Abstract
Objective: GlucoTrack is a non-invasive device that indirectly measures glucose fluctuation in the earlobe tissue. Thus, its accuracy may be subjected to a time lag between glucose concentration in blood and tissue. This time lag was shown to depend on individual characteristics related to microvascular complications, such as diabetes duration, HbA1c level, and smoking history. Therefore, the current study investigated the effects of these factors on GlucoTrack performance.
Research design and methods: Clinical trials were conducted on 114 people with type 2 diabetes. Device performance was clinically evaluated using Clarke error grid (CEG) analysis and numerically evaluated using the distribution of absolute relative difference (ARD) values.
Results: CEG analysis revealed that 98.0% of glucose readings were within the clinically acceptable CEG A + B zones. Total mean ARD was 22.7%. Clinical and numerical accuracies were comparable between never smokers and former/current smokers, but slightly reduced in the HbA1c ≥ 7.5% group and in the diabetes duration ≥15 years group. Yet, likelihood ratio and parametric bootstrap tests statistically demonstrated that ARD values did not depend on diabetes duration, HbA1c level, or smoking history.
Conclusions: GlucoTrack performance does not depend on diabetes duration, HbA1c level, and smoking history, indicating the device is suitable for various people with type 2 diabetes.
Keywords: Non-invasive glucose monitoring, GlucoTrack, diabetes, glucose self-monitoring
1. Introduction
Self-monitoring of blood glucose (SMBG) has been long recognized as a key component of treatment regimens and an essential requirement in diabetes management [1]. SMBG is important for determining medical treatment, for the detection of blood glucose levels outside of the target range and for determining the impact of lifestyle changes (nutrition, physical activity) on glucose levels. Accordingly, diabetes guidelines for SMBG even in non-insulin treated type 2 diabetes endorse SMBG as part of ongoing diabetes self-management to assist in better understanding the disease and provides a means to actively and effectively participate in its control and treatment [2,3]. These guidelines are supported by abundant evidence pointing to clinical benefits following frequent SMBG in type 1 [4], insulin-treated type 2 [5] and non-insulin treated type 2 diabetes mellitus [6,7].
However, the pain and invasiveness associated with current SMBG technology can lead to patient incompliance and insufficient number of daily measurements [8]. Accordingly, it has been recently proposed that non-invasive glucose monitoring may encourage more frequent estimation of glucose levels and thus improve glycemic control [9].
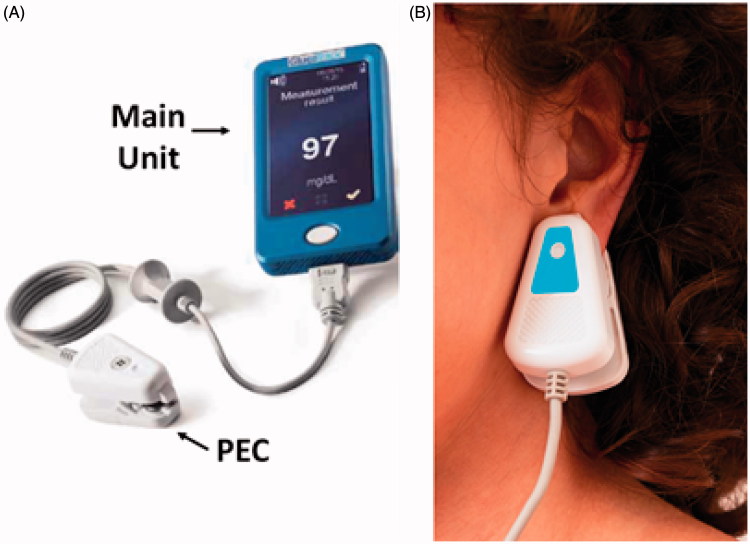
One commercially available non-invasive glucose monitoring device for home use is GlucoTrack (Figure 1) [10–12]. GlucoTrack tracks the physiological effects of glucose variations in the earlobe tissue using three independent technologies: ultrasonic, electromagnetic, and thermal in order to estimate tissue glucose by them. The device measures specific ultrasonic, electromagnetic and thermal parameters of the tissue, which change due to glucose-related shifts in ion concentration, density, compressibility and hydration of both cellular and extracellular compartments of the tissue [10,11].
