Abstract
Aim of the study
High-resolution ultrasonography of the tibial nerve is a fast and non invasive tool for diagnosis of diabetic peripheral neuropathy. Our study was aimed at finding out the correlation of the cross sectional area and maximum thickness of nerve fascicles of the tibial nerve with the presence and severity of diabetic peripheral neuropathy.
Material and methods
75 patients with type 2 diabetes mellitus clinically diagnosed with diabetic peripheral neuropathy were analysed, and the severity of neuropathy was determined using the Toronto Clinical Neuropathy Score. 58 diabetic patients with no clinical suspicion of diabetic peripheral neuropathy and 75 healthy non-diabetic subjects were taken as controls. The cross sectional area and maximum thickness of nerve fascicles of the tibial nerves were calculated 3 cm cranial to the medial malleolus in both lower limbs.
Results
The mean cross sectional area (22.63 +/− 2.66 mm2) and maximum thickness of nerve fascicles (0.70 mm) of the tibial nerves in patients with diabetic peripheral neuropathy compared with both control groups was significantly larger, and statistically significant correlation was found with the Toronto Clinical Neuropathy Score (p < 0.001). The diabetic patients with no signs of peripheral neuropathy had a larger mean cross sectional area (14.40 +/− 1.72 mm2) and maximum thickness of nerve fascicles of the tibial nerve (0.40 mm) than healthy non-diabetic subjects (12.42 +/− 1.01 mm2 and 0.30 mm respectively).
Conclusion
The cross sectional area and maximum thickness of nerve fascicles of the tibial nerve is larger in diabetic patients with or without peripheral neuropathy than in healthy control subjects, and ultrasonography can be used as a good screening tool in these patients.
Keywords: diabetes mellitus, tibial nerve, ultrasonography
Introduction
According to the World Health Organization, India had 69.2 million people living with diabetes as per the 2015 data, with more than 50% of the cases remaining undiagnosed. Diabetic peripheral neuropathy is one of the major complications of diabetes mellitus, with prevalence of approximately 30%. About half of the patients will develop neuropathy during the course of the disease(1). Poor glycemic control is a major risk factor in the development of diabetic polyneuropathy. Hyperglycemia leads to osmotic swelling of the nerves with injury to the axons and myelin sheath, which triggers the onset of neuropathy. Patients commonly present with complaints of tingling, numbness, or prickling sensations affecting the feet. The symptoms are often seen bilaterally and in an asymmetrical distribution. The most common sign is the absence of ankle reflexes. Sensory disturbance is also commonly seen, and the most common is the loss of vibration sense at the toes, followed by pinprick, temperature, and light touch sensations. The symptoms, sensory tests and reflex scores are part of clinical examination in the Toronto clinical neuropathy scoring system(2). Ultrasonography (US) of the tibial nerve is a fast real time tool for detecting diabetic neuropathy. There is no patient discomfort, nor any radiation exposure. The nerve conduction studies used by the neurologists for the diagnosis of peripheral neuropathy are defined by the presence of at least one abnormal parameter, including amplitude, latency and conduction velocity of the nerve, yet these studies are not available in every setup.
A few previous studies have analysed the relationship between diabetic neuropathy and cross-sectional area of peripheral nerves on ultrasonography(3,4), but a smaller sample size was a limitation(5).We have conducted a larger study, studying the cross sectional area and maximum thickness of nerve fascicles of the tibial nerve in diabetic patients with peripheral neuropathy.
