Abstract
Background
Recent studies on clinical chorioamnionitis at term suggest that some patients with this diagnosis have neither intra-amniotic infection nor intra-amniotic inflammation. A false-positive diagnosis of clinical chorioamnionitis in preterm gestation may lead to unwarranted preterm delivery.
Objective
To determine the frequency of intra-amniotic inflammation and microbiologically proven amniotic fluid infection in patients with preterm clinical chorioamnionitis.
Study design
Amniocentesis was performed in singleton pregnant women with preterm clinical chorioamnionitis (<36 weeks of gestation). Amniotic fluid was cultured for aerobic and anaerobic bacteria and genital mycoplasmas and assayed for matrix metalloproteinase-8 concentration. Microbial invasion of the amniotic cavity was defined as a positive amniotic fluid culture; intra-amniotic inflammation was defined as an elevated amniotic fluid matrix metalloproteinase-8 concentration of >23 ng/mL. Non-parametric and survival techniques were used for analysis.
Results
Among patients with preterm clinical chorioamnionitis, 24% (12/50) had microbiologic evidence of neither intra-amniotic infection nor intra-amniotic inflammation. Microbial invasion of the amniotic cavity was present in 34% (18/53) and intra-amniotic inflammation in 76% (38/50) of patients. The most common microorganisms isolated from the amniotic cavity were the Ureaplasma species. Finally, patients without microbial invasion of the amniotic cavity or intra-amniotic inflammation had significantly lower rates of adverse outcomes (including lower gestational age at delivery, a shorter amniocentesis-to-delivery interval, acute histologic chorioamnionitis, acute funisitis, and significant neonatal morbidity) than those with microbial invasion of the amniotic cavity and/or intra-amniotic inflammation.
Conclusion
Among patients with preterm clinical chorioamnionitis, 24% had no evidence of either intra-amniotic infection or intra-amniotic inflammation, and 66% had negative amniotic fluid cultures, using standard microbiologic techniques. These observations call for a re-examination of the criteria used to diagnose preterm clinical chorioamnionitis.
Keywords: amniocentesis, matrix metalloproteinase-8 (MMP-8), microbial invasion of the amniotic cavity (MIAC), pregnancy, preterm delivery
Introduction
Clinical chorioamnionitis is the most frequent pregnancy-related infection diagnosed in labor and delivery units worldwide [1]. The prevalence of this condition in term gestations ranges from 5% to 12% [2], while in preterm gestations, it is diagnosed in 20% of patients with preterm premature rupture of the membranes (PROM) [3–5] and in about 10% of patients with preterm labor and intact membranes [6–11]. Clinical chorioamnionitis is a well-known risk factor for adverse maternal and perinatal outcomes [12–14]. Affected mothers have an increased rate of endometritis [2, 15], post-operative wound infections [2, 16], sepsis [17, 18], disseminated intravascular coagulation [19], septic pelvic thrombophlebitis [20, 21], postpartum hemorrhage [22], pelvic abscess [2], and other complications [2, 4, 23–26]. Neonatal complications include congenital sepsis [11, 25, 27–34] as well as localized infections such as pneumonia [27, 28], dermatitis [35], and otitis media [36]. In addition, clinical chorioamnionitis has been associated with an increased risk of long-term complications such as cerebral palsy [30, 37–44].
Clinical chorioamnionitis at term is frequently diagnosed during labor, and treatment consists of antibiotic administration and acceleration of the labor process to reduce the risk of exposure to infection [2, 4, 5, 24, 28, 44]. In the context of preterm gestation, the diagnosis of clinical chorioamnionitis is an indication for delivery to spare an immature neonate the serious consequences of congenital neonatal sepsis, multiple organ involvement, and the long-term adverse effects of exposure to infection [5, 24]. Thus, clinical chorioamnionitis in preterm gestation is frequently associated with either the spontaneous onset of preterm labor or the induction of preterm labor.
Most studies of clinical chorioamnionitis have focused on term gestation. However, there is a paucity of studies done in the context of preterm gestation. Recent evidence derived from studies on clinical chorioamnionitis at term, as well as some early studies in preterm labor with intact membranes, suggests that a fraction of patients with this diagnosis have neither intra-amniotic infection nor intra-amniotic inflammation [45, 46]. In other words, some patients with preterm clinical chorioamnionitis appear to have a false-positive diagnosis. The implications of a false-positive diagnosis in term gestations are less severe than those associated with the delivery of a preterm neonate. The objective of this study was to determine the prevalence of intra-amniotic infection and intra-amniotic inflammation in patients with the diagnosis of clinical chorioamnionitis in preterm gestation.
Material and methods
Study design
This was a retrospective cohort study conducted at the Seoul National University Hospital, Seoul, South Korea, from 1993 through 2012. Patients with the diagnosis of preterm clinical chorioamnionitis (gestational age <36 weeks) were identified. Clinical chorioamnionitis was diagnosed in the presence of a maternal temperature of ≥ 37.8°C and two or more of the following criteria: (1) uterine tenderness; (2) malodorous vaginal discharge; (3) maternal leukocytosis (white blood cell count of >15,000 cells/mm3); (4) maternal tachycardia (>100 beats/minute); and (5) fetal tachycardia (>160 beats/minute), following the recommendations of Gibbs et al. [47–50].
The criteria for inclusion in this study were as follows: (1) transabdominal amniocentesis performed <36 weeks of gestation to obtain amniotic fluid (AF) for microbiologic studies; (2) singleton gestation; (3) clinical chorioamnionitis diagnosed within 48 hours of amniocentesis (this criterion was used to preserve a meaningful relationship between the results of AF studies and clinical chorioamnionitis); and (4) no evidence of other causes of fever at the time of amniocentesis. Patients had no evidence of being immunocompromised (i.e., evidence of HIV or similar diseases). Corticosteroids (dexamethasone) and the selection of antimicrobial agents were at the discretion of the managing physician. Intra-amniotic inflammation was defined as an elevated concentration of AF matrix metalloproteinase-8 (MMP-8) (>23 ng/ml), according to our previous publications that described in detail the methodology for this test, performance characteristics, diagnostic indices, predictive values, and likelihood ratios in preterm and term gestations [51–53].
Microbial invasion of the amniotic cavity (MIAC) was diagnosed in the presence of a positive AF culture for microorganisms. The methodology for culture included both aerobic and anaerobic bacteria as well as genital mycoplasmas. We have reported extensively on these results and characterized the microbial burden of the amniotic cavity in preterm gestation with these techniques.
