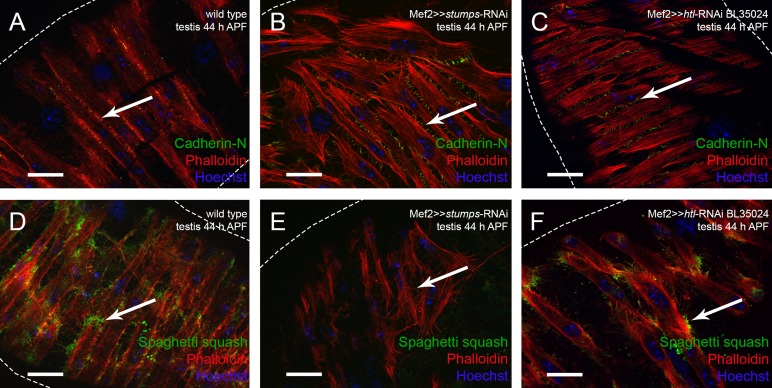
We observed Cad-N expression in nascent myotubes migrating along the testis and in the mature musculature (Fig. 2D-E). We asked whether reducing Heartless signaling impairs expression of Cad-N in pupal testes (arrows in Fig. 7A-C). Cad-N was expressed at the site of contact (Fig. 7A) in wild type and after knock-down of either Htl (Fig. 7C) or Stumps (Fig. 7B), which indicates that Cad-N expression is independent of Htl signaling. At 44 h APF in the Stumps knock-down mutant, nascent myotubes were less tightly packed and many Cad-N-positive filopodia were visible at the contact sites between neighboring myotubes (Fig. 7B).
Fig. 7.
Expression of the myosin II subunit Spaghetti squash is not detectable after knock-down of Stumps. (A-F) Visualization of muscles on testes by Phalloidin (red). (A-C) Expression of Cad-N in (A) wild type, (B) stumps knock-down, and (C) htl knock-down. (D-F) Sqh expression in (D) wild type (Z-stacks, 27 images with 0.48 µm per layer), (E) stumps knock-down (Z-stacks, 12 images with 0.52 µm per layer), and (F) htl knock-down (Z-stacks, 9 images with 0.24 µm per layer). Arrows in A-C indicate Cad-N and in D-F indicate Sqh; dotted lines indicate border of testis.
Next we asked whether Sqh, the light chain of myosin II, is expressed dependent on Htl signaling. At 44 h APF, Sqh localized to the filopodia of nascent myotubes on wild-type testes, and Sqh was enriched at the ends of stretching myotubes (Fig. 7D). In the htl-RNAi knock-down situation, however, nascent myotubes were less densely packed and had started to stretch while encircling the testis tube. In this situation, Sqh expression was not obviously disturbed (Fig. 7F). This is not surprising since in the less efficient htl-RNAi line showed a weak phenotype comparable to the dominant-negative version. By contrast, we did not detect Sqh in the poorly stretched nascent myotubes after efficient reduction of Stumps in the parallel experiment (Fig. 7E) (for knock down phenotype with respect to testis shape see Fig. 6E). We propose that Heartless signaling via Stumps directly or indirectly activates the synthesis of Sqh, the regulatory light chain of myosin II.
DISCUSSION
Wnt signaling does not directly affect testis myotube migration but affects determination of testis-relevant myoblasts
DWnt2 functions in pigment cell determination and possibly also in the migration of nascent myotubes onto pupal testes in addition to, or as a consequence of, the failure of pigment cell migration (Kozopas et al., 1998). We checked whether amorphic DWnt2 alleles have migration defects. In agreement with the results of Kozopas et al. (1998) and Linnemannstöns et al. (2014), an amorphic allelic combination of DWnt2 produced small testes of various sizes. Kozopas et al. (1998) observed only a small amount of muscles on adult DWnt2 mutant testes. We showed that smooth-like muscles were absent from the testis sheath. Surprisingly, a few striated muscles were visible on these testes.
