Abstract Abstract
Rodents constitute one of the most diversified mammalian orders. Due to the morphological similarity in many of the groups, their taxonomy is controversial. Karyotype information proved to be an important tool for distinguishing some species because some of them are species-specific. Additionally, rodents can be an excellent model for chromosome evolution studies since many rearrangements have been described in this group.This work brings a review of cytogenetic data of Brazilian rodents, with information about diploid and fundamental numbers, polymorphisms, and geographical distribution. We point out that, even with the recent efforts on cytogenetic studies in this group, many species lack karyotypic data. Moreover, we describe for the first time the karyotype of Carterodon sulcidens (Lund, 1838) (Family Echimyidae), a new fundamental number for an undescribed species of Neacomys Thomas, 1900 (Family Cricetidae, Subfamily Sigmodontinae), and illustrate the karyotype of a Brazilian specimen of Mus musculus Linnaeus, 1758 (Family Muridae). This review compiles the cytogenetic data on Brazilian rodents reported in the last three decades, after the last revision published in 1984, including synonyms, chromosomal variations, and geographic distribution. Additionally, it also reinforces that Brazilian biodiversity is still poorly known, considering the new data reported here.
Keywords: Chromosomes, Rodentia, karyotype evolution, Carterodon sulcidens, Neacomys
Introduction
More than three decades after the last revision of cytogenetics of Brazilian rodents (Kasahara and Yonenaga-Yassuda 1984), in which the karyotypes of approximately 60 species were reported, several new karyotypes and chromosomal rearrangements have been described. In the last 30 years, huge progress has been made, and up to this date, new species have frequently been described. However, as we shall explore herein, there still remain gaps in knowledge about many species.
Cytogenetic information on Brazilian rodents was firstly described by Cestari and Imada (1968) for the species referred to as Akodon arviculoides cursor Thomas, 1913. From then on, cytogenetic data confirmed the great chromosomal variability in rodents, especially after the advent of banding techniques in the beginning of the 1970s.
Throughout the following decades, several Master dissertations and PhD theses have addressed cytogenetic studies on Brazilian rodents. It became evident that karyotypic data could contribute to accurate taxonomic information, since different names were applied to groups that shared the same karyotype, and very distinct karyotypes were attributed to a single species. Additionally, major fieldwork efforts in Brazil (especially in unexplored areas) have led to the discovery of many new species.
The increasing number of cytogenetic studies on rodents resulted in the characterization of banding patterns, recognition of sex chromosomes, identification of supernumerary chromosomes, pericentric inversions and Robertsonian rearrangements, variations in the amount and localization of constitutive heterochromatin, and recognition of species (cytotaxonomy). These discoveries have led researchers to consider that rodents have undergone a “karyotypic explosion” process and that they stand out as an excellent group for chromosomal evolution studies, since they present many examples of chromosome rearrangements. These rearrangements may have played an important role in karyotype diversification and speciation, with the reduction of gene flow due to meiotic problems (King 1993, Rieseberg 2001, Patton 2004, Faria and Navarro 2010).
Previously, chromosome evolution studies were essentially based on the comparison of banding patterns (Yonenaga-Yassuda et al. 1975, 1987a, Leal-Mesquita et al. 1992, Silva and Yonenaga-Yassuda 1999). Later, the association of cytogenetics with molecular biology allowed for a new important approach for studying karyotype evolution. Notwithstanding, molecular cytogenetics allows the localization of specific DNA sequences in the chromosomes based on DNA denaturation and its subsequent annealing with complementary sequences. In Brazilian rodents, localization of specific sequences using fluorescence in situ hybridization (FISH) was specifically applied in the Akodontini and Oryzomyini tribes of the Family Cricetidae, Subfamily Sigmodontinae, which is traditionally divided into 10 tribes and one incertae sedis group (Pardiñas et al. 2015a). Nevertheless, this kind of approach is still lacking for the other tribes of Sigmodontinae, and the remaining rodent families, mainly because of the difficulty in obtaining specific probes.
FISH was first performed using telomeric sequence probes, revealing that, besides the telomeric position itself, the sequences could also be detected at telomeric interstitial sites (ITS), such as those present in the Sigmodontinae genus Akodon Meyen, 1833, Thaptomys Thomas, 1916, and Cerradomys Weksler, Percequillo & Voss, 2006 (Fagundes et al. 1997a, Fagundes and Yonenaga-Yassuda 1998, Silva and Yonenaga-Yassuda 1998a, Andrades-Miranda et al. 2002a, Ventura et al. 2004, 2006). These ITS were correlated with components of constitutive heterochromatin, amplification of TTAGGGn sequences, telomeres remnants after chromosomal rearrangements or reservoirs for future fission rearrangements. On the other hand, the absence of ITS in other Sigmodontinae species with chromosome polymorphisms, such as Oligoryzomys Bangs, 1900, and Rhipidomys Tschudi, 1845, was also described (Silva and Yonenaga-Yassuda 1997, 1999).
More recently, probes from entire chromosomes were obtained by microdissection or flow sorting, representing a breakthrough in evolutionary studies. The first Brazilian study employing this technique was published by Fagundes et al. (1997b), in which the largest pair (pair 1) of the karyotype of the rodent Akodon cursor (Winge, 1887) (Subfamily Sigmodontinae, tribe Akodontini) was obtained in order to investigate regions of homology between chromosomes of this species and Akodon montensis Thomas, 1913.
More than one decade later, Hass et al. (2008), using Mus musculus commercial chromosome probes, established chromosomal homology maps between five species of the tribe Akodontini, plus one Oryzomyini species. One year later, Ventura et al. (2009) performed chromosome painting using Akodon species-specific probes.
After the tribe Akodontini, Oryzomyini is the second most studied tribe by chromosome painting from the Subfamily Sigmodontinae. Comparisons between Hylaeamys megacephalus (G. Fischer, 1814) and Cerradomys langguthi Percequillo, Hingst-Zaher & Bonvicino, 2008 were performed by Nagamachi et al. (2013), and Di-Nizo et al. (2015) studied chromosome evolution within the genus Oligoryzomys. In addition, chromosome painting using Hylaeamys megacephalus probes was performed to compare the Akodontini and Oryzomyini tribes (Suárez et al. 2015, Pereira et al. 2016) and, more recently, two populations of Oecomys catherinae Thomas, 1909 were also evaluated (Malcher et al. 2017).
The role of cytogenetics in species recognition (cytotaxonomy) has been know for a while, considering that many rodents’ species are morphologically similar (Bonvicino and Weksler 1998, Christoff et al. 2000, Percequillo et al. 2008). In addition, molecular phylogenetics improved the possibility of recognizing monophyletic clades. In fact, the proper identification of undescribed species is only possible with the association of morphology, cytogenetics, geographic distribution and molecular phylogeny. Altogether, these different approaches are essential not only for identifying the cryptic Brazilian biodiversity but also for public health programs, since some rodents’ species are Hantavirus reservoirs (Souza et al. 2002, Lemos et al. 2004).
Therefore, the aim of this review is to compile all the cytogenetic data available for Brazilian rodents, presenting not only the diploid and fundamental numbers, but also the chromosomal polymorphisms, synonyms, and geographic distribution. In addition, we describe for the first time the karyotype of the monotypic species Carterodon sulcidens, and show the karyotype of Brazilian specimen of the introduced rodent Mus musculus for the first time. A new fundamental number for a putative undescribed species of Neacomys is also reported. In addition, to investigate phylogenetic relationships among Neacomys species, molecular analyses based on the gene cytochrome b were performed. This work discusses the most common rearrangements in each group, by pointing out the species which could represent complexes of species (thus needing revision) or present polymorphisms, as well as highlighting the species and families that lack cytogenetic information.
Material and methods
Literature revision
This review was done after an extensive revision of the literature, including Master’s and Ph.D. theses, when available (Table 1). Abstracts from congresses and conferences were not considered, since karyotype pictures were only available during the events and access to this kind of material is restricted. Chromosome rearrangements in Table 1 were named as described in the literature (for example Robertsonian rearrangement, centric fusion, etc.). However, in the text, we refer to centric fusion/fission as a synonym of Robertsonian rearrangement (Sumner 2003). Except for the species that have not been formally described (e.g. Thaptomys sp., Proechimys gr. goeldii, etc.), the taxonomical classification follows the one proposed by Patton et al. (2015) and Fabre et al. (2016), that recently included Myocastor Kerr, 1792 within the Family Echimyidae.
Table 1.
Compilation of cytogenetic data of Brazilian rodents, with the respective synonyms, diploid number (2n) and fundamental number (FN), karyotypic variation, localities (according to Bonvicino et al. 2008 and Patton et al. 2015) and references.
| Species | Synonyms | 2n | FN | Karyotypic Variations | Distribution | References | ||
|---|---|---|---|---|---|---|---|---|
| Family Caviidae | Cavia aperea Erxleben, 1777 | - | 64 | 116, 124 | - | PE, SE, AL, BA, MG, GO, MT, MS, MG, SP, PR and SC | Maia 1984, Bonvicino et al. 2008, Gava et al. 2011 | |
| Cavia fulgida Wagler, 1831 | - | 64 | 124 | - | Eastern Brazil, between MG and SC | Woods and Kilpatrick 2005, Walker et al. 2014 | ||
| Cavia intermedia Cherem, Olimpio, and Ximénez, 1999 | Cavia aff. magna | 62 | 108 | - | Endemic from SC (Ilhas Moleques do Sul) | Gava et al. 1998, Woods and Kilpatrick 2005 | ||
| Cavia magna Ximénez, 1980 | - | 62; 64 | 102; 124 | Pericentric inversions; addition and deletion of constitutive hetechromatin; Robertsonian rearrangement | RS and SC | Bonvicino et al. 2008, Gava et al. 2011, Walker et al. 2014 | ||
| Cavia porcellus (Linnaeus, 1758) | - | 64* | 100-102 | Polymorphism in chromosome 1 | All Brazilian States | Bonvicino et al. 2008, Walker et al. 2014 | ||
| Galea flavidens (Brandt, 1835) | - | N/A | N/A | - | Northwestern MG and Northeastern GO | Bonvicino et al. 2008 | ||
| Galea spixii (Wagler, 1831) | - | 64 | 118 | - | PA, MT, MG, BA, PE, PB, RN, CE, PI, MA and DF | Maia 1984, Bonvicino et al. 2008 | ||
| Hydrochoerus hydrochaeris (Linnaeus, 1766) | - | 66 | 102 | - | All Brazilian States, except CE | Wurster et al. 1971, Bonvicino et al. 2008 | ||
| Kerodon acrobata Moojen, 1997 | - | 52 | 92 | - | Northeastern GO | Bonvicino et al. 2008, Zappes et al. 2014 | ||
| Kerodon rupestris (Wied-Neuwied, 1820) | - | 52 | 92, 94 | Pericentric inversion | From PI and CE to Northern MG | Maia 1984, Bonvicino et al. 2008, Lessa et al. 2013 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Akodontini | Akodon azarae (J. B. Fischer, 1829) | - | 37-38 | 40-44 | Variation in the Y morphology; deletion of the X long arm | Southern Brazil | Kasahara and Yonenaga-Yassuda 1984, Vitullo et al. 1986, Sbalqueiro 1989, Pardiñas et al. 2015b |
| Akodon cursor (Winge, 1887) | Akodon arviculoides | 14-16 | 18-26 | Pericentric inversions in pairs 2, 4 and 6; centric fusion and pericentric inversion in pairs 1 and 3; trisomy of the pair 7; ITS | Atlantic Forest formations in Eastern Brazil from PB to PR and Eastern MG | Cestari and Imada 1968, Fagundes et al. 1997a, Fagundes et al. 1997b, Fagundes et al. 1998, Geise et al. 1998 | ||
| Akodon lindberghi Hershkovitz, 1990 | Akodon sp. | 42 | 42 | ITS | Cerrado habitat, Central and Southeastern Brazil | Svartman 1989, Svartman and Almeida 1994, Geise 1995 | ||
| Akodon montensis Thomas, 1913 | Akodon aff. arviculoides, Akodon sp. | 23; 24-26; 24/25; 23/24 | 40; 42; 44 | X monosomy; 1 or 2 B chromosomes; mosaicism; reciprocal translocation (1, 6); sex chromosome heteromorphism | Southeastern Brazil, from RJ to RS, including gallery Forest settings in MG and GO | Geise et al. 1998, Fagundes et al. 1997b, Fagundes et al. 2000 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Akodontini | Akodon mystax Hershkovitz, 1998 | - | 42, 44 | 42 | - | Pico da Bandeira, in the border of MG and ES | Musser and Carleton 2005, Gonçalves et al. 2007, Pardiñas et al. 2015b |
| Akodon paranaensis Christoff, Fagundes, Sbalqueiro, Mattevi and Yonenaga- Yassuda, 2000 | Akodon serrensis | 44 | 44 | Non-disjunction of the sex chromosomes (2n = 43 and 45) | Eastern RJ and SP and Southern Brazil | Christoff et al. 2000 | ||
| Akodon reigi E. M. González, Langguth & Oliveira, 1998 | - | 44 | 44 | - | Southernmost Brazil (RS) | Musser and Carleton 2005 | ||
| Akodon sanctipaulensis Hershkovitz, 1990 | - | N/A | N/A | - | Serra do Mar, Southeastern Brazil | Musser and Carleton 2005 | ||
| Akodon sp. n. | - | 9; 10 | 14-16 | X monosomy; pericentric inversion in pair 3; ITS | Only known from its type locality, MT | Silva and Yonenaga-Yassuda 1998a | ||
| Akodon toba Thomas, 1921 | Akodon varius | 40*; 42-43* | 40*; 44* | Karyotype of specimens from Paraguay | Southwestern MS | Bonvicino et al. 2008, Pardinãs et al. 2015a | ||
| Bibimys labiosus (Winge, 1887) | - | 70 | 80 | - | Northern RS, and Southeastern MG and RJ | Bonvicino et al. 2008, Gonçalves et al. 2007 | ||
| Blarinomys breviceps (Winge, 1887) | - | 28; 31 (29+2Bs); 34; 37 (36 + 1B); 43 (39 + 4Bs); 45 (44 + 1B); 52; 52 (50 + 2Bs) | 48, 50; 50; 50; 50; 50; 50, 51; 50; 50 | B chromosomes; Robertsonian rearrangement; ITS | Atlantic Forest regions of Southeastern Brazil (from BA to SP, and Eastern MG) | Silva et al. 2003, Musser and Carleton 2005, Geise et al. 2008, Ventura et al. 2012 | ||
| Brucepattersonius griserufescens Hershkovitz, 1998 | - | 52 | 52, 53 | Pericentric inversion in pair 2 | Eastern MG, and ES to RJ | Bonvicino et al. 1998a, Musser and Carleton 2005 | ||
| Brucepattersonius igniventris Hershkovitz, 1998 | - | N/A | N/A | - | Southeastern SP | Musser and Carleton 2005, Bonvicino et al. 2008, Rossi 2011 | ||
| Brucepattersonius iheringi (Thomas, 1896) | Oxymycterus iheringi | 52 | 52 | - | Southern Brazil | Musser and Carleton 2005, Vilela 2005 | ||
| Brucepattersonius soricinus Hershkovitz, 1998 | - | 52 | 52 | - | Eastern SP and PR | Musser and Carleton 2005, Di-Nizo et al. 2014 | ||
| Castoria angustidens (Thomas, 1902) | Akodon sp., A. leucogula, A. serrensis | 46 | 46 | ITS | Atlantic Forest from Southeastern ES to RS | Geise et al. 1998, Christoff et al. 2000, Abreu et al. 2014, Pardiñas et al. 2015b, Pardiñas et al. 2016a | ||
| Deltamys araucaria Quintela, Bertuol, González, Cordeiro-Estrela, Freitas, Gonçalves, 2017 | - | 34 | 34 | - | Only known from its type locality, São Francisco de Paula/RS | Quintela et al. 2017 | ||
| Deltamys kempi Thomas, 1917 | - | 35-38 | 38 | Centric fusion/fission; multiple sex determination system. | Eastern RS | Sbalqueiro et al. 1984, Castro et al. 1991, Musser and Carleton 2005, Bonvicino et al. 2008 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Akodontini | Deltamys sp. | - | 40 | 40 | - | Esmeralda (RS) | Ventura et al. 2011 |
| Gyldenstolpia fronto Winge, 1887 | Kunsia fronto | N/A | N/A | - | Lagoa Santa (MG) | Musser and Carleton 2005, Pardiñas et al. 2008, Pardiñas and Bezerra 2015 | ||
| Gyldenstolpia planaltensis (Avila-Pires, 1972) | Kunsia fronto planaltensis | N/A | N/A | - | Westcentral Brazil | Pardiñas and Bezerra 2015 | ||
| Juscelinomys candango Moojen, 1965 | - | N/A | N/A | - | DF | Musser and Carleton 2005 | ||
| Kunsia tormentosus (Lichtenstein, 1830) | - | 44 | 42 | - | Westcentral Brazil | Andrades-Miranda et al. 1999, Musser and Carleton 2005 | ||
| Necromys lasiurus (Lund, 1840) | Zygodontomys lasiurus, Bolomys lasiurus | 34, 33, 33/34 | 34 | Robertsonian rearrangement; centric fusion, X polymorphism; mosaicism (XX/X0) | Southern Amazon River, Brazil | Maia and Langguth 1981, Kasahara and Yonenaga-Yassuda 1983, Svartman and Almeida 1993a, Musser and Carleton 2005 | ||
| Necromys urichi (J. A. Allen & Chapman, 1897) | - | 18 | 30 | - | Northern Brazil | Reig et al. 1986, Musser and Carleton 2005 | ||
| Oxymycterus amazonicus Hershkovitz, 1994 | - | 54 | N/A | - | Lower Amazon Basin, Southern Amazon River, between Tocantins and Madeira Rivers, Central Brazil, Northwestern MT | Bonvicino et al. 1998a, Musser and Carleton 2005 | ||
| Oxymycterus caparoae Hershkovitz, 1998 | - | 54 | 64 | - | Eastern MG and ES to RJ | Bonvicino et al. 1998a, Musser and Carleton 2005 | ||
| Oxymycterus dasytrichus (Schinz, 1821) | Oxymycterus angularis, Oryzomys hispidus, Oryzomys roberti | 54 | 64 | - | Atlantic and interior forest of Eastern Brazil (PE, AL, SE, BA, MG, ES, RJ, SP and PA) | Musser and Carleton 2005, Moreira et al. 2009 | ||
| Oxymycterus delator Thomas, 1903 | Oxymycterus sp., Oxymycterus roberti | 54 | 62 | - | Southcentral Brazil | Svartman and Almeida 1993b , Bonvicino et al. 2005a, Musser and Carleton 2005 | ||
| Oxymycterus inca Thomas, 1900 | - | 54 | N/A | - | Acre | Bonvicino et al. 1998a, Bonvicino et al. 2008 | ||
| Oxymycterus nasutus (Waterhouse, 1837) | - | 54 | 64 | - | Eastern RS to Eastern SP | Musser and Carleton 2005, Quintela et al. 2012 | ||
| Oxymycterus quaestor Thomas, 1903 | Oxymycterus judex | 54 | N/A | - | Eastern Brazil, from RS to SP, and Serra dos Órgãos (RJ) | Bonvicino et al. 1998a, Bonvicino et al. 2008, Oliveira and Gonçalves 2015 | ||
| Oxymycterus rufus (G. Fischer, 1814) | - | 54 | 66 | - | Southeastern MG | Geise 1995 | ||
| Podoxymys roraimae Anthony, 1929 | - | 16 | 26 | - | RR | Pérez-Zapata et al. 1992, Musser and Carleton 2005, Bonvicino et al. 2008 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Akodontini | Scapteromys aquaticus Thomas, 1920 | - | 32 | 40 | - | Westernmost RS | Bonvicino et al. 2013 |
| Scapteromys meridionalis Quintela, Gonçalves, Althoff, Sbalqueiro, Oliveira, Freitas, 2014 | Scapteromys sp. 1, Scapteromys sp. 2 | 34, 36 | 40 | Centric fusion | Southern Brazil | Freitas et al. 1984, Bonvicino et al. 2013, Quintela et al. 2014 | ||
| Scapteromys tumidus (Waterhouse, 1837) | - | 24 | 40 | - | Southernmost Brazil (RS) | Brum-Zorrilla et al. 1986, Musser and Carleton 2005 | ||
| Thalpomys cerradensis Hershkovitz, 1990 | - | 36 | 34 | - | Cerrado of Central Brazil | Andrade et al. 2004, Musser and Carleton 2005 | ||
| Thalpomys lasiotis Thomas, 1916 | Akodon reinhardti | 37, 38 | 38 | Centric fusion/fission; heterochromatin variation in an autosomal pair | Cerrado of Central Brazil | Yonenaga-Yassuda et al. 1987b | ||
| Thaptomys nigrita (Lichtenstein, 1829) | Akodon (Thaptomys) nigrita | 52 | 52 | - | Southeastern Brazil, BA to RS | Yonenaga 1972, Yonenaga 1975, Souza 1981, Castro 1989, Fagundes 1993, Geise 1995 | ||
| Thaptomys sp. | - | 50 | 48 | ITS | Only known from its type locality - BA | Ventura et al. 2004 | ||
| Tribe Ichthyomyini | Neusticomys ferreirai Percequillo, Carmignotto & Silva, 2005 | - | 92 | 98 | - | Amazonian lowland of MT and PA | Percequillo et al. 2005 | |
| Neusticomys oyapocki (Dubost & Petter, 1979) | - | N/A | N/A | - | Amazonian of Northern Brazil (AP and PA) | Voss 2015a | ||
| Tribe Oryzomyini | Cerradomys akroai Bonvicino, Casado & Weksler, 2014 | - | 60 | 74 | - | TO | Bonvicino et al. 2014 | |
| Cerradomys goytaca Tavares, Pessôa & Gonçalves, 2011 | - | 54 | 62, 63, 66 | Different interpretation of morphology of small pairs and pericentric inversion in small chromosome | Northeastern littoral of RJ and Southern littoral of ES (Restinga region) | Tavares et al. 2011, Bonvicino et al. 2014 | ||
| Cerradomys langguthi Percequillo, Hingst- Zaher, and Bonvicino, 2008 | Oryzomys sp. B | 46, 48, 49, 50 | 56 | Centric fusion/ fission; Y polymorphism; ITS | PE, MA, PB and CE | Maia and Hulak 1981, Percequillo et al. 2008, Nagamachi et al. 2013 | ||
| Cerradomys maracajuensis (Langguth & Bonvicino, 2002) | - | 56 | 58 | - | Central MT and MS | Langguth and Bonvicino 2002, Bonvicino et al. 2008, Bonvicino et al. 2014 | ||
| Cerradomys marinhus (Bonvicino, 2003) | - | 56 | 54 | - | GO and Southeatern BA | Bonvicino 2003, Bonvicino et al. 2008 | ||
