Abstract
New methods, derived from animal work, for measuring food reward value (i.e. reinforcing value of food), and motivation (i.e. strength of desire) to consume, in humans are described and validated. A sipping device (sipometer) was developed that permits access to a liquid food or beverage on two reward schedules: continuous reinforcement (CR) and progressively increasing time spent exerting pressure on a straw (PR-schedule). In addition, a pictorial scale showing a cup, from which the ‘amount wanted’ could be marked was used to pre-test potential consumption. Intake, time spent sipping, breakpoint, and pressure exerted were the main dependent variables measured. Three pilot experiments were conducted. In Experiment 1, participants (n = 8) consumed yogurt shakes after a 1-h or 21-h food deprivation period on both schedules. In Experiment 2, participants (n = 8) sham-consumed (i.e. spit out) sweet and non-sweet beverages, utilizing both schedules. In Experiment 3, sham-consuming sweet and non-sweet beverages on both schedules and working for shake on the PR schedule were repeated, after three nights of either habitual sleep or short sleep duration (n = 7) in a crossover design. In Experiment 1, participants sipped longer after 21-h vs. 1-h of food deprivation (13 ± 3.0 vs. 8.0 ± 2.1 s; p = 0.04), on the PR schedule. In Experiment 2, sham-intake (p = 0.01) and sipping time (p = 0.04) were greater for sweet than non-sweet beverages on the PR schedule and a similar, though not conventionally significant, effect was observed for exerted pressure (p = 0.09). In both Experiment 2 and Experiment 3 after habitual sleep, on the PR schedule, cumulative pressure difference between sweet and non-sweet beverage increased with difference in amount wanted in the taste test. In contrast, after short sleep participants were less willing to work for sweet taste as their wanting increased, suggesting that sleep deprivation raises desire, but lowers behavioral output. Taken together these results demonstrate that the sipometer and associated ratings are reliable and useful measures of motivation to consume and reward value in humans. Participants were more motivated to obtain access to sweet beverages, especially when these were better liked than to obtain access to non-sweet beverages.
Keywords: Reward, Reinforcing value, Motivation, Consummatory behavior, Liking wanting
1. Introduction
The aim of the experiments described in this paper was to validate new methods for measuring a person’s motivation to eat and the reward value. We combined a progressive ratio (PR) sipping task and a scaling procedure for the participant to indicate the amount of reinforcer he/she wanted to consume. During the sipping task, participants consumed a beverage on different reward schedules from a specially designed sipping device (the ‘sipometer’). This task was based on animal studies [1,2] in which the reinforcer was either continuously available (continuous reinforcement, CR) or required extra effort to obtain access to it (a PR schedule of reinforcement). The size of a liquid portion the participant “wanted” to consume was assessed by means of a pictorial method that we expected would predict the amount of effort the participant would expend. A visual analogue scale (VAS) was used to assess hedonic evaluation as a separate predictor of effort.
Both pleasantness of a food (i.e. ‘liking’, a hedonic sensation) and the motivation to engage in eating or drinking (i.e. ‘wanting’) are important determinants of food intake [3,4] (see [5] for some current thoughts about motivation). In humans, increases in food deprivation or food palatability increased PR responding for snack food rewards [6–8], and PR responding was elevated in obese compared to normal weight individuals and in individuals with binge eating disorder (BED [9]) and bulimia [6,10] compared to healthy individuals. Pleasantness of food and motivation to consume, also varied across individuals with different dopamine D2 receptor genotypes (16). Thus, reward value, measured by the PR response task is influenced by both the “liking” and the “wanting” components of reward, and which one predominates is determined by the independent variables (e.g. internal signals vs external stimuli).
Following Stellar [11], we consider the amount of motivated behavior for food or beverage (reinforcers) as a reflection of the amount of neural activity in certain parts of the brain (not exclusively hypothalamus as in the original formulation) needed to perform a specific behavior in order to obtain the reinforcer. To distinguish behavior driven mainly by internal stimuli from behavior driven mainly by external stimuli, in this paper, we refer to “motivation” as behavior resulting from changes in the individual’s internal environment, and to “reinforcing value”, or “reward value”, by changes in the sensory properties of the reinforcer. “Reward value” is related to motivation, but refers to the idea that the behavior is based on the evaluation [12] of the reinforcer by the individual that is being tested. Thus, the method for verifying the reward or reinforcing value is behavioral. In the seminal study that led to this idea, Sheffield and Roby [13] examined responsiveness to non-nutritive solutions in rats and concluded that “…when total food intake is held constant, the reward value of eating is a function of the amount of consummatory activity required to ingest the food”. The strength of each of these constructs is typically measured by comparing effort expended to obtain the reinforcer under at least two different experimental conditions.
A commonly used measure of motivation is the PR task [14]. In animal studies, rats are typically trained to press levers to obtain small quantities of food in operant reinforcement paradigms. Similar measures have been applied in humans by means of games or procedures in which the participant must progressively increase the number of button presses to obtain food rewards [12,15,16]. However, these procedures introduce artificial elements in the behavioral chain of eating activities. Consequently, Sclafani and Ackroff [2] utilized a PR task in which they replaced lever pressing activity by licking, a behavior that is a natural part of the rodent’s consummatory behavior. An equally natural analog from human ingestive behavior that would measure human motivation is sipping on a straw to obtain a beverage.
To test whether this analog to animal behavior was valid and useful, three pilot experiments were conducted. In the first, we measured food intake and effort to obtain it after different periods of fasting (1 h vs. 21 h), in order to manipulate the physiological state motivation to work for a food [17–20]. Thus, to demonstrate the proof of principle, our first hypothesis is that effort to obtain a food will increase with increased duration of food deprivation.
In the second experiment, we measured reward value for sweet and non-sweet beverages by means of a modified sham-feeding method [21]. Modified sham-feeding is used to assess the orosensory contribution of sweet taste to intake in the absence of postingestive stimulation, because the stimulus is limited to the mouth. This procedure was adopted for several reasons: 1) It is possible to test several beverages in the same session without the participant feeling full. 2) The procedure may be able to discriminate responses of normal and obese participants [22]. 3) In previous studies in which modified sham-feeding was used with similar beverages, intake was a function of sweetener concentration [21,23,24]. 4) The inference from these studies is that sweet taste, by itself, is a reinforcer that motivates consumption in humans, and motivation is measured by the participant’s display of increased effort. Thus, the second hypothesis is that more effort will be exerted to obtain access to sweet, than to non-sweet, drinks. Two schedules of reinforcement were utilized, continuous reinforcement (CR) and progressive ratio (PR). There were two reasons for use of the CR schedule. First, we wanted to determine whether introduction of sipping from the device without any extra effort would enable us to replicate a previously demonstrated increase in sham-intake of a sweet tasting beverage without any special device [24]. In that study participants sipped each beverage for 3 one-minute periods. Since we could only do one period, because of the other trials, we opted for a single 2 min time period for CR. Second, comparison of PR and CR schedules on various responses (e.g. intake, pressure exerted, time spent sipping) to sweet tasting beverages enabled the investigators to discriminate the effects of introducing effort, compared to no effort, to obtain the beverages.
In the final experiment, we applied the sham-feeding measure with the sipometer to investigate motivation for access to sweet and non-sweet beverages in a situation associated with increased food intake: short sleep duration. Short sleep duration and/or total sleep deprivation is positively associated with weight gain and body mass index (BMI) [25,26], increases in appetite [27], portion size [28], food intake [29–31], circulating ghrelin levels [28,32,33], and increased brain activity in response to presentation of food pictures [34–36]. The selection of larger portions of especially snack foods [28,30] and the greater brain activation when participants viewed “unhealthy” compared to “healthy” foods [34–36] further suggested that sleep restriction increased sensitivity to food reward. With these results in mind, we predicted that individuals would be willing to work harder for a reward following sleep loss (compared to a period of habitual sleep). We predicted increased motivation for reward after sleep loss.
2. Methods
2.1. General methods
2.1.1. Sipping device: the sipometer and reward schedules
In all experiments, the beverages were consumed from the ‘sipometer’. Two models of the sipometer were used. For Experiment 1 the sipping device consisted of a plastic “straw” attached to food-grade tubing that passed through a solenoid controlled (24 V dc) pressure-operated valve into a reservoir containing the liquid test diet. When the solenoid was in the open position, fluid could flow through the straw when the participant sipped; when it was in the closed position, the tubing was compressed and fluid could not flow through. The straw was attached to a swivel that closed a micro-switch when the participant pulled the tube forward with his/her mouth for sipping. The micro-switch was interfaced with a computer, and a program written in Basic controlled the valve.
Beverages were consumed on two different reward schedules. During the CR schedule, the solenoid remained open as long as the participant was sipping, allowing for ad libitum intake. During the PR schedule, the solenoid was open at the start of the test meal, but was then opened and closed during the meal, resulting in limited access. The sipping time required to obtain access to the food was progressively increased by 3 s increments. On both schedules, sipping time was recorded by the computer.
In the second model (see Fig. 1 right side), the switch was replaced by a pressure sensor in line with tubing connected to the straw. When the participant exerted pressure exceeding 0.5 PSI, for the requisite amount of time (0 s for CR, vs. increasing durations between reinforcements for PR), the computer activated a compressed air-controlled valve. This version of the sipometer was invented by Anthony Sclafani and designed and fabricated at the John B Pierce Laboratory in collaboration with Anthony Sclafani, Harry Kissileff and Dana Small. The device is currently being patented and developed for dissemination. For more information please contact Dana Small (dana.small@yale.edu).
Fig. 1.
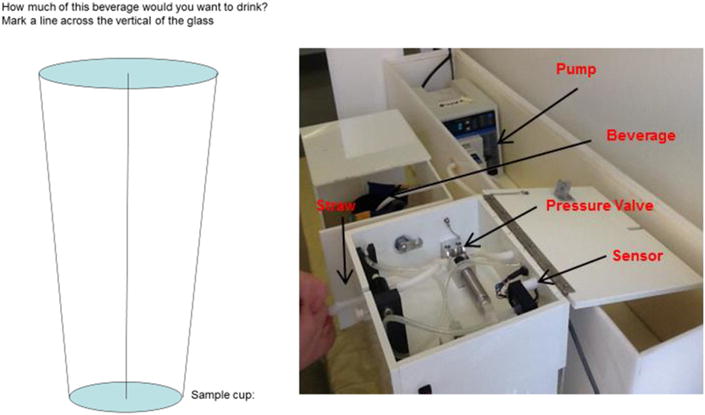
Left: Wanting scale. The dimensions of the cup shown to the participant were 18.3 cm high × upper diameter 4.9 cm lower diameter 3.1 cm. The volume (in ml) was computed by applying the conic formula (3.1417 is pi, A2 is the height of the cup marked by the participant): Volume = 3.1417 × ((3.12) × (A2) + (3.1 × (4.9 − 3.1) × ((A2)2)) / 18.3 + (((4.9 − 3.1)2) × ((A2)3) / (3 × (18.3)2))). Right: Final model of the sipometer.