The results presented here led us to tentatively conclude that DWnt2 affects cell fate determination of testis-relevant myoblasts. Indeed, it has been shown that Wnt signaling specifies cell identity of a subset of somatic muscle founder cells in Drosophila embryogenesis (Baylies et al., 1995) and during many other determinations of the fate of the cell (Bertrand, 2016; Munoz-Descalzo et al., 2015). However, an additional later role in the migration of testis myotubes cannot be excluded.
Multiple roles of Heartless-dependent FGF signaling during the development of the male reproductive system
The FGF receptor Btl is required for migration of glia and tracheal cells (Klämbt et al., 1992). In our study, down-regulation of the FGFR Btl, specifically in myoblasts, did not result in migration defects of testis myotubes. Conversely, knock-down of htl led to adult testes of a severely reduced size that remained free of muscles or to less drastic phenotypes, depending on the RNAi fly line chosen.
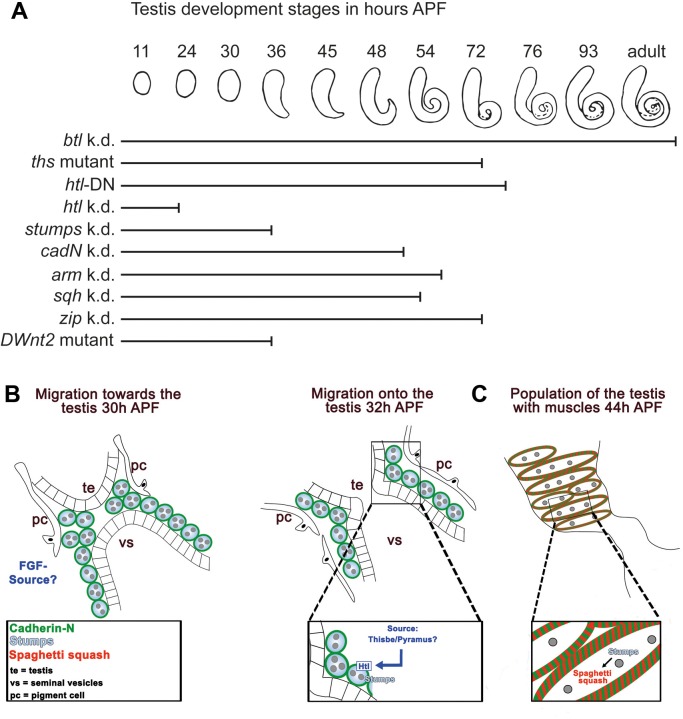
The htl knock-down yielded two further phenotypes. One phenotype was the drastically reduced number of non-testes-relevant myoblasts building the genital discs 24 h APF, while testis-relevant myoblasts were present on the developing seminal vesicles. This might be due to decreased proliferation of myoblasts on genital discs, since myoblasts undergo mitosis around 16 h APF (Kuckwa et al., 2016). Alternatively, this might be due to survival or determination defects. The second phenotype was the lack of a connection between the seminal vesicles and the testes. Knock-down of htl by one RNAi line caused testes development to stop early, whereas the dominant-negative version of Htl (Fig. 8A) and a weaker RNAi line (BL 35024) resulted in testes shapes that pointed towards a much later stop in development. Testes of hypomorph mutants of the Htl ligand Ths (Fig. 8A) showed a remarkably similar phenotype. This suggests that Ths is an important ligand of Htl in this system.
Fig. 8.
Model of testis myotube migration. (A) Summary of shaping defects of knock-down/mutant testes. Stages of testis development in wild-type males during metamorphosis, from 11 h APF to adult. Bars indicate the stage to which to mutant testes develop [myoblast-specific knock-down (k.d.) mutants, mutant testes expressing the dominant-negative (DN) Htl protein, or a DWnt2 mutant]. According to the affected gene or genetic manipulation, testis development correlates to different stages of wild-type development. For example, down-regulation of btl does not interrupt testis development, whereas htl knock-down leads to a very early stop in development. (B,C) Two-phase model of testis myotube migration. (B) Phase one represents the Htl dependent migration of nascent myotubes onto the testis before and after fusion of the epithelium of the seminal vesicle (vs) and the terminal epithelium (te) of the testes. Pigment cells (pc) migrated towords the vs. The FGF ligand Ths and possibly Pyr are secreted by an unknown source. (C) In phase two, nascent myotubes are already on the testis and migrate further towards the tip. Cad-N mediates adhesion between adjacent myotubes. Stumps might control the migration process, likely via non-muscle myosin II. Sqh expression depends on Stumps, which suggests Sqh regulation by Htl signaling. Note that after an initial phase one, both phases run in parallel until all myotubes reach the testis.