| Cerradomys scotti (Langguth & Bonvicino, 2002) | Oryzomys gr. subflavus | 58 | 70-72 | Pericentric inversion in small chromosome pair; X and Y polymorphisms | GO, Southern MT, Southeastern RO, Northern MS, Western MG and BA, Southeastern TO and Southern PI | Langguth and Bonvicino 2002, Bonvicino et al. 2008 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Oryzomyini | Cerradomys subflavus (Wagner, 1842) | - | 54; 55; 56 | 62; 63; 64 | Robertsonian rearrangement; pericentric inversion in pair 5; X and Y polymorphisms; ITS | PB, PE, AL, BA, MG and SP | Almeida and Yonenaga-Yassuda 1985, Bonvicino et al. 2008 |
| Cerradomys vivoi Percequillo, Hingst- Zaher & Bonvicino, 2008 | Oryzomys gr. subflavus | 50 | 62, 63 | Pericentric inversion; ITS | MG, BA and SE | Andrades-Miranda et al. 2002a, Percequillo et al. 2008 | ||
| Drymoreomys albimaculatus Percequillo, Weksler & Costa, 2011 | - | 62 | 62 | ITS | Atlantic Forest of SP | Percequillo et al. 2011, Suárez-Villota et al. 2013 | ||
| Euryoryzomys emmonsae (Musser, Carleton, Brothers & Gardner, 1998) | Oryzomys emmonsae | 80 | 86 | - | Centraleastern PA | Musser et al. 1998, Bonvicino et al. 2008 | ||
| Euryoryzomys lamia (Thomas, 1901) | - | 58; 60, 64 | 82, 84; 84 | One name with different karyotypes associated | Western MG and Eastern GO | Bonvicino et al. 1998b, Andrades-Miranda et al. 2000, Bonvicino et al. 2008 | ||
| Euryoryzomys macconnelli (Thomas, 1910) | Oryzomys macconnelli | 64; 58 | 70; 90 | One name with different karyotypes associated | Northern Brazil | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Euryoryzomys nitidus (Thomas, 1884) | Oryzomys nitidus | 80 | 86 | - | AC, RO, Western MT and Southern AM | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Euryoryzomys russatus (Wagner, 1848) | Oryzomys capito, Oryzomys nitidus, O. intermedius, Oryzomys russatus | 80; 80/81 | 86 | Dissociation of the X chromosome; X and Y polymorphisms | Southeastern Brazil from BA to RS | Yonenaga et al. 1976, Almeida 1980, Zanchin 1988, Silva 1994, Geise 1995, Musser and Carleton 2005, Bonvicino et al. 2008 | ||
| Euryoryzomys sp. | - | 76 | 86 | - | Only known from its type locality - CE | Silva et al. 2000 | ||
| Holochilus brasiliensis (Desmarest, 1819) | - | 55; 56-58 | 56 | Centric fusion; 0 to 2 B chromosomes | Southern and Southeastern Brazil | Freitas et al. 1983, Yonenaga-Yassuda et al. 1987a, Bonvicino et al. 2008 | ||
| Holochilus chacarius Thomas, 1906 | - | 48-56* | 56-60* | Centric fusion, inversion and B chromosomes | Western MS | Vidal et al. 1976, Bonvicino et al. 2008, Gonçalves et al. 2015 | ||
| Holochilus sciureus Wagner, 1842 | Holochilus brasiliensis | 55-56 | 56 | Centric fusion and heteromorphism in pair 1 | Northern, Northeastern and Central Brazil | Freitas et al. 1983, Patton et al. 2000, Bonvicino et al. 2008 | ||
| Holochilus vulpinus (Brants, 1827) | Holochilus brasiliensis vulpinus | 40 | 56 | - | Western RS | Freitas et al. 1983, Bonvicino et al. 2008 | ||
| Hylaeamys laticeps (Lund, 1840) | Oryzomys capito, O. c. laticeps, Oryzomys megacephalus, Hylaeamys laticeps | 48 | 60 | - | Eastern Atlantic Forest, from BA to Northern RJ | Percequillo 2015b | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Oryzomyini | Hylaeamys megacephalus (G. Fischer, 1814) | Oryzomys capito, O. c. laticeps, Oryzomys megacephalus; | 54 | 62 | - | Northern and Central Brazil | Musser et al. 1998, Patton et al. 2000, Musser and Carleton 2005 |
| Hylaeamys oniscus (Thomas, 1904) | Oryzomys capito oniscus | 52 | 62 | - | Northern Rio São Francisco, in PB, PE and AL | Maia 1990, Brennand et al. 2013 | ||
| Hylaeamys perenensis (J. A. Allen, 1901) | Oryzomys perenensis | 52 | 62 | - | Western Brazil | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Hylaeamys seuanezi (Weksler, Geise & Cerqueira, 1999) | Oryzomys capito, O. c. oniscus, Oryzomys laticeps | 48 | 60 | - | Southern Rio São Francisco, from BA to RJ | Brennand et al. 2013 | ||
| Hylaeamys yunganus (Thomas, 1902) | Oryzomys yunganus | 52-60 | 62-67 | Chromosome polymorphisms within and between western and eastern population | Northern Brazil | Musser et al. 1998, Patton et al. 2000, Bonvicino et al. 2008 | ||
| Lundomys molitor (Winge, 1887) | Holochilus magnus | 52 | 58 | Variation in the X chromosome | Central RS | Freitas 1980, Freitas et al. 1983, Bonvicino et al. 2008 | ||
| Microakodontomys transitorius Hershkovitz, 1993 | - | 38 | 46 | - | DF | Musser and Carleton 2005, Bonvicino et al. 2008, Paresque and Hanson 2015 | ||
| Neacomys amoenus amoenus Thomas, 1903 | Neacomys spinosos amoenus | 64 | 68 | - | Northwestern Brazil | Patton et al. 2000, Bonvicino et al. 2008, Hurtado and Pacheco 2017 | ||
| Neacomys dubosti Voss, Lunde & Simmons, 2001 | - | 62, 64 | 68 | Robertsonian rearrangement | Northern AP | Voss et al. 2001, Musser and Carleton 2005, Bonvicino et al. 2008, Silva et al. 2015 | ||
| Neacomys guianae Thomas, 1905 | - | 56 | N/A | - | Northern Brazil | Musser and Carleton 2005, Silva et al. 2015 | ||
| Neacomys minutus Patton, da Silva & Malcolm, 2000 | - | 35-36 | 40 | Robertsonian rearrangement | Southwestern AM | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Neacomys musseri Patton, da Silva & Malcolm, 2000 | - | 34 | 64-68 | Pericentric inversion | Westernmost AC | Patton et al. 2000, Musser and Carleton 2005, Bonvicino et al. 2008 | ||
| Neacomys paracou Voss, Lunde & Simmons, 2001 | - | 56 | 62, 66 | Pericentric inversion | Northernmost Brazil | Voss et al. 2001, Bonvicino et al. 2008, Silva et al. 2015 | ||
| Neacomys sp. | - | 58 | 64, 66, 70 | Differences in amount of heterochromatin, pericentric inversion | PA and MT | Silva et al. 2015, present study | ||
| Nectomys apicalis Peters, 1861 | - | 42 | 40 | - | Westernmost Brazil, AC and AM | Patton et al. 2000, Musser and Carleton 2005 | ||
| Nectomys rattus Pelzeln, 1883 | Nectomys squamipes, N. mattensis | 52-55 | 52, 54, 56 | B chromosomes; X and Y polymorphisms | Northern, Northeastern and Central Brazil | Furtado 1981, Maia et al. 1984, Yonenaga-Yassuda et al. 1988, Zanchin 1988, Svartman 1989, Bonvicino 1994, Bonvicino et al. 1996, Silva and Yonenaga-Yassuda 1998b, Silva 1999, Lima-Rosa et al. 2000, Patton et al. 2000, Bonvicino et al. 2008 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Oryzomyini | Nectomys squamipes Brants, 1827 | - | 56-59; 55; 56/57 | 56-58; 60; 62 | B chromosomes; fusion/fission of autosomes; X monossomy; X and Y polymorphisms | Southeastern Brazil from PE to Northern RS | Yonenaga 1972, Yonenaga et al. 1976, Freitas 1980, Furtado 1981, Maia et al. 1984, Yonenaga-Yassuda et al. 1988, Zanchin 1988, Silva 1994, Geise 1995, Bonvicino et al. 1996, Silva 1999, Bonvicino et al. 2008 |
| Oecomys auyantepui Tate, 1939 | - | 64; 66; 72 | 110; 114; 80 | One name with different karyotypes associated | Northern AP and PA | Bonvicino et al. 2008, Lira 2012, Gomes Jr. et al. 2016 | ||
| Oecomys bahiensis (Hershkovitz, 1960) | Oecomys concolor bahiensis | 60 | 62 | - | BA, PE (uncertain distribution) | Langguth et al. 2005, Flores 2010, Gomes Jr. et al. 2016 | ||
| Oecomys bicolor (Tomes, 1860) | - | 80 | 140; 142 | - | Northern and Central Brazil | Suárez-Villota et al. 2017 | ||
| Oecomys catherinae Thomas, 1909 | - | 60 | 62; 64 | - | Atlantic forest from PB to SC, and Cerrado and Caatinga regions of BA, GO and MG | Musser and Carleton 2005, Bonvicino et al. 2008, Suárez-Villota et al. 2017 | ||
| Oecomys cleberi Locks, 1981 | - | 80; 82 | 124, 134, 140, 142; 116 | One name with different karyotypes associated | DF, PN Emas (GO), and São Joaquim da Barra and Guará (SP) | Lira 2012, Suárez-Villota et al. 2017 | ||
| Oecomys concolor (Wagner, 1845) | Oryzomys (Oecomys) concolor | 60 | 62 | - | Northwestern Brazil | Furtado 1981, Svartman 1989, Lima-Rosa et al. 2000, Musser and Carleton 2005 | ||
| Oecomys franciscorum Pardiñas, Teta, Salazar-Bravo, Myers & Galliari, 2016 | - | 72 | 90 | - | Pantanal | Pardiñas et al. 2016b, Suárez-Villota et al. 2017 | ||
| Oecomys mamorae (Thomas, 1906) | - | N/A | N/A | - | Westcentral Brazil | Musser and Carleton 2005, Suárez-Villota et al. 2017 | ||
| Oecomys paricola (Thomas, 1904) | - | 68; 70 | 72; 72, 74, 76 | One name with different karyotypes associated | Central Brazil, Southern Amazon River | Musser and Carleton 2005, Suárez-Villota et al. 2017 | ||
| Oecomys rex Thomas, 1910 | - | 62 | 80 | - | Northern Amazon Rio (AP and AM) | Musser and Carleton 2005, Lira 2012, Gomes Jr. et al. 2016 | ||
| Oecomys roberti (Thomas, 1904) | - | 80; 82 | 114; 106 | - | Amazon region of AM, RO and MT | Musser and Carleton 2005, Patton et al. 2000, Suárez-Villota et al. 2017 | ||
| Oecomys rutilus Anthony, 1921 | - | 54 | 82, 90 | - | Eastern AM | Voss et al. 2001, Gomes Jr. et al. 2016 | ||
| Oecomys superans Thomas, 1911 | - | 80 | 108 | - | Western AM | Patton et al. 2000 | ||
| Oecomys trinitatis (J. A. Allen & Chapman, 1893) | - | 58 | 96 | - | Northern AC, AM and RR, and Northwestern PA | Bonvicino et al. 2008 | ||
| Oecomys sp. | - | 86 | 98 | - | AM | Patton et al. 2000, Suárez-Villota et al. 2017 | ||
| Oecomys sp. | Oecomys cf. bicolor | 80 | 124 | - | MT | Lima-Rosa et al. 2000, Andrades-Miranda et al. 2001a | ||
| Oecomys sp. 1 | - | 54 | 54 | - | MT | Suárez-Villota et al. 2017 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Oryzomyini | Oecomys sp. 2 | - | 60 | 62 | - | Aripuanã (MT) | Suárez-Villota et al. 2017 |
| Oecomys sp. 3 | - | 60 | 62 | - | São Joaquim da Barra (SP) | Suárez-Villota et al. 2017 | ||
| Oecomys sp. 4 | - | 62 | 62 | - | Vila Rica (MT), Parauapebas (PA) | Suárez-Villota et al. 2017 | ||
| Oligoryzomys chacoensis (Myers & Carleton, 1981) | - | 58 | 74 | - | Centraleastern Brazil | Myers and Carleton 1981, Bonvicino and Geise 2006 | ||
| Oligoryzomys flavescens (Waterhouse, 1837) | - | 64-68 | 66-72 | 1 to 4 B chromosomes; sex chromosome polymorphisms | Eastern Brazil, from BA to RS | Sbalqueiro et al. 1991, Bonvicino et al. 2008, Di-Nizo 2013 | ||
| Oligoryzomys mattogrossae (J. A. Allen, 1916) | Oligoryzomys eliurus, O. fornesi | 62 | 64-66 | Pericentric inversion in small acrocentric pair | DF, Northern MG, GO, BA and Western PE | Bonvicino and Weksler 1998, Andrades-Miranda et al. 2001a, Bonvicino et al. 2008 | ||
| Oligoryzomys messorius (Thomas, 1901) | - | 66 | 74 | - | Northern Brazil (RO) | Andrades-Miranda et al. 2001a, Weksler and Bonvicino 2015 | ||
| Oligoryzomys microtis (J. A. Allen, 1916) | - | 64 | 64, 66 | Pericentric inversion in pair 1; X polymorphism | Amazon Basin of Brazil | Aniskin and Voloboeuv 1999, Patton et al. 2000, Musser and Carleton 2005, Di-Nizo et al. 2015 | ||
| Oligoryzomys moojeni Weksler & Bonvicino, 2005 | Oligoryzomys sp. | 70 | 72, 74, 76 | Pericentric inversion in small acrocentric pairs; sex chromosome polymorphisms | Southern TO, Northern GO, e Northwestern MG | Lima et al. 2003, Weksler and Bonvicino 2005, Bonvicino et al. 2008, Di-Nizo 2013 | ||
| Oligoryzomys nigripes (Olfers, 1818) | Oligoryzomys delticola, Oryzomys eliurus | 61, 62 | 78-82 | Pericentric inversions in pairs 2, 3, 4 and 8; Sex chromosome polymorphism; mosaicism (XX/X0) | PB to Northern RS, MG and DF | Almeida and Yonenaga-Yassuda 1991, Paresque et al. 2007, Bonvicino et al. 2008, Di-Nizo 2013 | ||
| Oligoryzomys rupestris Weksler & Bonvicino, 2005 | Oligoryzomys sp. 1 | 46 | 52 | - | high altitudes in GO and BA | Silva and Yonenaga-Yassuda 1997, Weksler and Bonvicino 2005 | ||
| Oligoryzomys stramineus Bonvicino and Weksler, 1998 | - | 52 | 68-70 | Pericentric inversion in one small acrocentric pair | Cerrado (GO and MG) and Caatinga (PB, PI e PE) | Bonvicino and Weksler 1998, Weksler and Bonvicino 2005 | ||
| Oligoryzomys utiaritensis J. A. Allen, 1916 | Oligoryzomys nigripes | 72 | 76 | - | MT and PA (Transition of Cerrado and Amazon) | Agrellos et al. 2012 | ||
| Oligoryzomys sp. | Oligoryzomys cf. messorius | 56 | 58 | - | AP | Andrades-Miranda et al. 2001a, Miranda et al. 2008, Weksler and Bonvicino 2015 | ||
| Oligoryzomys sp. 2 | - | 44; 45 | 52; 53 | Mosaicism of a small acrocentric pair; X chromosome polymorphisms | Only known from its type locality (Serra do Cipó, MG) | Silva and Yonenaga-Yassuda 1997 | ||
| Pseudoryzomys simplex (Winge, 1887) | - | 56 | 54; 55 | Addition of constitutive heterochromatin in pair 17 | Central Brazil (MT, TO, GO, MG, SP, BA, AL and PE) | Bonvicino et al. 2008, Moreira et al. 2013 | ||
| Scolomys ucayalensis Pacheco, 1991 | Scolomys juruaense | 50 | 68 | - | Westernmost Brazil (AC and AM) | Patton and da Silva 1995, Musser and Carleton 2005, Patton 2015 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Oryzomyini | Sooretamys angouya (G. Fischer, 1814) | - | 57-60 | 60-64 | 0-2 B chromosomes | Southeastern Brazil, from ES to RS | Almeida 1980, Zanchin 1988, Silva 1994, Geise 1995, Musser and Carleton 2005, Bonvicino et al. 2008 |
| Zygodontomys brevicauda (J. A. Allen & Chapman, 1893) | - | 86; 84; 82 | 96-100; 96-98; 94 | One name with different karyotypes associated | Northernmost Brazil (AM, RR, PA and AP) | Mattevi et al. 2002, Bonvicino et al. 2009, Voss 2015b | ||
| Tribe Phyllotini | Calassomys apicalis Pardiñas, Lessa, Salazar-Bravo & Câmara, 2014 | - | 62 | 116 | - | Only known in three localities in Central MG | Pardiñas et al. 2014 | |
| Calomys aff. expulsus | - | 64 | 66 | - | GO | Mattevi et al. 2005 | ||
| Calomys callidus (Thomas, 1916) | - | 48 | 66 | - | Western Brazil (RO to MT) | Mattevi et al. 2005, Bonvicino et al. 2010 | ||
| Calomys callosus (Rengger, 1830) | - | 50 | 66 | - | Western MS | Bonvicino et al. 2008, Bonvicino et al. 2010 | ||
| Calomys cerqueirai Bonvicino, Oliveira & Gentile, 2010 | - | 36; 38 | 66 | Centric Fusion | MG and ES | Bonvicino et al. 2010, Colombi and Fagundes 2014 | ||
| Calomys expulsus (Lund, 1840) | - | 66 | 68 | - | Caatinga and Cerrado formations from PE to GO | Musser and Carleton 2005, Bonvicino and Almeida 2000 | ||
| Calomys laucha (G. Fisher, 1814) | - | 64 | 68 | - | Southermost RS | Bonvicino et al. 2008, Mattevi et al. 2005 | ||
| Calomys tener (Winge, 1887) | - | 64; 66 | 64; 66 | One name with different karyotypes associated | Atlantic Forest region and habitats bordering the Cerrado, Southeastern Brazil (GO, MG, ES, SP, BA and DF) | Bonvicino and Almeida 2000, Mattevi et al. 2005, Musser and Carleton 2005, Bonvicino et al. 2008, Salazar-Bravo 2015 | ||
| Calomys tocantinsi Bonvicino, Lima & Almeida, 2003 | Calomys sp. | 46 | 66 | - | Cerrado habitats MT, TO and GO | Bonvicino et al. 2003a, Musser and Carleton 2005, Bonvicino et al. 2008 | ||
| Tribe Reithrodontini | Reithrodon typicus Waterhouse, 1837 | - | 28 | 40 | - | Boundary between RS and Uruguay | Freitas et al. 1983, Pardiñas et al. 2015c | |
| Tribe Sigmodontini | Sigmodon alstoni (Thomas, 1881) | - | 78, 80, 82* | N/A | Robertsonian polymorphisms; Karyotype of specimens from Venezuela | Northernmost Brazil (RR, AP and PA) | Voss 1992, Bonvicino et al. 2008 | |
| Tribe Thomasomyini | Rhagomys rufescens (Thomas, 1886) | - | 36 | 50 | - | RJ, SP and MG | Bonvicino et al. 2008, Testoni et al. 2010 | |
| Family Cricetidae - Subfamily Sigmodontinae | Tribe Thomasomyini | Rhipidomys cariri Tribe, 2005 | R. cariri baturiteensis | 44 | 48, 50 | FN=50 (type locality), FN=48 (R. cariri baturiteensis) | CE, PE and BA | Tribe 2005, Bonvicino et al. 2008, Thomazini 2009,Carvalho et al. 2012, Geise et al. 2010 |
| Rhipidomys emiliae (J. A. Allen, 1916) | - | 44 | 46, 52, 64 | Pericentric inversion | Eastern PA, MT (Serra do Roncador) and Western MA | Silva and Yonenaga-Yassuda 1999, Bonvicino et al. 2008, Tribe 2015 | ||
| Rhipidomys gardneri Patton, da Silva & Malcolm, 2000 | - | 44 | 50 | - | Northwestern AC | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Rhipidomys ipukensis R. G. Rocha, Costa & Costa, 2011 | - | N/A | N/A | - | Endemic to the Araguaia-Tocantins basin | Rocha et al. 2011, Tribe 2015 | ||
| Rhipidomys itoan B. M. de A. Costa, Geise, Pereira and L. P. Costa, 2011 | - | 44 | 48-50 | Pericentric inversion | RJ and Eastern SP to Southern Serra da Mantiqueira | Costa et al. 2011 | ||
| Rhipidomys leucodactylus (Tschudi, 1845) | - | 44 | 46, 48, 52 | Pericentric inversion | Northwestern Brazil (AM, AC, MT, RO, RR, AP and PA) | Zanchin et al. 1992, Silva and Yonenaga-Yassuda 1999, Patton et al. 2000, Bonvicino et al. 2008, Tribe 2015 | ||
| Rhipidomys macconnelli de Winton, 1900 | - | 44* | 50* | Karyotype of specimens from Venezuela | AM (Serra da Neblina) and Western RR, above 1.000m of altitude | Aguilera et al. 1994, Bonvicino et al. 2008 | ||
| Rhipidomys macrurus (P. Gervais, 1855) | - | 44 | 48-52 | Pericentric inversion | Cerrado and Caatinga biomes, from CE to MT, and MG | Zanchin et al. 1992, Silva and Yonenaga-Yassuda 1999, Musser and Carleton 2005, Carvalho et al. 2012 | ||
| Rhipidomys mastacalis (Lund, 1840) | - | 44 | 70, 74, 76, 80 | Pericentric inversion | Atlantic Forest region, from PE to PR | Zanchin et al. 1992, Andrades-Miranda et al. 2002b, Paresque et al. 2004, Musser and Carleton 2005, Sousa 2005, Bonvicino et al. 2008, Carvalho et al. 2012, Tribe 2015 | ||
| Rhipidomys nitela Thomas, 1901 | Rhipidomys sp. B | 48; 50 | 68; 71, 72 | Pericentric inversion in pair 8, addition and deletion of constitutive hetechromatin | Northcentral Brazil (AM, MT, AP, RR, PA, TO and GO) | Silva and Yonenaga-Yassuda 1999, Andrades-Miranda et al. 2002b, Tribe 2015 | ||
| Rhipidomys tribei B. M. de A. Costa, Geise, Pereira and L. P. Costa, 2011 | - | 44 | 50 | - | Serra do Caraça, Southern MG | Zanchin et al. 1992, Costa et al. 2011 | ||
| Rhipidomys wetzeli A. L. Gardner, 1990 | - | N/A | N/A | - | Northern Brazil | Fonseca et al. 1996, Tribe 2015 | ||
| Tribe Wiedomyini | Wiedomys cerradensis P. R. Gonçalves, Almeida & Bonvicino, 2005 | - | 60 | 88 | - | Only known from its type locality (Southwestern BA) | Gonçalves et al. 2005 | |
| Wiedomys pyrrhorhinos (Wied-Neuwied, 1821) | - | 62 | 86, 90, 104 | Pericentric inversion in the smallest pairs | Southern CE, Southeastern PI, and Western PB, PE, AL, BA and Northern MG | Maia and Langguth 1987, Gonçalves et al. 2005, Bonvicino et al. 2008, Souza et al. 2011 | ||
| Family Cricetidae - Subfamily Sigmodontinae | Incertae sedis | Abrawayaomys ruschii F. Cunha & Cruz, 1979 | - | 58 | N/A | - | ES, RJ, SP, MG and SC | Bonvicino et al. 2008, Pereira et al. 2008 |
| Delomys altimontanus Gonçalves & Oliveira, 2014 | - | 82 | 86 | - | Disjunction distribution in Itatiaia (RJ) and Caparaó (MG) | Gonçalves and Oliveira 2014 | ||
| Delomys dorsalis (Hensel, 1872) | Thomasomys dorsalis collinus, D. collinus | 82 | 80 | - | Atlantic Forest of Southeastern Brazil, from MG and ES to RS | Musser and Carleton 2005, Gonçalves and Oliveira 2014 | ||
| Delomys sublineatus (Thomas, 1903) | - | 72 | 90 | - | Atlantic Forest of Southeastern Brazil, from MG and ES to SC | Musser and Carleton 2005, Gonçalves and Oliveira 2014 | ||
| Juliomys ossitenuis L. P. Costa, Pavan, Leite, and Fagundes, 2007 | - | 20 | 36 | - | Southern ES, and Eastern SP and MG | Costa et al. 2007, Bonvicino et al. 2008 | ||
| Juliomys pictipes (Osgood, 1933) | Wilfredomys pictipes | 36 | 34 | - | Southeastern Brazil, from MG to RS | Bonvicino and Otazu 1999, Musser and Carleton 2005 | ||
| Juliomys rimofrons J. A. Oliveira & Bonvicino, 2002 | - | 20 | 34 | - | High altitudes at Serra da Mantiqueira, in SP, RJ and MG | Oliveira and Bonvicino 2002, Bonvicino et al. 2008 | ||
| Juliomys sp. | - | 32 | 48 | - | Aparados da Serra National Park, ES | Paresque et al. 2009 | ||
| Phaenomys ferrugineus (Thomas, 1917) | - | 78 | 114 | - | Restricted areas from Serra do Mar, in RJ and SP | Bonvicino et al. 2001b, Musser and Carleton 2005 | ||
| Wilfredomys oenax (Thomas, 1928) | - | N/A | N/A | - | Southern Brazil and Southeastern SP | Bonvicino et al. 2008 | ||
| Family Ctenomyidae | Ctenomys bicolor Miranda-Ribeiro, 1914 | - | 40 | 64 | - | RO | Stolz 2012 | |
| Ctenomys flamarioni Travi, 1981 | - | 48 | 50-78 | Variation in the amount of constitutive heterochromatin | Eastern RS | Massarini and Freitas 2005, Bonvicino et al. 2008 | ||
| Ctenomys ibicuiensis T. R. O. Freitas, Fernandes, Fornel & Roratto, 2012 | - | 50 | 68 | - | Western RS | Bidau 2015 | ||
| Ctenomys lami T. R. O. Freitas, 2001 | - | 54-58 | 74-82; 84 | Centric fusion/ fission in pairs 1 and 2; pericentric inversion | RS (Coxilha das Lombas, Northeastern Guaiba River to Southwestern Banks of Barros Lake) | Woods and Kilpatrick 2005, Freitas 2007 | ||
| Ctenomys minutus Nehring, 1887 | - | 42, 43, 44; 45; 46-51; 49-51; 48-51; 51; 52 | 74; 75/76; 77; 78; 78, 80; 79 | Robertsonian rearrengements and tandem fusions | Eastern RS and SC | Freitas 1997, Gava and Freitas 2002, Freygang et al. 2004, Bonvicino et al. 2008 | ||
