Abstract
Although certain risk factors can identify individuals who are most likely to develop chronic pain, few interventions to prevent chronic pain have been identified. To facilitate the identification of preventive interventions, an IMMPACT meeting was convened to discuss research design considerations for clinical trials investigating the prevention of chronic pain. We present general design considerations for prevention trials in populations that are at relatively high risk for developing chronic pain. Specific design considerations included subject identification, timing and duration of treatment, outcomes, timing of assessment, and adjusting for risk factors in the analyses. We provide a detailed examination of 4 models of chronic pain prevention (i.e., chronic post-surgical pain, postherpetic neuralgia, chronic low back pain, and painful chemotherapy-induced peripheral neuropathy). The issues discussed can, in many instances, be extrapolated to other chronic pain conditions. These examples were selected because they are representative models of primary and secondary prevention, reflect persistent pain resulting from multiple insults (i.e., surgery, viral infection, injury, and toxic/noxious element exposure), and are chronically painful conditions that are treated with a range of interventions. Improvements in the design of chronic pain prevention trials could improve assay sensitivity and thus accelerate the identification of efficacious interventions. Such interventions would have the potential to reduce the prevalence of chronic pain in the population. Additionally, standardization of outcomes in prevention clinical trials will facilitate meta-analyses and systematic reviews and improve detection of preventive strategies emerging from clinical trials.
Keywords: Prevention trial design, Chronic post-surgical pain, Postherpetic neuralgia, Chronic low back pain, Chemotherapy-induced peripheral neuropathy, Risk factors
1. Introduction
Chronic pain is highly prevalent and difficult to treat [81]. Moreover, it is a costly public health problem, contributing to high health care costs and lost productivity [11,17,20,56,100]. Although certain risk factors can identify individuals who are most likely to develop chronic pain, very few interventions to prevent chronic pain have been identified, adopted for use in clinical practice, or approved by regulatory agencies. Chronic pain that develops after an injury has resolved, a toxicity or noxious element has been removed, or an infection has resolved have all been hypothesized, at least in part, to be mediated by nerve damage either from the insult itself or from an increase in the excitability and responsiveness of neurons in the spine (i.e., central sensitization) due to severe acute pain [179]. If nerve damage during the initiating insult contributes to chronic pain, minimizing that damage as early as possible will likely decrease both acute and chronic pain. If the persistent pain is, at least in part, caused by central sensitization, preventing or minimizing acute pain at the time of insult may prevent the development of chronic pain. In other instances, such as chronic low back pain (CLBP), preventing re-injury could provide an approach for preventing chronic or recurrent pain.
In this article, we discuss research design considerations for clinical trials that evaluate both primary and secondary preventive interventions that target mechanisms that putatively contribute to the development of chronic pain. We focus on research design issues that can be applied to prevention trials for any chronic pain condition in which a patient population at high risk for developing chronic pain can be identified. We have selected 4 models of chronic pain prevention to discuss in detail: chronic post-surgical pain (CPSP), postherpetic neuralgia (PHN), CLBP, and chemotherapy-induced peripheral neuropathy (CIPN). Since estimates of pain prevalence vary greatly depending on how the data are collected, it is difficult to determine which pain conditions have the greatest personal and public health impact. Thus, these four examples were chosen because they (1) represent models that could be amenable to primary or secondary prevention; (2) are conditions that are treated with a range of modalities including pharmacological, invasive, and non-pharmacological / non-invasive interventions; and (3) represent pain induced by multiple etiologies. Specifically, we chose models of surgical trauma, viral infection, injury, and a toxicant, as these models are most commonly discussed in the context of preventing chronic pain. This article is not a systematic review of all published chronic pain prevention studies in these 4 fields and will not comment on efficacy of any given preventive treatment based on single studies.
2. Methods
An Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) meeting, including a diverse group of participants from universities, government agencies, industry, and a patient advocacy group, with 25 representatives from the United States, 2 from Canada, and 4 from Europe, was held to discuss research design considerations for chronic pain prevention trials. Participants were selected to represent a broad range of relevant topics, areas of expertise, and disciplines, while the number of participants was limited to promote productive and efficient discussion.
To facilitate discussion, background lectures were presented that examined (1) neurobiologic aspects of preventing acute and chronic pain (CJW); (2) risk factors for the development of chronic pain, including genetic predisposition (RBF); (3) research design considerations for clinical trials of perioperative analgesic medications to optimize acute postoperative pain management and recovery (SNR); (4) chronic post-surgical pain (IG); (5) postherpetic neuralgia (RHD); (6) painful diabetic peripheral neuropathy (DZ); (7) HIV and chemotherapy-associated peripheral neuropathies (MJP); (8) complex regional pain syndrome (CRPS) type 1 (ALO); (9) chronic neuropathic low back pain (LBP) (JDM); (10) chronic musculoskeletal LBP (JPR); and (11) critical methodologic aspects of clinical trials to prevent chronic pain (JF).
To supplement the background presentations, a review was performed for each of the prevention models discussed here to identify any recent randomized clinical trials (RCTs) with relevant prevention design features that could be described as examples of the recommendations that were formulated through the meeting and subsequent discussions. PubMed was searched for articles published between June 2009 and December 2014 using the search strategies outlined in Appendix 1.
Ideally, recommendations for clinical trial designs should be based on systematic studies; however, because relatively few randomized clinical trials investigating preventive interventions for chronic pain have been conducted (e.g., see Chaparro et al. [30] and Han et al. [68]), information that would make it possible to attempt to attribute falsely negative trial results to methodological issues is lacking. The design recommendations presented in this article are based primarily on existing literature along with the experience and expertise of the authors, the background presentations, the discussions that subsequently occurred during the meeting, circulation of a draft manuscript to all authors, and iterative revision of the draft manuscript until approval of all authors for submission for publication was achieved. We focus on design considerations that are particularly relevant to prevention trials. Previous IMMPACT recommendations as well as the CONSORT statement and comprehensive text books are available for guidance on more general methodological issues of clinical trials (e.g., randomization procedures, choice of active vs. inactive placebo) [49,62,145,149]. Figure 1 presents a general research design schema for chronic pain prevention trials that provides a framework for many of the specific considerations discussed below. We believe that implementation of these recommendations in future chronic pain prevention trials would expedite the development of effective prevention strategies and facilitate the preparation of more informative systematic reviews and meta-analyses.
Figure 1. General chronic pain prevention design schema.
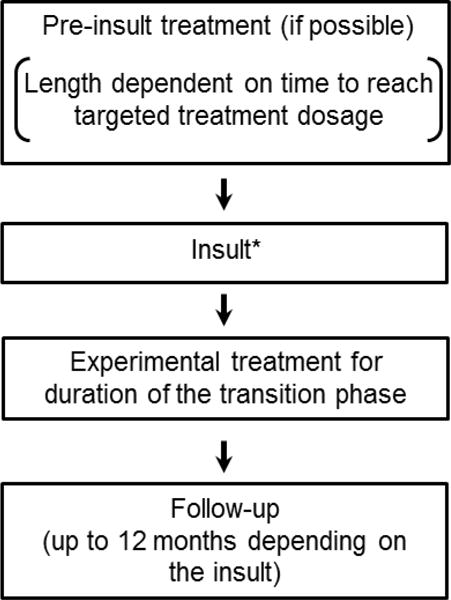
*Insult models include surgery, disease (e.g., herpes zoster), injury (e.g., acute low back injury), and toxic exposure (e.g., chemotherapy).
3. General considerations
3.1. Acute pain severity
One prominent hypothesis regarding mechanisms underlying the development of chronic pain is that nociceptive processes, which also cause acute pain, can cause peripheral or central sensitization, leading to the initiation and maintenance of chronic pain [90,110,180]. Thus, one investigational approach has been aimed at reducing acute pain during or shortly after an inciting painful insult in order to reduce both acute pain and the potential development of chronic pain [5, 30]. Monitoring acute pain intensity allows one to test the hypothesis that the effect of treatment on preventing chronic pain is mediated by its effect on acute pain. For example, if the preventive analgesia does not decrease either acute pain or chronic pain, further efforts to decrease acute pain with other agents or different dosages of the same agent as a method to prevent chronic pain would be warranted. If acute pain severity is lower in the treated group than in the placebo group, but no difference in chronic pain is found between groups, decreasing acute pain may not be sufficient to prevent chronic pain, a possibility that has been discussed by Katz and Seltzer [91]. Alternatively, it is possible that the threshold of acute pain that triggers central sensitization is even lower than the level experienced by the treated group. When deciding whether to further pursue preventive treatments that target acute pain levels, investigators should consider the degree to which the acute pain was decreased in the treatment group and whether a larger decrease in acute pain to reach a possibly lower acute pain threshold is a realistic goal. For trials that use prevalence of any chronic pain as the primary outcome, an acute pain severity measure that assesses a similar type of pain as in the chronic pain outcome (e.g., burning pain, pain on movement) should be used.
3.2. Outcome measures
Previous IMMPACT recommendations suggested including pain intensity and physical and emotional functioning as core outcome domains in chronic pain trials [162]. Our recommendations emphasize pain outcomes, including presence and severity, to illustrate various methodological issues in the prevention setting. However, these considerations are also generally applicable to other outcomes that can be important to include in prevention trials, including physical and emotional functioning and sleep, which we also discuss when their assessment is particularly important. When assessing pain severity it is important to consider the time frame (e.g., a single time point after randomization or a combination of multiple assessments). Presence outcomes include any pain versus no pain or pain above or below a “clinically meaningful” or moderate pain intensity level (e.g., average pain intensity of 3 out of 10 or greater). Although an analysis based on “clinically meaningful” pain levels may have a higher impact from a clinical and public health perspective, it may also have less power because the incidence of moderate to severe pain will be lower than the incidence of any pain. Furthermore, it may be difficult to identify clinically meaningful levels of pain for the different chronic pain conditions given that there has been little systematic examination of patient-reported assessments of the long-term impact of different levels of pain. Future studies should investigate patient opinions regarding the minimal pain intensity and duration that would be considered to be clinically meaningful in relation to the probability of developing such chronic pain as well as risks and costs of the potential preventive treatment (e.g., what level and nature of side effects would the patient be willing to tolerate for an intervention that reduced the probability of a certain intensity of pain in the future by a specified amount or time period). Better understanding of how to define the minimal threshold of chronic pain that would be considered clinically meaningful will allow researchers to more accurately determine the necessary sample sizes for RCTs of preventive analgesic treatments. It is important to note that such clinically meaningful differences should only be used to define responders at the individual level, and should not be extrapolated to a required minimum difference between groups in pain intensity [47]. Issues regarding outcome measurement and timing in specific prevention models are discussed further below.
Post-randomization initiation of non-study pain medications can complicate the interpretation of pain severity ratings. Initiation of such pain medications is likely to be more common in prevention trials than in treatment trials in which patients have been in pain for several months or more and often are already taking pain medications at stable dosages. Because prevention trials start prior to or soon after pain onset, it is generally not possible to require that patients use only those non-study pain medications they were taking regularly before the initiation of the trial. Therefore, innovative ways to manage post-randomization initiation of non-study pain medications in the analyses are especially important in prevention trials. As a potential solution to this problem, the U.S. Food and Drug Administration (FDA) [166] recently recommended including an outcome that would jointly assess pain and rescue medication in analgesic trials. For example, a responder analysis could be conducted in which participants are considered responders if they report chronic pain less than a pre-specified value and take less than a pre-specified amount of rescue medication. The reliability and responsiveness of such a composite outcome measure has yet to be established and therefore this approach cannot be suggested for primary outcome measures. However, considering that there is no existing evidence-based solution for this problem, and that these types of measures have face validity, we recommend that investigators consider including some type of composite measure as a secondary outcome. The inclusion of such measures in future trials will provide data to examine the reliability, responsiveness, and validity of such approaches. Another option for prevention trials is an analysis comparing treatment groups with respect to the presence or absence of pain of any level of intensity at a pre-specified extended, post-insult time point (i.e., at time point when the pain is considered to be chronic). This analysis would likely not be complicated by the use of rescue or concomitant analgesic medication because it is rare that chronic pain is completely eliminated by medication.
