Abstract
Background
Placental lesions consistent with maternal vascular underperfusion (MVU) are thought to be pathogenically linked to preeclampsia, small-for-gestational-age newborns, fetal death, and spontaneous preterm labor and delivery; yet, these lesions cannot be diagnosed antenatally. We previously reported that patients with such conditions and lesions have an abnormal profile of the angiogenic placental growth factor (PlGF) and anti-angiogenic factors [e.g., soluble vascular endothelial growth factor receptor-1 (sVEGFR-1)].
Objectives
The objectives of this study were to: 1) examine the relationship between the maternal plasma PlGF/sVEGFR-1concentration ratio (referred to herein as angiogenic index-1) and the burden of histologic placental features consistent with MVU; and 2) test the hypothesis that angiogenic index-1 can identify patients in the midtrimester who are destined to deliver before 34 weeks of gestation with multiple (i.e., 3 or more) histologic placental features consistent with MVU.
Study Design
A two-stage case-cohort sampling strategy was used to select participants from among 4,006 women with a singleton gestation enrolled from 2006 through 2010 in a longitudinal study. Maternal plasma angiogenic index-1 ratios were determined using enzyme-linked immunosorbent assays. Placentas underwent histologic examination according to standardized protocols by experienced pediatric pathologists who were blinded to clinical diagnoses and pregnancy outcomes. The diagnosis of lesions consistent with MVU was made using criteria proposed by the Perinatal Section of the Society for Pediatric Pathology. Weighted analyses were performed to reflect the parent cohort, ‘n*’ is used to reflect weighted frequencies.
Results
1) Angiogenic index-1 (PlGF/sVEGFR-1) concentration ratios were determined in 7,560 plasma samples collected from 1,499 study participants; 2) the prevalence of lesions consistent with MVU was 21% (n* = 833.9/3,904) and 27% (n* = 11.4/42.7) of women with 3 or more MVU lesions delivered before 34 weeks of gestation; 3) a low angiogenic index-1 (<2.5th quantile for gestational age) in maternal plasma samples obtained within 48 hours of delivery had a sensitivity of 73% [n* = 8.3/11.4; 95% confidence interval (CI), 47%–98%], a specificity of 94% (n* = 3130.9/3316.2; 95% CI, 94%–95%), a positive likelihood ratio (LR+) of 12.2, and a negative likelihood ratio (LR-) of 0.29 in the identification of patients who delivered placentas with 3 or more MVU lesions at <34 weeks; 4) prospectively, at 20–23 weeks of gestation, a maternal plasma concentration of angiogenic index-1 below the 2.5th quantile identified 70% (n* = 7.2/10.3; 95% CI, 42%–98%) of patients who delivered placentas with 3 or more MVU lesions before 34 weeks [specificity, 97% (n* = 2831.3/2918; 95% CI, 96%–98%); LR+, 23; LR−, 0.31]; and 5) among women without obstetrical complications who delivered at term, angiogenic index-1 was lower in women with compared to those without placental lesions consistent with MVU (p < 0.05).
Conclusion
Maternal plasma angiogenic index-1 (PlGF/sVEGFR-1) is the first biomarker for the burden of placental lesions consistent with MVU. We propose that an accumulation of these lesions in placentas delivered before 34 weeks is a histologic counterpart of an anti-angiogenic profile.
Keywords: acute atherosis, basal plate, fetal death, intervillous fibrin, persistent muscularization of the arteries, placental pathology, preeclampsia, preterm delivery, small for gestational age, soluble Flt-1, syncytial knot, villous infarction
Introduction
The uteroplacental circulation is established around the end of the first trimester [1–14]. Physiologic transformation of the spiral arteries increases the size of these vessels, allowing blood to flow into the intervillous space [15–22], where oxygen and nutrients are transported to the fetus [23–26]. Disruption of maternal vascular development is thought to result in reduced blood supply to the placenta. Histologic placental features consistent with maternal vascular underperfusion (MVU) are associated with preeclampsia [27–55], intrauterine growth restriction [35, 50, 56–67], fetal death [50, 68–81], and delivery of small-for-gestational-age (SGA) newborns [81–83]. These conditions contribute to a substantial fraction of perinatal morbidity and mortality, often mediated by indicated preterm delivery [84–87]. Uteroplacental vasculopathy is also associated with spontaneous preterm labor (PTL) with intact membranes and preterm prelabor rupture of the membranes (PPROM) [50, 88–92]; thus, maternal vascular obstructive lesions, bleeding/vessel integrity, and lack of physiologic conversion of maternal spiral arteries might constitute or interact in pathways to spontaneous as well as indicated preterm delivery [93], possibly contributing to an even larger fraction of adverse pregnancy outcomes [94]. Indeed, we propose that processes resulting in maternal vascular lesions contribute to many of the “great obstetrical syndromes” [22, 95, 96].
Our group demonstrated that an imbalance in maternal plasma concentrations of the angiogenic placental growth factor (PlGF) and anti-angiogenic factors [e.g., soluble vascular endothelial growth factor receptor-1 (sVEGFR-1)] is characteristic of a large fraction of women who have or will develop preeclampsia [44, 97–111]. We also found significant differences in angiogenic and anti-angiogenic factor distributions among women with uncomplicated pregnancies and those who are or will be affected by spontaneous PTL with intact membranes [112], fetal death [109, 113, 114], massive perivillous fibrin deposition [115], twin-to-twin transfusion syndrome [116], and delivery of SGA newborns [103, 117, 118]. Moreover, we reported that differences in angiogenic and anti-angiogenic factor concentrations are greater when patients with these obstetrical syndromes have placental lesions consistent with MVU [44, 109, 111]. Therefore, we hypothesize that the ratio of angiogenic to anti-angiogenic factor concentrations in maternal plasma reflects the burden of lesions consistent with MVU.
The objectives of this study were to: 1) examine the relationship between the maternal plasma PlGF/sVEGFR-1 concentration ratio (referred to herein as angiogenic index-1) and the burden of histologic placental features consistent with MVU, irrespective of clinical diagnosis; and 2) test the hypothesis that angiogenic index-1 can identify patients in the mid-trimester who are destined to deliver before 34 weeks of gestation with multiple (i.e., 3 or more) histologic placental features consistent with MVU.
Materials and Methods
Study design and participants
A two-stage case-cohort [119] sampling strategy was used to select participants into this prospective nested longitudinal case-cohort study from among 4,006 women with a singleton gestation. These women, who had been enrolled between 6 and 22 weeks of gestation at Hutzel Women’s Hospital, Detroit, MI, from 2006 through 2010, were followed up until delivery. Exclusion criteria were multiple gestations and any of the following at enrollment: active vaginal bleeding, obstetrical complications, serious medical illness (renal insufficiency, congestive heart disease, and chronic respiratory insufficiency), chronic hypertension requiring medication, asthma requiring systemic steroids, requirement of anti-platelet or non-steroidal anti-inflammatory drugs, active hepatitis, or fetal anomalies identified.
Figure 1 describes the selection of study participants. In the first sampling stage, 1,000 women were randomly selected from among 2,893 who had venipuncture samples collected in at least three of seven pre-defined gestational-age intervals (8–15.9, 16–19.9, 20–23.9, 24–27.9, 28–31.9, 32–36.9, and ≥ 37 weeks). In the second sampling stage, all remaining women who had any of the following diagnoses at delivery were selected from among the 3,006 women who were not selected in the first stage of sampling: preeclampsia, PTL, fetal death, PPROM, and delivery of a newborn weighing less than the 5th centile for gestational age [120]. The most centrally located venipuncture sample within each of the seven intervals defined by gestational age for each patient was used for analysis, and in cases of a tie, the first sample obtained was selected.
Figure 1. Flow diagram describing selection of participants.
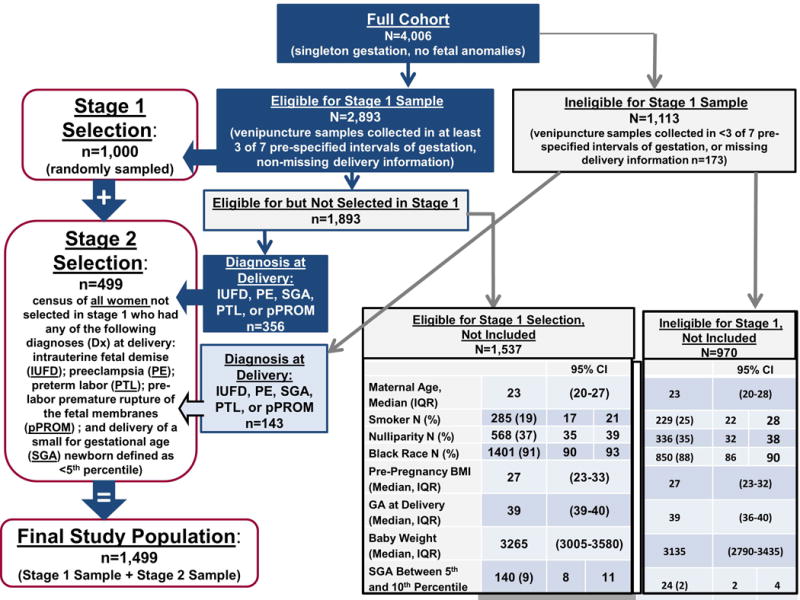
Flow diagram describing the two-stage selection of study participants.
BMI: body mass index; CI: confidence interval; GA: gestational age; IQR: interquartile range.
All patients provided written informed consent, and the use of clinical data and biological specimens for research purposes was approved by the Institutional Review Boards of Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS).
Sample collection and immunoassays
Venipuncture was performed and blood was collected into tubes containing EDTA at enrollment and during subsequent examinations scheduled every four weeks until the 24th week of gestation, and bi-weekly thereafter until delivery. Samples were centrifuged at 4°C, pipetted, and stored at −70°C. Maternal plasma concentrations of PlGF and sVEGFR-1 were determined by immunoassays as described previously (R&D Systems, Minneapolis, MN, USA) [105]. The inter- and intra-assay coefficients of variation were 1.4% and 3.9% for sVEGFR-1 and 6.02% and 4.8% for PlGF, respectively. The sensitivities of the assays were 16.97 pg/mL for sVEGFR-1 and 9.52 pg/mL for PlGF. Laboratory personnel performing the assays were blinded to clinical information.
Histologic examination
Placentas were examined histologically according to standardized protocols by perinatal pathologists blinded to clinical diagnoses and obstetrical outcomes. Briefly, three to nine sections of the placenta were examined, including at least two full-thickness sections of the placental disc, two umbilical cord sections, and one membrane roll from the extraplacental membranes. At least one full-thickness section was taken from the center of the placenta; others were taken randomly from the placental disc. Placental features consistent with MVU (referred to herein as MVU lesions) were diagnosed using criteria established by the Perinatal Section of the Society for Pediatric Pathology [121], as described in prior studies [122, 123] (Supplemental Table 1).