Material and methods
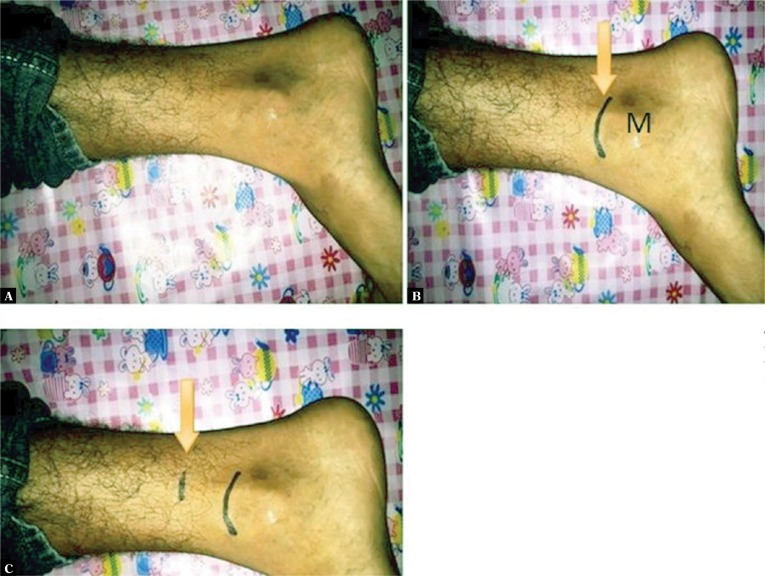
The study was conducted at Sri Guru Ram Das Institute of Medical Sciences and Research, Sri Amritsar and at Nijjar Scan Centre Sri Amritsar. 133 type 2 diabetic patients referred to the Department of Radio Diagnosis and Imaging were recruited for the study, and provided their written informed consents. They were divided into two groups. Diabetic patients with clinical signs and symptoms of peripheral neuropathy were assigned to Group I, consisting of 75 patients. Group II comprised 58 diabetic patients with no clinical signs and symptoms of peripheral neuropathy. 75 healthy volunteers were also recruited for the study, and assigned to Group III. Ultrasonography was performed with VOLUSON E8 EXPERT BT09 (GE) using a linear transducer (4–10 MHz), or with SP-10-16 D (GE) using a linear transducer (7–18 MHz), or Hitachi HIVISION using a linear transducer (7–18 MHz). The examination was performed with the patient lying in a lateral position for an easy assessment of the medial aspect of the ankle and distal leg. The cross sectional area and maximum thickness of nerve fascicles of bilateral tibial nerves were recorded 3 cm above the medial malleolus (Fig. 1). The cross sectional area was calculated by manual tracing. The maximum thickness of the nerve fascicle was calculated by the largest antero-posterior dimension of the largest hypoechoic area in the short axis view of the tibial nerve.
Fig. 1.
Patient position for focusing the tibial nerve (A). Arrow in B demonstrates the marking of the upper end of the medial malleolus. Arrow in C marks the position of the probe, 3 cm proximal to the upper end of the medial malleolus. M – the medial malleolus
The peripheral neuropathy status of the patients was calculated using the Toronto Clinical Neuropathy Score (TCNS) by the referring physicians. The severity of neuropathy was determined with TCNS (out of 19 points, 0–5: absent; 6–11: mild-moderate; >12: severe)(5,6). Patients with neuropathy due to causes other than diabetes mellitus were excluded from the study. The radiologist was blinded to HbA1c levels, the duration of the disease and TCNS of the patient. Demographic information, including age, sex, weight, systolic blood pressure, diastolic blood pressure and HbA1c levels was recorded for all patients. The cross sectional area (CSA) and maximum thickness of nerve fascicles (MTNF) were found to be statistically correlated with the TCNS score and HbA1c levels. All the patients underwent neurological and sonographic examination during one visit.
Statistical analysis
The mean cross sectional area and maximum thickness of nerve fascicles of the tibial nerve in all three groups were compared using IBM SPSS 20.0 software, with ANOVA and post hoc tests performed. The correlations with TCNS and HbA1c levels were identified using Pearson correlation coefficient.