Written informed consent was obtained from all subjects to donate AF for research purposes. The Institutional Review Board of the Seoul National University Hospital approved the collection and use of these samples and the information for research purposes. The Seoul National University Hospital has received a Federalwide Assurance with the Office for Human Research Protection of the U. S. Department of Health and Human Services.
Amniotic fluid analysis
Amniotic fluid was retrieved by transabdominal amniocentesis. The fluid was then immediately transported to the Department of Laboratory Medicine of our hospital, using specific transport media, and cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas (Ureaplasma species and Mycoplasma hominis). The culture techniques are similar to those previously described [54]. An aliquot of AF was transported to the laboratory and examined in the hemocytometer chamber to determine the AF white blood cell count. Amniotic fluid not used for diagnostic studies was centrifuged for 10 minutes at 4°C and stored at −70°C until assayed. MMP-8 concentrations were measured by a commercially available enzyme-linked immunosorbent assay (R&D Systems, Inc., Minneapolis, MN, USA). Inter-assay and intra-assay coefficients of variation were <10% for each.
Placental pathology, diagnosis of acute histologic chorioamnionitis, acute funisitis, and neonatal morbidity
Acute histologic chorioamnionitis was defined as the presence of acute inflammatory changes characterized by the infiltration of neutrophils in the choriodecidua and amnion [55, 56], respectively; acute funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly, using previously published criteria [55–60]. Significant neonatal morbidity was defined as the presence of any of the following conditions: respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage (grade ≥II), periventricular leukomalacia, proven congenital neonatal sepsis, or necrotizing enterocolitis. The definitions of each of these complications have been previously described [61].
Statistical analysis
Mann-Whitney U tests were used to compare continuous non-parametric variables, and Fisher’s exact tests were used for comparison of proportions. Survival analysis was performed with the Kaplan-Meier method to compare the amniocentesis-to-delivery interval for patients with and without MIAC and/or intra-amniotic inflammation. Patients delivered for maternal or fetal indications were treated as censored observations with a censoring time equal to the amniocentesis-to-delivery interval. A Cox proportional hazards model was used to examine the relationship between the interval from amniocentesis to delivery and the presence or absence of MIAC and/or intra-amniotic inflammation after adjusting for gestational age. Logistic regression analysis was used to examine the relationship between the presence or absence of MIAC and/or intra-amniotic inflammation and the pregnancy outcome of interest after adjustment for gestational age; the Firth method was used if the outcome variable was perfectly separated by the presence or absence of MIAC and/or intra-amniotic inflammation [62]. A probability value <0.05 was considered as significant. SPSS Version 22.0 for Windows (IBM, Armonk, NY, USA) and SAS 9.3 (SAS Institute, Cary, NC, USA) were used for statistical analyses.
Results
The frequency of microbial invasion of the amniotic cavity and intra-amniotic inflammation in preterm clinical chorioamnionitis
A total of 53 patients with clinical chorioamnionitis met the entry criteria. The prevalence of a positive AF culture for microorganisms was 34% (18/53). Thus, 66% (35/53) of patients with the diagnosis of clinical chorioamnionitis had a negative AF culture for microorganisms. Ureaplasma species were the most frequent microorganisms isolated from the amniotic cavity (n = 8). Other isolates included Streptococcus anginosus (n = 3), Escherichia coli (n = 2), Candida species (n = 2), Mycoplasma hominis (n = 2), Klebsiella pneumoniae (n = 1), Listeria monocytogenes (n = 1), and Gardnerella vaginalis (n = 1).
Intra-amniotic inflammation (defined as an MMP-8 >23 ng/mL) was present in 76% (38/50) of patients. Amniotic fluid was not available from three women for MMP-8 determinations (two women with a positive AF culture and 1 with a negative AF culture); therefore, results about the frequency of inflammation are based on 50 patients. Importantly, 24% (12/50) of patients with the clinical diagnosis of chorioamnionitis did not have either bacteria in the AF or an elevated MMP-8 concentration. These patients could be considered to have a false-positive diagnosis of preterm clinical chorioamnionitis.
All patients with bacteria in the AF (n = 16) had an elevated AF MMP-8 concentration, and 44% (22/50) of patients had an elevated MMP-8 concentration with a negative AF culture, indicating that intra-amniotic inflammation was more frequent than proven intra-amniotic infection. Importantly, there were no patients who had bacteria in the AF without intra-amniotic inflammation.
Characteristics of the study population according to the presence or absence of microbial invasion of the amniotic cavity and/or intra-amniotic inflammation
Table 1 compares the characteristics of the study population according to the presence or absence of MIAC and/or intra-amniotic inflammation. Patients with MIAC and/or intra-amniotic inflammation had a significantly lower median gestational age at amniocentesis than those without MIAC or intra-amniotic inflammation (25.8 weeks [interquartile range (IQR), 23.7–30.0 weeks] versus 31.4 weeks [IQR, 28.3–33.3 weeks], P = .006). There were no significant differences in the median maternal body temperature and maternal white blood cell count between the two study groups (P > 0.1).
Table 1.
Clinical characteristics of the study population according to the presence or absence of microbial invasion of the amniotic cavity and/or intra-amniotic inflammation
| MIAC and/or IAI (n=40) |
MIAC (−)/IAI (−) (n=12) |
P | |
|---|---|---|---|
| Maternal age, years | 31.5 (28.5–36) | 29.5 (27–31) | .112 |
| Nulliparity | 40 (16/40) | 66.7 (8/12) | .186 |
| Gestational age at amniocentesis, weeks | 25.8 (23.7–30.0) | 31.4 (28.3–33.3) | .006 |
| Diagnosis | |||
| Preterm PROM | 32.5 (13/40) | 8.3 (1/12) | .144 |
| Preterm labor | 47.5 (19/40) | 75.0 (9/12) | .133 |
| Acute cervical insufficiency | 10.0 (4/40) | 0 (0/12) | .562 |
| Clinical chorioamnionitis alone | 10.0 (4/40) | 16.7 (2/12) | .612 |
| Maternal body temperature, °C | 38.2 (38.0–38.3) | 38.3 (38.0–38.6) | .758 |
| Maternal blood WBC count, × 103 cells/mm3 | 15.2 (12.3–17.7) | 16.3 (8.5–18.0) | .862 |
| Antibiotic use | 95.0 (38/40) | 69.2 (8/12) | .021 |
| Before amniocentesis | 55.0 (22/40) | 25.0 (3/12) | .101 |
| Corticosteroid use | 42.5 (17/40) | 41.7 (5/12) | 1.000 |
| Before amniocentesis | 32.5 (13/40) | 25.0 (3/12) | .733 |
Data are presented as median (interquartile range) or % (n/N).
