Abstract
Background
The mechanisms by which persistent AF terminates via localized ablation are not well understood. To address the hypothesis that sites where localized ablation terminates persistent AF have characteristics identifiable with activation mapping during AF, we systematically examined activation patterns acquired only in cases of unequivocal termination by ablation.
Methods and Results
We recruited 57 patients with persistent AF undergoing ablation, in whom localized ablation terminated AF to sinus rhythm or organized tachycardia. For each site, we performed an offline analysis of unprocessed unipolar electrograms collected during AF from multipolar basket catheters using the maximum –dV/dt assignment to construct isochronal activation maps for multiple cycles. Additional computational modeling and phase analysis were used to study mechanisms of map variability. At all sites of AF termination, localized repetitive activation patterns were observed. Partial rotational circuits were observed in in 26/57 (46%) cases, focal patterns in 19/57 (33%), and complete rotational activity in 12/57 (21%) cases. In computer simulations, incomplete segments of partial rotations coincided with areas of slow conduction characterized by complex, multicomponent electrograms, and variations in assigning activation times at such sites substantially altered mapped mechanisms.
Conclusions
Local activation mapping at sites of termination of persistent AF showed repetitive patterns of rotational or focal activity. In computer simulations, complete rotational activation sequence was observed but was sensitive to assignment of activation timing particularly in segments of slow conduction. The observed phenomena of repetitive localized activation and the mechanism by which local ablation terminates putative AF drivers require further investigation.
Journal Subject Terms: Arrhythmias, Atrial Fibrillation, Electrophysiology, Catheter Ablation, Implantable Cardioverter-Defibrillator
Keywords: atrial fibrillation, ablation, mapping, electrophysiology mapping, rotor, mechanism, phase, computer modeling
Introduction
Therapy for persistent atrial fibrillation (AF) is limited by uncertainty about sustaining mechanisms 1. A wide variety of putative mechanisms of persistence of AF have been observed or inferred 2, with reported outcomes of ablation based on these concepts. Different ablation strategies include the creation of empiric lines 3, targeting of fragmented electrograms 4 and ablation of AF triggering sites. 5 Difficulty in reconciling the putative mechanisms has been associated with lack of consistency of reported outcomes from ablation 6–8.
We used the universal observation that local ablation can terminate AF 9–11 as a starting point to assess whether these sites could reveal a potential common mechanism or identifiable activation pattern. We hypothesized that repetitive activation patterns would be observed at sites where AF terminated, unlike remote regions demonstrating disorganized electrical activity.
We therefore analyzed mapping data by systematically examining the unprocessed pre-ablation electrograms and activation patterns at and around sites of unequivocal termination by ablation for consecutive patients who met this criterion. Computer modeling was used to give further insight and to identify possible unifying characteristics of these unique sites apparently necessary for driving persistent AF.
Methods
Selected data from this study are freely available online (http://narayanlab.stanford.edu) and are part of a separate AF mapping analysis12. Other data, analytic methods, and study materials will be made available to other researchers on request for the purpose of reproducing the results or replicating the procedure.
Subjects Included in the Study Cohort
We identified 57 patients with persistent AF at 5 teaching hospitals (Stanford University Hospital, Palo Alto, CA; Veterans Affairs Medical Center, San Diego, CA; University of Colorado Hospital, Aurora, CO; Indiana University, Indianapolis, IN and Klinikum Coburg, Germany), in whom localized ablation terminated persistent AF to sinus rhythm or atrial tachycardia (AT). Persistent AF was defined as AF for >7 days without self-termination, and longstanding persistent AF as continuous for >12 months (n=6) 1. Each patient had unipolar electrogram data collected during electrophysiological study. This population was derived from a total of 559 presenting to the 5 centers for ablation of AF, and excludes N=52 AF terminations when mapping was performed after pulmonary vein isolation (PVI), and in patients with bipolar only data for analysis. The study was approved by an institutional review committee and that the subjects gave informed consent.
Electrophysiological Study and Ablation
Patients on anti-arrhythmic medications had these agents withheld for 5 half-lives (median 228, range 90–1580 days, in 28 patients with prior amiodarone use). Heparin was infused to maintain activated clotting time > 300 seconds. A 64-pole basket (Constellation, Boston Scientific or FIRMap, Topera, Inc.) was used to map both the right (RA) and left (LA) atrium during AF. In each case a region of interest, identified prospectively by mapping of repetitive activation patterns11, was targeted for RF ablation by applying contiguous lesions in localized areas of <3–4 cm2 10,13. The location of each ablation site was referenced to known electrodes, and their anatomical locations were verified by 3-dimensional electroanatomic imaging (NavX, St Jude Medical, St Paul, MN; Carto, Biosense-Webster, Diamond Bar, CA). Contact force sensing catheters were used in 27 cases (47%).
Data Acquisition and Analysis
One-minute electrogram segments used to map AF and guide prospective ablation were recorded on digital recorders (Bard LabSystem Pro, or GE Prucka Cardiolab) at 1000Hz sampling rate, filtered at 0.05 to 500 Hz, exported for offline analysis. The exported segment was the one used in each case to identify regions of interest and before any ablation in that atrium.
Electrograms were analyzed blinded to the site where ablation terminated AF. Four second epochs were analyzed cycle-by-cycle in each case. AF cycle length was measured from the most rapid coronary sinus electrograms averaged over 10 cycles of AF during the epoch exported for offline electrogram analysis. The present study used only non-proprietary methods for signal processing for noise reduction, filtering, QRS subtraction or phase analysis. Activation times were determined objectively by software written in Matlab (Mathworks, Natick, MA) as follows:
In biphasic unipolar electrograms, we used the minimum dV/dt (i.e. maximum negative slope) within an interval of 50ms starting from the unipolar local absolute maximum as local activation 14. We applied a blanking period of 100ms to avoid repeat annotation within repolarization 15.
Activation time of monophasic unipolar signals was assigned by peak amplitude as recently described 8, with a 50ms interval centered on the local maximum to detect peak-to-peak maxima 14.
Supplementary figure 1 shows that isochronal maps using both criteria show good agreement in the same datasets. To ensure consistency, one method was used per patient.