Results
The demographic, clinical, biochemical and US characteristics of all the three groups are presented in Tab. 1. Male predominance was observed in all three groups. Weight, systolic blood pressure and diastolic blood pressure were the highest in Group I. The mean CSA of the tibial nerve was significantly larger in Group I subjects compared with Groups II and III, with p < 0.001 (Tab. 1). The mean CSA of the tibial nerve was also larger in Group II than in Group III, with p < 0.001. The mean MTNF of the tibial nerve was higher in Group I than Groups II and III, with p < 0.001. The mean MTNF was higher in Group II compared with Group III, with p < 0.05 (significant). The mean HbA1c level in Group I was higher than in the other groups, with a significant statistical correlation with CSA (r = 0.683 ; p <0.001) and MTNF (r = 0.613; p < 0.001) identified. Compared with Groups II and III, Group I subjects showed the highest mean TCNS, with a good correlation with CSA (r = 0.785 ; p <0.001) and MTNF (r = 0.761; p <0.001) of the tibial nerve.
Tab. 1.
Clinical and biochemical characteristics of patients, CSA, MTNF and TCNS results. Group I – diabetic patients with peripheral neuropathy, Group II – diabetic patients without peripheral neuropathy, Group III – non-diabetic subjects
| Clinical and biochemical characteristics | Group I (N = 75) |
Group II (N = 58) |
Group III (N = 75) |
|---|---|---|---|
| Age in years (Mean +/− SD) Range |
59.30 +/− 12.11 (40–89) |
46.98 +/− 6.82 (33–60) |
39.54 +/− 10.12 (23–72) |
| Duration of diabetes in years (Mean +/− SD) Range |
13.98 +/− 7.06 (5–38) |
7.53 +/− 4.26 (1–19) |
− |
| Male : Female | 46 : 29 | 32 : 26 | 44 : 31 |
| Weight in kg (mean +/− SD) Range |
78.52 +/− 9.60 (53–96) |
63.24 +/− 9.89 (40–80) |
63.34 +/− 10.33 (40–85) |
| Systolic blood pressure in mmHg (mean +/− SD) Range |
138.48 +/− 10.37 (110–160) |
121.48 +/− 9.58 (100–146) |
120.77 +/− 9.34 (100–140) |
| Diastolic blood pressure in mmHg (mean +/− SD) Range |
83.46 +/− 6.54 (68–94) |
76.68 +/− 6.19 (64–90) |
76.26 +/− 6.09 (60–86) |
| HbA1c levels in % (mean +/− SD) Range |
7.84 +/− 1.20 (6.10–12.50) |
5.92 +/− 0.69 (5.0–7.30) |
5.23 +/− 0.23 (5.0–5.92) |
| CSA of the tibial nerve in mm2 (mean +/− SD) Range |
22.63 +/− 2.66 (18.5–29) |
14.40 +/− 1.72 (12–18.5) |
12.42 +/− 1.10 (10–14) |
| MTNF of the tibial nerve in mm (mean +/− SD) Range |
0.47 +/− 0.09 (0.30–0.70) |
0.23 +/− 0.06 (0.10–0.40) |
0.20 +/− 0.05 (0.10–0.30) |
| TCNS (mean +/− SD) Range |
10.14 +/− 3.45 (6.0–18.5) |
1.67 +/− 1.61 (0–5) |
− |
CSA – cross sectional area; DN – diabetic neuropathy; HRUS – high resolution ultrasonography; MTNF – maximum thickness of nerve fascicles; MRI – magnetic resonance imaging; NCS – nerve conduction study; r – Pearson correlation coefficient value; TCNS – Toronto Clinical Neuropathy score; US – ultrasonography
Discussion
Before the introduction of imaging methods, the diagnostic work-up of peripheral nerve disorders was based only on the evaluation of the clinical history, neurological examination and other diagnostic tests, such as nerve conduction studies, F-waves and electromyography(1). Nerve imaging has become an important method in patient management by providing information on lesion morphology, anatomical location, relationship of the lesions to the adjacent structures, and evaluation of areas not accessible with electrodiagnostic tests. Imaging can also identify peripheral nerve space-occupying lesions that are not apparent on electrodiagnostic tests. High resolution ultrasound (HRUS) and magnetic resonance imaging (MRI) are the most commonly used methods for visualizing peripheral nerves. US is used as an adjuvant in the neurological assessment even in day-to-day practice, and this confirms the increasing interest in US for the evaluation of the peripheral nervous system disorders(7).