IAI: intra-amniotic inflammation, defined as an amniotic fluid matrix metalloproteinase-8 concentration >23 ng/mL.
MIAC: microbial invasion of amniotic cavity, defined as a positive amniotic fluid culture.
PROM: preterm rupture of the membranes.
WBC: white blood cell.
Outcomes according to the presence or absence of microbial invasion of the amniotic cavity and/or intra-amniotic inflammation
Table 2 compares the pregnancy outcomes according to the presence or absence of MIAC and/or intra-amniotic inflammation. Women with MIAC and/or intra-amniotic inflammation had a significantly higher rate of amniocentesis-to-delivery intervals of <7 days and <14 days and preterm delivery (including spontaneous preterm delivery) <34 weeks and <37 weeks of gestation, even after adjusting for gestational age at amniocentesis.
Table 2.
Pregnancy outcomes of the study population according to the presence or absence of microbial invasion of the amniotic cavity and/or intra-amniotic inflammation
| MIAC and/or IAI (n=40) |
MIAC (−)/IAI (−) (n=12) |
P | Adjusted Pa |
|
|---|---|---|---|---|
| Gestational age at delivery, weeks | 26.0 (23.8–30.2) | 34.8 (30.2–37.8) | <.001 | |
| Amniocentesis-to-delivery interval | ||||
| <48 hours | 77.5 (31/40) | 58.3 (7/12) | .267 | - |
| <7 days | 95.0 (38/40) | 58.3 (7/12) | .005 | .004 |
| <14 days | 100 (40/40) | 58.3 (7/12) | <.001 | .011b |
| Amniocentesis-to-spontaneous-delivery interval | ||||
| <48 hours | 71.9 (23/32) | 28.6 (2/7) | .075 | - |
| <7 days | 92.9 (26/28) | 28.6 (2/7) | .001 | .005 |
| <14 days | 100 (26/26) | 28.6 (2/7) | <.001 | .004b |
| Preterm delivery | ||||
| <30 weeks | 96.7 (29/30) | 60 (3/5) | .047 | .106 |
| <34 weeks | 97.4 (37/38) | 41.7 (5/12) | <.001 | .008 |
| <37 weeks | 100 (40/40) | 66.7 (8/12) | .002 | .015b |
| Spontaneous preterm delivery | ||||
| <30 weeks | 94.4 (17/18) | 33.3 (1/3) | .041 | .991 |
| <34 weeks | 95.8 (23/24) | 22.2 (2/9) | <.001 | .005 |
| <37 weeks | 100 (26/26) | 33.3 (2/6) | <.001 | .006b |
| Acute histologic chorioamnionitis | 91.2 (31/34) | 25 (2/8) | <.001 | .003 |
| Acute funisitis | 57.6 (19/33) | 0 (0/8) | .004 | .024b |
Data are presented as median (interquartile range) or % (n/N).
IAI, intra-amniotic inflammation, defined as an amniotic fluid matrix metalloproteinase-8 concentration >23 ng/mL.
MIAC, microbial invasion of amniotic cavity, defined as a positive amniotic fluid culture.
Logistic regression analysis was used to adjust gestational age at amniocentesis.
Firth’s method was used in the analysis.
Figure 1 illustrates the interval from amniocentesis to delivery according to the presence or absence of MIAC and/or intra-amniotic inflammation. Of the 23 cases in which delivery resulted from induction of labor or cesarean delivery before the onset of labor, this interval was censored. Multivariate survival analysis demonstrated that women with MIAC and/or intra-amniotic inflammation had a significantly shorter median amniocentesis-to-delivery interval than those without MIAC or intra-amniotic inflammation, after adjusting for the gestational age at amniocentesis (hazards ratio, 7.55; 95% confidence interval, 1.74–32.8; P < 0.01 by the Cox proportional hazards model analysis).
Figure 1.
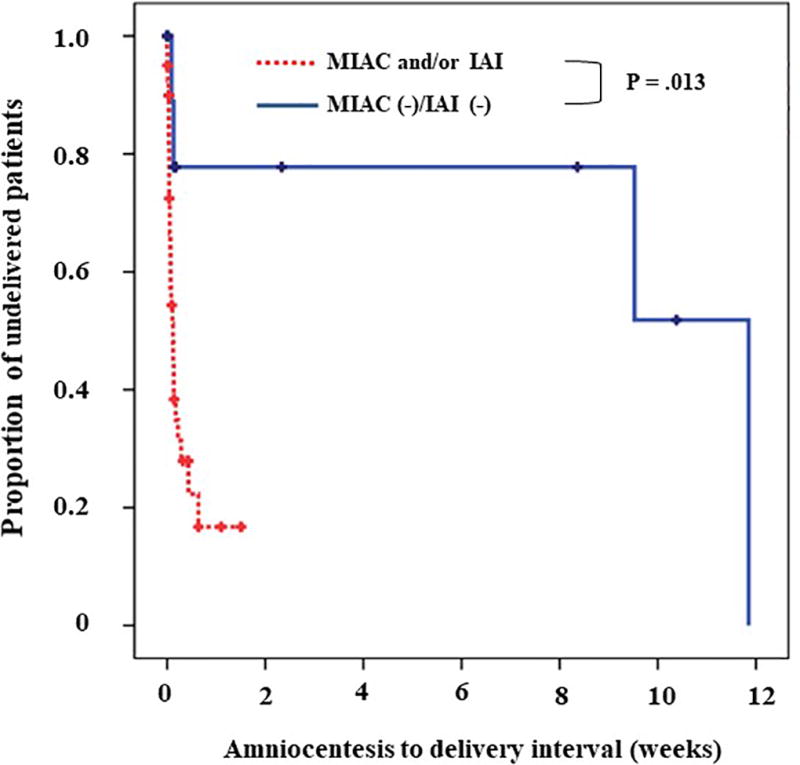
Survival analysis of the amniocentesis-to-delivery intervals, according to the presence or absence of microbial invasion of the amniotic cavity (MIAC) and/or intra-amniotic inflammation (IAI) (median 0.1 [range, 0–1.5 vs. median 4.8 range 0–11.8 weeks, respectively; P = .013).
Neonatal outcomes
Table 3 compares neonatal outcomes according to the presence or absence of MIAC and/or intra-amniotic inflammation. All fetal deaths (n = 3) were observed in mothers with MIAC and/or intra-amniotic inflammation. Neonates born to mothers with MIAC and/or intra-amniotic inflammation had a significantly higher rate of serious neonatal morbidity than those born to mothers without MIAC or intra-amniotic inflammation. Among the 40 women with MIAC and/or intra-amniotic inflammation, 78% (31/40) delivered within two days of amniocentesis. Among the remaining nine women, two women remained undelivered for more than seven days. However, one patient had a fetal death and the other underwent induction of labor at a non-viable gestational age due to maternal sepsis.