Contour (isochronal) maps of AF were created by marking regions activated at the same time as contiguous colors in 10ms bins, each displaying the entire mapped atrium as a grid with red/blue indicating early/late activation respectively.
Contour Maps of AF Mechanisms at AF-Terminating Sites
Once isochronal maps were generated, the site where ablation terminated AF in each case was unblinded. Spatio-temporal maps at sites of AF termination were classified using previously described patterns 8:
Partial rotational circuits: consistent spatial locations with non-contiguous activation due to activation from a focal or breakthrough site encountering a line of block 8, spanning < 70% of the AF cycle length 16.
Repetitive foci: site(s) of earliest activation with centrifugal spread during AF 8.
Complete rotational circuit: stable repetitive earliest meets latest interaction on isochronal maps with electrograms spanning >70% of AF cycle length 16.
A source was only counted consistently stable if spatio-temporal patterns were present for >3 reconstructed isochronal maps 8,17 during the 1 minute time period examined.
We graded agreement between mapped mechanisms by 3 investigators (JZ, TB, MA) for these categories. For 68% of the patients, there was complete agreement between the 3 raters in terms of whether there was full, partial or no rotations, and in the remaining 32% of the patients there was agreement between 2 of 3 raters. There were no cases where each rater ranked something completely different. The overall kappa score was 0.56 (p=0.001).
Computational Modeling
We also used computer models to test mechanistic hypotheses in relation to mapping methods. Simulations were carried out 18,19 in 2-dimensional sheets using the monodomain equation:
| (1) |
Here, V is the membrane voltage, Cm=1μF/cm2 the membrane capacitance, D the diffusion tensor with diagonal entries of D0=0.001 cm2/ms, and Iion the membrane currents. Membrane currents were implemented by the Fenton-Karma (FK) model 18,20,21 with parameter values chosen to reproduce AF dynamics. Eq. 1 was simulated on a square grid using a standard finite difference scheme with spatial discretization of 0.025cm and time-step of 0.05ms. In one set of simulations, the tissue conductivity was homogeneous (D0=0.001 cm2/ms), while in the other the conductivity was heterogeneous (D0=0.001 cm2/ms in one part and D0=0.0002 cm2/ms in the other). Reentry was initiated using a standard cross-stimulation protocol. Phase analysis methods are described in supplementary material.
Statistical Analysis
We used SPSS version 23 (IBM, New York) and Prism 5.0 (Graphpad, La Jolla, CA). Continuous data are represented as mean±SD. Normality was evaluated using the Kolmogorov-Smirnov test. Comparisons between two groups were made with Student’s t test if normally distributed or, if not normally distributed, evaluated with the Mann-Whitney U test. Analysis of variance (ANOVA) was used to compare variables between patients with different AF mechanisms, with post-hoc Bonferroni corrections to identify the differences between group pairs. Nominal values were expressed as n (%) and compared with chi-square or Fisher’s exact test when expected cell frequency was <5. A probability of < 0.05 was considered statistically significant.
Results
Clinical and Electrophysiological Characteristics of the Study Cohort
Table 1 shows subject characteristics. Patients had an AF history of 5.9±4.6 years, LA diameters of 49±9mm and CHADSVASc (congestive heart failure, hypertension, age, diabetes, vascular, age, sex) score of 2.2±1.5. Each patient had acute AF termination by prospective localized ablation prior to PVI. Persistent AF terminated to sinus rhythm (n=28, 49%) or atrial tachycardia/flutter (n=29, 51%) in all subjects included in the cohort. Figure 1 illustrates two cases where ablation at the first targeted site terminated persistent AF, prior to PVI, which occurred in 14 (25%) of patients. Overall, patients had 4.4±1.9 AF sources identified.
Table 1.
Study population
| Patient Characteristics (n=57) | |
|---|---|
| Age (years) | 62.9±10.0 |
| Male/Female (%) | 80/20 |
| Paroxysmal/Persistent (%) | 0/100 |
| Mode of termination (%SR/AT) | 49/51 |
| AF History (years) | 5.85±4.57 |
| Left atrial diameter (mm) | 48.8 ± 8.59 |
| LV ejection fraction (%) | 51.8±13.1 |
| Coronary artery disease (%) | 34.9 |
| Valvular disease (%) | 20.8 |
| Body Mass Index (kg/m2) | 30.9±5.93 |
| CHADS2VASc score | 2.2±1.5 |
| Number of previously failed anti-arrhythmic drugs | 1.6±1.1 |
| Previous RF ablation for AF | 51% |
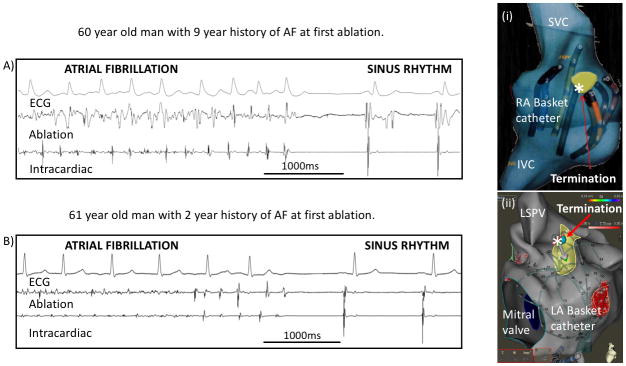
Figure 1. Acute termination in patients with persistent AF by ablation at sites marked (*).
A) Acute termination of AF in a 60-year-old man to sinus rhythm by ablation, depicted on the electroanatomic right atrial shell (i). B) Acute termination of AF in a 61-year-old with the first ablation lesion applied in the left atrium (ii). This study was designed to investigate these scenarios.
Mapping of Repetitive Activation Patterns during Persistent AF
Activation mapping at sites of AF termination revealed partial rotational circuits, transient foci with or without intermittent disorganized fibrillatory patterns or stable complete rotational activation.