Figure 1.
GlucoTrack non-invasive monitoring device. A. The device includes a main unit and three different sensor pairs, one per each of the three technologies, all located at the tip of a personal ear clip (PEC). B. Illustration of glucose measurement performance using GlucoTrack. The PEC is clipped to the earlobe for spot measurement.
Specifically, GlucoTrack measures the physiological effects that occur in the whole earlobe tissue rather than directly sampling the blood or the subcutaneous interstitial fluid (ISF). The earlobe tissue is a skin tissue, composed of the epidermis, dermis and subcutaneous layers, each composed of varying volumes of blood plasma, lymph fluid, ISF and cells [13]. While all of these components are involved in glucose handling, their glucose concentrations vary in a given time [13]. Indeed, the presence of a lag in glucose concentration in all skin layers ISF relative to the blood has been widely reported and acknowledged [14,15], and has been a major issue previously confronted by continuous glucose monitoring systems (CGMs) [13,15,16]. Since the time lag of glucose dynamics varies depending on the properties of the skin layer (e.g. volumes of interstitial fluid, cells, and blood) [13], estimating glucose from the whole tissue subjects GlucoTrack accuracy to suffer from a time lag between the glucose concentration in blood and ISF of all skin layers. Thus, the accuracy of GlucoTrack may be affected by a general blood-tissue time lag that depends on a total lag between ISF and blood.
The total duration of the physiological lag between blood and ISF is mostly determined by blood perfusion at the measured site [17]. Although GlucoTrack mitigates some of this lag through a unique algorithm [10,11], this perfusion-related delay can be considerably different among individuals with different clinical characteristics. We have recently shown that age, gender, body mass and the presence of ear piercing do not have an effect on GlucoTrack performance [18], despite their potential effects on tissue characteristics (e.g. structure and hydration status) [19,20]. However, the time lag was shown to depend on other individual characteristics, specifically those related to microvascular complications. For example, diabetes duration, higher levels of HbA1c and smoking history were previously associated with risk of microvascular events [21–23]. By exerting effects on the time lag, these clinical characteristics may postpone the changes in tissue glucose levels and as a result their corresponding tissue parameters. Thus, there might be discrepancies between actual blood glucose levels and the tissue glucose estimated by these tissue parameters. Therefore, the current study evaluated GlucoTrack performance as a function of diabetes duration, smoking history and HbA1c levels.
2. Subjects, materials and methods
2.1. Participants
135 subjects with type 2 diabetes were screened. 21 subjects did not complete the clinical trial mainly due to unavailability of the subject or technical malfunctions during data acquisition. Thus, the present study evaluated GlucoTrack performance on 114 people with type 2 diabetes above the age of 18 according to the device intended use. Exclusion criteria included earlobe scratches, birthmarks, multiple piercing or any other condition that may hamper the contact between the personal ear clip (PEC) and the earlobe. Due to constrains derived from the mechanical shape and size of the sensors’ assembly, subjects with earlobes of less than 14 mm or above 25 mm in diameter and subjects with earlobe thickness lower than 3 mm or above 6 mm were excluded from the study. Participants on dialysis and pregnant or nursing women were also excluded from the study due to imbalance in their water and mineral state [24,25].
2.2. Clinical characteristics
Sixty-five subjects were only on oral medication (57%), 6 subjects were only on insulin (5.3%), 40 subjects were on both insulin and oral medication (35.1%) and 3 subjects were not on either oral or insulin treatments (2.6%). Diabetes duration, smoking history and HbA1c level were chosen to represent the perfusion-related clinical characteristics that may affect the measured tissue parameters. The clinical characteristics of the subjects are summarized in Table 1.
Table 1.
Participants' clinical characteristics and the number of paired GlucoTrack-invasive readings.
| Category | No. of subjects | No. of paired readings |
|---|---|---|
| Smoking history | ||
| Former/current smokers | 44 | 2002 |
| Never smokers | 70 | 3111 |
| HbA1c level (%) a | ||
| ≤7.5 | 58 | 2588 |
| >7.5 | 42 | 1872 |
| Diabetes duration (years) | ||
| 0-5 | 10 | 801 |
| 5–10 | 25 | 1404 |
| 10–15 | 25 | 1605 |
| >15 | 29 | 1303 |
| Total | 114 | 5113 |
The data was available from 100 participants.