Table 3.
Neonatal outcomes of the study population according to the presence or absence of microbial invasion of the amniotic cavity and/or intra-amniotic inflammation
| MIAC and/or IAI (n=38)a |
MIAC (−)/IAI (−) (n=12) |
P | Adjusted Pb |
|
|---|---|---|---|---|
| 1-min Apgar score <7c | 89.2 (33/37) | 41.7 (5/12) | .002 | .035 |
| 5-min Apgar score <7c | 70.3 (26/37) | 25.0 (3/12) | .008 | .185 |
| Fetal death in utero | 7.9 (3/38) | 0 (0/12) | 1.000 | - |
| Neonatal death and/or stillbirthc | 40.5 (15/37) | 16.7 (2/12) | .175 | - |
| Significant morbidityc,d | 84.6 (22/26) | 33.3 (4/12) | .003 | .015 |
| Respiratory distress syndrome | 34.6 (9/26) | 33.3 (4/12) | 1.000 | - |
| Bronchopulmonary dysplasia | 52.2 (12/23) | 0 (0/10) | .005 | .120e |
| Intraventricular hemorrhage | 26.9 (7/26) | 0 (0/12) | .074 | - |
| Periventricular leukomalacia | 19.2 (5/26) | 0 (0/12) | .158 | - |
| Necrotizing enterocolitis | 7.7 (2/26) | 0 (0/12) | 1.000 | - |
| Proven early neonatal sepsis | 7.7 (2/26) | 0 (0/12) | 1.000 | - |
Data are presented as n/N (%).
MIAC: microbial invasion of amniotic cavity, defined as a positive amniotic fluid culture.
IAI: intra-amniotic inflammation, defined as an amniotic fluid matrix metalloproteinase-8 concentration >23 ng/mL.
Two cases with congenital anomaly were excluded from the analysis.
Logistic regression analysis was used to adjust gestational age at amniocentesis.
One case with unavailable data was excluded from the analysis.
Eleven neonates who died in utero (n=3) or immediately after birth because of extreme prematurity (n=8) and thus could not be evaluated with respect to the presence or absence of complications were excluded from the analysis.
Firth’s method was used in the analysis.
Amniotic fluid analysis, placental pathology, and pregnancy outcome
Table 4 displays the clinical characteristics, AF analyses, histopathologic characteristics of the placenta, and pregnancy and neonatal outcomes of 12 patients without MIAC and/or intra-amniotic inflammation. One-third (4/12) of women without MIAC or intra-amniotic inflammation delivered their neonates at term, and two-thirds (8/12) of neonates did not have any significant neonatal morbidity. The rate of histologic chorioamnionitis was 25% (2/8); however, acute funisitis was not found in any case.
Table 4.
Cases without microbial invasion of the amniotic cavity or intra-amniotic inflammation
| No | Gestational age at amniocentesis (weeks) |
Gestational age at birth (weeks) |
Interval from amniocentes is(days) |
Diagnosis | Birth- weight (gram) |
Maternal white blood cell count (cells/mm3) |
Amniotic fluid MMP-8 concentration (ng/mL) |
Acute histologic chorioamnionitis |
Acute funisitis |
Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28.9 | 29 | 1 | PROM | 950 | 18400 | 5.8 | Present | Absent | Maternal preeclampsia, RDS, neonatal death due to pulmonary hemorrhage |
| 2 | 25.1 | 25.3 | 2 | PTL | 911 | 15500 | 1.7 | Present | Absent | Progression of spontaneous labor, RDS, neonatal death due respiratory failure |
| 3 | 35.4 | 35.4 | 0 | PTL | 2390 | 18200 | 0.5 | Absent | Absent | Delivery due to sustained fever, survival without significant morbidity |
| 4 | 32.4 | 42.4 | 70 | CCA | 4040 | 18100 | 1.4 | N/A | N/A | Survival without significant morbidity |
| 5 | 33.7 | 36 | 16 | PTL | 2970 | 8000 | 0.3 | Absent | Absent | Cesarean delivery due to placenta previa |
| 6 | 27.6 | 38.1 | 74 | PTL | 2450 | 6000 | 0.5 | N/A | N/A | Survival without significant morbidity |
| 7 | 34.1 | 34.1 | 0 | PTL | 2010 | 8900 | 0.7 | Absent | Absent | Cesarean delivery due to breech presentation and spontaneous labor progression |
| 8 | 31.3 | 31.3 | 0 | PTL | 1700 | 17090 | 0.3 | Absent | Absent | Cesarean delivery due to maternal bleeding, RDS |
| 9 | 25.7 | 37.4 | 83 | PTL | 2850 | 17860 | 0.5 | Absent | Absent | Survival without significant morbidity |
| 10 | 31.4 | 41 | 67 | CCA | 3320 | 7000 | 0.3 | N/A | N/A | Survival without significant morbidity |
| 11 | 29.1 | 29.1 | 0 | PTL | 1140 | 15030 | 0.3 | Absent | Absent | Cesarean delivery due to non-reassuring fetal status, RDS |
| 12 | 32.9 | 33 | 1 | PTL | 1980 | 17460 | 2.1 | N/A | N/A | Spontaneous labor progression |
CCA: clinical chorioamnionitis without preterm rupture of the membranes or preterm labor; MMP-8: matrix metalloproteinase-8; N/A: not assessed; PTL: preterm labor; PROM: premature rupture of the membranes; RDS: respiratory distress syndrome;
Comment
Principal findings of the study
The principal findings of the study are as follows: 1) among patients with preterm clinical chorioamnionitis, 24% had neither culture-proven microbiologic evidence of intra-amniotic infection nor intra-amniotic inflammation; 2) demonstrable intra-amniotic infection was present in 34% of cases (18/53); 3) the most common microorganisms isolated from the amniotic cavity were the Ureaplasma species; 4) patients without MIAC or intra-amniotic inflammation had significantly better outcomes than those with MIAC and/or intra-amniotic inflammation. The main conclusion of this study is that the current clinical criteria used for the diagnosis of clinical chorioamnionitis in preterm gestation may result in unnecessary preterm deliveries and, therefore, neonatal morbid events. There is an urgent need to improve the diagnosis of this condition in preterm gestation.