Stumps was expressed in FC-like myoblasts and nascent myotubes adjacent to the epithelium on genital discs and testes. In addition, down-regulation of the adaptor protein stumps yielded drastic defects in covering the testes with myotubes. This could be caused by a reduced number of myotubes or by a migration defect. We observed a fairly normal amount of myoblasts lying over the seminal vesicles. This and the disruption of migration of these myotubes in ex vivo cultures of testes argue for a migration defect in addition to morphology defects. stumps knock-down pupal testes 40 h APF exhibited many nascent myotubes, which indicated that initial migration occurred. However, we cannot exclude that we were unable to detect a low level of Stumps in immunofluorescence assays and that such a low amount could be sufficient for initial migration onto the testes.
Our results led us to conclude that migration of nascent myotubes on the testis depends on FGF signaling via Ths, Htl, and Stumps. Future research will focus on unravelling the distinct functions of FGF signaling via Heartless during muscle development in the male reproductive system.
Non-muscle myosin II drives the migration of nascent testis myotubes dependent on Heartless signaling
Down-regulation of non-muscle myosin II (i.e. zip or sqh) resulted in adult testes that were not fully covered with muscles, which indicated that non-muscle myosin II is necessary for the migration of nascent testis myotubes. Myosin II is also required for proper detachment and migration of border cells during egg chamber development (Combedazou et al., 2016; Majumder et al., 2012), and Drosophila tubulogenesis (Saxena et al., 2014; Nie et al., 2014). Signaling through epidermal growth factor receptor activates non-muscle myosin II (Saxena et al., 2014; Combedazou et al., 2016). Here, we propose that Heartless-mediated FGF signaling directly or indirectly induces non-muscle myosin II, which promotes migration of nascent testis myotubes. This might explain migration and morphological defects of myotubes in vivo and ex vivo.
Cadherin-N and Armadillo are expressed during cell migration and for stabilizing the testis muscle sheath
Cad-N was expressed in myoblasts and nascent myotubes of genital discs and pupal testes as well as in the membranes of adult testis myotubes. In contrast to the epithelial-mesenchymal transition during embryogenesis (Leptin and Grunewald, 1990), no change in expression from Shg to Cad-N was observed when the nascent myotubes started to migrate. During the initial migration onto and along the testis, Cad-N was expressed, which suggests a collective mode of cell migration. We propose that lack of Cad-N or Arm leads to disturbances in collective cell migration. As a consequence, far fewer myotubes spread over the testes, and residual myotubes cannot yield a tight sheath of muscles. This leads to thickened areas in the testes, where the population of muscles is low. Therefore, we in turn propose that Cad-N in cooperation with Arm is furthermore essential for mediating cell adhesion between single testis myotubes, thereby stabilizing the testis muscle sheath and the tubular and coiled shape of the testes. In contrast, to Sqh we gained no evidence for Htl dependent expression of Cad-N.
Testis shaping depends on an intact muscle sheath
Shaping of adult testes was disturbed to varying degrees depending on the genetic background (Fig. 8). The shape resembled that of different developmental time points in the wild type (Fig. 8A). Mutants with amorphic DWnt2 alleles, htl knock-down, or stumps knock-down showed hardly any shaping. These phenotypes correlate with the total absence or presence of only a few testis muscles. Hypomorph Ths mutants as well as the expression of a dominant-negative version of Htl led to a less severe phenotype that matched the shaping defects produced by down-regulating htl with another RNAi fly line. These adult testes resembled those of the wild type at 72 h APF. Knock-down of cadN, arm, sqh, or zip led to adult testes whose shape, but not the degree of coverage of the testes with muscles, resembled that of wild-type testes at 54–72 h APF. At 42 h APF, nascent myotubes reach the testis tip in the wild type (Kuckwa et al., 2016). This is also the case when cadN, arm, sqh, or zip is down-regulated specifically in myoblasts. However, already at this stage, fewer myotubes were present on knock-down testes (Fig. 7), which supports our conclusions that Cad-N and non-muscle myosin II are required for the migration of testis myotubes and that an intact muscle sheath is required for the shaping of the testis.