The analyses of the HbA1c data were conducted on 100 participants due to unreported data by 14 subjects. To note, subjects’ stratification was conducted retroactively and their enrolment was only based on the exclusion/inclusion criteria aforementioned and did not depend on these clinical characteristics. Participants were stratified based on diabetes duration by 5 year strata, as has been previously done [21]. This previous study has shown that for each 5 year increase in diabetes duration, the risk of microvascular events was increased by 28%. The threshold of HbA1c categorization was set to 7.5%. This threshold was determined based on research showing that in participants with type 2 diabetes, a higher level of HbA1c is significantly associated with higher risks of microvascular events in threshold above 7.0% [26] and that individuals with HbA1c in the range of 7.0–7.9% have nine-fold higher prevalence of mild and moderate retinopathy than people with HbA1c ≤ 6.9% [22]. Smoking history was dichotomized to never smokers and former/current smokers, as has been previously done in epidemiological studies [27].
2.3. Procedure
Clinical trials were conducted in the diabetes unit of the Soroka University Medical Center, Be’er Sheva, Israel. The study protocol was approved by the local ethics committee and all participants signed an informed consent form. Trial Registration: ClinicalTrials.gov Identifier: NCT00889668 (http://www.clinicaltrials.gov).
On the first day of the study participants underwent individual PEC adjustment to ensure comfort, optimal fit and good sensor-to-tissue contact for each ear width. The PEC was then individually calibrated to establish an individual baseline for the detection of physiological changes. The calibration process involved three paired measurements of GlucoTrack and an invasive reference, with 10 minute intervals between each pair. The invasive blood glucose measurements were obtained from finger capillary blood using the HemoCue® Glucose 201 RT system (Ängleholm, Sweden). GlucoTrack spot measurements were conducted by placing the PEC on the participants’ earlobes. Each measurement took about 1 minute. After completing the measurement, the PEC was removed, the glucose level was displayed on the screen of the device and recorded in the clinical research form.
The study involved two to three non-consecutive days of sampling in the course of one month. Each trial-day continued for 8 to 10 hours (between 8:00 AM to 6:00 PM) and included about 16 simultaneous paired measurements with GlucoTrack and HemoCue. On each trial day, subjects received meals and fruits in order to produce variability in their glucose profiles. Paired GlucoTrack-HemoCue measurements were taken before and between 30 to 210 minutes after food consumption [12].
2.4. Evaluation methods
GlucoTrack performance was evaluated using clinical and numerical accuracy methods [28]. Clinical accuracy was assessed using Clarke error grid (CEG) analysis that evaluates medical importance of the differentiations between GlucoTrack and the established invasive blood glucose reference method [29]. In this analysis, a grid breaks down a scatterplot of a reference glucose monitoring device and an evaluated glucose monitoring device into five regions: region A includes values within 20% of the reference, region B contains points that are outside of 20% but would not lead to inappropriate treatment, region C consists of points leading to unnecessary treatment, region D includes points indicating a potentially dangerous failure to detect hypoglycemia or hyperglycemia, and region E contains points that would confuse treatment of hypoglycemia for hyperglycemia and vice versa. Region A is considered clinically accurate and region B is considered clinically acceptable. Numerical accuracy was assessed using absolute relative difference (ARD) of paired GlucoTrack-HemoCue measurement readings, calculated as follows: ARD=|GlucoTrack-HemoCue|/HemoCue*100[%], where GlucoTrack refers to measurement result of GlucoTrack and HemoCue refers to measurement result of HemoCue. The distribution of ARD values was used to gain statistical insights on the device performance across HbA1c level, diabetes duration and smoking history.
