Abstract
Purpose
Anticancer T-cell responses can control tumors, but immunosuppressive mechanisms in vivo prevent their function. The role of regulatory T cells (Tregs) in metastatic colorectal cancer is unclear. We have previously shown depletion of Tregs enhances colorectal cancer–specific effector T-cell responses. Low-dose cyclophosphamide targets Tregs in animal models and some human studies; however, the effect of cyclophosphamide in metastatic colorectal cancer is unknown.
Experimental Design
Fifty-five patients with metastatic colorectal cancer were enrolled in a phase I/II trial and randomly assigned to receive 2-week-long courses of low-dose (50 mg twice a day) cyclophosphamide or not. The absolute number, phenotype, and antitumor function of peripheral blood–derived lymphocyte subsets were monitored throughout treatment, as well as during 18-month follow-up.
Results
Initially, cyclophosphamide reduced proliferation in all lymphocyte subsets; however, a rapid mobilization of effector T cells overcame this decrease, leading to increased absolute T-cell numbers. In contrast, a reduction in proportional and absolute Treg, B-cell, and NK-cell numbers occurred. The expansion and subsequent activation of effector T cells was focused on tumor-specific T cells, producing both granzyme B and IFNγ. Cyclophosphamide-treated patients demonstrating the most enhanced IFNγ+ tumor-specific T-cell responses exhibited a significant delay in tumor progression [HR = 0.29; 95% confidence interval (CI), 0.12–0.69; P = 0.0047), compared with nonresponders and no-treatment controls.
Conclusions
Cyclophosphamide-induced Treg depletion is mirrored by a striking boost in antitumor immunity. This study provides the first direct evidence of the benefit of naturally primed T cells in patients with metastatic colorectal cancer. Our results also support the concept that nonmutated self-antigens may act as useful targets for immunotherapies.
Introduction
Metastatic colorectal cancer remains the second leading cause of death from cancer (1). The current care of patients revolves around excision of the tumor, histopathologic staging, and 5-fluorouracil–based chemotherapy, either as adjuvant with a curative aim, or as palliative chemotherapy for patients with advanced metastatic disease. Chemotherapy has a significant morbidity (and even mortality) associated with its use, and approximately 40% of patients with "curative" treatment relapse and succumb to the cancer. Hence, there is a need to develop less toxic and more targeted therapies.
Correctly harnessing a patient's immune system to target and kill cancerous cells has enormous potential but has thus far proven challenging, with immune-based therapies for colorectal cancer in particular lacking in efficacy (2). One potential obstacle to achieving objective antitumor responses is the suppression of tumor-specific T cells by CD4+CD25hiFoxp3+ regulatory T cells (Tregs; refs. 3, 4). Cancer-bearing individuals have increased frequencies of Tregs, both in peripheral blood, and enriched within the tumor microenvironment (5). We have previously shown that colorectal cancer can drive this expansion of Tregs that control antitumor immune responses (6–10). Specifically, when peripheral blood–derived Tregs from colorectal cancer patients are depleted in vitro, we found tumor antigen (5T4, CEA)-specific IFNγ+ responses are enhanced (11, 12).
The utilization of metronomic low-dose cyclophosphamide was initially proposed as a salvage therapy in end-stage cancer patients, aimed at inhibiting tumor angiogenesis (13–15), although the immunomodulatory effects of cyclophosphamide had been noted decades earlier in animal models (16, 17). Currently, there is a revived interest in using cyclophosphamide for the purpose of depleting Tregs in humans (refs. 8, 18 and reviewed in ref. 19). Tregs are thought to be more susceptible to the toxic effects of cyclophosphamide due to their low levels of intracellular ATP and glutathione, relative to other T cells (20); glutathione is required to counteract the toxic effects of cyclophosphamide on cellular DNA, thus giving effector T-cell populations a survival advantage. Profound effects on the function of other immune cells, including NK cells (21), dendritic cells (22), and MDSCs (23) have also been noted. However, at least one group did not observe Treg depletion using a low-dose metronomic dosing regimen (24), and a lack of consensus exists due to the concurrent use of other immune-potentiating therapies; the heterogeneous methods used to identify Tregs; the study days on which immunologic responses were monitored; and the cohorts of cancer patients studied. In addition, some groups have administered low doses of cyclophosphamide intravenously, and it is possible differences may result from pharmacodynamic effects when compared with oral dosing (21, 25).
A metronomic, oral dosage of 50mg twice-a-day cyclophosphamide has previously been established as a dose that depletes Tregs whilst preserving overall lymphocyte numbers (21). Here, we sought to determine the effect of this dosage of cyclophosphamide on lymphocyte subsets in patients with metastatic colorectal malignancy, as part of a randomized phase I/II clinical trial. A primary objective was to identify whether cyclophosphamide treatment induced oncofetal antigen (5T4)-specific lymphocyte responses; the secondary objectives included measurements of the effects of cyclophosphamide on T-cell subsets (i.e., number, phenotype and function) and tumor progression.
Materials and Methods
Patients
Fifty-five subjects with inoperable metastatic colorectal cancer were enrolled on to the phase I/II clinical trial, TaCTiCC (TroVax and Cyclophosphamide Treatment in Colorectal Cancer; ref. 26). Patients receiving prior first-line chemotherapy had to show evidence of responding or stable disease within 4 weeks of trial entry, as demonstrated by CT scan in comparison with pretreatment CT scan using RECIST criteria to measure quantitative tumor burden. Additional inclusion criteria included age ≥18 years, WHO performance status 0–2, lymphocyte count ≥ 500/μL, neutrophil count > 1,200/μL, and platelet count > 100,000/μL. Key exclusion criteria included life expectancy <3 months, evidence of actively progressing malignancy, bilirubin level >50 μmol/L, completion of first-line chemotherapy less than 2 weeks from start of treatment, clinically apparent/active autoimmune disease or those receiving immunosuppressants.
All patients gave written, informed consent personally prior to trial inclusion. Results reported in this manuscript are derived from a clinical trial approved by the Cardiff and Vale Ethics committee and the Gene Therapy Advisory Committee (GTAC 175). Trial authorization was granted from the Medicines and Healthcare products Regulatory Authority.
Study design and treatment
This trial was a randomized, open-label study carried out from September 2012 to May 2016. Randomization was undertaken at the Clinical Trials Office, University Hospital of Wales (Wales, United Kingdom), using an unstratified balanced block design, with the outcome communicated to the attending physician immediately upon randomization. Patient characteristics are shown in Table 1. Upon enrolment, 27 patients were randomized to a cyclophosphamide group, and 28 were randomized to a non-cyclophosphamide group: one patient withdrew consent one day postrandomization and two patients were later found to have had a curative procedure preenrolment. Thus, 25 patients were analyzed from this group and reported in this article, as shown in the CONSORT flow diagram (Supplementary Fig. S1). Of these patients, 17 received subsequent immunotherapy and were excluded from survival analyses, and the remaining eight were randomized to a "watch & wait" control arm of the trial and did not receive any subsequent treatment.