Conclusions and model
Based on our results, we propose a model in which testis myotube migration is divided into two Htl- and Cad-N-dependent phases. (i) After myoblast fusion, when Stumps and Cad-N are distributed along the plasma membrane of nascent testis myotubes that are in close contact to the adjacent epithelium of the prospective seminal vesicles, FGF signaling initiates collective migration of nascent testis myotubes from the prospective seminal vesicle onto the testis (Fig. 8B). (ii) The coverage of the testes with muscles is achieved by collective cells migrating towards the testis tip, and this migration requires myosin II and the formation of a network of nascent myotubes with Cad-N-positive connections (Fig. 8C).
We propose that the ligand Ths and possibly also Pyr binds Htl in nascent myotubes and thereby activates FGF signaling via Stumps during both phases of migration; the source of these ligands needs to be determined. Downstream, components of MAPK signaling, such as Erk, could be activated, which might cause changes in transcription of different target genes. Indeed, we observed Htl-dependent expression of Sqh. We hypothesize that Thisbe/Heartless-induced MAPK signaling might link to the actin cytoskeleton via non-muscle myosin II, thereby facilitating cytoskeletal changes needed for cell migration (Fig. 8B).
Our data provide first insights into the Thisbe/Heartless-dependent regulation of myotube migration via myosin II expression from the seminal vesicle to and along the testes, which is dependent on successful connection of these tissues. Future studies aiming at identifying missing components and functions to fully elucidate testis myoblast specification and myotube migration will require the establishment of more specific tools, such as driver lines specifically active in testes myoblasts and myotubes.
MATERIALS AND METHODS
Fly stocks
Flies were kept and RNAi crossings were carried out on standard medium at 25°C. w1118 (BL6326) was used as the wild-type reference. The following transgenic flies were used: Mef2-Gal4 (Ranganayakulu et al., 1995), Htl-Gal4 (BL40669), UAS-Dcr-2;;Mef2-Gal4 (BL25756), UAS-htl-DN (BL5366), and UAS-mCD8-GFP (BL32186). For RNAi experiments, the following fly lines were used: UAS-cadN-RNAi (v1092, v1093, v101642), UAS-arm-RNAi (BL31304, BL35004), UAS-sqh-RNAi (BL32439, BL33892), UAS-zip-RNAi (BL36727, BL37480), UAS-btl-RNAi (BL40871, v27106), UAS-htl-RNAi (BL35024, v6692), and UAS-stumps-RNAi (v21317, v105603). We conducted all RNAi experiments with at least two different fly lines per gene; the differences were in knock-down efficiencies or effects of second site insertions, as described in Vissers et al. (2016). An overview of all produced phenotypes is given in Table S1. The ths mutant was generated by crossing thse02026/CyO.actin-GFP (Stathopoulos et al., 2004) and Df(2R)ths238/CyO.actin-GFP (Kadam et al., 2009). For DWnt2 experiments, DWnt2L (BL6909, Kozopas et al., 1998) and DWnt2O (BL6958, Kozopas et al., 1998) flies were used. BL flies were ordered from Bloomington Drosophila Stock Center (Bloomington, IN, USA), and v flies were obtained from Vienna Drosophila RNAi Center (Vienna, Austria).
Fertility tests
One adult male of the F1 generation from RNAi crossings was mated with three virgin females (w1118) over seven days. After two weeks, the efficiency of the matings was examined. All fertility tests were carried out at 25°C.