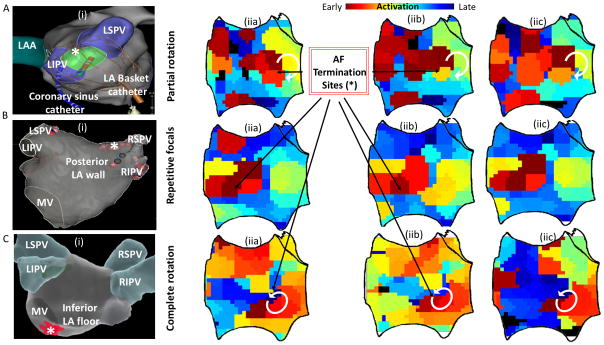
Incomplete rotations at the site of termination were observed for more than 3 AF cycles in 26/57 (46%) of patients in contour maps. Figure 2A(iia-c) shows an example of a partial rotation on isochronal mapping.
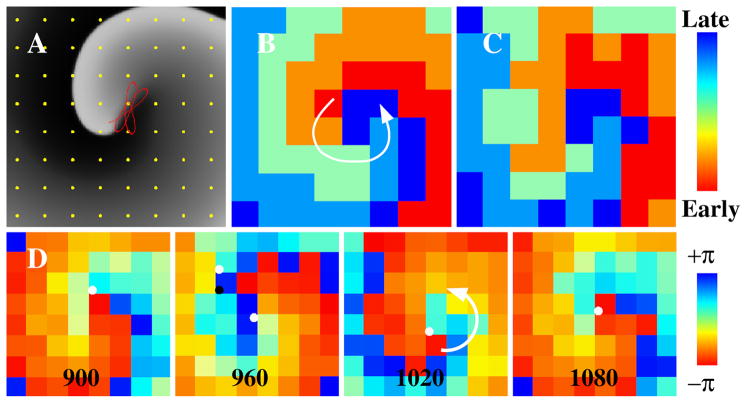
Figure 2. Consecutive isochronal maps at sites of AF termination.
A) Partial rotational circuit with a termination site near the left pulmonary vein carina. B) Repetitive focal activity from site of termination on the LA roof. C) Complete rotational circuit with a termination site near the mitral valve. Isochronal maps (iia, iib, iic) are shown for three consecutive cycles of AF.
Focal activation was found in 19/57 (33%) of patients. Of these, there were 2 sites demonstrating consistent presence in all maps analyzed during the one minute mapping period. Figure 2B(iia–c) shows repetitive foci on consecutive AF cycles at the site of AF termination.
Stable rotational sources were seen at the site of AF termination in 12/57 (21%) patients on contour maps. Figures 2C and 3 illustrate rotational circuits in persistent AF. For each, electrograms spanned 100% of the AF cycle length and activation patterns were stable for multiple cycles.
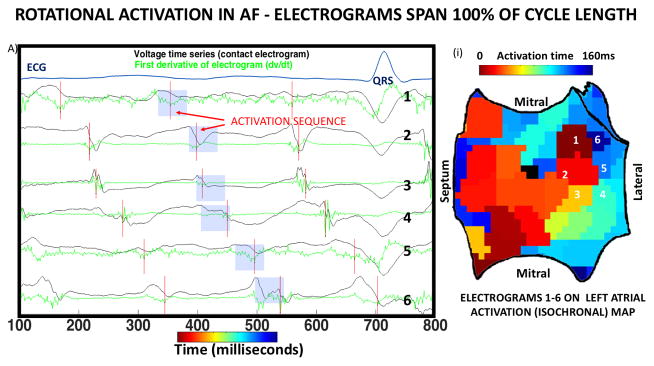
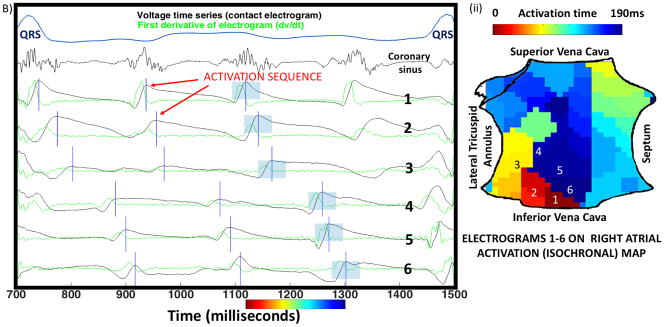
Figure 3. Complete rotational circuits at sites of termination of persistent AF.
A) Three cycles of AF in a 66-year-old man, with unipolar electrograms spanning 100% of the AF cycle length (160ms). Dashed line shows the first derivative (-dv/dt), with red vertical lines indicating activation at each AF cycle (maximum –dv/dt). Blue boxes highlight a 50ms window used to detect local activation. Numbered traces on left atrial isochrones (i) show rotational circuits with head-meets-tail interaction. B) Three cycles of AF in a 54-year-old man with persistent AF annotated using blue vertical lines (local peak-to-peak amplitude). Coronary sinus recording confirms AF. (ii) Isochronal activation map shows rotational activation in right atrium.
Computer Modeling for Assessment of Accurate Activation Timing Assignment
Multicomponent electrograms are often seen in AF, and can alter activation marking and hence contour maps. We modeled how assigning different deflections for activation time may impact mapped AF mechanisms. Figure 4A shows a simulated stable spiral wave and rotational activity in an isochronal map (figure 4B). Shifting the activation time of each electrode by ±10%, allocated randomly, yields a contour map (figure 4C) that no longer shows rotational activity. Phase mapping using these same noisy activation times still shows rotational activity (figure 4D), with additional adjacent singularities of opposite chirality which are transient yet represent false positives 22.
Figure 4. Phase mapping and isochronal mapping of AF.
(A) Snapshot of a computer simulation of a spiral wave with period 180ms and a meandering tip trajectory in red. (B) Isochronal map using the activation times at the 8×8 grid shown in yellow in (A), identifying the spiral wave. (C) As in (B) but now with variations of the relative timing of each electrode chosen at random between ±20ms (± 10% of cycle length 180ms). Altered activation times alter the spiral wave in the isochronal map. (D) Sequence of phase maps spanning the time interval of (B). Phase singularities reveal a counter-clockwise rotation at these same activation times. See supplementary materials for phase methods.