| Ctenomys nattereri Wagner, 1848 | Ctenomys boliviensis | 36 | 64 | - | Southwestern MT and Southeastern RO | Anderson et al. 1987, Bonvicino et al. 2008, Stolz 2012 | ||
| Ctenomys rondoni Miranda-Ribeira, 1914 | - | N/A | N/A | - | MT and RO | Bidau 2015 | ||
| Family Ctenomyidae | Ctenomys torquatus Lichtenstein, 1830 | - | 40, 42, 44, 46 | 72 | Robertsonian fusion; Variation in the amount of constitutive heterochromatin; secondary constricton | Southeastern RS | Freitas and Lessa 1984, Bonvicino et al. 2008, Fernandes et al. 2009 | |
| Family Cuniculidae | Cuniculus paca (Linnaeus, 1766) | - | 74 | 98 | - | All Brazilian States | Giannoni et al. 1991, Bonvicino et al. 2008 | |
| Family Dasyproctidae | Dasyprocta azarae Lichtenstein, 1823 | Dasyprocta aurea | 64 | 122 | - | Southcentral Brazil, MG and SP | Souza et al. 2007, Bonvicino et al. 2008 | |
| Dasyprocta croconota Wagler, 1831 | - | N/A | N/A | - | Northeastern PA, Northwestern CE and Northermost TO | Bonvicino et al. 2008, Patton and Emmons 2015 | ||
| Dasyprocta fuliginosa Wagler, 1832 | - | 64; 65 | 116; 122 | B chromosome | AM, AC, RO and Northwestern MT | Lima and Langguth 1998, Ramos et al. 2003, Bonvicino et al. 2008 | ||
| Dasyprocta iacki Feijó & Langguth, 2013 | Dasyprocta aguti | 64 | 122 | - | Littoral zone in PB and PE | Lima and Langguth 1998, Feijó and Langguth 2013, Patton and Emmons 2015 | ||
| Dasyprocta leporina Linnaeus, 1758 | - | 64, 65 | 122-124 | B chromosome | Northermost Brazil (AM, RR, AP and PA) | Ramos et al. 2003, Bonvicino et al. 2008, Patton and Emmons 2015 | ||
| Dasyprocta prymnolopha Wagler, 1831 | Dasyprocta nigriclunis | 64, 65 | 122 | B chromosome | Northeastern Brazil, and Northern MG | Ramos et al. 2003, Woods and Kilpatrick 2005, Bonvicino et al. 2008 | ||
| Dasyprocta punctata Gray, 1842 | - | N/A | N/A | - | Southeastern Brazil | Woods and Kilpatrick 2005, Patton and Emmons 2015 | ||
| Dasyprocta variegata Tschudi, 1845 | - | 64* | 124 | - | Western Brazil | Patton and Emmons 2015 | ||
| Dasyprocta sp. | - | 64, 65 | 124 | B chromosome | unknown distribution | Ramos et al. 2003 | ||
| Myoprocta acouchy (Erxleben, 1777) | - | 62 | 118 | - | RR, and Northeastern AM and PA | Hsu and Benirschke 1968, Bonvicino et al. 2008, Patton and Emmons 2015 | ||
| Myoprocta pratti Pocock, 1913 | - | N/A | N/A | - | AC and Western AM | Bonvicino et al. 2008, Patton and Emmons 2015 | ||
| Family Dinomyidae | Dinomys branickii Peters, 1873 | - | 64 | 98 | - | AC and Southwesternmost AM | Bonvicino et al. 2008, Vargas and Ortiz 2010 | |
| Family Echimyidae | Callistomys pictus (Pictet, 1843) | - | 42 | 76 | - | Southeastern BA | Bonvicino et al. 2008, Ventura et al. 2008, Emmons and Leite 2015 | |
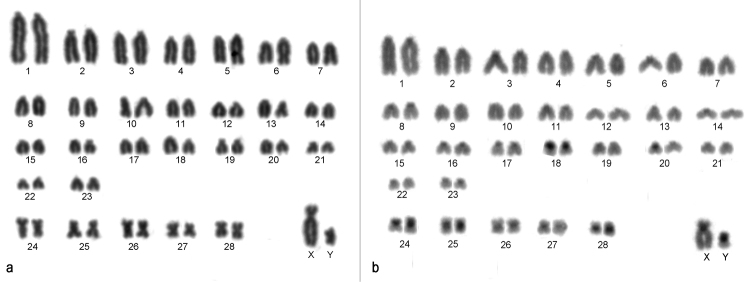
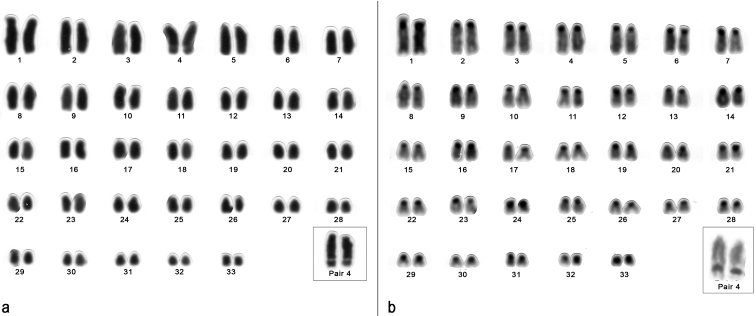
| Carterodon sulcidens (Lund, 1838) | - | 66 | N/A | Secondary constriction in the forth largest pair | DF, GO, MT and MG | Carmignotto 2005, Bezerra and Bonvicino 2015, Present study | ||
| Family Echimyidae | Clyomys laticeps (Thomas, 1909) | Clyomys bishopi | 34; 32 | 58, 60, 62; 54 | Pericentric inversion; Robertsonian rearrangement; secondary constriction in pair 1; addition of constitutive heterochromatin | MT, MS, GO, DF, SP and MG | Souza and Yonenaga-Yassuda 1984, Svartman 1989, Bonvicino et al. 2008, Bezerra et al. 2012 | |
| Dactylomys boliviensis Anthony, 1920 | - | 118 | 168 | - | AC | Dunnum et al. 2001, Woods and Kilpatrick 2005 | ||
| Dactylomys dactylinus (Desmarest, 1817) | - | 94 | 144 | - | AM, PA, RR, TO and Northern GO | Aniskin 1993, Bonvicino et al. 2008 | ||
| Echimys chrysurus (Zimmermann, 1780) | - | N/A | N/A | - | Southern AP, Northeastern PA and Northwestern MA | Bonvicino et al. 2008 | ||
| Echimys vieirai Iack-Ximenes, de Vivo & Percequillo, 2005 | - | N/A | N/A | - | Central-Easternmost AM and Central-Westernmost PA | Bonvicino et al. 2008 | ||
| Euryzygomatomys spinosus (G. Fischer, 1814) | - | 46 | 82 | - | Eastern MG, SP and RJ, PR and Northern RS | Yonenaga 1975, Bonvicino and Bezerra 2015 | ||
| Isothrix bistriata Wagner, 1845 | - | 60 | 116 | - | Northern AC and RO, Northeastern MT, and Southern AM | Leal-Mesquita 1991, Bonvicino et al. 2008 | ||
| Isothrix negrensis Thomas, 1920 | - | 60 | 112 | - | Northern AM | Bonvicino et al. 2003b, Bonvicino et al. 2008 | ||
| Isothrix pagurus Wagner, 1845 | - | 22 | 38 | - | Northeastern AM | Patton and Emmons 1985, Bonvicino et al. 2008 | ||
| Kannabateomys amblyonyx (Wagner, 1845) | - | 98 | 126 | - | Eastern Brazil, from ES to RS | Paresque et al. 2004, Bonvicino et al. 2008 | ||
| Lonchothrix emiliae Thomas, 1920 | - | N/A | N/A | - | Eastern AM | Bonvicino et al. 2008 | ||
| Makalata didelphoides (Desmarest, 1817) | - | 66 | 106 | Secondary constriction in pair 11 | AP, RR, Eastern AM, Western PA and TO, and Northern MT | Lima et al. 1998, Bonvicino et al. 2008 | ||
| Makalata macrura (Wagner, 1842) | - | N/A | N/A | - | AM and AC | Bonvicino et al. 2008 | ||
| Makalata obscura (Wagner, 1840) | - | N/A | N/A | - | Eastern PA and Westernmost MA | Bonvicino et al. 2008 | ||
| Mesomys hispidus (Desmarest, 1817) | - | 60 | 116 | - | Northern Brazil, and Northwestern MT | Leal-Mesquita 1991, Bonvicino et al. 2008 | ||
| Mesomys occultus Patton, da Silva & Malcolm, 2000 | - | 42 | 54 | Secondary constriction in the smallest biarmed pair | Central AM | Patton et al. 2000, Woods and Kilpatrick 2005 | ||
| Mesomys stimulax Thomas, 1911 | - | 60 | 116 | - | Eastern PA | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Myocastor coypus (G. I. Molina, 1782) | - | 42 | 76 | - | RS | González and Brum-Zorilla 1995, Bonvicino et al. 2008, Fabre et al. 2016 | ||
| Family Echimyidae | Phyllomys blainvillii (Jourdan, 1837) | - | 50 | 88, 94-96 | Pericentric inversion | BA, SE, AL and PE, Southern CE, and Northern MG | Souza 1981, Leite 2003, Bonvicino et al. 2008, Machado 2010 | |
| Phyllomys brasiliensis Lund, 1840 | - | N/A | N/A | - | Central MG | Bonvicino et al. 2008 | ||
| Phyllomys dasythrix Hensel, 1872 | - | 72 | 108 | - | Southern PR to RS | Leite 2003, Woods and Kilpatrick 2005, Machado 2010 | ||
| Phyllomys kerri (Moojen, 1950) | - | N/A | N/A | - | Ubatuba (SP) | Woods and Kilpatrick 2005 | ||
| Phyllomys lamarum (Thomas, 1916) | - | 56 | 102 | - | Eastern Brazil, from PB to MG | Woods and Kilpatrick 2005, Araújo et al. 2014 | ||
| Phyllomys lundi Y. L. R. Leite, 2003 | - | N/A | N/A | - | Southern MG to RJ | Bonvicino et al. 2008 | ||
| Phyllomys mantiqueirensis Y. L. R. Leite, 2003 | - | N/A | N/A | - | Serra da Mantiqueira (MG) | Bonvicino et al. 2008 | ||
| Phyllomys medius (Thomas, 1909) | - | 96 | 108 | - | From RJ to RS | Sbalqueiro et al. 1989, Bonvicino et al. 2008 | ||
| Phyllomys nigrispinus (Wagner, 1842) | - | 84, 85 | N/A | Secondary constriction in one acrocentric pair | Coast from RJ to PR, extending to inland Western SP | Leite 2003, Woods and Kilpatrick 2005, Delciellos et al. 2017 | ||
| Phyllomys pattoni Emmons, Leite, Kock & Costa, 2002 | - | 72; 76; 80 | 114; 148; 100, 108, 112 | Pericentric inversion; centric fusion/ fission | From PB to Northeastern SP | Zanchin 1988, Leite 2003, Paresque et al. 2004, Woods and Kilpatrick 2005, Leite and Loss 2015 | ||
| Phyllomys sulinus Y. L. R. Leite, Christoff & Fagundes, 2008 | - | 92 | 102 | - | Southern Brazil, from SP to RS | Yonenaga 1975, Leite 2003, Leite and Loss 2015 | ||
| Phyllomys thomasi (Ihering, 1897) | - | N/A | N/A | - | Ilha de São Sebastião (SP) | Woods and Kilpatrick 2005, Leite and Loss 2015 | ||
| Phyllomys unicolor (Wagner, 1842) | - | N/A | N/A | - | Southernmost BA | Bonvicino et al. 2008, Leite and Loss 2015 | ||
| Proechimys brevicauda (Günther, 1876) | - | 28 | 48-50 | Variations in FN due to difficulty in classifying the morphology of the small pairs | AC and Southern AM | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Proechimys cuvieri Petter, 1978 | - | 28 | 46-48 | Differences in the number of subtelocentrics and acrocentrics | Northern Brazil | Maia and Langguth 1993, Patton et al. 2000, Bonvicino et al. 2008 | ||
| Proechimys echinotrix M. N. F. da Silva, 1998 | - | 32 | 60 | - | Northwestern AM | da Silva 1998, Bonvicino et al. 2008 | ||
| Proechimys gardneri M. N. F. da Silva, 1998 | - | 40 | 54, 56 | Pericentric inversion; secondary constriction in the smallest submetacentric pair | Southern AM | da Silva 1998, Bonvicino et al. 2008, Eler et al. 2012 | ||
| Proechimys goeldii Thomas, 1905 | - | 24 | 44 | - | Easternmost AM and Northwestern PA | Machado et al. 2005, Patton and Leite 2015 | ||
| Proechimys gr. goeldii | - | 15 | 16 | - | MT | Machado et al. 2005 | ||
| Family Echimyidae | Proechimys guyannensis (I. Geoffrey St.-Hilaire, 1803) | - | 38, 44 | 52 | One name with different karyotypes associated | Northeastern AM, Northern PA, Southeastern RR and AP | Machado et al. 2005, Bonvicino et al. 2008 | |
| Proechimys hoplomyoides Tate, 1939 | - | N/A | N/A | - | Northernmost RR | Bonvicino et al. 2008 | ||
| Proechimys kulinae M. N. F. da Silva, 1998 | - | 34 | 52 | - | Southeastern AM | da Silva 1998, Patton et al. 2000, Bonvicino et al. 2008 | ||
| Proechimys longicaudatus (Rengger, 1830) | - | 28 | 48-50 | Pericentric inversion of pairs 3 and 11; addition/deletion of constitutive heterochromatin | MT | Machado et al. 2005, Bonvicino et al. 2008 | ||
| Proechimys cf. longicaudatus | - | 16, 17 | 14 | Robertsonian rearrangement between X and the largest acrocentric chromosome; Multiple sex chromosome system (XX, XY1Y2) | MT | Amaral et al. 2013 | ||
| Proechimys pattoni M. N. F. da Silva, 1998 | - | 40 | 56 | - | Western AC | Patton and Gardner 1972, da Silva 1998, Bonvicino et al. 2008 | ||
| Proechimys quadruplicatus Hershkovitz, 1948 | - | 28 | 42 | - | Northcentral AM | Patton et al. 2000, Bonvicino et al. 2005b, Bonvicino et al. 2008 | ||
| Proechimys roberti Thomas, 1901 | - | 30 | 54-56 | Pericentric inversion of pairs 13 and 14 | Eastern PA, TO and GO, and Western MG and MA | Svartman 1989, Leal-Mesquita 1991, Machado et al. 2005, Ribeiro 2006, Bonvicino et al. 2008 | ||
| Proechimys simonsi Thomas, 1900 | Proechimys hendeei | 32 | 56-58 | Pericentric inversion; secondary constriction in pair 8 of the karyotype with NF=56 | AC and Southwestern AM | Patton and Gardner 1972, Gardner and Emmons 1984, Patton et al. 2000, Bonvicino et al. 2008 | ||
| Proechimys steerei Goldman, 1911 | - | 24 | 40-42 | Pericentric inversion in pair 3 (smallest metacentric), with homo or heterozigous chromosomes | AC and Southwestern AM | Patton et al. 2000, Bonvicino et al. 2008 | ||
| Proechimys sp. | Proechimys gr. longicaudatus | 30 | 52 | - | Rio Jamari, RO | Leal-Mesquita 1991, Patton and Leite 2015 | ||
| Proechimys sp. A | Proechimys gr. goeldii | 38 | 52 | - | Rio Negro-Rio Aracá, AM | Bonvicino et al. 2005b | ||
| Proechimys sp. B | - | 46 | 50 | - | RR and Northern AM | Bonvicino et al. 2005b, Bonvicino et al. 2008 | ||
| Thrichomys apereoides (Lund, 1839) | - | 28 | 50, 52 | Secondary constriction in pair 2 | MG, Eastern GO and Western BA | Bonvicino et al. 2002a, Pessôa et al. 2004 | ||
| Thrichomys inermis (Pictet, 1843) | - | 26 | 48 | Secondary constriction in pair 2 | BA and TO | Pessôa et al. 2004, Bonvicino et al. 2008 | ||
| Thrichomys laurentius Thomas, 1904 | - | 30 | 54 | Secondary constriction in pair 1 | Northeastern Brazil, except MA | Souza and Yonenaga-Yassuda 1982, Bonvicino et al. 2008 | ||
| Family Echimyidae | Thrichomys aff. laurentius | - | 30 | 56 | Secondary constriction in pair 1 | Central Brazil | Bonvicino et al. 2002a, Braggio and Bonvicino 2004 | |
| Thrichomys pachyurus Wagner, 1845 | - | 34 | 64 | Secondary constriction in pair 2 | Southern MT, and MS | Pessôa et al. 2004, Bonvicino et al. 2008 | ||
| Trinomys albispinus (I. Geoffrey St.-Hilaire, 1838) | - | 60 | 116 | Secondary constriction in pair 10 | BA, SE and MG | Leal-Mesquita et al. 1993, Souza et al. 2006, Pessôa et al. 2015 | ||
| Trinomys dimidiatus (Günther, 1876) | - | 60 | 116 | Secondary constriction in pair 10 | RJ and Northern SP | Pessôa et al. 2004, Bonvicino et al. 2008 | ||
| Trinomys eliasi (Pessôa & Reis, 1993) | - | 38 | 112 | Secondary constriction in pair 10 | RJ | Pessôa et al. 2005, Bonvicino et al. 2008 | ||
| Trinomys gratiosus (Moojen, 1948) | Trinomys gr. bonafidei | 56 | 108 | Secondary constriction in pair 10 | Southcentral ES to Southwestern RJ | Zanchin 1988, Woods and Kilpatrick 2005 | ||
| Trinomys iheringi (Thomas, 1911) | Proechimys iheringi iheringi | 60-65 | 116 | 1 to 5 B chromosomes; secondary constriction in pair 7 | Coast from Southern RJ to Northern PR | Yonenaga-Yassuda et al. 1985, Fagundes et al. 2004, Bonvicino et al. 2008 | ||
| Trinomys mirapitanga Lara, Patton and Hingst- Zaher, 2002 | - | N/A | N/A | - | BA | Lara et al. 2002, Woods and Kilpatrick 2005 | ||
| Trinomys moojeni (Pessôa, Oliveira & Reis, 1992) | - | 56 | 106 | - | Only known from the type locality (MG) | Corrêa et al. 2005, Woods and Kilpatrick 2005 | ||
| Trinomys paratus (Moojen, 1948) | - | 58 | 112 | Secondary constriction in long arm of a median size autosome | South-central ES and easternmost MG | Bonvicino et al. 2008, Lazar et al. 2017 | ||
| Trinomys setosus (Desmarest, 1817) | Trinomys s. setosus and Trinomys s. elegans | 56 | 108, 104 | NFs refer to each subspecies, respectively | Eastern Brazil, from SE to ES and MG | Bonvicino et al. 2008, Pêssoa et al. 2015 | ||
| Trinomys yonenagae (P. L. B. Rocha, 1996) | - | 54 | 104 | Secondary constriction in pair 10 | BA, left bank of Rio São Francisco | Leal-Mesquita et al. 1992, Bonvicino et al. 2008 | ||
| Toromys grandis (Wagner, 1845) | - | N/A | N/A | - | Eastern AM and PA | Bonvicino et al. 2008 | ||
| Family Erethizontidae | Chaetomys subspinosus Olfers, 1818 | - | 52 | 76 | - | ES and Southeastern BA | Bonvicino et al. 2008, Vilela et al. 2009 | |
| Coendou insidiosus (Olfers, 1818) | Sphiggurus insidiosus | 62 | 76 | - | Eastern Brazil, from CE to ES | Lima 1994, Bonvicino et al. 2008, Voss 2015c | ||
| Coendou melanurus (Wagner, 1842) | Sphiggurus melanurus | 72 | 76 | - | Northernmost Brazil (AM, RR, AP and PA) | Bonvicino et al. 2002b, Bonvicino et al. 2008, Voss 2015c | ||
| Coendou nycthemera (Olfers, 1818) | - | N/A | N/A | - | Easternmost AM and PA | Bonvicino et al. 2008, Voss 2015c | ||
| Coendou prehensilis (Linnaeus, 1758) | - | 74 | 82 | - | From Northern to Southeastern Brazil | Lima 1994, Bonvicino et al. 2008, Voss 2015c | ||
| Coendou roosmalenorum Voss and da Silva, 2001 | Sphiggurus roosmalenorum | N/A | N/A | - | Centraleastern AM | Bonvicino et al. 2008, Voss 2015c | ||
| Family Echimyidae | Coendou speratus Mendes Pontes, Gadelha, Melo, de Sá, Loss, Caldara Junior, Costa & Leite, 2013 | - | N/A | N/A | - | Eastern PE and AL | Mendes-Pontes et al. 2013, Voss 2015c | |
| Coendou spinosus (F. Cuvier 1823) | Sphiggurus spinosus, S. villosus | 42 | 76 | - | Southern Brazil, Southeastern MG, and Eastern SP and RJ | Mendes-Pontes et al. 2013, Voss 2015c | ||
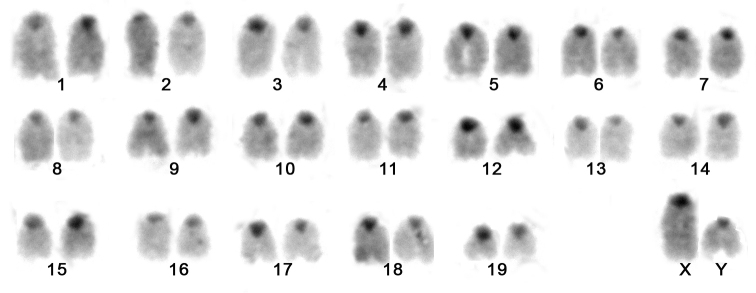
| Family Muridae | Mus musculus Linnaeus, 1758 | - | 40 | 38 | - | All Brazilian States | Bonvicino et al. 2008, present study | |
| Rattus rattus Linnaeus, 1758 | - | 38 | 58-59 | Pericentric inversion in pair 8 | All Brazilian States | Kasahara and Yonenaga-Yassuda 1981, Kasahara and Yonenaga-Yassuda 1984, Bonvicino et al. 2008 | ||
| Rattus norvegicus Berkenhout, 1769 | - | 42 | 64 | - | All Brazilian States | Bianchi et al. 1969, Bonvicino et al. 2008 | ||
| Family Sciuridae | Guerlinguetus aestuans (Linnaeus, 1766) | Guerlinguetus gilvigularis, G. poaiae | N/A | N/A | - | RR, AP, AM, PA and Central MT | Bonvicino et al. 2008, De Vivo and Carmignotto 2015 | |
| Guerlinguetus brasiliensis (Gmelin, 1788) | Guerlinguetus alphonsei, G. henseli, G. ingrami | 40 | 74, 76 | Pericentric inversions | Disjunct distribution of Amazonian, Caatinga, and Coastal Brazil | Lima and Langguth 2002, Fagundes et al. 2003, De Vivo and Carmignotto 2015 | ||
| Hadrosciurus igniventris (Wagner, 1842) | Sciurus igniventris | N/A | N/A | - | Northern Brazil, Southern Amazon River | Bonvicino et al. 2008, De Vivo and Carmignotto 2015 | ||
| Hadrosciurus pyrrhinus (Thomas, 1898) | Sciurus igniventris, S. pyrrhonotus, S. pyrrhinus | N/A | N/A | - | Western Brazilian Amazonia | Patton et al. 2015 | ||
| Hadrosciurus spadiceus (Olfers, 1818) | Sciurus spadiceus | 40 | 76 | - | Central to Southern AM, AC, RO, and Western PA and MT | Lima and Langguth 2002, Bonvicino et al. 2008, De Vivo and Carmignotto 2015 | ||
| Microsciurus flaviventer (Gray, 1867) | - | N/A | N/A | - | Northern Amazon River, Brazil | Bonvicino et al. 2008 | ||
| Notosciurus pucheranii (Fitzinger, 1867) | Guerlinguetus ignitus | N/A | N/A | - | Northwestern MT, Western AC and Southwestern AM | Bonvicino et al. 2008 | ||
| Sciurillus pusillus (I. Geoffrey St.-Hilaire, 1803) | - | N/A | N/A | - | Eastern AM and Western PA | Bonvicino et al. 2008 | ||
Abbreviations: Brazilian states AC: Acre; AL: Alagoas; AP: Amapá; AM: Amazonas; BA: Bahia; CE: Ceará; DF: Distrito Federal; ES: Espírito Santo; GO: Goiás; MA: Maranhão; MG: Minas Gerais; MS: Mato Grosso do Sul; MT: Mato Grosso; PA: Pará; PB: Paraíba; PE: Pernambuco; PI: Piauí; PR: Paraná; RJ: Rio de Janeiro; RN: Rio Grande do Norte; RO: Rondônia; RR: Roraima; RS: Rio Grande do Sul; SC: Santa Catarina; SE: Sergipe; SP: São Paulo; TO: Tocantins. N/A means that information is not available and (*) means that data do not refer to Brazilian specimens.
Sampling
The single female of Carterodon sulcidens (lab number: CIT787/ field number: APC58) was captured in Serra da Mesa, State of Goiás, Brazil (13°53'S, 48°19'W), a region characterized by the Cerrado biome. Additionally, five males of Mus musculus (field number: PCH4078, 4079, 4094–96) were captured in Guará, São Paulo State, Brazil (20°29'S, 47°51'W), a transitional region between the Cerrado and Atlantic Forest.
Regarding Neacomys, four specimens of N. amoenus amoenus Thomas, 1903 were captured in Mato Grosso State, Brazil, in a transitional area between Cerrado and Amazonian Rainforest. Two specimens of Neacomys sp. were captured, one at Vila Rica (Mato Grosso State), and the other at Igarapé-Açu (Amazonas State), Brazil (field number, locality, and coordinates are presented in Suppl. material 1).
Cytogenetic preparation
Chromosome preparations of Carterodon sulcidens, the five samples of Mus musculus, four Neacomys a. amoenus, and a specimen of Neacomys from Vila Rica, Mato Grosso State, were obtained in vivo from bone marrow and spleen, following Ford and Hamerton (1956) or in vitro from fibroblast culture (Freshney 1986). Conventional Giemsa staining was performed to determine the diploid and fundamental numbers, and C-banding and Ag-NOR were performed according to Sumner (1972) and Howell and Black (1980), respectively.
Molecular phylogeny analyses of Neacomys
DNA was extracted from the liver or muscle with Chelex 5% (Bio-Rad) (Walsh et al. 1991) of five specimens of Neacomys. DNA of the specimen from Vila Rica, Mato Grosso State, was extracted from fibroblast cell culture using DNeasy Blood and Tissue kit (Qiagen, catalog number 69506).
PCR was performed in a thermal cycler (Eppendorf Mastercycler ep Gradient, Model 5341) using primers MVZ05 (5-CGA AGC TTG ATA TGA AAA ACC ATC GTT G-3) and MVZ16 (5-AAA TAG GAA RTA TCA YTC TGG TTT RAT-3) (Irwin et al. 1991, Smith and Patton 1993, respectively). PCR mixture contained 30 ng of DNA, 25 pmol of each primer, 0.2 mM of dNTP, 2.52 µL of reaction buffer (50 mM KCl, 2.5 mM MgCl2, 10 mM Tris-HCl; pH 8.8) and 0.2 units of Taq DNA polymerase (Invitrogen). Thirty-nine amplification cycles were performed, consisting of denaturation at 94 °C for 30 s, annealing at 48 °C for 45 s, extension at 72 °C for 45 s and the final extension at 72 °C for 5 min. The PCR products were separated using 1% agarose gel in TAE buffer. Sequencing was conducted using BigDye (DNA “Big Dye Terminator Cycle Sequencing Standart,” Applied Biosystems) and an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). All sequences were submitted to a comparative similarity search on BLAST (Basic Local Alignment Search Tool) before the alignment. Alignments were performed by using Muscle (Edgar, 2004) implemented in Geneious 4.8.5 (Biomatters). GenBank access numbers are provided in Suppl. material 1.
Models of nucleotide substitution were selected using Bayesian Information Criterion (BIC), implemented in PartitionFinder, version 1.1.1 (Lanfear et al. 2012). Approximately 673 bp were used to perform Maximum Likelihood (ML) in GARLI 2.0 (Bazinet et al. 2014) and Bayesian Inference (BI) in MrBayes 3.04b (Ronquist and Huelsenbeck 2003), using 69 additional Neacomys sequences downloaded from GenBank, plus sequences of Euryoryzomys russatus (Wagner, 1848), Holochilus brasiliensis (Desmarest, 1819) and Oligoryzomys nigripes (Olfers, 1818) as the outgroup (see Suppl. material 1).
