Abstract
Objective
To examine within-person variation in dietary self-monitoring during a 6-month technology-supported weight loss trial as a function of time-varying factors including time in the study, day of the week, and month of the year.
Methods
Smartphone self-monitoring data was examined from 31 obese adults (18-60 years) who participated in a 6-month technology-supported weight loss program. Multilevel modeling was used to examine within-person variation in dietary self-monitoring.
Results
Participants recorded less as time in the study progressed. Fewer foods were reported on the weekends compared to weekdays. More foods were self-monitored in January compared to October, however a seasonal effect was not observed.
Conclusions and Implications
The amount of time in a study and day of the week were associated with dietary self-monitoring, but not season. Future studies should examine factors that influence the variations in self-monitoring and identify methods to improve technology-supported dietary self-monitoring adherence.
Keywords: Self-monitoring, weight loss, adherence, diet, obesity, technology
INTRODUCTION
Dietary self-monitoring is a key component of successful behavioral weight loss interventions1-3 and is essential for facilitating other behavior change techniques (e.g. setting goals, providing behavioral feedback).4 As a part of self-regulation, individuals monitor their behavior, evaluate how that behavior compares to behavioral goals, and then use methods of self-control and reinforcement to modify behaviors and reduce future discrepancies between the goal and actual behaviors.5,6 Daily dietary self-monitoring generally entails tracking all foods and drinks consumed, the portion size of each item, and the corresponding calorie and fat gram totals. Ideally, this recording occurs as foods are consumed, yet, in practice, many people do not record near the time they ate.7 Awareness of caloric intake is expected to align eating behaviors with goals to create a negative energy balance and facilitate weight loss.
Patterns in short-term dietary intake have also been identified, including consuming more calories8,9 and fat10 on the weekends, particularly among those who are overweight/obese, high-income, or between the ages of 18-64 years.11 In addition to these increases in calories and fat, diet quality is poorer on the weekends.11 The increase in caloric and fat intake corresponds with weekly fluctuations in weight – weight is higher on Sundays and Mondays and decreases as the end of the week nears.12 In contrast to the increase in caloric consumption and body weight, self-monitoring was recently found to be less frequent on weekends than weekdays.13 The holidays are another time when increased caloric intake and weight gains of 0.4-0.7 kg are seen.14-16 Self-monitoring consistency during this high risk time is associated with better weight management,2,17 yet it is unclear whether self-monitoring patterns vary during the winter months. Few studies have examined weekly and seasonal patterns of dietary self-monitoring, particularly when using a smartphone application, in individuals enrolled in a weight loss trial.
The purpose of the current study was to examine the temporal patterning of dietary self-monitoring across multiple time scales (i.e., time since the start of intervention, day of week, and month of year). To accomplish these goals, we evaluated self-monitoring records from adults participating in a technology-supported weight loss program.18 Adjusting for age, sex, baseline weight, and daily weight change, we hypothesized that participants would report fewer food items, calories, and fat as their time in the study progressed (due to a combination of the effects of the intervention and fatigue with protocol demands; hypothesis 1). In addition, we hypothesized that participants would self-monitor less on weekends than weekdays due to changes in typical week day routines (hypothesis 2) and less during the winter months as compared to the summer months due to the holiday season (hypothesis 3). By identifying time-varying factors that influence self-monitoring, behavioral interventions may be refined to reduce the impact of these factors on the frequency and comprehensiveness of self-monitoring.
METHODS
Study Design
Participants were randomized into one of three weight loss conditions as part of the ENGAGED study: (1) technology-supported, (2) standard behavioral weight loss, or (3) self-guided behavioral weight loss.18 Only participants in the technology-supported intervention were asked to self-monitor dietary intake on a study smartphone application. Thus, the current analyses focus on the self-monitoring records obtained from participants using the smartphone application.
Participants
Participants (n=32) were adults between the ages of 18-60 years with a body mass index (BMI) between 30-40 kg/m2. All participants were weight stable, not enrolled in a weight management program, and did not have any unstable medical conditions. In addition, participants were not pregnant or could not be taking any medications that may influence weight. A full list of inclusion/exclusion criteria is described elsewhere.18 Participants were recruited in two cohorts and started the intervention in either September, 2011 or April, 2012. All participants provided written informed consent and the study was approved by the Northwestern University Institutional Review Board.