Selecting a different component of complex electrograms altered AF contour maps. Figure 5 illustrates a 54-year-old man with persistent AF in whom localized ablation near electrode D3 terminated AF to sinus rhythm. Electrograms in the vicinity are largely easy to mark, yet selecting which of the 3 deflections on electrode D3 to mark alters maps. Selecting the first deflection by maximum -dv/dt produces a focal map (figure 5(i)). Selecting the second deflection produces a partial rotational map (figure 5(ii)), with a line of conduction block at D3 preventing completion of a full rotational circuit. Adjustment of marking to select the third deflection on D3 based on computational data recreated complete rotation spanning > 70% of AF cycle (figure 5(iii)). Ambiguity in marking electrogram was common in patients with partial rotations on AF contour maps (group 1).
Figure 5. Importance of timing assignment in multiple component electrograms in AF.
A) Persistent AF in a 54-year-old man, with electrode D3 indicating 3 possible deflections. Annotating the first (minimum dv/dt; red lines) portrays a focal source at the termination site (i). Annotating the middle deflection (green) indicates a partial rotation (green box, ii). Annotating the third deflection (blue) gives a complete rotational circuit (blue box, iii). B) Termination of AF with ablation at D3.
Isochronal Mapping and Choice of Analysis Interval
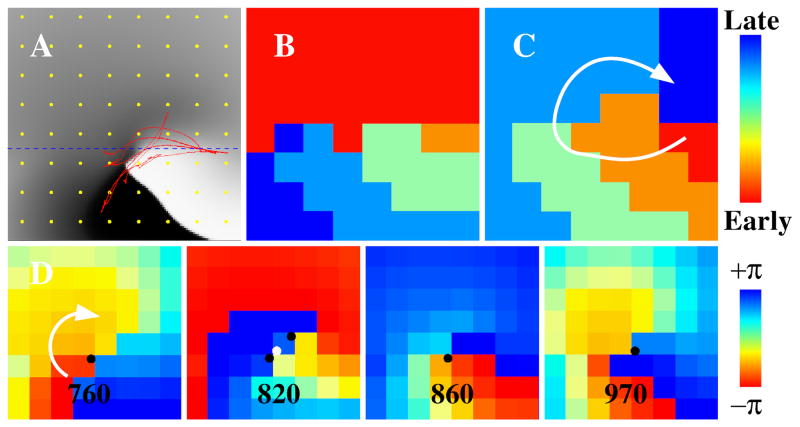
Producing a contour map for any rhythm requires assigning a mapping window to start (and stop) activation time marking. This is challenging in AF because the cycle length varies across the atrium with differing earliest activation sites. We used computer modeling to study the impact of different mapping windows on resulting AF maps, and related this to our clinical series.
We hypothesized that variations in AF cycle length reflect heterogeneous conduction. Figure 6 shows a spiral wave (figure 6A) in a domain where the upper half has higher conductivity and wavefront speed than the lower half. For certain start times, activation times of the upper half coalesce, making it challenging to identify rotations (figure 6B). However, starting the window 50ms later (figure 6C) uncovers clear rotational activity on activation maps. Phase maps of these same data reveal rotational activity despite non-uniform conduction (figure 6D; singularities shown as black or white dots, depending on chirality). The predominant singularity in successive phase snapshots indicates consistent reentry.
Figure 6. Importance of selection of time window for analysis in computer simulation.
(A) Snapshot of a computer simulation of a spiral wave and a meandering tip trajectory in red. Tissue is heterogeneous with a larger (smaller) conductivity above (below) the dashed line. Membrane potential is indicated using a gray scale with activated/deactivated tissue shown in white/black. (B) Isochronal map using activation times on the 8×8 grid shown in yellow in (A). No rotation is seen. (C) As in (B) using a time window shifted by 50ms. Rotation is identified. (D) Phase maps spanning the time interval of (B) using the activation times of the grid, with phase singularities shown as black (clockwise rotations) or white (counter-clockwise rotations) symbols.
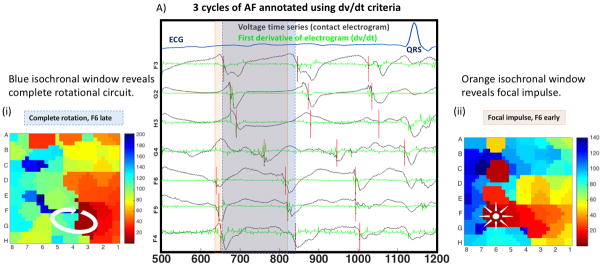
Figure 7 illustrates AF in a 70-year-old lady in whom contour maps are affected by window selection. Shifting the onset time of the analysis window by 15ms (AF cycle length 190ms), from the blue box to the orange box, alters the mapped AF mechanism from a complete rotational circuit to a focal or partial rotation pattern. In simulations phase analysis showed a consistent singularity. Ablation at the site indicated by the blue dashed box terminated persistent AF.
Figure 7. Clinical Impact of timing window on AF maps.
A) Three consecutive cycles of AF are shown in a 70-year-old female, as voltages and –dv/dt. The blue box represents one AF cycle (190ms), with electrograms spanning 100% of the AF cycle in all channels in a rotational circuit (i). Moving this box 15ms earlier (orange box) now shows focal activity (starting with F6) with activity spanning only 140ms (ii). Morphology (qS) did not indicate focal activity (cycles 2 and 3). Ablation at FG34 (from blue window) acutely terminated AF.
Adjustment of electrogram annotation was most impactful on mechanism at sites where multiphasic electrograms were adjacent to those with monophasic morphology. Isochronal window adjustment impacted sites where multiple AF wavefronts coalesced, occupying a large proportion of the activation cycle. Both occurred more frequently at sites of rotational activation than of focal activity.
Discussion
This study reconstructed activation maps for persistent AF, in a cohort of patients in whom localized ablation terminated persistent AF. Repetitive activation patterns were observed at all sites where AF terminated. Activation maps at site of AF termination were, in order of prevalence, partial rotations, repetitive foci, and stable consistent rotational activity. To the best of our knowledge, this latter group is the first demonstration of putative rotational drivers for human persistent AF by activation mapping, without the use of signal processing. In combined clinical and computational studies, maps were altered by the window selected for analysis and which of multiple electrogram deflections were selected for activation marking. In regions of potential slow conduction, we illustrate cases in which assignment of alternative electrogram deflections clarified complete rotational maps at sites where ablation terminated AF. This approach of mapping sites of clinical relevance to AF provides a novel foundation that may ultimately improve approaches to therapy.