2.4.1. Statistical analysis of ARD values
An adequate statistical evaluation requires consideration of the nested nature of the data (data for each subject are organized in multiple levels) and residual distribution of the outcome (ARD). Hence, statistical framework of generalized linear mixed effects models was used to identify the model with the best fit to the data. The Akaike Information Criterion (AIC) was used to choose the best model that fits the data: gamma residual distribution with log link function. Repeated measurements were nested within corresponding days and the latter were nested within subjects. The differences between clinical characteristics categories were examined using two different statistical tests: the likelihood ratio test (LRT) and the parametric bootstrap test (PBT) [30]. Two statistical tests were used to ensure the robustness of the findings. All statistical analyses were performed using R software (version 3.2.3) and the Ime4 package [31].
3. Results
Overall GlucoTrack accuracy
CEG analysis showed that 98.0% of the measurements (5,113 cases) were in the clinically acceptable zones A and B, with 52.3% in the clinically accurate zone A. 2.0% were in zone D. No data points fell in zones C or E. Total mean ARD was 22.7% and total median ARD was 19.0%.
Diabetes duration
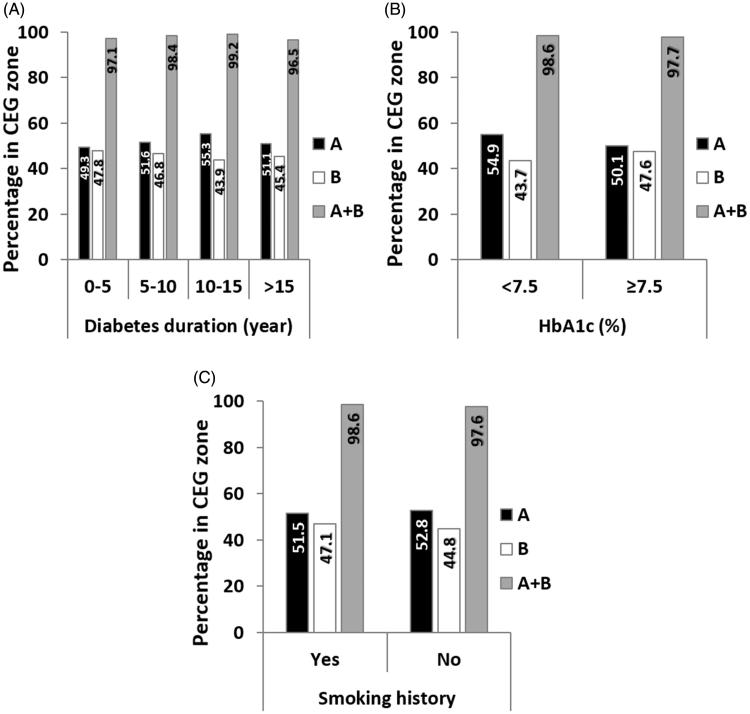
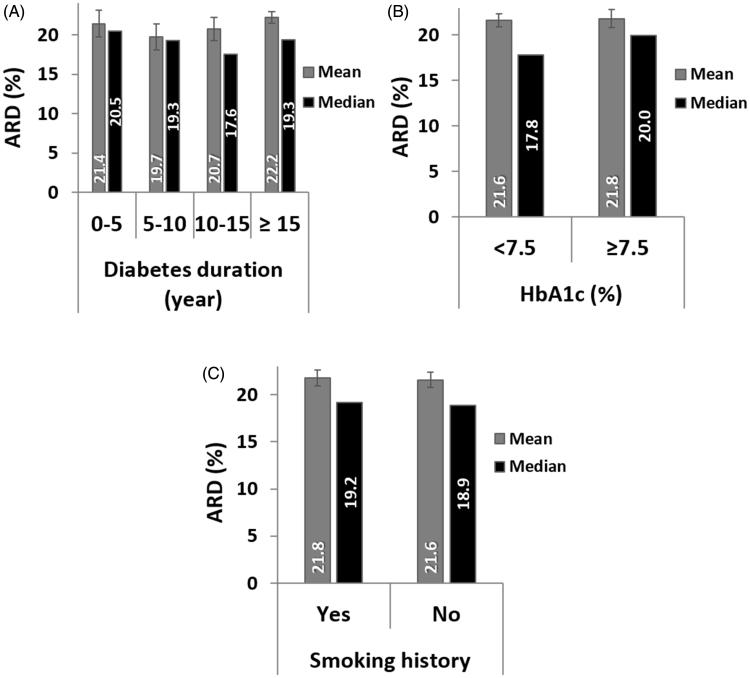
For each group between 801 and 1,605 paired GlucoTrack-HemoCue readings were obtained (Table 1). Clinical accuracy measured by CEG was comparable for all duration groups, with a marginal decrease for above 15 years of diabetes duration (Figure 2(A)). Comparison of ARD values within each group revealed similar mean and median values (Figure 3(A)). According to LRT and PBT, no significant relationship was found between ARD and diabetes duration (χ2 (3) = 2.26, pLRT = 0.52, pPBT = 0.56).