3.3. Challenges of evaluating assay sensitivity
One challenge of conducting chronic pain prevention studies is that very few, if any, hypothesized interventions have shown replicated evidence of efficacy; therefore, active comparators are not readily available to assess the assay sensitivity of particular outcome measures or trial designs. Once an efficacious preventive intervention is identified for a condition, the sensitivity of the outcomes proposed in this review can more easily be evaluated. Another challenge occurs when pain existing prior to the insult cannot easily be distinguished from the pain subsequently caused by the insult. For example, patients who have burning pain in the feet from diabetic peripheral neuropathy (DPN) are likely to find it impossible to distinguish this pain from burning pain in the feet from CIPN. Additionally, patients undergoing surgery for back pain may not be able to distinguish between chronic pain caused by the surgery and residual unresolved back pain. In these instances, it may be advantageous to exclude such patients from prevention studies. In contrast, patients with burning pain in the feet from DPN can probably distinguish their DPN pain from new onset thoracic PHN pain. In such cases, patients with existing pain can be included in the trials, but existing chronic pain should be examined in the analyses in a similar manner to risk factors described in Section 3.4.
3.4. Pre-specified adjustment or stratification for risk factors
Adjustment for a limited number of well-established risk factors for the development of chronic pain could increase the ability to detect a preventive effect [86,166]. In order to control type I error, any covariate adjustment made in the primary analysis or secondary analyses should be specified before the treatment assignments are revealed. Data from such pre-specified analyses could also be used as further evidence to address the validity of the proposed risk factors. However, it is important to note that cohorts participating in a clinical trial may be quite different from the target population, and therefore associations found using clinical trial data may not necessarily reflect the associations that exist in the population. Furthermore, including interactions between treatment and potential risk factors for the development of chronic pain can identify possible sub-groups for which a preventive intervention is more likely to be efficacious. Such findings would generally be considered to be hypothesis generating, unless incorporated in the primary analysis through pre-planned adjustment for multiplicity. Researchers should also consider using well-established risk factors as stratification variables in randomization plans.
A potential alternative to adjustment for chronic pain risk factors is to consider risk factors in the study entry criteria to increase the incidence of chronic pain in the sample population. This is a common practice in PHN prevention trials that often include only herpes zoster patients older than 50 years of age. CIPN prevention trials always enroll patients receiving chemotherapy treatments with the highest risk of CIPN, including taxanes, platinum agents, and vinca alkaloids. In other conditions in which risk factors for chronic pain have less evidential support, this may not be appropriate. However, greater acute pain intensity (i.e., severity) has frequently been found to increase the risk of chronic pain and should therefore be considered as a possible inclusion criterion or covariate in secondary prevention trials of chronic pain. The extent to which more restrictive eligibility criteria would hinder recruitment rates should be considered before their implementation. Furthermore, such eligibility criteria would decrease the generalizability of the findings to only those patients who are identified as high risk for the development of chronic pain. On the other hand, a trial that includes only those patients considered to be at high risk will likely require a smaller sample size.
In general, the number of adjustments made in the primary analysis should be limited, because covariates that are not predictive of the outcome can decrease power, especially for studies with a small number of participants. It is also important to note that it is generally not appropriate to adjust for a risk factor that is measured post-baseline because it could be altered by the experimental treatment. For example, adjusting for cumulative dosage of chemotherapy in a trial of a preventive treatment for CIPN might produce misleading results because the treatment may have an effect on both the cumulative dosage of chemotherapy and the development of CIPN. An exception to this occurs when one is interested in testing specific hypotheses concerning factors that may mediate the effect of a treatment on prevention of pain. For example, structural equation models [117,135] can be used to examine whether the effect of a treatment on prevention of chronic pain is mediated through its effect on acute pain. It is beyond the scope of this work to perform a systematic review of the literature to identify and evaluate the level of evidence for risk factors for each of the 4 chronic pain models discussed here. However, investigators should review the literature when planning chronic pain prevention trials to identify evidence-based risk factors that should be considered when developing the study design and analyses.
3.5. Genetic Factors
Genetic factors contribute significantly to the variability in both pain and analgesic responses; therefore, consideration of genetics is important in the design and conduct of chronic pain prevention trials. Multiple candidate genes have been associated with laboratory measures of pain sensitivity and with clinical pain [38,39]. In particular, three genes have shown consistent associations with both experimental and clinical pain responses: the catechol-O-methyl transferase gene (COMT), the mu-opioid receptor gene (OPRM1), and the GTP-cyclohydrolase gene (GCH1) [115]. Regarding COMT, polymorphisms and haplotypes have been associated with laboratory pain sensitivity, and acute and chronic clinical pain [13,40], including long-term outcomes after back surgery [143]. Similarly, the A118G polymorphism of OPRM1 has been associated with experimental pain sensitivity and with clinical pain [53,71,123], including long-term outcomes of acute back pain [124]. Finally, GCH1 genotypes have been associated with experimental and clinical pain responses across several cohorts [15,28,159,160], and GCH1 genotypes have predicted outcomes from lumbar spine surgery [96,160]. Genetic factors can also impact postoperative analgesic responses, which may impact CPSP since acute pain severity is among the strongest predictors of CPSP. In addition to multiple genes that impact drug metabolism [144], COMT [29,36] and OPRM1 [80,152] have been associated with postoperative opioid analgesic requirements.
Importantly, these genes can interact with each other and with non-genetic factors to influence pain responses and analgesic requirements. For example, previous studies have reported that COMT, OPRM1, and GCH1 show sex-specific associations with pain responses [14,15,124]. In addition, COMT haplotypes have been shown to interact with psychological functioning (i.e., pain catastrophizing) to predict both experimental and clinical shoulder pain outcomes [58,60]. Research has shown that OPRM1 and COMT exerted combined effects on the total dosage of morphine consumed by patients with chronic cancer-related pain [138]. Specifically, individuals who were homozygous for both the Val allele of COMT Val158Met polymorphism and the 118A allele of OPRM1 required significantly lower morphine dosages compared to all other groups. Genetic factors can also directly impact responses to pharmacologic interventions; therefore, genetic variables should be considered for incorporation into trials designed to reduce the risk of chronic pain as potential covariates to increase power or allow examination of differential treatment response in patient subgroups.
Although some trials may be developed to address genetic factors, many trials may not be designed or have sufficient power to test genetic hypotheses. In these cases, collection and storage of biological samples for future genotyping would be an ideal approach. This practice could create a rich resource for future analyses designed to identify genetic influences on the development of chronic postoperative pain and its prevention. The added logistical and administrative burden is relatively small in proportion to the potential scientific and translational value that such information could provide.
4. Design considerations in the context of 4 illustrative prevention models
4.1. Chronic post-surgical pain (CPSP)
Several definitions of CPSP have been proposed, including a recent proposal based on research findings and current knowledge of pathophysiologic mechanisms [172]. CPSP occurs after the damaged tissue has healed and thus a large component is thought to be neuropathic [70]. Although CPSP is generally thought to be a result of surgery-induced nerve damage, the exact mechanisms are unknown and whether the mechanisms are similar between different surgery types is also unknown. Potential causes include peripheral and central sensitization [21,105,110,179,180]. It is important to note that in specific circumstances, pain is likely not neuropathic. For example, postherniotomy pain could be due to irritation from the mesh and once that mesh is removed the pain could subside. CPSP has been considered an excellent model to study chronic pain prevention because the exact timing of the injury is known, allowing for primary prevention. One potential drawback of CPSP models is that surgery is often used to treat painful conditions and it can be difficult to avoid confounding from pre-surgery pain in the outcome analyses. CPSP models with little to no pre-surgical pain (e.g., thoracotomy) can be used to prevent confounding.
Preventive efficacy of a compound for CPSP should first be studied in surgical pain models with higher incidences of CPSP (i.e., 20%-60%), such as mastectomy, thoracotomy, amputation, and inguinal hernia repair [21,70] to decrease necessary sample sizes. Pain prevention studies using similar pharmaceutical agents in different post-operative models have had inconsistent results [27,120,121,150], suggesting that different mechanisms may be involved compared to other persistent postoperative pain states. This inconsistency could be due to variable efficacy of similar drugs in different models, or could represent false negative results due to low power or other methodological issues. Therefore, any interventions found to be efficacious in surgeries with high rates of CPSP should be tested in other models before being used clinically in other surgical settings [89,99].
4.1.1. Treatment timing
Primary chronic pain prevention, which is treatment initiated before exposure to the pain causing agent or event, is ideal. Primary preventive treatments for CPSP should generally be initiated so that treatments are ideally at their effective dosage before the surgery begins. This may require days or even weeks of titration to target dosage with such medications as duloxetine, gabapentin, or pregabalin. Many prevention studies for CPSP have initiated gabapentin and other oral medications between 1 hour and 1 day prior to surgery [3,12,24,25,27,33,51,52,57,66,95,97,98,101,108,116,131,133,147,148,165]. One study initiated treatment with an antidepressant 2-3 weeks prior to coronary artery bypass surgery; however, chronic pain was only assessed as part of a composite quality of life measure [31].
Acute post-surgical pain includes all pain that occurs before healing of the tissue damage from the surgery [106]. When feasible and reasonable, preventive interventions for CPSP should generally be continued as long as the tissue damage from the surgery is present and for the entire duration of acute post-operative pain. This type of pain, however, can last for months for certain surgeries (e.g., thoracotomy or total knee replacement). Depending on the type of investigational intervention, it may not be feasible or sensible to extend the treatment for the full duration of the acute pain period. For example, an intravenous (IV) or subcutaneous analgesic that is given throughout the surgery and / or post-operative stay will likely not cover the entire duration of the acute pain period, but may still be effective at reducing chronic pain. Several prevention trials have investigated IV or subcutaneous medications administered before, during, and between 1 and 4 days after surgery [34,42,65,72,158,171]. An oral analgesic, however, can be administered throughout the entire acute pain period, in both clinical trials and in clinical practice, thus continuing such an intervention throughout the entire acute pain recovery period should be considered. This recommendation is based on the hypothesis that chronic pain is caused, at least in part, by pathophysiologic mechanisms associated with acute pain. If another mechanism (e.g., nerve injury) is responsible for the development of CPSP, analgesic treatment for the entire duration of tissue damage may not be necessary, especially in instances where continued treatment with the experimental intervention is associated with unacceptable adverse events or high cost. We suggest that the time period in which post-surgical pain is considered acute should be based on natural history data specific to each surgery model or, in the absence of epidemiological data, the clinical expertise of the investigator. Several clinical trials have extended the investigational treatment between 5 and 14 days post-surgery [3,27,51,52,67,66,95,108,120,133].