Immunofluorescence
Pupae selection, genital disc dissection, and immunofluorescence were carried out as described elsewhere (Kuckwa et al., 2016) and were repeated at least three times. The following antibodies were used: anti Duf/Kirre (1:500, Kreisköther et al., 2006), anti-Cadherin-N (1:100, DSHB DN-Ex #8), anti-Shotgun (1:100, DSHB DCAD2), anti-Stumps (Dof, 1:1000; gift from Maria Leptin, University of Cologne, Germany; Vincent et al., 1998), antiSqh [1:10, 64 h incubation time; anti Phospho-Myosin Light Chain 2 (ser19), #3671 Cell Signaling; dilution according to Saxena et al., 2014; Nie et al., 2014] and anti-Trol (Perlecan domain V, 1:2000; gift from Stefan Baumgartner, Lund University, Sweden; Friedrich et al., 2000). The following secondary antibodies were used: anti-rat Cy3 (1:500; Jackson ImmunoResearch Laboratories), anti-rat Alexa Fluor® 488 (1:500; Jackson ImmunoResearch Laboratories), anti-rabbit DyLight 488 (1:500; Vector Laboratories), anti-rabbit DyLight 549 (1:500; Vector Laboratories). For visualization of F-actin, we used Phalloidin-Atto 565 (4 nmol l−1; 94072, Sigma-Aldrich, St. Louis, MO, USA); to visualize nuclei, Hoechst 33342 was used (3.2 µg ml−1; 62249, Thermo Fisher Scientific, Waltham, MA, USA).
Live imaging of ex vivo cultures
Male white prepupae were collected at 0 h APF and aged on a moistened filter. Genital discs and pupal testes were dissected in culture medium and transferred into a tissue culture treated µ-Dish35mm,high (81156, ibidi, Martinsried, Germany) containing 2 ml culture medium Shields and Sang M3 Insect Medium (S8398, Sigma-Aldrich) was made according to Aldaz et al. (2010) by filtering and supplementing with 2% fetal bovine serum (S0113, Biochrom AG, Berlin, Germany), 0.5% penicillin-streptomycin (P11-010, PAA, Cambridge, UK), and 0.1 µg ml−1 Ecdysone (H5142, Sigma-Aldrich). Culture medium worked successfully for up to 4 days when stored at 4°C. A Zeiss AxioObserver Z.1 inverse microscope was used for live imaging. A Z-stack was acquired every 15 min for up to 10 h.
Image acquisition and processing
Conventional fluorescent images and optical sections were captured with a Zeiss AxioObserver Z.1 inverse microscope with ApoTome function. Images were taken and processed with AxioVision LE64 (Carl Zeiss Microscopy GmbH, Jena, Germany), figures were assembled with Adobe Photoshop CS6 (Adobe Systems Incorporated, San José, CA, USA), and models were generated in Adobe Illustrator CS6 (Adobe Systems Incorporated). Charts were generated in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA).
Supplementary Material
Acknowledgements
We thank Katja Gessner for secretarial assistance, Peer Fender for graphic design expertise, Ljubinka Cigoja for help in dissecting and fly work, Hedda Wassum for initial experiments, Karen Linnemannstöns for advice on ex vivo testes cultures, Angelike Stathopoulos for fly lines, Maria Leptin and Stefan Baumgartner for antibodies, and Karen A. Brune for linguistic revision of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.R.-F., K.F., R.R.-P.; Methodology: S.R.-F., K.F., M.C.B.; Validation: S.R.-F., K.F., M.C.B., D.B.; Investigation: S.R.-F., K.F., M.C.B., D.B.; Writing - original draft: S.R.-F., K.F., R.R.-P.; Writing - review & editing: R.R.-P.; Visualization: M.C.B., D.B., S.F.O.; Supervision: S.F.O., R.R.-P.; Project administration: R.R.-P.; Funding acquisition: R.R.-P.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft [Re 628/16-1, GRK 1216 and GRK 2213].