Table 1.
Patient baseline characteristics
| Cyclophosphamide (n = 27) |
No cyclophosphamide (n = 25) |
|
|---|---|---|
| Sex | ||
| Female | 9 (33%) | 5 (20%) |
| Male | 18 (67%) | 20 (80%) |
| Mean age, y | 65.2 | 63.1 |
| Site of primary | ||
| Right | 5 (19%) | 4 (16%) |
| Left | 9 (33%) | 12 (48%) |
| Sigmoid/rectum | 10 (37%) | 9 (36%) |
| Not identified | 3 (11%) | 0 (0%) |
| Site of metastases | ||
| Liver | 19 (70%) | 14 (56%) |
| Lung | 11 (41%) | 13 (52%) |
| Peritoneum | 11 (41%) | 8 (32%) |
| WHO Performance status | ||
| 0 | 15 (56%) | 10 (40%) |
| 1 | 12 (44%) | 14 (56%) |
| 2 | 0 (0%) | 1 (4%) |
| Previous treatment | ||
| Primary colectomy | 15 (56%) | 17 (68%) |
| Capecitabine | 27 (100%) | 25 (100%) |
| 5-Fluorouracil | 24 (89%) | 24 (96%) |
| Irinotecan | 11 (41%) | 11 (44%) |
| Oxaliplatin | 9 (33%) | 9 (36%) |
| Cetuximab | 3 (11%) | 0 (0%) |
| Aflibercept | 2 (7%) | 1 (4%) |
Orally administered 50 mg cyclophosphamide was taken twice-a-day on treatment days 1–7 and 15–21; no cyclophosphamide was taken on treatment days 8–14 or 22–106, or until patient relapsed. Peripheral blood samples (10–40 mL) were taken at regular intervals during therapy, as shown in the schematic (Fig. 1A). Blood samples were subsequently taken at treatment days 29, 43, 64, 78, and 106. All patients were clinically examined throughout the trial, with progression-free survival (PFS) monitored.
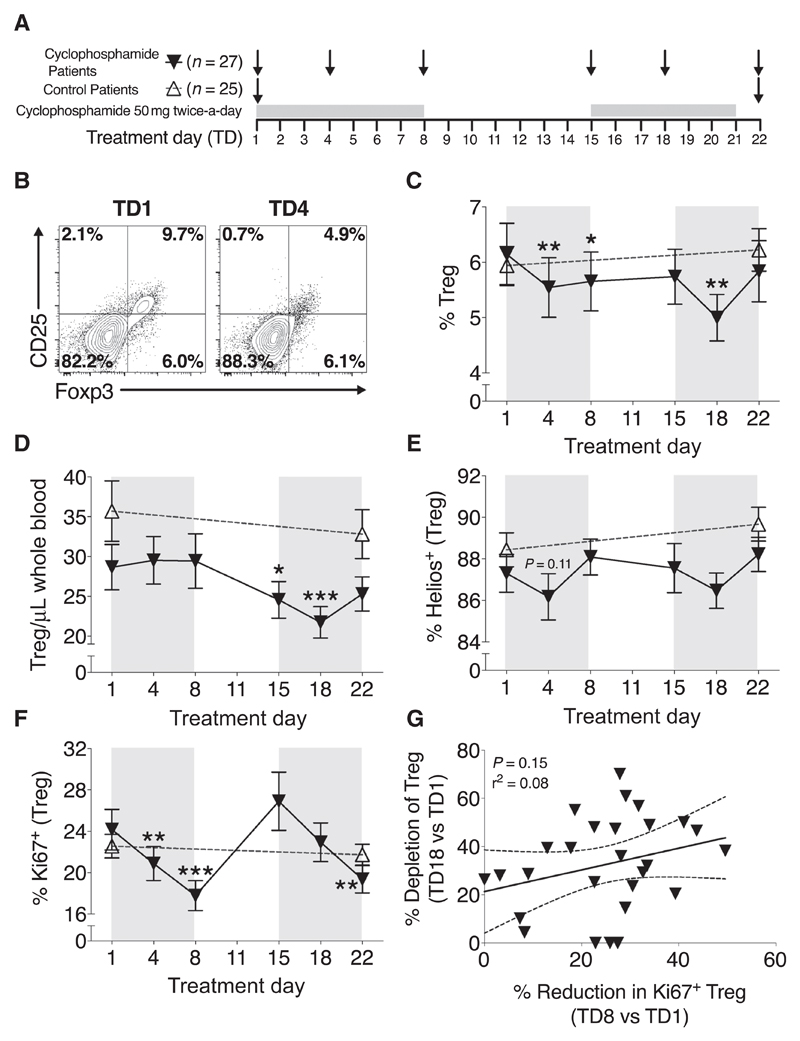
Figure 1.
The effect of metronomic, low-dose cyclophosphamide on CD4+CD25hiFoxp3+ Treg. A, 50 mg cyclophosphamide was administered twice daily to 27 colorectal cancer patients on treatment days 1–7 and 15–21, with blood samples being taken at regular intervals, as shown in this schematic. B, Example FACS plots of CD25hi- and Foxp3-expressing CD4+ T cells at TD1 and TD4, and the gating used to denote the Treg percentage (top right quadrant). C, The percentage of Tregs was measured at the indicated time points throughout treatment. D, Peripheral Treg numbers were derived from the number of CD3+CD4+ T cells from whole blood samples and the proportion of CD4+ T cells expressing CD25hiFoxp3 in subsequent phenotypic analysis. The percentage of Tregs expressing Helios (E) and Ki67 (F) was measured at the indicated time points throughout treatment. G, The percent depletion of Treg numbers at treatment day 18 in comparison with treatment day 1 was correlated with the percent reduction in Ki67-expressing Tregs at treatment day 8 (TD8) in comparison with treatment day 1 (TD1). Black triangles/lines indicate patients taking cyclophosphamide (n = 27); clear triangles/dashed lines indicate control patients at the same stage of tumor progression (n = 25). Significant differences are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Lymphocyte purification and culture
Peripheral blood samples were collected in 10-mL lithium heparin tubes (BD Biosciences). Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation of heparinized blood over Lymphoprep (Axis-Shield). Cells were then washed and resuspended in advanced RPMI (Life Technologies) supplemented with 5% batch-tested, pooled human AB serum (Welsh Blood Service), L-glutamine, and penicillin/streptomycin. PBMCs were plated in 96-well plates (Nunc) and cultured in triplicate wells with specific antigens for 14 days, supplemented with 10 μL CellKine media (Helvetica Healthcare) on day 3 and fresh media containing 20 IU/mL IL2 on days 7 and 10.