Figure 2.
Clinical accuracy assessed by CEG as function of A. Diabetes duration, B. HbA1c, and C. Smoking history.
Figure 3.
Numerical accuracy as function of A. Diabetes duration, B. HbA1c, and C. Smoking history: Mean ARD, its model-based upper and lower 95% confidence intervals and median ARD.
HbA1c levels
Above 1,800 paired GlucoTrack-HemoCue readings were obtained for both HbA1c level groups. The clinical accuracy was slightly better in the HbA1c < 7.5% group (Figure 2(B)). However, the mean and median ARD values were similar across groups (Figure 3(B)), and there was no statistical difference between HbA1c groups and ARD values (χ2 (1) = 0.026, pLRT = 0.87, pPBT = 0.89).
Smoking history
Clinical accuracy did not differ between smoking groups (Figure 2(C)). Mean and median ARD were also similar across groups (Figure 3(C)) and it did not depend on smoking history (χ2 (1) = 0.025, pLRT = 0.87, pPBT = 0.93).
4. Discussion
Non-invasive glucose monitoring holds great promise for improved management of diabetes, since it may alleviate the pain associated with frequent skin pricking. The accuracy is an essential requirement from emerging non-invasive systems for people with diabetes. However, showing overall accuracy is insufficient. It is also important to evaluate how clinical characteristics of its users affect device performance to ensure its suitability for a broad range of people [32]. In the present study, we investigated the performance of GlucoTrack among people with type 2 diabetes with different clinical characteristics that may influence the accuracy of GlucoTrack by affecting the time lag between blood and tissue glucose and thus changing the levels of glucose estimated from the tissue. Overall, our results show that the accuracy of the device does not depend on diabetes duration, smoking history or HbA1c level. These results extend previous findings, showing that demographic factors such as age, gender, body mass and the presence of ear piercing do affect GlucoTrack performance [18], further advocating GlucoTrack’s suitability for type 2 diabetes mellitus population.
GlucoTrack estimates glucose from the whole earlobe tissue. The epidermis, dermis and subcutaneous layers of the skin have been shown to vary in volumes of blood plasma and ISF, which affect the time lag of glucose dynamics [13]. Thus, an important determinant of GlucoTrack accuracy is the total physiological time lag of glucose transport from the vascular to the interstitial space of all three skin layers of the earlobe. This physiological delay mainly depends on blood perfusion which influences vascular permeability [17]. In this study we focused on three clinical characteristics that have been associated with microvascular complications: diabetes duration [21], HbA1c levels [26] and smoking history [23]. To comprehensively assess GlucoTrack performance as a function of these factors, accuracy was evaluated both numerically (ARD distribution) and clinically (CEG) [28]. Our results show that GlucoTrack numerical and clinical accuracies were not affected by smoking history. In contrast, the clinical accuracy was slightly reduced in people with diabetes duration above 15 years as well as in people with HbA1C levels higher that 7.5%, but were all acceptable (above 95% in A + B zones). These results correspond with previous studies demonstrating that higher HbA1c levels and longer disease duration affect vascular permeability, thus influencing the time lag between tissue and blood and consequently GlucoTrack estimated glucose levels. Nonetheless, despite these small differences, statistical tests on ARD values showed independency between these examined clinical characteristics and device accuracy. Taken together, these findings indicate that GlucoTrack accuracy is consistent across individuals with different perfusion-related clinical characteristics.