Clinical diagnosis of chorioamnionitis
The most widely used definition of clinical chorioamnionitis was proposed by Gibbs et al. [47–50]. This definition uses a combination of clinical and laboratory parameters derived from the mother or the fetus. Maternal fever, tachycardia, and an elevation of white blood cells in the peripheral blood count reflect signs of maternal systemic inflammation. In contrast, uterine tenderness and foul-smelling amniotic fluid/discharge are indicative of a localized response to microbial invasion or uterine inflammation. Fetal tachycardia could reflect a response to infection/inflammation or, alternatively, a physiologic response to a change in maternal temperature, as the fetus is less able to dissipate heat than the mother [24, 63].
The sensitivity and specificity of each clinical and laboratory sign of clinical chorioamnionitis in preterm gestation has not been examined in the context of AF cultures and intra-amniotic inflammation. The original diagnostic indices were based on the results of AF microbiologic studies obtained using a transcervical catheter in term gestations [49]. Retrieval of AF via such a catheter is susceptible to contamination of the specimen with vaginal/cervical bacteria. In support of this concept is the study of Gibbs et al. [49]; there were 52 patients with clinical chorioamnionitis, and 52 others matched for gestational age without this diagnosis. Notably, approximately 75% of samples from patients without clinical chorioamnionitis had >102 colony-forming units/mL in AF cultures. These findings sharply contrast with a systematic study of the amniotic cavity in women in spontaneous labor at term with intact membranes, in which only 17% had positive AF cultures for microorganisms in the absence of clinical signs of infection. Collectively, this evidence suggests that samples obtained by transvaginal/transcervical catheterization are prone to bacterial contamination and, therefore, cannot be relied on to accurately represent the microbial state of the amniotic cavity.
A recent study of the accuracy of the clinical signs proposed by Gibbs et al. [47–50] in clinical chorioamnionitis at term using AF obtained by transabdominal amniocentesis indicated that all clinical signs have an accuracy of around 50% for the identification of intra-amniotic infection (presence of bacteria and intra-amniotic inflammation). The implications of a high inaccuracy rate in the diagnosis of preterm clinical chorioamnionitis could be serious because the standard intervention is the induction of labor [64].
False-positive diagnosis of clinical chorioamnionitis
While 76% (38/50) of patients with the clinical diagnosis of chorioamnionitis had MIAC and/or intra-amniotic inflammation, it is noteworthy that 24% had neither; therefore, the diagnoses should be considered as false-positive. This observation was unexpected because there is a widespread belief among clinicians that the diagnosis of clinical chorioamnionitis may not be sensitive to the detection of intra-amniotic infection in preterm gestations, but is quite specific for the diagnosis of intra-amniotic infection. Indeed, in a study in which serial amniocenteses were performed in patients with preterm PROM, the frequency of MIAC increased as a function of time, and, in particular, when patients with preterm PROM began to contract (i.e. evidence of preterm labor, even in the absence of clinical signs of infection).
Our results call for a re-examination of the reliability of the criteria because a clinical diagnosis is frequently used as an indication for delivery. The consequences of preterm delivery because of a false-positive diagnosis could be serious. Future studies are required to determine the mechanisms of disease that mimic the clinical syndrome of chorioamnionitis. Also, examination of AF retrieved by a transvaginal collector [65] in patients with preterm PROM and the determination of inflammatory cytokines may dramatically improve the accuracy of the diagnosis of “true” intra-amniotic inflammation without resorting to an invasive procedure.
Microbiologically proven clinical chorioamnionitis
Of the cases with clinical chorioamnionitis, 34% (18/53) had microbiologic evidence of infection. The most common microorganisms isolated were the Ureaplasma species—this is consistent with previous reports [4, 24, 45, 53, 54, 66–77]. We reported that the intensity of the systemic fetal inflammatory response in cases with a positive AF culture is greater than that observed in cases of intra-amniotic inflammation with a negative culture [78].
False-negative diagnosis of intra-amniotic infection
A consistent observation across many studies, including the present one, is that the frequency of intra-amniotic inflammation is greater than that of AF cultures positive for microorganisms [7, 52, 53, 79–81]. The mere presence of microbial footprints, using molecular microbiologic techniques in a fraction of these patients, is a poor prognostic factor [76, 81–87]. Therefore, failure to grow microorganisms in the laboratory should not be interpreted as the absence of bacteria in vivo. Cultivation techniques are helpful when the results are positive, but negative culture results when inflammation is present should be interpreted with caution [81, 85, 86]. The possibility that some intra-amniotic infections may be caused by viruses or other organisms requiring special isolation techniques should not be overlooked, and future studies are required to identify novel pathogens—thus far, our studies have not identified viruses as major organisms causative of intra-amniotic infection. Another potential limitation is that antibiotics were used to treat one-half of the patients prior to amniocentesis. It is possible that this could have reduced the rate of positive AF cultures. Further studies using molecular microbiologic techniques, which are able to identify bacterial footprints even after treatment with antibiotics, would be required to address this question. However, our study spans 20 years, and during much of this time, molecular microbiologic techniques were not available, and they are still not part of routine practice in obstetrics. Finally, inflammation is a non-specific mechanism of host defense and can be caused by non-infection-related insults, which we have termed “sterile” inflammation [81, 88, 89]. The precise nature of these “danger signals” has not been identified, but there is evidence that they may engage alarmins and the inflammasome system in the onset of term or preterm labor [90–94].
Strengths and limitations
The current study represents the most comprehensive examination of AF analysis for microorganisms using cultivation techniques, assessment of intra-amniotic inflammation, neonatal outcome, and placental pathology in preterm gestation. The focus of this study—preterm clinical chorioamnionitis—is one of the major indications for the induction of labor in preterm gestations. The major finding of the study—that a substantial number of patients diagnosed with preterm clinical chorioamnionitis had no evidence of either intra-amniotic infection or intra-amniotic inflammation—is unexpected and calls for very careful documentation of the criteria used for diagnosis and intervention in daily clinical practice. A limitation of this study is that it was conducted over a long period of time, given the low prevalence of proven clinical chorioamnionitis in preterm gestations. However, we used a uniform protocol for the diagnosis of intra-amniotic infection, as the cultivation techniques used in our unit have been in place for more than 20 years. Moreover, we have used objective criteria for the diagnosis of intra-amniotic inflammation, and samples have been stored under optimal conditions (−70° C). The criteria for the diagnosis of placental pathologic lesions have been used by our placental pathologists for more than 25 years as well as being the subject of extensive publication. The main message of this article is to alert clinicians to the potential diagnosis of preterm clinical chorioamnionitis, and such a conclusion is solidly derived from the findings reported herein.