Antigens
Forty-one 20-mer peptides overlapping by 10 amino acids covering the entire human 5T4 protein were synthesized by Fmoc chemistry to >95% purity (GL Biochem). The peptides were divided into 13 pools (Supplementary Fig. S2). Whole 5T4 protein was produced as described previously (27). The recall antigens tuberculin purified protein derivative (PPD; Statens Serum Institut) and hemagglutinin (HA; gift from Dr. John Skehel, National Institute of Medical Research, London, United Kingdom), and the T-cell mitogen PHA (Sigma) were used as positive controls. All antigens were used at a final concentration of 5 μg/mL.
ELISpot assays
Polymer-backed 96-well filtration plates (MAIPS4510; Merck Millipore) were used for all ELISpot assays. Antibodies and alkaline phosphatase substrate kits were obtained from Mabtech. The concentrations of antibodies used and washing steps were according to the manufacturer's instructions; all antibody incubations were with 50 μL/well. Cells were pooled from triplicate wells in identical culture conditions, washed, resuspended, and counted before being plated with or without the corresponding 5T4 peptide pool for direct comparison. The plate was incubated at 37°C, 5% CO2 for 24 hours. Cytokine-producing T cells were enumerated at the single-cell level by counting the number of spots per well using an automated ELISpot plate reader (ImmunoSpot S6 Ultra; CTL Europe GmbH). Positive responses were identified as having at least 20 spot-forming cells (SFC) per 105 cultured cells, and at least double the number of spots above background.
FluoroSpot assays
PVDF 96-well filtration plates designed for low autofluorescence (IPFL; Millipore) were used for all FluoroSpot assays. Antibodies to IFNγ and granzyme B, and fluorescence enhancer kits were obtained from Mabtech. All antibody incubations were with 50 μL/well. Frozen PBMCs were thawed and rested overnight in advanced RPMI + 5% AB serum. Cells were then washed, plated, and stimulated with 5 μg/mL antigen in duplicate wells. Plates were incubated at 37°C, 5% CO2 for 24 hours. Cytokine-producing T cells were enumerated using Smart Count settings on an automated FluoroSpot plate reader (ImmunoSpot S6 Ultra; CTL Europe GmbH), allowing for an assessment of single and dual cytokine–producing cells. Positive responses were identified as having at least 5 SFC/2 × 105 PBMCs, and at least double that of the negative (no antigen) control.
Flow cytometry
A no-wash, single platform approach was utilized to perform T-cell, B-cell, and NK-cell counts. Three microliters of CD3-APC (Miltenyi Biotec), CD4-PE (BioLegend), CD8-PerCPCy5.5 (BioLegend), CD19-FITC (BioLegend), and CD56-Brilliant Violet-421 (BD Biosciences) antibodies were added to a FACS tube, followed by 50 μL of whole heparinized blood using a reverse pipetting technique. Samples were left at 4°C for 30 minutes, before addition of 450 μL red blood cell lysis buffer (BioLegend). After 10 minutes, 50 μL of CountBright Absolute Counting Beads (Life Technologies) were reverse pipetted into the lysed blood sample before acquisition on a FACSCanto II (BD Biosciences). Forward scatter and side scatter threshold parameters were set on the instrument to exclude debris and a minimum of 5,000 beads were collected. FACS samples were analyzed on FlowJo version 10 (TreeStar), and cell counts calculated on the basis of number of gated events (i.e., cells) divided by number of gated beads in sample, multiplied by number of beads/μL in CountBright vial.
To analyze phenotypic markers expressed on freshly isolated PBMC subsets, two panels were devised; a regulatory T-cell panel consisted of CD4-APCh7 (BD Biosciences), CD25-Brilliant Violet-421 (BioLegend), Foxp3-APC (eBioscience), Helios-FITC (BioLegend), Ki67-PE (BioLegend), HLA-DR-PECy7 (BioLegend), and ICOS-PerCPCy5.5 (BioLegend) antibodies. The second panel, to study cytotoxic lymphocytes, consisted of CD3-APCh7 (BD Biosciences), CD56-Brilliant Violet-421 (BD Biosciences), CD8-PerCPCy5.5 (BioLegend), Perforin-FITC (BioLegend), Granzyme B-Alexa Fluor 647 (BioLegend), HLA-DR-PECy7 (BioLegend) and Ki67-PE (BioLegend) antibodies. Live/Dead-Aqua (Life Technologies) was used in both panels to exclude dead cells. Surface staining was performed in PBS, 2.5% FCS, and 5 mmol/L EDTA; intracellular staining was performed in permeabilization buffer plus 2% normal rat serum. Samples were acquired on a FACS-Canto II (BD Biosciences) and data analyzed using FlowJo version 10 (TreeStar).
Statistical analysis
Follow-up was complete by December 2016, by which point all patients had progressed or were receiving other treatments. Datasets were tested for normality, and appropriate tests were used to compare patient immune responses at specified time points during the trial (nonparametric: Wilcoxon signed rank test, parametric: paired t test). Error bars represent mean ± SEM where appropriate. PFS was analyzed using log-rank tests and displayed using Kaplan–Meier plots. P < 0.05 was considered statistically significant and all tests of significance were two-sided. Analyses were performed using SAS v9.4 and GraphPad Prism v7.
Trial registration
This trial is registered with European Union Drug Regulating Authorities Clinical Trial (2010-024380-41), the ISRCTN registry (ISRCTN54669986) and was conducted in compliance to ICH-GCP regulatory requirements.
Results
Patient baseline characteristics
Three patients were not included in the analyses of immune responses (see Materials and Methods); however, an additional patient was recruited and was included in the analyses, hence 52 patients could be evaluated. All metastatic colorectal cancer patients presented with stable metastatic disease in at least one location, primarily liver, lung, and peritoneal metastases; further baseline characteristics of the patients randomized to each group are shown in Table 1.
Effects of cyclophosphamide on lymphocyte subset frequency and activation
Over the 22-day course of low-dose cyclophosphamide treatment, 40-mL blood samples were collected at days 1, 8, 15, and 22 for analysis of 5T4-specific T-cell and antibody responses, and 10-mL blood samples collected at days 1, 4, 8, 15, 18, and 22 to analyze phenotype and function of lymphocytes. Forty-milliliter blood samples were taken from control patients at treatment days 1 and 22, as shown in the treatment overview (Fig. 1A).