4.1.2. Outcome measures and assessment timing
Whether using a continuous pain intensity measure (e.g., numeric rating scale (NRS) or visual analog scale) or a binary any pain vs. no pain measure, “pain intensity upon movement” should be included in CPSP trials as a component of the acute and chronic pain assessments [93]. Various trials have assessed CPSP with less specific movement, for instance “while coughing” [125,165], “during daily activities” [12], “while moving” [88,170] or during a defined movement protocol [3]. Procedure-specific validated assessments of pain-related functional consequences, such as those described for hernia surgery [54], thoracic surgery [140], and breast [4] surgery, have been developed. Inclusion of these measures in future studies could help characterize their responsivity to change, which has yet to be investigated. Pain measures that assess both neuropathic and non-neuropathic pain qualities such as the Short-form McGill Pain Questionnaire-2 [50], or that only assess neuropathic pain qualities such as the Neuropathic Pain Symptom Inventory [22], can also be included to assess the nature of the CPSP [106]. If the pain qualities are different before and after surgery, the pain can more confidently be attributed to the surgery. Multiple studies have included neuropathic pain measures as CPSP study outcomes [12,19,27,33,51,65,67,78,88,108].
The primary assessment time for CPSP should be assessed at a point after surgery when the tissue damage would be expected to have healed, but not so long that the prevalence of the CPSP is too low to detect a difference between groups with a reasonable number of study subjects. Early CPSP assessments should be administered shortly after the surgical tissue damage is expected to heal. In order to improve opportunities for future meta-analyses, all CPSP trials should also include 3, 6, and 12 month assessments, whenever possible. Acute pain outcomes (e.g., 24 - 48 hours post-surgery) and use of non-study analgesic treatments should be collected even though the primary aim of the study is to investigate the prevention of CPSP. These data can contribute to our understanding of whether acute pain predicts chronic pain and whether different acute factors contribute to the transition from acute to chronic pain (i.e., 24 – 72 hours to 3 months) than those that contribute to the persistence of chronic pain (i.e., 6 months to 1 year), which has been investigated in previous studies [87,146].
4.2. Postherpetic neuralgia (PHN)
PHN is persistent pain that occurs following herpes zoster (HZ) infection (i.e., shingles), which may be caused by central sensitization from acute HZ pain [142]. HZ causes a unilateral, dermatomal, usually painful, vesicular rash [45]. The 3 best-established risk factors for PHN after HZ infection are advanced age (> 50 years), greater HZ rash severity, and more severe pain during the HZ infection [46]. Approximately 20% of patients who have HZ and are over age 50 develop PHN even with antiviral therapy, which is the only preventive treatment for PHN that has shown replicated efficacy [18,44,83,163,164,178]. We recommend that investigators include only patients over 50 years of age in these studies to maximize power. Furthermore, they should consider using a minimum acute pain score (e.g., greater than 3 on a 0-10 NRS) at the time of enrollment to further increase the number of patients who will develop PHN [32,85,111,118,175].
A vaccine is available that decreases the risk of HZ infection and in so doing decreases the risk of developing PHN. However, the vaccine did not decrease the risk of developing PHN in those patients who developed HZ after being vaccinated [127] and is therefore less directly relevant to the prevention of chronic pain.
4.2.1. Treatment timing
Prior to the HZ infection, it is difficult to predict which individuals will develop PHN therefore, only secondary prevention (i.e., prevention of transition to chronic pain after exposure to a pain causing agent or event) is possible without recruiting an extremely large sample of participants. Ideally, preventive treatment should be started as soon as possible after the initial insult, in this case the HZ infection and rash onset. Patient recruitment is often a limiting factor in how early in the course of HZ progression the preventive treatment can be initiated. Patients typically do not visit their doctor until after the rash appears and HZ is usually treated by primary care physicians. Thus, close collaborations with primary care physicians are encouraged [126]. Investigators should also identify a maximum duration of HZ rash for study eligibility. Cut-offs of 3 [174,178], 6 [43] or 7 days [84,130,168] following rash onset have been used in previous trials. Because antiviral treatment decreases HZ severity and the duration of PHN [163], all subjects in PHN prevention trials should be treated with an antiviral agent in addition to either the investigational treatment or placebo.
The natural history of HZ infection and progression of PHN has been studied in detail [6,45]. Pain existing up to 30 days after HZ rash onset is considered acute and preventive treatment throughout this time period in clinical trials is encouraged. This could include oral medications taken daily for up to 30 days (but probably for not less than 2 weeks) as well as single or intermittent treatments whose effects are thought to persist beyond their administration (e.g., nerve blocks of various types). Investigation of treatments that target the infection (e.g., novel antiviral therapies or treatments that enhance immune function) is an exception to these considerations. These treatments would likely only be beneficial while the HZ infection is active, although their effects on reducing nerve damage could have long-term benefits. Seven days of antiviral treatment was shown to decrease the duration of PHN in a study in which the median time to last positive viral culture was 2 days in the placebo group; this suggests that 7 days likely covers the period of active infection in the majority of patients and would be a reasonable treatment time for interventions that target the acute infection [163].
4.2.2. Outcome measures and assessment timing
The frequency with which PHN eventually resolves depends on how long after rash onset the HZ-associated pain has persisted. The rate of pain resolution in patients whose pain has persisted at least 4 months after rash onset appears to be less than the rate of resolution in patients whose pain has lasted less than 4 months; thus, it has been suggested that PHN can be defined as pain persisting at least 4 months after rash onset [45]. This 4 month time point can be considered for primary analyses in PHN prevention trials, although pain persisting for 90 days after rash onset – the definition of PHN used in the Shingles Prevention Study of vaccination – is also reasonable [127].
Prevalence of any persisting HZ-associated pain at a pre-specified time point after rash-onset has been used to assess efficacy of PHN prevention treatments [23,84,107,130,168]. Whether using a continuous pain intensity measure or a binary pain prevalence outcome measure, participants in PHN prevention studies should be instructed to only consider the pain that is located in the area that their HZ rash had been present. If overall pain intensity is assessed using a NRS or other rating scale, participants should also be asked separately about pain in response to non-painful stimuli, especially from light touch (i.e., allodynia).
4.3. Chronic low back pain (CLBP)
CLBP was recently defined by the NIH task force on research standards for CLBP as pain occurring between the lower posterior region of the rib cage and the horizontal gluteal fold that has lasted every day for at least 3 months and occurred on at least half the days for a minimum of 6 months [37]. A recent global evaluation of disease burden found that LBP was the greatest contributor to disability of the 291 diseases studied [79]. The strongest predictor of developing CLBP is experiencing an acute LBP episode. Studies suggest that only 25% to 58% of patients with acute LBP will fully recover within 12 months of the original episode, with the remaining patients experiencing recurring episodes of acute pain or persistent chronic pain [35,76,94,132]. Predicting which acute back pain patients will develop chronic pain is challenging, which can make it difficult to identify patients to enroll in a prevention trial.
The STarT Back Tool (SBT) combines 9 repeatedly identified risk factors (i.e., leg pain, comorbid pain, 2 disability items, bothersomeness, catastrophizing, fear, anxiety, and depression). Considering that multiple studies demonstrate the ability of the SBT to predict chronic pain or disability [16,77,169], investigators could consider including this as a covariate in the primary analyses of prevention trials. This practice would also better characterize the prognostic value of the SBT for different populations of acute back pain patients. The SBT was shown to have relatively high specificity when predicting poor outcomes in 2 studies [77,169]; therefore, investigators could consider using the SBT to identify patients likely to experience CLBP for enrollment in prevention trials. However, because of the relatively low sensitivity of the SBT, even in the studies demonstrating its prognostic value, many patients who would be excluded from a clinical trial using this measure would develop CLBP, potentially limiting the generalizability of the trial’s results. It is important to note, however, that the optimal balance between sensitivity and specificity of a screening tool depends on the purpose of the screening [119].
Optimal participants for a CLBP prevention trial would be patients who are experiencing their first LBP episode; however, these patients are relatively rare in the adult population and such an inclusion criterion could be expected to increase the enrollment time and cost of a trial. In order to minimize the number of patients included in a prevention trial who already frequently experience recurrent LBP episodes, investigators could consider limiting recruitment to patients who have not visited a clinician for LBP or experienced a recurrence of back pain for some period of time before the current episode, for example, 6 months or 1 year [169].
The pathophysiological mechanisms of nonspecific LBP are largely unknown. Like the other chronic pain conditions discussed here, severe acute LBP has been shown to predict CLBP. Thus, controlling acute LBP initially could potentially prevent chronic back pain by decreasing central sensitization or other peripheral or central mechanisms. However, evidence suggests that CLBP also has a large psychosocial component. Furthermore, physical therapy and spinal manipulation are often used to promote healing of acute LBP in clinical practice. As a result, the types of interventions used to prevent CLBP often include non-pharmacologic treatments. The type and mechanism of an intervention intended to prevent the development of CLBP in patients with acute back pain must be carefully considered when applying these recommendations to trials designed to test these interventions.
4.3.1. Treatment timing
The CLBP study designs proposed are trials of secondary prevention and thus the acceptable length of time after initiation of the acute pain episode needs to be considered. Assuming that decreasing acute LBP truly prevents the transition to CLBP, enrolling participants as close as possible to the start of their pain episode would be optimal. Data from a prospective study by Croft et al. [35] suggest that the earlier patients present at the clinic for treatment, the more likely they are to recover by 3 and 6 months. Previous prospective studies have recruited LBP patients experiencing a LBP episode of less than 2 [73], 3 [55,153], and 6 weeks [176].
The exact time point at which an acute back pain injury begins and is resolved is difficult to identify and likely differs greatly among patients. Research suggests that few patients who still experience LBP at 3 months after the initial episode will fully recover [26,35,82,94,132], suggesting that by 3 months acute LBP has likely become chronic, which is consistent with the NIH task force definition of CLBP [37]. Thus, administering preventive treatments for 3 months after enrollment would likely be sufficient to cover the time period of most patients’ acute injury pain. A full 3-month treatment may not be necessary, however, for interventions with lasting effects such as self-hypnosis or relaxation training. The duration of the preventive interventions should be based on previous studies of similar interventions in order to avoid unnecessary burden on patients and reduce study costs.
4.3.2. Outcome measures and assessment timing
The NIH task force recommends that all research studies involving participants with LBP should include a minimum dataset with the domains of physical function, depression, sleep disturbance, and catastrophizing [37]. The presentation of CLBP is variable; patients can experience a consistent level of pain or recurring episodes of severe pain interspersed with periods of moderate pain intensity or even no symptoms [94]. This variable pattern can make it difficult to capture the pain experience for all patients in a single primary endpoint if the intervention is targeted at preventing both steady chronic and episodic pain. CLBP prevention studies have used outcomes such as pain intensity (with various recall periods) at a pre-specified time point [2,59,61,109,157,161,173]. Although these outcomes can provide valuable information, if an individual experiences variable pain, the outcome will be very different depending on whether the pre-specified assessment point falls during a period of high or low pain [119]. Similarly, a time to recovery outcome (used in [69]), may not reflect severity for episodic pain patients. A recent study by Williams et al. [176] used a time to recovery of at least 7 days, which could increase the validity of this type of outcome measure for episodic patients whose pain fluctuates frequently. The number of low back pain recurrences or painful months in a pre-specified time period (used in [41,103]) will more accurately represent episodic or variable pain. These outcomes, however, may minimize the pain experience of an individual who consistently experiences moderate to severe pain. By monitoring back pain on a daily basis, from baseline until 6 months, patients could be stratified into 2 groups: those that meet the NIH task force definition of CLBP (i.e., pain every day for 3 months or for half of the days for 6 months) and those that do not. This type of outcome measure would include both chronic and frequent episodic pain, but would have limited power due to its dichotomous nature and the fact that it does not account for pain severity. The area under the curve (AUC) of periodic pain intensity assessments over a pre-specified period of time would represent pain severity in individuals who experience different pain patterns and would be a continuous outcome variable, thus potentially increasing the power of the analysis. This type of outcome variable was used in the Shingles Prevention Study to characterize the overall severity and persistence of HZ pain [127]. The frequency of assessment for an AUC outcome should be determined based on consideration of patient burden and reasonable recall periods. Suni et al. [157] required patients to keep a weekly diary for 12 months, suggesting that frequent data collection is possible; however, the level of diary compliance was not reported.