This study focused on the extent to which inter-subject variability in constant time lags alter GlucoTrack performance. Thus, the consistency in GlucoTrack performance across clinical characteristics can probably be accredited to an effective individual calibration, which establishes a baseline for the detection of physiological changes that are not expected to alter substantially during the device calibration period (6 months). Future studies should also examine if the accuracy of GlucoTrack depends on intra-subject variability in the physiological delay imposed by factors such as food intake.
It should be noted that the performance of GlucoTrack is inferior to that of current SMBG and CGMs, mainly due to the indirect non-invasive nature of the measurement that subjects it to suffer from a relatively low signal-to-noise ratio. For this reason, GlucoTrack should not be used for diagnosis and medications intake or treatment decisions should not be based only on measurements obtained by it. Moreover, GlucoTrack device is under constant improvements, aiming to further advance its performance by significantly improving its algorithms, software and features.
There are also several limitations to this study. First, there are other clinical characteristics known to affect perfusion (e.g. cardiovascular and renal disease [33]), that should be addressed in future studies. Second, HbA1c data were missing from 14 subjects, however it is a small proportion of the total population, thus conclusions could have been made. Additionally, due to relatively small sample size we could not distinguish between current and former smokers. Furthermore, a more systematic smoking history assessment (e.g. packs of cigarettes per day) could contribute to more specific insights in this regard. Evaluating GlucoTrack performance in future large-scale studies that assess detailed smoking history may shed light on this issue. Finally, GlucoTrack results were compared against HemoCue, rather than comparing them against gold standard reference samples. This, however, should not affect the interpretation of our results.
In conclusion, GlucoTrack accuracy was comparable across individuals with different clinical characteristics, indicating it is suitable for a variety of people with type 2 diabetes mellitus. We believe that due to its non-invasive nature, the device will encourage frequent glucose monitoring, and thus improve glycemic control and consequentially reduce diabetes-related complications.
Acknowledgements
The authors thank Dr. Pavel Goldstein for helpful statistical consultation.
Funding Statement
This study is funded by Integrity Applications Ltd, Ashdod, Israel.
Transparency
Declaration of funding
This study was funded by Integrity Applications Ltd, Ashdod, Israel.
Declaration of financial/other relationships
TL, YM, and KB are/were full time employees of Integrity Applications, Ashdod, Israel at the time of the analysis and may potentially own stocks or hold stock options in the company. JDA peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1. Bergenstal RM, Gavin JR.. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med. 2005;118:1–6. [DOI] [PubMed] [Google Scholar]
- 2. Gagliardino JJ, Bergenstal R, Colagiuri S, et al. IDF Guideline on self-monitoring of blood glucose in non-insulin treated type 2 diabetes. Int Diabetes Fed. IDF Brus, Int Diabetes Fed. 2009. [Google Scholar]
- 3. American Diabetes Association Standards of medical care in diabetes: 2016. Diabetes Care. 2016;29(Suppl 1):S1–S112. [Google Scholar]
- 4. Haller MJ, Stalvey MS, Silverstein JH.. Predictors of control of diabetes: monitoring may be the key. J Pediatr. 2004;144:660–661. [DOI] [PubMed] [Google Scholar]
- 5. Schütt M, Kern W, Krause U, et al. . Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24 500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Amp Diabetes. 2006;114:384–388. [DOI] [PubMed] [Google Scholar]
- 6. Towfigh A, Romanova M, Weinreb JE, et al. . Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: a meta-analysis. Am J Manag Care. 2008;14:468–475. [PubMed] [Google Scholar]