Clinical implications
The clinical diagnosis of chorioamnionitis is thought to be a reliable indicator of intra-amniotic infection. While most such infections are known to be sub-clinical, false-positive diagnoses of chorioamnionitis are believed to be rare. Our observations call for a re-examination of this view. This is particularly the case in preterm gestation when an erroneous diagnosis may lead to unwarranted induction of labor or cesarean delivery. Amniotic fluid analysis, including rapid tests for the detection of inflammation [95–98] and molecular microbiologic methods [34, 45, 72, 81–87, 99–107], are likely to become important in the management of preterm clinical chorioamnionitis. Recent observations suggest that assessment of AF retrieved using an AF collector [65] and the measurement of inflammatory mediators could be of value in the assessment of patients with preterm PROM [108].
Research implications
The findings of this study have implications for both clinical and translational research. Clinical research is necessary to estimate the impact of interventions based on false-positive diagnoses of clinical chorioamnionitis. Outcomes could include neonatal morbidity and the cost of care for an unindicated preterm delivery. The causes of the clinical syndrome that resembles chorioamnionitis in these patients need to be determined. Although, in the context of PROM, the onset of preterm contractions in a patient who has been quiescent in the antepartum period has been associated with MIAC [3], it is now clear that the syndrome of clinical chorioamnionitis can occur without microbial invasion or even intra-amniotic inflammation. Although negative cultures could be explained by treatment with antibiotics, this is unlikely to be the explanation for the absence of intra-amniotic inflammation in our study.
The gold standard used for the diagnosis of intra-amniotic infection/inflammation in our study required examination of AF obtained by amniocentesis. Recently, a device was developed for the retrieval of AF in patients with preterm PROM [65]. The assay of such fluid for inflammatory markers may help in the identification of patients with true intra-amniotic inflammation [109–111]. In patients with preterm labor with intact membranes or acute cervical insufficiency, examination of AF retrieved by amniocentesis is probably the best method to assess the true state of the amniotic cavity. Emphasis needs to be placed on the development of bedside tests that allow a rapid answer [95–98, 112, 113]. Research in non-invasive methods to assess the state of the amniotic cavity is desirable. For example, it would be ideal if assessment of cervical fluid could provide information without requiring amniocentesis.
Acknowledgments
We are grateful to the patients who volunteered for the study, attending physicians, residents, faculty, nursing staff, and administrative personnel at the Seoul National University for the contributions they have made over the years to the conduct of these and many other studies related to clinical chorioamnionitis.
Source of Funding: This work was supported in part, by the SNUH research fund (Grant No. 04-2016-0920), and in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, Chaiyasit N, Yeo L, Shaman M, Lannaman K, Cher B, Hassan SS, Gomez-Lopez N. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med. 2017;45:523–538. doi: 10.1515/jpm-2016-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouse DJ, Landon M, Leveno KJ, Leindecker S, Varner MW, Caritis SN, O’Sullivan MJ, Wapner RJ, Meis PJ, Miodovnik M, Sorokin Y, Moawad AH, Mabie W, Conway D, Gabbe SG, Spong CY. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. The maternal-fetal medicine units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol. 2004;191:211–216. doi: 10.1016/j.ajog.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, Hobbins JC. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–666. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 4.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37:339–354. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinelli P, Sarno L, Maruotti GM, Paludetto R. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med. 2012;(25 Suppl 4):29–31. doi: 10.3109/14767058.2012.714981. [DOI] [PubMed] [Google Scholar]
- 6.Coultrip LL, Lien JM, Gomez R, Kapernick P, Khoury A, Grossman JH. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 1994;171:901–911. doi: 10.1016/s0002-9378(94)70057-5. [DOI] [PubMed] [Google Scholar]
- 7.Yoon BH1, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 8.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 9.Nasef N, Shabaan AE, Schurr P, Iaboni D, Choudhury J, Church P, Dunn MS. Effect of clinical and histological chorioamnionitis on the outcome of preterm infants. Am J Perinatol. 2013;30:59–68. doi: 10.1055/s-0032-1321501. [DOI] [PubMed] [Google Scholar]
- 10.Bastek JA, Weber AL, McShea MA, Ryan ME, Elovitz MA. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol. 2014;210:450. doi: 10.1016/j.ajog.2013.12.024. e1–e10. [DOI] [PubMed] [Google Scholar]
- 11.Sung JH, Choi SJ, Oh SY, Roh CR, Kim JH. Revisiting the diagnostic criteria of clinical chorioamnionitis in preterm birth BJOG. BJOG. 2017;124:775–783. doi: 10.1111/1471-0528.14176. [DOI] [PubMed] [Google Scholar]
- 12.Russell KP, Anderson GV. The aggressive management of ruptured membranes. Am J Obstet Gynecol. 1962;83:930–937. doi: 10.1016/0002-9378(62)90089-3. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs CE, Locke WE. Maternal deaths in Texas, 1969 to 1973 A report of 501 consecutive maternal deaths from the Texas Medical Association’s Committee on Maternal Health. Am J Obstet Gynecol. 1976;126:687–692. [PubMed] [Google Scholar]
- 14.Mertz KJ, Parker AL, Halpin GJ. Pregnancy-related mortality in New Jersey, 1975 to 1989. Am J Public Health. 1992;82:1085–1088. doi: 10.2105/ajph.82.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey BM, Cox SM. Chorioamnionitis and endometritis. Infect Dis Clin North Am. 1997;11:203–222. doi: 10.1016/s0891-5520(05)70349-4. [DOI] [PubMed] [Google Scholar]
- 16.Dotters-Katz SK, Feldman C, Puechl A, Grotegut CA, Heine RP. Risk factors for post-operative wound infection in the setting of chorioamnionitis and cesarean delivery. J Matern Fetal Neonatal Med. 2016;29:1541–1545. doi: 10.3109/14767058.2015.1058773. [DOI] [PubMed] [Google Scholar]
- 17.Lee W, Clark SL, Cotton DB, Gonik B, Phelan J, Faro S, Giebel R. Septic shock during pregnancy. Am J Obstet Gynecol. 1988;159:410–416. doi: 10.1016/s0002-9378(88)80096-6. [DOI] [PubMed] [Google Scholar]
- 18.Chebbo A, Tan S, Kassis C, Tamura L, Carlson RW. Maternal sepsis and septic shock. Crit Care Clin. 2016;32:119–135. doi: 10.1016/j.ccc.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Kadar N, Vaisbuch E, Hassan SS. Maternal death following cardiopulmonary collapse after delivery: amniotic fluid embolism or septic shock due to intrauterine infection? Am J Reprod Immunol. 2010;64:113–125. doi: 10.1111/j.1600-0897.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol. 1984;64:615–620. [PubMed] [Google Scholar]