In the pilot study for Experiment 2, the participant could obtain different amounts of food under the two different reward conditions, either by sipping harder when a sweet reinforcer was provided, or by letting the non-sweet reinforcer fall back into the reservoir. Therefore, in the second and third experiments, a peristaltic pump was added to the sipping device to control the amount of beverage that participants could access when the valve was open. Consequently, the reward size was the same for each reinforcer (7 ml in Experiment 2 and 10 ml in Experiment 3), thereby eliminating the possibility that differences in sip size (following larger or longer sips) would affect the results [37–39]. The second model also provided a continuous record of both the cumulative intake, by recording the weight of the test food in the reservoir every 0.1 s, as had been previously recorded in experiments from this laboratory [40], and the pressure exerted on the straw. The pressure recording permitted the controlling program (written in MATLAB) to track whether the participants were actually sipping, rather than merely pulling or placing their mouths on the straw. This recording allowed the experimenters both to set criteria for the intensity and duration of sipping needed to obtain the reinforcer, and to utilize the sipping pattern as a measure of the motivation to consume.
Measures of interest provided by the computerized programs included the total intake of beverage, the sipping time, cumulative pressure on the straw (obtained by summing only positively increasing pressures every tenth of a second), total sipping time, the breakpoint (i.e. the highest ratio of sipping for a food reward completed before quitting the task), and total number of sips (uninterrupted bouts of pressure exerted).
In order to prevent post-ingestive effects of the reinforcer, a modified sham-feeding method for use in humans [21,23,24] was utilized for the sweet and non-sweet beverages in Experiments 2 and 3 (i.e. participants expectorated these beverages). Prior to the experimental session, participants engaged in a brief training session during which they practiced the sham-feeding procedure with water on a CR schedule for 1 min. In all experiments, participants were tested individually in a quiet room with the sipometer that was connected with the computer in the adjacent room. The experimenter ensured that participants completed the pre-meal questionnaires and was familiar with the procedures (see “instructions” below), and then left to the adjacent room.
2.1.2. Participants (general)
Healthy women were recruited via online advertisements and flyers in and around the St. Luke’s/Roosevelt hospital. Participants had to be non-smokers, and without history or current diagnosis of eating disorders or phenylketonuria. Participants were excluded if they had scores >11 on the Stunkard and Messick scale of restrained eating [41]. All experimental procedures were approved by the St. Luke-Roosevelt’s Institutional Review Board and conducted according to NIH guidelines of Good Clinical Practice.
2.1.3 Instructions to the participants
In all experiments, the participants were familiarized with the sipometer prior to the test sessions. They were informed that in some trials, the reinforcement might stop periodically but if they continued to sip, it would again be available. During the testing session, this instruction for the PR-schedule was provided via tape recordings: “If the shake isn’t available keep sucking and it will become available again. The shake will sometimes be available, and sometimes will not be available. This is purposeful and is not due to any malfunctioning of the equipment. Continue to suck until you have had enough.” Additionally, participants in Experiments 2 and 3 were instructed to “remember to spit out into the container after each sip” when sham-consuming the sweet and non-sweet beverages. For the CR-schedules in Experiments 2 and 3, participants were instructed to “sip the beverage and remember to spit out into the container after each sip. The beverage will be delivered automatically to your mouth and you will need to sip in order to activate the delivery. Sip as much of the beverage as you want.” The sipping time was limited to 2 min by the experimenter remotely activating a tape recording which sounded a tone and instruction to stop sipping.
2.1.3. Questionnaires for assessment of feelings and dispositions related to motivation
Before and after consumption, participants rated the perceived intensity of sweetness and liking of the beverage, and the extent to which they enjoyed consuming the shake on Likert scales and lines marked with anchors (see legends below tables for specific information from each variable measured). The test beverages used in each experiment are described below and details are provided in Tables 1 and 2.
Table 1.
Yogurt shake nutrient composition and preparation for Experiment 1.
| Ingredient | Weight (g) |
Protein (g) |
Carbohydrate (g) |
Fat (g) |
|---|---|---|---|---|
| Dannon lowfat plain yogurt | 843.8 | 54.6 | 79.4 | 17.4 |
| 10% Maltodextrin solution | 337.5 | 0 | 135 | 0 |
| Cream | 37.5 | 0.8 | 1.1 | 12.9 |
| Equal | 12 | 0 | 0 | 0 |
| Flavoring | 22.5 | 0 | 0 | 0 |
| Total | 1253.3 | 55.3 | 215.5 | 30.2 |
| Total kcal | 1355.7 | 221.4 | 862.2 | 272.2 |
| Energy density (kcal) / weight (g) | 1.1 | 16.3 | 63.6 | 20.1 |
Table 2.
Composition of sweet and non-sweet chocolate shake.
| Ingredients | Weight (g) |
Protein (g) |
Carbohydrate (g) |
Fat (g) |
|---|---|---|---|---|
| Nonfat dry milk | 46 | 16.7 | 23 | 0 |
| Water | 328 | 0 | 0 | 0 |
| Domino’s sugar (only for sweet shake) | 75 | 0 | 75 | 0 |
| Heavy cream | 150 | 3.1 | 4.2 | 55.5 |
| Hershey’s unsweetened cocoa | 12 | 2.4 | 7.2 | 1.2 |
| Maltodextrin (only for non-sweet shake) | 75 | 0 | 75 | 0 |
| Xanthan gum | 1 | 0 | 0 | 0 |
| Total g | 612.0 | 22.2 | 109.4 | 56.7 |
| Total kcal | 998.1 | 88.7 | 437.5 | 510.3 |
| Energy density (kcal/g) | 1.6 | 8.6 | 42.2 | 49.2 |
2.2. Experiment 1: Measurement of motivation for food (shake) after food deprivation
2.2.1. Study design
Eight healthy normal-weight females (age: 24 ± 2.0 y, BMI: 21.8 ± 0.5 kg/m2) completed four visits to the Ingestive Behavior Core Laboratory of the New York Obesity Research Center, at St. Luke’s/Roosevelt Hospital, following a screening trial. At each visit, they consumed a sweet yogurt shake test meal by means of the first model of the sipometer, in which a 2 × 2 (food deprivation × reward schedule) Latin square-balanced design was employed, to reduce the possibility that trial effects would confound the experimental treatments (see Figure 2). These visits were scheduled either 1 h (‘sated’) or 21 h (‘food deprived’) after a standardized meal (see next section). The test meal (see next section) was consumed on a sipping reward schedule that followed either CR (i.e., ad libitum intake) or PR (i.e., exert effort for the food reinforcement). On the screening trial scheduled 3 h after the standardized meal, participants had to rate liking of the shake with at least 6 on a 9-point Likert-scale [42] during a taste test and eat a minimum of 250 g of the shake in order to ensure they were taking the test seriously and consuming the shake as a meal.
Fig. 2.
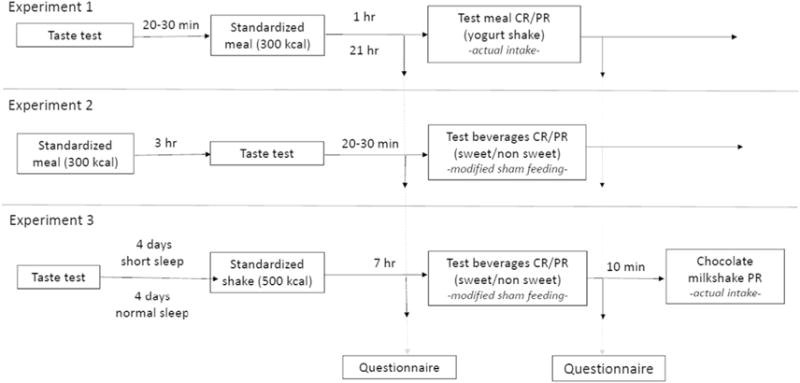
Time line of experimental procedures in Experiment 1, Experiment 2, and Experiment 3. Boxes show each procedure and times above arrows the intervals between them. See text for details of procedures in each box. ‘Questionnaire’ refers to the ratings that the participants completed in all experiments. Ratings included hunger, fullness, taste intensity, sweetness, liking and the extent of enjoying the consumption. CR = continuous reinforcement schedule (ad libitum intake), PR = progressive ratio schedule.
2.2.2. Procedures and foods
Either 1 h or 21 h before their test meal (see Table 1 for composition), participants consumed, in the laboratory, a standardized 300-kcal “snack”, which consisted of an ‘Oatmeal Raisin Walnut’ Clifbar (Clif Bar & Company, Emeryville, USA) and 110 g of apple juice (Red Cheek, Fleetwood, USA) and a separate cup with 129 g water. Before this test meal, glucose levels were determined via finger prick test to assess compliance.
During the PR schedule, the solenoid was opened for 2 sec-periods when the required sip time was reached. The time between reinforcements when the participant was required to hold the straw in the “on” position was progressively increased by 5 s, after each reinforcement. Time spent sipping accumulated, so participants did not have to sip on the straw continuously in order to reach the PR requirement. On the CR schedule the reinforcer was continuously available as long as the straw was in the “on” position.
2.3. Experiment 2: measurement of motivation for sweet and non-sweet beverages
2.3.1. Procedures, foods, and participants
On their trial day, healthy female participants, whose BMI was between 18 and 26 kg/m2, and age between 18 and 35 y, first underwent a taste test, in which they rated “liking” and “wanting” of the sweet and non-sweet beverages. The purpose of this test was to ensure that participants’ differences in liking would lead to significant differences in intake as this laboratory previously reported that a 2 out of 9 unit difference in liking between two beverages led to a significant 200 g difference in intake [43]. A difference in liking ratings between the sweet and non-sweet beverages of at least 45 mm on a 190 mm line (corresponds to 2 units on the classical 9 point scale used by Peryam and Pilgrim [42]) was therefore required. In the taste test participants sampled eight beverages: distilled water, 6.1% sucrose solution, 34% sucrose solution, and 0%, 2.5%, 5%, 10%, and 20% sucrose-equivalents of cherry-flavored unsweetened Kool-Aid (Kraft Foods, Inc., Northfield, USA), sweetened with aspartame. The 10% sucrose equivalent sweetened Kool-Aid solution was prepared by the experimenter’s dissolving 375 g aspartame in 500 ml volumetric flask with distilled water plus 0.951 g of cherry-flavored unsweetened Kool-Aid, and diluting for the lower concentrations, and by dissolving 0.75 g in 500 ml for 20% sucrose equivalent. The unsweetened version was made by simply dissolving the cherry-flavored Kool-Aid in distilled water, without the aspartame. The participants were served 5 ml of each beverage at approximately 50 °F.