Technology-Supported Intervention
Participants were given a 7% weight loss goal and encouraged to meet a calorie goal of between 1200-2000 kcal/day based on starting body weight and fat gram goal of 25% of total calories. They were also encouraged to progressively engage in 175 minutes/week of moderate-intensity physical activity. Participants attended weekly in-person group sessions during weeks 1-8 and attended sessions on Monday, Tuesday, or Wednesday based on their availability. Each session lasted approximately 90 minutes and discussed topics similar to the Diabetes Prevention Program (i.e. problem solving, stimulus control, healthy eating).19 In addition to the group sessions, participants received regular telephone calls (Monday-Friday) from a coach during the 6 month intervention.
Participants were loaned a smartphone that contained the ENGAGED application at the first group session. The ENGAGED smartphone application allows participants to self-monitor daily dietary intake, using a food database of over 50,000 generic and name brand foods. Participants received visual feedback on calories and fat grams consumed and were encouraged to self-monitor everything that they ate and drank on the application during months 1-6. Additional capabilities of the ENGAGED application have been described previously.18 Participants received brief training at the first session on how to use the application and how to best estimate portion sizes.
Measures
Baseline body weight was measured using a calibrated balance beam scale, with participants wearing light weight clothing without shoes. Daily dietary self-monitoring data was obtained from the ENGAGED smartphone application over the 6 month study period. For each day in the study, the number of foods, calories, and fat grams that were recorded was summed over each day and a daily average for each participant was calculated. Daily self-weighing measurements were also obtained from the ENGAGED smartphone application to adjust for daily weight changes.
Data Analysis
Descriptive statistics were calculated for all study variables. The data had a hierarchical structure with multiple days nested within each participant so random intercept multilevel models were estimated to accommodate the dependencies between observations from each person over time.20 The day-of-week and month-of-year for each Level-1 observation were recoded as two sets of six and 11 dummy variables, respectively. The reference day and month were selected to indicate high (unadjusted) dietary self-monitoring so the remaining model coefficients would reveal significant deviations from the average timing of peak recording. For example, participants reported the lowest fat and calorie values on Fridays and in March so they became the reference day and month in the models of those outcomes. Likewise, participants reported the greatest number of foods on Mondays and in October so they became the reference day and month in that model. Daily self-reported weights were transformed into two variables using established techniques: a starting weight on the first day (between-person variable) and a daily weight-loss progress representing the difference between a participant’s starting weight and their daily weight (within-person variable).21 The number of foods reported was transformed into two variables: a person-mean centered variable representing the average number of foods reported across days (between-person variable) and a daily deviation from that person-mean centered score representing whether a person reported more or fewer foods than usual for them that day. All analyses were completed using MPlus version 7.31 (Los Angeles, CA).
RESULTS
A total of 31 participants provided self-monitoring data during the 6 month intervention. Participants were 90% female, 42% Black, with an average age of 40.7 (SD = 10.8) years and body mass index (BMI) of 34.7 (2.8) kg/m2. The number of food items was correlated with calories and fat grams both within and between subjects (P<0.01); however, fat grams were only associated with calories within-person (Table 1). Participants recorded an average of 8.3 ± 6.1 food items, 1116 ± 408 calories, and 32.9 ± 16.6 grams of fat per day over the 6 month intervention (see Table 1).
Table 1.
Daily Self-Monitoring Characteristics and Correlations (Between- and Within-Person) for the Number of Foods, Calories, and Fat Grams Reported During the 6 Month Intervention
| Daily Self-Monitoring Characteristics |
Correlation Between Self-Monitoring
Variables Reported |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Total
Number of Person Days |
Mean |
Standard
Deviation |
Median | Mode | Range |
Number of
Food Items |
Calories |
Fat
Grams |
|
|
Number of
Food Items |
4774 | 8.3 | 6.1 | 8.0 | 0.0 | 0.0-39.0 | 0.66** | 0.53** | |
| Calories | 4439 | 1116.2 | 408.1 | 1160.6 | 205.0 | 4.0-3333.4 | 0.67** | 0.17 | |
| Fat Grams | 4439 | 32.9 | 16.6 | 32.6 | 0.0 | 0.0-327.3 | 0.47** | 0.19** | |
p<0.01
Note. Between-person and within-person correlations appear in the upper and lower halves of the correlation matrices, respectively.