Clinical definitions
Preeclampsia was defined as new-onset hypertension that developed after 20 weeks of gestation and with proteinuria. Hypertension was defined as systolic ≥140 and/or diastolic ≥90 mmHg blood pressure, measured at two occasions, four hours to one week apart [124, 125]. Proteinuria was defined as a urine protein of ≥300 mg in a 24-hour urine collection, or two random urine specimens, obtained four hours to one week apart, showing ≥1+ by dipstick [125, 126]. Gestational hypertension was defined as blood pressure ≥140/90 mm Hg detected at >20 weeks of gestation without proteinuria, and without emergence of preeclampsia by 12 weeks’ postpartum [47]. Delivery of SGA neonates was classified by birth weight <10th percentile for gestational age at delivery according to a U.S. reference population and did not include co-occurring preeclampsia [120]. The diagnosis of PPROM was determined with a sterile speculum examination with documentation of either vaginal pooling or positive nitrazine or ferning tests. Spontaneous PTL was defined as the spontaneous onset of labor with intact membranes and delivery that occurred before the 37th week of gestation. Fetal death was defined as intrapartum or antepartum death of a fetus prior to delivery at 20 or more weeks of gestation, which was not a consequence of an induced termination of pregnancy [126].
Statistical analysis
The quantreg package [127, 128] under the R statistical environment [129] was used to fit locally weighted linear quantile regression models to estimate reference biomarker quantiles (i.e., centiles) as a function of gestational age at venipuncture, using information from women selected in the first stage of sampling who did not develop preeclampsia or deliver SGA newborns. A total of 100 models were fit to estimate quantiles for 100 equally spaced gestational ages occurring between the maximum and minimum observed values. Each model, for each specific gestational age estimate, used the entirety of data (i.e., measurements across all gestational ages); yet, different weights were used for each measurement in each model. The weights for each measurement were determined using a Gaussian distribution, so that it decreased exponentially as a function of the distance between the gestational age of each measurement and that for which the quantiles were estimated. The spread of the Gaussian distribution was set such that the weights of measurements that were three weeks away from the gestational age for which centiles were estimated were about 50% of that for measurements at the targeted gestational age. The resulting locally estimated centiles were then smoothed using a 5th-degree polynomial function of gestational age, to allow interpolation of the centiles at any desired value of gestational age.
Weighting was used to reflect the parent cohort: 723 of 747 patients with conditions identified in the parent cohort and over-selected into the case-cohort had venipuncture samples available, and these patients received a weight of 1.03; 776 of 3,259 patients without over-selected conditions were included in the case-cohort study, and these patients received a weight of 4.20. The sum of weighted frequencies is equal to the total sample size of the parent cohort (n = 4,006); ‘n*’ is used to denote weighted frequencies, whereas ‘n’ is used to denote unweighted frequencies.
Locally weighted regression models were fit for descriptive purposes to show between-group differences in biomarker concentration ratio (PlGF/sVEGFR-1) distributions expressed as a function of gestational age at venipuncture. These models were not weighted by inverse sampling probability, since we wanted to show actual patient-specific biomarker profiles expressed as a function of gestational age, and thought it most appropriate that the accompanying group mean patterns directly characterize the observed patient-specific profiles [hence, confidence intervals (CI) are wider and, thus, more likely to overlap, indicating bias toward the null hypothesis—no association). Otherwise, analyses were performed with inverse probability weighting.
Generalized linear models with robust estimators of variance were fit to examine differences in biomarker ratios adjusting for potentially confounding factors. Proportions and 95% CIs or medians and interquartile ranges were calculated to describe categorical and arithmetic variables, respectively. Binomial and multinomial logistic regression models were fit to determine magnitudes of association. A positive likelihood ratio (LR+) was calculated as, sensitivity ÷ (1-specificity). A negative likelihood ratio (LR-) was calculated as (1-sensitivity) ÷ specificity. Statistical significance was defined using a 5% threshold for Type I error [i.e., p < 0.05 and 95% CI for magnitudes of association that do not include the null hypothesis (i.e., an odds ratio (OR) of 1.0)]. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA) [130].
Results
Descriptive characteristics
The maternal plasma angiogenic index-1 concentration ratio was determined in 7,560 venipuncture samples collected across gestation from the 1,499 women included in this study (Figure 1). Descriptive characteristics of the case-cohort and the parent cohort study populations are presented in Table 1: 92% of patients selected into the case-cohort were identified as African American, 39% were nulliparous, and their median gestational age at delivery was 39.3 weeks, closely reflecting the distributions in the parent cohort (91%, 37%, and 39.1, respectively). Subsequent analyses used information from the case-cohort study population.
Table 1.
Descriptive characteristics of the study population
| Stage 1 random sample | Stage 2 over-sample | Weighted case-cohorta | Parent cohort | |
|---|---|---|---|---|
| Descriptive Characteristic | (n = 1,000) | (n = 499) | (n = 1,499) | (n = 4,006) |
| Age, y, median (IQR) | 23 (20–27) | 23 (20–28) | 23 (20–27) | 23 (20–27) |
| African American race | 93% (n = 27) | 93% (n = 463) | 92% (n* = 3700) | 91% (n = 3641) |
| Nulliparity | 38% (n = 384) | 39% (n = 195) | 39% (n* = 1,545) | 37% (n = 1483) |
| Pre-pregnancy BMI, median (IQR) | 26.6 (22.5–32.5) | 26.8 (22.7–32.5) | 26.8 (22.6–32.6) | 26.8 (22.6–32.4) |
| Any tobacco smoking during pregnancy | 21% (n = 207) | 25% (n = 124) | 20% (n* = 801) | 22% (n = 845) |
| PTL with intact membranes | 7% (n=65) | 28% (n=137) | 5% (n=209.7) | 5% (n=209) |
| PPROM | 3% (n=34) | 16% (n=78) | 3% (n*=115.7) | 3% (n=115) |
| Preeclampsia | 8% (n=77) | 30% (n=147) | 6% (n*=231) | 6% (n=224) |
| SGA newborn | 15% (n=149) | 37% (n=185) | 14% (n*=541) | 13% (n=502) |
| Gestational hypertension | 9% (n=94) | 5% (n=26) | 10% (n*384) | 8% (n=341) |
| GA at delivery, wk, median (IQR) | 39.1 (37.9–40.1) | 36.4 (33.6–38.4) | 39.3 (38–40.3) | 39.1 (37.7–40.1) |
| Birthweight, g, median (IQR) 3170 | 3170 (2792–3482) | 2370 (1800–2745) | 3190 (2830–3490) | 3150 (2765–3475) |
n* denotes weighting to reflect the parent cohort.
BMI: body mass index; GA: gestational age; IQR: interquartile range; PPROM: preterm prelabor rupture of the membranes; PTL: preterm labor; SGA: small for gestational age.
Column proportions or median plus IQR ranges are reported.
The prevalence of having 1, 2, or 3 or more placental lesions consistent with MVU was 15.9% (n* = 620/3904), 4.3% (n* = 171.2/3904), and 1.1% (n* = 42.7/3904), respectively; Table 2 shows the specific lesions and clinical diagnoses of women in each of these groups. Increased syncytial knots and increased intervillous fibrin were the lesions consistent with MVU most frequently diagnosed among women with 2 or more of such histologic placental features.
Table 2.
Characteristics of patients who deliver a placenta with 0, 1, 2, or ≥3 placental features consistent with MVU
| Histologic placental feature/clinical diagnosis | No. of MVU features identified | |||
|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | |
| n* = 3070 | n* = 620 | n* = 171 | n* = 43 | |
| % | % | % | % | |
| Villous changes | ||||
| Remote villous infarct, n* = 60.5 | — | 5.4 | 9.1 | 26.8 |
| Recent villous infarct, n* = 32.3 | — | 2.4 | 7.3 | 12.1 |
| Increased syncytial knots, n* = 356.2 | — | 30.5 | 76.3 | 85.3 |
| Villous agglutination, n* = 43.4 | — | 1.5 | 13.4 | 26.9 |
| Increased intervillous fibrin, n* = 260 | — | 22.4 | 58.0 | 44.0 |
| Distal villous hypoplasia, n* = 14.5 | — | 0.3 | 3.0 | 16.9 |
| Vascular lesions | ||||
| Persistent muscularization of the basal plate arteries, n* = 223.7 | — | 29.0 | 13.4 | 48.9 |
| Mural hypertrophy of the decidual arterioles, n* = 61.6 | — | 5.1 | 11.6 | 24.4 |
| Acute atherosis of the basal plate arteries and/or decidual arterioles, n* = 45.98 | — | 3.4 | 7.9 | 26.8 |
| Clinical diagnosis at delivery | ||||
| Preeclampsia, n* = 231.4 | 4.0 | 8.0 | 16.9 | 48.4 |
| Small for gestational age, n* = 541.1 | 10.9 | 19.5 | 26.7 | 46.1 |
| Preterm labor with intact membranes, n* = 209.7 | 4.5 | 7.7 | 6.0 | 4.8 |
| Preterm prelabor rupture of the membranes, n* = 115.7 | 2.6 | 3.5 | 4.2 | 0.0 |
| Fetal death, n* = 24.8 | 0.4 | 1.3 | 1.8 | 4.8 |
n* Denotes weighted frequencies; column proportions are reported; the higher the proportion, the more frequent the characteristic was identified among women in each column.
MVU: maternal vascular underperfusion.
Burden of histologic placental features consistent with MVU and gestational age at delivery
We first tested the hypothesis that an accumulating burden (i.e., increasing number) of MVU lesions in the placenta would be associated with increasing risk of early versus late preterm delivery. Table 3 shows the magnitudes of association (OR) between the number of histologic placental features consistent with MVU and gestational age at delivery. The greater the number of histologic features consistent with maternal vascular obstruction, the stronger the magnitude of association (OR) with having delivered preterm, particularly before 34 weeks of gestation. Women whose placentas had three or more histologic features consistent with MVU were 2.8 (95% CI, 1.1–7), 13.4 (95% CI, 5.9–30), and 8.1 (95% CI, 2.4–28) times more likely than those without such features to have delivered preterm at 34.1–36.9, 28–34.0, or 20–27.9 weeks of gestation, respectively. Women with two placental features consistent with MVU were 1.8 (95% CI, 1.1–3), 4.9 (95% CI, 2.7–9), and 7.2 (95% CI, 3.8–14) more likely than those without such lesions to have delivered at 34.1–36.9, 28–34.0, or 20–27.9 weeks. Multivariable adjustment for maternal age, race, nulliparity, and pre-pregnancy body mass index did not appreciably alter the association between burden of MVU and gestational age at delivery (see Supplemental Table 2).