Beverages were kept cold after they were removed from a refrigerator by placement of the 30 ml serving cups over cups of ice in a wooden or Styrofoam block to ensure constant temperature (45–50 °F) during the test. The order was 6.1% sucrose first, to adapt participants to the testing environment, followed by distilled water. The next six items were presented in an order counterbalanced by Latin squares for every 6 participants. Participants were instructed by a timed tape recording to drink each item at 1 min intervals, to mark their rating of liking (see Table 4 for results) after 15 s, and to rinse their mouth with water at 20 and 40 s after drinking. Participants were instructed to mark how much they liked or disliked each item on a 190 mm horizontal line with two anchors, “strongest imagined liking of any kind” at the right, and “strongest imagined disliking of any kind” on the left. This scale was described by Kalva et al. [44] and modified by Hayes et al. [45] who suggested removal of intermediate marks, and Bartoshuk [46] who suggested “imaginable” wasn’t necessary but that scales anchored by “most imaginable” and “most experienced” correlated [47], although the evidence for the correlation was never published [48]. We, therefore, removed the intermediate marks.
Table 4.
Comparison of taste test ratings for Experiments 2 and 3.
| Liking ratings taste test (mm)
|
Wanting ratings taste test (ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Exp. 2 | Exp. 3 | ΔExp. 2 and 3 | p-Value Δ | Exp. 2 | Exp. 3 | ΔExp. 2 and 3 | p-Value Δ | |
| Water | 106 ± 14 | 90 ± 8.7 | 17 ± 16 | 0.29 | 430 ± 135 | 231 ± 61 | 199 ± 129 | 0.14 |
| 6.1% Sucrose | 86 ± 12 | 80 ± 8.7 | 5.4 ± 15 | 0.72 | 273 ± 63 | 92 ± 24 | 181 ± 57 | <0.001 |
| 34% Sucrose | 118 ± 11 | 81 ± 13 | 37 ± 19 | 0.06 | 480 ± 46 | 72 ± 24 | 408 ± 47 | <0.001 |
| Kool-Aid 0% Sucr Eq (non-sweet) | 48 ± 8.5 | 57 ± 7.4 | −9.1 ± 12 | 0.45 | 52 ± 28 | 39 ± 9 | 12 ± 24 | 0.61 |
| Kool-Aid 10% Sucr Eq (sweet) | 127 ± 8.4 | 114 ± 7.4 | 12 ± 12 | 0.30 | 346 ± 87 | 244 ± 46 | 102 ± 89 | 0.26 |
| Kool-Aid 20% Sucr Eqa | 117 ± 17 | 108 ± 9.4 | 9.8 ± 18 | 0.59 | 312 ± 68 | 225 ± 32 | 86 ± 66 | 0.21 |
This level was not used in the behavior tests, only in the taste test. Mean (±SEM) of ratings of liking (mm out of 190 with scale anchors described in text) and ‘wanting’ (amount wanted, volume calculated from the rating by means of the formula given in legend of Fig. 1 where the scale was described) during the taste test preceding Experiment 2 (n = 8) and Experiment 3 (n = 7) and differences in ratings between Experiments 2 and 3, and p-values of these differences (t-test).
For the wanting ratings, the participants were instructed to mark how much of the beverage they wanted to drink “right now” on a vertical 183 mm line, superimposed inside an image of a 32-oz. truncated cup (see Fig. 1, left side). A sample cup was available to visualize the size of the cup. The “amount wanted” was calculated from this rating, by means of the conic formula (see legend of Fig. 1 for details).
In the morning on the day of their trial, participants consumed a standardized breakfast consisting of an English muffin (Thomas’; Bimbo Bakeries USA, Horsham, USA) with a teaspoon of salted butter (Land O’Lakes, Saint Paul) and 177 g (6 oz.) of apple juice (Mott’s LLP, General Mills, Plano, USA) 3 h before their test session. Appointments were scheduled between 11 AM and 2 PM.
In Experiments 2 and 3, participants received the 10% sucrose equivalent aspartame-flavored beverage when the sipping pressure was above 0.5 psi. During the CR-schedule, the valve remained open as long as participants sipped, for a fixed time of 2 min. During the PR schedule, participants had to keep the pressure above the threshold for progressively longer periods of time in order to receive the reward: the valve was opened after 3, 6, 9, 12, etc. seconds of sipping for the successive reinforcements. After 2 min of non-sipping, the experimenter returned to the room and told the participant that this phase of the trial was completed, and that they would continue with the next beverage.
2.3.2. Study design
Participants completed the four conditions/beverages (sweet and non-sweet beverage, on both the CR and PR schedule) on a single trial day (see Figure 2). They were adapted to the procedure with a 1 min trial with water as the reinforcer, followed by sham-consumption of the four beverages from the sipometer. The two schedules of each beverage were presented sequentially. After two trials on one beverage the tubing was switched to the other reservoir, for the second two trials. The trials were counterbalanced so within each beverage presentation, half the participants received sweet beverage first, with PR and CR counterbalanced, followed by non-sweet, with PR and CR counterbalanced. The other half of the participants received non-sweet, followed by sweet with PR and CR counterbalanced, as well.
2.4. Experiment 3: measuring motivation after sleep restriction
2.4.1. Study design
Twelve healthy female participants were enrolled in a study of the effect of sleep restriction on energy expenditure [49]. Participant enrolment and characteristics and study design have been described in more detail elsewhere [49]. Briefly, this was a laboratory-based randomized, crossover study with two sleep conditions: short (4 h/night; 0100–0500 h) and habitual (8 h/night; 2300–0700 h) sleep duration. Each condition lasted 4 days (including 3 nights of short or habitual sleep) that were scheduled to start approximately 4 weeks apart. Participants entered the laboratory for a 4-d inpatient stay. The participants remained on site until bedtime (2300 or 0100) and slept in a metabolic chamber. Study personnel ensured that participants remained awake during the duration of scheduled wakefulness by regular visual inspection through the chamber window and phone calls. Participants were awakened at either 0700 or 0500 the following morning. Sleep duration was objectively monitored with the use of wrist-actigraphy worn during the sleep episodes while participants were in the laboratory [50]. The participants slept for 7.35 ± 0.09 h during the habitual sleep condition and 3.73 ± 0.04 h during the sleep restriction condition [49]. Participants were asked to undergo in an additional test using the sipometer following the sleep conditions. Seven females (n = 7, age: 23 ± 0.8 y, BMI: 21.3 ± 0.5 kg/m2) completed the sipometer testing in both sleep conditions.
2.4.2. Procedures and foods
Prior to the start of each sleep condition (day 1), participants performed the taste test as described in Experiment 2. Liking ratings were, however, not used as inclusion criteria; inclusion criteria were already set with the primary aim to recruit healthy participants with normal sleeping behaviors [49]. During the first 3 days, participants consumed a weight-maintenance diet with fixed meal times. Throughout the study, daytime naps were prohibited. Study personnel were instructed to monitor participants to ensure wakefulness throughout all scheduled wake episodes. Participants were prohibited from engaging in exercise or strenuous physical activity for the duration of the study. On day 4 of each condition, participants remained in a small whole-room indirect calorimeter from 7:30 AM to 2:20 PM. At 8:15 AM, all participants consumed 325 ml Original Rich Chocolate BOOST nutritional drink [Nestle USA, Glendale, CA] to which 19.5 g Bertolli Extra Light Tasting Olive Oil [Bertolli, Unilever, Englewood Cliffs, USA] was added. The breakfast shake provided 500 kcal with 50% of energy from fat. After the recording period, participants came to the Ingestive Behavior Core laboratory to complete the trial with the sipometer.
Procedures with the sipometer were conducted as described in Experiment 2, with some modifications (see Figure 2). First, participants consumed the high-fat liquid meal in the morning of their sipometer trial, instead of the muffin and apple juice. Second, following the sham-feeding trials with sweet and non-sweet beverages on the CR and PR schedules, all participants consumed a chocolate milkshake (see Table 2 for composition) on the PR schedule in order to assess actual intake of food. In addition, the 2.5% and 5% Kool-Aid beverages in the taste test were replaced by a sweet and a non-sweet chocolate shake, consisting of non-sweet cocoa powder [The Hershey Company, USA], heavy cream [Tuscan Dairy Farms, USA], non-fat dry milk [Alba Drinks, The Hain Celestial Group, Inc. USA] and either sugar [sweet shake; Domino Foods Inc., USA] or maltodextrin [non-sweet shake, “Star Dri 100”, Tate & Lyle]. The sipometer trials were scheduled around 3:00 PM, 7 h after participants had the BOOST meal. The pre-meal taste test was conducted on a separate day before each of the sleep phases, and the anchors on the liking scale were “experienced” instead of “imagined” based on advice received from Linda Bartoshuk (personal communication) and [47].
2.5. Data analysis
Because these are pilot studies, as first tests on the utility of the sipometer, adjustments for multiple measures were not done, and results should be considered as guidelines for future work rather than definitive. In Experiment 1, effects of duration of food deprivation (1 h/21 h) and reward schedule (CR/PR) on sipping time and intake were tested by means of ANOVA (SAS 9.3 PROC MIXED) with repeated measures across the four beverage-schedule combinations followed by planned comparisons (t-tests with appropriate error terms from ANOVA) between schedule and deprivation. In Experiment 2, the effects of beverage (sweet/non-sweet) and reward schedule (CR/PR) on all variables were tested by means of ANOVA (PROC MIXED with repeated measures on participants as a random factor) followed by planned comparisons between schedule and taste. Variance structures of each variable were tested for homogeneity across treatment conditions, and where they were homogenous, structured covariance was used; otherwise, unstructured (t-test) was used. The type of variance can be seen in the results by the degrees of freedom (21 for structured, 7 for unstructured). In Experiment 3, the effect of sleep duration (habitual/short) was crossed with the effects of beverage (sweet/non-sweet) crossed with reward schedule (CR/PR) on all variables by means of ANOVA (PROC MIXED with repeated measures on participants as a random factor). Planned comparisons included effects of sleep condition averaged over the four beverage conditions and for the shake. Because comparisons between conditions can be strongly affected, within individuals, by their hedonic and motivational reactions to the beverages, liking and wanting ratings were covaried with the other dependent variables. In addition, the effect of differences between liking and wanting ratings and differences between dependent variables indicating reward value and motivation were obtained by regression analysis (PROC MIXED without class statements) in order to determine the sizes of the effects of cognitive variables (i.e. ratings) on the sizes of the effects on performance (i.e. working to obtain reinforcement). Data analysis was performed using SAS 9.3 (SAS Institute, Inc., Cary, NC, USA). Means and differences are always presented with their standard errors.