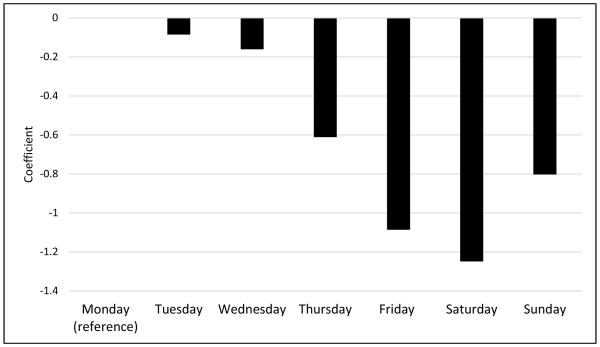
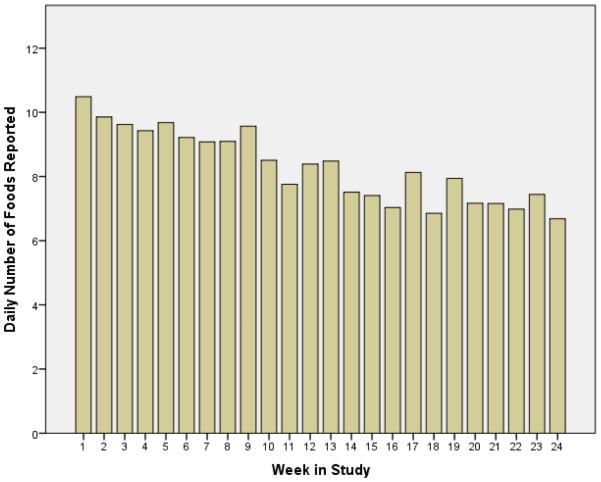
Coefficients from the multilevel model of daily food items self-monitored are presented in Table 2. A reduction in the number of foods reported within each person was seen with each successive day in the intervention (P<0.01). Figure 1 illustrates the mean number of foods recorded over time in the intervention. Similar patterns were seen with calories and fat, in that fewer calories and fat grams were reported as time in the intervention progressed (Table 3). There was also a weekend effect such that participants reported significantly fewer foods between Thursday and Sunday relative to Monday (See Figure 2). After adjusting for time in the study, the day of week, and weight change to date, participants reported more foods in January compared to the reference month of October (P <0.05); however an overall seasonality effect (winter vs. summer months) was not observed.
Table 2.
Multi-Level Model Coefficients Based on Daily Food Items Self-Monitored
| B | SE | P | |
|---|---|---|---|
| Intercept | 12.35 | 1.89 | <0.01 |
| Intervention day | −0.02 | 0.01 | <0.01 |
| Weight Lost | 0.08 | 0.06 | 0.17 |
| Sunday | −0.78 | 0.38 | 0.04 |
| Tuesday | −0.06 | 0.23 | 0.79 |
| Wednesday | −0.14 | 0.20 | 0.49 |
| Thursday | −0.61 | 0.26 | 0.02 |
| Friday | −1.07 | 0.34 | 0.002 |
| Saturday | −1.25 | 0.29 | <0.01 |
| Within-person residual variance | 15.93 | 2.08 | <0.01 |
| Age | 0.08 | 0.08 | 0.30 |
| Sex | −1.06 | 1.16 | 0.36 |
| Baseline weight | −0.03 | 0.04 | 0.35 |
| Between-person residual variance | 18.36 | 6.55 | 0.01 |
Figure 1.
The Number of Food Items Recorded Daily Decreased During a 6-Month Technology-Supported Behavioral Weight Loss Intervention
Table 3.
Multi-level modeling coefficients for both daily caloric and fat gram intake adjusting for demographics and number of daily food items reported
| Caloric Intake | Fat Gram Intake | |||||
|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | |
| Intercept | 7.52 | 1.44 | <0.01 | 29.55 | 3.72 | <0.01 |
| Intervention day | −0.01 | 0.01 | 0.04 | −0.04 | 0.02 | 0.02 |
| Daily deviations in the number of food items reported |
0.40 | 0.05 | 0.00 | 0.82 | 0.17 | <0.01 |
| Daily weight change | −0.02 | 0.03 | 0.56 | −0.06 | 0.14 | 0.67 |
| Within-person residual variance | 10.39 | 1.04 | <0.01 | 230.49 | 33.07 | <0.01 |
| Usual number of food items reported daily |
3.12 | 0.77 | <0.01 | 3.40 | 2.60 | 0.19 |
| Age | −0.04 | 0.02 | 0.07 | −0.07 | 0.09 | 0.43 |
| Sex | 1.24 | 1.07 | 0.25 | 2.29 | 2.28 | 0.32 |
| Baseline weight (kg) | 0.02 | 0.01 | 0.15 | 0.01 | 0.05 | 0.81 |
| Between-person residual variance | 1.85 | 0.39 | <0.01 | 27.94 | 11.71 | 0.02 |
Figure 2.
