Abstract
Objective
Pfirrmann disc grade is a useful scoring tool for evaluating disc degeneration, but normal values according to aging process has not been elucidated. This study was conducted to identify the prevalence and pattern of whole spine disc degeneration according to ages and gender differences.
Methods
Total 653 patients (336 male and 317 female patients, 48.1±58.7 years old) who took whole spine magnetic resonance images were enrolled in this study. There were 19 cases in their 2nd decades and 74 cases in 3rd decades, 141 cases in 4th decades, 129 cases in 5th decades, 139 cases in 6th decades, and 93 cases in 7th decades, 58 cases in over 8th decades. Pfirrmann disc grades were measured according to sex and ages by 2 neurosurgeons that were blind to this study.
Results
All spinal disc degeneration grades were correlated with ageing. The Pfirrmann disc grades of degeneration in all spine levels showed the statistically significant difference according to the ages (p<0.001). The common Pfirrmann disc grades according to the ages were grade 3 among 2nd to 5th decades, and grade 4 was more common than 6th decades. The lower cervical level (C2–3 to C4–5) and lumbar level (L1–2 to L5–S1) were happened relatively early severe disc degeneration compared to other levels. The intersexual differences were increased after 6th decades.
Conclusion
Disc degeneration is natural course after one’s 2nd decades. And its incidence and grade were increased with age, and more affected by sexual difference after 6th decades.
Keywords: Pfirrmann disc grades, Disc degeneration, Spine, Age, Decades
INTRODUCTION
Disc degeneration in modern humans is often attributed to our upright, bipedal locomotion that is thought to place huge mechanical stresses on the vertebral column. Interestingly, in the analyze of the 1.5-million-year-old boy Homo erectus who died at an age of approximately 8 years, the indirect evidence of possible juvenile disc herniation (disc degeneration) represents the earliest known case of this typical human ailment that is intricately linked to upright bipedalism14). Nowadays, disc degeneration is a leading cause of lower back pain and a significant societal and economic problem world widely, and its prevalence are increasing due to the gradually lifestyle changes and the increased the lifespan in the modern civilized society compared to the previous period10). In fact, the relationship between ageing and disc degeneration is a matter of natural course, but, the comparison study for disc degeneration on imaging studies and ageing were relatively not well reported in the literature. Therefore, the authors conducted this study to identify the natural course of whole spine disc degeneration, and standard disc degeneration grade according to the ages.
MATERIALS AND METHODS
Total of all 653 patients who took whole spine magnetic resonance (MR) images between November 2012 and January 2013 at single spine institution were entrolled in this study to get permission of Institutional Review Board in Inha University Hospital (3190493AN01-201210-HR-003). The exclusion criteria for this study were lumbosacral abnormalities such as lumbarization or sacralization of the lumbosacral junction. The mean age was 48.1±58.7 years old (range, 12–95 years), and the subjects were composed with 336 male and 317 female patients. There were 19 cases in their 2nd decades and 74 cases in 3rd decades, 141 cases in 4th decades, 129 cases in 5th decades, 139 cases in 6th decades, and 93 cases in 7th decades, 58 cases in over 8th decades.
Whole spine disc degenerations were graded by 2 neurosurgeons that were blind to this study using the Pfirrmann classification (Fig. 1)23,24). If the measured grades were differently checked depending on different physicians, the grades were rechecked, and the agreed grades by 2 observers were selected. The Pfirrmann classification is one of useful tool to assess the degree of disc degeneration from T2-weighted images as it summarized in Table 1: grade 1, normal shape, no horizontal bands, clear distinction of nucleus and annulus; grade 2, nonhomogeneous shape with horizontal bands, some blurring between nucleus and annulus; grade 3, nonhomogeneous shape with blurring between nucleus and annulus, annulus shape still recognizable; grade 4, nonhomogeneous shape with hypointensity, annulus shape not intact and distinction between nucleus and annulus impossible, disc height usually decreased; and grade 5, same as grade 4 but with collapsed disc space. Grades 1 to 2 were classified as normal discs, while grades 3 to 5 were defined as degenerated17,23,27). All MR examinations were performed by 1.5 Tesla MR System(Magnetom Essenza M-1.5-S, Siemens, Munich, Germany), and only the sagittal T2-weighted image in whole spine MR were included in this analysis.
Fig. 1.