The regulatory T-cell proportion was identified by FACS, first gating on live lymphocytes, CD3+CD4+ T cells, then drawing a quadrant to distinguish CD25hiFoxp3+, a representative example of which is shown in Fig. 1B. There was a significant decrease in the proportion of CD4+ T cells expressing CD25hi/Foxp3 at treatment day 4 in comparison with baseline (TD1 mean 6.1% ± 0.6% vs. TD4 5.5% ± 0.5%, P = 0.0085); however, this began recovering toward baseline levels by treatment day 8 (Fig. 1B and C). The greatest proportional reduction was noted at treatment day 18 (TD1 vs. TD18, 5.1% ± 0.4%, P = 0.0014), before again rebounding to baseline by TD22. While the reduction on Treg proportion was transient, a decrease in absolute peripheral Treg number was only observed in the second week of treatment. Significant decreases were observed at treatment days 15 (TD1 mean 28.7 ± 2.9 cells/μL vs. TD15 24.2 ± 2.4 cells/μL, P = 0.042) and 18 (TD1 vs. TD18, 21.7 ± 2.1 cells/μL, P = 0.0003) before starting to recover toward pretreatment numbers at day 22 (Fig. 1D).
The proportion of Tregs expressing Helios, a putative marker of thymus-derived Tregs (28), did not vary dramatically during treatment, suggesting that neither thymus-derived nor peripherally induced Tregs were preferentially targeted (Fig. 1E). However, small reductions in Helios+ Tregs were noted at TD4 and TD18, with each of these dips followed temporally 4 days later by a significant decrease in the proportions of proliferating Tregs, as demonstrated by Ki67 staining (TD1 24.2% ± 2.0% vs. TD8 17.8% ± 1.5%, P < 0.0001; TD1 vs. TD22 19.4% ± 1.3%, P = 0.0026; Fig. 1F). Reduction in the proliferating subset of Tregs during the first treatment week does not appear to be responsible for the depletion of Treg numbers during the second week (P = 0.15; Fig. 1G). Despite this, Tregs with the greatest proliferative capacity demonstrate a greater susceptibility to the cytotoxic effects of cyclophosphamide.
The relationship between the proportion (percentage) of Ki67+ cells in blood and absolute cell numbers is more complex for CD3+CD4+(Foxp3−) T-cells, CD3+CD8+ T cells, NK cells, and B cells. During the first cycle of cyclophosphamide in week one, all populations, except B cells, show an increase in blood cell numbers (Fig. 2A–C; Supplementary Fig. S3), this rise being most marked for CD3+CD8+ T-cells (TD1 mean 331.6 ± 41.5 cells/μL vs. TD4 457.5 ± 81.4 cells/μL, P = 0.0039; Fig. 2B); this corresponded with a fall in Ki67 expression in all lymphocyte populations (Fig. 2D–F). This inverse relationship is different to that observed within the Treg population and indicates early mobilization into blood from a reservoir occurs for this rapid increase in cell numbers. These numbers quickly returned to baseline, and this inverse effect was not repeated in the second cycle of cyclophosphamide as the %Ki67 expression now increased for all three cell populations (in contrast to Tregs where %Ki67 expression falls during second cyclophosphamide cycle). Given that Treg numbers remained stable but CD4+Foxp3− T-cell numbers increase at TD4, this contributes to the proportional reduction in Tregs as described above.
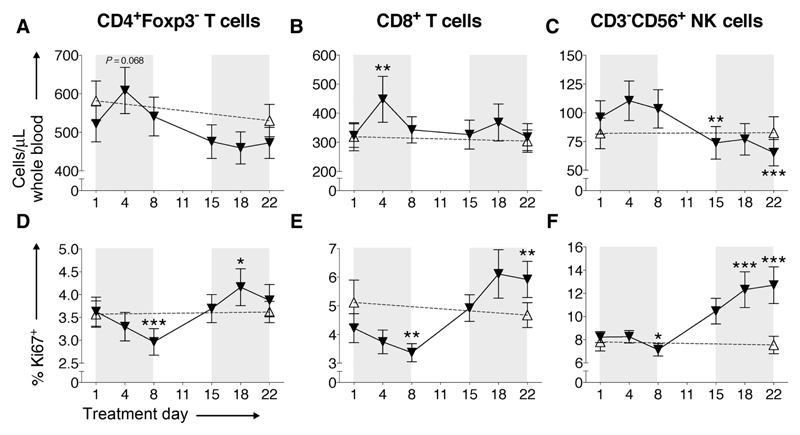
Figure 2.
T- and NK-cell number and proliferation in response to cyclophosphamide. Absolute numbers of CD3+CD4+(Foxp3−) T-cells (A), CD3+CD8+ T cells (B), and CD3−CD56+ NK-cells (C) per microliter of whole blood were analyzed at indicated time points. The expression of Ki67 in CD4+(Foxp3−) T cells (D), CD8+ T cells (E), and CD3−CD56+ NK cells (F) was monitored throughout cyclophosphamide treatment. Black triangles/lines indicate patients taking cyclophosphamide (n = 27); clear triangles/dashed lines indicate control patients at the same stage of tumor progression (n = 25). Significant differences are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
While effector T-cell numbers remained relatively stable from treatment day 8 onwards, the numbers of peripheral CD3−CD56+ NK cells steadily decreased after the first week (TD1 mean 97.0 ± 14.6 cells/μL vs. TD15 73.8 ± 14.2, P = 0.0036, TD1 vs. TD22 65.3 ± 11.5 cells/μL, P = 0.0002; Fig. 2C). Overall, these data mirror the time points at which significant depletion of Treg numbers was also observed; hence, cyclophosphamide does not appear to only selectively deplete Tregs. A similar profile was noted among B cells, with a significant depletion at TD18 (TD1 mean 124.0 ± 33.6 cells/μL vs. TD18 82.1± 23.1 cells/μL, P = 0.025; Supplementary Fig. S3).
As mentioned above, the proportion of potentially proliferative Ki67+ T and NK cells all decrease during the first cyclophosphamide treatment week, but then reversed and dramatically increased from day 8 and continued to increase into the second cycle of cyclophosphamide (TD15–22; Fig. 2D–F), with significant increases in %Ki67 seen in CD4+Foxp3− T cells (TD1 mean 3.7% ± 0.3% vs. TD18 4.3% ± 0.4%, P = 0.045; Fig. 2D); CD8+ T cells (TD1 mean 4.2% ± 0.5% vs. TD22 5.9% ± 0.6%, P = 0.0012; Fig. 2E) and NK cells (TD1 mean 8.2% ± 0.5% vs. TD22 12.7% ± 1.6%, P = 0.001; Fig. 2F). This effect was observed in 23 of 27 patients and is mirrored by the absolute reduction in Treg numbers at TD15–22 (Fig. 1D), raising the possibility that Tregs are controlling homeostatic proliferation/activation of these other cell populations. The striking increases in %Ki67 corresponded to T-cell activation demonstrated by increased expression of HLA-DR, ICOS, and CD25 (Supplementary Fig. S4).