Results
The current review encompasses all rodent species which up to the present have been reported in Brazil, comprising 271 species from 10 families (Musser and Carleton 2005, Patton et al. 2015, Fabre et al. 2016). Diploid number ranges from 2n = 9, 10 in Akodon sp. n. to 2n = 118 in Dactylomys boliviensis Anthony, 1920 (Table 1). It is noteworthy that 38 species (14%) lack any cytogenetic data. Besides, nine species present only the diploid number with no information about the fundamental number.
Many species show chromosome rearrangements leading to variation in diploid and fundamental numbers. Also, more than one diploid number was associated with one single species, suggesting that they could represent species’ complexes. Additionally, new karyotypes were assigned to 22 species highlighting them as candidate species, which have not been formally described yet.
All comments below refer to the data compiled and presented in Table 1.
Family Caviidae
From a total of ten species, cytogenetic data is lacking for only one: Galea flavidens (Brandt, 1835). The diploid number varied from 2n = 52 in Kerodon acrobata Moojen, 1997 and K. rupestris (Wied-Neuwied, 1820) to 2n = 66 in Hydrochoerus hydrochaeris (Linnaeus, 1766). Currently, polymorphism of autosomal chromosomes has been described for Cavia porcellus (Linnaeus, 1758), pericentric inversions for C. magna Ximénez, 1980 and K. rupestris, and Robertsonian rearrangement for C. magna (Maia 1984, Gava et al. 2011) (Table 1).
Family Cricetidae
Subfamily Sigmodontinae
Tribe Akodontini
This is the second most diverse tribe in the subfamily Sigmodontinae. Only five out of 42 species (D’Elía and Pardiñas 2015) that occur in Brazil lack diploid number information (Table 1). However, for one species, Akodon toba Thomas, 1921, such information is available only for Paraguayan specimens. In addition to the species on which there is no information on the diploid number, four species of the genus Oxymycterus Waterhouse, 1837 have not had their fundamental number established, yet.
In this tribe, the diploid number varied from 2n = 9, 10 in Akodon sp. n. to 2n = 70 in Bibimys labiosus (Winge, 1887). B chromosomes are found in Akodon montensis and Blarinomys breviceps (Winge, 1887). Also, pericentric inversions were described in three species of the tribe, Robertsonian rearrangements in six, and reciprocal translocation in one. These rearrangements are reported for Akodon cursor (although some authors consider A. cursor as a species complex, because of the molecular phylogeny – see Geise et al. 2001, Silva et al. 2006), Akodon sp. n., Akodon montensis, Blarinomys breviceps, Brucepattersonius griserufescens Hershkovitz, 1998, Deltamys kempi Thomas, 1917, Necromys lasiurus (Lund, 1840), Scapteromys meridionalis Quintela, Gonçalves, Althoff, Sbalqueiro, Oliveira, & Freitas, 2014, and Thalpomys lasiotis Thomas, 1916.
Sex chromosome variation is also common, occurring in six species. It is also remarkable that Deltamys kempi is one of the few rodents to which multiple sex system has been described (X1X1X2X2/ X1X2Y) (Sbalqueiro et al. 1984).
Cytogenetic studies have proved to be a useful tool in the recognition of species, mainly in the case of the cryptic and sympatric species as Akodon cursor and A. montensis. On the other hand, karyotype was less variable in some other Akodontini genus (for instance Brucepattersonius and Oxymycterus), and in this case, they could not be distinguished cytogenetically. This reveals the need for gathering cytogenetic, molecular and morphological data in taxonomic studies.
Tribe Ichthyomyini
Two species of Neusticomys, N. oyapocki (Dubost & Petter, 1979) and N. ferreirai Percequillo, Carmignotto & Silva, 2005, occur in Brazil and karyotype information is available only for N. ferreirai (Table 1). Karyotype shows 2n = 92, FN = 98, and autosomes consist of four biarmed pairs and 41 acrocentrics. X chromosome is a large metacentric and Y is the largest acrocentric (Percequillo et al. 2005).
Tribe Oryzomyini
Comprising 73 species up to now, this tribe alone comprises about 47% of the Sigmodontinae diversity. Notwithstanding, it is one of the best cytogenetically studied taxa of Brazilian rodents, and cytogenetic information on fundamental number lacks for only one species: Neacomys guianae Thomas, 1905. In Brazilian representatives the diploid number varied from 2n = 34 in Neacomys musseri Patton, da Silva & Malcolm, 2000 to 2n = 86 in Zygodontomys brevicauda (J. A. Allen & Chapman, 1893).
Pericentric inversion (n = 13) and Robertsonian rearrangements (n = 8) are common rearrangements, as well as sex chromosomes variations, that were described in 12 species and correlated to addition/deletion of constitutive heterochromatin and pericentric inversions.
Besides, Oryzomyini is also the tribe with more species having supernumerary chromosomes (n = 6). Remarkably, B chromosomes in this tribe present different morphology and composition, not only between, but also within the same species. For instance, Nectomys squamipes Brants, 1827 presents from one to three supernumeraries that could be large/medium submetacentric or medium acrocentric, with interstitial or entire long arm C-banded, with late or early replication and with or without interstitial telomeric sites (Silva and Yonenaga-Yassuda 1998b). Differences were also described in Bs of Holochilus brasiliensis, Nectomys rattus Pelzeln, 1883, and Oligoryzomys flavescens (Waterhouse, 1837) (Silva and Yonenaga-Yassuda 2004). Recently, FISH with Holochilus brasiliensis probes of sex chromosomes (X and Y) and both supernumeraries (B1 and B2) were performed, revealing positive signal on sex chromosome of 12 Oryzomyini species and Bs of Holochilus brasiliensis, Nectomys rattus and N. squamipes (Ventura et al. 2015). No signal was observed in Bs of Oligoryzomys flavescens and Sooretamys angouya (G. Fischer, 1814), though, corroborating that supernumeraries in this group may have had independent origins (Ventura et al. 2015).
Karyotype information proved to be important in this tribe, since many species present species-specific karyotypes. For example, species of the genus Oligoryzomys are morphologically very similar but they present different karyotypes: Oryzomys mattogrossae (J. A. Allen, 1916) (2n = 62, FN = 64), Oryzomys microtis (J. A. Allen, 1916) (2n = 64, FN = 64,66), Oryzomys moojeni Weksler & Bonvicino, 2005 (2n = 70, FN = 72, 74, 76), Oryzomys nigripes (2n = 62, FN = 80-82), Oryzomys stramineus Bonvicino & Weksler, 1998 (2n = 52, FN = 68-70), Oryzomys utiaritensis J. A. Allen, 1916 (2n = 72, FN = 76) (Almeida and Yonenaga-Yassuda 1991, Bonvicino and Weksler 1998, Andrades-Miranda et al. 2001a, Agrellos et al. 2012, Di-Nizo 2013).
Chromosome data also show evidence that distinctive karyotypes are being attributed to the same name, for instance Euryoryzomys macconnelli (Thomas, 1910), E. lamia (Thomas, 1901), Hylaeamys yunganus (Thomas, 1902), Oecomys cleberi Locks, 1981, Oecomys paricola (Thomas, 1904), Oecomys roberti (Thomas, 1904) and Zygodontomys brevicauda (Andrades-Miranda et al. 2000, Patton et al. 2000, Suárez-Villota et al. 2017).
Additionally, some species could not be identified by chromosome data alone, because they share the same karyotype. This is the case of Cerradomys marinhus (Bonvicino, 2003) and Pseudoryzomys simplex (Winge, 1887) (2n = 56, FN = 54 - except for the morphology of the Y); Euryoryzomys emmonsae (Musser et al., 1998), E. russatus and E. nitidus (Thomas, 1884) (2n = 80, FN = 86); Hylaeamys laticeps (Lund, 1840) and H. seuanezi (Weksler et al., 1999) (2n = 48, FN = 60); H. oniscus (Thomas, 1904) and H. perenensis (J. A. Allen, 1901) (2n = 52, FN = 62); Neacomys dubosti Voss et al., 2001 and N. amoenus (2n = 64, FN = 68); Oecomys bahiensis (Hershkovitz, 1960), Oecomys catherinae, and Oecomys concolor (Wagner, 1845), Oecomys sp. 2 and sp. 3 (2n = 60, FN = 62); Drymoreomys albimaculatus Percequillo, Weksler & Costa, 2011 and Oecomys sp. 4 (2n = 62, FN = 62 - although ITS was observed in Drymoreomys but not in Oecomys – see Suárez-Villota et al. 2013 and Malcher et al. 2017); and Holochilus brasiliensis and Nectomys squamipes (standard karyotypes: 2n = 56, FN = 56). Also, although not distributed in Brazil, Oligoryzomys brendae Massoia, 1998 is found sympatric to Oryzomys chacoensis (Myers & Carleton, 1981) in Argentina and both possess 2n = 58, FN = 74.
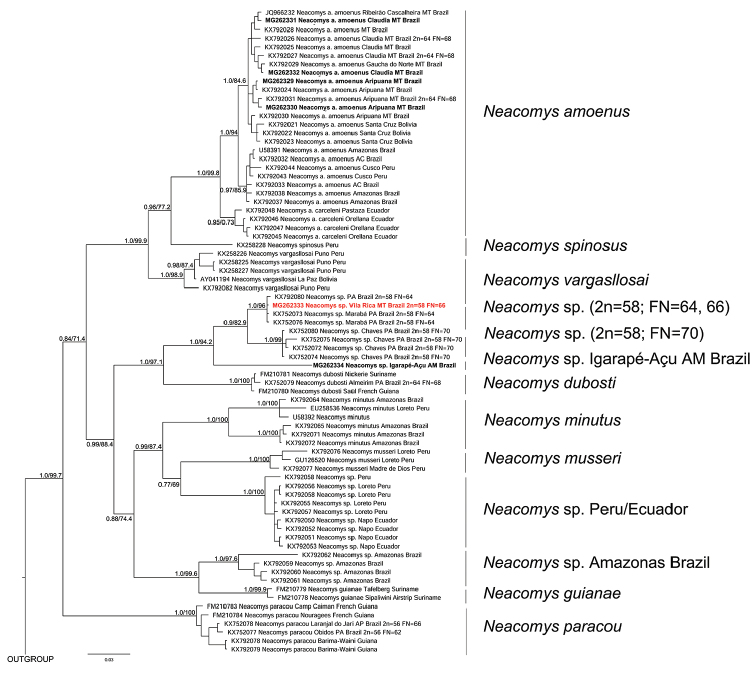
Just as in all hierarchical levels of rodents’ taxonomy, cytogenetic diversity is underestimated in this tribe. For instance, recently, Silva et al. (2015) described two new cytotypes for Neacomys: 2n = 58, FN = 64, from samples collected in Marabá, and 2n = 58, FN = 70, from samples collected in Chaves, Marajó Island, localities from Pará State. According to the authors, both cytotypes differed in the number of biarmed pairs due to amplification/deletion of constitutive heterochromatin in the short arms of pairs 24, 26, and 27 (from Marajó Island) and pericentric inversion involving pairs 28 (metacentric) and 24 (acrocentric) from Marajó Island and Marabá, respectively. These karyotypes could not be assigned to any species described so far, and molecular phylogeny of these samples corroborates the cytogenetic data that it might be a new species (Silva et al. 2015).
Herein, we describe the same diploid (2n = 58), but with a different fundamental number (66) to Neacomys collected in Vila Rica, Mato Grosso State (approximately 700 km from those samples described by Silva et al. 2015). The karyotype comprises 23 acrocentric pairs decreasing in size (pair 1 is the largest of the complement), and five small biarmed pairs. The X chromosome is a large submetacentric, and the Y is a small submetacentric (Fig. 1a). The C-banding pattern shows constitutive heterochromatin at the pericentromeric regions of all autosomes, and in the short arm of both X and Y (Fig. 1b).
Figure 1.
Karyotype of a male of Neacomys 2n=58, FN=66, from Vila Rica, Mato Grosso State, Brazil. a Giemsa-staining b C-banding.
For phylogenetic analyses, the best model selected for the mitochondrial gene (cyt-b) was GTR+I+G. Our molecular phylogeny suggests that this specimen with 2n = 58, FN = 66, from Vila Rica may be an undescribed species that belongs to the same one reported by Silva et al. (2015) with 2n = 58, FN = 64, but with a new fundamental number, probably due to pericentric inversions (Fig. 2). Two structured clades of Neacomys with 2n = 58 were recovered: one with samples with FN = 70, and the other with FN = 64 and 66. Additionally, a sample from Igarapé-Açu (MTR12842), Rio Abacaxis (Amazonas, Brazil) was recovered as the sister group of these two clades. Although the phylogenetic reconstruction lacks N. tenuipes Thomas, 1900 (because the unique sequence available in GenBank has only 177pb), it is unlikely that samples with 2n = 58 belong to N. tenuipes once this species is distributed in Colombia and Venezuela and did not nest in the clade of N. tenuipes of the molecular phylogeny presented by Silva et al. (2015). In addition, our phylogenetic reconstruction recovered Neacomys as monophyletic with high support values (1PP/ 99ML). ML and IB analyses recovered the same topology.
Figure 2.
Bayesian phylogenetic hypothesis of Neacomys based on cyt-b. Numbers in the nodes indicate BI posterior probability (PP) and bootstrap support (ML), respectively. Individual from Vila Rica, Mato Grosso State with 2n=58, FN=66, is highlighted in red and the other samples analysed in this work are in bold.
Tribe Phyllotini
In Brazil, this tribe was initially composed only of the genus Calomys Waterhouse, 1837. However, due to sampling efforts, a new genus was recently added, Calassomys Pardiñas, Lessa, Salazar-Bravo & Câmara, 2014. The diploid number varied from 2n = 36 in Calomys cerqueirai to 2n = 66 in Calomys tener and Calomys expulsus, although the latter presents two different diploid numbers and karyotypes associated to its name, therefore highlighting the need for further investigation (Bonvicino and Almeida 2000, Mattevi et al. 2005). Cytogenetic information is available for all the representatives, and it is an important tool for the recognition of species (cytotaxonomy). One species presents centric fusion (Calomys cerqueirai) (Colombi and Fagundes 2014).
Tribe Reithrodontini
In Brazil, the only representative of this tribe is Reithrodon typicus Waterhouse, 1837. This species possesses a low diploid number (2n = 28) and occurs on the border of Uruguay (Freitas et al. 1983, Pardiñas et al. 2015c) (Table 1).
Tribe Sigmodontini
Only one species of this tribe can be found in Brazil, Sigmodon alstoni (Thomas, 1881). Voss (1992) karyotyped 11 specimens from three localities at Venezuela with 2n = 78, 80 and 82, but the picture of the karyotypes and the fundamental numbers were not reported. Also, the author suggested that Robertsonian rearrangement is a plausible explanation for the variation observed. There have been no Brazilian representatives of this species karyotyped so far.
Tribe Thomasomyini
This tribe is represented by only two genera in Brazil: Rhipidomys Tschudi, 1845 and Rhagomys Thomas, 1886. The diploid number varied from 2n = 36 in Rhagomys rufescens (Thomas, 1886) to 2n = 50 in Rhipidomys nitela Thomas, 1901. Apart from R. nitela, which possesses 2n = 48 (samples from Roraima State) or 50 (samples from Manaus, Amazonia State), in general, the karyotype is not informative for Rhipidomys, since nine species present the same diploid number (2n = 44), and two species lack karyotype data (Silva and Yonenaga-Yassuda 1999, Tribe 2005). In fact, Tribe (2015) provisionally inserted the 2n = 50 samples in R. nitela but reiterated that they need taxonomic revision. Pericentric inversion, found in six species, plays an important role in the genus, and this is reflected in the variation of the fundamental number. Two species lack cytogenetic data: Rhipidomys ipukensis R. G. Rocha, Costa & Costa, 2011 and R. wetzeli A. L. Gardner, 1990.
Tribe Wiedomyini
This tribe is composed of two species: Wiedomys pyrrhorhinos (Wied- Neuwied, 1821) and W. cerradensis P. R. Gonçalves, Almeida & Bonvicino, 2005. Both occur in Brazil with disjunctive distribution (W. pyrrhorhinos at Caatinga, and W. cerradensis at Cerrado) and possess different karyotypes (2n = 62 and 60, respectively) (Maia and Langguth 1987, Gonçalves et al. 2005). Recent molecular studies indicate that W. pyrrhorhinos, may represent a species complex with Rio São Francisco acting as a barrier to the populations from both river banks (Di-Nizo in prep.). Pericentric inversions have also been described for this species.
Incertae sedis
This group comprises the genera Abrawayaomys F. Cunha & Cruz, 1979, Delomys Thomas, 1917, Juliomys E. M. González, 2000, Phaenomys Thomas, 1917, and Wilfredomys Avila-Pires, 1960, which could not be inserted into any other tribes, according to phylogenetic and morphological analyses (Musser and Carleton 2005, Patton et al. 2015). Cytogenetic information is available for all species, except one, Wilfredomys oenax (Thomas, 1928), and is helpful for distinguishing species of the genus Delomys and Juliomys.
Family Ctenomyidae
This family comprises a single genus, Ctenomys, which presents a great variation in diploid numbers, especially C. lami T. R. O. Freitas, 2001, C. minutus Nehring, 1887 and C. torquatus Lichtenstein, 1830 for which Robertsonian rearrangements and in tandem fusions were described (Freitas and Lessa 1984, Fernandes et al. 2009). The diploid number varied from 36 in Ctenomys nattereri Wagner, 1848 to 58 in C. lami. Only one species out of eight lacks karyotype information. Cytogenetic data was useful for recognizing Ctenomys bicolor Miranda- Ribeiro, 1914, C. ibicuiensis T. R. O. Freitas, Fernandes, Fornel & Roratto, 2012 and C. nattereri, because it presents exclusive karyotype (Stoulz 2012). Pericentric inversion has been described for C. lami and in tandem fusions for C. minutus.
Family Cuniculidae
This family is represented by a single species, Cuniculus paca (Linnaeus, 1766), with a wide distribution and unique karyotype (2n = 74, FN = 98) (Giannoni et al. 1991, Bonvicino et al. 2008).
Family Dasyproctidae
This family comprises two genera: Dasyprocta Illiger, 1811, with nine species, and Myoprocta Thomas, 1903, with two species (Patton and Emmons 2015). There is no cytogenetic data known for three species (Table 1). The diploid number in the Family varied from 62 to 65, and in the genus Dasyprocta, from 64 to 65, due to the presence of B chromosomes in four species (Ramos et al. 2003).
Family Dinomyidae
This family possesses only one species, Dinomys branickii Peters, 1873, to which the karyotype is 2n = 64, FN = 98 (Table 1).
Family Echimyidae
Even being the second largest Brazilian rodent family, a remarkable gap regarding cytogenetic data of this family still remains, with 14 species out of 68 lacking such information. This represents about 37% of all the unknown karyotypic information of all Brazilian rodents.
Diploid numbers varied from 2n = 15 in Proechimys goeldii Thomas, 1905 to 118 in Dactylomys boliviensis. B chromosomes have been described for one species: Trinomys iheringi (Thomas, 1911) (Yonenaga-Yassuda et al. 1985), pericentric inversion for seven species, and Robertsonian rearrangement for three. A multiple sex chromosome system was described for Proechimys cf. longicaudatus (Amaral et al. 2013), and addition/deletion of constitutive heterochromatin was described for Clyomys laticeps (Thomas, 1909) and P. longicaudatus (Rengger, 1830) (Souza and Yonenaga-Yassuda 1984, Bezerra et al. 2012, Machado et al. 2005). Secondary constriction is a characteristic feature of several species, occurring in Carterodon sulcidens (this work), Clyomys laticeps, Mesomys occultus Patton, da Silva & Malcolm, 2000, Makalata didelphoides (Desmarest, 1817), Proechimys gardneri M. N. F. da Silva, 1998, all five Thrichomys E.- L. Trouessart, 1880 species, and seven species of Trinomys Thomas, 1921.
Within this family, there are also cases in which different diploid numbers are assigned to the same name. In the case of Clyomys laticeps, the 2n = 34, FN = 58, 60, 62 and 2n = 32, FN = 54, the karyotypes are very similar, and differ by a Robertsonian rearrangement and pericentric inversion (2n = 32). Also, species such as Phyllomys pattoni Emmons, Leite, Kock & Costa, 2002 and Proechimys guyannensis E. Geoffroy, 1803 should be investigated by molecular phylogeny and morphology, because they are prone to either represent species-complex or have taxonomic misidentification.
In this work, the karyotype of Carterodon sulcidens is being described for the first time, showing 2n = 66. Since the animal was a female, it was not possible to recognize the X chromosomes and the exact morphology of the small pair, so we could not establish the fundamental number. Karyotype is composed of 32 acrocentric pairs decreasing in size and presumably one biarmed pair (pair 33). Also, the fourth largest pair possesses a remarkable secondary constriction (Fig. 3a). Constitutive heterochromatin is located in the pericentromeric region of all autosomes (Fig. 3b). Ag-NOR showed signals in the secondary constriction of pair 4 (Fig. 3b inset).
Figure 3.
Karyotype of a female of Carterodon sulcidens with 2n=66 from Serra da Mesa, Goiás State, Brazil. a Giemsa-staining. Inset: Pair 4 with evident secondary constriction b C-banding. Inset: Pair 4 after silver nitrate staining.
Within the Echimyidae Family, the only other species with 2n = 66 described so far is Makalata didelphoides, but its karyotype presents 20 pairs of metacentric chromosomes, which clearly differs from the karyotype of Carterodon sulcidens.
Family Erethizontidae
Three out of eight species lack cytogenetic information. The diploid number varied from 42 in Coendou spinosus (F. Cuvier, 1823) to 74 in C. prehensilis (Linnaeus, 1758) (Lima 1994, Mendes-Pontes et al. 2013) (Table 1).
Family Muridae
This family (represented by the genera Mus and Rattus) was introduced from Europe, and even though it is not a native, it is currently widespread throughout Brazil (Musser and Carleton 2005).
Little is known about the cytogenetics of the Mus musculus Brazilian populations because this species seems to be negglected. The present paper features the first picture of Mus musculus karyotype from Brazil. This species presented 2n = 40, FN = 38, with all chromosomes acrocentrics. C-banding was restricted to the centromeric region of all chromosomes (Fig. 4). Sex chromosomes could only be recognized after G-banding (not showed) because they have similar morphology compared to the autosomes.
Figure 4.
Karyotype after C-banding of a male of Mus musculus with 2n=40, FN=38, from Guará, São Paulo State, Brazil.
For the black rat Rattus rattus Linnaeus, 1758, diploid number of South America population is the same as those from Oceania (2n = 38), and Kasahara and Yonenaga-Yassuda (1981) described pericentric inversion for individuals from São Paulo, Brazil.
Family Sciuridae
Cytogenetic data is unknown for almost the entire family. For the two species to which chromosome information is known, diploid number is 2n = 40, and pericentric inversion has been described for one of them, Guerlinguetus brasiliensis (Gmelin, 1788) (Lima and Langguth 2002, Fagundes et al. 2003) (Table 1).
Discussion
Advances since the last revision
The last cytogenetic revision on Brazilian rodents, published in 1984, described the karyotype of 62 species, mainly from South and Southeast Brazil (Kasahara and Yonenaga-Yassuda 1984). This paper compiles the karyotype of 271 species distributed throughout Brazil, representing an increase of more than 300%.
Since then, new cytotypes have been attributed to already known species. For instance, new diploid numbers were described for Ctenomys torquatus and new fundamental numbers for Oligoryzomys nigripes (described as Oryzomys nigripes – see references in Table 1). B chromosomes were described for Sooretamys angouya and also for four species of Dasyprocta. Undescribed rearrangements, including multiple sex chromosome system, were also detected (see Table 1). Moreover, new karyotypes that could not be correlated to any name were published, evidencing the possibility that an undescribed species may exist (e.g.: Akodon sp. n., Deltamys sp., Thaptomys sp., Euryoryzomys sp., Neacomys sp., Oecomys sp. 1 – 4, Oligoryzomys sp., Juliomys sp., Dasyprocta sp. Proechimys sp. – see Table 1). Additionally (as we will mention below) there are many species with a different diploid number associated that do not represent polymorphisms, which need to be revised (e.g. Euryoryzomys lamia, Euryoryzomys macconnelli, Hylaeamys yunganus, Oecomys auyantepui, Oecomys cleberi, Oecomys paricola, Oecomys roberti, Zygodontomys brevicauda, Rhipidomys nitela, Phyllomys pattoni, Proechimys guyannensis, etc.).
Since 1984, many species’ names have been redescribed or validated (e.g. Zygodontomys lasiurus was named as Bolomys lasiurus for a long time, and nowadays is Necromys lasiurus – see synonyms of Table 1). Also, due to the progress of molecular biology during the 1990, associated to morphological information, the number of species described has increased exponentially. It is important to emphasize that molecular phylogeny hitherto has contributed to better understand the cryptic diversity of Brazilian rodents, recognizing monophyletic clades. For instance, new candidate species of Akodon (Silva and Yonenaga-Yassuda 1998a, Silva et al. 2006), Oecomys (Suárez-Villota et al. under revision), Oligoryzomys (Andrades-Miranda et al. 2001a, Miranda et al. 2008), Neacomys (Silva et al. 2015, present paper), Thaptomys (Ventura et al. 2004, 2010), etc. were recognized based on new karyotypes associated to the monophyly of the samples. Even new genera were described based on multidisciplinary approaches: Drymoreomys (Percequillo et al. 2011) and Calassomys (Pardiñas et al. 2014).
Technological advances with fluorescent in situ hybridization (developed at the end of 1980’s but more used during 2000’s to date), made it possible to characterize chromosome rearrangements more precisely.