- 21.Witlin AG, Mercer BM, Sibai BM. Septic pelvic thrombophlebitis or refractory postpartum fever of undetermined etiology. J Matern Fetal Med. 1996;5:355–358. doi: 10.1002/(SICI)1520-6661(199611/12)5:6<355::AID-MFM12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol. 2013;209:51. doi: 10.1016/j.ajog.2013.03.011. e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauth JC, Gilstrap LC, 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol. 1985;66:59–62. [PubMed] [Google Scholar]
- 24.Westover T, Knuppel RA. Modern management of clinical chorioamnionitis. Infect Dis Obstet Gynecol. 1995;3:123–132. doi: 10.1155/S1064744995000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mark SP, Croughan-Minihane MS, Kilpatrick SJ. Chorioamnionitis and uterine function. Obstet Gynecol. 2000;95:909–912. [PubMed] [Google Scholar]
- 26.Rojas-Suarez J, Paternina-Caicedo AJ, Miranda J, Mendoza R, Duenas-Castel C, Bourjeily G. Comparison of severity-of-illness scores in critically ill obstetric patients: a 6-year retrospective cohort. Crit Care Med. 2014;42:1047–1054. doi: 10.1097/CCM.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 27.Yoder PR, Gibbs RS, Blanco JD, Castaneda YS, St Clair PJ. A prospective, controlled study of maternal and perinatal outcome after intra-amniotic infection at term. Am J Obstet Gynecol. 1983;145:695–701. doi: 10.1016/0002-9378(83)90575-6. [DOI] [PubMed] [Google Scholar]
- 28.Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 1993;36:795–808. doi: 10.1097/00003081-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol. 1996;87:188–194. doi: 10.1016/0029-7844(95)00402-5. [DOI] [PubMed] [Google Scholar]
- 30.Alexander JM, Gilstrap LC, Cox SM, McIntire DM, Leveno KJ. Clinical chorioamnionitis and the prognosis for very low birth weight infants. Obstet Gynecol. 1998;91:725–729. doi: 10.1016/s0029-7844(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 31.Ladfors L, Tessin I, Mattsson LA, Eriksson M, Seeberg S, Fall O. Risk factors for neonatal sepsis in offspring of women with prelabor rupture of the membranes at 34–42 weeks. J Perinat Med. 1998;26:94–101. doi: 10.1515/jpme.1998.26.2.94. [DOI] [PubMed] [Google Scholar]
- 32.Volante E, Moretti S, Pisani F, Bevilacqua G. Early diagnosis of bacterial infection in the neonate. J Matern Fetal Neonatal Med. 2004;(16 Suppl 2):13–16. doi: 10.1080/14767050410001727116. [DOI] [PubMed] [Google Scholar]
- 33.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK. Canadian Neonatal Network. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009;200:372. doi: 10.1016/j.ajog.2008.11.034. e1–e6. [DOI] [PubMed] [Google Scholar]
- 34.Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, Walsh MC, Das A, Hale EC, Newman NS, Higgins RD. Eunice Kennedy Shriver National Institute of Child Health, Human Development Neonatal Research Network. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168:137–147. doi: 10.1001/jamapediatrics.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–514. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Felice C1, De Capua B, Costantini D, Martufi C, Toti P, Tonni G, Laurini R, Giannuzzi A, Latini G. Recurrent otitis media with effusion in preterm infants with histologic chorioamnionitis--a 3 years follow-up study. Early Hum Dev. 2008;84:667–671. doi: 10.1016/j.earlhumdev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–211. [PubMed] [Google Scholar]
- 38.Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000;13:133–139. doi: 10.1097/00019052-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 40.Nelson KB. The epidemiology of cerebral palsy in term infants. Ment Retard Dev Disabil Res Rev. 2002;8:146–150. doi: 10.1002/mrdd.10037. [DOI] [PubMed] [Google Scholar]
- 41.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–29. doi: 10.1002/mrdd.10003. [DOI] [PubMed] [Google Scholar]
- 42.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 43.Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116:387–392. doi: 10.1097/AOG.0b013e3181e90046. [DOI] [PubMed] [Google Scholar]
- 44.Fishman SG, Gelber SE. Evidence for the clinical management of chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:46–50. doi: 10.1016/j.siny.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Docheva N, Martinez-Varea A, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44:5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol. 1977;1:71–77. [PubMed] [Google Scholar]
- 48.Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol. 1980;136:709–713. doi: 10.1016/0002-9378(80)90445-7. [DOI] [PubMed] [Google Scholar]
- 49.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 50.Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988;72:823–828. doi: 10.1097/00006250-198812000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, Ko EM. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–1161. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 52.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 53.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633. doi: 10.1016/j.ajog.2007.11.047. e1–e8. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 55.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–S52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Kusanovic JP, Yoon BH, Kim JS, Chaiyasit N, Ahmed AI, Qureshi F, Jacques SM, Kim CJ, Hassan SS, Chaiworapongsa T, Yeo L, Kim YM. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44:33–51. doi: 10.1515/jpm-2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 58.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Society for Pediatric Pathology, Perinatal Section, Amniotic Fluid Infection Nosology Committee Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 59.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19:693–697. doi: 10.1080/14767050600927353. [DOI] [PubMed] [Google Scholar]
- 60.Mi Lee S, Romero R, Lee KA, Jin Yang H, Joon Oh K, Park CW, Yoon BH. The frequency and risk factors of funisitis and histologic chorioamnionitis in pregnant women at term who delivered after the spontaneous onset of labor. J Matern Fetal Neonatal Med. 2011;24:37–42. doi: 10.3109/14767058.2010.482622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 62.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002;21:2409–2419. doi: 10.1002/sim.1047. [DOI] [PubMed] [Google Scholar]
- 63.Herbst A, Wolner-Hanssen P, Ingemarsson I. Maternal fever in term labour in relation to fetal tachycardia, cord artery acidaemia and neonatal infection. BJOG. 1997;104:363–366. doi: 10.1111/j.1471-0528.1997.tb11469.x. [DOI] [PubMed] [Google Scholar]
- 64.Romero R, Chaemsaithong P, Korzeniewski SJ, Kusanovic JP, Docheva N, Martinez-Varea A, Ahmed AI, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med. 2016;44:23–32. doi: 10.1515/jpm-2015-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SM, Romero R, Park JS, Chaemsaithong P, Jun JK, Yoon BH. A transcervical amniotic fluid collector: a new medical device for the assessment of amniotic fluid in patients with ruptured membranes. J Perinat Med. 2015;43:381–389. doi: 10.1515/jpm-2014-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sperling RS, Newton E, Gibbs RS. Intraamniotic infection in low-birth-weight infants. J Infect Dis. 1988;157:113–117. doi: 10.1093/infdis/157.1.113. [DOI] [PubMed] [Google Scholar]
- 67.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol. 1992;167:1092–1095. doi: 10.1016/s0002-9378(12)80044-5. [DOI] [PubMed] [Google Scholar]