Adjusted Multilevel Model Coefficients by Day of the Week Based on the Number of Foods Self-Monitored
DISCUSSION
Dietary self-monitoring is a commonly-used strategy within behavioral weight loss programs; however few studies have examined the weekly and seasonal patterns of dietary technology-based self-monitoring in individuals enrolled in a weight loss trial. The current study examined within-person variations in dietary self-monitoring as a function of time-varying factors during a 6 month technology-supported weight loss intervention. Specifically, the results suggested that participants recorded fewer items and less calories and fat as time in the study progressed and on the weekends. Additionally, although participants self-monitored more food in January, there was no association between season and self-monitoring.
Consistent with our hypotheses, participants self-monitored fewer food items on the weekends as compared to the weekdays. While we speculate that the fewer items reported may be a result of lower adherence to dietary self-monitoring, we are not truly able to distinguish this from actually consuming less on the weekends. Other behaviors, such as physical activity, which can be objectively measured, are typically lower on the weekends than the weekdays.22 Additionally, diet quality has also been shown to be poorer on the weekends, including in those with overweight/obesity.11 While we know health behaviors often cluster,23 we are unable to fully explain why fewer items are self-monitored on the weekends without an objective measure of dietary intake. Self-monitoring on the weekdays as well as on the weekends are independent predictors of weight loss;13 thus, interventions may need to place additional emphasis on the importance of self-monitoring, not only on the weekdays, but also on the weekends. Text messages, for example, which have the ability to provide feedback, reminders, and encouragement to self-monitor in real-time, have shown promise to help with weight loss and self-monitoring adherence.24-26 Providing these prompts on the weekends may help improve adherence to self-monitoring recommendations.
The decrease in self-monitoring throughout an intervention is similar to previous findings.13,27-29 With each successive day in the intervention, fewer food items were recorded. It is unknown whether the number of food items reduced over time due to fatigue with the intervention demands or because individuals have been successful with weight loss, are eating fewer foods, and thus have fewer items to record each day. Future studies are needed to examine innovative ways to objectively determine exactly what an individual consumes in real-time and whether the food item is actually self-monitored.30 Advances in technology may soon allow passive monitoring of eating behaviors which will provide the ability to determine the ground truth of whether food consumption occurs.31 Further, these temporal changes in self-monitoring, and likely dietary intake, may warrant adapting behavioral strategies in real-time based on an individual’s adherence to self-monitoring or weight changes observed.
Contrary to our hypothesis, our results did not indicate that self-monitoring was lower during the winter months as compared to the summer months. Instead, the results suggested that self-monitoring did not vary across seasons, with the exception of the finding that there was an increase in the number of foods self-monitored in January as compared to the reference month of October. It is well established that adults generally will gain weight during the holidays32,33 and self-monitoring can help to better manage weight during this period.17,34 Weight loss is the most common New Year’s resolution35 and may explain the increased number of foods reported in January; however, the typical pattern of self-monitoring during the holidays is not well established. Additional studies should be completed to gain a better understanding of the more comprehensive self-monitoring observed in January in the current trial.
The results examining variations in self-monitoring due to time varying factors is not without limitations. The participants in this analysis participated in a 6 month technology-supported behavioral weight loss program and the results may not be generalizable to other populations or other modes of self-monitoring including paper or online. Additionally, the number of foods recorded may be influenced by the type and total number of foods available in the nutrition and food database used. Interventions using different food databases may have different results.
In conclusion, several time-varying factors including time in a study and day of the week are associated with individuals’ dietary self-monitoring patterns. Factors that influence these variations warrant further investigation in order to identify methods and additional strategies to better understand and improve dietary self-monitoring adherence. Current technology may enable researchers to examine intensive behavioral data to elucidate temporal relationships between behaviors, monitoring, and outcomes and how they may interact with individual differences. Next generation technology-supported interventions may leverage this information to personalize treatments, optimizing self-regulatory behaviors in a time dependent way.
IMPLICATIONS FOR RESEARCH AND PRACTICE.