In this paper, we provide a new fundamental number for an undescribed species of Neacomys. The karyotype presented here (FN = 66) is similar to the one described by Silva et al. (2015) with FN = 64, except that we found five biarmed pairs and the distribution of constitutive heterochromatin in autosomes was restricted to pericentric regions. We suggest that differences in fundamental numbers are due to pericentric inversions in a small pair, since C-banding evidenced constitutive heterochromatin at the pericentromeric regions, and the morphology of chromosomes was accurately defined. Sex chromosomes presented the same morphology, although the Y was heterochromatic in the short arm (present paper), while it was entirely heterochromatic in the samples described by Silva et al. (2015).
Karyotype information was the first to point out that this specimen may represent a new species, since 2n = 58, FN = 66, has never been described for any Neacomys species. Although we used only one molecular marker (incomplete cyt-b), which was the same used by Silva et al. (2015), the phylogeny corroborates this information, since all samples with 2n = 58 clustered in a monophyletic high supported the clade. This included two well-supported structured clades, one with samples with FN = 70 (Chaves, Marajó Island) and the other with samples with FN = 64 and 66 (Marabá, Pará State and Vila Rica, Mato Grosso State, respectively), both sister clade to the sample from Igarapé-Açu, Amazonas State. Whether these samples belong to the same undescribed entity with strong population structure or whether they represent at least three different species must be clarified with further phylogeographic and morphological studies, including samples from other localities. This shows the importance of integrative approaches.
In fact, Neacomys have a greater diversity than previously known. Recently, based on morphology and molecular phylogeny, Hurtado and Pacheco (2017) demonstrated that Neacomys spinosus is a species complex and considered the subespecies Neacomys spinosus amoenus a valid species. After this revision, Neacomys spinosus is restricted to populations from Peruvian Amazon, and Neacomys amoenus encompasses two subspecies: Neacomys a. amoenus (from Brazilian Cerrado and Bolivia) and Neacomys a. carceleni (from Amazon basin of Ecuador, Brazil and Peru). Thus, sequences related to N. spinosus from central Brazil, and transition areas of Cerrado and Amazonia correspond to N. amoenus. Also, a new species, N. vargasllosai, from southern Peru and Bolivia was described. In this same revision, authors recovered three new species pending formal description (the first from Pará, Brazil, the second from Amazonas, Brazil, and the third from Peru and Ecuador). The one from Pará corresponds to the clade composed of samples with 2n = 58 (Fig. 2), reiteraiting the lack of knowledge in this genus.
The description of the karyotype of Carterodon sulcidens (a rare species) also corroborates the lack of knowledge for some species, and the importance of fieldwork in discovering new data.
We also show the picture of the karyotype of the exotic species Mus musculus for the first time. Despite the noteworthy variation in diploid numbers in Western Europe and Mediterranean populations because of Robertsonian rearrangements (Nachman et al. 1994), in Brazil, the only diploid number described was the standard one (2n = 40).
Progress in cytogenetics: the molecular era
During the beginning of the 1970s (although banding techniques had already been described), karyotypes of Brazilian rodents were studied mainly through conventional staining and the description was limited to diploid and fundamental numbers. Even so, the idea of a wide chromosomal variability already existed. From the 1980s until now, comparative cytogenetics with chromosome banding persists and contributed for elucidating these variations, being that G and C-banding and Ag-NORs are the commonest and cheapest banding techniques.
In fact, the distribution of constitutive heterochromatin and Ag-NORs can be markers in some species. For example, large blocks of constitutive heterochromatin were detected in Clyomys laticeps (family Echimyidae) (Souza and Yonenaga-Yassuda 1984, Bezerra et al. 2012) and a huge heterochromatic arm in Pseudoryzomys simplex (family Cricetidae, subfamily Sigmodontinae, tribe Oryzomyini) (Moreira et al. 2013). C-band pattern is also an important technique for recognizing sex chromosomes, especially within the subfamily Sigmodontinae (Silva 1994, Di-Nizo 2013). Regarding the nucleolus organizer region, it seems that secondary constriction is a characteristic feature of the family Echimyidae and, as with other vertebrates, may be an important marker. However, chromosomal comparison is now passing from banding patterns to the use of higher resolution innovation of molecular cytogenetics using FISH.
FISH using chromosome painting allows a comparison in a wide genomic scale, revealing a greater number of chromosome changes, unrevealed by the commonest banding techniques, especially in the tribes Akodontini and Oryzomyini of the Subfamily Sigmodontinae. For instance, G-banding pattern showed several rearrangements between Akodon species (Tribe Akodontini) (Geise et al. 1998, Silva et al. 2006), but much more complex rearrangements within this genus were observed after cross-species chromosome painting (Ventura et al. 2009).
Extensive chromosomal rearrangements such as Robertsonian, in tandem fusion/fission and pericentric inversion, were also observed within the genus Oligoryzomys (Tribe Oryzomyini), after chromosome painting. Using a molecular phylogeny as a reference, it was also possible to detect the direction of the rearrangements and to infer that fission events were as common as fusion events (Di-Nizo et al. 2015). Moreover, Robertsonian rearrangement between Oryzomys rupestris Weksler & Bonvicino, 2005 (referred as Oligoryzomys sp. 1), 2n = 46, FN = 52, and Oligoryzomys sp. 2, 2n = 46, FN = 52 was firstly detected by using classic cytogenetic and FISH with telomeric probes (Silva and Yonenaga-Yassuda 1997) and later corroborated by chromosome painting (Di-Nizo et al. 2015). However further studies with molecular phylogeny and morphology are necessary to clarify if both entities represent a single species (with a polymorphism spread in the population) or two different species (in the case of this rearrangement resulted in reproductive incompatibilities leading to the speciation of ancestral population).
The advent of chromosome painting made it possible to compare not only related species but also distant ones, something which is difficult to achieve with banding patterns. Hass et al. (2008) compared Mus musculus (family Muridae) to Akodon species (family Cricetidae); Nagamachi et al. (2013) compared two different, unrelated genera of the tribe Oryzomiyni (Cerradomys and Hylaeamys) and Suárez et al. (2015) and Pereira et al. (2016) compared homologies between the tribes Akodontini and Oryzomyini.
Despite the ‘modern cytogenetics era’, chromosome banding is still an important tool for animal cytogenetic studies, not only because FISH cannot reveal chromosome inversions, but also because it is still a difficult and expensive technique to use.
Chromosome rearrangements and speciation
Rodents proved to be a good model for chromosome evolution studies. Cytogenetics associated with molecular or morphological phylogenetic reconstruction broke cytogeneticist paradigms that fusion rearrangement is more common than fission, and that the reduction in 2n is the expected pattern (e.g. Di-Nizo et al. 2015).
Chromosomal rearrangement could possibly be the cause of reproductive isolation in many Brazilian rodents’ species, leading to speciation. The main rearrangements that lead to species formation are Robertsonian, in tandem fusion/fission and pericentric inversion, while the variability in constitutive heterochromatin does not seem to create a reproductive barrier and consequent speciation (King 1993, Romanenko and Voloboeuv 2012).
For a long time, it was thought that chromosomal structural rearrangements promoted speciation by generating gametes with duplications and deficiencies, therefore, causing less adaptability of the heterozygotes, but this model was rejected because it lacked theoretical support (Rieseberg 2001, Patton 2004, Jackson 2011). Recently, a different model of chromosome speciation was proposed in which the gene flow is reduced because of recombination-suppression in rearranged regions (Noor et al. 2001, Rieseberg 2001).
In fact, normal meiotic behavior with suppression of crossing over in inverted segments of heteromorphic chromosomes caused by pericentric inversions of Akodon cursor and Oligoryzomys nigripes was observed, with non-selective disadvantages in heterozygous carries (Fagundes et al. 1998, Bonvicino et al. 2001a). Some genetic mechanisms seem to be responsible for overcoming meiotic errors in heterozygous individuals, such as the occurrence of heterosynapsis and the low frequency of chiasm between the inverted segments.
A remarkable chromosome variation can be found in the semi- and fossorial Brazilian rodents Blarinomys breviceps (in which molecular phylogeny demonstrated two structured clades – see Ventura et al. 2012), Clyomys laticeps and Ctenomys minutus. Their species status, and whether their chromosome variation is adaptative and correlated with ecological patterns should be evaluated.
For example, a very well-known case of chromosome speciation due to population adaptation to climatic stress and ecological unpredictability was described in the subterranean rodent Spalax ehrenbergi (Family Spalacidae) found in Israel, in which diploid numbers increase coincidently with geographic regions of high aridity (Wahrman et al. 1969). The weak dispersion pattern of this fossorial rodent may have contributed to the fixation of adaptative chromosome change (Árnason 1972).
Cytotaxonomy
Cytotaxonomy is the use of chromosome data as the first clue in the identification of species. Since many Brazilian rodent species present species-specific karyotype and show morphological similarities, chromosome information showed to be useful in the diagnosis of species.
The present revision showed that the delimitation of species based on chromosome data (cytotaxonomy) is essential for recognizing some species of the genera Akodon, Calomys, Cerradomys, Euryoryzomys, Delomys, Hylaeamys, Juliomys, Neacomys, Oecomys, Oligoryzomys (family Cricetidae, subfamily Sigmodontinae), Ctenomys (family Ctenomyidae), and Thrichomys and Trinomys (family Echimyidae).
On the other hand, since rates of karyotype evolution differ in distinct branches of the rodents’ phylogeny, some species present identical diploid and fundamental numbers, and they cannot be identified solely through chromosome data. This is the case of the following species: (i) Cavia aperea, Cavia fulgida and Cavia magna; (ii) Kerodon acrobata and Kerodon rupestris (Family Caviidae); (iii) Akodon lindberghi and A. mystax; (iv) Akodon paranaensis and A. reigi; (v) Brucepattersonius griserufescens, B. iheringi, B. soricinus and Thaptomys nigrita; (vi) Oxymycterus caparoae, Oxymycterus dasytrichus, Oxymycterus nasutus and Oxymycterus roberti (the other four species of Oxymycterus also have the same diploid number but lacks information on FN) (Family Cricetidae, Subfamily Sigmodontinae, Tribe Akodontini); (vii) Cerradomys marinhus and Pseudoryzomys simplex; (viii) Drymoreomys albimaculatus and Oecomys sp. 4; (vix) Euryoryzomys emmonsae, E. nitidus and E. russatus (despite E. nitidus and E. russatus have disjunction distribution); (x) Holochilus brasiliensis and Nectomys squamipes; (xi) Hylaeamys laticeps and Hylaeamys seuanezi; (xii) Hylaeamys oniscus and H. perenensis; (xiii) Oecomys bahiensis, Oecomys concolor, Oecomys sp. 2 and sp. 3; (xiv) Neacomys dubosti and N. amoenus (family Cricetidae, Subfamily Sigmodontinae, tribe Oryzomyini); (xv) Rhipidomys cariri, R. gardneri, R. tribei, R. itoan and R. macconnelli (family Cricetidae, Subfamily Sigmodontinae, Tribe Thomasomyini); (xvi) Dasyprocta azarae, D. iacki, D. fuliginosa, D. leporina, D. prymnolopha, D. variegata and Dasyprocta sp. (family Dasyproctidae); (xvii) Isothrix bistriata, Mesomys hispidus, M. stimulax, Trinomys albispinus and T. dimidiatus; (xviii) Proechimys brevicauda and Proechimys cuvieri; (xix) Proechimys gardneri and Proechimys pattoni (family Echimyidae) and (xx) Guerlinguetus brasiliensis and Hadrosciurus spadiceus (family Sciuridae) (Table 1).
Furthermore, some unrelated species, that belong to different tribes, or even families, present the same diploid and fundamental number, suggesting a homoplastic character: (i) Hylaeamys megacephalus and Oxymycterus delator; (ii) Juliomys pictipes and Thalpomys cerradensis; (iii) Calomys laucha and Neacomys amoenus (although there are differences in the size of the biarmed chromosomes); (iv) Oecomys franciscorum and Delomys sublineatus (despite the first acrocentric pair in D. sublineatus is bigger than in Oryzomys franciscorum as well as the biarmed pair in the last species); (v) Coendou melanurus and Oligoryzomys utiaritensis; (vi) Ctenomys ibicuiensis and Scolomys ucayalensis and (vii) Callistomys pictus, Coendou spinosus and Myocastor coypus.
Interdisciplinarity
Since the beginning of the cytogenetic studies in Brazilian rodents, there have been cases in which different karyotypes were assigned to one species or the same karyotype was referred to in different species. In fact, many of these cases were solved after the integration of different disciplines. For instance, for many years cytogenetic information indicated that the previous “Oryzomys subflavus” could, in fact, be more than one species, since nine different karyotypes were attributed to a single taxonomic entity (Maia and Hulak 1981, Almeida and Yonenaga-Yassuda 1985, Svartman and Almeida 1992, Silva 1994). Nowadays, after interdisciplinary studies with morphology and molecular phylogeny, it is possible to recognize eight species (Weksler et al. 2006, Percequillo et al. 2008, Tavares et al. 2011, Bonvicino et al. 2014). Moreover, for a long time Nectomys was represented by only one species in Brazil, with two diploid numbers (2n = 52 + 1 to 3 Bs and 2n = 56 + 1 to 3 Bs). Nevertheless analyses of the spermatogenesis in hybrids and the sterility of crosses between both cytotypes indicated that Nectomys should be considered two distinct species: Nectomys rattus (2n = 52) and Nectomys squamipes (2n = 56) (Bonvicino et al. 1996).
The opposite occurred in the genus Oligoryzomys since the same karyotype (2n = 62, FN = 80-82) was attributed to different names (Oryzomys nigripes, Oryzomys delticola, and Oryzomys eliurus). After molecular and morphology integration, Oryzomys delticola and Oryzomys eliurus were considered as a junior synonym of Oryzomys nigripes (Bonvicino and Weksler 1998).
Some of these cases persist until today, for instance, more than one karyotype was described for Euryoryzomys macconnelli and E. lamia (Table 1). Molecular phylogeny and morphology corroborate the species complex status of both entities (Almeida 2014, Percequillo 2015a). Similarly, Oecomys roberti, Oryzomys paricola, and Oryzomys catherinae are probably species complexes, not only because of their variability in diploid number, but also because of phylogenetic reconstruction and morphological studies (Suárez-Villota et al. 2017). Ctenomys minutus, C. torquatus, Hylaeamys yunganus, Rhipidomys nitela, Sigmodon alstoni and Zygodontomys brevicauda also deserve taxonomic attention because they may represent cases in which different diploid numbers are attributed to the same names. Similarly, Blarinomys breviceps has a variable diploid number and two geographic structured clades were recovered in the molecular phylogeny (Ventura et al. 2012), indicating that a morphological revision is needed.
Remarkably, such examples can also be found in the family Echimyidae. The need to use different approaches for taxonomic revision is clear in order to investigate whether Phyllomys blainvillii, Phyllomys pattoni, and Proechimys guyannensis represent species complexes, given the fact that they have more than one karyotype associated.
Interdisciplinary approaches, including cytogenetic, molecular phylogeny, morphology and geographic distribution are essential for accessing the limits of Brazilian rodents’ species. One of the best-known examples was the old genera Oryzomys, considered the most complex and composing almost half of the species of the tribe Oryzomyini (Musser and Carleton 1993). The current genera Melanomys, Microryzomys, Nesoryzomys, Oecomys, and Oligoryzomys, were first considered a subgenus of Oryzomys and later elevated to the category of genus after morphology, chromosomal and molecular analyses (Myers et al. 1995, Smith and Patton 1999, Bonvicino and Moreira 2001). Another outstanding example of an integrative approach was the study in which ten new genera were described for species that were previously referred to as Oryzomys (Weksler et al. 2006), corroborating the cryptic diversity in Oryzomyini previously indicated by cytogenetic data.
Within the Family Echimyidae, the association of morphology and molecular analysis was essential for elevating Trinomys (considered subgenus of Proechimys) to the genus category (Lara et al. 1996, Leite and Patton 2002).
Perspectives
Despite the new technological approaches, chromosome characterization with conventional staining and banding pattern is still important, mainly because 38 species lack any karyotype information (Table 1). From this amount, 16 are distributed in the Amazonian biome, evidencing the lack of knowledge for this region. The fieldwork is very important and must be encouraged not only because new species and even genera are constantly being described but also because cytogenetic and distribution information of several species are poorly known.
Concerning the family Echimyidae, it is noteworthy that cytogenetic information is lacking for more than 20% of its species. Eleven out of 17 echimyid genera which occur in Brazil are arboreal (Galewski et al. 2005, Emmons et al. 2015). The issues for sampling small arboreal mammals and the consequent low number of studies with this approach have already been highlighted in the literature (Malcolm 1991, Taylor and Lowman 1996, Graipel et al. 2003). In this sense, it can be inferred that this deficiency in echimyid cytogenetic knowledge may be related to sampling scarcity.
The future of molecular biology is promising, with next-generation sequencing (NGS) technology and mitogenomics hopefully providing more robust phylogenetic studies. This new approach was performed with the Family Echymyidae, revealing new supported nodes and clarifying some aspects of the group’s taxonomy (Fabre et al. 2016).
However, it is important to reiterate the heterogeneity of characters since DNA, chromosomes, morphology, and behavior are not evolving at the same rate. This particularity may imply in different taxonomic interpretations, with a population being identified as a unique species by one character and two or more species by another, especially in the cases of recent or ongoing speciation. The consequences can be taxonomic inflation or underestimation of the biodiversity, and that is why interdisciplinary approaches are crucial to better understand the biological diversity of rodents.
Acknowledgments
The authors would like to thank Dr. Ana Paula Carmignotto, Pedro Luís B. da Rocha, Leonora P. Costa, and Miguel T. Rodrigues for collecting samples and donating tissues, and Yatiyo Yonenaga-Yassuda for infrastructure and for reading the first version of the manuscript. CAPES and FAPESP (2014/02885-2 for MJJS) supported this work.
Citation
Di-Nizo CB, Banci KRS, Sato-Kuwabara Y, Silva MJJ (2017) Advances in cytogenetics of Brazilian rodents: cytotaxonomy, chromosome evolution and new karyotypic data. Comparative Cytogenetics 11(4): 833–892. https://doi.org/10.3897/CompCytogen.v11i4.19925
Supplementary materials
Table S1
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Camilla Bruno Di-Nizo, Karina Rodrigues da Silva Banci, Yukie Sato Kuwabara, Maria José de J. Silva
Data type: molecular data
Explanation note: Sequences analysed for phylogenetic reconstruction (Maximum likelihood and Bayesian Inference) of Neacomys, with species, GenBank and lab/ field number, diploid and fundamental number (when available), locality and reference.
Abbreviations: N/A means that the information is not available. *Cytogenetic data analysed in this work. In bold, sequences obtained in this work. Coordinates for Neacomys specimens studied herein: Amazonas State: Igarapé-Açu (04°20'S, 58°38'W); Mato Grosso State: Aripuanã (10°10'S, 59°27"W); Cláudia (11°30'S, 54°53'W); Vila Rica (09°54'S, 51°12'W). Museum and collector acronyms for specimens studied herein: APC (Ana Paula Carmignotto), CIT (Laboratório de Citogenética de Vertebrados - IBUSP), MTR (Miguel Trefaut Rodrigues), MZUSP (Museu de Zoologia, Universidade de São Paulo, Brazil) and PEU (Pedro Luís Bernardo da Rocha).
References
- Abreu MS, Christoff AU, Valiati VH, de Oliveira LR. (2014) New distribution records of Serra do Mar Grass Mouse Akodon serrensis Thomas, 1902 (Mammalia: Rodentia: Sigmodontinae) in the southernmost Brazil. Check List 10(3): 655–659. http://dx.doi.org/10.15560/10.3.655 [Google Scholar]
- Aguilera M, Pérez-Zapata MA, Martino A, Barros MA, Patton JL. (1994) Karyosystematics of Aepeomys and Rhipidomys (Rodentia: Cricetidae). Acta Cientifica Venezolana 48: 247–48. [Google Scholar]
- Agrellos R, Bonvicino CR, Rosa EST, Marques AAR, D’Andrea PS, Weksler M. (2012) The taxonomic status of the Castelo dos Sonhos Hantavirus reservoir, Oligoryzomys utiaritensis Allen 1916 (Rodentia: Cricetidae: Sigmodontinae). Zootaxa 3220: 1–28. [Google Scholar]
- Almeida EJC. (1980) Variabilidade citogenética nos gêneros Oryzomys e Thomasomys (Cricetidae, Rodentia). Ph.D. Thesis, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 160 pp. [In Portuguese] [Google Scholar]
- Almeida EJC, Yonenaga-Yassuda Y. (1985) Robertsonian fusion, pericentric inversion and sex chromosome heteromorphisms in Oryzomys subflavus (Cricetidae, Rodentia). Caryologia 38: 129–137. http://dx.doi.org/10.1080/00087114.1985.10797737 [Google Scholar]
- Almeida EJC, Yonenaga-Yassuda Y. (1991) Pericentric inversions and sex chromosome heteromorphisms in Oryzomys nigripes (Rodentia, Cricetidae). Caryologia 44(1): 63–73. https://doi.org/10.1080/00087114.1991.10797020 [Google Scholar]
- Almeida KA. (2014) Filogenia, filogeografia e avaliação do código de barras de DNA em roedores do gênero Euryoryzomys (Sigmodontinae: Oryzomyini). Master’s Dissertation, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 153 pp. [In Portuguese] [Google Scholar]
- Amaral PJ, Nagamachi CY, Noronha RC, Costa MJ, Pereira AL, Rossi RV, Pieczarka JC. (2013) Proechimys (Rodentia, Echimyidae): characterization and taxonomic considerations of a form with a very low diploid number and a multiple sex chromosome system. BMC Genetics 14(1): 21. https://doi.org/10.1186/1471-2156-14-21 [DOI] [PMC free article] [PubMed]
- Anderson S, Yates TL, Cook JA. (1987) Notes on Bolivian mammals 4. The genus Ctenomys (Rodentia, Ctenomyidae) in the eastern lowlands. American Museum Novitates 2891: 1–20. http://hdl.handle.net/2246/5157 [Google Scholar]
- Andrade AFB, Bonvicino CR, Briani DC, Kasahara S. (2004) Karyologic diversification and phylogenetics relationships of the genus Thalpomys (Rodentia, Sigmodontinae). Acta Theriologica 49: 181–90. https://doi.org/10.1007/BF03192519 [Google Scholar]
- Andrades-Miranda J, Nunes AP, Oliveira LBV, Mattevi MS. (1999) The karyotype of the South American rodent Kunsia tormentosus (Lichtenstein, 1830). Cytobios 98: 137–147. [PubMed] [Google Scholar]
- Andrades-Miranda J, Zanchin NIT, Oliveira LFB, Langguth AR, Mattevi MS. (2000) Cytogenetic studies in nine taxa of the genus Oryzomys (Rodentia, Sigmodontinae) from Brazil. Mammalia 65(4): 461–472. https://doi.org/10.1515/mamm.2001.65.4.461 [Google Scholar]
- Andrades-Miranda J, Oliveira LFB, Lima-Rosa AV, Nunes AP, Zanchin NIT, Mattevi MS. (2001a) Chromosome studies of seven species of Oligoryzomys (Rodentia: Sigmodontinae) from Brazil. Journal of Mammalogy 82: 1080–1091. https://doi.org/10.1644/1545-1542(2001)082<1080:CSOSSO>2.0.CO;2 [Google Scholar]
- Andrades-Miranda J, Oliveira LF, Zanchin NI, Mattevi MS. (2001b) Chromosomal description of the rodent genera Oecomys and Nectomys from Brazil. Acta Theriologica 46(3): 269–278. https://doi.org/10.1007/BF03192433 [Google Scholar]
- Andrades-Miranda J, Zanchin NI, Oliveira LF, Langguth AR, Mattevi MS. (2002a) (T2AG3)n Telomeric sequence hybridization indicating centric fusion rearrangements in the karyotype of the rodent Oryzomys subflavus. Genetica 114(1): 11–16. https://doi.org/10.1023/A:1014645731798 [DOI] [PubMed] [Google Scholar]
- Andrades-Miranda J, de Oliveira LF, Lima-Rosa CAV, Sana DA, Nunes AP, Mattevi MS. (2002b) Genetic studies in representatives of genus Rhipidomys (Rodentia, Sigmodontinae) from Brazil. Acta Theriologica 47(2): 125–135. https://doi.org/10.1644/1545-1542(2001)082<1080:CSOSSO>2.0.CO;2 [Google Scholar]
- Aniskin VM. (1993) New karyotypes of prickly chinchillas of the family Echimyidae (Rodentia). Genetika 29(9): 1500–1507. [Google Scholar]
- Aniskin VM, Volobouev VT. (1999) Comparative chromosome banding of two South-American species of rice rats of the genus Oligoryzomys (Rodentia, Sigmodontinae). Chromosome Research 7: 557–562. https://doi.org/10.1023/A:1009245729902 [DOI] [PubMed] [Google Scholar]
- Araújo NP, Loss AC, Cordeiro-Junior DA, da Silva KR, Leite YL, Svartman M. (2014) New karyotypes of Atlantic tree rats, genus Phyllomys (Rodentia: Echimyidae). Genome 57(1): 1–8. https://doi.org/10.1139/gen-2013-0168 [DOI] [PubMed] [Google Scholar]
- Árnason Ú. (1972) The role of chromosomal rearrangement in mammalian speciation with special reference to Cetacea and Pinnipedia. Hereditas 70(1): 113–118. https://doi.org/10.1111/j.1601-5223.1972.tb00999.x [PubMed] [Google Scholar]
- Bazinet AL, Zwickl DJ, Cummings MP. (2014) A gateway for phylogenetic analysis powered by grid computing featuring GARLI 2.0. Systematic Biology 63(5): 812–818. https://doi.org/10.1093/sysbio/syu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra AMR, Pagnozzi J, Carmignotto AP, Yonenaga-Yassuda Y, Rodrigues FHG. (2012) A new karyotype for the spiny rat Clyomys laticeps (Thomas, 1909) (Rodentia, Echimyidae) from Central Brazil. Comparative Cytogenetics 6(2): 153–161. doi: 10.3897/CompCytogen.v6i2.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra AMR, Bonvicino CR. (2015) Genus Carterodon In: Patton, JL, Pardiñas, UFJ, D’Elía, G (Eds) Mammals of South America, Volume 2: Rodents University of Chicago Press, 933–935.