Table 3.
Magnitudes of association between gestational interval at preterm delivery and the number of histologic placental features consistent with maternal vascular underperfusion
| No. of histologic features consistent with MVU, n | GA 20–27.9 weeks n* = 80.7 |
GA 28–33.9 weeks n* = 133.4 |
GA 34–36.9 weeks n* = 292.3 |
|||
|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| 0 (n*=3070) | 1 | reference | ||||
| 1 (n*=620) | 2.7 | (1.6–4.7) | 3.4 | (2.3–5) | 1.6 | (1.2–2.2) |
| 2 (n*=171.2) | 7.2 | (3.8–14) | 4.9 | (2.7–8.8) | 1.8 | (1.1–3.1) |
| ≥3 (n*=42.7) | 8.1 | (2.4–28) | 13.4 | (5.9–30) | 2.8 | (1.1–7) |
n* denotes weighted frequencies; the reported magnitudes of association (OR) were calculated using a multinomial logistic regression model that estimates the relative odds of delivering placentas before 34 weeks of gestation with 0, 1, 2, or ≥3 lesions consistent with MVU (i.e., reference is delivery ≥34 weeks) comparing women with low angiogenic index-1 ratios to those with higher ratios determined at 20–23 weeks of gestation. CIs that do not include “1.0” are statistically significant.
CI: confidence interval; GA: gestational age; MVU: maternal vascular underperfusion; OR, odds ratio.
Angiogenic index-1 and MVU lesions in patients who deliver within 48 hours of venipuncture
To determine whether there was a relationship between angiogenic index-1 and the burden of placental lesions consistent with MVU, we first restricted the analysis to women who had blood samples collected within 48 hours of delivery to maintain a meaningful temporal relationship between maternal plasma concentrations of angiogenic and anti-angiogenic factors and histologic findings in the placenta. Of the 1,499 women in this study, 1,204 (80%) had samples obtained within 48 hours of delivery. The weighted proportions of these women who had 1, 2, or 3 or more lesions consistent with MVU were 16% (n* = 541.8/3327.6), 4% (n* = 145.2/3327.6), and 1.2% (n* = 38.6/3327.6), respectively.
The odds of having 0, 1, 2, or 3 or more placental lesions comparing women with and without angiogenic index-1 ratios below the 10th reference quantile for gestational age within 48 hours of delivery differed significantly before 34 weeks and after 34 weeks of gestation, with and without multivariable adjustment for potential confounders (p < 0.0001 for both). Patients with angiogenic index-1 ratios below the 10th reference quantile for gestational age within 48 hours of delivery were 2 (95% CI, 1.3–4), 8 (95% CI, 5–15), 10 (95% CI, 4–24), and 17 (95% CI, 5–64) times more likely than those with higher ratios to deliver before 34 weeks with 0, 1, 2, or 3 or more placental lesions consistent with MVU, adjusting for maternal age, race, nulliparity, and pre-pregnancy body mass index. Figure 2 illustrates the weighted proportions of patients who delivered placentas with 0, 1, 2, or 3 or more MVU lesions before 34 weeks and after 34 weeks of gestation with angiogenic index-1 ratios below the 2.5th reference quantile for gestational age. The greater the number of MVU lesions, the higher the proportion of patients with low angiogenic index-1 ratios (Cochran-Armitage Trend Test; p < 0.0001 for both comparisons). The angiogenic index-1 ratio was below the 2.5th reference quantile for gestational age within 48 hours of delivery in 73% of patients with three or more lesions consistent with MVU who delivered before 34 weeks [(n* = 8.3/11.4; 95% CI. 47%–98%), specificity, 94% (n* = 3130.9/3316.2; 95% CI, 94%–95%); LR+, 12.2; LR− 0.29)]. In contrast to women who delivered before 34 weeks, a smaller fraction of those who delivered after 34 weeks with MVU lesions had angiogenic index-1 ratios below the 2.5th reference quantile for gestation within 48 hours of delivery (Figure 2).
Figure 2. Proportion with low biomarker ratios who do and do not deliver before 34 weeks by number of placental malperfusion features.
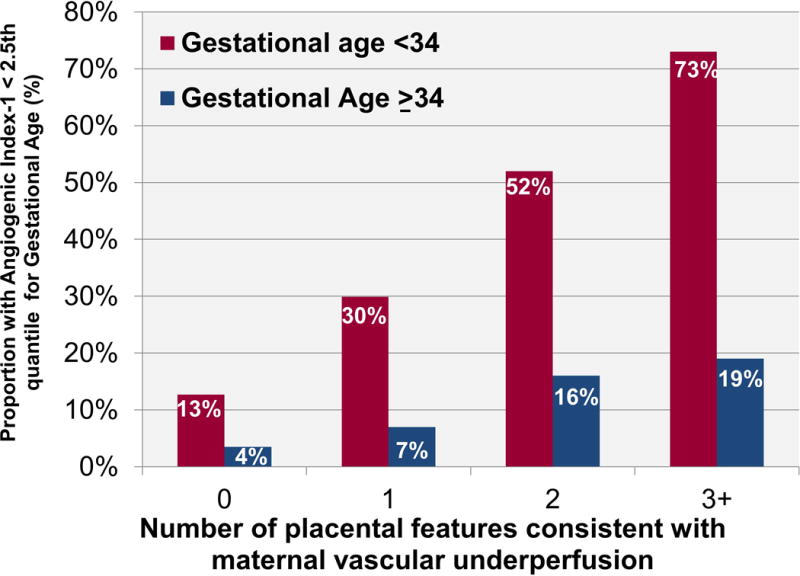
Weighted percentage of patients who delivered placentas <34 weeks and at ≥ 34 weeks of gestation with 0, 1, 2, or 3 or more histologic placental features consistent with maternal vascular underperfusion with angiogenic index-1 ratios within 48 hours of delivery below the 2.5th reference quantile for gestational age.
Plasma angiogenic index-1 concentration ratios and delivery of placentas with histologic features consistent with MVU before 34 weeks
Given the association between the increasing burden of MVU and early preterm delivery (i.e., before 34 weeks) and the association between low angiogenic index-1 ratios within 48 hours of delivery and the increasing burden of MVU, we sought to determine whether low mid-pregnancy angiogenic index-1 ratios are associated with higher risks of delivering placentas at <34 weeks with the increasing burden of MVU. Table 4 describes the relative odds of delivering placentas before 34 weeks of gestation with 0, 1, 2, or 3 or more histologic placental features consistent with MVU, comparing women with low (i.e., <2.5th or <10th reference quantile) to those with higher plasma angiogenic index-1 ratios at 20–23 weeks of gestation. The magnitude of association (OR) with a low angiogenic index-1 strengthened with increasing numbers of histologic indicators of MVU in the placenta (i.e., dose-response association). Women with biomarker ratios below the 10th reference quantile at 20–23 weeks of gestation were 6.6 (95% CI, 3.7–12), 7.4 (95% CI, 3–18), and 19 (95% CI, 5–73) times more likely than those with higher ratios to deliver placentas before 34 weeks with 1, 2, or 3 or more placental features consistent with MVU, respectively. Multivariable adjustment for potential confounders did not meaningfully alter the estimated magnitudes of association (Supplemental Table 3). When excluding women who developed preeclampsia or delivered an SGA newborn, those with low compared to high biomarker ratios were still significantly more likely to deliver a placenta before 34 weeks with features consistent with MVU (OR, 4.4; 95% CI, 2.3–8.5), but not without placental features consistent with MVU (OR, 1.4; 95% CI, 0.7–2.9).
Table 4.
Magnitudes of association between plasma angiogenic index-1 (PlGF/sVEGFR-1) concentration quantile at 20–23 weeks of gestation and delivery of placentas before 34 weeks of gestation with 0, 1, 2, or ≥3 histologic features consistent with MVU
| Gestational age at delivery before 34 weeks with | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 MVU | 1 MVU | 2 MVU | ≥3 MVU | |||||
| n* = 77.6 | n* = 46.6 | n* = 19.6 | n* = 10.3 | |||||
| Angiogenic index-1 ratio | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) |
| ˂10th quantile n* = 348 | 1.4 | (0.7–2.7 | 6.6 | (3.7–12) | 7.4 | (3.0–18) | 19.3 | (5.1–73 |
| ≥10th quantile n* = 2580.3 | 1 | Reference | ||||||
| ˂2.5th quantile n* = 93.8 | 1.6 | (0.5–5.2) | 7.2 | (3.1–16) | 18 | (6.7–48) | 91 | (24–352) |
| ≥2.5th quantile n* = 2834.4 | 1 | Reference | ||||||
Cis that do not include “1.0” are statistically significant.
CI: confidence interval; MVU: maternal vascular underperfusion; OR: odds ratio; PlGF: placental growth factor; sVEGFR-1: soluble vascular endothelial growth factor receptor-1.
The specific lesions consistent with MVU most strongly associated with low angiogenic index-1 at 20–23 weeks of gestation were recent villous infarct, distal villous hypoplasia, and acute atherosis of the basal plate arteries and/or decidual arterioles (Supplemental Table 4). These three lesions were also more common among those with three or more histologic features consistent with MVU who delivered before 34 weeks as compared to those who delivered after 34 weeks [recent villous infarct: 27%, n* = 3.1/11.4 versus 7%, n* = 2.1/31.3; distal villous hypoplasia: 55%, n* = 6.2/11.4 versus 3%, n* = 1/31.3; and acute atherosis of the basal plate arteries and/or decidual arterioles: 36%, n* = 4.1/11.6 versus 23%, n* = 7.3/31.3].