US examination is currently increasingly used for the imaging of peripheral nerves, supplementing the physical examination. An important break-through in the ultrasound diagnostics of peripheral nerves occurred with the introduction of US probes with high frequencies (greater than 12-15 MHz). Peripheral nerves have a typical US pattern that correlates with their histological structure: hypoechoic areas are separated by hyperechoic bands. The hypoechoic areas correspond to neuronal fascicles(8,9) (Fig. 2). The sural nerve is affected earliest in diabetic neuropathy, and is detected on nerve conduction studies. The tibial nerve at the level of the medial malleolus may be similarly affected in this disease(10,11). Due to high spatial resolution of US, the modality is useful for studying the morphology of the peripheral nerves, with the tibial nerve being a good choice for this investigation(12).
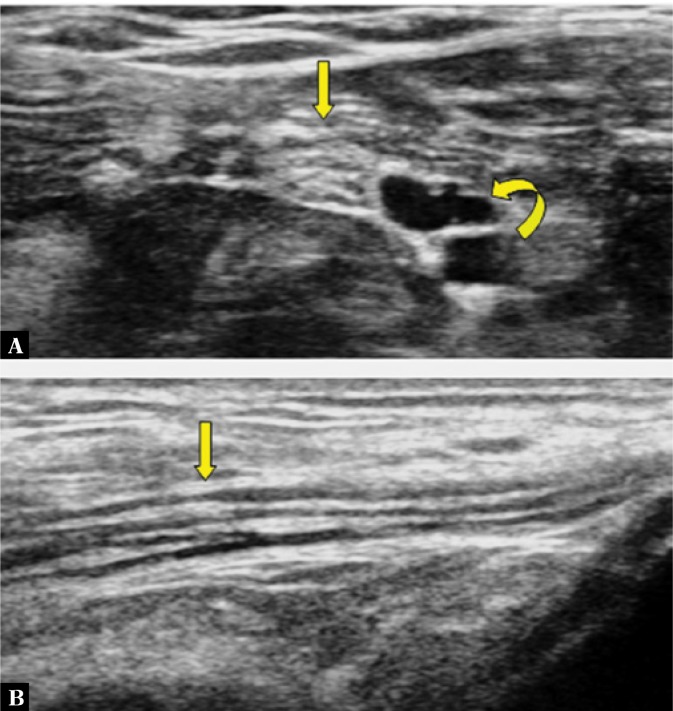
Fig. 2.
A transverse view of the tibial nerve, showing a honeycomb pattern (straight arrow in A) due to hypoechoic areas (nerve fascicle groups) distributed over a hyperechoic background (perineurium). The echopoor areas are posterior tibial vessels (curved arrow in A). In the longitudinal view, the nerve appears as the long, slim structure with alternate hypoechoic and hyperechoic stripes (Straight arrow in B).
The vessels accompanying the nerves are taken as anatomical reference points during US examination. The tibial nerve is the thicker terminal branch of the sciatic nerve. At the level of the medial malleolus, it is accompanied by the posterior tibial artery and veins, proximally on its anterior side, and distally along its medial aspect(13). The diagnosis of diabetic neuropathy can be confirmed with NCS, however it is time-consuming and invasive, and is not feasible for repeated evaluations. US, on the other hand, can be performed at the patient’s little or no discomfort, and has already been used for evaluating peripheral neural pathologies(14,15). The symptoms of DPN usually first appear in the toes or the soles of the feet(16,17). This was another reason why we used the tibial nerve as the studied structure in our study.