Investigators should consider assessing the efficacy of CLBP preventive treatments at 3, 6, and 12 months or a period that encompasses these time points. These time points are consistent with the NIH task force [37] definition of CLBP as well as with the many epidemiologic studies that investigate persistent CLBP at 12 months. Study designs should avoid assessing the outcome too close to the end of the preventive intervention in order to clearly distinguish prevention as opposed to management of symptoms. For example, if the investigational treatment lasts for 3 months, investigators should consider measuring the primary outcome variable at 6 or 12 months.
4.4. Chemotherapy-induced peripheral neuropathy (CIPN)
CIPN is caused by various neurotoxic chemotherapy agents (e.g., platinum agents, taxanes, vinca alkaloids, bortezomib) and includes sensory symptoms ranging from numbness and tingling to pain and allodynia [7, 10]. Few clinical studies have investigated chronic CIPN, or CIPN that persists after completion of chemotherapy. The estimated prevalence of chronic CIPN symptoms persisting 5-15 years after completion of treatment with platinum agents ranges from 13%-35% [64,156]. One study found that 32% of patients taking vincristine still reported neuropathy symptoms after completing vincristine therapy, with a median duration of follow-up of 34 months; however, no patients who were 40 months post-treatment reported neuropathy symptoms [134]. Ten percent of patients who received bortezomib developed ≥ 2 grade neuropathy that persisted for at least 1 year [139]. However, none of these studies investigated rates of persistent painful neuropathy, specifically. One study showed that 18% of patients receiving paclitaxel for breast cancer reported neuropathic pain after an average of 9 years and that patients who had experienced CIPN during chemotherapy were 3 times more likely to report neuropathic pain after chemotherapy completion [137]. However, they also found that diabetes and osteoarthritis were associated with neuropathic pain in these cancer survivors [137].
Many previous studies that examined preventive treatments for CIPN investigated prevention of continually occurring acute symptoms present during ongoing chemotherapy administration and did not investigate prevention as it is classically defined (i.e., assessing outcomes after the preventive treatment is terminated) [74]. Therefore, although these trials are different from treatment trials because the investigational treatments are started either prior to or at the same time as chemotherapy with the goal of preventing neuropathy symptoms, we will not discuss these designs in this article.
Eligible patients for CIPN prevention trials should be patients scheduled to receive a neurotoxic chemotherapy (e.g., platinum agents, taxanes, vinca alkaloids, bortezomib). Patients should also have a life expectancy that is at least 6-12 months longer than their scheduled course of chemotherapy to minimize trial attrition. Investigators should consider excluding patients who have diabetes, HIV, or alcoholism, all of which can also cause a peripheral neuropathy. Inclusion of these patients in trials can make it difficult to determine whether CIPN, a neuropathy from another cause, or the combination of multiple types of neuropathy is being investigated in the trial. Patients with diabetes have been excluded from previous CIPN prevention studies [75,141].
The specific pathophysiological mechanism of nerve damage associated with CIPN varies depending on the neurotoxic agent [7], but acute nerve damage at the time of infusion may transition into sub-acute and chronic pain, and it is possible that minimizing acute pain may limit this transition. It is also possible, however, that minimizing acute pain will allow patients to receive a higher cumulative dosage of chemotherapy, which could actually lead to increased severity of chronic painful CIPN. Other interventions could target putative mechanisms of nerve damage, such as oxidative stress. Clinical trial designs for CIPN prevention should be tailored based on the treatments’ proposed mechanism(s) of action.
4.4.1. Treatment timing
Primary prevention can be studied in CIPN by initiating the investigational treatment on or before the first day of chemotherapy. Although achieving the target dosage of the investigational medication before chemotherapy is administered would be ideal, this may not be feasible if, for example, chemotherapy must be administered very soon after a cancer diagnosis and the medication must be titrated slowly to the effective dosage, such as would likely be true of anti-epileptics. In certain cases, investigators may choose to investigate secondary prevention of painful CIPN by enrolling patients who develop early CIPN symptoms such as numbness and tingling after the initiation of chemotherapy. Doing so would increase the percentage of participants who will develop chronic painful CIPN in the sample, but such timing could also attenuate the beneficial effects of a truly preventive treatment that requires very early administration to prevent nerve damage.
It would generally be recommended that preventive treatments should be administered throughout the duration of the insult that causes chronic pain; however, CIPN is unique in that the pain-inducing insult occurs at multiple intervals over a period of time that often lasts for months [10,122,136,177]. Whether the treatment would likely be best given throughout the full duration of the prescribed chemotherapy or only proximal to each chemotherapy infusion depends on the proposed mechanism and mode of administration of the investigational drug. For example, a bolus intravenous dose of an anti-inflammatory or other agent hypothesized to prevent nerve damage may be best administered directly before or after each chemotherapy infusion as was performed in multiple studies [102,104,113,141,155]. In fact, daily infusions of an investigational prophylactic CIPN treatment throughout the full course of chemotherapy are not likely to be feasible. On the contrary, oral or topical medications that target the nervous system – such as anti-epileptics, anti-depressants, or anti-inflammatory agents that are being used to decrease pain or augment pain inhibition – would likely best be initiated before the start of chemotherapy and continued until the end of chemotherapy (e.g., [1,75,92]) or for 2 weeks to 3 months after chemotherapy completion (e.g., [8,9,63,128,129]).
4.4.2. Outcome measures and assessment timing
As with the other prevention models, possible outcome measures include the intensity or incidence of any pain or minimal pain intensity associated with chemotherapy at a pre-specified time following cessation of chemotherapy. Previous studies of CIPN prevention, have focused on composite, and sometimes crude, measures of neuropathy symptoms, such as the National Cancer Institute - Common Toxicity Criteria (NCI-CTC) and European Organization for Research and Treatment of Cancer- Quality of Life Questionnaire- Chemotherapy-induced Peripheral Neuropathy-20 (EORTC-QLQ-CIPN20) scales and not pain specifically [74]. This could explain why trials of gabapentin and amitriptyline, which are known to work for other neuropathic pain conditions, have failed to detect a preventive treatment effect in CIPN [92,114] and why many other trials have also failed [74]. A recent CIPN treatment trial by Smith et al. [154] of patients with established painful CIPN found an effect of duloxetine on pain severity, suggesting that focusing on pain rather than neuropathy in general could improve assay sensitivity. Of course, the fact that the trial by Smith et al. found an effect of duloxetine could be due to multiple factors other than the primary outcome measure, such as trial size and the fact that it was a treatment trial executed solely after chemotherapy was discontinued, eliminating the variability in neuropathy symptoms caused by changes in chemotherapy dosing during treatment. Cancer patients are often treated with other agents that can cause pain, such as antiestrogen or radiation therapy. In order to minimize the effect that such treatments have on outcome measures of CIPN, patients should be educated on the types of pain caused by neuropathy and instructed to consider only these types of pain, for example, only pain localized to their hands and feet.
A recent meta-analysis by Seretny et al. [151] found that the average reported prevalences of CIPN at 3 and 6 months after termination of chemotherapy were 60% (95% CI: 36% - 82%) and 30% (95% CI: 6.4% - 54%), respectively. However, these estimates were based on relatively few studies (i.e., 4 and 5, respectively) and are highly variable. These data suggest that CIPN symptoms are still naturally decreasing at 3 months after chemotherapy; therefore, 6 months after chemotherapy would be a reasonable time point to assess the effects of a CIPN prevention treatment, although some patients might still have resolution of their pain after this point. However, because these studies did not assess pain specifically, which could have a lower prevalence at both 3 and 6 months after chemotherapy, 3 months should also be considered as an assessment time point given that 3 months is largely considered the minimum duration of pain that is considered to be chronic [48,112]. Previous CIPN prevention studies have assessed neuropathy at 1 month [63, 128], 6 weeks [167], and 3 months [8,9,129,141] after the cessation of chemotherapy. However, only one of these trials declared the chronic CIPN outcome at the chosen time point the primary outcome of the study [141]. An interesting secondary analysis could also examine the “time to recovery” of neuropathic pain after cessation of chemotherapy within the subset of patients that developed a pre-specified level of pain. This type of outcome was used to assess chronicity of neuropathy up to 20 months after cessation of chemotherapy with concurrent gabapentin or placebo treatment [114].
A challenge that is unique to the CIPN prevention model is that although everyone in the study may be prescribed the same or one of a few chemotherapy regimens, chemotherapy is often modified or stopped because of side effects, including neuropathy. The cumulative dosage of chemotherapy is highly associated with neuropathy [7,177] and thus termination of chemotherapy after different dosages can introduce variability in neuropathy outcomes, including pain, at any given time after cessation of chemotherapy. Some studies have dealt with this by eliminating participants who do not receive a pre-specified minimum dosage of chemotherapy [63,128,129,167]. However, this practice could bias the treatment effect estimate because modifications in cumulative chemotherapy dosage could be affected by the investigational treatment; this issue should be carefully considered when developing the statistical analysis plan.
5. Conclusions
Although the preventing chronic pain would have substantial public health benefits, few preventive interventions have been developed. To facilitate the design of clinical trials that would examine the efficacy of such interventions, we have reviewed and discussed research designs and other considerations for such trials. We highlighted 4 models, but these considerations are widely applicable to any pain prevention model in which patients at relatively high risk of developing chronic pain can be identified (see Table 1 for a summary of major considerations). It must be emphasized that many of these recommendations are not based on systematic research, but on published chronic pain prevention and treatment trials and the experience and expertise of the meeting participants. The fact that the majority of the meeting participants were from North America could be considered a limitation. However, we feel that this is unlikely given that the recommendations focus on trial design rather than treatment recommendations or policy considerations. We hope that the suggestions made in this article will stimulate interest in the prevention of chronic pain and facilitate the development of efficacious and safe preventive interventions.
Table 1.