Women who delivered placentas with 1, 2, or 3 or more histologic features consistent with MVU before 34 weeks had plasma angiogenic index-1 concentration ratios around the expected median before 15 weeks of gestation (Figure 3A). By contrast, at 20–23 weeks, 70% of patients with samples in this interval who delivered placentas with 3 or more features consistent with MVU before 34 weeks had biomarker ratios below the 2.5th reference quantile (Figure 3B) ([n* = 7.2/10.3; 95% CI, 42%–98%]; specificity, 97% [n = 2831.3/2918; 95% CI, 96%–98%]; LR+, 23; LR−, 0.31). No patient who delivered a placenta before 34 weeks with three or more histologic indicators of MVU had sVEGFR-1 concentrations below the 2.5th reference quantile, and only half had PlGF concentrations below the 2.5th reference quantile. The pattern of association between angiogenic index-1 at 24–28 weeks and subsequent delivery of placentas before 34 weeks with two MVU features was similar to the pattern observed at 20–23 weeks for patients whose placenta had three or more MVU lesions: 63% (n* = 10.3/19.6; 95% CI, 31%–75%) of women who delivered a placenta with two MVU lesions before 34 weeks of gestation had an angiogenic index-1 below the 10th reference quantile (Figure 3C), and 53% (n* = 10.3/19.6; 95% CI, 31%–74%) had ratios below the 2.5th reference quantile for gestational age. None of the patients who delivered a placenta before 34 weeks with two MVU lesions had sVEGFR-1 concentrations below the 2.5th reference quantile, and 47% had PlGF concentrations below the 2.5th reference quantile.
Figure 3. Biomarker ratios of patients with multiple placental lesions who deliver before 34 weeks.
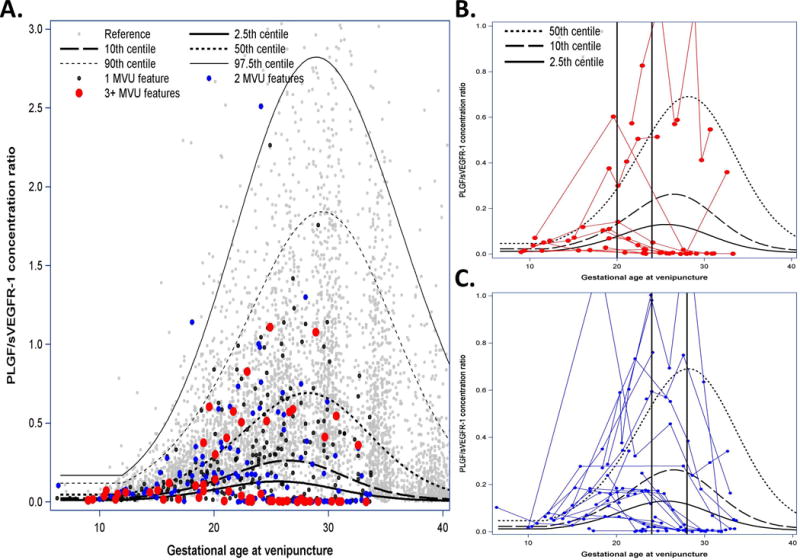
Angiogenic index-1 [placental growth factor (PlGF)/soluble vascular endothelial growth factor receptor-1 (sVEGFR-1)] concentration ratios among women destined to deliver a placenta before 34 weeks of gestation with 1, 2, or 3 or more histologic features consistent with maternal vascular underperfusion (MVU). Vertical axis represents the maternal plasma PlGF/sVEGFR-1 ratio concentration; horizontal axis represents gestational age at venipuncture; black lines represent estimated reference population quantiles for gestational age at venipuncture; and light gray dots represent reference population measurements. A. Black, blue, and red dots represent biomarker ratio concentrations of patients destined to deliver a placenta before 34 weeks of gestation with 1, 2, or 3 or more histologic features consistent with MVU. B. Red lines describe patient-specific profiles over time for patients who delivered a placenta before 34 weeks with 3 or more histologic features consistent with MVU. C. Blue lines indicate patients delivering a placenta before 34 weeks with 2 such histologic features. Vertical black lines are provided for reference pertaining to descriptions in the main text.
Plasma angiogenic index-1 concentration ratios and delivery of placentas with histologic features consistent with MVU at ≥34 weeks
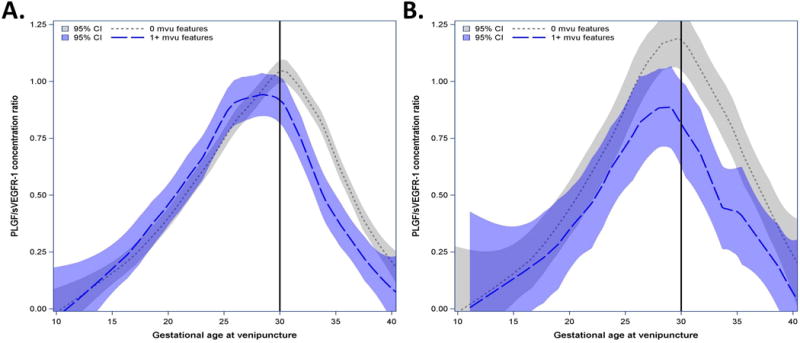
The profile of angiogenic index-1 ratios also differed over time among patients who delivered at ≥34 weeks of gestation as a function of the burden of lesions consistent with MVU (Figure 4A). Two parameters can be used to describe the angiogenic index-1 ratio profile: the magnitude of the peak and the gestational age at which such a peak occurs. Patients without MVU lesions reached a peak of the ratio of angiogenic index-1 of 1.0 at 30 weeks of gestation. In contrast, patients with one or two lesions had a mean peak ratio of 0.77 observed at 28 weeks. Patients with three or more MVU lesions reached a lower peak ratio (0.62) at an earlier gestational age (27 weeks). Figures 4B and 4C display serial measurements for patients who had three or more, or one to two, placental lesions consistent with MVU, respectively; by comparing Figures 3B and 4B, and by comparing Figures 3C and 4C, it is apparent that the decrease in angiogenic index-1 occurs later in gestation in patients who deliver at ≥34 weeks than in those who deliver before 34 weeks.
Figure 4. Biomarker ratios of patients with multiple placental lesions who deliver ≥ weeks.
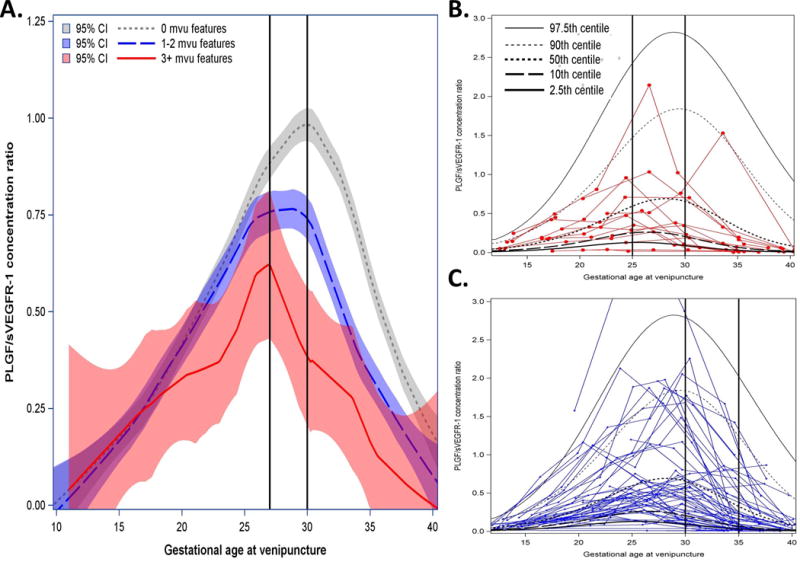
Mean maternal plasma angiogenic index-1 [placental growth factor (PlGF)/soluble vascular endothelial growth factor receptor-1 (sVEGFR-1)] concentration ratios with 95% confidence intervals (CI) estimated by locally weighted regression as a function of gestational age at venipuncture and patient-specific profiles for those who delivered a placenta with B, ≥3, or C, 2 maternal vascular underperfusion (MVU) features. Vertical axis represents the maternal plasma PlGF/sVEGFR-1 ratio concentration; horizontal axis represents gestational age at venipuncture. A. Estimated mean biomarker ratio for women who deliver a placenta at >34 weeks with 0, 1–2, or ≥3 histologic features consistent with MVU. B. Red lines show patient-specific profiles over time for patients who delivered a placenta ≥34 weeks with ≥3 histologic features consistent with MVU. C. Blue lines for patients delivering placentas ≥34 weeks with 2 such histologic features. Vertical black lines are provided for reference pertaining to descriptions in the main text.
Plasma angiogenic index-1 concentration ratios among patients without obstetrical complications who delivered a placenta at term with and without histologic features consistent with MVU
Lastly, to determine whether angiogenic index-1 ratios reflect the burden of MVU among women without clinical diagnoses, we examined differences in patients with and without such lesions in two sub-cohorts: 1) among women without major obstetrical complications (n = 560, n* = 2323.4), and 2) further restricting the analysis to women without any obstetrical complications or other medical conditions (n = 111, n* = 466.2). Major pregnancy complications warranting exclusion from the first sub-cohort included the following: PTL, PPROM, SGA, preeclampsia, eclampsia/HELLP syndrome, fetal death, acute pyelonephritis, placental abruption, gestational hypertension, and gestational or pre-diabetes mellitus. The second sub-cohort was restricted to women who met the following conditions: absence of any obstetrical complications throughout gestation; absence of chronic disease (e.g., chronic hypertension, diabetes, kidney disease, thyroid disease, asthma, autoimmune disease, coagulopathies); absence of active infection (i.e., sexually transmitted diseases or clinical chorioamnionitis); and no illicit drug use (narcotics, barbiturates or opiates).
Among women without major obstetrical complications (Figure 5A), the mean angiogenic index-1 ratios were significantly lower after 30 weeks of gestation among women with term deliveries whose placentas would have one or more versus no histologic placental features consistent with MVU, as indicated by non-overlapping CIs from 30–38 weeks of gestation. A generalized linear model with inverse sampling probability weighting showed that angiogenic index-1 ratios remained lower from 30 weeks of gestation onward among women with rather than those without lesions consistent with MVU (p = 0.003), adjusting for maternal age, race, nulliparity, and pre-pregnancy body mass index. Figure 5B shows that a consistent pattern was observed when the analysis was further restricted to patients who had neither obstetrical nor other medical complications (i.e., non-overlapping CIs from 30–35 weeks of gestation; p < 0.05 in inverse sampling probability weighted analysis testing whether biomarker ratios were lower from 30 weeks of gestation onward among women with rather than without MVU lesions).
Figure 5. Biomarker ratios of patients delivered at term without complications who did or did not have placental lesions.
Mean maternal plasma angiogenic index-1 [placental growth factor (PlGF)/soluble vascular endothelial growth factor receptor-1 (sVEGFR-1)] concentration ratios with 95% confidence intervals (CI) estimated by locally weighted regression as a function of gestational age at venipuncture for patients without major obstetrical complications. Vertical axis represents the maternal plasma PlGF/sVEGFR-1 ratio concentration; horizontal axis represents gestational age at venipuncture. A. Estimated mean biomarker concentration ratios for women who deliver at term without major complications with and without histologic features consistent with maternal vascular underperfusion (MVU). B. After excluding patients with any obstetrical or other medical conditions across gestation.