Sheila Riazi et al. studied 98 diabetic patients classified by NCS. The severity of neuropathy was determined using TCNS. The cross-sectional area of the tibial nerve was measured at 1, 3, and 5 cm proximal to the level of the medial malleolus. They concluded that CSA measured at 3 cm above the medial malleolus had an optimal threshold value for diagnosing diabetic peripheral neuropathy, with a sensitivity and specificity of 0.69 and 0.77 respectively(5). In our study, we measured CSA at the level of 3cm proximal to the medial malleolus, with a cutoff value of 18.5 mm2 in Group II patients and 14 mm2 in Group III subjects observed.
Fukashi Ishibashi et al. studied CSA, hypoechoic area and MTNF of the median and posterior tibial nerves in patients with or without diabetic neuropathy. CSA was measured by direct tracing. They concluded that the morphological changes in peripheral nerves of type 2 diabetic patients were seen even prior to the clinical onset of neuropathy, and were closely correlated with the severity of the disease. Moreover, CSA and MTNF in patients with neuropathy were larger than those in the controls, with significant p-values(17). The findings were similar in our study, where CSA and MTNF in Group I patients were larger than in the other two groups, with p <0.001. There was also a statistically significant difference between MTNF of Group II and Group III, with p < 0.05. Hence, the morphological changes in the tibial nerve can be detected on HRUS even before the onset of neuropathy.
There was no statistically significant difference between CSA and MTNF of the tibial nerves in right (respectively) and left lower limbs (respectively). Also, CSA of the tibial nerve was larger in males than in females, both in the affected patients as well as controls. Similar findings were observed in the study conducted by Fukashi Ishibashi et al., where there was no difference in CSA of the median nerve between right and left hands, neither in controls, nor in diabetic patients, and CSA was larger in men than in women(17).
As far as HbA1c is concerned, it is an index of the average blood glucose level over the preceding weeks to months. Quarterly HbA1c determination is an important measure of glycemic control of the patient. Poor glycaemic control can result in the onset as well as progression of diabetic peripheral neuropathy(18).
Watanabe et al. studied the role of ultrasonography in diabetic peripheral neuropathy, concluding that there was no statistically significant correlation between HbA1c levels and CSA of the peripheral nerves(4). The likely reason may be the small sample size in their study. In our study, a significant correlation was observed between these two parameters, with p < 0.001 in Group I and 0.008 in Group II.
The size of the nerve fascicles is one of the determinants of the hypoechoic area in CSA of peripheral nerves. MTNF in non-neuropathic diabetic patients was larger compared with control subjects, with statistically significant correlation between MTNF and the severity of neuropathy, as observed by Ishibashi. The morphological changes in the peripheral nerves of diabetic patients were detected even before the onset of neuropathy, and were closely correlated with the severity of the disease(17). Similar findings were observed in our study, as CSA of the tibial nerve was at its maximum in the diabetic patients with diabetic neuropathy, followed by diabetic patients without neuropathy and the non-diabetic healthy volunteers (Fig. 3, 4; Tab. 1). Thus, CSA of the tibial nerve may be used as a marker for detecting DN and also in the grading of its severity.
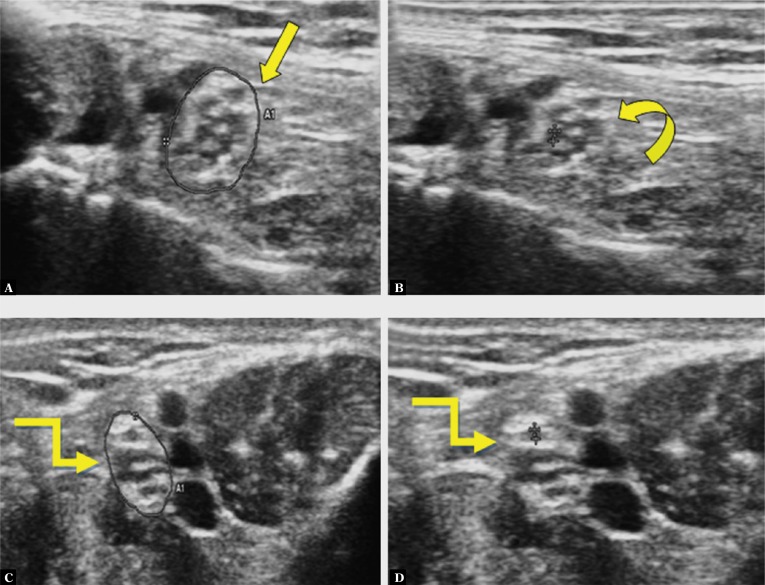
Fig. 3.