3. Results
3.1. Experiment 1: Measurement of motivation for food (shake) after food deprivation
Glucose levels indicated compliance with the fasting procedures before both the CR (125 ± 10.1 mg/dL after 1 h and 96 ± 3.7 mg/dL after 21 h of food deprivation) and the PR schedule (133 ± 12.3 mg/dL after 1 h and 97 ± 3.2 mg/dL after 21 h of food deprivation). Hunger and fullness ratings were different between deprivation times (see Table 3). We can, therefore, conclude that the participants complied with the deprivation instruction. Sipping time differed across conditions (F (1,7) = 11.92, p < 0.001) As we expected, participants sipped longer during the PR schedule than during the CR schedule ((t(21)) = −5.52, p < 0.001). In addition, during the PR schedule, participants sipped longer on the straw after the 21-h food deprivation period than after the 1-h deprivation period (t(21) = 2.25, p = 0.035). This difference was not observed in during CR (see Table 3 for mean values and differences (t(21) = 0.4, p = 0.69)). Shake intake also differed across conditions. Intake was higher when the shake was consumed on the CR than on the PR schedule (p = 0.006). Also, ad libitum intake (CR) was greater after the 21-h food deprivation period than after the 1-h deprivation period (p = 0.01), while intake after 21 h vs. 1 h of food deprivation was not different on the PR schedule (p = 0.17). Ratings of liking, enjoyment, and sweetness of the shake did not differ across conditions (Table 3).
Table 3.
Mean (±SEM) intake, sipping time and eating rate and appetite and liking/enjoy ratings for the shakes following consumption in the continuous reinforcement (CR) and progressive reinforcement (PR) schedules in Experiment 1 (n = 8).
| Continuous reinforcement
|
Progressive ratio
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Food deprivation period
|
Food deprivation period
|
|||||||
| 1 h | 21 h | Δ21 h–1 h | p-Value Δ | 1 h | 21 h | Δ21 h–1 h | p-Value Δ | |
| Behavioral measures | ||||||||
| Intake (g) | 318 ± 50a | 491 ± 93b | 173 ± 60 | 0.01a | 192 ± 41 | 277 ± 70 | 86 ± 60 | 0.17 |
| Sipping time (min) | 1.9 ± 0.4 | 2.7 ± 0.6 | 0.81 ± 2.0 | 0.69 | 8.0 ± 2.1 | 12.6 ± 3.0 | 4.6 ± 1.4 | 0.04 |
| Duration (min) | 20 ± 15 | 6.8 ± 1.1 | −13.4 ± 11 | 0.22 | 9.8 ± 2.2 | 15.6 ± 3.6 | 5.8 ± 11 | 0.59 |
| Number of reinforcements | 13 ± 1.8 | 16 ± 2.2 | 3.4 ± 1.7 | 0.06 | ||||
| Eating rate (g/min) | 68 ± 15 | 88 ± 18 | 20 ± 14 | 0.17 | 21 ± 2.8 | 18 ± 2.4 | −3 ± 10 | 0.88 |
| Ratings | ||||||||
| Hunger (pre-meal, mm)b | 46 ± 12 | 117 ± 6.7 | 71 ± 14 | <0.001 | 36 ± 12 | 113 ± 13 | 77 ± 14 | <0.001 |
| Fullness (pre-meal, mm)b | 68 ± 17 | 10 ± 2.3 | −58 ± 16 | <0.001 | 59 ± 16 | 11 ± 3.1 | −48 ± 16 | <0.001 |
| Hunger (post-meal, mm)b | 13 ± 5 | 42 ± 16 | 29 ± 14 | 0.05 | 16 ± 5 | 61 ± 15 | 45 ± 14 | <0.001 |
| Fullness (post-meal, mm)b | 111 ± 7 | 95 ± 15 | −16 ± 12 | 0.19 | 100 ± 8.7 | 67 ± 12 | −33 ± 12 | 0.01 |
| Liking (Likert, 1–9 points)c | 5.9 ± 0.7 | 6.3 ± 0.7 | 0.30 ± 0.33 | 0.37 | 6.3 ± 0.7 | 6.5 ± 0.8 | 0.25 ± 0.31 | 0.44 |
| Liking (gLMS)d | 82 ± 12 | 92 ± 13 | 9.6 ± 7.7 | 0.23 | 83 ± 9.9 | 82 ± 13 | 0.75 ± 7.7 | 0.93 |
| Enjoy (mm)e | 76 ± 12 | 80 ± 17 | 3.5 ± 10 | 0.73 | 71 ± 15 | 86 ± 16 | 15 ± 10 | 0.16 |
| Sweetness intensity (mm)f | 48 ± 7.5 | 58 ± 7.5 | 10 ± 6.3 | 0.86 | 53 ± 5.4 | 54 ± 6.4 | 1.4 ± 6.3 | 0.21 |
P-values indicate significance of differences in behavioral measures or ratings between the 1-h and 21-h deprivation periods (Δ21 h–1 h). Ratings were assessed by means of:
VAS (visual analogue scale, 150 mm anchored by “Not at all” and “Extremely” in response to the question “How hungry/full do you feel”).
9-point Likert scale of dislike (1–4 extremely, very much, moderately, slightly) 5 = neutral and like (6–9 inverse of 1–4) and
gVAS (general visual analogue scale, 190 mm, anchored by “strongest imaginable disliking of any kind” at the left, “strongest imaginable liking of any kind” at the right)
Rating was made by marking 150 mm line: “How much did you enjoy what you’ve eaten?” on 150 mm line anchored “Not at all” and “Extremely”
Rating was made by marking a 100 mm line with instructions: “Using the scale below indicate where on this scale you would place the described sensation. Please rate the intensity of that sensation by placing a vertical stroke (∣) anywhere through the horizontal line “How sweet did you find this shake?” with left anchor no sensation at all right anchor “strongest imaginable sensation of any kind”.
3.2. Experiment 2: measuring motivation for sweet and non-sweet beverages
3.2.1. Participant characteristics
Eight of nine screened participants met the taste test criterion for acceptance (difference in liking ratings between the sweet and non-sweet beverages of 45 out of 190 mm). All of them were normal-weight healthy females (age: 23 ± 0.8 y, BMI: 21.3 ± 0.5 kg/m2).
3.2.2. Behavioral measures
Sham-intake was significantly higher (t(21) = 3.66, p = 0.008) and sipping time longer by 30 s ± 12 SE (t(7) = 2.56, p = 0.04) for the sweet beverage than the non-sweet beverage on the PR schedule (see Table 5 for means and SE). Remarkably, this finding was reversed on the CR schedule (t(21) = −2.55, p = 0.04 for sham-intake; t(7) = −2.30, p = 0.06 for sipping time). As expected, participants sipped longer on the PR schedule than on the CR schedule when the beverage was sweet (difference = 29.8 s ± 10.6 SE, (t(7) = −2.8, p = 0.027), but not when it was not sweet. On the PR schedule, cumulative pressure was somewhat, but not significantly, higher for the sweet than the non-sweet beverages. Participants reached a significantly higher mean breakpoint for the sweet beverage than the non-sweet beverage.
Table 5.
Mean (±SEM) of behavioral measures and appetite and sensory ratings for sham-consumption of non-sweet and sweet beverages on a continuous reinforcement (CR) and a progressive reinforcement (PR) schedule in Experiment 2 (n = 8).
|
Continuous reinforcement
|
Progressive ratio
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-sweet beverage | Sweet beverage | Δtaste | p-Value Δ | Non-sweet beverage | Sweet beverage | Δtaste | p-Value Δ | |
| Behavioral measures | ||||||||
| Sham-intake (g) | 84 ± 3.5 | 65 ± 6.7 | −19 ± 7.3 | 0.04a | 23 ± 3.1 | 37 ± 4.3 | 14 ± 3.9 | <0.01 |
| Cum. pressure (psi) | 90 ± 10 | 122 ± 26 | 31 ± 24 | 0.23 | 64 ± 16 | 273 ± 111 | 209 ± 107 | 0.09 |
| Sipping time (sec) | 25 ± 1.2 | 19 ± 2.2 | −5.4 ± 2.3 | 0.06 | 19 ± 4.3 | 49 ± 12 | 30 ± 12 | 0.04 |
| Number of reinforcements | 12 ± 0.6 | 9.5 ± 1.1 | −2.6 ± 1.2 | 0.06 | 3.3 ± 0.4 | 5.3 ± 0.7 | 2.0 ± 0.7 | 0.02 |
| Breakpoint (s) | 2.0 ± 0 | 2.0 ± 0 | 0 ± 1.6 | 1.0 | 8.8 ± 1.2 | 15 ± 2.0 | 6.0 ± 1.6 | <0.01 |
| Duration (s) | 270 ± 31 | 261 ± 36 | −9 ± 26 | 0.74 | 332 ± 44 | 393 ± 59 | 61 ± 45 | 0.22 |
| Sipping rate (g/s) | 0.3 ± 0.04 | 0.3 ± 0.06 | −0.04 ± 0 | 0.29 | 0.07 ± 0.01 | 0.1 ± 0.01 | 0.02 ± 0.0 | 0.58 |
| Reinforcement size (g) | 7.0 ± 0.1 | 7.5 ± 0.4 | 0.5 ± 0.4 | 0.20 | 6.9 ± 0.2 | 7.5 ± 0.5 | 0.6 ± 0.4 | 0.09 |
| Ratings | ||||||||
| Hunger, post-drink (VAS, mm)b | 66 ± 15 | 54 ± 17 | −13 ± 9.1 | 0.17 | 74 ± 15 | 60 ± 16 | −13 ± 9.1 | 0.16 |
| Fullness, post-drink (VAS, mm)b | 45 ± 15 | 48 ± 19 | 3.1 ± 6.4 | 0.63 | 47 ± 20 | 51 ± 19 | 4.2 ± 6.4 | 0.52 |
| Liking (Lickert,1–9 points)c | 3.3 ± 0.6 | 5.0 ± 0.6 | 1.8 ± 0.7 | 0.02 | 3.1 ± 0.4 | 4.6 ± 0.6 | 1.5 ± 0.7 | 0.04 |
| Liking (gLMS, 1–150 mm)d | 40 ± 11 | 71 ± 11 | 29 ± 13 | 0.04 | 46 ± 19 | 69 ± 12 | 25 ± 13 | 0.08 |
| Enjoy (VAS, mm)e | 27 ± 11 | 54 ± 15 | 27 ± 14 | 0.07 | 22 ± 8.9 | 53 ± 16 | 31 ± 14 | 0.04 |
| Wanting, intensity (VAS, mm)f | 57 ± 12 | 45 ± 14 | −12 ± 12 | 0.33 | 38 ± 10 | 43 ± 13 | 5.7 ± 12 | 0.64 |
| Wanting intensity (gLMS, mm)g | 22 ± 6.6 | 34 ± 8.2 | 13 ± 7.7 | 0.12 | 26 ± 7.0 | 32 ± 5.4 | 5.6 ± 7.7 | 0.47 |
| Wanting, quantity (VAS, mm)h | 41 ± 19 | 44 ± 10 | 2.5 ± 16 | 0.88 | 29 ± 11 | 38 ± 11 | 9.5 ± 16 | 0.56 |
| Sweetness intensity (VAS, mm)i | 25 ± 6.4 | 55 ± 6.4 | 31 ± 7.9 | <0.01 | 31 ± 7.0 | 55 ± 5.7 | 26 ± 8.4 | 0.02 |
| Ideal sweetness (VAS, mm)j | 44 ± 11 | 89 ± 6.7 | 45 ± 12 | <0.01 | 42 ± 12 | 94 ± 5.5 | 53 ± 12 | <0.01 |
p-Value indicate significance of differences in behavioral measures or ratings between the sweet and non-sweet beverages (Δtaste = sweet − non-sweet).