Recommendations.
| Models | Treatment timing | Outcome measures | Assessment timing |
|---|---|---|---|
| CPSP | • Preoperative • Peri-operative • Duration of acute pain recovery (based on natural history of recovery for each surgery) |
• Presence vs. absence of pain • Presence vs. absence of “clinically meaningful” pain • Pain intensity at rest • Pain intensity upon movement and specific activities (well defined) • Pain qualities Secondary endpoints: physical and emotional functioning |
• 24-48 hours post-surgery • 3, 6, and 12 months • Surgery-specific times based on natural history of acute to chronic pain transition |
| PHN | • As soon as possible after rash onset (but ≤ 7 days) • Duration of acute HZ pain (≤ 30 days from rash onset) |
• Presence vs. absence of pain in the area of the rash • Presence vs. absence of “clinically meaningful” pain in the area of the rash • Pain intensity at HZ rash location • Pain qualities at HZ rash location Secondary endpoints: physical and emotional functioning |
• 3-4 months after rash onset |
| CLBP | • As soon as possible after an acute back pain episode (within 3 weeks) • Duration of acute pain (~ 3 months) |
• Presence vs. absence of chronic pain as defined by NIH Task Force [37] • Pain intensity • AUC of pain assessments between 3 months and final time point Secondary endpoints: physical and emotional functioning |
• 3, 6, and 12 months |
| Painful CIPN | • Pre-chemotherapy • Duration of chemotherapy (either daily or only proximal to chemotherapy infusions) |
• Presence vs. absence of pain • Presence vs. absence of “clinically meaningful” pain Secondary endpoints: physical and emotional functioning |
• 3 and 6 months |
Chronic post-surgical pain (CPSP), postherpetic neuralgia (PHN), herpes zoster (HZ), chronic low back pain (CLBP), chemotherapy-induced peripheral neuropathy (CIPN), area under the curve (AUC)
Supplementary Material
Acknowledgments
At the time of the meeting on which this article is based, several authors were employed by pharmaceutical companies and others had received consulting fees or honoraria from one or more pharmaceutical or device companies. Authors of this article who were not employed by industry or government at the time of the meeting received travel stipends, hotel accommodations, and meals during the meeting from the University of Rochester Office of Continuing Professional Education with funds from unrestricted grants to support the activities of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) provided by multiple pharmaceutical companies. Preparation of background literature reviews and draft manuscripts was supported by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the US Food and Drug Administration (FDA), which has received research contracts, grants, or other revenue from the FDA, multiple pharmaceutical and device companies, and other sources. No official endorsement by the FDA, US Department of Veterans Affairs, US National Institutes of Health, or the pharmaceutical and device companies that have provided unrestricted grants to support the activities of IMMPACT and ACTTION should be inferred.
Footnotes
Conflict of interest statement
The views expressed in this article are those of the authors, none of whom have financial conflicts of interest specifically related to the issues discussed in this article.
References
- 1.Afonseca SO, Cruz FM, Cubero Dde I, Lera AT, Schindler F, Okawara M, Souza LF, Rodrigues NP, Giglio A. Vitamin E for prevention of oxaliplatin-induced peripheral neuropathy: a pilot randomized clinical trial. Sao Paulo Med J. 2013;131:35–8. doi: 10.1590/S1516-31802013000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandre NM, De Moraes MA, Correa Filho HR, Jorge SA. Evaluation of a program to reduce back pain in nursing personnel. Rev Saude Publica. 2001;35:356–61. doi: 10.1590/s0034-89102001000400004. [DOI] [PubMed] [Google Scholar]
- 3.Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26:381–5. doi: 10.1097/AJP.0b013e3181cb406e. [DOI] [PubMed] [Google Scholar]
- 4.Andersen KG, Christensen KB, Kehlet H, Bidstrup PE. The effect of pain on physical functioning after breast cancer treatment. Development and validation of an assessment tool. Clin J Pain. 2015 doi: 10.1097/AJP.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 5.Andreae MH, Andreae DA. Local anaesthetics and regional anaesthesia for preventing chronic pain after surgery. Cochrane Database Syst Rev. 2012;(10):CD007105. doi: 10.1002/14651858.CD007105.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arani RB, Soong SJ, Weiss HL, Wood MJ, Fiddian PA, Gnann JW, Whitley R. Phase specific analysis of herpes zoster associated pain data: a new statistical approach. Stat Med. 2001;20:2429–39. doi: 10.1002/sim.851. [DOI] [PubMed] [Google Scholar]
- 7.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Argyriou AA, Chroni E, Koutras A, Ellul J, Papapetropoulos S, Katsoulas G, Iconomou G, Kalofonos HP. Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology. 2005;64:26–31. doi: 10.1212/01.WNL.0000148609.35718.7D. [DOI] [PubMed] [Google Scholar]
- 9.Argyriou AA, Chroni E, Koutras A, Iconomou G, Papapetropoulos S, Polychronopoulos P, Kalofonos HP. Preventing paclitaxel-induced peripheral neuropathy: a phase II trial of vitamin E supplementation. J Pain Symptom Manage. 2006;32:237–44. doi: 10.1016/j.jpainsymman.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol. 2008;66:218–28. doi: 10.1016/j.critrevonc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin ML. Reducing the costs of work-related musculoskeletal disorders: targeting strategies to chronic disability cases. J Electromyogr Kinesiol. 2004;14:33–41. doi: 10.1016/j.jelekin.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg. 2009;109:240–4. doi: 10.1213/ane.0b013e3181a4928d. [DOI] [PubMed] [Google Scholar]
- 13.Belfer I, Segall S. COMT genetic variants and pain. Drugs Today (Barc) 2011;47:457–467. doi: 10.1358/dot.2011.47.6.1611895. [DOI] [PubMed] [Google Scholar]
- 14.Belfer I, Segall SK, Lariviere WR, Smith SB, Dai F, Slade GD, Rashid NU, Mogil JS, Campbell CM, Edwards RR, Liu Q, Bair E, Maixner W, Diatchenko L. Pain modality- and sex-specific effects of COMT genetic functional variants. Pain. 2013;154:1368–76. doi: 10.1016/j.pain.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belfer I, Youngblood V, Darbari DS, Wang Z, Diaw L, Freeman L, Desai K, Dizon M, Allen D, Cunnington C, Channon KM, Milton J, Hartley SW, Nolan V, Kato GJ, Steinberg MH, Goldman D, Taylor JGT. A GCH1 haplotype confers sex-specific susceptibility to pain crises and altered endothelial function in adults with sickle cell anemia. Am J Hematol. 2014;89:187–93. doi: 10.1002/ajh.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beneciuk JM, Bishop MD, Fritz JM, Robinson ME, Asal NR, Nisenzon AN, George SZ. The STarT back screening tool and individual psychological measures: evaluation of prognostic capabilities for low back pain clinical outcomes in outpatient physical therapy settings. Phys Ther. 2013;93:321–33. doi: 10.2522/ptj.20120207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5:143–9. doi: 10.1016/j.jpain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–53. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bollag L, Richebe P, Siaulys M, Ortner CM, Gofeld M, Landau R. Effect of transversus abdominis plane block with and without clonidine on post-cesarean delivery wound hyperalgesia and pain. Reg Anesth Pain Med. 2012;37:508–14. doi: 10.1097/AAP.0b013e318259ce35. [DOI] [PubMed] [Google Scholar]
- 20.Borghouts JA, Koes BW, Vondeling H, Bouter LM. Cost-of-illness of neck pain in The Netherlands in 1996. Pain. 1999;80:629–36. doi: 10.1016/S0304-3959(98)00268-1. [DOI] [PubMed] [Google Scholar]
- 21.Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. 2013;257:403–12. doi: 10.1097/SLA.0b013e3182701a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–57. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327–31. doi: 10.1016/s0885-3924(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 24.Brogly N, Wattier JM, Andrieu G, Peres D, Robin E, Kipnis E, Arnalsteen L, Thielemans B, Carnaille B, Pattou F, Vallet B, Lebuffe G. Gabapentin attenuates late but not early postoperative pain after thyroidectomy with superficial cervical plexus block. Anesth Analg. 2008;107:1720–5. doi: 10.1213/ane.0b013e318185cf73. [DOI] [PubMed] [Google Scholar]
- 25.Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg. 2010;110:1180–5. doi: 10.1213/ANE.0b013e3181cf949a. [DOI] [PubMed] [Google Scholar]
- 26.Burton AK, Waddell G, Tillotson KM, Summerton N. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine (Phila Pa 1976) 1999;24:2484–91. doi: 10.1097/00007632-199912010-00010. [DOI] [PubMed] [Google Scholar]
- 27.Buvanendran A, Kroin JS, Della Valle CJ, Kari M, Moric M, Tuman KJ. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110:199–207. doi: 10.1213/ANE.0b013e3181c4273a. [DOI] [PubMed] [Google Scholar]
- 28.Campbell CM, Edwards RR, Carmona C, Uhart M, Wand G, Carteret A, Kim YK, Frost J, Campbell JN. Polymorphisms in the GTP cyclohydrolase gene (GCH1) are associated with ratings of capsaicin pain. Pain. 2009;141:114–118. doi: 10.1016/j.pain.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candiotti KA, Yang Z, Buric D, Arheart K, Zhang Y, Rodriguez Y, Gitlin MC, Carvalho E, Jaraba I, Wang L. Catechol-o-methyltransferase polymorphisms predict opioid consumption in postoperative pain. Anesth Analg. 2014;119:1194–200. doi: 10.1213/ANE.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 30.Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;(7):CD008307. doi: 10.1002/14651858.CD008307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chocron S, Vandel P, Durst C, Laluc F, Kaili D, Chocron M, Etievent JP. Antidepressant therapy in patients undergoing coronary artery bypass grafting: the MOTIV-CABG trial. Ann Thorac Surg. 2013;95:1609–18. doi: 10.1016/j.athoracsur.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Choo PW, Galil K, Donahue JG, Walker AM, Spiegelman D, Platt R. Risk factors for postherpetic neuralgia. Arch Intern Med. 1997;157:1217–24. [PubMed] [Google Scholar]
- 33.Clarke H, Pereira S, Kennedy D, Andrion J, Mitsakakis N, Gollish J, Katz J, Kay J. Adding gabapentin to a multimodal regimen does not reduce acute pain, opioid consumption or chronic pain after total hip arthroplasty. Acta Anaesthesiol Scand. 2009;53:1073–83. doi: 10.1111/j.1399-6576.2009.02039.x. [DOI] [PubMed] [Google Scholar]
- 34.Cohen SP, Galvagno SM, Plunkett A, Harris D, Kurihara C, Turabi A, Rehrig S, Buckenmaier CC, 3rd, Chelly JE. A multicenter, randomized, controlled study evaluating preventive etanercept on postoperative pain after inguinal hernia repair. Anesth Analg. 2013;116:455–62. doi: 10.1213/ANE.0b013e318273f71c. [DOI] [PubMed] [Google Scholar]
- 35.Croft PR, Macfarlane GJ, Papageorgiou AC, Thomas E, Silman AJ. Outcome of low back pain in general practice: a prospective study. BMJ. 1998;316:1356–9. doi: 10.1136/bmj.316.7141.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Gregori M, Garbin G, De Gregori S, Minella CE, Bugada D, Lisa A, Govoni S, Regazzi M, Allegri M, Ranzani GN. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur J Clin Pharmacol. 2013;69:1651–8. doi: 10.1007/s00228-013-1523-7. [DOI] [PubMed] [Google Scholar]