- Bianchi NO, Paulete-Vanrell J, de Vidal Rioja LA. (1969) Complement with 38 chromosomes in two South American populations of Rattus rattus. Cellular and Molecular Life Sciences 25(10): 1111–1112. https://doi.org/10.1007/BF01901465 [DOI] [PubMed] [Google Scholar]
- Bidau CJ. (2015) Family Ctenomyidae. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 818–876.
- Bonvicino CR. (1994) Especiação do rato d’água Nectomys (Rodentia, Cricetidae) Abordagem cariológica, morfológica e geográfica. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 265 pp. [In Portuguese] [Google Scholar]
- Bonvicino CR, D’Andrea PS, Cerqueira R, Seuánez HN. (1996) The chromosomes of Nectomys (Rodentia, Cricetidae) with 2n = 52, 2n = 56, and interspecific hybrids (2n = 54). Cytogenetics Cell Genetics 73: 190–193. https://doi.org/10.1159/000134337 [DOI] [PubMed] [Google Scholar]
- Bonvicino CR, Weksler M. (1998) A new species of Oligoryzomys (Rodentia, Sigmodontinae) from northeastern and central Brazil. Zeitschrift für Säugetierkunde 63(2): 90–103. [Google Scholar]
- Bonvicino CR, Penna-firme V, Seuanez HN. (1998a) The karyotype of Brucepattersonius griserufescens Hershkovitz, 1998 (Rodentia, Sigmodontinae) with comments on distribution and taxonomy. Zeitschrift für Säugetierkunde 63: 329–35. [Google Scholar]
- Bonvicino C, Otazu I, Weksler M. (1998b) Oryzomys lamia Thomas, 1901 (Rodentia, Cricetidae): karyotype, geographic distribution and conservation status. Mammalia 62(2): 253–258. https://doi.org/10.1515/mamm.1998.62.2.253 [Google Scholar]
- Bonvicino CR, Otazu I. (1999) The Wilfredomys pictipes (Rodentia, Sigmodontinae) karyotype with comments on the karyosystematics of Brazilian Thomasomyini. Acta Theriologica 44: 329–332. https://doi.org/10.4098/AT.arch.99-31 [Google Scholar]
- Bonvicino C, Almeida FC. (2000) Karyotype, morphology and taxonomic status of Calomys expulsus (Rodentia: Sigmodontinae). Mammalia 64(3): 339–352. https://doi.org/10.1515/mamm.2000.64.3.339 [Google Scholar]
- Bonvicino CR, Moreira MAM. (2001) Molecular phylogeny of the genus Oryzomys (Rodentia: Sigmodontinae) based on cytochrome b DNA sequences. Molecular Phylogenetics and Evolution 18(2): 282–292. https://doi.org/10.1006/mpev.2000.0878 [DOI] [PubMed] [Google Scholar]
- Bonvicino CR, D’Andrea PS, Borodin PM. (2001a) Pericentric inversion in natural populations of Oligoryzomys nigripes (Rodentia: Sigmodontinae). Genome 44(5), 791–796. https://doi.org/10.1139/g01-080 [DOI] [PubMed]
- Bonvicino CR, Oliveira JA, D’Andrea PS, Carvalho RD. (2001b) The endemic Atlantic forest rodent Phaenomys ferrugineus (Thomas, 1894) (Sigmodontinae): New data on its morphology and karyology. Boletim do Museu Nacional 467: 1–12. [Google Scholar]
- Bonvicino CR, Otazu IB, D’Andrea PS. (2002a) Karyologic evidence of diversification of the genus Thrichomys (Rodentia, Echimyidae). Cytogenetic and Genome Research 97(3-4): 200–204. https://doi.org/10.1159/000066613 [DOI] [PubMed] [Google Scholar]
- Bonvicino CR, Penna-Firme V, Braggio E. (2002b) Molecular and karyologic evidence of the taxonomic status of Coendou and Sphiggurus (Rodentia: Hystricognathi). Journal of Mammalogy 83(4): 1071–1076. https://doi.org/10.1644/1545-1542(2002)083<1071:MAKEOT>2.0.CO;2 [Google Scholar]
- Bonvicino CR. (2003) A new species of Oryzomys (Rodentia, Sigmodontinae) of the subflavus group from the Cerrado of Central Brazil. Mammalian Biology 68: 78–90. https://doi.org/10.1078/1616-5047-00066 [Google Scholar]
- Bonvicino CR, Lima JF, Almeida FC. (2003a) A new species of Calomys Waterhouse (Rodentia, Sigmodontinae) from the Cerrado of central Brazil. Revista Brasileira de Zoologia 20(2): 301–307. http://dx.doi.org/10.1590/S0101-81752003000200021 [Google Scholar]
- Bonvicino CR, Menezes AREAN, Oliveira JA. (2003b) Molecular and karyologic variation in the genus Isothrix (Rodentia, Echimyidae). Hereditas 139(3): 206–211. https://doi.org/10.1111/j.1601-5223.2003.01807.x [DOI] [PubMed] [Google Scholar]
- Bonvicino CR, Lemos B, Weksler M. (2005a) Small mammals of Chapada dos Veadeiros National Park (Cerrado of Central Brazil): ecologic, karyologic, and taxonomic considerations. Brazilian Journal of Biology 65(3): 395–406. http://dx.doi.org/10.1590/S1519-69842005000300004 [DOI] [PubMed] [Google Scholar]
- Bonvicino CR, Otazú IB, Vilela JF. (2005b) Karyologic and molecular analysis of Proechimys Allen, 1899 (Rodentia, Echimyidae) from the Amazonian region. Arquivos Museu Nacional 63: 191–200. [Google Scholar]
- Bonvicino CR, Geise L. (2006) Relevância dos estudos cariológicos na taxonomia de alguns gêneros de Oryzomyini (Rodentia, Sigmodontinae). In: Freitas TRO, Vieira E, Pacheco S, Christoff AU. (Eds) Mamíferos do Brasil: genética, sistemática, ecologia e conservação. São Carlos: Sociedade Brasileira de Genética, Suprema, 27–37.
- Bonvicino CR, Oliveira JA, D’Andrea PS. (2008) Guia dos roedores do Brasil, com chaves para gêneros baseada em caracteres externos de Janeiro: Centro Pan-Americano de Febre Aftosa – OPAS/OMS, 120 pp.
- Bonvicino CR, Gonçalves PR, de Oliveira JA, de Oliveira LFB, Mattevi MS. (2009) Divergence in Zygodontomys (Rodentia: Sigmodontinae) and distribution of Amazonian savannas. Journal of Heredity 100(3): 322–328. https://doi.org/10.1093/jhered/esn105 [DOI] [PubMed] [Google Scholar]
- Bonvicino CR, Oliveira JAD, Gentile R. (2010) A new species of Calomys (Rodentia: Sigmodontinae) from eastern Brazil. Zootaxa 2336: 19–25. https://doi.org/10.5281/zenodo.193184 [Google Scholar]
- Bonvicino CR, Fernandes FA, Viana MC, Bernardo RT, D’Andrea PS. (2013) Scapteromys aquaticus (Rodentia: Sigmodontinae) in Brazil with comments on karyotype and phylogenetics relationships. Zoologia (Curitiba) 30(2): 242–247. http://dx.doi.org/10.1590/S1984-46702013000200016 [Google Scholar]
- Bonvicino CR, Casado F, Weksler M. (2014) A new species of Cerradomys (Mammalia: Rodentia: Cricetidae) from Central Brazil, with remarks on the taxonomy of the genus. Zoologia (Curitiba) 31(6): 525–540. http://dx.doi.org/10.1590/S1984-46702014000600002 [Google Scholar]
- Bonvicino CR, Bezerra AMR. (2015) Genus Euryzygomatomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 937–939.
- Braggio E, Bonvicino CR. (2004) Molecular divergence in the genus Thrichomys (Rodentia, Echimyidae). Journal of Mammalogy 85(2): 316–320. https://doi.org/10.1644/1545-1542(2004)085<0316:MDITGT>2.0.CO;2 [Google Scholar]
- Brennand PG, Langguth A, Percequillo AR. (2013) The genus Hylaeamys Weksler, Percequillo, and Voss 2006 (Rodentia: Cricetidae: Sigmodontinae) in the Brazilian Atlantic Forest: geographic variation and species definition. Journal of Mammalogy 94(6): 1346–1363. https://doi.org/10.1644/12-MAMM-A-312.1 [Google Scholar]
- Brum-Zorrilla N, Oliver G, de Fronza TG, Wainberg R. (1986) Karyological studies of South American rodents (Rodentia: Cricetidae) I Comparative chromosomic analysis in Scapteromys taxa. Caryologia 39(2): 131–142. http://dx.doi.org/10.1080/00087114.1986.10797774 [Google Scholar]
- Carmignotto AP. (2005) Pequenos mamíferos terrestres do bioma Cerrado: padrões faunísticos locais e regionais. Ph.D. Thesis, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 404 pp. [In Portuguese] [Google Scholar]
- Carvalho AH, Lopes MOG, Svartman M. (2012) A new karyotype for Rhipidomys (Rodentia, Cricetidae) from Southeastern Brazil. Comparative Cytogenetics 6(3): 227. https://doi.org/10.3897/compcytogen.v6i3.2432 [DOI] [PMC free article] [PubMed]
- Castro EC. (1989) Ocorrência e caracterização cromossômica de roedores Akodontinos do Rio Grande do Sul. Master’s Dissertation, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 207 pp. [In Portuguese] [Google Scholar]
- Castro EC, Mattevi MS, Maluf SW, Oliveira LFB. (1991) Distinct centric fusions in different populations of Deltamys kempi (Rodentia, Cricetidae) from South America. Cytobios 68: 153–159. [PubMed] [Google Scholar]
- Catzeflis F, Tilak MK. (2009) Molecular systematics of Neotropical spiny mice (Neacomys: Sigmodontinae, Rodentia) from the Guianan Region. Mammalia 73(3): 239–247. https://doi.org/10.1515/MAMM.2009.037 [Google Scholar]
- Cestari NA, Imada J. (1968) Os cromossomos do roedor Akodon arviculoides cursor Winge, 1888 (Cricetidae, Rodentia). Ciência e Cultura 20: 758–762. [Google Scholar]
- Christoff AU, Fagundes V, Sbalqueiro YJ, Mattevi MS, Yonenaga-Yassuda Y. (2000) description of a new species of Akodon (Rodentia: Sigmodontinae) from Southern Brazil. Journal of Mammalogy 81(3): 838–851. https://doi.org/10.1644/1545-1542(2000)081<0838:DOANSO>2.3.CO;2 [Google Scholar]
- Colombi VH, Fagundes V. (2014) First record of Calomys cerqueirai (Rodentia: Phyllotini) in Espírito Santo (Brazil) with description of the 2n = 36, FNA = 66 karyotype. Mammalia 79(4): 479–486. https://doi.org/10.1515/mammalia-2014-0076 [Google Scholar]
- Corrêa MMDO, Lopes MOG, Câmara EV. (2005) The karyotypes of Trinomys moojeni (Pessôa, Oliveira & Reis, 1992) and Trinomys setosus elegans (Lund, 1841) (Rodentia, Echimyidae) from Minas Gerais, eastern Brazil. Arquivos do Museu Nacional 63(1): 169–174. [Google Scholar]
- Costa LP, Pavan SE, Leite YL, Fagundes V. (2007) A new species of Juliomys (Mammalia: Rodentia: Cricetidae) from the Atlantic forest of southeastern Brazil. Zootaxa 1463(1): 21–37. [Google Scholar]
- Costa BMA, Geise L, Pereira LG, Costa LP. (2011) Phylogeography of Rhipidomys (Rodentia: Cricetidae: Sigmodontinae) and description of two new species from southeastern Brazil. Journal of Mammalogy 92(5): 945–962. https://doi.org/10.1644/10-MAMM-A-249.1 [Google Scholar]
- da Silva MNF. (1998) Four new species of spiny rats of the genus Proechimys (Rodentia: Echimyidae) from the western Amazon of Brazil. Proceedings of the Biological Society of Washington 111(2): 436–471. http://repositorio.inpa.gov.br/handle/123/2787 [Google Scholar]
- De Vivo M, Carmignotto AP. (2015) Family Sciuridae In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America, Volume 2: Rodents. University of Chicago Press, 3–47.
- D’Elía G, Pardiñas UFJ. (2015) Tribe Akodontini. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 140–279.
- Delciellos AC, Loss AC, Aguieiras M, Geise L, Rocha-Barbosa O. (2017) Syntopy of cryptic Phyllomys (Rodentia: Echimyidae) species: description of the karyotype of Phyllomys nigrispinus and an expansion of the geographic distribution of Phyllomys sulinus Mammalia https://doi.org/10.1515/mammalia-2016-0149
- Di-Nizo CB. (2013) Citotaxonomia e evolução cromossômica em Oligoryzomys (Rodentia, Sigmodontinae). Master’s Dissertation, Universidade de São Paulo, São Paulo, Brazil, 165 pp. [In Portuguese] [Google Scholar]
- Di-Nizo CB, Neves CL, Vilela JF, Silva MJJ. (2014) New karyologycal data and cytotaxonomic considerations on small mammals from Santa Virgínia (Parque Estadual da Serra do Mar, Atlantic Forest, Brazil). Comparative Cytogenetics 8(1): 11–30. https://doi.org/10.3897/compcytogen.v8i1.6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Nizo CB, Ventura K, Ferguson-Smith MA, O’Brien PCM, Yonenaga-Yassuda Y, Silva MJJ. (2015) Comparative chromosome painting in six species of Oligoryzomys (Rodentia, Sigmodontinae) and the karyotype evolution of the genus. PLOS One 10(2): e0117579. https://doi.org/10.1371/journal.pone.0117579 [DOI] [PMC free article] [PubMed]
- Dunnum JL, Salazar-Bravo J, Yates TL. (2001) The Bolivian bamboo rat, Dactylomys boliviensis (Rodentia: Echimyidae), a new record for chromosome number in a mammal. Mammalian Biology 66(2): 121–126. [Google Scholar]
- Edgar RC. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32(5): 1792–1797. https://doi.org/10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eler ES, Da Silva MNF, Silva CEF, Feldberg E. (2012) Comparative cytogenetics of spiny rats of the genus Proechimys (Rodentia, Echimyidae) from the Amazon region. Genetics and Molecular Research 11(2): 830–846. http://dx.doi.org/10.4238/2012.April.3.5 [DOI] [PubMed] [Google Scholar]
- Emmons LH, Leite YLR. (2015) In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 889–890.
- Emmons LH, Leite YRL, Patton JL. (2015) Genus Toromys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 929–931.
- Fabre PH, Upham NS, Emmons LH, Justy F, Leite YL, Loss AC, Orlando L, Tilak MK, Patterson BD, Douzery EJ. (2016) Mitogenomic phylogeny, diversification, and biogeography of South American spiny rats. Molecular Biology and Evolution 34(3): 613–633. https://doi.org/10.1093/molbev/msw261 [DOI] [PubMed] [Google Scholar]
- Fagundes V. (1993) Análises cromossômicas e dos complexos sinaptonêmicos em roedores brasileiros das famílias Cricetidae e Echimydae. Master’s Dissertation Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 194 pp [In Portuguese]
- Fagundes V, Vianna-Morgante AM, Yonenaga-Yassuda Y. (1997a) Telomeric sequences localization and G-banding patterns in the identification of a polymorphic chromosomal rearrangement in the rodent Akodon cursor (2n = 14, 15 and 16). Chromosome Research 5(4): 228–232. https://doi.org/10.1023/A:1018463401887 [DOI] [PubMed] [Google Scholar]
- Fagundes V, Scalzi-Martin JM, Sims K, Hozier J, Yonenaga-Yassuda Y. (1997b) ZOO-FISH of a microdissection DNA library and G-banding patterns reveal the homeology between the Brazilian rodents Akodon cursor and A. montensis. Cytogenetic and Genome Research 78(3-4): 224–228. https://doi.org/10.1159/000134662 [DOI] [PubMed] [Google Scholar]
- Fagundes V. (1998) Evolutionary conservation of whole homeologous chromosome arms in the akodont rodents Bolomys and Akodon (Muridae, Sigmodontinae): maintenance of interstitial telomeric segments (ITBS) in recent event of centric fusion. Chromosome Research 6(8): 643–648. https://doi.org/10.1023/A:1009213712370 [DOI] [PubMed] [Google Scholar]
- Fagundes V, Christoff AU, Yonenaga-Yassuda Y. (1998) Extraordinary chromosomal polymorphism with 28 different karyotypes in the neotropical species Akodon cursor (Muridae, Sigmodontinae), one of the smallest diploid number in rodents (2n = 16, 15 and 14). Hereditas 129(3): 263–74. https://doi.org/10.1111/j.1601-5223.1998.00263.x [DOI] [PubMed] [Google Scholar]
- Fagundes V, Christoff AU, Scalzi-Martin J, Hozier J, Moreira CA, Yonenaga-Yassuda Y. (2000) X,Y translocation revealed by chromosome microssection and FISH in fertile XY females in the Brazilian rodent Akodon montensis. Cytogenet and Cell Genetics 8: 124–128. https://doi.org/10.1159/000015504 [DOI] [PubMed] [Google Scholar]
- Fagundes V, Christoff AU, Amaro-Ghilardi RC, Scheibler DR, Yonenaga-Yassuda Y. (2003) Multiple interstitial ribosomal sites (NORs) in the Brazilian squirrel Sciurus aestuans ingrami (Rodentia, Sciuridae) with 2n = 40: An overview of Sciurus cytogenetics. Genetics and Molecular Biology 26(3): 253–257. http://dx.doi.org/10.1590/S1415-47572003000300007 [Google Scholar]
- Fagundes V, Camacho JPM, Yonenaga-Yassuda Y. (2004) Are the dot-like chromosomes in Trinomys iheringi (Rodentia, Echimyidae) B chromosomes? Cytogenetic and Genome Research 106(2-4): 159–164. https://doi.org/10.1159/000079282 [DOI] [PubMed]
- Faria R, Navarro A. (2010) Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends in Ecology & Evolution 25(11): 660–669. https://doi.org/10.1016/j.tree.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Feijó A, Langguth A. (2013) Mamíferos de médio e grande porte do Nordeste do Brasil: distribuição e taxonomia, com descrição de novas espécies. Revista Nordestina de Biologia 22(1): 3–225. http://periodicos.ufpb.br/ojs/index.php/revnebio/article/view/16716 [Google Scholar]
- Fernandes FA, Gonçalves GL, Ximenes SS, de Freitas TR. (2009) Karyotypic and molecular polymorphisms in Ctenomys torquatus (Rodentia: Ctenomyidae): taxonomic considerations. Genetica 136(3): 449–459. https://doi.org/10.1007/s10709-008-9345-8 [DOI] [PubMed] [Google Scholar]
- Flores TA. (2010) Diversidade morfológica e molecular do gênero Oecomys Thomas, 1906 (Rodentia: Cricetidae) na Amazônia oriental brasileira. Ph.D. Thesis, Universidade Federal do Pará, Belém, Brazil, 103 pp. [In Portuguese] [Google Scholar]
- Fonseca GAB, Hermann G, Leite YLR, Mittermeier RA, Rylands AB, Patton JL. (1996) Lista anotada dos mamíferos do Brasil. Ocassional Papers in Conservation Biology Conservation Biology 4: 1–38. [Google Scholar]
- Ford CE, Hamerton JL. (1956) A colchicine hypotonic-citrate squash sequence for mammalian chromosome. Stain Technology 31(6): 247–251.http://dx.doi.org/10.3109/10520295609113814 [DOI] [PubMed] [Google Scholar]
- Freitas TRO. (1980) Estudos citogenéticos em roedores do Sul do Brasil. Master’s Dissertation Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 140 pp. [In Portuguese] [Google Scholar]
- Freitas TRO, Mattevi MS, Oliveira LFB, Souza MJ, Yonenaga-Yassuda Y, Salzano FM. (1983) Chromosome relationship in three representatives of the genus Holochilus (Rodentia, Cricetidae) from Brazil. Genetica 61: 13–20. https://doi.org/10.1007/BF00563228 [Google Scholar]
- Freitas TRO, Lessa EP. (1984) Cytogenetics and morphology of Ctenomys torquatus (Rodentia-Octodontidae). Journal of Mammalogy 65: 637–42. https://doi.org/10.2307/1380845 [Google Scholar]
- Freitas TRO, Mattevi MS, Oliveira LFB. (1984) Unusual C-band patterns in three karyotypically rearranged forms of Scapteromys (Rodentia, Cricetidae) from Brazil. Cytogenetic and Genome Research 38(1): 39–44. https://doi.org/10.1159/000132027 [DOI] [PubMed] [Google Scholar]
- Freitas TRO. (1997) Chromosome polymorphism in Ctenomys minutus (Rodentia-Octodontidae). Brazilian Journal of Genetics 20(1). http://dxdoiorg/10.1590/S0100-84551997000100001
- Freitas TRO. (2007) Ctenomys lami: The highest chromosome variability in Ctenomys (Rodentia, Ctenomyidae) due to a centric fusion/fission and pericentric inversion system. Acta Theriologica 52(2): 171–180.https://doi.org/10.1007/BF03194212 [Google Scholar]
- Freshney RI. (1986) Animal cell culture: a practical approach. IRL press, Oxford, 397 pp. [Google Scholar]
- Freygang CC, Marinho JR, Freitas TR. (2004) New karyotypes and some considerations about the chromosomal diversification of Ctenomys minutus (Rodentia: Ctenomyidae) on the coastal plain of the Brazilian state of Rio Grande do Sul. Genetica 121(2): 125–132. https://doi.org/10.1023/B:GENE.0000040376.56321.be [DOI] [PubMed] [Google Scholar]
- Furtado VV. (1981) Diversidade cromossômica em roedores das famílias Cricetidae e Caviidae em Pernambuco, Brasil. Ph.D. Thesis, Universidade do Rio Grande do Sul, Porto Alegre, Brazil, 185 pp. [In Portuguese] [Google Scholar]
- Galewski T, Mauffrey JF, Leite YRL, Patton JL, Douzery EJP. (2005) Ecomorphological diversification among South American spiny rats (Rodentia; Echimyidae): a phylogenetic and chronological approach. Molecular Phylogenetics and Evolution 34: 601–615. https://doi.org/10.1016/j.ympev.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Gardner AL, Emmons LH. (1984) Species groups in Proechimys (Rodentia, Echimyidae) as indicated by karyology and bullar morphology. Journal of Mammalogy 65(1): 10–25. https://doi.org/10.2307/1381195 [Google Scholar]
- Gava A, Freitas TRO, Olimpio J. (1998) A new karyotype for the genus Cavia from a southern island of Brazil (Rodentia-Caviidae). Genetics and Molecular Biology 21(1): 77–80. http://dxdoiorg/101590/S1415-47571998000100013 [Google Scholar]
- Gava A, Freitas TRO. (2002) Characterization of a hybrid zone between chromosomally divergent populations of Ctenomys minutus (Rodentia: Ctenomyidae). Journal of Mammalogy 83(3): 843–851. https://doi.org/10.1644/1545-1542(2002)083<0843:COAHZB>2.0.CO;2 [Google Scholar]
- Gava A, dos Santos MB, Quintela FM. (2011) A new karyotype for Cavia magna (Rodentia: Caviidae) from an estuarine island and C. aperea from adjacent mainland. Acta Theriologica 57(1): 9–14. https://doi.org/10.1007/s13364-011-0042-0 [Google Scholar]
- Geise L. (1995) Os roedores Sigmodontinae (Rodentia, Muridae) do Estado do Rio de Janeiro Sistemática, citogenética, distribuição e variação geográfica. Ph.D. Thesis, Departamento de Genética, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 388 pp. [In Portuguese] [Google Scholar]
- Geise L, Canavez FC, Seuánez HN. (1998) Comparative karyology in Akodon (Rodentia, Sigmodontinae) from Southeastern Brazil. Journal of Heredity 89(2): 158–163. https://doi.org/10.1093/jhered/89.2.158 [DOI] [PubMed] [Google Scholar]
- Geise L, Smith MF, Patton JL. (2001) Diversification in the genus Akodon (Rodentia: Sigmodontinae) in southeastern South America: mitochondrial DNA sequence analysis. Journal of Mammalogy 82(1): 92–101. https://doi.org/10.1644/1545-1542(2001)082<0092:DITGAR>2.0.CO;2 [Google Scholar]
- Geise L, Bergallo HG, Esberard CE, Rocha CF, Sluys MV. (2008) The karyotype of Blarinomys breviceps (Mammalia: Rodentia: Cricetidae) with comments on its morphology and some ecological notes. Zootaxa 1907: 47–60. [Google Scholar]
- Geise L, Paresque R, Sebastião H, Shirai LT, Astúa D, Marroig G. (2010) Non-volant mammals, Parque Nacional do Catimbau, Vale do Catimbau, Buíque, state of Pernambuco, Brazil, with karyologic data. Check List 6(1): 180. http://dx.doi.org/10.15560/6.1.180
- Giannoni ML, Duarte JMB, Ribeiro RP, Lui JF, Tosta P. (1991) Cytogenetic research in wild animals at FCAVJ, Brazil. I Mammals. Genetics Selection Evolution 23(1): 123–125. https://doi.org/10.1186/1297-9686-23-S1-S123 [Google Scholar]
- Gomes Jr. RG, Schneider CH, Lira T, Carvalho NDM, Feldberg E, Silva MNF, Gross MC. (2016) Intense genomic reorganization in the genus Oecomys (Rodentia, Sigmodontinae): comparison between DNA barcoding and mapping of repetitive elements in three species of the Brazilian Amazon. Comparative Cytogenetics 10(3): 401–426. https://doi.org/10.3897/CompCytogen.v10i3.8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves PR, Almeida FC, Bonvicino CR. (2005) A new species of Wiedomys (Rodentia: Sigmodontinae) from Brazilian Cerrado. Mammalian Biology-Zeitschrift für Säugetierkunde 70(1): 46–60. https://doi.org/10.1078/1616-5047-00175 [Google Scholar]
- Gonçalves PR, Myers P, Vilela JF, Oliveira JA. (2007) Systematics of species of the genus Akodon (Rodentia: Sigmodontinae) in Southeastern Brazil and implications for the biogeography of the campos de Altitude. Museum of Zoology, University of Michigan, 197: 23 pp.