Comment
Principal findings
The central finding of this study is that maternal plasma concentrations of angiogenic index-1 (PlGF/sVEGFR-1) reflect the burden of lesions consistent with MVU in the placenta. This is the first report of a biomarker measured in maternal plasma that can identify patients who will deliver a placenta before 34 weeks of gestation with an accumulation of these specific lesions, irrespective of clinical diagnosis. Importantly, the PlGF/sVEGFR-1 ratio (or angiogenic index-1 ratio) can identify such patients in advance of early preterm delivery, and this has potential diagnostic, prognostic, and therapeutic value.
An abnormal angiogenic/anti-angiogenic profile in maternal blood, pregnancy complications, and MVU
There is a growing body of evidence indicating that an imbalance in maternal plasma concentrations of angiogenic and anti-angiogenic factors is characteristic of a substantial fraction of women who have or will develop preeclampsia [44, 97–107, 110, 111, 130–168], fetal death [114, 115, 169–173], SGA [64, 102–104, 117, 173–178], massive perivillous fibrin deposition [115, 178], twin-to-twin transfusion syndrome [118, 179, 180], and mirror syndrome [181]. We have also found significant differences in angiogenic marker distributions among uncomplicated pregnancies and those who are or will be affected by spontaneous PTL with intact membranes [112]. Histologic placental features consistent with MVU are also more common among women with such conditions, particularly those with preeclampsia who deliver before 34 weeks of gestation [40, 41, 182]. These histologic features are also found in placentas delivered by women with spontaneous PTL with intact membranes or PPROM [50, 88–92]. It is possible that extensive lesions predispose to reduced perfusion of the intervillous space, ischemia, and compensatory maternal hypertension, whereas less extensive lesions consistent with MVU that are insufficient to induce maternal hypertension may predispose to PTL/preterm delivery [22]. In this view, both PTL with intact membranes and PPROM might involve subgroups formed according to vascular pathways, infection, or both [88, 183]. Indeed, maternal vascular obstructive lesions, bleeding/vessel integrity, and lack of physiologic conversion of maternal spiral arteries might constitute or interact in pathways to both spontaneous and indicated preterm delivery [93].
In this longitudinal case-cohort study, we examined the relationship between maternal plasma angiogenic/anti-angiogenic factor concentration ratios and the presence or accumulation of histologic placental features consistent with MVU. We found two compelling dose-response relationships: 1) the greater the number of MVU lesions, the stronger the association with early preterm delivery (i.e., <34 weeks), and 2) low mid-trimester maternal plasma angiogenic index-1 concentration ratios (i.e., <10th or <2.5th reference quantiles) were strongly associated with increasing odds of delivering placentas before 34 weeks with 1, 2, or 3 or more histologic features consistent with MVU. Moreover, we found significant differences in distributions of plasma angiogenic index-1 concentration ratios among women with compared to those without such histologic features in placentas delivered at term, even when excluding women with any obstetrical complication or other medical conditions. Maternal plasma angiogenic index-1 concentration ratios thus seem to reflect the burden of uteroplacental vasculopathy indicated by histologic examination of the placenta, irrespective of clinical diagnosis. The time order between changes in plasma angiogenic index-1 concentration ratios and placental features consistent with maternal vasculopathy, however, remains to be determined.
Our results are consistent with evidence suggesting different histologic features related to placental disease in early versus late gestations [43, 65, 184, 185]. The results of this study also support the view that angiogenic markers reflect pathways to preterm delivery that do and do not relate to preeclampsia [186].
Strengths and weaknesses
The major strength of this study is the use of a prospective nested longitudinal case-cohort design that allowed inclusion of all 4,006 women in the parent cohort who developed major pregnancy complications of interest. Another strength is that the placentas of women without indications underwent histologic examination by pediatric pathologists blinded to clinical information and pregnancy outcome, unlike in most centers. Limitations include that some patients with venipuncture samples collected in less than three of seven gestational intervals who were ineligible for the first sampling stage possibly introduced bias. However, we included a complete census of all patients in the parent cohort who developed any of the “great obstetrical syndromes,” regardless of the number of samples available, and the reference population was selected irrespective of pregnancy outcome, reducing the chance of differential selection bias. Limitations rooted in missing data, the number of patients whose placentas had three or more histologic features consistent with uteroplacental underperfusion, unknown generalizability to dissimilar socio-demographic populations, and the inability to discern causation from association are also acknowledged.
Conclusion
Maternal plasma angiogenic index-1 (PlGF/sVEGFR-1) reflects the burden of placental lesions consistent with MVU. We propose that an accumulation of these lesions in placentas delivered before 34 weeks is a histologic counterpart of an anti-angiogenic profile.
Supplementary Material
Acknowledgments
Financial Support: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
Footnotes
Disclosure: The authors report no conflict of interest
References
- 1.HUSTIN J, SCHAAPS JP. Echographic [corrected] and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol. 1987;157:162–168. doi: 10.1016/s0002-9378(87)80371-x. [DOI] [PubMed] [Google Scholar]
- 2.HUSTIN J, SCHAAPS JP, LAMBOTTE R. Anatomical studies of the utero-placental vascularization in the first trimester of pregnancy. Troph Res. 1988;1988:49–60. [Google Scholar]
- 3.JAUNIAUX E, JURKOVIC D, CAMPBELL S. In vivo investigations of the anatomy and the physiology of early human placental circulations. Ultrasound Obstet Gynecol. 1991;1:435–445. doi: 10.1046/j.1469-0705.1991.01060435.x. [DOI] [PubMed] [Google Scholar]
- 4.JAFFE R, WARSOF SL. Transvaginal color Doppler imaging in the assessment of uteroplacental blood flow in the normal first-trimester pregnancy. Am J Obstet Gynecol. 1991;164:781–785. doi: 10.1016/0002-9378(91)90515-s. [DOI] [PubMed] [Google Scholar]
- 5.JAUNIAUX E, JURKOVIC D, CAMPBELL S, HUSTIN J. Doppler ultrasonographic features of the developing placental circulation: correlation with anatomic findings. Am J Obstet Gynecol. 1992;166:585–587. doi: 10.1016/0002-9378(92)91678-4. [DOI] [PubMed] [Google Scholar]
- 6.JAFFE R, WOODS JR., Jr Color Doppler imaging and in vivo assessment of the anatomy and physiology of the early uteroplacental circulation. Fertil Steril. 1993;60:293–297. doi: 10.1016/s0015-0282(16)56100-7. [DOI] [PubMed] [Google Scholar]
- 7.COPPENS M, LOQUET P, KOLLEN M, DE NEUBOURG F, BUYTAERT P. Longitudinal evaluation of uteroplacental and umbilical blood flow changes in normal early pregnancy. Ultrasound Obstet Gynecol. 1996;7:114–121. doi: 10.1046/j.1469-0705.1996.07020114.x. [DOI] [PubMed] [Google Scholar]
- 8.MERCE LT, BARCO MJ, BAU S. Color Doppler sonographic assessment of placental circulation in the first trimester of normal pregnancy. J Ultrasound Med. 1996;15:135–142. doi: 10.7863/jum.1996.15.2.135. [DOI] [PubMed] [Google Scholar]
- 9.VALENTIN L, SLADKEVICIUS P, LAURINI R, SODERBERG H, MARSAL K. Uteroplacental and luteal circulation in normal first-trimester pregnancies: Doppler ultrasonographic and morphologic study. Am J Obstet Gynecol. 1996;174:768–775. doi: 10.1016/s0002-9378(96)70462-3. [DOI] [PubMed] [Google Scholar]
- 10.KURJAK A, KUPESIC S. Doppler proof of the presence of intervillous circulation. Ultrasound Obstet Gynecol. 1996;7:463–464. doi: 10.1046/j.1469-0705.1996.07060461-3.x. [DOI] [PubMed] [Google Scholar]
- 11.JAFFE R, JAUNIAUX E, HUSTIN J. Maternal circulation in the first-trimester human placenta—myth or reality? Am J Obstet Gynecol. 1997;176:695–705. doi: 10.1016/s0002-9378(97)70572-6. [DOI] [PubMed] [Google Scholar]
- 12.BURTON GJ, JAUNIAUX E, WATSON AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 13.JAUNIAUX E, BURTON GJ. Oxygen delivery at the deciduoplacental interface. In: Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders: basic science and its translation to obstetrics. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- 14.BERNIRSCHKE K, BURTON GJ, BAERGEN RN. Pathology of the human placenta. Cambridge, UK: Springer; 2012. Early development of the human placenta. [Google Scholar]
- 15.BROSENS I, ROBERTSON WB, DIXON HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 16.PIJNENBORG R, DIXON G, ROBERTSON WB, BROSENS I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- 17.PIJNENBORG R, BLAND JM, ROBERTSON WB, DIXON G, BROSENS I. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta. 1981;2:303–e16. doi: 10.1016/s0143-4004(81)80027-6. [DOI] [PubMed] [Google Scholar]
- 18.PIJNENBORG R, BLAND JM, ROBERTSON WB, BROSENS I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 19.BROSENS JJ, PIJNENBORG R, BROSENS IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 20.BROSENS I. Unraveling the anatomy. In: Pijnenborg R, Romero R, editors. Placental bed disorders: basic science and its translation to obstetrics. Cambridge, UK: Cambridge University Press; 2010. [Google Scholar]
- 21.KHONG Y, BROSENS I. Defective deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25:301–311. doi: 10.1016/j.bpobgyn.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 22.BROSENS I, PIJNENBORG R, VERCRUYSSE L, ROMERO R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WANG Y, ZHAO S. Vascular biology of the placenta. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 2, Placental blood circulation. [PubMed] [Google Scholar]
- 24.KAY HH, NELSON MD, WANG Y. Development and anatomy of the human placenta The placenta: from development to disease. Singapore: Blackwell Publishing Ltd; 2011. [Google Scholar]
- 25.BRETT KE, FERRARO ZM, YOCKELL-LELIEVRE J, GRUSLIN A, ADAMO KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci. 2014;15:16153–16185. doi: 10.3390/ijms150916153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CARTER AM. Placental gas exchange and the oxygen supply to the fetus. Compr Physiol. 2015;5:1381–1403. doi: 10.1002/cphy.c140073. [DOI] [PubMed] [Google Scholar]