See corresponding notes in Table 3.
Rating marked on 150 mm line: “How much did you enjoy what you’ve tasted?” anchored “Not at all” and “Extremely”.
Rating marked on 150 mm line: “How strongly do you want to drink this beverage right now?” anchored “Not at all” and “Extremely”.
Rating marked on 100 mm line: “Please rate the intensity of how strongly you want to consume the beverage” anchored by “no sensation (left) and strongest experienced sensation of any kind” (right).
Rating marked on 150 mm line: “What quantity of the beverage do you want to drink right now?” anchored by “none at all” (left) and “as much as possible” (right).
Identical to 6in Table 3 but right hand anchor substituted “experienced” for “imaginable”.
Rating marked on 150 mm line: “How sweet did you find the beverage?” anchored by So far BELOW the right sweetness that I’d never drink it” (left), “So far ABOVE the right sweetness that I’d never drink it (right) and “At this sweetness I’d always drink it”, in the middle.
3.2.3. Ratings and their prediction of behavioral variables
Hunger and fullness ratings did not differ across conditions. Wanting and liking ratings during the taste test (Table 4) and liking and enjoyment ratings following sham-consumption (Table 5) were all higher for the sweet beverages than for the non-sweet beverages.
On the PR schedule, differences in ratings between sweet and non-sweet beverages for liking and wanting in the taste test, and enjoyment after consumption significantly (or in two cases marginally) predicted differences in all the behavioral variables, except for cumulative pressure from liking differences in the taste test on the PR schedule (p = 0.19, see Fig. 3 for typical graphs and Table 6 for regression statistics for all significant slopes). On the PR schedule, the strongest predictor of the various behavioral responses was “wanting” in the taste test (R2’s ranged 0.67–0.85), while the weakest was “liking” in the taste test, for which two of the three predicted variables were only marginally significant, and the third not at all. On the CR schedule, the only significant predictors were differences in enjoyment rating and liking, which predicted differences in sham-intake and sipping time. Thus, variables that reflected pleasure, rather than motivation, predicted intake and sipping time.
Fig. 3.
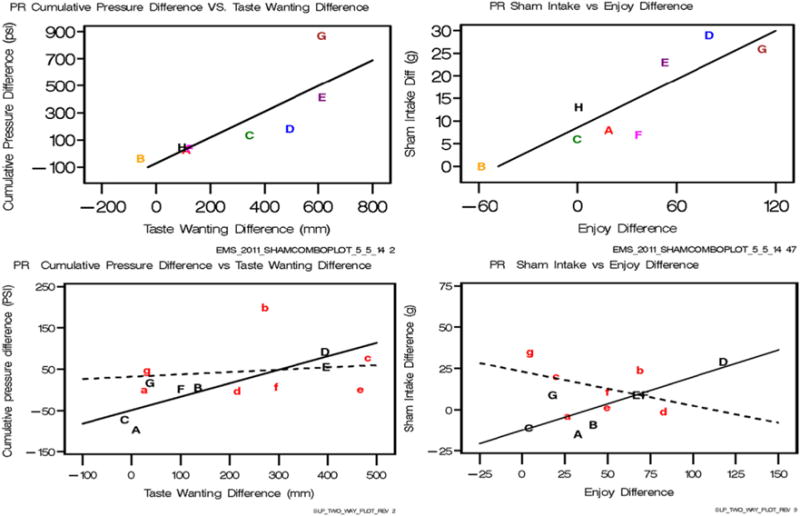
Left: Regressions on the PR schedule of differences between sweet and non-sweet fluids in pressure exerted between reinforcements predicted from differences between sweet and non-sweet fluids in amount wanted during the taste test before the trial, for Experiment 2 (top row) and Experiment 3 (bottom row). Right: Regressions of differences in sham-intakes between sweet and non-sweet fluids predicted from differences in hedonic (“how much did you enjoy”) ratings between sweet and non-sweet beverage after consuming it during the test for Experiment 2 (top row) and Experiment 3 (bottom row) Parameters of the lines for Experiment 2 are shown in lines 1 and 2 of Table 6 and for Experiment 3 lines 1 and 4 Table 10. Each letter shows an individual participant so that responses of the same participants across variables can be compared. Note in Experiment 2, that one participant (“B”) wanted more of the non-sweet than sweet and this reversal of the expected difference (i.e. sweet < non-sweet) was also seen on the predicted variables. For Experiment 3 (bottom row) the dotted line, lower case letters is for short sleep and the solid line, uppercase letters for habitual sleep.
Table 6.
Regression statistics with significant slopes for Experiment 2.
| Reward schedule/line # | Behavioral measure (dependent) | Rating (dependent) | Intercept | p-Value intercept | Slope | p-Value slope | R-squared |
|---|---|---|---|---|---|---|---|
| PR 1 | Cumulative pressure | Amount wanted in a taste test | −70.5 ± 103.8 | 0.52 | 0.95 ± 0.27 | 0.01 | 0.67 |
| PR 2 | Cumulative pressure | Enjoyment rating after sham-drink | 73.0 ± 84.4 | 0.42 | 4.47 ± 1.46 | 0.02 | 0.61 |
| PR 3 | Sham-intake | Amount wanted in a taste test | 3.6 ± 3.2 | 0.31 | 0.04 ± 0.01 | 0.006 | 0.74 |
| PR 4 | Sham-intake | Enjoyment rating after sham-drink | 8.6 ± 2.3 | 0.009 | 0.18 ± 0.04 | 0.004 | 0.77 |
| PR 5 | Sham-intake | Liking rating in a taste test | −3.0 ± 7.6 | 0.70 | 0.22 ± 0.09 | 0.05 | 0.50 |
| PR 6 | Time spent sipping | Amount wanted in a taste test | −4.8 ± 7.3 | 0.54 | 0.12 ± 0.02 | 0.0009 | 0.86 |
| PR 7b | Time spent sipping | Enjoyment rating after sham-intake | 13.0 ± 7.0 | 0.11 | 0.55 ± 0.12 | 0.004 | 0.77 |
| PR 8 | Time spent sipping | Liking rating in a taste test | −19.4 ± 24.4 | 0.46 | 0.62 ± 0.29 | 0.07 | 0.44 |
| CR 9 | Sham-intake | Liking rating in a taste test | −54.1 ± 13.9 | 0.008 | 0.45 ± 0.16 | 0.03 | 0.56 |
| CR 10 | Sham-intake | Enjoyment rating after sham-intake | −30.0 ± 7.4 | 0.007 | 0.41 ± 0.17 | 0.05 | 0.49 |
| CR 11 | Time spent sipping | Liking rating in a taste test | −16.8 ± 4.2 | 0.007 | 0.15 ± 0.05 | 0.03 | 0.59 |
| CR 12 | Time spent sipping | Enjoyment rating after sham-intake | −9.2 ± 2.2 | 0.006 | 0.14 ± 0.05 | 0.03 | 0.56 |
3.3. Experiment 3: measuring motivation after short sleep duration
3.3.1. Behavioral measures and ratings
There were no significant differences in behavioral variables (e.g. in take, pressure, breakpoint etc.) between sweet and non-sweet beverages for either schedule (see Behavioral Measures in Tables 7 and 8), but participants exerted slightly, but not significantly (ps = 0.08) more pressure and sipped longer when they were sham-consuming the sweet beverage vs. the non-sweet beverage on the PR-schedule after three nights of short sleep (see second and third data lines in Table 8). There were differences between schedules and significant differences between ratings of sweet and non-sweet beverages (e.g. liking, sweetness, enjoyment), and there was one significant difference between sleep conditions: participants rated a higher desire to binge after three nights of restricted (i.e. “short”), compared to three nights of habitual sleep duration. The mean of four “desire to binge” ratings per participant at each sleep condition was 38.0 mm ± 9.2 SE during short sleep, but only 14.3 mm ± 3.7 SE during habitual sleep, and the difference was 23.7 mm ± 6.6 SE (t(42) = 3.60; p = 0.001). Participants tended to be hungrier after short sleep (mean = 83.4 mm ± 9.4 SE vs habitual sleep mean = 65.6 mm ± 7 SE, difference = 17.7 mm ± 9.2 SE, (t(42) = 1.92; p = 0.06). All behavioral variables except cumulative pressure were significantly higher for the CR than PR schedules (see Table 9).
Table 7.
Mean (±SEM) of behavioral measures and appetite and sensory ratings for sham-consumption of non-sweet and sweet beverages on a continuous reinforcement (CR) in Experiment 3 (n = 7).