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.025940.supplemental
References
- Ahmad S. M. and Baker B. S. (2002). Sex-specific deployment of FGF signaling in Drosophila recruits mesodermal cells into the male genital imaginal disc. Cell 109, 651-661. 10.1016/S0092-8674(02)00744-4 [DOI] [PubMed] [Google Scholar]
- Aldaz S., Escudero L. M. and Freeman M. (2010). Live imaging of Drosophila imaginal disc development. Proc. Natl. Acad. Sci. USA 107, 14217-14222. 10.1073/pnas.1008623107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awe S. and Renkawitz-Pohl R. (2010). Histone H4 acetylation is essential to proceed from a histone- to a protamine-based chromatin structure in spermatid nuclei of Drosophila melanogaster. Syst. Biol. Reprod. Med. 56, 44-61. 10.3109/19396360903490790 [DOI] [PubMed] [Google Scholar]
- Baylies M. K., Martinez Arias A. and Bate M. (1995). wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development 121, 3829-3837. [DOI] [PubMed] [Google Scholar]
- Bertrand V. (2016). Beta-catenin-driven binary cell fate decisions in animal development. Wiley Interdiscip. Rev. Dev. Biol. 5, 377-388. 10.1002/wdev.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenstein D. (1950). The postembryonic development of Drosophila. In Biology of Drosophila (ed. Demerec M.), pp. 275-367. New York: John Wiley & Sons, Inc. [Google Scholar]
- Bulgakova N. A., Klapholz B. and Brown N. H. (2012). Cell adhesion in Drosophila: versatility of cadherin and integrin complexes during development. Curr. Opin. Cell Biol. 24, 702-712. 10.1016/j.ceb.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone K. G. and Welch M. D. (2010). A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11, 237-251. 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combedazou A., Choesmel-Cadamuro V., Gay G., Liu J., Dupre L., Ramel D. and Wang X. (2016). Myosin II governs collective cell migration behaviour downstream of guidance receptor signalling. J. Cell Sci. 130, 97-103. 10.1242/jcs.179952 [DOI] [PubMed] [Google Scholar]
- Estrada B., Casares F. and Sánchez-Herrero E. (2003). Development of the genitalia in Drosophila melanogaster. Differentiation 71, 299-310. 10.1046/j.1432-0436.2003.03017.x [DOI] [PubMed] [Google Scholar]
- Friedrich M. V. K., Schneider M., Timpl R. and Baumgartner S. (2000). Perlecan domain V of Drosophila melanogaster. Sequence, recombinant analysis and tissue expression. Eur. J. Biochem. 267, 3149-3159. 10.1046/j.1432-1327.2000.01337.x [DOI] [PubMed] [Google Scholar]
- Gärtner S. M. K., Rathke C., Renkawitz-Pohl R. and Awe S. (2014). Ex vivo Culture of Drosophila pupal testis and single male germ-line cysts: dissection, imaging, and pharmacological treatment. J. Vis. Exp. 91, e51868 10.3791/51868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselbrecht S., Skeath J. B., Doe C. Q. and Michelson A. M. (1996). heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 10, 3003-3017. 10.1101/gad.10.23.3003 [DOI] [PubMed] [Google Scholar]
- Glazer L. and Shilo B. Z. (1991). The Drosophila FGF-R homolog is expressed in the embryonic tracheal system and appears to be required for directed tracheal cell extension. Genes Dev. 5, 697-705. 10.1101/gad.5.4.697 [DOI] [PubMed] [Google Scholar]
- Greig S. and Akam M. (1995). The role of homeotic genes in the specification of the Drosophila gonad. Curr. Biol. 5, 1057-1062. 10.1016/S0960-9822(95)00210-7 [DOI] [PubMed] [Google Scholar]
- Gryzik T. and Müller H.-A. J. (2004). FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Curr. Biol. 14, 659-667. 10.1016/j.cub.2004.03.058 [DOI] [PubMed] [Google Scholar]