- 27.MAQUEO M, CHAVEZAZUELA J, DOSALDELAVEGA M. Placental pathology in eclampsia and preeclampsia. Obstet Gynecol. 1964;24:350–356. [PubMed] [Google Scholar]
- 28.BROSENS IA, ROBERTSON WB, DIXON HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 29.BROSENS I, RENAER M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw. 1972;79:794–799. doi: 10.1111/j.1471-0528.1972.tb12922.x. [DOI] [PubMed] [Google Scholar]
- 30.DE WOLF F, ROBERTSON WB, BROSENS I. The ultrastructure of acute atherosis in hypertensive pregnancy. Am J Obstet Gynecol. 1975;123:164–174. doi: 10.1016/0002-9378(75)90522-0. [DOI] [PubMed] [Google Scholar]
- 31.ROBERTSON WB, BROSENS I, DIXON G. Uteroplacental vascular pathology. Eur J Obstet Gynecol Reprod Biol. 1975;5:47–65. doi: 10.1016/0028-2243(75)90130-6. [DOI] [PubMed] [Google Scholar]
- 32.ROBERTSON WB, BROSENS I, DIXON G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976;5:115–127. [PubMed] [Google Scholar]
- 33.KITZMILLER JL, WATT N, DRISCOLL SG. Decidual arteriopathy in hypertension and diabetes in pregnancy: immunofluorescent studies. Am J Obstet Gynecol. 1981;141:773–779. doi: 10.1016/0002-9378(81)90703-1. [DOI] [PubMed] [Google Scholar]
- 34.HUSTIN J, FOIDART JM, LAMBOTTE R. Maternal vascular lesions in pre-eclampsia and intrauterine growth retardation: light microscopy and immunofluorescence. Placenta. 1983;4:489–498. [PubMed] [Google Scholar]
- 35.KHONG TY, DE WOLF F, ROBERTSON WB, BROSENS I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 36.KHONG TY, PEARCE JM, ROBERTSON WB. Acute atherosis in preeclampsia: maternal determinants and fetal outcome in the presence of the lesion. Am J Obstet Gynecol. 1987;157:360–363. doi: 10.1016/s0002-9378(87)80172-2. [DOI] [PubMed] [Google Scholar]
- 37.FRUSCA T, MORASSI L, PECORELLI S, GRIGOLATO P, GASTALDI A. Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. Br J Obstet Gynaecol. 1989;96:835–839. doi: 10.1111/j.1471-0528.1989.tb03324.x. [DOI] [PubMed] [Google Scholar]
- 38.KHONG TY. Acute atherosis in pregnancies complicated by hypertension, small-for-gestational-age infants, and diabetes mellitus. Arch Pathol Lab Med. 1991;115:722–725. [PubMed] [Google Scholar]
- 39.MEEKINS JW, PIJNENBORG R, HANSSENS M, VAN ASSCHE A, MCFADYEN IR. Immunohistochemical detection of lipoprotein(a) in the wall of placental bed spiral arteries in normal and severe preeclamptic pregnancies. Placenta. 1994;15:511–524. doi: 10.1016/s0143-4004(05)80420-5. [DOI] [PubMed] [Google Scholar]
- 40.MOLDENHAUER JS, STANEK J, WARSHAK C, KHOURY J, SIBAI B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189:1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 41.VAN DER MERWE JL, HALL DR, WRIGHT C, SCHUBERT P, GROVE D. Are early and late preeclampsia distinct subclasses of the disease—what does the placenta reveal? Hypertens Pregnancy. 2010;29:457–467. doi: 10.3109/10641950903572282. [DOI] [PubMed] [Google Scholar]
- 42.STAFF AC, DECHEND R, PIJNENBORG R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia—novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56:1026–1034. doi: 10.1161/HYPERTENSIONAHA.110.157743. [DOI] [PubMed] [Google Scholar]
- 43.Oggé G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39:641–652. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, Hassan SS, Kim CJ, Chaiworapongsa T. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25:498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.STEVENS DU, AL-NASIRY S, BULTEN J, SPAANDERMAN ME. Decidual vasculopathy in preeclampsia: lesion characteristics relate to disease severity and perinatal outcome. Placenta. 2013;34:805–809. doi: 10.1016/j.placenta.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 46.STAFF AC, DECHEND R, REDMAN CW. Review: preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: two new hypotheses. Placenta. 2013;34(Suppl):S73–S78. doi: 10.1016/j.placenta.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 47.CHAIWORAPONGSA T, CHAEMSAITHONG P, YEO L, ROMERO R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.STAFF AC, JOHNSEN GM, DECHEND R, REDMAN CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. J Reprod Immunol. 2014:101–102. 120–126. doi: 10.1016/j.jri.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Baltajian K, Hecht JL, Wenger JB, Salahuddin S, Verlohren S, Perschel FH, Zsengeller ZK, Thadhani R, Karumanchi SA, Rana S. Placental lesions of vascular insufficiency are associated with anti-angiogenic state in women with preeclampsia. Hypertens Pregnancy. 2014;33:427–439. doi: 10.3109/10641955.2014.926914. [DOI] [PubMed] [Google Scholar]
- 50.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, Qureshi F, Jacques SM, Ahmed AI, Chaiworapongsa T, Hassan SS, Yeo L, Korzeniewski SJ. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015;28:2001–2009. doi: 10.3109/14767058.2014.976198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NELSON DB, ZIADIE MS, MCINTIRE DD, ROGERS BB, LEVENO KJ. Placental pathology suggesting that preeclampsia is more than one disease. Am J Obstet Gynecol. 2014;210:66.e1–e7. doi: 10.1016/j.ajog.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Kim YM1, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, Qureshi F, Jacques SM, Ahmed AI, Chaiworapongsa T, Hassan SS, Yeo L, Korzeniewski SJ. Placental lesions associated with acute atherosis. J Matern Fetal Neonatal Med. 2015;28:1554–1562. doi: 10.3109/14767058.2014.960835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.QUINN MJ. Preeclampsia: 2 placental phenotypes, 1 etiology? Am J Obstet Gynecol. 2014;211:313–314. doi: 10.1016/j.ajog.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 54.NELSON DB, MCINTIRE DD, LEVENO KJ. Reply: To PMID 24036400. Am J Obstet Gynecol. 2014;211:314. doi: 10.1016/j.ajog.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 55.MCDONNOLD M, SAADE G, COSTANTINE M. Reply: To PMID 24096182. Am J Obstet Gynecol. 2014;211:572–573. doi: 10.1016/j.ajog.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 56.BROSENS I, DIXON HG, ROBERTSON WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 57.DE WOLF F, BROSENS I, RENAER M. Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br J Obstet Gynaecol. 1980;87:678–685. doi: 10.1111/j.1471-0528.1980.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 58.SHEPPARD BL, BONNAR J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:695–705. doi: 10.1111/j.1471-0528.1981.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 59.LABARRERE C, ALONSO J, MANNI J, DOMENICHINI E, ALTHABE O. Immunohistochemical findings in acute atherosis associated with intrauterine growth retardation. Am J Reprod Immunol Microbiol. 1985;7:149–155. doi: 10.1111/j.1600-0897.1985.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 60.ALTHABE O, LABARRERE C, TELENTA M. Maternal vascular lesions in placentae of small-for-gestational-age infants. Placenta. 1985;6:265–276. doi: 10.1016/s0143-4004(85)80056-4. [DOI] [PubMed] [Google Scholar]
- 61.Park S-Y, Kim MY, Kim YJ, Chun YK, Kim HS, Kim HS, Hong SR. Placental pathology in intrauterine growth retardation. Korean J Pathol. 2002;36:30–37. [Google Scholar]
- 62.Kovo M, Schreiber L, Ben-Haroush A, Cohen G, Weiner E, Golan A, Bar J. The placental factor in early- and late-onset normotensive fetal growth restriction. Placenta. 2013;34:320–324. doi: 10.1016/j.placenta.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Parra-Saavedra M, Crovetto F, Triunfo S, Savchev S, Peguero A, Nadal A, Parra G, Gratacos E, Figueras F. Placental findings in late-onset SGA births without Doppler signs of placental insufficiency. Placenta. 2013;34:1136–1141. doi: 10.1016/j.placenta.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 64.Triunfo S, Lobmaier S, Parra-Saavedra M, Crovetto F, Peguero A, Nadal A, Gratacos E5, Figueras F. Angiogenic factors at diagnosis of late-onset small-for-gestational age and histological placental underperfusion. Placenta. 2014;35:398–403. doi: 10.1016/j.placenta.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 65.MIFSUD W, SEBIRE NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn Ther. 2014;36:117–128. doi: 10.1159/000359969. [DOI] [PubMed] [Google Scholar]
- 66.KOVO M, SCHREIBER L, ELYASHIV O, BEN-HAROUSH A, ABRAHAM G, BAR J. Pregnancy outcome and placental findings in pregnancies complicated by fetal growth restriction with and without preeclampsia. Reprod Sci. 2015;22:316–321. doi: 10.1177/1933719114542024. [DOI] [PubMed] [Google Scholar]
- 67.Parra-Saavedra M, Simeone S, Triunfo S, Crovetto F, Botet F, Nadal A, Gratacos E, Figueras F. Correlation between histological signs of placental underperfusion and perinatal morbidity in late-onset small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2015;45:149–155. doi: 10.1002/uog.13415. [DOI] [PubMed] [Google Scholar]
- 68.HOVATTA O, LIPASTI A, RAPOLA J, KARJALAINEN O. Causes of stillbirth: a clinicopathological study of 243 patients. Br J Obstet Gynaecol. 1983;90:691–696. doi: 10.1111/j.1471-0528.1983.tb09296.x. [DOI] [PubMed] [Google Scholar]
- 69.Korteweg FJ, Erwich JJ, Holm JP, Ravisé JM, van der Meer J, Veeger NJ, Timmer A. Diverse placental pathologies as the main causes of fetal death. Obstet Gynecol. 2009;114:809–817. doi: 10.1097/AOG.0b013e3181b72ebe. [DOI] [PubMed] [Google Scholar]
- 70.Reddy UM, Goldenberg R, Silver R, Smith GC, Pauli RM, Wapner RJ, Gardosi J, Pinar H, Grafe M, Kupferminc M, Hulthén Varli I, Erwich JJ, Fretts RC, Willinger M. Stillbirth classification—developing an international consensus for research: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2009;114:901–914. doi: 10.1097/AOG.0b013e3181b8f6e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.HEAZELL AE, MARTINDALE EA. Can post-mortem examination of the placenta help determine the cause of stillbirth? J Obstet Gynaecol. 2009;29:225–228. doi: 10.1080/01443610802716042. [DOI] [PubMed] [Google Scholar]
- 72.KIDRON D, BERNHEIM J, AVIRAM R. Placental findings contributing to fetal death, a study of 120 stillbirths between 23 and 40 weeks gestation. Placenta. 2009;30:700–704. doi: 10.1016/j.placenta.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 73.AMIR H, WEINTRAUB A, ARICHA-TAMIR B, APEL-SARID L, HOLCBERG G, SHEINER E. A piece in the puzzle of intrauterine fetal death: pathological findings in placentas from term and preterm intrauterine fetal death pregnancies. J Matern Fetal Neonatal Med. 2009;22:759–764. doi: 10.3109/14767050902929396. [DOI] [PubMed] [Google Scholar]
- 74.Shehata F, Levin I, Shrim A, Ata B, Weisz B, Gamzu R, Almog B. Placenta/birthweight ratio and perinatal outcome: a retrospective cohort analysis. BJOG. 2011;118:741–747. doi: 10.1111/j.1471-0528.2011.02892.x. [DOI] [PubMed] [Google Scholar]
- 75.TELLEFSEN CH, VOGT C. How important is placental examination in cases of perinatal deaths? Pediatr Dev Pathol. 2011;14:99–104. doi: 10.2350/10-07-0870-OA.1. [DOI] [PubMed] [Google Scholar]