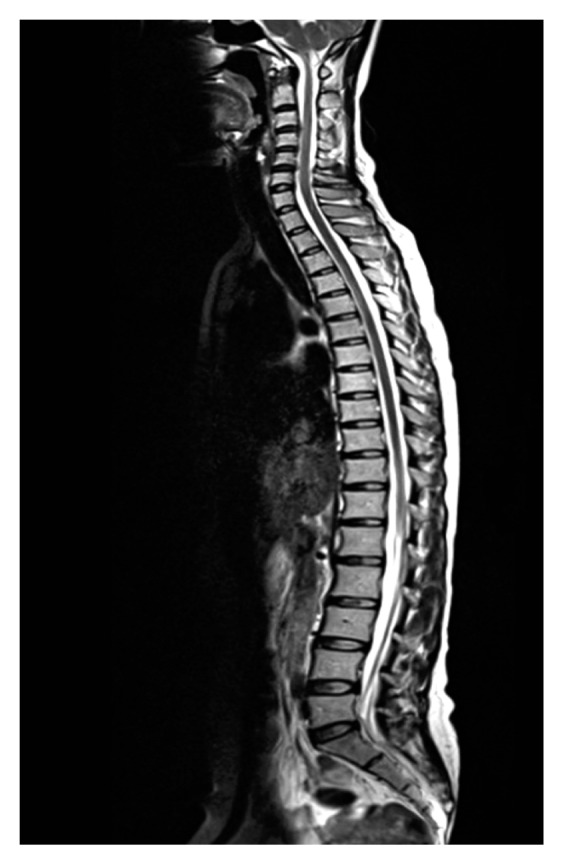
Whole spine sagittal T2-weighted image was analyzed to check the Pfirrmann disc grade.
Table 1.
The classification of Pfirrmann disc degeneration grade
| Grade | Structure | Distinction of nucleus and anulus | Signal intensity | Height of Intervertebral disc |
|---|---|---|---|---|
| 1 | Homogeneous, bright white | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| 2 | Inhomogeneous with or without horizontal bands | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| 3 | Inhomogeneous, gray | Unclear | Intermediate | Normal to slightly decreased |
| 4 | Inhomogeneous, gray to black | Lost | Intermediate to hypointense | Normal to moderately decreased |
| 5 | Inhomogeneous, black | Lost | Hypointense | Collapsed |
Grade 1, normal shape, no horizontal bands, clear distinction of nucleus and annulus; grade 2, nonhomogeneous shape with horizontal bands, some blurring between nucleus and annulus; grade 3, nonhomogeneous shape with blurring between nucleus and annulus, annulus shape still recognizable; grade 4, nonhomogeneous shape with hypointensity, annulus shape not intact and distinction between nucleus and annulus impossible, disc height usually decreased; grade 5, same as grade 4 but with collapsed disc space.
The Pfirrmann disc grades according to all disc levels from C2–3 to L5–S1 of 653 patients were analyzed including the sex and age variables, so total 15,019 disc levels were included in this study. The Levene test for equality of variances, Spearman rank correlation coefficient test and Mann-whitney U analysis were used to determine the significances of differences between age or sex and disc degeneration. SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used throughout, and statistical significance was accepted for p-values of <0.050.
RESULTS
Intraclass correlation between 2 different observers was found to be 0.891. In the Levene test for equality of variances, the equal variance of disc degeneration was observed according age in both male and female group (p=0.487), and the distribution was also not different between 2 groups (p=0.170). All checked Pfirrmann grades of disc degeneration at each disc levels were summarized in Table 2. The mean age was 48.1±58.7 years old (range, 12–95 years), and the subjects were composed with 336 male and 317 female patients. The most common Pfirrmann disc grades of disc degeneration without regarding of age was Pfirrmann disc grade 3 (45.5%, 1,782 among 3,918 discs) following Pfirrmann disc grade 4 (38.7%, 1,517 among 3,918 discs) and grade 3 (11.9%, 468 among 3,918 discs) in cervical regions, Pfirrmann disc grade 3 (52.5%, 4,115 among 7,836 discs) following Pfirrmann disc grade 2 (24.3%, 1,901 among 7,836 discs) and grade 4 (22.0%, 1,727 among 3,918 discs) in thoracic regions, and Pfirrmann disc grade 3 (40.6%, 1,327 among 3,265 discs) following Pfirrmann disc grade 4 (30.7%, 1,002 among 3,265 discs) and grade 3(22.4%, 732 among 3,265 discs) in lumbar regions.
Table 2.