- Gonçalves PR, De Oliveira JA. (2014) An integrative appraisal of the diversification in the Atlantic forest genus Delomys (Rodentia: Cricetidae: Sigmodontinae) with the description of a new species. Zootaxa 3760(1): 1–38. https://doi.org/10.11646/zootaxa.3760.1.1 [DOI] [PubMed] [Google Scholar]
- Gonçalves PR, Teta P, Bonvicino CR. (2015) Genus Holochilus. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 325–334.
- González S, Brum-Zorrilla N. (1995) Karyological studies of the South American rodent Myocastor coypus Molina 1782 (Rodentia: Myocastoridae). Revista Chilena de Historia Natural 68: 215–226. [Google Scholar]
- Graipel ME, Cherem JJ, Miller PRM, Glock L. (2003) Trapping small mammals in the forest understory: a comparison of three methods. Mammalia 67(4): 551–558. https://doi.org/10.1515/mamm-2003-0409 [Google Scholar]
- Hass I, Sbalqueiro IJ, Müller S. (2008) Chromosomal phylogeny of four Akodontini species (Rodentia, Cricetidae) from Southern Brazil established by Zoo-FISH using Mus musculus (Muridae) painting probes. Chromosome Research 16(1): 75–88. https://doi.org/10.1007/s10577-007-1211-5 [DOI] [PubMed] [Google Scholar]
- Howell WT, Black DA. (1980) Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Cellular and Molecular Life Sciences 36(8): 1014–1015. https://doi.org/10.1007/BF01953855 [DOI] [PubMed] [Google Scholar]
- Hsu TC, Benirschke K. (1968) Myoprocta acouchy (Red acouchy). In: An Atlas of Mammalian Chromosomes. Springer, New York, 97–99. https://doi.org/10.1007/978-1-4615-6424-9_25
- Hurtado N, Pacheco VR. (2017) Revision of Neacomys spinosus (Thomas, 1882) (Rodentia: Cricetidae) with emphasis on Peruvian populations and the description of a new species. Zootaxa 4242(3): 401–440. http://dx.doi.org/10.11646/zootaxa.4242.3.1 [DOI] [PubMed] [Google Scholar]
- Irwin DM, Kocher TD, Wilson AC. (1991) Evolution of the cytochrome b gene of mammals. Journal of Molecular Evolution 32(2): 128–144. https://doi.org/10.1007/BF02515385 [DOI] [PubMed] [Google Scholar]
- Jackson BC. (2011) Recombination-suppression: how many mechanisms for chromosomal speciation? Genetica 139(3): 393–402. https://doi.org/10.1007/s10709-011-9558-0 [DOI] [PubMed]
- Kasahara S, Yonenaga-Yassuda Y. (1981) Chromosome variability in Brazilian specimens of Rattus rattus (2n = 38). Cellular and Molecular Life Sciences 37(1): 31–32. https://doi.org/10.1007/BF01965551 [Google Scholar]
- Kasahara S, Yonenaga-Yassuda Y. (1983) Sex chromosome variability in Zygodontomys lasiurus (Rodentia, Cricetidae). Cytologia 48: 569–576. http://doi.org/10.1508/cytologia.48.569 [DOI] [PubMed] [Google Scholar]
- Kasahara S, Yonenaga-Yassuda Y. (1984) A progress report of cytogenetic data on Brazilian rodents São Paulo. Revista Brasileira de Genética 3: 509–533. http://hdl.handle.net/11449/63661 [Google Scholar]
- King M. (1993) Species evolution: the role of chromosome change. Cambridge University Press, 336 pp.
- Lanfear R, Calcot B, Ho SY, Guindon S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29(6): 1695–1701. https://doi.org/10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Langguth A, Bonvicino CR. (2002) The Oryzomys subflavus species group, with description of two new species (Rodentia, Muridae, Sigmodontinae). Arquivos do Museu Nacional 60: 285–294. [Google Scholar]
- Langguth A, Maia V, Mattevi MS. (2005) Karyology of large size Brazilian species of the genus Oecomys Thomas, 1906 (Rodentia, Muridae, Sigmodontinae). Arquivos do Museu Nacional 63(1): 183–190. [Google Scholar]
- Lara MC, Patton JL, da Silva MNF. (1996) The simultaneous diversification of South American echimyid rodents (Hystricognathi) based on complete cytochrome b sequences. Molecular Phylogenetics and Evolution 5(2): 403–413. https://doi.org/10.1006/mpev.1996.0035 [DOI] [PubMed] [Google Scholar]
- Lara M, Patton JL, Hingst-Zaher E. (2002) Trinomys mirapitanga, a new species of spiny rat (Rodentia: Echimyidae) from the Brazilian Atlantic Forest. Mammalian Biology-Zeitschrift für Säugetierkunde 67(4): 233–242. https://doi.org/10.1078/1616-5047-00034 [Google Scholar]
- Lazar A, Nacif C, Weksler M, Bonvicino CR. (2017) The karyotype of Trinomys paratus (Rodentia: Echimyidae) with comments about its phylogenetic relationship. Mammalia 2017: 2016–0131. https://doi.org/10.1515/mammalia-2016-0131 [Google Scholar]
- Leal-Mesquita ER. (1991) Estudos citogenéticos em dez espécies de roedores brasileiros da família Echimyidae. Master’s Dissertation, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 167 pp. [In Portuguese] [Google Scholar]
- Leal-Mesquita ER, Yonenaga-Yassuda Y, Chu TH, Da Rocha PLB. (1992) Chromosomal characterization and comparative cytogenetic analysis of two species of Proechimys (Echimyidae, Rodentia) from the Caatinga domain of the State of Bahia, Brazil. Caryologia 45(2): 197–212. http://dx.doi.org/10.1080/00087114.1992.10797223 [Google Scholar]
- Leal-Mesquita ER, Fagundes V, Yonenaga-Yassuda Y, Rocha PLB. (1993) Comparative cytogenetic studies of two karyomorphs of Trichomys apereoides (Rodentia, Echimydae). Revista Brasileira de Genética 16(3): 639–651. [Google Scholar]
- Leite YL, Patton JL. (2002) Evolution of South American spiny rats (Rodentia, Echimyidae): the star-phylogeny hypothesis revisited. Molecular Phylogenetics and Evolution 25(3): 455–464. https://doi.org/10.1016/S1055-7903(02)00279-8 [DOI] [PubMed] [Google Scholar]
- Leite YL. (2003) Evolution and systematics of the Atlantic tree rats, genus Phyllomys (Rodentia, Echimyidae), with description of two new species. University of California Press, 133 pp.
- Leite YL, Loss AC. (2015) Genus Phyllomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 915–927. https://doi.org/10.1525/california/9780520098497.001.0001
- Lemos ERSD, D’Andrea PS, Bonvicino CR, Famadas KM, Padula P, Cavalcanti AA, Schatzmayr HG. (2004) Evidence of hantavirus infection in wild rodents captured in a rural area of the state of São Paulo, Brazil. Pesquisa Veterinária Brasileira 24(2): 71–73. http://dx.doi.org/10.1590/S0100-736X2004000200004 [Google Scholar]
- Lessa G, Corrêa MM, Pessôa LM, Zappes IA. (2013) Chromosomal differentiation in Kerodon rupestris (rodentia: Caviidae) from the Brazilian semi-arid region. Mastozoología Neotropical 20(2): 399–405. [Google Scholar]
- Lima FS. (1994) Cariótipos em espécies de Dasyproctidae e Erethizontidae, com discussão da evolução cromossômica (Rodentia, Caviomorpha). Brazilian Journal of Genetics 17: 135.
- Lima FS, Bonvicino CR, Kasahara S. (2003) A new karyotype of Oligoryzomys (Sigmodontinae, Rodentia) from central Brazil. Hereditas 139: 1–6. https://doi.org/10.1111/j.1601-5223.2003.01706.x [DOI] [PubMed] [Google Scholar]
- Lima JDS, Langguth A. (1998) The karyotypes of three Brazilian species of the genus Dasyprocta (Rodentia, Dasyproctidae). Iheringia Serie Zoologia 85: 141–145. [Google Scholar]
- Lima JDS, Langguth A, Sousa LCD. (1998) The karyotype af Makalata didelphoides (Rodentia, Echimyidae). Zeitschrift fur Saugetierkunde 63(5): 315–318. [Google Scholar]
- Lima JFD, Langguth A. (2002) Karyotypes of Brazilian squirrels: Sciurus spadiceus and Sciurus alphonsei (Rodentia, Sciuridae). Folia Zoologica 51: 201–204. [Google Scholar]
- Lima-Rosa CAV, Hutz MH, Oliveira LFB, Andrades-Miranda J, Mattevi MS. (2000) Heterologous amplification of microsatellite loci from mouse and rat in Oryzomyine and Thomasomyine South American rodents. Biochemical Genetics 38: 97–108. https://doi.org/10.1023/A:1002767930781 [DOI] [PubMed] [Google Scholar]
- Lira T. (2012) Citogenética clássica e molecular de alguns representantes da tribo Oryzomyini (Rodentia, Cricetidae) da Amazônia Central. Master’s Dissertation, Instituto Nacional de Pesquisas da Amazônia, Amazônia, Brazil, 59 pp. [In Portuguese] [Google Scholar]
- Machado LF, Leite YL, Christoff AU, Giugliano LG. (2014) Phylogeny and biogeography of tetralophodont rodents of the tribe Oryzomyini (Cricetidae: Sigmodontinae). Zoologica Scripta 43(2): 119–130. https://doi.org/10.1111/zsc.12041 [Google Scholar]
- Machado MX. (2010) Diversidade citogenética e citotaxonomia de Phyllomys (Rodentia, Echimyidae). Master Dissertation, Universidade Federal do Espírito Santo, Vitória, Brazil, 36 pp. [In Portuguese] [Google Scholar]
- Machado T, Silva MJJ, Leal-Mesquita ER, Carmignotto AP, Yonenaga-Yassuda Y. (2005) Nine karyomorphs for spiny rats of the genus Proechimys (Echimyidae, Rodentia) from North and Central Brazil. Genetics and Molecular Biology 28(4): 682–692. http://dx.doi.org/10.1590/S1415-47572005000500007 [Google Scholar]
- Maia V, Hulak A. (1981) Robertsonian polymorphism in chromosomes of Oryzomys subflavus (Rodentia, Cricetidae). Cytogenetic and Genome Research 31(2): 33–39. https://doi.org/10.1159/000131623 [DOI] [PubMed] [Google Scholar]
- Maia V, Langguth A. (1981) New karyotypes of Brazilian akodont rodents with notes on taxonomy. Zeitschrift für Säugetierkunde 46(4): 241–249. [Google Scholar]
- Maia V. (1984) Karyotypes of three species of Caviinae (Rodentia, Caviidae). Experientia 40(6): 564–566. https://doi.org/10.1007/BF01982332 [Google Scholar]
- Maia V, Yonenaga-Yassuda Y, Freitas JRO, Kasahara S, Mattevi MS, Oliveira LF, Galindo MA, Sbalqueiro IJ. (1984) Supernumerary chromosomes, Robertsonian rearrangements and variability of the sex chromosomes in Nectomys squamipes (Cricetidae, Rodentia). Genetica 63: 121–28. https://doi.org/10.1007/BF00605896 [Google Scholar]
- Maia V, Langguth A. (1987) Chromosomes of the Brazilian Cricetid Rodent Wiedomys pyrrhorhinos (Wied, 1821). Revista Brasileira de Genética 10(2): 229–233. [Google Scholar]
- Maia V. (1990) Karyotype of Oryzomys capito oniscus (Rodentia), from Northeastern Brazil. Revista Brasileira de Genética 13(2): 377–382. [Google Scholar]
- Maia V, Langguth A. (1993) Constitutive heterochromatin polymorphism and NORs in Proechimys cuvieri Petter, 1978 (Rodentia, Echimyidae). Revista Brasileira de Genética 16(1): 45–154. [Google Scholar]
- Malcher SM, Pieczarka JC, Geise L, Rossi RV, Pereira AL, O’Brien PCM, Asfora PH, Fônseca da Silva V, Sampaio MI, Ferguson-Smith MA, Nagamachi CY. (2017) Oecomys catherinae (Sigmodontinae, Cricetidae): Evidence for chromosomal speciation? PLOS One, 12(7): e0181434. https://doi.org/10.1371/journal.pone.0181434 [DOI] [PMC free article] [PubMed]
- Malcolm JR. (1991) Comparative Abundances of Neotropical Small Mammals by Trap Height. Journal of Mammalogy 72(1): 188–192. https://doi.org/10.2307/1381995 [Google Scholar]
- Massarini IA, Freitas TRO. (2005) Morphological and cytogenetics comparison in species of the mendocinus-group (genus Ctenomys) with emphasis in C. australis and C. flamarioni (Rodentia-Ctenomyidae). Caryologia 58(1): 21–27. http://dx.doi.org/10.1080/00087114.2005.10589427 [Google Scholar]
- Mattevi MS, Haag T, Nunes AP, Oliveira LFB, Cordeiro JLP. (2002) Karyotypes of Brazilian representatives of genus Zygodontomys (Rodentia, Sigmodontinae). Mastozoología Neotropical 9: 33–38. [Google Scholar]
- Mattevi MS, Haag T, Oliveira LD, Langguth AR. (2005) Chromosome characterization of Brazilian species of Calomys Waterhouse, 1837 from Amazon, Cerrado and Pampas domains (Rodentia, Sigmodontinae). Arquivos do Museu Nacional 63(1): 175–181. [Google Scholar]
- Mendes-Pontes AR, Gadelha JR, Melo ER, De Sa FB, Loss AC, Júnior VC, Costa L, Leite YL. (2013) A new species of porcupine, genus Coendou (Rodentia: Erethizontidae) from the Atlantic forest of northeastern Brazil. Zootaxa 3636(3): 421–438. http://dx.doi.org/10.11646/zootaxa.3636.3.2 [DOI] [PubMed] [Google Scholar]
- Miranda GB, Oliveira LFB, Andrades-Miranda J, Langguth A, Callegari-Jacques SM, Mattevi M. (2008) Phylogenetic and phylogeographic patterns in Sigmodontine rodents of the genus Oligoryzomys. Journal of Heredity 100: 309–321. https://doi.org/10.1093/jhered/esn099 [DOI] [PubMed] [Google Scholar]
- Moreira CN, Di-Nizo CB, Silva MJJ, Yonenaga-Yassuda Y, Ventura K. (2013) A remarkable autosomal heteromorphism in Pseudoryzomys simplex 2n = 56, FN = 54-55 (Rodentia, Sigmodontinae). Genetics and Molecular Biology 36(2): 201–206. https://doi.org/10.1590/S1415-47572013000200010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira JC, Manduca EG, Gonçalves PR, Morais Jr MD, Pereira RF, Lessa G, Dergam JA. (2009) Small mammals from Serra do Brigadeiro State Park, Minas Gerais, southeastern Brazil: species composition and elevational distribution. Arquivos do Museu Nacional 67: 103–118. [Google Scholar]
- Musser GG, Carleton MD. (1993) Family Muridae. In: Wilson DE, Reeder DM. (Eds) Mammals species of the world, A Taxonomic and Geographic Reference. 2nd ed. Smithsonian Institution Press, Washington, 501–756.
- Musser GG, Carleton MD, Brothers EM, Gardner AL. (1998) Systematic studies of Oryzomyine rodents (Muridae, Sigmodontinae): Diagnoses and distributions of species formally assigned to Oryzomys capito. Bulletin of the American Museum of Natural History 236: 1–376. http://hdl.handle.net/2246/1630 [Google Scholar]
- Musser GG, Carleton MD. (2005) Superfamily Muroidea. In: Wilson DE, Reeder DM. (Eds) Mammals species of the world, A Taxonomic and Geographic Reference. The Johns Hopkins University Press, Baltimore, 955–1186.
- Myers P, Carleton MD. (1981) The species of Oryzomys (Oligoryzomys) in Paraguay and the identity of Azara’s” rat sixième ou rat à tarse noir”. Museum of Zoology, University of Michigan 161: 1–41. [Google Scholar]
- Myers P, Lundrigan B, Tucker PK. (1995) Molecular phylogenetics of oryzomyine rodents: the genus Oligoryzomys. Molecular Phylogenetics and Evolution 4(4): 372–382. https://doi.org/10.1006/mpev.1995.1035 [DOI] [PubMed] [Google Scholar]
- Nachman MW, Boyer SN, Searle JB, Aquadro CF. (1994) Mitochondrial DNA variation and the evolution of Robertsonian chromosomal races of house mice, Mus domesticus. Genetics 136(3): 1105–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamachi CY, Pieczarka JC, O’Brien PCM, Pinto JA, Malcher SM, Pereira AL, Rissino JD, Mendes-Oliveira AC, Rossi RV, Ferguson-Smith MA. (2013) FISH with whole chromosome and telomeric probes demonstrates huge karyotypic reorganization with ITS between two species of Oryzomyini (Sigmodontinae, Rodentia): Hylaeamys megacephalus probes on Cerradomys langguthi karyotype. Chromosome Research 21(2): 107–119. https://doi.org10.1007/s10577-013-9341-4 [DOI] [PubMed] [Google Scholar]
- Noor MA, Grams KL, Bertucci LA, Reiland J. (2001) Chromosomal inversions and the reproductive isolation of species. Proceedings of the National Academy of Sciences 98(21): 12084–12088. https://doi.org 10.1073/pnas.221274498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira JA, Bonvicino CR. (2002) A new species of Sigmodontine rodent from the Atlantic forest of eastern Brazil. Acta Theriologica 47(3): 307–322. https://doi.org10.1007/BF03194149 [Google Scholar]
- Oliveira JA, Gonçalves PR. (2015) Genus Oxymycterus. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 247–267.
- Pardiñas UF, D’Elía G, Teta P. (2008) Una introducción a los mayores sigmodontinos vivientes: revisión de Kunsia Hershkovitz, 1966 y descripción de un nuevo género (Rodentia: Cricetidae). Arquivos do Museu Nacional 66(3-4): 509–594. [Google Scholar]
- Pardiñas UF, Lessa G, Teta P, Salazar-Bravo J, Câmara EM. (2014) A new genus of sigmodontine rodent from eastern Brazil and the origin of the tribe Phyllotini. Journal of Mammalogy 95(2): 201–215. https://doi.org/10.1644/13-MAMM-A-208 [Google Scholar]
- Pardiñas UFJ, Bezerra AMR. (2015) Genus Gyldenstopia. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 222–224.
- Pardiñas UFJ, Teta P, Salazar-Bravo J. (2015a) A new tribe of Sigmodontinae rodents (Cricetidae). Mastozoología Neotropical 22(1): 171–186. [Google Scholar]
- Pardiñas UFJ, Teta P, Alvarado-Serrano DF, Geise L, Jayat P, Ortiz PE, Gonçalves PRG, D’Elía G. (2015b) Genus Akodon. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 144–203.
- Pardiñas UFJ, Galliari CA, Teta P. (2015c) Tribe Reithrodontini. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 559–565.
- Pardiñas UF, Geise L, Ventura K, Lessa G. (2016a) A new genus for Habrothrix angustidens and Akodon serrensis (Rodentia, Cricetidae): again paleontology meets neontology in the legacy of Lund. Mastozoología Neotropical 23(1): 93–115. [Google Scholar]
- Pardiñas UFJ, Teta P, Salazar-Bravo J, Myers P, Galliari CA. (2016b) A new species of arboreal rat, genus Oecomys (Rodentia, Cricetidae) from Chaco. Journal of Mammalogy 97(4): 1177–1196. https://doi.org/10.1093/jmammal/gyw070 [Google Scholar]
- Paresque R, Souza WD, Mendes SL, Fagundes V. (2004) Composição cariotípica da fauna de roedores e marsupiais de duas áreas de Mata Atlântica do Espírito Santo, Brasil. Boletim Museu Biológico Mello Leitão 17: 5–33. [Google Scholar]
- Paresque R, Silva MJJ, Yonenaga-Yassuda Y, Fagundes V. (2007) Karyological geographic variation of Oligoryzomys nigripes Olfers, 1818 (Rodentia, Cricetidae) from Brazil. Genetics and Molecular Biology 30: 43–53. https://doi.org/10.1590/S1415-47572007000100010 [Google Scholar]
- Paresque R, Christoff AU, Fagundes V. (2009) Karyology of the Atlantic forest rodent Juliomys (Cricetidae): A new karyotype from southern Brazil. Genetics and Molecular Biology 32(2): 301–305. http://dx.doi.org/10.1590/S1415-47572009005000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paresque R, Hanson JD. (2015) Genus Microakodontomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 354–355.
- Patton JL, Gardner AL. (1972) Notes on the Systematics of Proechimys (Rodentia: Echimyidae): With Emphasis on Peruvian Forms. Louisiana State University 44: 30 pp.
- Patton JL, Emmons L. (1985) A review of the genus Isothrix (Rodentia, Echimyidae). American Museum of Natural History 2817: 1–14 http://hdlhandlenet/2246/5225 [Google Scholar]
- Patton JL, da Silva MNF. (1995) A review of the spiny mouse genus Scolomys (Rodentia: Muridae: Sigmodontinae) with the description of a new species from the western Amazon of Brazil. Proceedings of the Biological Society of Washington 108: 19–37. http://biostor.org/reference/74423 [Google Scholar]
- Patton JL, Da Silva MNF, Malcolm JR. (2000) Mammals of the Rio Jurua and the evolutionary and ecological diversification of Amazonia. Bulletin of the American Museum of Natural History 244: 306 pp. https://doi.org/10.1206/0003-0090(2000)244<0001:MOTRJA>2.0.CO;2
- Patton JL. (2004) Comparative genomics and the role of chromosomal rearrangements in species divergence: a paradigm revisited. Mastozoología Neotropical 11: 147–150. [Google Scholar]
- Patton JL. (2015) Genus Scolomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 445–447.
- Patton JL, Emmons LH. (2015) Family Dasyproctidae. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 733–761.
- Patton JL, Leite RN. (2015) Genus Proechimys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 950–988.
- Patton JL, Pardiñas UF, D’Elía G. (Eds) (2015) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 1336 pp. https://doi.org/10.7208/chicago/9780226169606.001.0001
- Percequillo AR, Carmignotto AP, Silva MJJ. (2005) . A new species of Neusticomys (Ichthyomyini, Sigmodontinae) from central Brazilian Amazonia. Journal of Mammalogy 86(5): 873–880. https://doi.org/10.1644/1545-1542(2005)86[873:ANSONI]2.0.CO;2
- Percequillo AR, Hingst-Zaher E, Bonvicino CR. (2008) Systematic Review of Genus Cerradomys Weksler, Percequillo & Voss, 2006 (Rodentia: Cricetidae: Sigmodontinae: Oryzomyini), with Description of Two New Species from Eastern Brazil. American Museum of Natural History 3622: 46 pp. https://doi.org/10.1206/495.1
- Percequillo AR, Weksler M, Costa LP. (2011) A new genus and species of rodent from the Brazilian Atlantic Forest (Rodentia: Cricetidae: Sigmodontinae: Oryzomyini), with comments on oryzomyine biogeography. Zoological Journal of the Linnean Society 161(2): 357–90. https://doi.org/10.1111/j.1096-3642.2010.00643.x [Google Scholar]
- Percequillo AR. (2015a) Genus Euryoryzomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America Volume 2: Rodents. University of Chicago Press, 312–319.
- Percequillo AR. (2015b) Genus Hylaeamys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 335–345.
- Pereira LG, Geise L, Cunha AA, Cerqueira R. (2008) Abrawayaomys ruschii Cunha & Cruz, 1979 (Rodentia, Cricetidae) no Estado do Rio de Janeiro, Brasil. Papéis Avulsos de Zoologia 48(5): 33–40. http://dx.doi.org/10.1590/S0031-10492008000500001 [Google Scholar]
- Pereira AL, Malcher SM, Nagamachi CY, O’Brien PCM, Ferguson-Smith MA, Mendes-Oliveira AC, Pieczarka JC. (2016) Extensive Chromosomal Reorganization in the Evolution of New World Muroid Rodents (Cricetidae, Sigmodontinae): Searching for Ancestral Phylogenetic Traits. PLOS One 11(1): e0146179. https://doi.org/10.1371/journal.pone.0146179 [DOI] [PMC free article] [PubMed]
- Pérez-Zapata A, Lew D, Aguilera M, Reig OA. (1992) New data on the systematics and karyology of Podoxymys roraimae (Rodentia, Cricetidae). Zeitschrift für Säugetierkunde 52: 216–224. [Google Scholar]
- Pessôa LM, de Oliveira JA, Lopes MOG. (2004) Karyological and morphometric variation in the genus Thrichomys (Rodentia: Echimyidae). Mammalian Biology-Zeitschrift für Säugetierkunde 69(4): 258–269. https://doi.org/10.1078/1616-5047-00141 [Google Scholar]
- Pessôa LM, Corrêa M, Bittencourt E, Reis S. (2005) Chromosomal characterization of taxa of the genus Trinomys Thomas, 1921 (Rodentia, Echimyidae) in the states of Rio de Janeiro and São Paulo. Arquivos do Museu Nacional 63(1): 161–8. [Google Scholar]
- Pessôa LM, Tavares WC, Oliveira JA, Patton JL. (2015) Genus Trinomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 999–1018.