- 76.Bonetti LR, Ferrari P, Trani N, Maccio L, Laura S, Giuliana S, Facchinetti F, Rivasi F. The role of fetal autopsy and placental examination in the causes of fetal death: a retrospective study of 132 cases of stillbirths. Arch Gynecol Obstet. 2011;283:231–241. doi: 10.1007/s00404-009-1317-4. [DOI] [PubMed] [Google Scholar]
- 77.Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Khong TY, Neilson J, Ezzati M, Koopmans L, Ellwood D, Fretts R, Frøen JF, Lancet’s Stillbirths Series steering committee Stillbirths: the way forward in high-income countries. Lancet. 2011;377:1703–1717. doi: 10.1016/S0140-6736(11)60064-0. [DOI] [PubMed] [Google Scholar]
- 78.Helgadóttir LB1, Turowski G, Skjeldestad FE, Jacobsen AF, Sandset PM, Roald B, Jacobsen EM. Classification of stillbirths and risk factors by cause of death—a case-control study. Acta Obstet Gynecol Scand. 2013;92:325–333. doi: 10.1111/aogs.12044. [DOI] [PubMed] [Google Scholar]
- 79.Pinar H, Goldenberg RL, Koch MA, Heim-Hall J, Hawkins HK, Shehata B, Abramowsky C, Parker CB, Dudley DJ, Silver RM, Stoll B, Carpenter M, Saade G, Moore J, Conway D, Varner MW, Hogue CJ, Coustan DR, Sbrana E, Thorsten V, Willinger M, Reddy UM. Placental findings in singleton stillbirths. Obstet Gynecol. 2014;123:325–336. doi: 10.1097/AOG.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.ROESCHER AM, TIMMER A, ERWICH JJ, BOS AF. Placental pathology, perinatal death, neonatal outcome, and neurological development: a systematic review. PLoS One. 2014;9:e89419. doi: 10.1371/journal.pone.0089419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.ALTSHULER G, RUSSELL P, ERMOCILLA R. The placental pathology of small-for-gestational-age infants. Am J Obstet Gynecol. 1975;121:351–359. doi: 10.1016/0002-9378(75)90011-3. [DOI] [PubMed] [Google Scholar]
- 82.SALIM R, JUBRAN J, OKOPNIK M, GARMI G. Do placental lesions among term small for gestational age newborns differ according to the clinical presentation? Eur J Obstet Gynecol Reprod Biol. 2014;173:38–42. doi: 10.1016/j.ejogrb.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Parra-Saavedra M, Crovetto F, Triunfo S, Savchev S, Peguero A, Nadal A, Gratacós E, Figueras F. Association of Doppler parameters with placental signs of underperfusion in late-onset small-for-gestational-age pregnancies. Ultrasound Obstet Gynecol. 2014;44:330–337. doi: 10.1002/uog.13358. [DOI] [PubMed] [Google Scholar]
- 85.GETAHUN D, OYELESE Y, SALIHU HM, ANANTH CV. Previous cesarean delivery and risks of placenta previa and placental abruption. Obstet Gynecol. 2006;107:771–778. doi: 10.1097/01.AOG.0000206182.63788.80. [DOI] [PubMed] [Google Scholar]
- 85.INSTITUTE OF MEDICINE. Preterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 86.BERG CJ, CALLAGHAN WM, SYVERSON C, HENDERSON Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–1309. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 87.MWANIKI MK, ATIENO M, LAWN JE, NEWTON C. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 89.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, Thaler HT, Romero R. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 90.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 91.Kovo M, Schreiber L, Ben-Haroush A, Asalee L, Seadia S, Golan A, Bar J. The placental factor in spontaneous preterm labor with and without premature rupture of membranes. J Perinat Med. 2011;39:423–429. doi: 10.1515/jpm.2011.038. [DOI] [PubMed] [Google Scholar]
- 92.ARMSTRONG-WELLS J, POST MD, DONNELLY M, MANCO-JOHNSON MJ, FISHER BM, WINN VD. Patterns of placental pathology in preterm premature rupture of membranes. J Dev Orig Health Dis. 2013;4:249–255. doi: 10.1017/S2040174413000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kelly R, Holzman C, Senagore P, Wang J, Tian Y, Rahbar MH, Chung H. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009;170:148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.GOLDENBERG RL, CULHANE JF, IAMS JD, ROMERO R. Preterm birth 1—epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neona. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 96.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 97.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Gonçalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. discussion, 1547–1550. [DOI] [PubMed] [Google Scholar]
- 98.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Gonçalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 99.PARK CW, PARK JS, SHIM SS, JUN JK, YOON BH, ROMERO R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193:984–989. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 100.Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, Espinoza J, Gonçalves LF, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 101.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 102.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, Mittal P, Mazaki-Tovi S, Than NG, Gomez R, Hassan SS. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, Pineles BL, Papp Z, Hassan S. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, Yeo L, Conde-Agudelo A, Hassan SS. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaiworapongsa T, Romero R, Tarca AL, Kusanovic JP, Gotsch F, Mittal P, Kim SK, Vaisbuch E, Mazaki-Tovi S, Erez O, Dong Z, Kim CJ, Yeo L, Hassan SS. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol. 2010;202:550.e1–e10. doi: 10.1016/j.ajog.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, Dong Z, Tarca A, Gaurav B, Hassan SS. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187–1207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Romero R, Chaiworapongsa T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest. 2013;123:2775–2777. doi: 10.1172/JCI70431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, Dong Z, Than NG, Yeo L, Hernandez-Andrade E, Conde-Agudelo A, Hassan SS. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208:287.e1–e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaiworapongsa T, Romero R, Korzeniewski SJ, Cortez JM, Pappas A, Tarca AL, Chaemsaithong P, Dong Z, Yeo L, Hassan SS. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med. 2014;27:132–144. doi: 10.3109/14767058.2013.806905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.CHAIWORAPONGSA T, CHAEMSAITHONG P, KORZENIEWSKI SJ, YEO L, ROMERO R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol. 2014;10:531–540. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chaiworapongsa T1, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, Pacora P, Ogge G, Dong Z, Kim CJ, Yeo L, Hassan SS. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med. 2009;22:1122–1139. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, Kusanovic JP, Erez O, Bujold E, Gonçalves LF, Gomez R, Edwin S. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med. 2007;20:495–507. doi: 10.1080/14767050701413022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, Mittal P, Ogge G, Vaisbuch E, Mazaki-Tovi S, Dong Z, Kim SK, Yeo L, Hassan SS. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010;23:1384–1399. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Whitten AE, Romero R, Korzeniewski SJ, Tarca AL, Schwartz AG, Yeo L, Dong Z, Hassan SS, Chaiworapongsa T. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. Am J Obstet Gynecol. 2013;208:310.e1–e11. doi: 10.1016/j.ajog.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, Edwin S, Erez O, Gotsch F, Mazaki-Tovi S, Than NG, Soto E, Camacho N, Gomez R, Quintero R, Hassan SS. Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol. 2008;198:382.e1–e8. doi: 10.1016/j.ajog.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.SAVVIDOU MD, YU CK, HARLAND LC, HINGORANI AD, NICOLAIDES KH. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am J Obstet Gynecol. 2006;195:1668–1673. doi: 10.1016/j.ajog.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 118.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, Hassan SS, Romero R. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prentice R. A case-cohort design for epidemiologic cohort ctudies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 120.ALEXANDER GR, HIMES JH, KAUFMAN RB, MOR J, KOGAN M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 121.REDLINE RW, HELLER D, KEATING S, KINGDOM J. Placental diagnostic criteria and clinical correlation—a workshop report. Placenta. 2005;26(Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Oggé G, Romero R, Lee DC, Gotsch F, Than NG, Lee J, Chaiworapongsa T, Dong Z, Mittal P, Hassan SS, Kim CJ. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. J Pathol. 2011;223:553–565. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 124.VON DADELSZEN P, MAGEE LA, ROBERTS JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 125.MACDORMAN MF, KIRMEYER S. The challenge of fetal mortality. NCHS data brief. 2009:1–8. [PubMed] [Google Scholar]
- 126.KOENKER R. Quantreg: Quantile Regression. 2007 R package version 4.10. [Google Scholar]
- 127.KOENKER RW. Quantile regression. Cambridge: Cambridge U Press; 2005. [Google Scholar]
- 128.TEAM RDC. R: a language and environment for statistical computing. R foundation for statistical computing; Vienna, Austria: 2009. [Google Scholar]
- 129.TORRY DS, WANG HS, WANG TH, CAUDLE MR, TORRY RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 130.SAS, version 9.4. SAS Institute; Cary NC, USA: 2013. [Google Scholar]
- 131.REUVEKAMP A, VELSING-AARTS FV, POULINA IE, CAPELLO JJ, DUITS AJ. Selective deficit of angiogenic growth factors characterizes pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1999;106:1019–1022. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 132.TIDWELL SC, HO HN, CHIU WH, TORRY RJ, TORRY DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 133.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.TAYLOR RN, GRIMWOOD J, TAYLOR RS, MCMASTER MT, FISHER SJ, NORTH RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 135.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 136.LUTTUN A, CARMELIET P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest. 2003;111:600–602. doi: 10.1172/JCI18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 138.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 139.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, CPEP Study Group Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 140.ROBINSON CJ, JOHNSON DD, CHANG EY, ARMSTRONG DM, WANG W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–259. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 141.UNAL ER, ROBINSON CJ, JOHNSON DD, CHANG EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol. 2007;197:211.e1–e4. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 142.LINDHEIMER MD, ROMERO R. Emerging roles of antiangiogenic and angiogenic proteins in pathogenesis and prediction of preeclampsia. Hypertension. 2007;50:35–36. doi: 10.1161/HYPERTENSIONAHA.107.089045. [DOI] [PubMed] [Google Scholar]