All Pfirrmann grades of disc degeneration at each disc levels
| Level | Grade | ||||
|---|---|---|---|---|---|
|
| |||||
| 1 | 2 | 3 | 4 | 5 | |
| C2–3 | 6 (0.9) | 54 (8.3) | 318 (48.7) | 269 (41.2) | 6 (0.9) |
| C3–4 | 1 (0.2) | 49 (7.5) | 317 (48.5) | 279 (42.7) | 7 (1.1) |
| C4–5 | 3 (0.5) | 60 (9.2) | 312 (47.8) | 265 (40.6) | 13 (2.0) |
| C5–6 | 2 (0.3) | 45 (6.9) | 270 (41.3) | 275 (42.1) | 61 (9.3) |
| C6–7 | 3 (0.5) | 93 (14.2) | 262 (40.1) | 259 (39.7) | 36 (5.5) |
| C7–T1 | 6 (0.9) | 167 (25.6) | 303 (46.4) | 170 (26.0) | 7 (1.1) |
| T1–2 | 2 (0.3) | 148 (22.7) | 346 (53.0) | 150 (23.0) | 7 (1.1) |
| T2–3 | 1 (0.2) | 144 (22.1) | 344 (52.7) | 161 (24.7) | 3 (0.5) |
| T3–4 | 1 (0.2) | 126 (19.3) | 340 (52.1) | 179 (27.4) | 7 (1.1) |
| T4–5 | 1 (0.2) | 128 (19.6) | 319 (48.9) | 193 (29.6) | 12 (1.8) |
| T5–6 | 1 (0.2) | 125 (19.1) | 315 (48.2) | 201 (30.8) | 11 (1.7) |
| T6–7 | 0 (0) | 139 (21.3) | 315 (48.2) | 192 (29.4) | 7 (1.1) |
| T7–8 | 0 (0) | 139 (21.3) | 344 (52.7) | 161 (24.7) | 3 (0.5) |
| T8–9 | 1 (0.2) | 155 (23.7) | 354 (54.2) | 137 (21.0) | 5 (0.8) |
| T9–10 | 1 (0.2) | 170 (26.0) | 359 (55.0) | 120 (18.4) | 7 (1.1) |
| T10–11 | 2 (0.3) | 184 (28.2) | 359 (55.0) | 101 (15.5) | 3 (0.5) |
| T11–12 | 2 (0.3) | 216 (33.1) | 354 (54.2) | 76 (11.6) | 6 (0.9) |
| T12–L1 | 1 (0.2) | 227 (34.8) | 366 (56.0) | 56 (8.6) | 9 (1.4) |
| L1–2 | 0 (0) | 222 (34.0) | 333 (31.0) | 78 (11.9) | 20 (3.1) |
| L2–3 | 0 (0) | 197 (29.7) | 307 (47.0) | 122 (18.7) | 30 (4.6) |
| L3–4 | 0 (0) | 160 (24.5) | 284 (43.5) | 187 (28.6) | 22 (3.4) |
| L4–5 | 0 (0) | 82 (12.6) | 202 (30.9) | 302 (46.2) | 67 (10.3) |
| L5–S1 | 0 (0) | 71 (10.9) | 201 (30.8) | 313 (47.9) | 68 (10.4) |
Values are presented as number (%)
Grade 1, normal shape, no horizontal bands, clear distinction of nucleus and annulus; grade 2, nonhomogeneous shape with horizontal bands, some blurring between nucleus and annulus; grade 3, nonhomogeneous shape with blurring between nucleus and annulus, annulus shape still recognizable; grade 4, nonhomogeneous shape with hypointensity, annulus shape not intact and distinction between nucleus and annulus impossible, disc height usually decreased; grade 5, same as grade 4 but with collapsed disc space.