Prior Human Persistent AF Mapping Studies
Mapping this cohort of patients builds upon prior classical mapping studies of AF. In keeping with prior studies, the commonest group of mechanisms were partial rotations and multiple foci. However, our finding of stable rotations in 21% of patients is higher than prior studies reporting essentially no 6,8,23,24 rotations or transient rotations in 12% 25 to 17% 17.
This difference may reflect our study design of examining cases in whom AF terminated by ablation, in contrast to prior studies of patients at valvular surgery with incidental permanent AF 8,17,23. This difference may also be reconciled technically. Original reports 26 state that drifting AF sources (e.g. spiral wave) may be missed by wave mapping 23,24 in which “implicit assumption of stationarity over the time window of the analysis” may fragment the circuit into 2 wavefronts 26. Bipolar electrograms used in other reports are impacted by wavefront direction 27 which may reduce the stability of pivoting propagation patterns 17,25 unlike the analysis of unipolar electrograms in our study. Another factor may be our wide mapped areas which account for potential “drifting” sources 13,28. Whereas most of these prior studies mapped epicardium 29, we mapped endocardium. In recent optical mapping of human AF 30,31, endocardial mapping revealed differences between surfaces with stable rotations in AF endocardially that were manifest as sporadic breakthroughs epicardially.
This study adds to the growing body of evidence for organized activation patterns during persistent atrial fibrillation. This was first described using signal processing and vector analysis of electrograms collected on multipolar catheters to identify periods of “transient linking.”32 Lin, et al. described the use of signal processing and phase mapping to identify transient repetitive focal or rotational activity 33. The spatio-temporal dispersion of electrograms on a Pentarray was used in a recent study with similar termination rates of persistent AF to ours, prior to PVI34. These studies corroborate our findings of transient organization of atrial activation during persistent atrial fibrillation. In every patient with a targeted site leading to AF termination, a repetitive activation pattern was identified and mapped, suggesting that this phenomenon is more than a pattern of passive activation 35.
Mechanistic Interpretation at Sites of AF Termination
Further studies are required to explain how local ablation may terminate AF, and generally how ablation of any form terminates AF. The most common maps in our study showed partial rotations with conduction block (46%) and repetitive foci (33%), suggesting that ablation at structural elements causing repetitive conduction block or slowing may contribute to termination 36,37. In the cases that demonstrated full rotations at sites of termination, local ablation at these sites may anchor rotation to convert AF to atrial tachycardia or eliminated non-uniform excitability or small isthmuses of conduction to terminate AF to sinus rhythm 18. Further studies to define the mechanisms of AF termination by ablation may explain the relative contribution of proposed mechanisms including widening an excitable gap to allow invasion by a fibrillatory wave, and whether multiple mechanisms are operative in any one patient 18. It is notable that we observed cases where adjusting activation time assignment, in potential regions of slow conduction or fibrosis, or adjusting isochronal time windows for analysis, converted maps of partial rotations or transient foci to complete rotations.
AF Sources and Termination of Persistent AF by Targeted Ablation
Acute AF termination by ablation is not clearly linked to long-term outcome 38 for unclear reasons that may include lesion durability, incomplete lesion set delivery or progression of AF mechanisms. Nevertheless, recent independent reports of targeted ablation terminating persistent AF 34,39,40 corroborate the deterministic nature of this clinical phenomenon and support the mechanistic importance of our findings. The observation is consistent with a hierarchical model of human AF, with spiral wave(s) or repetitive foci driving fibrillatory conduction. Alternatively, autonomic modulation 41 or stretch induced remote effects could also explain a global effect of a local intervention in non-hierarchical AF models. However, contact force was monitored in half of cases and maintained within nominal limits, and there were no vagal responses suggesting acute autonomic modulation. Reduction of the critical mass required for multiple wavelets is a less likely mechanism, since the total amount of tissue ablated in these cases was often <5% of atrial mass (always <10–20%42) and unlikely to be sufficient for debulking.
These results may help us to refine electrogram marking rules to reduce inconsistencies between activation and phase mapping, which may help in AF mapping to guide ablation. Speculatively, sites where ablation terminates AF may identify specific mechanistic phenotypes, and such sites may warrant additional ablation, and such patients may be offered different post-ablation management than patients in whom AF did not terminate.
Limitations
This study has many important limitations. By specifically examining sites at which focal ablation terminated AF, the selected population consisting only of patients with acute termination may not be representative of all patients with persistent AF. We did not focus on sites where ablation has no acute impact on AF, which should be further studied based on the results of this work. We chose this design because AF termination is one of the few ways to probe acute impact of ablation on AF. We included termination of AF to sinus rhythm, but also termination (conversion) of AF to non-fibrillatory atrial tachycardia. While such AT may be symptomatic if not eliminated, termination still indicates elimination of the fibrillatory process to an organized circuit. Notably, each termination in this study occurred by brief targeted ablation prior to PVI, and not by extensive ablation. Overall, driver ablation does not appear to be pro-arrhythmic 43, and analyzing both termination modes in this work may help to explain prior studies 44,45.
Detection of AF wavefronts is dependent on spatial resolution. Whilst basket catheters used in this study have sufficient resolution to detect spiral waves in human AF 46, as predicted by the minimum wavelength of human atrial action potential and conduction velocity 19, and as shown in human optical imaging of human AF 30, false positives and negatives may occur 46. Accordingly, some of the partial rotations in this study may have appeared complete, and some focal mechanisms may have been micro-reentry had resolution been sufficient. Conversely, higher resolution may also reveal some spiral waves not to be complete, although when many cycles are present particularly by more than one mapping method 12 this would implicate a consistent driver region. Finally, high resolution optical mapping in human AF also shows stable microreeentry 30,31 which correlates with AF drivers identified simultaneously by clinical tools 47. Thus, this study, by itself cannot advocate for the use of multipolar baskets for AF mapping as it is merely describing the observations of apparently successful mapping attempts without regard to possible mapping failures. Nonetheless, the study does identify a possible strategy for identifying AF drivers noting that repetitive patterns were observed at all sites examined.