- 68.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, Baumann P, Araneda H, Kenney JS, Cotton DB, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 69.Averbuch B, Mazor M, Shoham-Vardi I, Chaim W, Vardi H, Horowitz S, Shuster M. Intra-uterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol. 1995;62:25–29. doi: 10.1016/0301-2115(95)02176-8. [DOI] [PubMed] [Google Scholar]
- 70.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, Kim KS. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–1260. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 71.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77–82. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 72.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokström H, Holst RM, Nikolaitchouk N, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–431. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 73.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong JS, Park KH, Noh JH, Suh YH. Cervical length and the risk of microbial invasion of the amniotic cavity in women with preterm premature rupture of membranes. J Korean Med Sci. 2007;22:713–717. doi: 10.3346/jkms.2007.22.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kirchner L, Helmer H, Heinze G, Wald M, Brunbauer M, Weninger M, Zaknun D. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol. 2007;134:44–50. doi: 10.1016/j.ejogrb.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, Yoon BH. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:211. doi: 10.1016/j.ajog.2010.03.035. e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294. doi: 10.1016/j.ajog.2007.07.006. e1–e6. [DOI] [PubMed] [Google Scholar]
- 79.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, Miller H, Luthy D, Pereira L, Nageotte M, Robilio PA, Fortunato S, Simhan H, Baxter JK, Amon E, Franco A, Trofatter K, Heyborne K. ProteoGenix/Obstetrix Collaborative Research Network. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125. doi: 10.1016/j.ajog.2013.11.032. e1–e15. [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014:1–17. doi: 10.3109/14767058.2014.954243. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–474. doi: 10.1111/aji.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, Jun JK. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–1137. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 83.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919–924. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 84.Kim M, Kim G, Romero R, Shim SS, Kim EC, Yoon BH. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J Perinat Med. 2003;31:146–152. doi: 10.1515/JPM.2003.020. [DOI] [PubMed] [Google Scholar]
- 85.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzún E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, Kim YM. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–1409. doi: 10.3109/14767058.2014.958463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–1455. doi: 10.3109/14767058.2011.591460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol. 2016;75:3–7. doi: 10.1111/aji.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, Chaemsaithong P, Chaiworapongsa T, Dong Z, Tarca AL, Abrahams VM, Yeo L, Hassan SS. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod Sci. 2017;24:934–953. doi: 10.1177/1933719116675058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM, Yeo L, Hassan SS. A role for the inflammasome in spontaneous preterm labor with acute histologic chorioamnionitis. Reprod Sci. 2017;24:1382–1401. doi: 10.1177/1933719116687656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM, Hassan SS, Yeo L, Gomez-Lopez N. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. 2016 Mar 8; doi: 10.1111/aji.12440. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nien JK, Yoon BH, Espinoza J, Kusanovic JP, Erez O, Soto E, Richani K, Gomez R, Hassan S, Mazor M, Edwin S, Bahado-Singh R, Romero R. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol. 2006;195:1025–1030. doi: 10.1016/j.ajog.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 96.Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, Yoon BH. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292. doi: 10.1016/j.ajog.2007.06.040. e1–e5. [DOI] [PubMed] [Google Scholar]
- 97.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497–502. doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim SM, Romero R, Lee J, Chaemsaithong P, Lee MW, Chaiyasit N, Lee HJ, Yoon BH. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. J Matern Fetal Neonatal Med. 2016;29:2414–2421. doi: 10.3109/14767058.2015.1094049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jalava J, Mäntymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O, Alanen A. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. BJOG. 1996;103:664–669. doi: 10.1111/j.1471-0528.1996.tb09835.x. [DOI] [PubMed] [Google Scholar]
- 100.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN, Eschenbach DA. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24:1228–1232. doi: 10.1086/513669. [DOI] [PubMed] [Google Scholar]
- 101.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG. 2002;109:527–533. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 102.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 103.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokström H, Holst RM, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–128. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 104.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382–1386. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 105.Yi J, Yoon BH, Kim EC. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes. 2005;19:255–260. doi: 10.1016/j.mcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodriguez N, Fernandez C, Zamora Y, Berdasquera D, Rivera JA. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:47–50. doi: 10.3109/14767058.2010.482609. [DOI] [PubMed] [Google Scholar]
- 108.Kunze M, Klar M, Morfeld CA, Thorns B, Schild RL, Markfeld-Erol F, Rasenack R, Proempeler H, Hentschel R, Schaefer WR. Cytokines in noninvasively obtained amniotic fluid as predictors of fetal inflammatory response syndrome. Am J Obstet Gynecol. 2016;215:96. doi: 10.1016/j.ajog.2016.01.181. e1–e8. [DOI] [PubMed] [Google Scholar]
- 109.Musilova I, Bestvina T, Hudeckova M, Michalec I, Cobo T, Jacobsson B, Kacerovsky M. Vaginal fluid interleukin-6 concentrations as a point-of-care test is of value in women with preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2016;215:619. doi: 10.1016/j.ajog.2016.07.001. e1–e12. [DOI] [PubMed] [Google Scholar]
- 110.Musilova I, Bestvina T, Hudeckova M, Michalec I, Cobo T, Jacobsson B, Kacerovsky M. Vaginal fluid IL-6 concentrations as a point-of-care test is of value in women with preterm PROM. Am J Obstet Gynecol. 2016;215:619. doi: 10.1016/j.ajog.2016.07.005. e1–e12. [DOI] [PubMed] [Google Scholar]
- 111.Kacerovsky M, Musilova I, Jacobsson B, Drahosova M, Hornychova H, Janku P, Prochazka M, Simetka O, Andrys C. Vaginal fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor membrane ruptures. J Matern Fetal Neonatal Med. 2015;28:392–398. doi: 10.3109/14767058.2014.917625. [DOI] [PubMed] [Google Scholar]
- 112.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29:360–367. doi: 10.3109/14767058.2015.1006621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, Yeo L. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2016;29:349–359. doi: 10.3109/14767058.2015.1006620. [DOI] [PMC free article] [PubMed] [Google Scholar]