- 37.Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, Carrino J, Chou R, Cook K, Delitto A, Goertz C, Khalsa P, Loeser J, Mackey S, Panagis J, Rainville J, Tosteson T, Turk D, Von Korff M, Weiner DK. Report of the NIH Task Force on research standards for chronic low back pain. J Pain. 2014;15:569–85. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. 2013;10 doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23:605–613. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 41.Donchin M, Woolf O, Kaplan L, Floman Y. Secondary prevention of low-back pain. A clinical trial. Spine (Phila Pa 1976) 1990;15:1317–20. doi: 10.1097/00007632-199012000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Duale C, Sibaud F, Guastella V, Vallet L, Gimbert YA, Taheri H, Filaire M, Schoeffler P, Dubray C. Perioperative ketamine does not prevent chronic pain after thoracotomy. Eur J Pain. 2009;13:497–505. doi: 10.1016/j.ejpain.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Dworkin RH, Barbano RL, Tyring SK, Betts RF, McDermott MP, Pennella-Vaughan J, Bennett GJ, Berber E, Gnann JW, Irvine C, Kamp C, Kieburtz K, Max MB, Schmader KE. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain. 2009;142:209–17. doi: 10.1016/j.pain.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Dworkin RH, Boon RJ, Griffin DR, Phung D. Postherpetic neuralgia: impact of famciclovir, age, rash severity, and acute pain in herpes zoster patients. J Infect Dis. 1998;178(Suppl 1):S76–80. doi: 10.1086/514260. [DOI] [PubMed] [Google Scholar]
- 45.Dworkin RH, Gnann JW, Jr, Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9:S37–44. doi: 10.1016/j.jpain.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW, Nurmikko TJ, Oaklander AL, Oxman MN, Pavan-Langston D, Petersen KL, Rowbotham MC, Schmader KE, Stacey BR, Tyring SK, Van Wijck AJ, Wallace MS, Wassilew SW, Whitley RJ. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 47.Dworkin RH, McDermott MP, Raja SN. Preventing chronic postsurgical pain: how much of a difference makes a difference? Anesthesiology. 2010;112:516–8. doi: 10.1097/ALN.0b013e3181cf4253. [DOI] [PubMed] [Google Scholar]
- 48.Dworkin RH, Turk DC, Basch E, Berger A, Cleeland C, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Markman J, Porter L, Raja SN, Ross E, Todd K, Wallace M, Woolf CJ. Considerations for extrapolating evidence of acute and chronic pain analgesic efficacy. Pain. 2011;152:1705–8. doi: 10.1016/j.pain.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 49.Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, Chappell A, Chartier K, Cleeland CS, Costello A, Cowan P, Dimitrova R, Ellenberg S, Farrar JT, French JA, Gilron I, Hertz S, Jadad AR, Jay GW, Kalliomaki J, Katz NP, Kerns RD, Manning DC, McDermott MP, McGrath PJ, Narayana A, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Reeve BB, Rhodes T, Sampaio C, Simpson DM, Stauffer JW, Stucki G, Tobias J, White RE, Witter J. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149:177–93. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce-Sandner S, Bhagwat D, Everton D, Burke LB, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA, Melzack R. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2) Pain. 2009;144:35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Eisenberg E, Pud D, Koltun L, Loven D. Effect of early administration of the N-methyl-d-aspartate receptor antagonist amantadine on the development of postmastectomy pain syndrome: a prospective pilot study. J Pain. 2007;8:223–9. doi: 10.1016/j.jpain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Fassoulaki A, Patris K, Sarantopoulos C, Hogan Q. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth Analg. 2002;95:985–91. doi: 10.1097/00000539-200210000-00036. [DOI] [PubMed] [Google Scholar]
- 53.Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Franneby U, Gunnarsson U, Andersson M, Heuman R, Nordin P, Nyren O, Sandblom G. Validation of an Inguinal Pain Questionnaire for assessment of chronic pain after groin hernia repair. Br J Surg. 2008;95:488–93. doi: 10.1002/bjs.6014. [DOI] [PubMed] [Google Scholar]
- 55.Fritz JM, Delitto A, Erhard RE. Comparison of classification-based physical therapy with therapy based on clinical practice guidelines for patients with acute low back pain: a randomized clinical trial. Spine (Phila Pa 1976) 2003;28:1363–71. doi: 10.1097/01.BRS.0000067115.61673.FF. discussion 1372. [DOI] [PubMed] [Google Scholar]
- 56.Gannon B, Finn DP, O’gorman D, Ruane N, McGuire BE. The cost of chronic pain: an analysis of a regional pain management service in Ireland. Pain Med. 2013;14:1518–28. doi: 10.1111/pme.12202. [DOI] [PubMed] [Google Scholar]
- 57.George RB, McKeen DM, Andreou P, Habib AS. A randomized placebo-controlled trial of two doses of pregabalin for postoperative analgesia in patients undergoing abdominal hysterectomy. Can J Anaesth. 2014;61:551–7. doi: 10.1007/s12630-014-0147-4. [DOI] [PubMed] [Google Scholar]
- 58.George SZ, Dover GC, Wallace MR, Sack BK, Herbstman DM, Aydog E, Fillingim RB. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict pain ratnigs. Clin. J Pain. 2008;24:793–801. doi: 10.1097/AJP.0b013e31817bcb65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.George SZ, Fritz JM, Bialosky JE, Donald DA. The effect of a fear-avoidance-based physical therapy intervention for patients with acute low back pain: results of a randomized clinical trial. Spine (Phila Pa 1976) 2003;28:2551–60. doi: 10.1097/01.BRS.0000096677.84605.A2. [DOI] [PubMed] [Google Scholar]
- 60.George SZ, Parr JJ, Wallace MR, Wu SS, Borsa PA, Dai Y, Fillingim RB. Biopsychosocial influence on exercise-induced injury: genetic and psychological combinations are predictive of shoulder pain phenotypes. J Pain. 2014;15:68–80. doi: 10.1016/j.jpain.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.George SZ, Zeppieri G, Jr, Cere AL, Cere MR, Borut MS, Hodges MJ, Reed DM, Valencia C, Robinson ME. A randomized trial of behavioral physical therapy interventions for acute and sub-acute low back pain ( NCT00373867) Pain. 2008;140:145–57. doi: 10.1016/j.pain.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gewandter JS, Dworkin RH, Turk DC, McDermott MP, Baron R, Gastonguay MR, Gilron I, Katz NP, Mehta C, Raja SN, Senn S, Taylor C, Cowan P, Desjardins P, Dimitrova R, Dionne R, Farrar JT, Hewitt DJ, Iyengar S, Jay GW, Kalso E, Kerns RD, Leff R, Leong M, Petersen KL, Ravina BM, Rauschkolb C, Rice AS, Rowbotham MC, Sampaio C, Sindrup SH, Stauffer JW, Steigerwald I, Stewart J, Tobias J, Treede RD, Wallace M, White RE. Research designs for proof-of-concept chronic pain clinical trials: IMMPACT recommendations. Pain. 2014;155:1683–95. doi: 10.1016/j.pain.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, Hashemzade S, Asghari Jafarabadi M, Montazeri V, Keshavarz SA, Darabi M. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer. 2012;12:355. doi: 10.1186/1471-2407-12-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, Huddart RA. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116:2322–31. doi: 10.1002/cncr.24981. [DOI] [PubMed] [Google Scholar]
- 65.Grigoras A, Lee P, Sattar F, Shorten G. Perioperative intravenous lidocaine decreases the incidence of persistent pain after breast surgery. Clin J Pain. 2012;28:567–72. doi: 10.1097/AJP.0b013e31823b9cc8. [DOI] [PubMed] [Google Scholar]
- 66.Grosen K, Drewes AM, Hojsgaard A, Pfeiffer-Jensen M, Hjortdal VE, Pilegaard HK. Perioperative gabapentin for the prevention of persistent pain after thoracotomy: a randomized controlled trial. Eur J Cardiothorac Surg. 2014;46:76–85. doi: 10.1093/ejcts/ezu032. [DOI] [PubMed] [Google Scholar]
- 67.Grosen K, Drewes AM, Hojsgaard A, Pfeiffer-Jensen M, Hjortdal VE, Pilegaard HK. Perioperative gabapentin for the prevention of persistent pain after thoracotomy: a randomized controlled trial. Eur J Cardiothorac Surg. 2014;46:76–85. doi: 10.1093/ejcts/ezu032. [DOI] [PubMed] [Google Scholar]
- 68.Han Y, Zhang J, Chen N, He L, Zhou M, Zhu C. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2013;(3):CD005582. doi: 10.1002/14651858.CD005582.pub4. [DOI] [PubMed] [Google Scholar]
- 69.Hancock MJ, Maher CG, Latimer J, McLachlan AJ, Cooper CW, Day RO, Spindler MF, McAuley JH. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomised controlled trial. Lancet. 2007;370:1638–43. doi: 10.1016/S0140-6736(07)61686-9. [DOI] [PubMed] [Google Scholar]
- 70.Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154:95–102. doi: 10.1016/j.pain.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 71.Hastie BA, Riley JL, Iii, Kaplan L, Herrera DG, Campbell CM, Virtusio K, Mogil JS, Wallace MR, Fillingim RB. Ethnicity interacts with the OPRM1 gene in experimental pain sensitivity. Pain. 2012 doi: 10.1016/j.pain.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hayes C, Armstrong-Brown A, Burstal R. Perioperative intravenous ketamine infusion for the prevention of persistent post-amputation pain: a randomized, controlled trial. Anaesth Intensive Care. 2004;32:330–8. doi: 10.1177/0310057X0403200305. [DOI] [PubMed] [Google Scholar]
- 73.Henschke N, Maher CG, Refshauge KM, Herbert RD, Cumming RG, Bleasel J, York J, Das A, McAuley JH. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ. 2008;337:a171. doi: 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32:1941–67. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 75.Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L, Wade JL, 3rd, Wong SF, Hortobagyi GN, Meyskens FL, Albain KS. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol. 2013;31:2627–33. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hestbaek L, Leboeuf-Yde C, Manniche C. Low back pain: what is the long-term course? A review of studies of general patient populations. Eur Spine J. 2003;12:149–65. doi: 10.1007/s00586-002-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, Hay EM. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59:632–41. doi: 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- 78.Ho KY, Tay W, Yeo MC, Liu H, Yeo SJ, Chia SL, Lo NN. Duloxetine reduces morphine requirements after knee replacement surgery. Br J Anaesth. 2010;105:371–6. doi: 10.1093/bja/aeq158. [DOI] [PubMed] [Google Scholar]
- 79.Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J, Murray C, Burstein R, Buchbinder R. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73:968–74. doi: 10.1136/annrheumdis-2013-204428. [DOI] [PubMed] [Google Scholar]
- 80.Hwang IC, Park JY, Myung SK, Ahn HY, Fukuda K, Liao Q. OPRM1 A118G gene variant and postoperative opioid requirement: a systematic review and meta-analysis. Anesthesiology. 2014;121:825–34. doi: 10.1097/ALN.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 81.Institute, Relieving pain in America: a blueprint for trasforming prevention, care, education, and research. Washington DC: 2011. [PubMed] [Google Scholar]