| Habitual sleep duration
|
Short sleep duration
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-sweet beverage | Sweet beverage | Δtaste | p-Value Δtaste | Non-sweet beverage | Sweet beverage | Δtaste | p-Value Δtaste | |
| Behavioral measures | ||||||||
| Intake (g) | 93 ± 7.4 | 98 ± 6.0 | 5.1 ± 10 | 0.62 | 81 ± 14.1 | 92 ± 7.8 | 11 ± 10 | 0.29 |
| Cum. pressure (psi) | 130 ± 13 | 118 ± 14 | −12 ± 26 | 0.65 | 90 ± 17 | 102 ± 14 | 12 ± 26 | 0.64 |
| Sipping time (s) | 28 ± 2.8 | 24 ± 2.5 | −3.9 ± 6.8 | 0.57 | 20 ± 4.1 | 23 ± 3.2 | 2.8 ± 6.8 | 0.69 |
| Number of reinforcements | 9.4 ± 0.8 | 9.7 ± 0.6 | 0.3 ± 0.9 | 0.76 | 7.9 ± 1.4 | 9.1 ± 0.9 | 1.3 ± 0.9 | 0.18 |
| Breakpoint (s) | 4.1 ± 1.7 | 2.4 ± 0.2 | −1.7 ± 1.8 | 0.36 | 2.4 ± 0.2 | 2.4 ± 0.2 | 0.0 ± 1.8 | 1.00 |
| Duration (s) | 156 ± 2.7 | 152 ± 3.5 | −3.7 ± 50 | 0.94 | 156 ± 3.2 | 152 ± 4.3 | −4.3 ± 50 | 0.93 |
| Sipping rate (g/s) | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.1 ± 0.1 | 0.40 | 0.5 ± 0.1 | 0.6 ± 0.0 | 0.1 ± 0.1 | 0.23 |
| Reinforcement size (g) | 10 ± 0.4 | 10 ± 0.6 | 0.3 ± 0.7 | 0.73 | 10 ± 0.4 | 10 ± 0.5 | −0.2 ± 0.7 | 0.77 |
| Ratingsa | ||||||||
| Hunger (post-meal, mm) | 69 ± 15 | 63 ± 15 | −6.0 ± 18 | 0.75 | 81 ± 21 | 84 ± 20 | 3.3 ± 18 | 0.86 |
| Fullness (post-meal, mm) | 31 ± 8.9 | 35 ± 13 | 4.7 ± 12 | 0.69 | 34 ± 14 | 29 ± 13 | −5.3 ± 12 | 0.65 |
| Liking (1–9 points) | 3.4 ± 0.7 | 6.1 ± 0.4 | 2.7 ± 0.6 | <0.01 | 3.0 ± 0.6 | 6.0 ± 0.8 | 3.1 ± 0.6 | <0.01 |
| Liking (GLMS) | 32 ± 11 | 80 ± 4.4 | 48 ± 13 | <0.01 | 32 ± 14 | 88 ± 15 | 56 ± 14 | <0.01 |
| Enjoy (mm) | 29 ± 18 | 70 ± 12 | 41 ± 11 | <0.01 | 21 ± 13 | 84 ± 15 | 62 ± 11 | <0.01 |
| Wanting, intensity (mm) | 32 ± 20 | 78 ± 12 | 46 ± 15 | <0.01 | 33 ± 20 | 85 ± 17 | 52 ± 15 | <0.01 |
| Wanting, intensity (gLMS) | 41 ± 12 | 57 ± 8.4 | 15 ± 9.8 | 0.13 | 45 ± 14 | 56 ± 9.3 | 11 ± 9.8 | 0.25 |
| Wanting, quantity (mm) | 26 ± 14 | 65 ± 10 | 39 ± 10 | <0.01 | 22 ± 15 | 69 ± 14 | 46 ± 10 | <0.01 |
| Sweetness intensity (mm) | 11 ± 2.5 | 40 ± 5.0 | 29 ± 7.6 | <0.01 | 9 ± 4.1 | 50 ± 8.7 | 40 ± 7.9 | <0.01 |
| Ideal sweetness (mm) | 16 ± 5.9 | 72 ± 6.7 | 57 ± 9.1 | <0.01 | 10 ± 6.6 | 75 ± 4.2 | 64 ± 9.1 | <0.01 |
| Desire to binge (mm) | 9.3 ± 4.1 | 18 ± 9.9 | 9.1 ± 13 | 0.49 | 40 ± 20 | 36 ± 20 | −4.4 ± 13 | 0.74 |
See Table 5 notes for all rating responses except “desire to binge” which was marked on 150 mm line: “How strongly do you want to binge right now?”, anchored by “not at all” (left) and “extremely” (right).
Table 8.
Mean (±SEM) of behavioral measures and appetite and sensory ratings for sham-consumption of non-sweet and sweet beverages and milkshake consumption on a progressive reinforcement (PR) schedule in Experiment 3 (n = 7).
| Habitual sleep duration
|
Short sleep duration
|
Milkshake consumption
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-sweet beverage |
Sweet beverage |
Δtaste | p-Value Δtaste |
Non-sweet beverage |
Sweet beverage |
Δtaste | p-Value Δtaste |
Habitual sleep |
Short sleep |
Δsleep durationa |
p-Value Δsleep |
|
| Behavioral measures | ||||||||||||
| Intake (g) | 35 ± 5.2 | 37 ± 4.2 | 2.1 ± 10.3 | 0.84 | 35 ± 5.4 | 48 ± 7.4 | 13 ± 10 | 0.21 | 45 ± 13 | 52 ± 11 | 7.5 ± 9.1 | 0.45 |
| Cum. pressure (psi) | 108 ± 27 | 109 ± 15 | 0.9 ± 26 | 0.97 | 75 ± 16 | 122 ± 36 | 47 ± 26 | 0.08 | 109 ± 44 | 96 ± 21 | −14 ± 21 | 0.47 |
| Sipping time (s) | 30 ± 11 | 26 ± 5.2 | −3.6 ± 6.8 | 0.60 | 25 ± 8.3 | 37 ± 9.5 | 12 ± 6.8 | 0.08 | 48 ± 13 | 59 ± 21 | 11 ± 12.2 | 0.43 |
| Number of reinforcements | 3.7 ± 0.7 | 3.7 ± 0.4 | 0 ± 0.9 | 1.00 | 3.4 ± 0.6 | 4.4 ± 0.6 | 1.0 ± 0.9 | 0.0.29 | 5.0 ± 1.0 | 5.5 ± 1.1 | 0.5 ± 0.62 | 0.46 |
| Breakpoint (s) | 11 ± 2.3 | 11 ± 1.3 | 0 ± 1.8 | 1.00 | 9.7 ± 2.0 | 13 ± 1.9 | 3.0 ± 1.8 | 0.11 | 14 ± 2.7 | 16 ± 3.2 | 2 ± 2.0 | 0.46 |
| Duration (s) | 214 ± 50 | 206 ± 46 | −8.3 ± 50 | 0.87 | 202 ± 52 | 255 ± 60 | 53 ± 50 | 0.0.29 | 183 ± 23 | 281 ± 46 | 98 ± 45 | 0.08 |
| Sipping rate (g/s) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0 ± 0.1 | 0.54 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.1 | 0.0.82 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.04 ± 0.03 | 0.20 |
| Reinforcement size (g) | 9.9 ± 0.5 | 10 ± 0.6 | 0.3 ± 0.7 | 0.71 | 11 ± 0.6 | 11 ± 1.3 | 0.4 ± 0.7 | 0.0.57 | 9.2 ± 0.4 | 9.8 ± 0.9 | 0.6 ± 0.73 | 0.45 |
| Ratingsb | ||||||||||||
| Hunger (post-meal, mm) | 61 ± 14 | 69 ± 16 | 7.4 ± 18 | 0.69 | 81 ± 19 | 87 ± 20 | 5.6 ± 18 | 0.76 | 76 ± 20 | 105 ± 19 | 29 ± 22 | 0.25 |
| Fullness (post-meal, mm) | 40 ± 11 | 38 ± 13 | −1.7 ± 12 | 0.88 | 27 ± 12 | 31 ± 12 | 4.0 ± 12 | 0.73 | 35 ± 15 | 26 ± 13 | −9.2 ± 18 | 0.63 |
| Liking (1–9 points)c | 3.4 ± 0.7 | 6 ± 0.4 | 2.6 ± 0.6 | <0.01 | 3.0 ± 0.6 | 5.7 ± 0.7 | 2.7 ± 0.6 | <0.01 | ||||
| Liking (GLMS) | 52 ± 13 | 82 ± 8.4 | 30 ± 13 | 0.03 | 51 ± 13 | 92 ± 7.2 | 41 ± 14 | <0.01 | 88 ± 11 | 93 ± 18 | 4.3 ± 7.5 | 0.59 |
| Enjoy (mm)c | 28 ± 18 | 73 ± 15 | 45 ± 11 | <0.01 | 20 ± 16 | 69 ± 15 | 49 ± 11 | <0.01 | 65 ± 19 | 68 ± 21 | 3.8 ± 7.4 | 0.63 |
| Wanting intens. VAS (mm) | 40 ± 20 | 70 ± 16 | 31 ± 15 | <0.04 | 20 ± 15 | 65 ± 20 | 44 ± 15 | <0.01 | 78 ± 19 | 81 ± 24 | 3.3 ± 13 | 0.81 |
| Wanting intens. GLMS mm) | 29 ± 12 | 55 ± 10 | 26 ± 9.8 | 0.01 | 45 ± 14 | 54 ± 9.9 | 9.4 ± 9.8 | 0.34 | c | |||
| Wanting, quantity (mm) | 27 ± 19 | 70 ± 16 | 42 ± 10 | <0.01 | 20 ± 17 | 55 ± 17 | 35 ± 10 | <0.01 | 76 ± 17 | 105 ± 19 | 28 ± 20 | 0.21 |
| Sweetness intensity (mm) | 22 ± 6.8 | 44 ± 6.6 | 22 ± 7.6 | <0.01 | 7.7 ± 2.1 | 47 ± 6.4 | 39 ± 8.2 | <0.01 | 52. ±7.7 | 55 ± 6.5 | 3 ± 4.2 | 0.51 |
| Ideal sweetness (mm) | 19 ± 6.8 | 78 ± 10 | 59 ± 9.1 | <0.01 | 12 ± 7.1 | 80 ± 7.8 | 69 ± 9.1 | <0.01 | 76 ± 6.8 | 70 ± 3.9 | −5.7 ± 5.4 | 0.34 |
| Desire to binge (mm) | 22 ± 9.8 | 8.4 ± 3.8 | −13 ± 13 | 0.34 | 37 ± 20 | 39 ± 18 | 1.4 ± 13 | 0.91 | c | |||
Δ = Short minus habitual.
Ratings made as described in Table 7.
Question not used for shake consumption.
Table 9.