- Quintela FM, Santos MB, Christoff AU, Gava A. (2012) Non-volant small mammals (Didelphimorphia, Rodentia) in two forest fragments in Rio Grande, Rio Grande do Sul Coastal Plain, Brazil. Biota Neotropica 12(1): 261–266. http://dxdoiorg/101590/S1676-06032012000100021 [Google Scholar]
- Quintela FM, Gonçalves GL, Althoff SL, Sbalqueiro IJ, Oliveira LFB, Freitas TRO. (2014) A new species of swamp rat of the genus Scapteromys Waterhouse, 1837 (Rodentia: Sigmodontinae) endemic to Araucaria angustifolia Forest in Southern Brazil. Zootaxa 3811(2): 207–225. http://dx.doi.org/10.11646/zootaxa.3811.2.3 [DOI] [PubMed] [Google Scholar]
- Quintela FM, Bertuol F, Gonzalez EM, Cordeio-Estrela P, Freitas TRO, Gonçalves GL. (2017) A new species of Deltamys Thomas, 1917 (Rodentia: Cricetidae) endemic to the Southern Brazilian Araucaria Forest and notes on the expanded phylogeographic scenario of D. kempi. Zootaxa 4294(1): 071–092. http://dx.doi.org/10.11646/zootaxa.4294.1.3 [Google Scholar]
- Ramos RS, Vale WG, Assis FL. (2003) Karyotypic analysis in species of the genus Dasyprocta (Rodentia: Dasyproctidae) found in Brazilian Amazon. Anais da Academia Brasileira de Ciências 75(1): 55–69. http://dx.doi.org/10.1590/S0001-37652003000100007 [DOI] [PubMed] [Google Scholar]
- Reig OA. (1986) Diversity patterns and differentiation of high Andean rodents In: Vuilleumier F, Monasterio (Eds) High altitude tropical biogeography Oxford University Press, New York, 404–439.
- Ribeiro NAB. (2006) Análises cromossômicas e filogenia de roedores do gênero Proechimys (Echimyidae, Rodentia). Ph.D. Thesis, Universidade Federal do Pará, Belém, Brazil, 95 pp. [In Portuguese] [Google Scholar]
- Rieseberg LH. (2001) Chromosomal rearrangements and speciation. Trends in Ecology & Evolution 16(7): 351–358. https://doi.org/10.1016/S0169-5347(01)02187-5 [DOI] [PubMed] [Google Scholar]
- Rocha RG, Ferreira E, Costa B, Martins I, Leite YL, Costa LP, Fonseca C. (2011) Small mammals of the mid-Araguaia River in central Brazil, with the description of a new species of climbing rat. Zootaxa 2789: 1–34. [Google Scholar]
- Romanenko SA, Volobouev V. (2012) Non-Sciuromorph rodent karyotypes in evolution. Cytogenetic and Genome Research 137(2-4): 233–245. https://doi.org/10.1159/000339294 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12): 1572–1574. https://doi.org/10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Rossi NF. (2011) Pequenos mamíferos não-voadores do Planalto Atlântico de São Paulo: identificação, história natural e ameaças Master Dissertation, Universidade de São Paulo, São Paulo, Brazil 388 pp. [In Portuguese]
- Salazar-Bravo J. (2015) Genus Calomys In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America, Volume 2: Rodents. University of Chicago Press, 481–507.
- Sbalqueiro IJ, Suñe-Mattevi M, Oliveira LFB. (1984) An X1X1X2X2/X1X2Y mechanism of sex determination in a South American Deltamys kempi (Rodentia, Cricetidae). Cytogenetics and Cell Genetics 38: 50–55. https://doi.org/10.1159/000132029 [DOI] [PubMed] [Google Scholar]
- Sbalqueiro IJ. (1989) Análises cromossômicas e filogenéticas em algumas espécies de roedores da região Sul do Brasil. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 297 pp. [In Portuguese] [Google Scholar]
- Sbalqueiro I, Mattevi M, Oliveira L, Solano MB. (1991) B chromosome system in populations of Oryzomys flavescens (Rodentia, Cricetidae) from southern Brazil. Acta Theriologica 36: 193–9. https://doi.org/10.4098/AT.arch.91-18, oai:rcin.org.pl:11751 [Google Scholar]
- Silva CR, Percequillo AR, Ximenes GI, De Vivo M. (2003) New distributional records of Blarinomys breviceps (Winge, 1888) (Sigmodontinae, Rodentia). Mammalia 67(1): 147–152. [Google Scholar]
- Silva MJJ. (1994) Estudos cromossômicos e de complexos sinaptonêmicos em roedores brasileiros da tribo Oryzomyini (Cricetidae, Rodentia). Master’s Dissertation, Universidade de São Paulo, São Paulo, Brazil, 168 pp. [In Portuguese] [Google Scholar]
- Silva MJJ, Yonenaga-Yassuda Y. (1997) New karyotypes of two related species of Oligoryzomys genus (Cricetidae, Rodentia) involving centric fusion with loss of NORs and distribution of telomeric (TTAGGG)n sequences. Hereditas 127: 217–229. https://doi.org/10.1111/j.1601-5223.1997.00217.x [DOI] [PubMed] [Google Scholar]
- Silva MJJ, Yonenaga-Yassuda Y. (1998a) Karyotype and chromosomal polymorphism of an undescribed Akodon from Central Brazil, a species with the lowest known diploid chromosome number in rodents. Cytogenetic and Genome Research 81(1): 46–50. https://doi.org/10.1159/000015006 [DOI] [PubMed] [Google Scholar]
- Silva MJJ, Yonenaga-Yassuda Y. (1998b) Heterogeneity and meiotic behaviour of B and sex chromosomes, banding patterns and localization of (TTAGGG)n sequences by FISH, in the Neotropical water rat Nectomys (Rodentia, Cricetidae). Chromosome Research 6: 455–462. https://doi.org/10.1023/A:1009248311530 [DOI] [PubMed] [Google Scholar]
- Silva MJJ. (1999) Estudo dos processos de diferenciação cariotípica, baseados em citogenética convencional e molecular, em quatro gêneros de roedores brasileiros. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 141 pp. [In Portuguese] [Google Scholar]
- Silva MJJ, Yonenaga-Yassuda Y. (1999) Autosomal and sex chromosomal polymorphism with multiple rearrangements and a new karyotype for the genus Rhipidomys (Sigmodontinae, Rodentia). Hereditas 131: 211–220. https://doi.org/10.1111/j.1601-5223.1999.00211.x [DOI] [PubMed] [Google Scholar]
- Silva MJJ, Percequillo AR, Yonenaga-Yassuda Y. (2000) Cytogenetics and systematic approach on a new Oryzomys species, of the nitidus group (Sigmodontinae, Rodentia) from Northeastern Brazil. Caryologia 53(3-4): 219–226. http://dx.doi.org/10.1080/00087114.2000.10589199 [Google Scholar]
- Silva MJJ, Yonenaga-Yassuda Y. (2004) B chromosomes in Brazilian rodents. Cytogenetic and Genome Research 106(2-4): 257–263. https://doi.org/10.1159/000079296 [DOI] [PubMed] [Google Scholar]
- Silva MJJ, Patton JL, Yonenaga-Yassuda Y. (2006) Phylogenetic relationships and karyotype evolution in the sigmodontine rodent Akodon (2n = 10 and 2n = 16) from Brazil. Genetics and Molecular Biology 29(3): 469–474. http://dx.doi.org/10.1590/S1415-47572006000300012 [Google Scholar]
- Silva WO, Pieczarka JC, Rossi RV, Schneider H, Sampaio I, Miranda CL, Silva CR, Cardoso EM, Nagamachi CY. (2015) Diversity and Karyotypic Evolution in the Genus Neacomys (Rodentia, Sigmodontinae). Cytogenetic and Genome Research 146: 296–305. https://doi.org/10.1159/000441173 [DOI] [PubMed] [Google Scholar]
- Smith MF, Patton JL. (1993) The diversification of South American murid rodents: evidence from mitochondrial DNA sequence data for the akodontine tribe. Biological Journal of the Linnean Society 50(3): 149–177. https://doi.org/10.1111/j.1095-8312.1993.tb00924.x [Google Scholar]
- Smith MF, Patton JL. (1999) Phylogenetics relationships and the radiation of Sigmodontinae Rodents in South America: Evidence from cytocrome b. Journal Mammalian Evolution 6: 89–128. https://doi.org/10.1023/A:1020668004578 [Google Scholar]
- Sousa MAN. (2005) Pequenos mamíferos (Didelphimorphia, Didelphidae e Rodentia, Sigmodontinae) de algumas áreas da Caatinga, Cerrado, Mata Atlântica e Brejo de Altitude do Brasil: considerações citogenéticas e geográficas. Ph.D. Thesis, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 143 pp. [In Portuguese] [Google Scholar]
- Souza LTMD, Suzuki A, Pereira LE, Ferreira IB, Souza RPD, Cruz AS, Ikeda TI, Moreira FG, Peres JB, Silva JG, Caldas EP, Dalmaso MH, Garrot PG, Torres EM, Castagen MC, Romano APM, Paula VR, Marques CCA. (2002) dentificação das espécies de roedores reservatórios de hantavírus no sul e sudeste do Brasil. Informe Epidemiológico do Sus 11(4): 249–251. http://dx.doi.org/10.5123/S0104-16732002000400008 [Google Scholar]
- Souza ALEG, Corrêa MMDO, Pessôa LM. (2006) Morphometric discrimination between Trinomys albispinus (Is. Geoffroy, 1838) and Trinomys minor (Reis & Pessôa, 1995) from Chapada Diamantina, Bahia, Brazil, and the karyotype of Trinomys albispinus (Rodentia, Echimyidae). Arquivos do Museu Nacional 64(4): 325–332. [Google Scholar]
- Souza MJ. (1981) Caracterização cromossômica em oito espécies de roedores brasileiros das famílias Cricetidae e Echimiydae. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil 198 pp.
- Souza MJ, Yonenaga-Yassuda Y. (1982) Chromosomal variability of sex chromosomes and NOR’s in Trichomys apereoides (Rodentia, Echimyidae). Cytogenetic and Cell Genetics 33: 197–203. https://doi.org/10.1159/000131755 [DOI] [PubMed] [Google Scholar]
- Souza MJ, Yonenaga-Yassuda Y. (1984) G- and C-band pattern and nucleolus organizer regions in somatic chromosomes of Clyomys laticeps laticeps (Rodentia, Echimyidae). Experientia 40: 96–97. https://doi.org/10.1007/BF01959122 [Google Scholar]
- Souza AL, Corrêa MM, Pessôa LM. (2007) The first description of the karyotype of Dasyprocta azarae Lichtenstein, 1823 (Rodentia, Dasyproctidae) from Brazil. Mastozoología Neotropical 14(2): 227–233. [Google Scholar]
- Souza AL, Corrêa MM, Aguilar CTD, Pessôa LM. (2011) A new karyotype of Wiedomys pyrrhorhinos (Rodentia: Sigmodontinae) from Chapada Diamantina, northeastern Brazil. Zoologia (Curitiba) 28(1): 92–96. http://dx.doi.org/10.1590/S1984-46702011000100013 [Google Scholar]
- Stolz JFB. (2012) Taxonomia e sistemática dos tuco-tucos do noroeste do Brasil (Rodentia-Ctenomyidae) e a descrição de uma nova espécie da Amazônia brasileira. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 118 pp. [In Portuguese] [Google Scholar]
- Suárez P, Nagamachi CY, Lanzone C, Malleret MM, O’Brien PCM, Ferguson-Smith MA, Pieczarka JC. (2015) Clues on syntenic relationship among some species of Oryzomyini and Akodontini tribes (Rodentia: Sigmodontinae). PLOS One 10(12): e0143482. https://doi.org/10.1371/journal.pone.0143482 [DOI] [PMC free article] [PubMed]
- Suárez-Villota EY, Di-Nizo CB, Neves CL, Silva MJJ. (2013) First cytogenetic information for Drymoreomys albimaculatus (Rodentia, Cricetidae), a recently described genus from Brazilian Atlantic Forest. ZooKeys 303: 65–76. https://doi.org/10.3897/zookeys.303.4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Villota EY, Carmignotto APC, Brandão MV, Percequillo AR, Silva MJJ. (2017) Species delimitation in the genus Oecomys (Sigmodontinae: Oryzomyini): phylogenetic, cytogenetic and morphological approaches reveal a cryptic diversity. Zoological Journal of the Linnean Society. [In press]
- Sumner AT. (1972) A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research 75: 304–306. https://doi.org/10.1016/0014-4827(72)90558-7 [DOI] [PubMed] [Google Scholar]
- Sumner AT. (2003) Chromosomes: organization and function. Blackwell Publishing, 287 pp.
- Svartman M. (1989) Levantamento cariotípico de roedores da região do Distrito Federal. Master’s Dissertation, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 63 pp. [In Portuguese] [Google Scholar]
- Svartman M, Almeida EJC. (1992) Sex chromosomes polymorphisms in Oryzomys aff. subflavus (Cricetidae, Rodentia) from central Brazil. Caryologia 45(3-4): 3 13–324. https://doi.org/10.1080/00087114.1992.10797234
- Svartman M, Almeida EJC. (1993a) Robertsonian fusion and X chromosome polymorphism in Zygodontomys (= Bolomys) lasiurus (Cricetidae, Rodentia) from Central Brazil Revista Brasileira. Genetica 16(1): 225–235. [Google Scholar]
- Svartman M, Almeida EJC. (1993b) Karyotype of Oxymycterus sp (Cricetidae, Rodentia) from Central Brazil. Experientia 49: 718–20. https://doi.org/10.1007/BF01923959 [Google Scholar]
- Svartman M, Almeida EJC. (1994) The karyotype of Akodon lindberghi Hershkovitz, 1990 (Cricetidae, Rodentia) from Central Brazil. Revista Brasileira de Genética 17: 963–972. [Google Scholar]
- Tavares WC, Pessôa LM, Gonçalves PR. (2011) New species of Cerradomys from coastal sandy plains of southeastern Brazil (Cricetidae: Sigmodontinae). Journal of Mammalogy 92: 645–58. https://doi.org/10.1644/10-MAMM-096.1 [Google Scholar]
- Taylor PH, Lowman MD. (1996) Vertical stratification of the small mammal community in a Northern hardwood forest Selbyana 17(1): 15–21. http://www.jstor.org/stable/41759919?seq = 1&cid = pdf- reference#references_tab_contents
- Testoni AF, Althoff SL, Nascimento AP, Steiner-Souza F, Sbalqueiro IJ. (2010) Description of the karyotype of Rhagomys rufescens Thomas, 1886 (Rodentia, Sigmodontinae) from Southern Brazil Atlantic forest. Genetics and Molecular Biology 33(3): 479–485. http://dx.doi.org/10.1590/S1415-47572010005000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomazini NB. (2009) Correlação entre estrutura cariotípica e filogenia molecular em Rhipidomys (Cricetidae, Rodentia) do leste do Brasil. Master’s Dissertation, Universidade Federal do Espírito Santo, Brazil, 95 pp. [In Portuguese] [Google Scholar]
- Tribe CJ. (2005) A new species of Rhipidomys (Rodentia, Muroidea) from north-eastern Brazil. Arquivos do Museu Nacional 63(1): 131–146. [Google Scholar]
- Tribe CJ. (2015) Genus Rhipidomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 583–616.
- Vargas LKU, López Ortiz JB. (2010) Caracterizacion Citogenética por Bandas R-Replicativas de la Guagua de Cola (Dynomis branickii). Revista Facultad Nacional de Agronomía 63(1): 5355–5362. [Google Scholar]
- Ventura K, Silva MJJ, Fagundes V, Pardini R, Yonenaga- Yassuda Y. (2004) An undescribed karyotype of Thaptomys (2n = 50) and the mechanism of differentiation from Thaptomys nigrita (2n = 52) evidenced by FISH and Ag-NORs. Caryologia 57: 89–97. http://dx.doi.org/10.1080/00087114.2004.10589376 [Google Scholar]
- Ventura K, Silva MJJ, Fagundes V, Christoff AU, Yonenaga-Yassuda Y. (2006) Non-telomeric sites as evidence of chromosomal rearrangement and repetitive (TTAGGG) n arrays in heterochromatic and euchromatic regions in four species of Akodon (Rodentia, Muridae). Cytogenetic and Genome Research 115(2): 169–175. https://doi.org/10.1159/000095238 [DOI] [PubMed] [Google Scholar]
- Ventura K, Ximenes GEI, Pardini R, Sousa MA, Yonenaga-Yassuda Y, Silva MJJ. (2008) Karyotypic analyses and morphological comments on the endemic and endangered Brazilian painted tree rat Callistomys pictus (Rodentia, Echimyidae). Genetics and Molecular Biology 31(3): 697–703. http://dx.doi.org/10.1590/S1415-47572008000400016 [Google Scholar]
- Ventura K, O’Brien PC, Yonenaga-Yassuda Y, Ferguson-Smith MA. (2009) Chromosome homologies of the highly rearranged karyotypes of four Akodon species (Rodentia, Cricetidae) resolved by reciprocal chromosome painting: the evolution of the lowest diploid number in rodents. Chromosome Research 17(8): 1063–1078. https://doi.org/10.1007/s10577-009-9083-5 [DOI] [PubMed] [Google Scholar]
- Ventura K, Silva MJJ, Yonenaga-Yassuda Y. (2010) Thaptomys Thomas 1915 (Rodentia, Sigmodontinae, Akodontini) with karyotypes 2n = 50, FN = 48, and 2n = 52, FN = 52: Two monophyletic lineages recovered by molecular phylogeny. Genetics and Molecular Biology 33(2): 256–261. https://doi.org/10.1590/S1415-47572010005000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura K, Fagundes V, D’Elía G, Christoff AU, Yonenaga-Yassuda Y. (2011) A new allopatric lineage of the rodent Deltamys (Rodentia: Sigmodontinae) and the chromosomal evolution in Deltamys kempi and Deltamys sp. Cytogenetic and Genome Research 135(2): 126–134. https://doi.org/10.1159/000331584 [DOI] [PubMed] [Google Scholar]
- Ventura K, Sato-Kuwabara Y, Fagundes V, Geise L, Leite YLR, Costa LP, Silva MJJ, Yonenaga-Yassuda Y, Rodrigues MT. (2012) Phylogeographic structure and karyotypic diversity of the Brazilian shrew mouse (Blarinomys breviceps, Sigmodontinae) in the Atlantic Forest. Cytogenetic and Genome Research 138(1): 19–30. https://doi.org/10.1159/000341887 [DOI] [PubMed] [Google Scholar]
- Ventura K, O’Brien PCM, Moreira CN, Yonenaga-Yassuda Y, Ferguson-Smith MA. (2015) .On the origin and evolution of the extant system of B chromosomes in Oryzomyini radiation (Rodentia, Sigmodontinae). PLOS One 10(8): e0136663. https://doi.org/10.1371/journal.pone.0136663 [DOI] [PMC free article] [PubMed]
- Vidal OR, Riva R, Baro NI. (1976) Los cromosomas del género Holochilus I Polimorfismo en H. chacarius Thomas (1906). Physis 35: 75–85. [Google Scholar]
- Vilela JF. (2005) Filogenia molecular de Brucepattersonius (Sigmodontinae: Akodontini) com uma análise morfométrica craniana do gênero. Ph.D. Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 67 pp. [In Portuguese] [Google Scholar]
- Vilela RV, Machado T, Ventura K, Fagundes V, Silva MJJ, Yonenaga-Yassuda Y. (2009) The taxonomic status of the endangered thin-spined porcupine, Chaetomys subspinosus (Olfers, 1818), based on molecular and karyologic data. BMC Evolutionary Biology 9(1): 29. https://doi.org/10.1186/1471-2148-9-29 [DOI] [PMC free article] [PubMed]
- Vitullo AD, Merani MS, Reig OA, Kajon AE, Scaglia O, Espinosa MB, Perez-Zapata A. (1986) Cytogenetics of South American akodont rodents (Cricetidae): new karyotypes and chromosomal banding patterns of Argentinian and Uruguayan forms. Journal of Mammalogy 67(1): 69–80. https://doi.org/10.2307/1381003 [Google Scholar]
- Voss RS. (1992) A revision of the South American species of Sigmodon (Mammalia: Muridae) with notes on their natural history and biogeography. American Museum Novitates 3050: 56 pp.
- Voss RS, Lunde DP, Simmons NB. (2001) The mammals of Paracou, French Guiana: a neotropical lowland rainforest fauna part 2 Nonvolant species. Bulletin of the American Museum of Natural History 263: 3–236. https://doi.org/10.1206/0003-0090(2001)263<0003:TMOPFG>2.0.CO;2 [Google Scholar]
- Voss RS. (2015a) Genus Neusticomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 287–292.
- Voss RS. (2015b) Genus Zygodontomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 460–464.
- Voss RS. (2015c) Genus Coendou. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 791–804.
- Walker LI, Soto MA, Spotorno ÁE. (2014) Similarities and differences among the chromosomes of the wild guinea pig Cavia tschudii and the domestic guinea pig Cavia porcellus (Rodentia, Caviidae). Comparative Cytogenetics 8(2): 153–167. https://doi.org/10.3897/CompCytogen.v8i2.7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh PS, Metzger DA, Higuchi R. (1991) Chelex 100 as a medium for simple extraction of DNA for PCR based typing from forensic material. Biotechniques 10: 506–513. [PubMed] [Google Scholar]
- Wahrman J, Goitein R, Nevo E. (1969) Mole rat Spalax: evolutionary significance of chromosome variation. Science 164(3875): 82–84. https://doi.org/10.1126/science.164.3875.82 [DOI] [PubMed] [Google Scholar]
- Weksler M, Bonvicino CR. (2005) Taxonomy of pigmy rice rats genus Oligoryzomys Bangs, 1900 (Rodentia, Sigmodontinae) of the Brazilian Cerrado, with the description of two new species. Arquivos do Museu Nacional 63(1): 113–30. [Google Scholar]
- Weksler M, Percequillo AR, Voss RS. (2006) Ten new genera of oryzomyine rodents (Cricetidae, Sigmodontinae). American Museum Novitates 3537. https://doi.org/10.1206/0003–0082(2006)3537[1:TNGOOR]2.0.CO;2
- Weksler M, Bonvicino CR. (2015) Genus Oligoryzomys. In: Patton JL, Pardiñas UFJ, D’Elía G. (Eds) Mammals of South America. Volume 2: Rodents. University of Chicago Press, 417–436.
- Woods CA, Kilpatrick CW. (2005) Infraorder Hystricognathi In: Wilson DE, Reeder DM. (Eds) Mammal Species of the World, A Taxonomic and Geographic Reference. The Johns Hopkins University Press, Baltimore, 1538–1600.
- Wurster DH, Snapper JR, Benirschke K. (1971) Unusually large sex chromosomes: new methods of measuring and description of karyotypes of six rodents (Myomorpha and Hystricomorpha) and one lagomorph (Ochotonidae). Cytogenetic and Genome Research 10(3): 153–176. https://doi.org/10.1159/000130136 [DOI] [PubMed] [Google Scholar]
- Yonenaga Y. (1972) Polimorfismos cromossômicos em roedores brasileiros. Ph.D. Thesis, Instituto de Biociências, Universidade de São Paulo, São Paulo, Brazil, 214 pp. [In Portuguese] [Google Scholar]
- Yonenaga Y. (1975) Karyotypes and chromosome polymorphisms in Brazilian rodents. Caryologia 28: 269–286. http://dx.doi.org/10.1080/00087114.1975.10796617 [Google Scholar]
- Yonenaga Y, Frota-Pessoa O, Kasahara S, Almeida EJC. (1976) Cytogenetic studies on Brazilian rodents. Ciência e Cultura 28: 202–211. [Google Scholar]
- Yonenaga-Yassuda Y, Souza MJ, Kasahara S, L’Abbate M, Chu HT. (1985) Supernumerary system in Proechimys iheringi iheringi (Rodentia, Echimyidae), from the state of Sao Paulo, Brazil. Caryologia 38(2): 179–194. http://dx.doi.org/10.1080/00087114.1985.10797742 [Google Scholar]
- Yonenaga-Yassuda Y, Prado RC, Mello DA. (1987a) Supernumerary chromosomes in Holochilus brasiliensis and comparative analysis with Nectomys squamipes (Cricetidae, Rodentia). Revista Brasileira de Genética (5): 209–220.
- Yonenaga-Yassuda Y, Pereira LA, Armada JL, L’Abbate M. (1987b) Chromosomal polymorphism in Akodon reinhardti Langguth, 1975 (Rodentia, Cricetidae). Revista Brasileira de Genética (2): 199–208.
- Yonenaga-Yassuda Y, Maia V, L’Abbate M. (1988) Two tandem fusions and supernumerary chromosomes in Nectomys squamipes (Cricetidae, Rodentia). Caryologia 41: 25–39. http://dx.doi.org/10.1080/00087114.1988.10797845 [Google Scholar]
- Zanchin NIT. (1988) Estudos cromossômicos em orizominos e equimídeos da Mata Atlântica Porto Alegre. Master’s Dissertation, Instituto de Biociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 162 pp. [In Portuguese] [Google Scholar]
- Zanchin NIT, Langguth A, Mattevi MS. (1992) Karyotypes of Brazilian species of Rhipidomys (Rodentia, Cricetidae). Journal of Mammalogy 73: 120–122. https://doi.org/10.2307/1381872 [Google Scholar]
- Zappes IA, Portella AS, Lessa GM. (2014) Description of Karyotype of Kerodon acrobata, an endemic rodent in Brazilian Cerrado. Brazilian Journal of Biology 74(1): 251–256. http://dx.doi.org/10.1590/1519-6984.23512 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Camilla Bruno Di-Nizo, Karina Rodrigues da Silva Banci, Yukie Sato Kuwabara, Maria José de J. Silva
Data type: molecular data
Explanation note: Sequences analysed for phylogenetic reconstruction (Maximum likelihood and Bayesian Inference) of Neacomys, with species, GenBank and lab/ field number, diploid and fundamental number (when available), locality and reference.
Abbreviations: N/A means that the information is not available. *Cytogenetic data analysed in this work. In bold, sequences obtained in this work. Coordinates for Neacomys specimens studied herein: Amazonas State: Igarapé-Açu (04°20'S, 58°38'W); Mato Grosso State: Aripuanã (10°10'S, 59°27"W); Cláudia (11°30'S, 54°53'W); Vila Rica (09°54'S, 51°12'W). Museum and collector acronyms for specimens studied herein: APC (Ana Paula Carmignotto), CIT (Laboratório de Citogenética de Vertebrados - IBUSP), MTR (Miguel Trefaut Rodrigues), MZUSP (Museu de Zoologia, Universidade de São Paulo, Brazil) and PEU (Pedro Luís Bernardo da Rocha).