- Imam F., Sutherland D., Huang W. and Krasnow M. A. (1999). stumps, a Drosophila gene required for fibroblast growth factor (FGF)-directed migrations of tracheal and mesodermal cells. Genetics 152, 307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry J. and Stathopoulos A. (2015). FGF signaling supports Drosophila fertility by regulating development of ovarian muscle tissues. Dev. Biol. 404, 1-13. 10.1016/j.ydbio.2015.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N. and Ornitz D. M. (2011). Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J. Biochem. 149, 121-130. 10.1093/jb/mvq121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S., McMahon A., Tzou P. and Stathopoulos A. (2009). FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development 136, 739-747. 10.1242/dev.027904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S., Ghosh S. and Stathopoulos A. (2012). Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands. Development 139, 699-708. 10.1242/dev.068791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Chang X., Edwards K. A., Kulkarni S., Aguilera I. and Kiehartt D. P. (1991). The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell 65, 1177-1189. 10.1016/0092-8674(91)90013-O [DOI] [PubMed] [Google Scholar]
- Klämbt C., Glazer L. and Shilo B.-Z. (1992). breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 6, 1668-1678. 10.1101/gad.6.9.1668 [DOI] [PubMed] [Google Scholar]
- Kozopas K. M., Samos C. H. and Nusse R. (1998). DWnt-2, a Drosophila Wnt gene required for the development of the male reproductive tract, specifies a sexually dimorphic cell fate. Genes Dev. 12, 1155-1165. 10.1101/gad.12.8.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisköther N., Reichert N., Buttgereit D., Hertenstein A., Fischbach K.-F. and Renkawitz-Pohl R. (2006). Drosophila Rolling pebbles colocalises and putatively interacts with alpha-Actinin and the Sls isoform Zormin in the Z-discs of the sarcomere and with Dumbfounded/Kirre, alpha-Actinin and Zormin in the terminal Z-discs. J. Muscle Res. Cell Motil. 27, 93-106. 10.1007/s10974-006-9060-y [DOI] [PubMed] [Google Scholar]
- Kuckwa J., Fritzen K., Buttgereit D., Rothenbusch-Fender S. and Renkawitz-Pohl R. (2016). A new level of plasticity: Drosophila smooth-like testes muscles compensate failure of myoblast fusion. Development 143, 329-338. 10.1242/dev.126730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptin M. and Grunewald B. (1990). Cell shape changes during gastrulation in Drosophila. Development 110, 73-84. [DOI] [PubMed] [Google Scholar]
- Lim J. and Thiery J. P. (2012). Epithelial-mesenchymal transitions: insights from development. Development 139, 3471-3486. 10.1242/dev.071209 [DOI] [PubMed] [Google Scholar]
- Linnemannstöns K., Ripp C., Honemann-Capito M., Brechtel-Curth K., Hedderich M. and Wodarz A. (2014). The PTK7-related transmembrane proteins off-track and off-track 2 are co-receptors for Drosophila Wnt2 required for male fertility. PLoS Genet. 10, e1004443 10.1371/journal.pgen.1004443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P., Aranjuez G., Amick J. and McDonald J. A. (2012). Par-1 controls myosin-II activity through myosin phosphatase to regulate border cell migration. Curr. Biol. 22, 363-372. 10.1016/j.cub.2012.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield G. S., Al-Shirawi D. Y., Ketchum A. S., Newbern C. E. and Kiehart D. P. (1996). Molecular organization and alternative splicing in zipper, the gene that encodes the Drosophila non-muscle myosin II heavy chain. J. Mol. Biol. 255, 98-109. 10.1006/jmbi.1996.0009 [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Gisselbrecht S., Buff E. and Skeath J. B. (1998a). Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development 125, 4379-4389. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Gisselbrecht S., Zhou Y., Baek K.-H. and Buff E. M. (1998b). Dual Functions of the heartless fibroblast growth factor receptor in development of the Drosophila embryonic mesoderm. Dev. Genet. 22, 212-229. [DOI] [PubMed] [Google Scholar]