Self-monitoring is a common and effective strategy for weight loss, yet little is known about the factors that influence self-monitoring consistency in adults with overweight/obesity participating in a weight management program. Time in the study and day of the week are linked with self-monitoring patterns so future research and weight loss program may need to place additional emphasis on the importance of maintaining self-monitoring on the weekends and as time in the program progresses to the extent that these behaviors are associated with weight control. If researchers can identify effective strategies for increasing self-monitoring during treatment, weight loss outcomes may be enhanced.
ACKNOWLEGEMENTS
This research was supported by grant RC1DK087126 from the National Institute of Diabetes and Digestive and in part through the Agency for Healthcare Research and Quality (K12HS023011). Support was also provided in part through the computational resources and staff contributions provided for the Social Sciences Computing cluster (SSCC) at Northwestern University. Recurring funding for the SSCC is provided by Office of the President, Weinberg College of Arts and Sciences, Kellogg School of Management, the School of Professional Studies, and Northwestern University Information Technology.
Funding: This project was supported by grant numbers RC1DK087126 from the National Institute of Diabetes and Digestive and Kidney Diseases and K12HS023011 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Baker R, Kirschenbaum D. Self-Monitoring may be necessary for successful weight control. Behav Ther. 1993;24:377–394. [Google Scholar]
- 2.Boutelle KN, Kirschenbaum DS. Further support for consistent self-monitoring as a vital component of successful weight control. Obes Res. 1998 May;6(3):219–224. doi: 10.1002/j.1550-8528.1998.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 3.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. J Am Diet Assoc. 2011 Jan;111(1):92–102. doi: 10.1016/j.jada.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009 Nov;28(6):690–701. doi: 10.1037/a0016136. [DOI] [PubMed] [Google Scholar]
- 5.Kanfer FH. Self-monitoring: Methodological limitations and clinical applications. J Consult Clin Psych. 1970;35(2):148–152. [Google Scholar]
- 6.Bandura A. Social cognitive theory of self-regulation. Organ Behav Hum Dec. 1991;50:248–287. [Google Scholar]
- 7.Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone A. Using instrumented paper diaries to document self-monitoring patterns in weight loss. Contemp Clin Trials. 2008 Mar;29(2):182–193. doi: 10.1016/j.cct.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haines PS, Hama MY, Guilkey DK, Popkin BM. Weekend Eating in the United States Is Linked with Greater Energy, Fat, and Alcohol Intake. Obes Res. 2003;11(8):945–949. doi: 10.1038/oby.2003.130. [DOI] [PubMed] [Google Scholar]
- 9.Jula A, Seppanen R, Alanen E. Influence of days of the week on reported food, macronutrient and alcohol intake among an adult population in south western Finland. Eur J Clin Nutr. 1999 Oct;53(10):808–812. doi: 10.1038/sj.ejcn.1600853. [DOI] [PubMed] [Google Scholar]
- 10.O'Dwyer NA, McCarthy SN, Burke SJ, Gibney MJ. The temporal pattern of the contribution of fat to energy and of food groups to fat at various eating locations: implications for developing food-based dietary guidelines. Public Health Nutr. 2005 May;8(3):249–257. doi: 10.1079/phn2004700. [DOI] [PubMed] [Google Scholar]
- 11.An R. Weekend-weekday differences in diet among U.S. adults, 2003–2012. Ann Epidemiol. 2016;26(1):57–65. doi: 10.1016/j.annepidem.2015.10.010. 1// [DOI] [PubMed] [Google Scholar]
- 12.Orsama AL, Mattila E, Ermes M, van Gils M, Wansink B, Korhonen I. Weight Rhythms: Weight Increases during Weekends and Decreases during Weekdays. Obesity Facts. 2014;7(1):36–47. doi: 10.1159/000356147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krukowski RA, Harvey-Berino J, Bursac Z, Ashikaga T, West DS. Patterns of success: online self-monitoring in a web-based behavioral weight control program. Health Psychol. 2013 Feb;32(2):164–170. doi: 10.1037/a0028135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoeller DA. The effect of holiday weight gain on body weight. Physiol Behav. 2014;134(0):66–69. doi: 10.1016/j.physbeh.2014.03.018. 7// [DOI] [PubMed] [Google Scholar]