High resolution US of a 44-year old male diabetic patient with no clinical signs of peripheral neuropathy (TCNS - 4 and DN score -0) shows CSA of 18 mm2 (straight arrow in A) and MTNF of 0.4 mm (curved arrow in B). US of a 36-year old healthy male shows CSA of 11 mm2 (elbow arrow in C) and MTNF of 0.2 mm (elbow arrow in D)
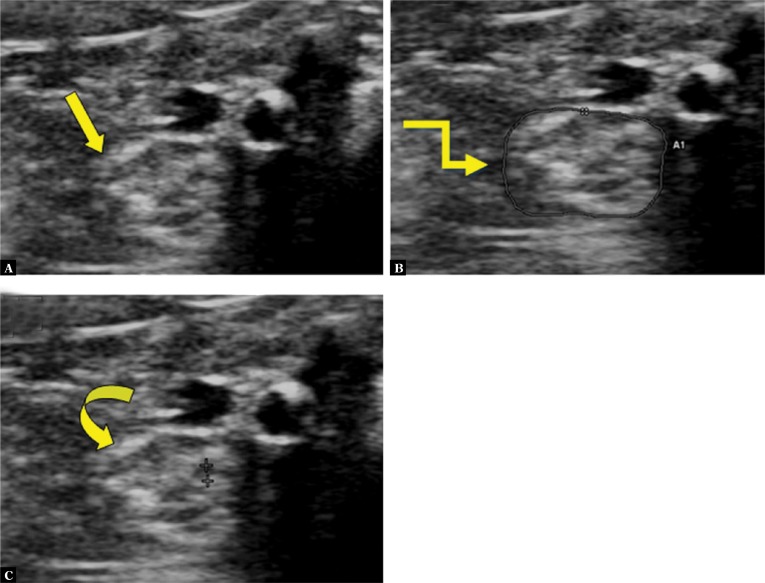
Fig. 4.
High resolution US of a 70-year old male diabetic patient with severe peripheral neuropathy (TCNS-15 and DN score -3) shows a swollen tibial nerve (straight arrow in A) with CSA of 29 mm2 (elbow arrow in B) and MTNF of 0.6 mm (curved arrow in C)
Kang et al. evaluated the role of multiple peripheral nerves in twenty diabetic neuropathy patients with twenty patients as controls. They concluded that CSAs of sural, tibial and median nerves show significant correlations with electrophysiological findings. CSA of sural nerve revealed a significant correlation with HbA1c levels(19). In our study, CSA of the tibial nerve correlated significantly with TCNS and HbA1c levels, at p <0.001.
Conclusion
Our study confirms that the cross sectional area and maximum thickness of nerve fascicles of the tibial nerve is larger in patients with diabetic peripheral neuropathy than healthy subjects or diabetic patients with no signs of neuropathy. Hence, ultrasonography is an excellent diagnostic tool for detecting morphological changes in the tibial nerves in diabetic patients, even before the clinical onset of peripheral neuropathy.
Conflict of interest
All the authors declare that they have no conflict of interest.
Acknowledgement
I would like to thank Dr. Harpreet Kaur (biostatistician, Department of Social and Preventive Medicine, SGRDIMSR, Sri Amritsar) for her help in the statistical analysis of the data.