- 143.Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197:244.e1–e8. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 144.WIDMER M, VILLAR J, BENIGNI A, CONDE-AGUDELO A, KARUMANCHI SA, LINDHEIMER M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007;109:168–180. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- 145.De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D’anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:837–842. doi: 10.1080/00016340802253759. [DOI] [PubMed] [Google Scholar]
- 146.Molvarec A, Szarka A, Walentin S, Szucs E, Nagy B, Rigó J., Jr Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res. 2010;33:892–898. doi: 10.1038/hr.2010.92. [DOI] [PubMed] [Google Scholar]
- 147.Powers RW, Jeyabalan A, Clifton RG, Van Dorsten P, Hauth JC, Klebanoff MA, Lindheimer MD, Sibai B, Landon M, Miodovnik M, Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units Network Soluble fms-like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PloS One. 2010;5:e13263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rana S, Hacker MR, Modest AM, Salahuddin S, Lim KH, Verlohren S, Perschel FH, Karumanchi SA. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60:451–458. doi: 10.1161/HYPERTENSIONAHA.112.195065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.GAROVIC VD. The role of angiogenic factors in the prediction and diagnosis of preeclampsia superimposed on chronic hypertension. Hypertension. 2012;59:555–557. doi: 10.1161/HYPERTENSIONAHA.111.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.GHOSH SK, RAHEJA S, TULI A, RAGHUNANDAN C, AGARWAL S. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet. 2012;285:417–422. doi: 10.1007/s00404-011-1960-4. [DOI] [PubMed] [Google Scholar]
- 151.HAGMANN H, THADHANI R, BENZING T, KARUMANCHI SA, STEPAN H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem. 2012;58:837–845. doi: 10.1373/clinchem.2011.169094. [DOI] [PubMed] [Google Scholar]
- 152.VERLOHREN S, STEPAN H, DECHEND R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond) 2012;122:43–52. doi: 10.1042/CS20110097. [DOI] [PubMed] [Google Scholar]
- 153.Rana S, Cerdeira AS, Wenger J, Salahuddin S, Lim KH, Ralston SJ, Thadhani RI, Karumanchi SA. Plasma concentrations of soluble endoglin versus standard evaluation in patients with suspected preeclampsia. PLoS One. 2012;7:e48259. doi: 10.1371/journal.pone.0048259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sibiude J1, Guibourdenche J, Dionne MD, Le Ray C, Anselem O, Serreau R, Goffinet F, Tsatsaris V. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PLoS One. 2012;7:e50208. doi: 10.1371/journal.pone.0050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vatten LJ, Åsvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta Obstet Gynecol Scand. 2012;91:1388–1394. doi: 10.1111/j.1600-0412.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- 156.McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, Parry S. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207:407.e1–e7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 157.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moore AG, Young H, Keller JM, Ojo LR, Yan J, Simas TA, Maynard SE. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J Matern Fetal Neonatal Med. 2012;25:2651–2657. doi: 10.3109/14767058.2012.713055. [DOI] [PubMed] [Google Scholar]
- 159.AGGARWAL PK, CHANDEL N, JAIN V, JHA V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26:236–241. doi: 10.1038/jhh.2011.29. [DOI] [PubMed] [Google Scholar]
- 160.Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, Redman CW, Shennan AH. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128:2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 161.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, Sabria J, Markfeld-Erol F, Galindo A, Schoofs K, Denk B, Stepan H. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352. doi: 10.1161/HYPERTENSIONAHA.113.01787. [DOI] [PubMed] [Google Scholar]
- 162.Goel A, Rana S. Angiogenic factors in preeclampsia: potential for diagnosis and treatment. Curr Opin Nephrol Hypertens. 2013;22:643–650. doi: 10.1097/MNH.0b013e328365ad98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moore Simas TA, Crawford SL, Bathgate S, Yan J, Robidoux L, Moore M, Maynard SE. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. J Matern Fetal Neonatal Med. 2014;27:1038–1048. doi: 10.3109/14767058.2013.847415. [DOI] [PubMed] [Google Scholar]
- 164.Rizos D, Eleftheriades M, Karampas G, Rizou M, Haliassos A, Hassiakos D, Vitoratos N. Placental growth factor and soluble fms-like tyrosine kinase-1 are useful markers for the prediction of preeclampsia but not for small for gestational age neonates: a longitudinal study. Eur J Obstet Gynecol Reprod Biol. 2013;171:225–230. doi: 10.1016/j.ejogrb.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 165.SCHAARSCHMIDT W, RANA S, STEPAN H. The course of angiogenic factors in early- vs. late-onset preeclampsia and HELLP syndrome. J Perinat Med. 2013;41:511–516. doi: 10.1515/jpm-2012-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maynard SE, Crawford SL, Bathgate S, Yan J, Robidoux L, Moore M, Moore Simas TA. Gestational angiogenic biomarker patterns in high risk preeclampsia groups. Am J Obstet Gynecol. 2013;209:53.e1–e9. doi: 10.1016/j.ajog.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 167.GRAY K, LIM KH, THOMAS A, PARRY S, MCELRATH TF. Maternal plasma levels of PlGF and sFlt-1 change throughout the 1st trimester and correlate with development of early-onset pre-eclampsia (abstract 633) Am J Obstet Gynecol. 2013;208:S268–S269. [Google Scholar]
- 168.Metz TD, Allshouse AA, Euser AG, Heyborne KD. Preeclampsia in high risk women is characterized by risk group-specific abnormalities in serum biomarkers. Am J Obstet Gynecol. 2014;211:512.e1–e6. doi: 10.1016/j.ajog.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, Connor JM, Dobbie R. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109:1316–1324. doi: 10.1097/01.AOG.0000265804.09161.0d. [DOI] [PubMed] [Google Scholar]
- 170.Chaiworapongsa T1, Kusanovic JP, Savasan ZA, Mazaki-Tovi S, Kim SK, Vaisbuch E, Tarca AL, Mittal P, Ogge G, Madan I, Dong Z, Yeo L, Hassan SS, Romero R. Fetal death: a condition with a dissociation in the concentrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern Fetal Neonatal Med. 2010;23:960–972. doi: 10.3109/14767050903410664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gotsch F, Romero R, Kusanovic JP, Chaiworapongsa T, Dombrowski M, Erez O, Than NG, Mazaki-Tovi S, Mittal P, Espinoza J, Hassan SS. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.ASVOLD BO, VATTEN LJ, ROMUNDSTAD PR, JENUM PA, KARUMANCHI SA, ESKILD A. Angiogenic factors in maternal circulation and the risk of severe fetal growth restriction. Am J Epidemiol. 2011;173:630–639. doi: 10.1093/aje/kwq373. [DOI] [PubMed] [Google Scholar]
- 173.Darling AM, McDonald CR, Conroy AL, Hayford KT, Liles WC, Wang M, Aboud S, Urassa WS, Kain KC, Fawzi WW. Angiogenic and inflammatory biomarkers in midpregnancy and small-for-gestational-age outcomes in Tanzania. Am J Obstet Gynecol. 2014;211:509.e1–e8. doi: 10.1016/j.ajog.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lobmaier SM, Figueras F, Mercade I, Perello M, Peguero A, Crovetto F, Ortiz JU, Crispi F, Gratacós E. Angiogenic factors vs Doppler surveillance in the prediction of adverse outcome among late-pregnancy small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2014;43:533–540. doi: 10.1002/uog.13246. [DOI] [PubMed] [Google Scholar]
- 175.DARLING AM, MCDONALD CR, CONROY AL, et al. Angiogenic and inflammatory biomarkers in midpregnancy and small-for-gestational-age outcomes in Tanzania. Am J Obstet Gynecol. 2014;211:29. doi: 10.1016/j.ajog.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.MULLERS S, UNTERSHEIDER J, DOYLE E, et al. Placental lesions in pregnancies complicated by intrauterine growth restriction and hypertension (Abstract 338) Am J Obstet Gynecol. 2014;210:S174–S175. [Google Scholar]
- 177.Chaiworapongsa T, Romero R, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, Segars JH, DeCherney AH, McCoy MC, Kim CJ, Yeo L, Hassan SS. Pravastatin to prevent recurrent fetal death in massive perivillous fibrin deposition of the placenta (MPFD) J Matern Fetal Neonatal Med. 2016;29:855–862. doi: 10.3109/14767058.2015.1022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fox CE, Lash GE, Pretlove SJ, Chan BC, Holder R, Kilby MD. Maternal plasma and amniotic fluid angiogenic factors and their receptors in monochorionic twin pregnancies complicated by twin-to-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2010;35:695–701. doi: 10.1002/uog.7515. [DOI] [PubMed] [Google Scholar]
- 179.Yinon Y, Ben Meir E, Berezowsky A, Weisz B, Schiff E, Mazaki-Tovi S, Lipitz S. Circulating angiogenic factors in monochorionic twin pregnancies complicated by twin-to-twin transfusion syndrome and selective intrauterine growth restriction. Am J Obstet Gynecol. 2014;210:141.e1–e7. doi: 10.1016/j.ajog.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 180.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, Kim CJ, Mittal P, Gotsh F, Erez O, Chaiworapongsa T, Hassan S. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome) J Matern Fetal Neonatal Med. 2006;19:607–613. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oggé G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39:641–652. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89:265–271. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 183.Sebire NJ, Goldin RD, Regan L. Term preeclampsia is associated with minimal histopathological placental features regardless of clinical severity. J Obstet Gynaecol. 2005;25:117–118. doi: 10.1080/014436105400041396. [DOI] [PubMed] [Google Scholar]
- 184.Apel-Sarid L, Levy A, Holcberg G, Sheiner E. Term and preterm (< 34 and < 37 weeks gestation) placental pathologies associated with fetal growth restriction. Arch Gynecol Obstet. 2010;282:487–492. doi: 10.1007/s00404-009-1255-1. [DOI] [PubMed] [Google Scholar]
- 185.Mijal RS, Holzman CB, Rana S, Karumanchi SA, Wang J, Sikorskii A. Mid-pregnancy levels of angiogenic markers as indicators of pathways to preterm delivery. J Matern Fetal Neonatal Med. 2012;25:1135–1141. doi: 10.3109/14767058.2011.625458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kraus FT, Redline R, Gersell DJ, Nelson MD, Dicke JM. Placental pathology. 1st. Washington DC: American Registry of Pathology; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