Of note, all electrogram notations were assigned objectively by algorithm. Separate from the core analysis, we explored manual adjustment of electrograms which in some patients produced substantial variations in mechanisms with relatively minor changes in activation time (figures 4 & 5). This emphasizes how sensitive activation mapping is to relatively small adjustments of choice of electrogram inflection and hence assigned activation timing (< 10% of AF cycle length), and the resulting findings generate hypotheses that require further systematic validation. Choice of ablation catheter and approach targeting localized ablation varied slightly between centers, but this is unlikely to explain the results of our study.
Conclusions
In this study, we successfully mapped persistent AF sources in a cohort of patients in whom localized ablation terminated persistent AF. All maps showed either repetitive partial rotations, focal activity, or complete rotational activation at sites of termination. Assignment of activation timings in multicomponent electrograms in regions of slow condition, and the precise definition of windows for analysis, substantially altered mapped mechanisms. The concept of using the mechanistically relevant clinical endpoint of termination of persistent AF by localized ablation provides a specific and objective endpoint on which to base clinical investigation of mapping, mechanism and ablation strategies.
Supplementary Material
What is Known?
Termination of persistent atrial fibrillation by localized ablation prior to pulmonary vein isolation may support the concept of AF drivers.
Traditional mapping (activation) shows partial rotational circuits, but these maps have never been reported at sites of potential mechanistic importance in patients undergoing ablation.
What This Study Adds?
In a multicenter registry representing one of the largest series of acute terminations of persistent AF prior to PVI (n=57), traditional mapping of sites of termination showed partial rotations (46%), focal patterns (33%) and, for the first time, complete rotational patterns (21%).
To examine how incomplete patterns may relate to AF termination, these data show that uncertainty in activation time assignment for multi-component electrograms, and variations in selecting an analysis window for isochronal maps, may change complete rotations to partial activation patterns.
Computer modeling demonstrated that phase based methods can overcome these limitations, and identify areas of stable rotation which appeared as partial rotations on traditional isochronal maps.
Acknowledgments
Sources of Funding: Dr Zaman is supported by British Heart Foundation Travel Grant and Fulbright British Heart Foundation scholarships and presented interim data at the Samuel A. Levine Young Clinical Investigator Final at the Scientific Sessions of the American Heart Association, Orlando, Florida, November 2015. Dr. Baykaner is supported by the Josephson and Wellens Heart Rhythm Society fellowship. This work was supported by NIH grants to Dr Narayan (HL70529, HL83359, HL103800) and Dr Rappel (HL123384). Professor Peters is supported by NIHR Biomedical Research Centre, BHF and ElectroCardioMaths Programme of the Imperial Centre for Cardiac Engineering.
Footnotes
Disclosures: Drs. Rappel and Narayan are co-authors of intellectual property owned by the University of California Regents, licensed to Topera Inc. and held equity in Topera. Dr. Narayan received honoraria from Medtronic and St. Jude Medical. Dr. Miller received honoraria from St. Jude Medical, Medtronic, Boston Scientific Corp., Biosense-Webster and served as an advisor to Topera Inc. The other authors report no conflicts.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol [Internet] 2014;64:2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Nishida K, Datino T, Macle L, Nattel S. Atrial Fibrillation Ablation. J Am Coll Cardiol [Internet] 2014;64:823–831. doi: 10.1016/j.jacc.2014.06.1172. [DOI] [PubMed] [Google Scholar]
- 3.Verma A, Jiang C, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo Ca, Haverkamp W, Weerasooriya R, Albenque J-P, Nardi S, Menardi E, Novak P, Sanders P. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N Engl J Med [Internet] 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 4.Wong KCK, Paisey JR, Sopher M, Balasubramaniam R, Jones M, Qureshi N, Hayes CR, Ginks MR. No Benefit OF Complex Fractionated Atrial Electrogram ( CFAE ) Ablation in Addition to Circumferential Pulmonary Vein Ablation and Linear Ablation: BOCA Study. Circ Arrhythmia Electrophysiol. 2015;8:1316–1324. doi: 10.1161/CIRCEP.114.002504. [DOI] [PubMed] [Google Scholar]
- 5.Dixit S, Marchlinski FE, Lin D, Callans DJ, Bala R, Riley MP, Garcia FC, Hutchinson MD, Ratcliffe SJ, Cooper JM, Verdino RJ, Patel VV, Zado ES, Cash NR, Killian T, Tomson TT, Gerstenfeld EP. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol [Internet] 2012;5:287–94. doi: 10.1161/CIRCEP.111.966226. [DOI] [PubMed] [Google Scholar]
- 6.de Groot N, van der Does L, Yaksh A, Lanters E, Teuwen C, Knops P, van de Woestijne P, Bekkers J, Kik C, Bogers A, Allessie M. Direct Proof of Endo-Epicardial Asynchrony of the Atrial Wall During Atrial Fibrillation in Humans. Circ Arrhythmia Electrophysiol [Internet] 2016;9:e003648. doi: 10.1161/CIRCEP.115.003648. [DOI] [PubMed] [Google Scholar]
- 7.Narayan SM, Krummen DE, Enyeart MW, Rappel W-J. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS One [Internet] 2012;7:e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL. Simultaneous Bi-Atrial High Density (510 – 512 electrodes) Epicardial Mapping of Persistent and Long-Standing Persistent Atrial Fibrillation in Patients: New Insights into the Mechanism of Its Maintenance. Circulation [Internet] 2015;132:2108–2117. doi: 10.1161/CIRCULATIONAHA.115.017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivkumar K, Ellenbogen KA, Hummel John D, Miller JM, Steinberg JS. Acute Termination of Human Atrial Fibrillation by Identification and Catheter Ablation of Localized Rotors and Sources: First Multicenter Experience of Focal Impulse and Rotor Modulation ( FIRM ) Ablation. J Cardiovasc Electrophysiol. 2012;23:1277–1285. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer P, Kircher S, Rolf S, John S, Arya A. Successful Repeat Catheter Ablation of Recurrent Longstanding Persistent Atrial Fibrillation with Rotor Elimination as the Procedural Endpoint: A Case Series. J Cardiovasc Electrophysiol. 2015;27:274–280. doi: 10.1111/jce.12874. [DOI] [PubMed] [Google Scholar]