Increased lymphocyte cytotoxicity with cyclophosphamide treatment
Cyclophosphamide treatment leads to a marked expansion of CD8+ T cells. To explore the impact of this treatment on the function of these cytotoxic T cells, we measured the cytolytic potential of peripheral CD8+ T cells by intracellular perforin and granzyme B, both of which play a major role in cell-mediated cytotoxicity of cancer cells (29). Corresponding with increased number, proliferation and activation of CD8+ T cells, the intracellular expression of both molecules significantly increased during cyclophosphamide treatment; representative examples of FACS staining are shown (Fig. 3A and B). This effect was noted for the majority (24/27) of patients taking cyclophosphamide (perforin TD1 mean 32.3% ± 4.4% vs. TD8 38.5% ± 4.4%, P = 0.0071; Fig. 3C, and granzyme B TD1 mean 53.4% ± 5.4% vs. TD8 56.8% ± 5.3%, P = 0.0084, vs. TD18 60.6% ± 5.4%, P = 0.0009; Fig. 3D). A similar increase in cytotoxicity potential (i.e., expression of granzyme B/perforin) was found in NK cells, an effect of cyclophosphamide not previously described (data not shown).
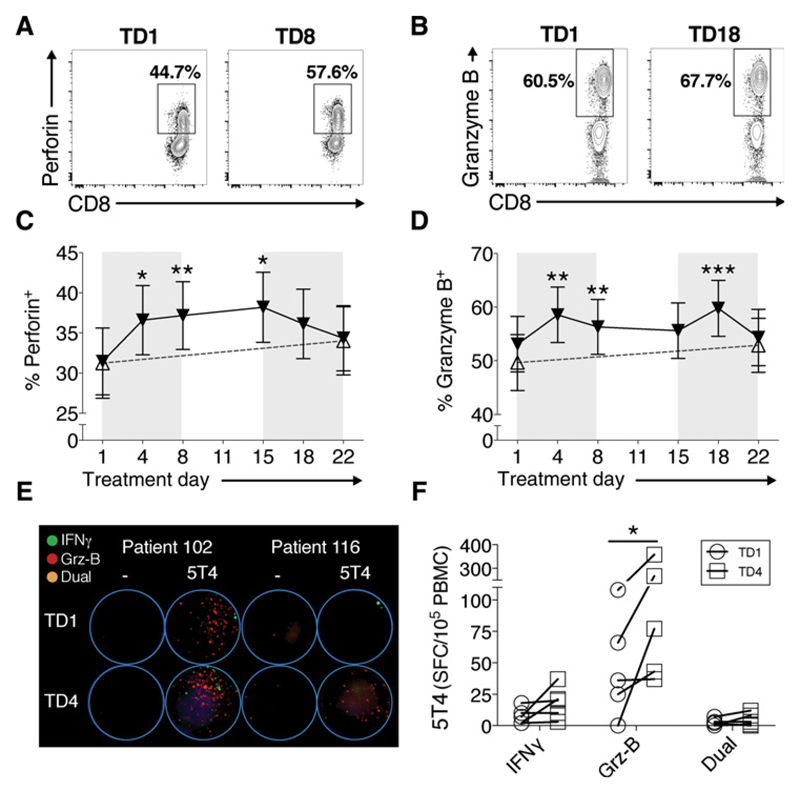
Figure 3.
Enhanced CD8+ T-cell function during cyclophosphamide treatment. Example FACS plots of perforin (A) and granzyme B (B) expression, and % expression of perforin (C) and granzyme B (Grz-B; D) in CD3+CD8+ T cells throughout cyclophosphamide treatment. Black triangles/lines indicate patients taking cyclophosphamide (n = 27); clear triangles/dashed lines indicate control patients at the same stage of tumor progression (n = 25). Significant differences are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Dual-color FluoroSpots analyzing the production of IFNγ (green spots), and granzyme B (red spots) were performed, and representative well images are shown from two patients before treatment (TD1) and during cyclophosphamide treatment (TD4), with PBMCs stimulated with 5T4 peptide pools (E). The average SFC/105 PBMCs from duplicated wells of single- (IFNγ/granzyme B) and dual-cytokine– producing T cells in response to 5T4 peptide pool stimulus in five cyclophosphamide-treated patients, is shown (F).
To assess whether the increased intracellular expression translated to an increase in functional cytotoxicity, that is, actual release of granzyme B, PBMCs from patients, identified as having an increased expression of granzyme B at TD4, were stimulated ex vivo with a peptide library covering the tumor antigen 5T4, in a FluoroSpot assay. This dual color (analyte) assay determined the production of the Th1 cytokines IFNγ (represented as green spots) alongside granzyme B (represented as red spots), allowing for cells with dual functionality to be enumerated. Representative FluoroSpot well images for two patients are shown (Fig. 3E). The number of 5T4-specific T cells producing granzyme B per 105 PBMCs increased significantly in the five cyclophosphamide-treated patients tested (P = 0.031; Fig. 3F), with smaller increases in single IFNγ producers and dual cytokine producers also noted. These data reveal effector antitumor Th1 function and cytolytic capability increases within a very short time frame after commencing cyclophosphamide.