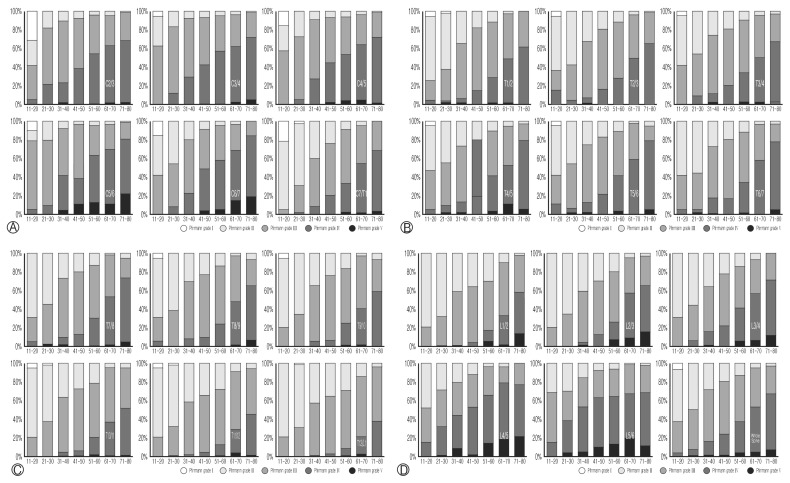
All spinal disc degeneration grades were correlated with ageing with the analysis using Spearmann rank correlation coefficient test. In this study, there were 19 cases in their 2nd decades and 74 cases in 3rd decades, 141 cases in 4th decades, 129 cases in 5th decades, 139 cases in 6th decades, and 93 cases in 7th decades, 58 cases in over 8th decades (Fig. 2). The Pfirrmann disc grades of degeneration in all spine levels showed the statistically significant difference according to the ages (p<0.001), and these were gradually increased from grade 1 to 5 with ageing process. The common Pfirrmann disc grades according to the ages were Grade 3 among 2nd to 5th decades and grade 4 was more common than 6th decades. The lower cervical level (from C5–6 to C6–7) and lumbar level (from L1–2 to L5–S1) were showed relatively high percentage of severe disc degeneration (Pfirrmann disc grade 5) in early stage (from 5th decades) compared to high cervical level (from C2–3 to C4–5) and thoracic level (from C7–T1 to T12–L1). Indeed, all single level disc degeneration at each level was significantly correlated with other disc degeneration at the different disc level in the analysis using Spearman rank correlation test (p<0.001).
Fig. 2.
Pfirrmann grade distribution by age at cervical (A), thoracic (B, C), lumbar levels and whole spine (D).
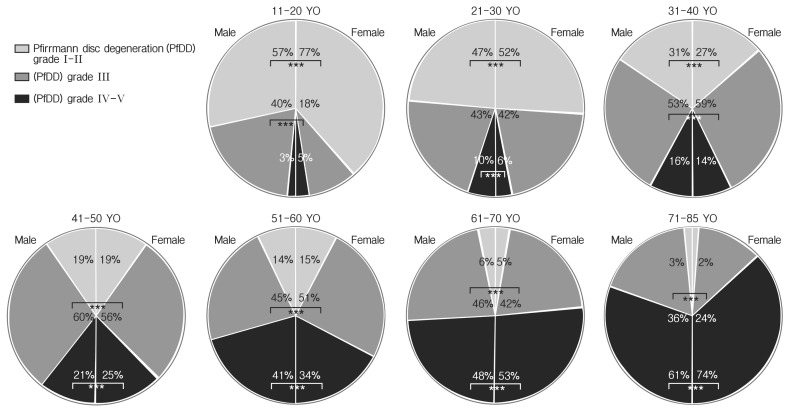
The relation between sex and Pfirrmann disc grade was analyzed by Mann-Whitney U-test (Table 3); it showed that no different disc degeneration among female population in all spine level with only except of the level of C2–3 (p=0.046), T6–7 (p= 0.050) and L4–5 (p=0.008). The composition of mild (Pfirrmann disc grades 1–2), moderate (Pfirrmann disc grade 3) and severe disc degeneration (Pfirrmann disc grades 4–5) is differently checked at all ages (Fig. 3). The intersexual difference were not definite until the 5th decades (significant differences were observed at the level of C3/4 and L5/S1 in 3rd decade, and L4/5 in 5th decade), but, the differences were frequently observed after one’s 6th decades (Fig. 4; significant differences were observed at the level of T8/9, T9/10, T12/L1, L1/2, L2/3, and L3/4 in 6th decade, T6/7 and T7/8 in 7th decade, and T6/7, T9/10, T10/11, and T11/12 in after 8th decade).
Table 3.
Comparison of Pfirrmann degeneration between sexes at each disc level in all age decades
| Level | p-value* |
|---|---|
| C2–3 | 0.046 |
| C3–4 | 0.782 |
| C4–5 | 0.349 |
| C5–6 | 0.667 |
| C6–7 | 0.678 |
| C7–T1 | 0.742 |
| T1–2 | 0.665 |
| T2–3 | 0.987 |
| T3–4 | 0.787 |
| T4–5 | 0.639 |
| T5–6 | 0.084 |
| T6–7 | 0.050 |
| T7–8 | 0.316 |
| T8–9 | 0.835 |
| T9–10 | 0.268 |
| T10–11 | 0.139 |
| T11–12 | 0.278 |
| T12–L1 | 0.358 |
| L1–2 | 0.783 |
| L2–3 | 0.315 |
| L3–4 | 0.713 |
| L4–5 | 0.008 |
| L5–S1 | 0.179 |
Mann-Whitney U-test
Fig. 3.