- 7. Karter AJ, Ackerson LM, Darbinian JA, et al. . Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry∗. Am J Med. 2001;111:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol. 2008;2:919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ong WM, Chua SS, Ng CJ.. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer. Adherence. 2014;8:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harman-Boehm I, Gal A, Raykhman AM, et al. . Noninvasive glucose monitoring: a novel approach. J Diabetes Sci Technol. 2009;3:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harman-Boehm I, Gal A, Raykhman AM, et al. . Noninvasive glucose monitoring: increasing accuracy by combination of multi-technology and multi-sensors. J Diabetes Sci Technol. 2010;4:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horman K, Mayzel Y, Gal A, et al. . Performance and user experience evaluation of a non-invasive glucose monitoring device. Int J Diabetes Metab Disord. 2016;1:1–7. [Google Scholar]
- 13. Groenendaal W, von Basum G, Schmidt KA, et al. . Quantifying the composition of human skin for glucose sensor development. J Diabetes Sci Technol. 2010;4:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cengiz E, Tamborlane WV.. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11(Suppl 1):S11–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stout PJ, Peled N, Erickson BJ, et al. . Comparison of glucose levels in dermal interstitial fluid and finger capillary blood. Diabetes Technol Ther. 2001;3:81–90. [DOI] [PubMed] [Google Scholar]
- 16. Cengiz E, Tamborlane WV.. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11:S1–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koschinsky T, Jungheim K, Heinemann L.. Glucose sensors and the alternate site testing-like phenomenon: relationship between rapid blood glucose changes and glucose sensor signals. Diabetes Technol Ther. 2003;5:829–842. [DOI] [PubMed] [Google Scholar]
- 18. Bahartan K, Horman K, Gal A, et al. . Assessing the performance of a non-invasive glucose monitor in people with type 2 diabetes with different demographic profiles. J Diabetes Res. 2017;2017:4393497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan CY, Statham B, Marks R, et al. . Skin thickness measurement by pulsed ultrasound: its reproducibility, validation and variability. Br J Dermatol. 1982;106:657–667. [DOI] [PubMed] [Google Scholar]
- 20. Mast TD. Empirical relationships between acoustic parameters in human soft tissues. Acoust Res Lett Online. 2000;1:37–42. [Google Scholar]
- 21. Zoungas S, Woodward M, Li Q, et al. . Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57:2465–2474. [DOI] [PubMed] [Google Scholar]
- 22. Sabanayagam C, Liew G, Tai ES, et al. . Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes? Diabetologia. 2009;52:1279–1289. [DOI] [PubMed] [Google Scholar]
- 23. Sinha RN, Patrick AW, Richardson L, et al. . A six-year follow-up study of smoking habits and microvascular complications in young adults with type 1 diabetes. Postgrad Med J. 1997;73:293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bergström J, Hultman E.. Water, electrolyte and glycogen content of muscle tissue in patients undergoing regular dialysis therapy. Clin Nephrol. 1973;2:24–34. [PubMed] [Google Scholar]
- 25. Lederman SA, Paxton A, Heymsfield SB, et al. . Body fat and water changes during pregnancy in women with different body weight and weight gain. Obstet Gynecol. 1997;90:483–488. [DOI] [PubMed] [Google Scholar]
- 26. Zoungas S, Chalmers J, Ninomiya T, et al. . Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55:636–643. [DOI] [PubMed] [Google Scholar]
- 27. Miller VA, Kris MG, Shah N, et al. . Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non–small-cell lung cancer. J Clin Oncol. 2004;22:1103–1109. [DOI] [PubMed] [Google Scholar]
- 28. Kovatchev B, Anderson S, Heinemann L, et al. . Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31:1160–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clarke WL. The original Clarke Error Grid Analysis (EGA). Diabetes Technol Ther. 2005;7:776–779. [DOI] [PubMed] [Google Scholar]
- 30. Halekoh U, Højsgaard S.. A Kenward-Roger approximation and parametric bootstrap methods for tests in linear mixed models: the R package pbkrtest. J Stat Softw. 2014;59:1–32.26917999 [Google Scholar]
- 31. Bates D, Maechler M, Bolker B, et al. . Fitting linear mixed-effects models using lme4. J Stat Softw. 2014;67:17487. [Google Scholar]
- 32. Lin T, Gal A, Mayzel Y, et al. . Non-invasive glucose monitoring: a review of challenges and recent advances. Curr Trends Biomed Eng Biosci. 2017;6:1–8. [Google Scholar]
- 33. O’hare AM, Glidden DV, Fox CS, et al. . High prevalence of peripheral arterial disease in persons with renal insufficiency results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;109:320–323. [DOI] [PubMed] [Google Scholar]