Augmented anti-5T4 T-cell responses during cyclophosphamide treatment correlate with prolonged PFS
Th1-type (IFNγ)-producing T cells are thought to be important mediators of beneficial antitumor immune responses (12). The oncofetal antigen 5T4 has been proposed as a target antigen given its expression on the majority of gastrointestinal adenocarcinomas, and the ability of 5T4-specific T cells to recognize and kill 5T4-expressing tumor cells (30). We have also found anti-5T4 CD4+ T-cell responses are associated with control of colorectal cancer, and are in turn regulated by tumor-driven Tregs (6, 8). Cyclophosphamide led to specific increases in T-cell responses to 5T4 in colorectal cancer patients. Example ELISpot well images from three patients taking cyclophosphamide demonstrate how a negative response at TD1 to 5T4 peptide pools (see Supplementary Fig. S2 for peptide sequences) became highly positive by TD8, indicative of an unmasked response to individual 5T4 epitopes (Fig. 4A). Significant increases in the overall magnitude of anti-5T4 T-cell responses were generated, peaking at day 15 (TD1 331.9 ± 65.1 SFC/105 vs. TD15 630.9 ± 95.7 SFC/105, P = 0.0013; numbers reflect cognate T-cell responses as measured by the number of 5T4-specific IFNγ spot-forming T cells i.e., SFC/105 cultured PBMCs; Fig. 4B). In patients followed up for a further 3 months after cyclophosphamide treatment, who were taking no other medication (schematic shown; Supplementary Fig. S5A), cyclophosphamide-induced 5T4-specific T-cell responses remained at a high level until treatment day 43; following this, responses returned to their baseline levels throughout the rest of the trial (Supplementary Fig. S5B). There was no evidence of immunocompromise in the patient groups (i.e., controls and cyclophosphamide-treated subjects) despite the advanced nature of the tumors; T cells remained responsive to the control recall antigens tuberculin PPD (Fig. 4C) and influenza HA (Fig. 4D).
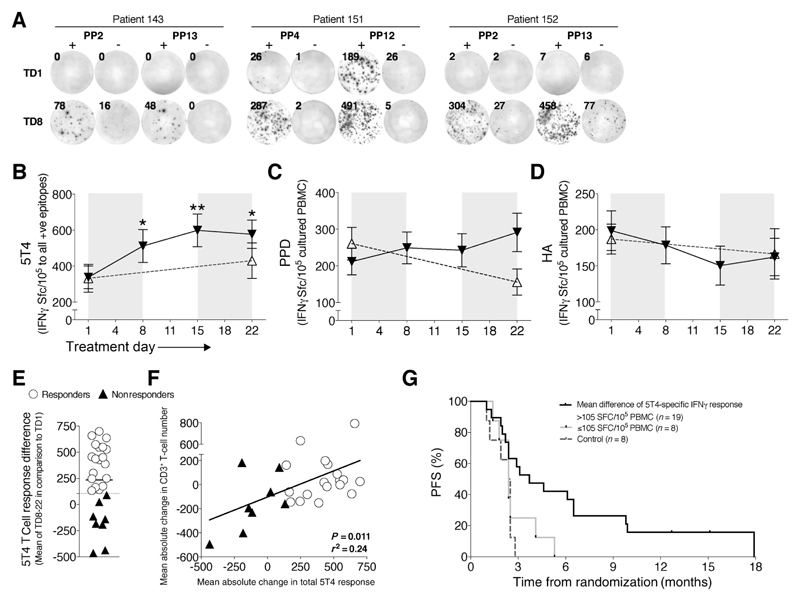
Figure 4.
Augmented antitumor (5T4)-specific responses during cyclophosphamide treatment associate with prolonged survival. PBMCs were cultured with 5T4 peptide pools (see Supplementary Fig. S2 for sequences), and IFNγ+ T-cell responses were measured by ELISpot. Representative examples from three patients at TD1 and TD8 are shown (A). The total number of spots to all positive identified 5T4 epitopes (B), tuberculin PPD (C), and HA (D) were enumerated and normalized to SFC/105 cultured PBMCs. Black triangles/lines indicate patients taking cyclophosphamide (n = 27); clear triangles/dashed lines indicate control patients at the same stage of tumor progression (n = 25). Significant differences are indicated (*, P < 0.05; **, P < 0.01). E, The mean change in 5T4-specific T-cell response at TD8–22 in comparison with TD1 was measured for all patients, with patients being separated into responders (white circles, n = 19) and nonresponders (black triangles, n = 8) below the 95% CI of responses at +105 SFC/105 cultured PBMCs. F, This change in 5T4 T-cell response was correlated with the patient's mean change in absolute peripheral CD3+ T-cell numbers during cyclophosphamide treatment. G, High anti-5T4 T-cell responses were associated with PFS. In addition, control patients who did not take any medication during the trial were also included for analysis (dashed lines; n = 8). 5T4 T-cell responders versus nonresponders and controls: HR, 0.29; 95% CI, 0.12–0.69; P = 0.0047.
Overall, 19 of 27 patients responded to cyclophosphamide with a mean increase (at TD8, 15, and 22) in total 5T4 T-cell responses above the lower 95% confidence interval (CI) of 105 IFNγ+ SFC/105 cultured PBMCs (Fig. 4E), and the magnitude of this response was so large that on an intention-to-treat basis, these increased responses remain highly significant even when including the nonresponders in the analysis (Fig. 4B). Increased anti-5T4 T-cell responses significantly correlated with mean increases in CD3+ T-cell numbers during treatment, whereby those patients with a tendency for lymphodepletion during cyclophosphamide treatment failed to mount robust anti-5T4 immune responses (P = 0.011; Fig. 4F).
We next looked at how patients responding to cyclophosphamide treatment with increased 5T4-specific immune responses fared clinically compared with subjects who did not respond to cyclophosphamide or who were randomized to the control group. Disease progression was assessed by radiologic progression of the tumor or metastases; increase in tumor markers; or clinical deterioration. Eight patients randomized to cyclophosphamide failed to respond (black triangles, Fig. 4E and F). Progression-free survival of both groups was assessed, based on the time taken from study enrolment to evidence of tumor progression, as assessed clinically, or from radiologic progression (31); the Kaplan–Meier curves are shown in Fig. 4G. A significant difference was found between cyclophosphamide responders and nonresponders plus 8 control metastatic colorectal cancer patients not taking cyclophosphamide or any other treatment (HR = 0.29; 95% CI, 0.12–0.69, P = 0.0047; Fig. 4G). Median time-to-progression of patients responding to cyclophosphamide in this manner was 3.7 months versus 2.4 months for nonresponders/controls.
One patient was offered eight repeat courses of low-dose cyclophosphamide given the beneficial immune responses generated when taking it. On every instance, 5T4 T-cell responses increased, Treg proportion decreased, and granzyme B expression in CD8+ T cells increased, demonstrating how this therapy is amenable to repeat dosing (Supplementary Fig. S6).
In summary, the most striking effects of cyclophosphamide therapy given to individuals with inoperable metastatic colorectal cancer, in terms of prolonging PFS, are seen when antitumor T-cell immune responses are induced via depletion of circulating Tregs.