References
- 1.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llewelyn JG. The diabetic neuropathies: types, diagnosis and management. J Neurol Neurosurg Psychiatry. 2003;74:ii15–ii19. doi: 10.1136/jnnp.74.suppl_2.ii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catwright MS, Passmore LV, Yoon JS, Brown ME, Caress JB, Walker FO. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve. 2008;37:566–571. doi: 10.1002/mus.21009. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Ito H, Sekine A, Katano Y, Nishimura T, Kato Y, et al. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med. 2010;29:697–708. doi: 10.7863/jum.2010.29.5.697. [DOI] [PubMed] [Google Scholar]
- 5.Riazi S, Bril V, Perkins BA, Abbas S, Chan VW, Ngo M, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy? A cross-sectional study. Diabetes Care. 2012;35:2575–2579. doi: 10.2337/dc12-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care. 2002;25:2048–2052. doi: 10.2337/diacare.25.11.2048. [DOI] [PubMed] [Google Scholar]
- 7.Bóhm J. High resolution sonography of peripheral nerves: normal values in healthy worth individuals and the role of sonography in rare disorders of peripheral nerves. doctoral dissertation. [Google Scholar]
- 8.Silvestri E, Martinoli C, Derchi LE, Bertolotto M, Chiaramondia M, Rosenberg I. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology. 1995;197:291–296. doi: 10.1148/radiology.197.1.7568840. [DOI] [PubMed] [Google Scholar]
- 9.Lawande AD, Warrier SS, Joshi MS. Role of ultrasound in evaluation of peripheral nerves. Indian J Radiol Imaging. 2014;24:254–258. doi: 10.4103/0971-3026.137037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins BA, Bril V. Diabetic neuropathy: a review emphasizing diagnostic methods. Clin Neurophysiol. 2003;114:1167–1175. doi: 10.1016/s1388-2457(03)00025-7. [DOI] [PubMed] [Google Scholar]
- 11.Arezzo JC. The use of electrophysiology for the assessment of diabetic neuropathy. Neurosci Res Commun. 1997;21:13–23. [Google Scholar]
- 12.Fantino O, Coillard JY, Borne J, Bordet B. Ultrasound of the tarsal tunnel: Normal and pathological imaging features. J Radiol. 2011;92:1072–1080. doi: 10.1016/j.jradio.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Bae JS, Kim BJ. Subclinical diabetic neuropathy with normal conventional electrophysiological study. J Neurol. 2007;254:53–59. doi: 10.1007/s00415-006-0261-5. [DOI] [PubMed] [Google Scholar]
- 14.Kowalska B, Sudot-Szopiñska I. Normal and sonography anatomy of selected peripheral nerves. Part III: Peripheral nerves of the lower limb. J Ultrason. 2012;12:148–163. doi: 10.15557/JoU.2012.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim WK, Kwon SH, Lee SH, Sunwoo I. Asymptomatic electrophysiologic carpal tunnel syndrome in diabetics: Entrapment or polyneuropathy. Yonsei Med J. 2000;41:123–127. doi: 10.3349/ymj.2000.41.1.123. [DOI] [PubMed] [Google Scholar]
- 16.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathic Study. Neurology. 1993;43:817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 17.Ishibashi F, Taniguchi M, Kojima R, Kawasaki A, Kosaka A, Uetake H. Morphological changes of the peripheral nerves evaluated by high-resolution ultrasonography are associated with the severity of diabetic neuropathy, but not corneal nerve fiber pathology in patients with type 2 diabetes. J Diabetes Investig. 2015;6:334–342. doi: 10.1111/jdi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang S, Kim SH, Yang SN, Yoon JS. Sonographic features of peripheral nerves at multiple sites in patients with diabetic polyneuropathy. J Diabetes Complications. 2016;30:518–523. doi: 10.1016/j.jdiacomp.2015.12.008. [DOI] [PubMed] [Google Scholar]