Differences in variables between schedules in Experiment 3.
| Schedule
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| CR
|
PR
|
Schedule difference | |||||||
| Mean | Std Err | Mean | Std Err | Difference (CR-PR) | SE | DF | t Value | p-Value | |
| A_ Intake (g) | 90.8 | 4.6 | 38.9 | 2.9 | 52 | 5.2 | 42 | 10.1 | <0.0001 |
| B_Cum. pressure (psi) | 110.2 | 7.5 | 103.4 | 12.2 | 6.8 | 12.9 | 42 | 0.5 | 0.6000 |
| C_Sipping time (sec) | 23.5 | 1.6 | 29.4 | 4.2 | −5.9 | 3.4 | 42 | −1.7 | 0.0880 |
| D_# of reinforcements | 9 | 0.5 | 3.8 | 0.3 | 5.2 | 0.5 | 42 | 11.2 | <0.0001 |
| E_Breakpoint (sec) | 2.9 | 0.4 | 10.9 | 0.9 | −8 | 0.9 | 42 | −8.7 | <0.0001 |
| F_Duration (sec) | 154 | 1.7 | 219.5 | 24.9 | −65.5 | 24.8 | 42 | −2.6 | 0.0120 |
| G_Sipping rate (g/s) | 0.6 | 0 | 0.2 | 0 | 0.4 | 0 | 42 | 12.3 | <0.0001 |
3.3.2. Significant predictors of sham-intake and pressure differences from differences in ratings between sleep conditions
There were four significant predictors of responses from experimental conditions, three after habitual, (two on PR and one on CR), and one after short (on PR), sleep. After habitual sleep, on the PR schedule, cumulative pressure difference between sweet and non-sweet beverage increased as difference in amount wanted in the taste test increased (see line 1 Table 10, pFig. 3 bottom left). The corresponding predictor for short sleep was not significant. In other words, sleep restriction appeared to prevent the behavioral response (pressure difference between sweet and non-sweet) to the cognitive variable (wanting difference between sweet and non-sweet). Also, after habitual sleep on PR, sham-intake difference increased as difference in enjoyment increased (line 4 Table 10, Fig. 3, lower right). However, after short sleep on PR, sham-intake difference reversed and decreased (slope = 0.21 g/mm ± 0.16 SE) as difference in enjoy rating increased, and the difference between the two sleep conditions (0.53 g/mm ± 0.21 SE) was significant (= 0.03). Both of these predictors during a period of habitual sleep are consistent with those in Experiment 2, but on the CR schedule, cumulative pressure difference decreased (i.e. slope became negative) as difference in amount wanted in the taste test (i.e. the slope) increased (line 3 Table 10). The decrease was significantly larger in absolute value by 0.14 psi/g ± 0.05 SE after short (0.06 psi/g ± 0.04 SE) than after habitual sleep (−0.2 psi/g ± 0.04 SE). After short sleep on the PR schedule, sham-intake differences decreased with differences in liking in the taste test (see line 2, Table 10).
Table 10.
Regression statistics for Experiment 3 from Fig. 3.
| Schedule | Sleep condition | Dependent | Independent | Intercept | Intercept p-Value | Slope | SLOPEP-value | R2 |
|---|---|---|---|---|---|---|---|---|
| PR | Habitual | Cumulative (sipping) pressure | Amount wanted − taste test | −48.7 ± 19.5 | 0.05 | 0.33 ± 0.09 | 0.01 | 0.73 |
| PR | Short | Sham-intake | Liking rating (GLMS) − taste test | 31.7 ± 7.0 | 0.006 | −0.32 ± 0.11 | 0.03 | 0.65 |
| CR | Habitual | Cumulative (sipping) pressure | Amount wanted − taste test | 18.8 ± 9.7 | 0.11 | −0.20 ± 0.04 | 0.006 | 0.81 |
| PR | Habitual | Sham-intake | Enjoyment after sham-intake | −12.5 ± 6.9 | 0.13 | 0.33 ± 0.12 | 0.04 | 0.59 |
3.3.3. Milkshake intake
The amount of chocolate milkshake that was actually consumed on a PR schedule did not differ between the habitual (45 ± 8 g) and the short sleep (52 ± 10 g) condition (t(6) = −0.82, p = 0.45). Total meal duration was slightly, but not significantly, longer after 3 nights of short sleep as compared to habitual sleep (t(6) = −2.2; p = 0.08), but we did not observe differences in any of the other behavioral measures during milkshake intake on a PR schedule following habitual or short sleep duration (Table 8).
3.4. Comparison taste test results from Experiments 2 and 3
Amounts wanted were significantly lower in Experiment 3 than 2 by 181 g ± 57 SE for 6.1% sucrose and by 408 g ± 47 SE for 34% sucrose, but liking for these two beverages was not significantly different (see Table 4 for means for each beverage and experiment). There was also a significant study × solution interaction (F(4,52) = 3.45, p = 0.0142) attributable to a greater difference in amount wanted (306 g ± 112 SE, t(52) = 2.73, p = 0.0086) between 34% sucrose, but not 6.1% between the two studies. Although there were no significant differences for either wanting or liking of the Kool-Aid beverages, sweetened with aspartame, as used in the experiments, lower motivation in Experiment 3 than 2 for sweet beverages could be attributable to the reduced wanting of very sweet taste (34% sucrose) seen in the taste tests by participants in Experiment 3 compared to those in Experiment 2.
4. Discussion
4.1. Demonstration of utility
Manipulations of deprivation state, sweetness intensity and sleep restriction demonstrate that the sipometer is useful for measuring motivation and reward value of beverages in humans, and is analogous to measures used in animal studies. We may conclude that the proof of principle of the device, as a valid measure of motivation and reward value, has been demonstrated. The increased effort displayed in Experiment 1 after 21 vs 1 h of deprivation and for sweet vs non-sweet beverages is consistent with previous studies in which an increase in the rewarding qualities of foods after food deprivation in both animals [51,52] and humans [20,53] were found. The ability to translate, to humans, the results of prior animal studies, in which motivation and reward value have been found to affect behavior, should enable parallel advancements in humans.
Since increases in effort are thought to indicate the reward value of a food [21,23,24,54], the observations from the sipometer successfully assessed a higher willingness to work for the sweet, more rewarding, beverages. Specifically, in a second experiment, differences in effort expended for tasting a sweet than a non-sweet beverage correlated with their differences in reported hedonic value and amount wanted than for a less-rewarding, non-sweet, beverage.
In Experiment 3, the laboratory-based sleep restriction paradigm, participants did not seem to be more motivated to obtain sweet (as compared to non-sweet) beverages following three nights of short sleep duration. These findings were initially surprising, but upon reflection, may well be consistent with previous work. It is possible that the increased ad libitum food intake after sleep restriction [30,31] is not necessarily “motivated”, but simply occurs because the food is easily accessible. These results are similar to findings of increased intake without motivation seen in rats with ventromedial hypothalamic lesions [55] and in some obese individuals who ate more than controls when food was readily available, but would not expend effort to obtain It [56]. Thus, sleep restriction might induce neurological changes similar to those seen after VMH lesions, in relation to food intake control. These observations were consistent with another study in which rapid eye movement (REM)-sleep-deprived rats decreased their lever-press response rates in a PR task. Decreased lever-pressing indicates motivation to work for food access following sleep loss could have been reduced by the manipulation. These results are consistent with the expectation that the sleep manipulation utilized here would reduce REM sleep expression [57].
4.2. Comparison of Experiments 2 and 3
In Experiment 3, differences in the behavioral measures between sweet and non-sweet beverages did not replicate the results of Experiment 2. However, and more importantly, the correlations of pressure and taste wanting with sham-intake and enjoy rating both replicated Experiment 2 in the habitual condition of Experiment 3, regardless of beverage sweetness. Although we are not sure why the behavioral means did not replicate, but the correlations did, there were differences in the selection process and responses in the taste test between the two studies. Thus, the participants in Experiment 2 were screened for liking of the beverages, whereas the participants in Experiment 3 were not. Although the mean “liking” ratings were not different between the two experiments for the beverages that were used as reinforcers, there was a difference in the profile of sweet “wanting” ratings, in that both mildly sweet and intensely sweet (6.1 and 34% sucrose, respectively) “wanting” ratings were significantly higher for participants in Experiment 2 than for participants in Experiment 3. The increased “wanting” differences between the groups in the extremes of sweet taste may be an indicator that the participants in Experiment 2 were more motivated to work at the sipometer task for sweet taste than were those in Experiment 3. It is also possible that fatigue, due to sleep restriction, reduced effort expanded to obtain a rewarding food. This is a proposition that deserves further exploration as sleepiness ratings and other measures of fatigue were not obtained in the context of this study.
4.3. Limitations
Some limitations in the current report should be considered. All experiments were conducted with a small group of healthy young women. We can therefore not say to what extent results are generalizable to populations with different weight, age or sex. The small sample may also have resulted in considerable variation. We tried to limit this by assessing the associations between differences in liking and wanting ratings of the sweet compared to non-sweet beverages and differences in the behavioral parameters for these beverages. Finally, one may argue whether sipping for >9 s to obtain a mouthful of beverage could introduce unnatural elements in the behavioral chain of eating. To limit this, introducing a variable ratio instead of a progressive ratio to obtain the next reinforcement should be considered in future studies. Nevertheless, the paradigm simulates the commonly observed behavior that people continue sipping beverages from cups when only a few drops are left.
4.4. Novelty and future directions
Although considered an area of interest for decades, an accurate and objective behavioral measure of motivation and food reward for liquids delivered, during the course of the experiment, has been lacking in humans. Nevertheless, methods for delivering rewards for solids, as well many indirect methods, in which the primary reinforcer was presented at the end of the experiment (e.g. [59]) have been published [16,58]. Many studies relied on questionnaires in which participants made ratings of their hunger/fullness, appetite, and desire to eat [60, 61]. Others have also utilized computerized portion size selection paradigms [62,63] as well as operant tasks that involve repeated pressing on a mouse or computer key to receive points toward a food or non-food item viewed on a computer screen [64]. While these previously described methods go beyond participants’ ratings to incorporate behaviors, the sipometer is a further improvement, because it is based on an ingestive behavior measured in response to the rewarding food cue.
The sipometer can be easily employed to investigate food reward systems in various settings and in different populations. Here, we have demonstrated some possibilities, namely exploring the effects of hunger, compared with satiation, and of experimental sleep restriction compared with habitual sleep. Future directions and applications of the sipometer can include determining the effects of pharmacological or psycho-behavioral interventions, different pathological states such as anorexia or bulimia, effects of different macronutrients and following weight loss. The combination of the sipometer with functional neuroimaging techniques will also be helpful in elucidating neural mechanisms involved in food intake reward and control.
4.5. Conclusion
In summary, the sipometer is a device that can directly assess motivation to obtain access to a liquid food or beverage by measuring sipping time, pressure exerted, and breakpoint in a PR schedule, in humans. It can be used to measure the differences in effort exerted for different reinforcers and under different physiological conditions. Participants were more willing to work for a sweet beverage that was better liked and wanted than a less-rewarding, non-sweet beverage. In addition, the indicated amount an individual wants to consume associates well with actual behavior. Sleep restriction, used as an early experimental demonstration in this study, did not increase motivation for sham-consumption of a sweet, rewarding beverage relative to a non-sweet beverage when compared to habitual sleep duration. Altogether, these results demonstrate that the sipometer and associated ratings are reliable and useful measures of motivation to consume and reward value in humans, and we recommend its use in future investigations of these measures.
HIGHLIGHTS.
A device to measure reward value of liquids in humans was validated.
Time spent sipping was longer after 21-h than 1-h of food deprivation.
Time spent sipping, and pressure exerted, were higher for sweet than non-sweet drinks.
Sweet-non-sweet differences in pressure exerted, and in “wanting”, were correlated.
Sleep-restricted subjects worked less for sweet reward, despite increased desire.