- Munoz-Descalzo S., Hadjantonakis A. K. and Arias A. M. (2015). Wnt/Beta-catenin signalling and the dynamics of fate decisions in early mouse embryos and embryonic stem (ES) cells. Semin. Cell Dev. Biol. 47-48, 101-109. 10.1016/j.semcdb.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda S., DeFalco T. J., Loh S. H. Y., Phochanukul N., Camara N., Van Doren M. and Russell S. (2009). Sox100B, a Drosophila group E Sox-domain gene, is required for somatic testis differentiation. Sex. Dev. 3, 26-37. 10.1159/000200079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Mahato S. and Zelhof A. C. (2014). The actomyosin machinery is required for Drosophila retinal lumen formation. PLoS Genet. 10, e1004608 10.1371/journal.pgen.1004608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M. and Itoh N. (2015). The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215-266. 10.1002/wdev.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M., McCrea P. D., Green K. J., Wieschaus E. and Gumbiner B. M. (1992). The Vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. Cell 118, 681-691. 10.1083/jcb.118.3.681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocha S. M. and Montell D. J. (2014). Cellular and molecular mechanisms of single and collective cell migrations in Drosophila : themes and variations. Annu. Rev. Genet. 48, 295-318. 10.1146/annurev-genet-120213-092218 [DOI] [PubMed] [Google Scholar]
- Ranganayakulu G., Zhao B., Dokidis A., Molkentin J. D., Olson E. N. and Schulz R. A. (1995). A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev. Biol. 171, 169-181. 10.1006/dbio.1995.1269 [DOI] [PubMed] [Google Scholar]
- Reim I., Hollfelder D., Ismat A. and Frasch M. (2012). The FGF8-related signals Pyramus and Thisbe promote pathfinding, substrate adhesion, and survival of migrating longitudinal gut muscle founder cells. Dev. Biol. 368, 28-43. 10.1016/j.ydbio.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P., Sunyer R. and Trepat X. (2013). Mechanical guidance of cell migration: lessons from chemotaxis. Curr. Opin. Cell Biol. 25, 543-549. 10.1016/j.ceb.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Rudolf A., Buttgereit D., Jacobs M., Wolfstetter G., Kesper D., Pütz M., Berger S., Renkawitz-Pohl R., Holz A. and Önel S. F. (2014). Distinct genetic programs guide Drosophila circular and longitudinal visceral myoblast fusion. BMC Cell Biol. 15, 27 10.1186/1471-2121-15-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., Denholm B., Bunt S. Bischoff M., VjiayRaghavan K. and Skaer H. (2014). Epidermal growth factor signalling controls myosin II planar polarity to orchestrate convergent extension movements during Drosophila tubulogenesis. PLoS Biol. 12, e1002013 10.1371/journal.pbio.1002013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B.-Z. (2016). New twists in Drosophila cell signaling. J. Biol. Chem. 291, 7805-7808. 10.1074/jbc.R115.711473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishido E., Higashijima S., Emori Y. and Saigo K. (1993). Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development 117, 751-761. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A., Tam B., Ronshaugen M., Frasch M. and Levine M. (2004). Pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 18, 687-699. 10.1101/gad.1166404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C. (1941). The Growth of Testes in Drosophila I. The relation between vas deferens and testis within various species. J. Exp. Zool. 87, 113-158. [Google Scholar]
- Susic-Jung L., Hornbruch-freitag C., Kuckwa J., Rexer K.-H., Lammel U. and Renkawitz-pohl R. (2012). Multinucleated smooth muscles and mononucleated as well as multinucleated striated muscles develop during establishment of the male reproductive organs of Drosophila melanogaster. Dev. Biol. 370, 86-97. 10.1016/j.ydbio.2012.07.022 [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Ma X., Adelstein R. S. and Horwitz A. R. (2009). Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778-790. 10.1038/nrm2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S., Wilson R., Coelho C., Affolter M. and Leptin M. (1998). The Drosophila protein Dof is specifically required for FGF signaling. Mol. Cell 2, 515-525. 10.1016/S1097-2765(00)80151-3 [DOI] [PubMed] [Google Scholar]
- Vissers J. H. A., Manning S. A., Kulkarni A. and Harvey K. F. (2016). A Drosophila RNAi library modulates Hippo pathway-dependent tissue growth. Nat. Commun. 7, 10368 10.1038/ncomms10368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt A., Pflanz R., Schäfer U. and Jäckle H. (2002). Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev. Dyn. 224, 403-412. 10.1002/dvdy.10120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.