Discussion
The clinical effectiveness of cancer immunotherapy is reliant upon a number of factors that allow an immune response to overcome the tumor. A major reason for failure despite the presence of antitumor responses is the existence of tumor-driven immunosuppressive mechanisms, mediated, at least in part, by CD25hiFoxp3+ Tregs. Cyclophosphamide has been used in preclinical and clinical studies (19), with it being examined for its immunologic effects at both high and low doses. While metronomic low-doses for cancer immunotherapy have been infrequently used, there are reports demonstrating prolonged Treg reduction (21, 32). One nonrandomized, retrospective study identified a possible small survival benefit in metastatic cancer patients when using low-dose cyclophosphamide (33). However, these studies highlighted the need for in-depth characterization of cyclophosphamide on immunologic findings, to optimize antitumor immunity in potential combination with other treatments.
Following initial cyclophosphamide administration to metastatic colorectal cancer patients, we demonstrated a short duration in the reduction in Treg proportion and overall Treg number. Cyclophosphamide acts very rapidly, within days (potentially hours): the first measurement on day 4 of treatment revealed cyclophosphamide had induced numerous immunologic perturbations, including significantly increased T-cell numbers resulting in fluctuations of CD4:CD8 T-cell ratio; enhanced CD8+ T-cell cytolytic function; and decreased expression of activation markers in CD25hiFoxp3+ Tregs. Despite such pronounced immunologic changes, nearly all patients taking cyclophosphamide did not report any side effects during treatment. While our understanding of the pharmacodynamics of cyclophosphamide with regards to its effect on lymphocytes remains incomplete, it appears that cyclophosphamide targets lymphocytes with greatest proliferative potential first, given the quite striking reductions in Ki67+ CD25hiFoxp3+ Tregs seen almost unanimously among the trial participants. Patients with a higher pretreatment expression of Helios (described as a marker of Treg activation and proliferation; ref. 34) or %Ki67-expressing Tregs, also exhibited a tendency for proportional Treg reduction and absolute Treg depletion during cyclophosphamide treatment. At the same time points (TD15–18), significant depletions of CD56+ NK cells and B cells were also observed. It should be noted that among lymphocytes, NK cells, B cells, and Tregs are highly dynamic proliferative populations in vivo, and homeostatic turnover is more rapid than for CD4+ and CD8+ effector T-cell populations (35–37). Thus, lymphocyte subsets with a relatively high proliferative capacity were more readily targeted by cyclophosphamide. In this case, however, reductions in absolute numbers of NK cells or B cells in the periphery did not appear to influence patient outcome, and NK-cell phenotype and cytolytic potential was actually augmented by cyclophosphamide (data not shown).
Cyclophosphamide leads to reduced numbers of Tregs in blood, and indirect evidence suggest this is due to increased cell death, combined with reduced proliferative potential (i.e., Ki67 positivity). Our experiments (and previous reports; ref. 32) suggest cyclophosphamide blocking of activation/proliferation of Tregs contributes to enhanced cytolytic T-cell function and increased CD4+ T-cell function. However, many of the effects seen on Tregs were transient and the Treg proportion rebounds during treatment. This could be an effort to maintain peripheral homeostasis in the face of reduced Treg function. It could also be the result of T cells initially being released at a greater rate from a reservoir (at TD1–4), before trafficking from the periphery (at TD5–8) and into secondary lymphoid tissues or tumors, as demonstrated when cyclophosphamide is administered in mouse tumor models (22). Absolute Treg numbers only significantly reduce at treatment days 15 and 18 before recovering, revealing the biphasic nature of the effects of cyclophosphamide on the Treg population, highlighting the importance of multiple immunologic measurements throughout the treatment course.
The boosting of antitumor immunity by cyclophosphamide may well extend beyond Treg depletion; in one mouse model of colon carcinoma, not only were significant effects on dendritic cell homeostasis noted, but enhanced antitumor responses mediated by CD8+ T cells were found after cyclophosphamide administration, despite only transient Treg depletions (22). However, this contrasts to a different mouse model employing melanoma, whereby perforin is downregulated in CD8+ TILs in mice receiving 2.5-mg cyclophosphamide (23). Here, we identified that the cytolytic potential and function of CD8+ T cells increased markedly during cyclophosphamide treatment in the majority of patients. Indeed, utilizing a dual-color FluoroSpot assay, we were able to measure increased numbers of granzyme B–producing 5T4-specific T cells just 4 days into cyclophosphamide treatment. These augmented responses result from enhanced function of T cells, as single- and dual-IFNγ and granzyme B–producing T-cell responses also increased in response to the T-cell mitogen PHA (data not shown). However, given there was no increase in T-cell responses to the control antigens, in contrast to the striking increase in responses directed against 5T4, this is indicative of immunosuppression specifically targeting antitumor immune responses.
Although many of the immunologic readouts returned to baseline by treatment day 8, crucially the antitumor immune response mediated by IFNγ-producing 5T4-specific T cells was enhanced over a more prolonged time period for the majority of patients, again indicative of reduced functional capacity of Tregs and augmented effector T-cell responses. The production of IFNγ by the host plays a crucial role in the ultimate success of therapy, and has a direct role in inducing permanent senescence of tumor cells (38). Here, the critical readout of whether such responses are beneficial is seen in prolonged PFS of the patients, whereby those patients mounting increased anti-5T4 IFNγ+ T-cell responses over the course of treatment had a significantly better clinical response in comparison with patients not responding to cyclophosphamide or those receiving no treatment. These data demonstrate the power of a targeted, Th1-based immune response to an antigen expressed on tumor cells, given that IFNγ responses to the irrelevant recall antigens had no effect on survival.
Low-dose cyclophosphamide appears to be a safe and beneficial drug for end-stage metastatic colorectal cancer patients with disseminated disease. Our ongoing studies aim to evaluate its usefulness in boosting the immunotherapeutic potential of anticancer vaccination.
Supplementary Material
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Translational Relevance.
Regulatory T cells (Treg) are enriched within colorectal cancer. The role they play within colorectal cancer is contentious; although some studies have identified a survival advantage in colorectal cancer patients with increased Treg numbers, depletion of these cells both in vitro and in animal models results in enhanced antitumor T-cell responses and better tumor control. Oral low-dose cyclophosphamide previously was noted to effectively deplete Tregs, but its effects have not been evaluated in the setting of a controlled, randomized trial. Here, we present a comprehensive profile of peripheral lymphocyte populations in patients with metastatic colorectal cancer enrolled in a randomized phase I/II trial of low-dose cyclophosphamide. We demonstrate that cyclophosphamide depletes Treg, B-, and NK-cell populations, with coincidental increases in polyfunctional tumor-specific T cells. Patients identified as having an increased antitumor immune response exhibited significantly longer progression-free survival. Hence, cyclophosphamide has a clear effect on colorectal cancer patients, warranting further investigation in patients with earlier stage disease to prevent relapse following primary resection.