- 82.Itz CJ, Geurts JW, Van Kleef M, Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain. 2013;17:5–15. doi: 10.1002/j.1532-2149.2012.00170.x. [DOI] [PubMed] [Google Scholar]
- 83.Jackson JL, Gibbons R, Meyer G, Inouye L. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis Arch Intern Med. 1997;157:909–12. [PubMed] [Google Scholar]
- 84.Ji G, Niu J, Shi Y, Hou L, Lu Y, Xiong L. The effectiveness of repetitive paravertebral injections with local anesthetics and steroids for the prevention of postherpetic neuralgia in patients with acute herpes zoster. Anesth Analg. 2009;109:1651–5. doi: 10.1213/ANE.0b013e3181b79075. [DOI] [PubMed] [Google Scholar]
- 85.Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545–51. doi: 10.1212/01.wnl.0000123261.00004.29. [DOI] [PubMed] [Google Scholar]
- 86.Kahan BC, Jairath V, Dore CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15:139. doi: 10.1186/1745-6215-15-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Katz J. One man’s risk factor is another man’s outcome: difference in risk factor profiles for chronic postsurgical pain maintenance vs transition. Pain. 2012;153:505–6. doi: 10.1016/j.pain.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 88.Katz J, Clairoux M, Redahan C, Kavanagh BP, Carroll S, Nierenberg H, Jackson M, Beattie J, Taddio A, Sandler AN. High dose alfentanil pre-empts pain after abdominal hysterectomy. Pain. 1996;68:109–18. doi: 10.1016/S0304-3959(96)03172-7. [DOI] [PubMed] [Google Scholar]
- 89.Katz J, Cohen L. Clinical Pain Management: Acute Pain. Hodder Arnold Ltd; London: 2008. Preventive analgesia and beyond: current status, evidence, and future directions; pp. 154–198. [Google Scholar]
- 90.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 91.Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9:723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- 92.Kautio AL, Haanpaa M, Leminen A, Kalso E, Kautiainen H, Saarto T. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res. 2009;29:2601–6. [PubMed] [Google Scholar]
- 93.Kehlet H, Dahl JB. Assessment of postoperative pain–need for action! Pain. 2011;152:1699–700. doi: 10.1016/j.pain.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Kent PM, Keating JL. The epidemiology of low back pain in primary care. Chiropr Osteopat. 2005;13:13. doi: 10.1186/1746-1340-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khurana G, Jindal P, Sharma JP, Bansal KK. Postoperative pain and long-term functional outcome after administration of gabapentin and pregabalin in patients undergoing spinal surgery. Spine (Phila Pa 1976) 2014;39:E363–8. doi: 10.1097/BRS.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 96.Kim DH, Dai F, Belfer I, Banco RJ, Martha JF, Tighiouart H, Tromanhauser SG, Jenis LG, Hunter DJ, Schwartz CE. Polymorphic variation of the guanosine triphosphate cyclohydrolase 1 gene predicts outcome in patients undergoing surgical treatment for lumbar degenerative disc disease. Spine (Phila Pa 1976) 2010;35:1909–14. doi: 10.1097/BRS.0b013e3181eea007. [DOI] [PubMed] [Google Scholar]
- 97.Kim SY, Jeong JJ, Chung WY, Kim HJ, Nam KH, Shim YH. Perioperative administration of pregabalin for pain after robot-assisted endoscopic thyroidectomy: a randomized clinical trial. Surg Endosc. 2010;24:2776–81. doi: 10.1007/s00464-010-1045-7. [DOI] [PubMed] [Google Scholar]
- 98.Kinney MA, Mantilla CB, Carns PE, Passe MA, Brown MJ, Hooten WM, Curry TB, Long TR, Wass CT, Wilson PR, Weingarten TN, Huntoon MA, Rho RH, Mauck WD, Pulido JN, Allen MS, Cassivi SD, Deschamps C, Nichols FC, Shen KR, Wigle DA, Hoehn SL, Alexander SL, Hanson AC, Schroeder DR. Preoperative gabapentin for acute post-thoracotomy analgesia: a randomized, double-blinded, active placebo-controlled study. Pain Pract. 2012;12:175–83. doi: 10.1111/j.1533-2500.2011.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kissin I. Preemptive analgesia: problems with assessment of clinical significance. Methods Mol Biol. 2010;617:475–82. doi: 10.1007/978-1-60327-323-7_34. [DOI] [PubMed] [Google Scholar]
- 100.Knight T, Schaefer C, Chandran A, Zlateva G, Winkelmann A, Perrot S. Health-resource use and costs associated with fibromyalgia in France, Germany, and the United States. Clinicoecon Outcomes Res. 2013;5:171–80. doi: 10.2147/CEOR.S41111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lakdja F, Dixmerias F, Bussieres E, Fonrouge JM, Lobera A. [Preventive analgesic effect of intraoperative administration of ibuprofen-arginine on postmastectomy pain syndrome] Bull Cancer. 1997;84:259–63. [PubMed] [Google Scholar]
- 102.Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber CH, Watanaboonyakhet P, Weiss M, Adams PT, Dockter TJ, Loprinzi CL. North Central Cancer Treatment Group/Alliance trial N08CA-the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled study. Cancer. 2014;120:1890–7. doi: 10.1002/cncr.28654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lonn JH, Glomsrod B, Soukup MG, Bo K, Larsen S. Active back school: prophylactic management for low back pain. A randomized, controlled, 1-year follow-up study. Spine (Phila Pa 1976) 1999;24:865–71. doi: 10.1097/00007632-199905010-00006. [DOI] [PubMed] [Google Scholar]
- 104.Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, Seisler D, Qamar R, Lewis GC, Grothey A. Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance) J Clin Oncol. 2014;32:997–1005. doi: 10.1200/JCO.2013.52.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br. 2008;90:166–71. doi: 10.1302/0301-620X.90B2.19640. [DOI] [PubMed] [Google Scholar]
- 106.Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- 107.Makharita MY, Amr YM, El-Bayoumy Y. Effect of early stellate ganglion blockade for facial pain from acute herpes zoster and incidence of postherpetic neuralgia. Pain Physician. 2012;15:467–74. [PubMed] [Google Scholar]
- 108.Martinez V, Szekely B, Lemarie J, Martin F, Gentili M, Ben Ammar S, Lepeintre JF, Garreau De Loubresse C, Chauvin M, Bouhassira D, Fletcher D. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. Pain. 2013;154:1197–203. doi: 10.1016/j.pain.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 109.Maul I, Laubli T, Oliveri M, Krueger H. Long-term effects of supervised physical training in secondary prevention of low back pain. Eur Spine J. 2005;14:599–611. doi: 10.1007/s00586-004-0873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McGreevy K, Bottros MM, Raja SN. Preventing chronic pain following acute pain: Risk factors, preventive strategies, and their efficacy. Eur J Pain Suppl. 2011;5:365–372. doi: 10.1016/j.eujps.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McKendrick MW, Wood MJ. Acyclovir and post-herpetic neuralgia. Two other participating study centres report different results. BMJ. 1995;310:1005. doi: 10.1136/bmj.310.6985.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merskey H, Bogduk N, editors. Classification of chronic pain: descriptions of chronic syndromes and definitions of pain terms. Seattle: IASP Press; 1994. [Google Scholar]
- 113.Milla P, Airoldi M, Weber G, Drescher A, Jaehde U, Cattel L. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs. 2009;20:396–402. doi: 10.1097/CAD.0b013e32832a2dc1. [DOI] [PubMed] [Google Scholar]
- 114.Mitchell PL, Goldstein D, Michael M, Beale P, Friedlander M, Zalcberg J, White S, Thomson JA, Clarke S. Addition of gabapentin to a modified FOLFOX regimen does not reduce oxaliplatin-induced neurotoxicity. Clin Colorectal Cancer. 2006;6:146–51. doi: 10.3816/CCC.2006.n.032. [DOI] [PubMed] [Google Scholar]
- 115.Mogil JS. Pain genetics: past, present and future. Trends Genet. 2012;28:258–266. doi: 10.1016/j.tig.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 116.Moore A, Costello J, Wieczorek P, Shah V, Taddio A, Carvalho JC. Gabapentin improves postcesarean delivery pain management: a randomized, placebo-controlled trial. Anesth Analg. 2011;112:167–73. doi: 10.1213/ANE.0b013e3181fdf5ee. [DOI] [PubMed] [Google Scholar]
- 117.F G, et al., editors. Longitudinal Data Analysis. Chapman and Hall/CRC; Boca Raton, FL: 2008. Growth mixture modeling: analysis with non-Gaussian random effects. [Google Scholar]
- 118.Nagasako EM, Johnson RW, Griffin DR, Dworkin RH. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46:834–9. doi: 10.1067/mjd.2002.120924. [DOI] [PubMed] [Google Scholar]
- 119.Nicholas MK, Linton SJ, Watson PJ, Main CJ. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther. 2011;91:737–53. doi: 10.2522/ptj.20100224. [DOI] [PubMed] [Google Scholar]
- 120.Nikolajsen L, Finnerup NB, Kramp S, Vimtrup AS, Keller J, Jensen TS. A randomized study of the effects of gabapentin on postamputation pain. Anesthesiology. 2006;105:1008–15. doi: 10.1097/00000542-200611000-00023. [DOI] [PubMed] [Google Scholar]
- 121.Nikolajsen L, Ilkjaer S, Christensen JH, Kroner K, Jensen TS. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet. 1997;350:1353–7. doi: 10.1016/S0140-6736(97)06315-0. [DOI] [PubMed] [Google Scholar]
- 122.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12:619–25. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 123.Ochroch EA, Vachani A, Gottschalk A, Kanetsky PA. Natural variation in the mu-opioid gene OPRM1 predicts increased pain on third day after thoracotomy. Clin J Pain. 2012;28:747–54. doi: 10.1097/AJP.0b013e3182442b1c. [DOI] [PubMed] [Google Scholar]
- 124.Olsen MB, Jacobsen LM, Schistad EI, Pedersen LM, Rygh LJ, Roe C, Gjerstad J. Pain Intensity the First Year after Lumbar Disc Herniation Is Associated with the A118G Polymorphism in the Opioid Receptor Mu 1 Gene: Evidence of a Sex and Genotype Interaction. J. Neurosci. 2012;32:9831–9834. doi: 10.1523/JNEUROSCI.1742-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Onan B, Onan IS, Kilickan L, Sanisoglu I. Effects of epidural anesthesia on acute and chronic pain after coronary artery bypass grafting. J Card Surg. 2013;28:248–53. doi: 10.1111/jocs.12086. [DOI] [PubMed] [Google Scholar]
- 126.Opstelten W, Van Wijck AJ, Moons KG. Design issues for studies into prevention of chronic pain: lessons from post-herpetic neuralgia. Anaesthesia. 2004;59:213–5. doi: 10.1111/j.1365-2044.2004.03702.x. [DOI] [PubMed] [Google Scholar]
- 127.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 128.Pace A, Giannarelli D, Galie E, Savarese A, Carpano S, Della Giulia M, Pozzi A, Silvani A, Gaviani P, Scaioli V, Jandolo B, Bove L, Cognetti F. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology. 2010;74:762–6. doi: 10.1212/WNL.0b013e3181d5279e. [DOI] [PubMed] [Google Scholar]
- 129.Pace A, Savarese A, Picardo M, Maresca V, Pacetti U, Del Monte G, Biroccio A, Leonetti C, Jandolo B, Cognetti F, Bove L. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. J Clin Oncol. 2003;21:927–31. doi: 10.1200/JCO.2003.05.139. [DOI] [PubMed] [Google Scholar]
- 130.Pasqualucci A, Pasqualucci V, Galla F, De Angelis V, Marzocchi V, Colussi R, Paoletti F, Girardis M, Lugano M, Del Sindaco F. Prevention of post-herpetic neuralgia: acyclovir and prednisolone versus epidural local anesthetic and methylprednisolone. Acta Anaesthesiol Scand. 2000;44:910–8. doi: 10.1034/j.1399-6576.2000.440803.x. [DOI] [PubMed] [Google Scholar]