- 11.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller JM. Treatment of Atrial Fibrillation by the Ablation of Localized Sources. J Am Coll Cardiol [Internet] 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhusseini M, Vidmar D, Meckler GL, Kowalewski CA, Shenasa F, Wang PJ, Narayan SM, Rappel WJ. Two Independent Mapping Techniques Identify Rotational Activity Patterns at Sites of Local Termination During Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol. 2017;28:615–622. doi: 10.1111/jce.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic Electrophysiological Mapping but not Electrogram Morphology Identifies Stable Sources for Human Atrial Fibrillation: Stable Atrial Fibrillation Rotors and Focal Sources Relate Poorly to Fractionated Electrograms. Circ Arrhythm Electrophysiol [Internet] 2013;6:58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konings KTS, Smeets JLRM, Penn OC, Wellens HJJ, Allessie MA. Configuration of Unipolar Atrial Electrograms During Electrically Induced Atrial Fibrillation in Humans. Circulation [Internet] 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 15.Lau DH, Maesen B, Zeemering S, Kuklik P, van Hunnik A, Lankveld TaR, Bidar E, Verheule S, Nijs J, Maessen J, Crijns H, Sanders P, Schotten U. Indices of Bipolar Complex Fractionated Atrial Electrograms Correlate Poorly with Each Other and Atrial Fibrillation Substrate Complexity. Hear Rhythm [Internet] 2015;12:1415–1423. doi: 10.1016/j.hrthm.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, Park C-I, Denis A, Jaïs P, Hocini M, Potocnik C, Allgeier J, Hochholzer W, Herrera-Sidloky C, Kim S, El Omri Y, Neumann F-J, Weber R, Haïssaguerre M, Arentz T. Ablation of Persistent Atrial Fibrillation Targeting Low-Voltage Areas With Selective Activation Characteristics. Circ Arrhythm Electrophysiol [Internet] 2016:9. doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 17.Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM, Kalman JM. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: Transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 18.Rappel W-J, Zaman JAB, Narayan SM. Mechanisms For the Termination of Atrial Fibrillation by Localized Ablation: Computational and Clinical Studies. Circ Arrhythmia Electrophysiol. 2015;8:1325–1333. doi: 10.1161/CIRCEP.115.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rappel W, Narayan S. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos An Interdiscip J … [Internet] 2013;23113:1–11. doi: 10.1063/1.4807098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenton F, Karma A. Vortex dynamics in three-dimensional continuous myocardium with fiber rotation: Filament instability and fibrillation. Chaos [Internet] 1998;8:20–47. doi: 10.1063/1.166311. [DOI] [PubMed] [Google Scholar]
- 21.Fenton FH, Cherry EM, Hastings HM, Evans SJ. Multiple mechanisms of spiral wave breakup in a model of cardiac electrical activity. Chaos. 2002;12:852–892. doi: 10.1063/1.1504242. [DOI] [PubMed] [Google Scholar]
- 22.Kuklik P, Zeemering S, van Hunnik A, Maesen B, Pison L, Lau D, Maessen J, Podziemski P, Meyer C, Schaffer B, et al. Identification of Rotors during Human Atrial Fibrillation using Contact Mapping and Phase Singularity Detection: Technical Considerations. IEEE Trans Biomed Eng. 2016:9294. doi: 10.1109/TBME.2016.2554660. [DOI] [PubMed] [Google Scholar]
- 23.Allessie MA, de Groot NMS, Houben RPM, Schotten U, Boersma E, Smeets JL, Crijns HJ. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol [Internet] 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 24.de Groot N, Houben R, Smeets J, Boersma E, Schotten U, Schalij M, Crijns H, Allessie M. Electropathological Substrate of Longstanding Persistent Atrial Fibrillation in Patients With Structural Heart DiseaseClinical Perspective. Circulation [Internet] 2010:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 25.Walters TE, Lee G, Morris G, Spence S, Larobina M, Atkinson V, Antippa P, Goldblatt J, Royse A, O’Keefe M, Sanders P, Morton JB, Kistler PM, Kalman JM. Temporal Stability of Rotors and Atrial Activation Patterns in Persistent Human Atrial Fibrillation: A High-Density Epicardial Mapping Study of Prolonged Recordings. JACC Clin Electrophysiol [Internet] 2015;1:14–24. doi: 10.1016/j.jacep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Rogers JM, Usui M, KenKnight BH, Ideker RE, Smith WM. Recurrent wavefront morphologies: a method for quantifying the complexity of epicardial activation patterns. Ann Biomed Eng. 1997;25:761–768. doi: 10.1007/BF02684160. [DOI] [PubMed] [Google Scholar]
- 27.Thompson NC, Stinnett-Donnelly J, Habel N, Benson B, Bates JHT, Sobel BE, Spector PS. Improved spatial resolution and electrogram wave direction independence with the use of an orthogonal electrode configuration. J Clin Monit Comput [Internet] 2013;28:157–163. doi: 10.1007/s10877-013-9508-8. [DOI] [PubMed] [Google Scholar]
- 28.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm [Internet] 2008;5:846–54. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berbari EJ, Lander P, Scherlag BJ, Lazzara R, Geselowitz DB. Ambiguities of epicardial mapping. J Electrocardiol [Internet] 1991;24:16–20. doi: 10.1016/s0022-0736(10)80007-x. [DOI] [PubMed] [Google Scholar]