- 15.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O'Neil PM, Sebring NG. A prospective study of holiday weight gain. N Engl J Med. 2000 Mar 23;342(12):861–867. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helander EE, Wansink B, Chieh A. Weight Gain over the Holidays in Three Countries. N Engl J Med. 2016 Sep 22;375(12):1200–1202. doi: 10.1056/NEJMc1602012. [DOI] [PubMed] [Google Scholar]
- 17.Boutelle KN, Kirschenbaum DS, Baker RC, Mitchell ME. How can obese weight controllers minimize weight gain during the high risk holiday season? By self-monitoring very consistently. Health Psychol. 1999 Jul;18(4):364–368. doi: 10.1037//0278-6133.18.4.364. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini CA, Duncan JM, Moller AC, et al. A smartphone-supported weight loss program: design of the ENGAGED randomized controlled trial. BMC public health. 2012 Nov 30;12(1):1041. doi: 10.1186/1471-2458-12-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes Prevention Program Research Group. The Diabetes Prevention Program Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999 Apr;22(4):623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snijders TAB, Bosker RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. Sage; Thousand Oaks, CA: 1999. [Google Scholar]
- 21.Bolger N, Davis A, Rafaeli E. Diary methods: capturing life as it is lived. Ann Rev Psychol. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- 22.Tudor-Locke C, Bassett DR, Swartz AM, et al. A Preliminary study of one year of pedometer self-monitoring. Ann Behav Med. 2004;28(3):158–162. doi: 10.1207/s15324796abm2803_3. 2004// [DOI] [PubMed] [Google Scholar]
- 23.Pronk NP, Anderson LH, Crain AL, et al. Meeting recommendations for multiple healthy lifestyle factors. Prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004 Aug;27(2 Suppl):25–33. doi: 10.1016/j.amepre.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 24.Norman GJ, Kolodziejczyk JK, Adams MA, Patrick K, Marshall SJ. Fruit and vegetable intake and eating behaviors mediate the effect of a randomized text-message based weight loss program. Prev Med. 2013;56(1):3–7. doi: 10.1016/j.ypmed.2012.10.012. 1// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro JR, Koro T, Doran N, et al. Text4Diet: A randomized controlled study using text messaging for weight loss behaviors. Prev Med. 2012;55(5):412–417. doi: 10.1016/j.ypmed.2012.08.011. 11// [DOI] [PubMed] [Google Scholar]
- 26.Burke LE, Styn MA, Sereika SM, et al. Using mHealth technology to enhance self-monitoring for weight loss: a randomized trial. Am J Prev Med. 2012 Jul;43(1):20–26. doi: 10.1016/j.amepre.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson ND, Middleton KR, Nackers LM, Medina KE, Milsom VA, Perri MG. Dietary self-monitoring and long-term success with weight management. Obesity. 2014;22(9):1962–1967. doi: 10.1002/oby.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke LE, Conroy MB, Sereika SM, et al. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011 Feb;19(2):338–344. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006 Aug 14-28;166(15):1620–1625. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 30.Scisco JL, Muth ER, Dong Y, Hoover AW. Slowing Bite-Rate Reduces Energy Intake: An Application of the Bite Counter Device. J Am Diet Assoc. 2011;111(8):1231–1235. doi: 10.1016/j.jada.2011.05.005. 8// [DOI] [PubMed] [Google Scholar]
- 31.Kalantarian H, Alshurafa N, Le T, Sarrafzadeh M. Monitoring eating habits using a piezoelectric sensor-based necklace. Comput Biol Med. 2015;58:46–55. doi: 10.1016/j.compbiomed.2015.01.005. 3/1/ [DOI] [PubMed] [Google Scholar]
- 32.Yanovski JA, Yanovski SZ, Sovik KN, Nguyen TT, O'Neil PM, Sebring NG. A Prospective Study of Holiday Weight Gain. NEJM. 2000;342(12):861–867. doi: 10.1056/NEJM200003233421206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hull HR, Radley D, Dinger MK, Fields DA. The effect of the Thanksgiving holiday on weight gain. Nutr J. 2006;5:29. doi: 10.1186/1475-2891-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker RC, Kirschenbaum DS. Weight control during the holidays: highly consistent self-monitoring as a potentially useful coping mechanism. Health Psychol. 1998 Jul;17(4):367–370. doi: 10.1037//0278-6133.17.4.367. [DOI] [PubMed] [Google Scholar]
- 35. Marist Poll: Holiday Spending Status Quo… Weight Loss Top Resolution for 2015. 2014.