Acknowledgments
The authors thank Laura North, Joanne Bagshawe, Georgina Radford, Marcia Short, and Helen Maule for collecting patient blood samples for use on this study; Helen Clark and Catherine Johnston for administrative and data assistance; and the patients and their families for their involvement in this study. We are also grateful for the support from the Clinical Research Facility (Cardiff and Vale University Health Board).
Grant Support
This trial was funded by Cancer Research Wales, the Institute for Cancer Vaccines & Immunotherapy, and the Cancer Research UK supported ECMC (Experimental Cancer Medicine Centre). Additional support was provided by the Cardiff and Vale University Health Board Research and Development, the Wales Cancer Research Centre, Cancer Research UK (C16731/A21200; to A. Gallimore), a Wellcome Trust university award (086983/Z/08/Z; A. Gallimore), and educational grants from the Welsh Assembly Government, the Wellcome Trust, and the Academy of Medical Sciences (to T. Pembroke).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
R. Harrop holds ownership interest (including patents) in Oxford BioMedica. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: M. Scurr, R. Adams, R. Harrop, A. Gallimore, A. Godkin
Development of methodology: M. Scurr, D. Roberts, R. Adams, R. Harrop, R. Hills, A. Gallimore, A. Godkin
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): M. Scurr, T. Pembroke, A. Bloom, D. Roberts, A. Thomson, R. Adams, A. Brewster, R. Jones, S. Gwynne, R. Harrop, A. Godkin
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): M. Scurr, T. Pembroke, A. Bloom, R. Adams, D. Blount, R. Hills, A. Gallimore, A. Godkin
Writing, review, and/or revision of the manuscript: M. Scurr, T. Pembroke, A. Bloom, D. Roberts, R. Adams, A. Brewster, D. Blount, R. Harrop, R. Hills, A. Gallimore, A. Godkin
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M. Scurr, A. Bloom, K. Smart, H. Bridgeman, D. Blount, A. Godkin
Study supervision: M. Scurr, R. Adams, A. Gallimore, A. Godkin
Other (development of laboratory and clinical protocols): D. Roberts
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–48. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, Galluzzi L, Zitvogel L, Fridman WH. Colorectal cancer: the first neoplasia found to be under immunosurveillance and the last one to respond to immunotherapy? Oncoimmunology. 2015;4:e1058597. doi: 10.1080/2162402X.2015.1058597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22:327–34. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM. Regulating the immune response to tumours. Adv Drug Deliv Rev. 2006;58:948–61. doi: 10.1016/j.addr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, et al. Suppression of tumour-specific CD4+ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61:1163–71. doi: 10.1136/gutjnl-2011-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke SL, Betts GJ, Plant A, Wright KL, El-Shanawany TM, Harrop R, et al. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scurr M, Bloom A, Pembroke T, Srinivasan R, Brown C, Smart K, et al. Escalating regulation of 5T4-specific IFN-gamma CD4 T cells distinguishes colorectal cancer patients from healthy controls and provides a target for therapy. Cancer Immunol Res. 2013;6:416–25. doi: 10.1158/2326-6066.CIR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scurr M, Gallimore A, Godkin A. T cell subsets and colorectal cancer: discerning the good from the bad. Cellular Immunol. 2012;279:21–4. doi: 10.1016/j.cellimm.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Scurr M, Ladell K, Besneux M, Christian A, Hockey T, Smart K, et al. Highly prevalent colorectal cancer-infiltrating LAP(+) Foxp3(-) T cells exhibit more potent immunosuppressive activity than Foxp3(+) regulatory T cells. Mucosal Immunol. 2014;7:428–39. doi: 10.1038/mi.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol. 2012;30:1835–41. doi: 10.1200/JCO.2011.40.2271. [DOI] [PubMed] [Google Scholar]
- 12.Scurr MJ, Brown CM, Costa Bento DF, Betts GJ, Rees BI, Hills RK, et al. Assessing the prognostic value of preoperative carcinoembryonic antigen-specific T-cell responses in colorectal cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv001. pii:djv00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O'Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86. [PubMed] [Google Scholar]
- 14.Glode LM, Barqawi A, Crighton F, Crawford ED, Kerbel R. Metronomic therapy with cyclophosphamide and dexamethasone for prostate carcinoma. Cancer. 2003;98:1643–8. doi: 10.1002/cncr.11713. [DOI] [PubMed] [Google Scholar]
- 15.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–40. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 16.Polak L, Turk JL. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature. 1974;249:654–6. doi: 10.1038/249654a0. [DOI] [PubMed] [Google Scholar]
- 17.Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 18.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2012;61:353–62. doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72:3439–44. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B. Selective depletion of CD4+CD25+Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res. 2010;70:4850–8. doi: 10.1158/0008-5472.CAN-10-0283. [DOI] [PubMed] [Google Scholar]
- 21.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radojcic V, Bezak KB, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, et al. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2010;59:137–48. doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevko A, Sade-Feldman M, Kanterman J, Michels T, Falk CS, Umansky L, et al. Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J Invest Dermatol. 2013;133:1610–9. doi: 10.1038/jid.2012.444. [DOI] [PubMed] [Google Scholar]
- 24.Ellebaek E, Engell-Noerregaard L, Iversen TZ, Froesig TM, Munir S, Hadrup SR, et al. Metastatic melanoma patients treated with dendritic cell vaccination, Interleukin-2 and metronomic cyclophosphamide: results from a phase II trial. Cancer Immunol Immunother. 2012;61:1791–804. doi: 10.1007/s00262-012-1242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 26.International Standard Randomized Controlled Trial Number. TroVax® and cyclophosphamide treatment in colorectal cancer. 2012 Available from: http://www.controlled-trials.com/ISRCTN54669986.
- 27.Harrop R, Ryan MG, Myers KA, Redchenko I, Kingsman SM, Carroll MW. Active treatment of murine tumors with a highly attenuated vaccinia virus expressing the tumor associated antigen 5T4 (TroVax) is CD4+ T cell dependent and antibody mediated. Cancer Immunol Immunother. 2006;55:1081–90. doi: 10.1007/s00262-005-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–23. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 30.Al-Taei S, Salimu J, Lester JF, Linnane S, Goonewardena M, Harrop R, et al. Overexpression and potential targeting of the oncofoetal antigen 5T4 in malignant pleural mesothelioma. Lung Cancer. 2012;77:312–8. doi: 10.1016/j.lungcan.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 33.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19:1737–46. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutz CT, Karapetyan A, Al-Attar A, Shelton BJ, Holt KJ, Tucker JH, et al. Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. J Immunol. 2011;186:4590–8. doi: 10.4049/jimmunol.1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–65. doi: 10.1038/ni.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–33. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.