- 30.Hansen BJ, Zhao J, Csepe Ta, Moore BT, Li N, Jayne La, Kalyanasundaram A, Lim P, Bratasz A, Powell Ka, Simonetti OP, Higgins RSD, Kilic A, Mohler PJ, Janssen PML, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J [Internet] 2015;36:2390–2401. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Hansen BJ, Csepe TA, Lim P, Hummel JD, Fedorov VV. Integration of High Resolution Optical Mapping and 3D Micro-CT Imaging to Resolve the Structural Basis of Atrial Conduction in the Human Heart. Circ Arrhythmia Electrophysiol. 2015:1–7. doi: 10.1161/CIRCEP.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerstenfeld EP, Sahakian aV, Swiryn S. Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation [Internet] 1992;86:375–382. doi: 10.1161/01.cir.86.2.375. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y-J, Lo M-T, Chang S-L, Lo L, Hu Y, Chao T, Chung F, Liao J, Lin C-Y, Kuo H, Chang Y, Lin C, Tuan T, Vincent Young H-W, Suenari K, Dan Do VB, Raharjo SB, Huang NE, Chen S. Benefits of Atrial Substrate Modification Guided by Electrogram Similarity and Phase Mapping Techniques to Eliminate Rotors and Focal Sources Versus Conventional Defragmentation in Persistent Atrial Fibrillation. JACC Clin Electrophysiol [Internet] 2016;2:667–678. doi: 10.1016/j.jacep.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Seitz J, Bars C, Théodore G, Beurtheret S, Lellouche N, Bremondy M, Ferracci A, Faure J, Penaranda G, Yamazaki M, Avula UMR, Curel L, Siame S, Berenfeld O, Pisapia A, Kalifa J. AF Ablation Guided by Spatiotemporal Electrogram Dispersion Without Pulmonary Vein Isolation: A Wholly Patient-Tailored Approach. J Am Coll Cardiol [Internet] 2017;69:303–321. doi: 10.1016/j.jacc.2016.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi Y, Hocini M, O’Neill MD, Sanders P, Rotter M, Rostock T, Jonsson A, Sacher F, Clémenty J, Jaïs P, Haïssaguerre M. Sites of focal atrial activity characterized by endocardial mapping during atrial fibrillation. J Am Coll Cardiol [Internet] 2006;47:2005–12. doi: 10.1016/j.jacc.2005.12.068. [DOI] [PubMed] [Google Scholar]
- 36.Krul SPJ, Berger WR, Smit NW, Van Amersfoorth SCM, Driessen AHG, Van Boven WJ, Fiolet JWT, Van Ginneken ACG, Van Der Wal AC, De Bakker JMT, Coronel R, De Groot JR. Atrial Fibrosis and Conduction Slowing in the Left Atrial Appendage of Patients Undergoing Thoracoscopic Surgical Pulmonary Vein Isolation for Atrial Fibrillation. Circ Arrhythmia Electrophysiol. 2015;8:997. doi: 10.1161/CIRCEP.115.003223. [DOI] [PubMed] [Google Scholar]
- 37.Ashihara T, Haraguchi R, Nakazawa K, Namba T, Ikeda T, Nakazawa Y, Ozawa T, Ito M, Horie M, Trayanova Na. The role of fibroblasts in complex fractionated electrograms during persistent/permanent atrial fibrillation: implications for electrogram-based catheter ablation. Circ Res [Internet] 2012;110:275–84. doi: 10.1161/CIRCRESAHA.111.255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elayi CS, Di Biase L, Barrett C, Ching CK, Al Aly M, Lucciola M, Bai R, Horton R, Fahmy TS, Verma A, Khaykin Y, Shah J, Morales G, Hongo R, Hao S, Beheiry S, Arruda M, Schweikert Ra, Cummings J, Burkhardt JD, Wang P, Al-Ahmad A, Cauchemez B, Gaita F, Natale A. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Hear Rhythm [Internet] 2010;7:1216–1223. doi: 10.1016/j.hrthm.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 39.Miller JM, Kalra V, Das MK, Jain R, Garlie JB, Brewster JA, Dandamudi G. Clinical Benefit of Ablating Localized Sources for Human Atrial Fibrillation. J Am Coll Cardiol [Internet] 2017;69:1247–1256. doi: 10.1016/j.jacc.2016.11.079. [DOI] [PubMed] [Google Scholar]
- 40.Lim HS, Hocini M, Dubois R, Denis A, Derval N, Zellerhoff S, Yamashita S, Berte B, Mahida S, Komatsu Y, Daly M, Jesel L, Pomier C, Meillet V, Amraoui S, Shah AJ, Cochet H, Sacher F, Jaïs P, Haïssaguerre M. Complexity and Distribution of Drivers in Relation to Duration of Persistent Atrial Fibrillation. J Am Coll Cardiol [Internet] 2017;69:1257–1269. doi: 10.1016/j.jacc.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Kapa S, Callans DJ. Looking beyond the ablation shore, treating atrial fibrillation from afar: Integrating anatomic, physiologic, neurologic, and cardiovascular principles into novel therapies. J Am Coll Cardiol [Internet] 2015;65:876–878. doi: 10.1016/j.jacc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Jadidi AS, Cochet H, Shah AJ, Kim SJ, Duncan E, Miyazaki S, Sermesant M, Lehrmann H, Lederlin M, Linton N, Forclaz A, Nault I, Rivard L, Wright M, Liu X, Scherr D, Wilton SB, Roten L, Pascale P, Derval N, Sacher F, Knecht S, Keyl C, Hocini M, Montaudon M, Laurent F, Haïssaguerre M, Jais P. Inverse Relationship between Fractionated Electrograms and Atrial Fibrosis in Persistent Atrial Fibrillation - A combined MRI and High Density Mapping. J Am Coll Cardiol [Internet] 2013;62:802–812. doi: 10.1016/j.jacc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 43.Ramirez FD, Birnie DH, Nair GM, Szczotka A, Redpath CJ, Sadek MM, Nery PB. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: A systematic review and meta-analysis. J Cardiovasc Electrophysiol [Internet] 2017:1–8. doi: 10.1111/jce.13313. [DOI] [PubMed] [Google Scholar]
- 44.Haïssaguerre M, Sanders P, Hocini M, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu L-F, Bordachar P, Reuter S, Roudaut R, Clémenty J, Jaïs P. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol [Internet] 2005;16:1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 45.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat M-Q, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation [Internet] 2014;130:530–8. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 46.Roney CH, Cantwell CD, Bayer JD, Qureshi NA, Lim PB, Tweedy JH, Kanagaratnam P, Peters NS, Vigmond EJ, Ng FS. Spatial Resolution Requirements for Accurate Identification of Drivers of Atrial Fibrillation. Circ Arrhythmia Electrophysiol [Internet] 2017;10:e004899. doi: 10.1161/CIRCEP.116.004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen BJ, Briggs C, Moore BT, Csepe TA, Li N, Zhao J, Garikipati NV, Janssen PM, Mohler PJ, Hummel JD, Fedorov VV. Abstract 18402: Human Atrial Fibrillation Drivers Seen Simultaneously by Focal Impulse and Rotor Mapping and High-resolution Optical Mapping. Circulation [Internet] 2015;132:A18402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.