- 131.Paul JE, Nantha-Aree M, Buckley N, Cheng J, Thabane L, Tidy A, Debeer J, Winemaker M, Wismer D, Punthakee D, Avram V. Gabapentin does not improve multimodal analgesia outcomes for total knee arthroplasty: a randomized controlled trial. Can J Anaesth. 2013;60:423–31. doi: 10.1007/s12630-013-9902-1. [DOI] [PubMed] [Google Scholar]
- 132.Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327:323. doi: 10.1136/bmj.327.7410.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pesonen A, Suojaranta-Ylinen R, Hammaren E, Kontinen VK, Raivio P, Tarkkila P, Rosenberg PH. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: a randomized placebo-controlled trial. Br J Anaesth. 2011;106:873–81. doi: 10.1093/bja/aer083. [DOI] [PubMed] [Google Scholar]
- 134.Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long-term effects of vincristine on the peripheral nervous system. J Neurooncol. 1993;15:23–7. doi: 10.1007/BF01050259. [DOI] [PubMed] [Google Scholar]
- 135.Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15:209–33. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- 136.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 137.Reyes-Gibby CC, Morrow PK, Buzdar A, Shete S. Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain. 2009;10:1146–50. doi: 10.1016/j.jpain.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reyes-Gibby CC, Shete S, Rakvag T, Bhat SV, Skorpen F, Bruera E, Kaasa S, Klepstad P. Exploring joint effects of genes and the clinical efficacy of morphine for cancer pain: OPRM1 and COMT gene. Pain. 2007;130:25–30. doi: 10.1016/j.pain.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, Blade J, Boccadoro M, Cavenagh JD, Boral AL, Esseltine DL, Wen PY, Amato AA, Anderson KC, San Miguel J. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144:895–903. doi: 10.1111/j.1365-2141.2008.07573.x. [DOI] [PubMed] [Google Scholar]
- 140.Ringsted TK, Wildgaard K, Kreiner S, Kehlet H. Pain-related impairment of daily activities after thoracic surgery: a questionnaire validation. Clin J Pain. 2013;29:791–9. doi: 10.1097/AJP.0b013e318278d4e2. [DOI] [PubMed] [Google Scholar]
- 141.Roberts JA, Jenison EL, Kim K, Clarke-Pearson D, Langleben A. A randomized, multicenter, double-blind, placebo-controlled, dose-finding study of ORG 2766 in the prevention or delay of cisplatin-induced neuropathies in women with ovarian cancer. Gynecol Oncol. 1997;67:172–7. doi: 10.1006/gyno.1997.4832. [DOI] [PubMed] [Google Scholar]
- 142.Rowbotham MC, Petersen KL. Zoster-associated pain and neural dysfunction. Pain. 2001;93:1–5. doi: 10.1016/S0304-3959(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 143.Rut M, Machoy-Mokrzynska A, Reclawowicz D, Sloniewski P, Kurzawski M, Drozdzik M, Safranow K, Morawska M, Bialecka M. Influence of variation in the catechol-O-methyltransferase gene on the clinical outcome after lumbar spine surgery for one-level symptomatic disc disease: a report on 176 cases. Acta Neurochir (Wien) 2014;156:245–52. doi: 10.1007/s00701-013-1895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics. 2012;13:1719–40. doi: 10.2217/pgs.12.152. [DOI] [PubMed] [Google Scholar]
- 145.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 146.Seebach CL, Kirkhart M, Lating JM, Wegener ST, Song Y, Riley LH, 3rd, Archer KR. Examining the role of positive and negative affect in recovery from spine surgery. Pain. 2012;153:518–25. doi: 10.1016/j.pain.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 147.Sen H, Sizlan A, Yanarates O, Emirkadi H, Ozkan S, Dagli G, Turan A. A comparison of gabapentin and ketamine in acute and chronic pain after hysterectomy. Anesth Analg. 2009;109:1645–50. doi: 10.1213/ANE.0b013e3181b65ea0. [DOI] [PubMed] [Google Scholar]
- 148.Sen H, Sizlan A, Yanarates O, Senol MG, Inangil G, Sucullu I, Ozkan S, Dagli G. The effects of gabapentin on acute and chronic pain after inguinal herniorrhaphy. Eur J Anaesthesiol. 2009;26:772–6. doi: 10.1097/EJA.0b013e32832ad2fa. [DOI] [PubMed] [Google Scholar]
- 149.Senn S. Statistical Issues in Drug Development. England: John Wiley & Sons, Ltd; 2007. [Google Scholar]
- 150.Senturk M, Ozcan PE, Talu GK, Kiyan E, Camci E, Ozyalcin S, Dilege S, Pembeci K. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002;94:11–5. doi: 10.1213/00000539-200201000-00003. table of contents. [DOI] [PubMed] [Google Scholar]
- 151.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, Macleod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 152.Sia AT, Lim Y, Lim EC, Ocampo CE, Lim WY, Cheong P, Tan EC. Influence of mu-opioid receptor variant on morphine use and self-rated pain following abdominal hysterectomy. J Pain. 2013;14:1045–52. doi: 10.1016/j.jpain.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 153.Sieben JM, Vlaeyen JW, Portegijs PJ, Verbunt JA, Van Riet-Rutgers S, Kester AD, Von Korff M, Arntz A, Knottnerus JA. A longitudinal study on the predictive validity of the fear-avoidance model in low back pain. Pain. 2005;117:162–70. doi: 10.1016/j.pain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 154.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–67. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smyth JF, Bowman A, Perren T, Wilkinson P, Prescott RJ, Quinn KJ, Tedeschi M. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: results of a double-blind, randomised trial. Ann Oncol. 1997;8:569–73. doi: 10.1023/a:1008211226339. [DOI] [PubMed] [Google Scholar]
- 156.Strumberg D, Brugge S, Korn MW, Koeppen S, Ranft J, Scheiber G, Reiners C, Mockel C, Seeber S, Scheulen ME. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13:229–36. doi: 10.1093/annonc/mdf058. [DOI] [PubMed] [Google Scholar]
- 157.Suni J, Rinne M, Natri A, Statistisian MP, Parkkari J, Alaranta H. Control of the lumbar neutral zone decreases low back pain and improves self-evaluated work ability: a 12-month randomized controlled study. Spine (Phila Pa 1976) 2006;31:E611–20. doi: 10.1097/01.brs.0000231701.76452.05. [DOI] [PubMed] [Google Scholar]
- 158.Suzuki M, Haraguti S, Sugimoto K, Kikutani T, Shimada Y, Sakamoto A. Low-dose intravenous ketamine potentiates epidural analgesia after thoracotomy. Anesthesiology. 2006;105:111–9. doi: 10.1097/00000542-200607000-00020. [DOI] [PubMed] [Google Scholar]
- 159.Tegeder I, Adolph J, Schmidt H, Woolf CJ, Geisslinger G, Lotsch J. Reduced hyperalgesia in homozygous carriers of a GTP cyclohydrolase 1 haplotype. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 160.Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lotsch J, Fillingim RB, Maixner W, Geisslinger G, Max MB, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 161.Traeger AC, Moseley GL, Hubscher M, Lee H, Skinner IW, Nicholas MK, Henschke N, Refshauge KM, Blyth FM, Main CJ, Hush JM, Pearce G, McAuley JH. Pain education to prevent chronic low back pain: a study protocol for a randomised controlled trial. BMJ Open. 2014;4:e005505. doi: 10.1136/bmjopen-2014-005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, Cleeland C, Dionne R, Farrar JT, Galer BS, Hewitt DJ, Jadad AR, Katz NP, Kramer LD, Manning DC, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robinson JP, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–45. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 163.Tyring S, Barbarash RA, Nahlik JE, Cunningham A, Marley J, Heng M, Jones T, Rea T, Boon R, Saltzman R. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89–96. doi: 10.7326/0003-4819-123-2-199507150-00002. [DOI] [PubMed] [Google Scholar]
- 164.Tyring SK, Beutner KR, Tucker BA, Anderson WC, Crooks RJ. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9:863–9. doi: 10.1001/archfami.9.9.863. [DOI] [PubMed] [Google Scholar]
- 165.Ucak A, Onan B, Sen H, Selcuk I, Turan A, Yilmaz AT. The effects of gabapentin on acute and chronic postoperative pain after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25:824–9. doi: 10.1053/j.jvca.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 166.United, Guidance for Industry Analgesic Indications: Developing Drug and Biologic Products. Sivler Spring, MD: 2014. [Google Scholar]
- 167.Van Kooten B, Van Diemen HA, Groenhout KM, Huijgens PC, Ossenkoppele GJ, Nauta JJ, Heimans JJ. A pilot study on the influence of a corticotropin (4-9) analogue on Vinca alkaloid-induced neuropathy. Arch Neurol. 1992;49:1027–31. doi: 10.1001/archneur.1992.00530340043016. [DOI] [PubMed] [Google Scholar]
- 168.Van Wijck AJ, Opstelten W, Moons KG, Van Essen GA, Stolker RJ, Kalkman CJ, Verheij TJ. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet. 2006;367:219–24. doi: 10.1016/S0140-6736(06)68032-X. [DOI] [PubMed] [Google Scholar]
- 169.Von Korff M, Shortreed SM, Saunders KW, Leresche L, Berlin JA, Stang P, Turner JA. Comparison of back pain prognostic risk stratification item sets. J Pain. 2014;15:81–9. doi: 10.1016/j.jpain.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 170.Wai EK, Sathiaseelan S, O’neil J, Simchison BL. Local administration of morphine for analgesia after autogenous anterior or posterior iliac crest bone graft harvest for spinal fusion: a prospective, randomized, double-blind, placebo-controlled study. Anesth Analg. 2010;110:928–33. doi: 10.1213/ANE.0b013e3181cb3f32. [DOI] [PubMed] [Google Scholar]
- 171.Weis F, Kilger E, Roozendaal B, De Quervain DJ, Lamm P, Schmidt M, Schmolz M, Briegel J, Schelling G. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg. 2006;131:277–82. doi: 10.1016/j.jtcvs.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 172.Werner MU, Kongsgaard UE. I. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113:1–4. doi: 10.1093/bja/aeu012. [DOI] [PubMed] [Google Scholar]
- 173.Whitfill T, Haggard R, Bierner SM, Pransky G, Hassett RG, Gatchel RJ. Early intervention options for acute low back pain patients: a randomized clinical trial with one-year follow-up outcomes. J Occup Rehabil. 2010;20:256–63. doi: 10.1007/s10926-010-9238-4. [DOI] [PubMed] [Google Scholar]
- 174.Whitley RJ, Weiss H, Gnann JW, Jr, Tyring S, Mertz GJ, Pappas PG, Schleupner CJ, Hayden F, Wolf J, Soong SJ. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376–83. doi: 10.7326/0003-4819-125-5-199609010-00004. [DOI] [PubMed] [Google Scholar]
- 175.Whitley RJ, Weiss HL, Soong SJ, Gnann JW. Herpes zoster: risk categories for persistent pain. J Infect Dis. 1999;179:9–15. doi: 10.1086/314562. [DOI] [PubMed] [Google Scholar]
- 176.Williams CM, Maher CG, Latimer J, McLachlan AJ, Hancock MJ, Day RO, Lin CW. Efficacy of paracetamol for acute low-back pain: a double-blind, randomised controlled trial. Lancet. 2014;384:1586–96. doi: 10.1016/S0140-6736(14)60805-9. [DOI] [PubMed] [Google Scholar]
- 177.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 178.Wood MJ, Kay R, Dworkin RH, Soong SJ, Whitley RJ. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341–7. doi: 10.1093/clinids/22.2.341. [DOI] [PubMed] [Google Scholar]
- 179.Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: implications for diagnosis and therapy. Life Sci. 2004;74:2605–10. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 180.Woolf CJ, Chong MS. Preemptive analgesia–treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