Acknowledgments
The authors thank Jessica Chen, Sarah McNally, and Lauren Hoffman for their help in conducting the experiments. P.S. Hogenkamp was supported by NWO Rubicon and SSMF H.R. Kissileff was supported by the NIH grant (NIH/NIDDK DK26687) during the execution and early writing of this project, conducted as a service of the Human Ingestive Behavior Core Laboratory of the New York Obesity Research Center, at Mt. Sinai-St. Luke’s/Roosevelt Hospital, and subsequently at Columbia University Medical Center, F.X. Pi-Sunyer, Director. The first sipometer was constructed by Anthony Sclafani at Brooklyn College, and the version used in Experiments 2 and 3 was designed by Anthony Sclafani and constructed by the machine shop at the Pierce Foundation, Yale University, New Haven, supervised by John Buckley. Dana Small, Pierce Foundation, Yale University, provided supervisory assistance with construction and design. The main part of the sleep study whose participants served in Experiment 3 was funded by a St Luke’s/Roosevelt Hospital Center Pilot & Feasibility grant (St. Onge as PI). We thank Daria Igudesman for helping to edit the manuscript.
Footnotes
Formerly when data were collected at St. Luke’s/Roosevelt Hospital.
References
- 1.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav. 2003;79:663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 3.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Finlayson G, King N, Blundell J. E. Liking vs. wanting food: importance for human appetite control and weight regulation. Neurosci Biobehav Rev. 2007;31:987–1002. doi: 10.1016/j.neubiorev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Kleinginna PR, Jr, Kleinginna AM. A categorized list of motivation definitions, with a suggestion for a consensual definition. Motiv Emot. 1981;5:263–291. [Google Scholar]
- 6.Bulik CM, Brinded EC. The effect of food deprivation on the reinforcing value of food and smoking in bulimic and control women. Physiol Behav. 1994;55:665–672. doi: 10.1016/0031-9384(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 7.Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav. 2003;78:221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- 8.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 9.Nasser JA, Evans SM, Geliebter A, Pi-Sunyer FX, Foltin RW. Use of an operant task to estimate food reinforcement in adult humans with and without BED. Obesity. 2008;16:1816–1820. doi: 10.1038/oby.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schebendach J, Broft A, Foltin RW, Walsh BT. Can the reinforcing value of food be measured in bulimia nervosa? Appetite. 2013;62:70–75. doi: 10.1016/j.appet.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stellar E. The physiology of motivation. Psychol Rev. 1954;61:5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 12.Epstein L, Leddy J, Temple J, Faith M. Food reinforcemnet and eating: a multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheffield FD, Roby TB. Reward value of a non-nutritive sweet-taste. J Comp Physiol Psychol. 1950;43:471–481. doi: 10.1037/h0061365. [DOI] [PubMed] [Google Scholar]
- 14.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134 doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 15.Havermans R, Janssen T, Giessen J, Roefs A, Jansen A. Food liking, food wanting, and sensory-specific satiety. Appetite. 2009;52:222–225. doi: 10.1016/j.appet.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T, et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr. 2012;96:467–473. doi: 10.3945/ajcn.112.036921. [DOI] [PubMed] [Google Scholar]
- 17.Epstein L, Truesdale R, Wojcik A, Paluch R, Raynor H. Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav. 2003;78:221–227. doi: 10.1016/s0031-9384(02)00978-2. [DOI] [PubMed] [Google Scholar]
- 18.Kissileff H, Thornton J. Facilitation and inhibition in the cumulative food intake curve in man. In: MAJ SP, editor. Changing Concepts of the Nervous System. Academic Press; New York: 1982. pp. 585–607. [Google Scholar]
- 19.Raynor H, Epstein L. The relative-reinforcing value of food under different levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 20.Beth Spitznagel M, Alosco M, Strain G, Devlin M, Cohen R, Paul R, et al. Cognitive function predicts 24-month weight loss success after bariatric surgery. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2013;9:765–770. doi: 10.1016/j.soard.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein DA, Schebendach JS, Devlin MJ, Smith GP, Walsh BT. Intake, sweetness and liking during modified sham feeding of sucrose solutions. Physiol Behav. 2006;87:602–606. doi: 10.1016/j.physbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Panek-Scarborough LM, Dewey AM, Temple JL. Sensation and perception of sucrose and fat stimuli predict the reinforcing value of food. Physiol Behav. 2012;105:1242–1249. doi: 10.1016/j.physbeh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Klein DA, Schebendach JE, Gerskovich M, Smith GP, Walsh BT. Modified sham feeding of sweet solutions in women with anorexia nervosa. Physiol Behav. 2010;101:132–140. doi: 10.1016/j.physbeh.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein DA, Schebendach JE, Brown AJ, Smith GA, Walsh BT. Modified sham feeding of sweet solutions in women with and without bulimia nervosa. Physiol Behav. 2009;96:44–50. doi: 10.1016/j.physbeh.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauerdale D, Knutson K, Rathouz P, Yan L, Hully S, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA sleep study. Am J Epidemiol. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogenkamp PS, Nilsson E, Nilsson VC, Chapman CD, Vogel H, Lundberg LS, et al. Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology. 2013;21:1548–1553. doi: 10.1016/j.psyneuen.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–273. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St-Onge M-P, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93:1229–1236. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- 34.Benedict C, Brooks SJ, O’Daly OG, Almèn MS, Morell A, Åberg K, et al. Acute sleep deprivation enhances the Brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–E447. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- 35.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–824. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St-Onge M, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond) 2014 doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weijzen PL, Smeets PA, de Graaf C. Sip size of orangeade: effects on intake and sensory-specific satiation. Br J Nutr. 2009;102:1091–1097. doi: 10.1017/S000711450932574X. [DOI] [PubMed] [Google Scholar]
- 38.Zijlstra N, de Wijk RA, Mars M, Stafleu A, de Graaf C. Effect of bite size and oral processing time of a semisolid food on satiation. Am J Clin Nutr. 2009;90:269–275. doi: 10.3945/ajcn.2009.27694. [DOI] [PubMed] [Google Scholar]
- 39.Bolhuis DP, Lakemond CMM, de Wijk RA, Luning PA, de Graaf C. Both longer oral sensory exposure to and higher intensity of saltiness decrease ad libitum food intake in healthy normal-weight men. J Nutr. 2011;141:2242–2248. doi: 10.3945/jn.111.143867. [DOI] [PubMed] [Google Scholar]
- 40.Kissileff HR, Klingsberg G, Van Itallie TB. Universal eating monitor for continuous recording of solid or liquid consumption in man. Am J Physiol. 1980;238:R14–R22. doi: 10.1152/ajpregu.1980.238.1.R14. [DOI] [PubMed] [Google Scholar]
- 41.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 42.Peryam DR, Pilgrim FJ. Hedonic scale method of measuring food preferences. Food Tech Supp. 1957;11:9–14. [Google Scholar]
- 43.Bobroff EM, Kissileff HR. Effects of changes in palatability on food intake and the cumulative food intake curve in man. Appetite. 1986;7:85–96. doi: 10.1016/s0195-6663(86)80044-7. [DOI] [PubMed] [Google Scholar]
- 44.Kalva JJ, Sims CA, Puentes LA, Snyder DJ, Bartoshuk LM. Comparison of the hedonic general labeled magnitude scale with the hedonic 9-point scale. J Food Sci. 2014;79:S238–S245. doi: 10.1111/1750-3841.12342. [DOI] [PubMed] [Google Scholar]
- 45.Hayes JE, Allen AL, Bennett SM. Direct comparison of the generalized visual analog scale (gVAS) and general labeled magnitude scale (gLMS) Food Qual Prefer. 2013;28:36–44. doi: 10.1016/j.foodqual.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartoshuk LM, Fast K, Snyder DJ. Differences in our sensory worlds invalid comparisons with labeled scales. Curr Dir Psychol Sci. 2005;14:122–125. [Google Scholar]
- 48.Fast K. Unpublished thesis. Yale University School of Medicine; New Haven, CT: 2004. A Scale to Measure just about Anything. [Google Scholar]
- 49.Shechter A, Rising R, Wolfe S, Albu J, St-Onge M. Postprandial thermogenesis and substrate oxidation are unaffected by sleep restriction. Int J Obes (Lond) 2014 doi: 10.1038/ijo.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013;98:1433–1439. doi: 10.3945/ajcn.113.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jewett D, Cleary J, Levine A, Schaal D, Thompson T. Effects of neuropeptide Y, insulin, 2-deoxyglucose, and food deprivation on food-motivated behavior. Psychopharmacol. 1995;120:267–271. doi: 10.1007/BF02311173. [DOI] [PubMed] [Google Scholar]
- 52.Moscarello J, Ben-Shahar O, Ettenberg A. Effects of food deprivation on goal-directed behavior, spontaneous locomotion, and c-Fos immunoreactivity in the amygdala. Behav Brain Res. 2009;197:9–15. doi: 10.1016/j.bbr.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drobes DJ, Miller EJ, Hillman CH, Bradley MM, Cuthbert BN, Lang PJ. Food deprivation and emotional reactions to food cues: implications for eating disorders. Biol Psychol. 2001;57:153–177. doi: 10.1016/s0301-0511(01)00093-x. [DOI] [PubMed] [Google Scholar]
- 54.Teitelbaum P. The use of operant methods in the assessment and control of motivational states. Operant behavior: areas of research and application. 1966:565–608. [Google Scholar]
- 55.Miller NE, Bailey CJ, Stevenson JAF. Decreased “hunger” but increased food intake resulting from hypothalamic lesions. Science. 1950;112:256–259. doi: 10.1126/science.112.2905.256. [DOI] [PubMed] [Google Scholar]
- 56.Schachter S. Obesity and Eating. Science. 1968;161:751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- 57.Shechter A, O’Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge M-P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303:R883–R889. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, et al. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology (Berl) 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- 59.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 60.Flint A, Raben A, Blundell J, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes (Lond) 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 61.Born JM, Lemmens SG, Martens MJ, Formisano E, Goebel R, Westerterp-Plantenga MS. Differences between liking and wanting signals in the human brain and relations with cognitive dietary restraint and body mass index. Am J Clin Nutr. 2011;94:392–403. doi: 10.3945/ajcn.111.012161. [DOI] [PubMed] [Google Scholar]
- 62.Brunstrom JM, Rogers PJ, Pothos EM, Calitri R, Tapper K. Estimating everyday portion size using a ‘method of constant stimuli’: in a student sample, portion size is predicted by gender, dietary behaviour, and hunger, but not BMI. Appetite. 2008;51:296–301. doi: 10.1016/j.appet.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Brunstrom JM, Rogers PJ. How many calories are on our plate? expected fullness, not liking, determines meal-size selection. Obesity. 2009;17:1884–1890. doi: 10.1038/oby.2009.201. [DOI] [PubMed] [Google Scholar]
- 64.Nasser JA, Evans SM, Geliebter A, Pi-Sunyer FX, Foltin RW. Use of an operant task to estimate food reinforcement in adult humans with and without BED. Obesity. 2008;16:1816–1820. doi: 10.1038/oby.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]