Pfirrmann grade distribution (PfDD) by sex. YO, years old. *** indicated statistically significant difference.
Fig. 4.
Pfirrmann grade distribution by sex and age. YO, years old. *** indicated statistically significant difference.
DISCUSSION
Intervertebral discs are complex anatomical structures that are essential for the mobility of intervertebral joints, and also participate in anchoring vertebrae together and distributing the pressure that results from movement of the entire trunk6). The first signs of disc degeneration begin to appear upon skeletal maturity in the nucleus pulposus3,6). Up until this time, 2 cell types populate the nucleus pulposus; chondrocyte-like cells and notochordal cells, and notochordal cells are responsible for maintaining homeostasis1,7–9). The loss of these cells during skeletal maturation might constitute one of the first changes that occur in the cascade of degenerative events. Although this degeneration arises during the natural aging process, pathological degeneration can also occur, which progresses in an accelerated and brutal manner6).
Back pain is strongly associated with disc degeneration and neurological dysfunction20,28), and is one of the leading chronic illnesses requiring physician care and utilization of the healthcare system4,11). Disc degeneration has been attributed to elevation in cytokine levels in the disc, which in turn elevates levels of aggrecanases and all matrix metalloproteinases that cleave aggrecan and other matrix components2). Fragmentation and loss of aggrecan, the most abundant proteoglycan present in the disc, results in loss of disc height, and its neurological sequelae lead to back pain5,25,26). The intervertebral disc becomes avascular by the fourth year of life, and it has been the major target of basic and clinical research on low back pain28). Despite the efficiency of pain-relieving treatments, the physicians had been seeked to develop innovative therapeutic approaches that might limit the use of invasive surgical procedures6). But, before a prerequisite to the development of these strategies, we should improve our fundamental knowledge regarding disc degeneration pathophysiology and its natural course.
Degenerative disc disease is part of the natural process of growing older, although it also can be caused by injury or trauma18). Indeed, recently, its relative other risk factors such as biomechanical18), biochemical21), genetic15), occupational29) disc nutritional factors12), were investigated and identified. Although definite answer for disc degeneration was not established yet, it was considered that ageing is a main pathogenesis of disc degeneration associated with multifactorial influence as our result indicated. In this study, the authors divided the cases according to the one’s decades of life, and the Pfirrmann disc grades in all spine levels showed that gradually of disc degeneration with ageing process. The common Pfirrmann disc grades were grade 3 in 2nd to 5th decades, and grade 4 was more common in over 6th decades. And it is well correlated with the data of previous systemic reviews13,18).
The Pfirrmann disc grades of degeneration in all spine levels showed the significant difference according to the ages, and all single level disc degeneration at each level was significantly correlated with other disc degeneration at the different disc level. This result was similar to previous study which reported a higher prevalence of disc degeneration of the cervical spine in patients with lumbar disc herniation compare to healthy volunteers22). In the consecutive studies about cervical and lumbar degeneration among asymptomatic Korean subjects, there are some similar result were observed: (1) high prevalence of annular fissure, nucleus degeneration, and extrusion at cervical and lumbar in asymptomatic subjects16,19); (2) high prevalence of bulging, protrusion, and annular fissure at L4/5 and L5/S1 and nucleus degeneration at L5/S1 in young ages16); and (3) close relationship between the cervical degeneration and lumbar degeneration19). Hence, the results of these studies suggest that disc degeneration appears to be a systemic phenomenon. And, it was showed that no different disc degeneration in all spine level between sexes (summarized all aging decades) with some exception of the level of C2–3, T6–7, and L4–5. This result was very similar to previous studies18,22), but each segment analysis with age and sex consideration showed that sexual difference according to the decades. The intersexual difference were not definite until the 5th decades (except at the level of C3/4, L5/S1, and L4/5), but, the differences were frequently observed after one’s 6th decades (especially at the level of T6/7–L3/4).
Some limitations could interfere this result. First, the Pfirrmann disc grades could over or underestimated by using whole spine MR image, because the image were could differently checkable by different image voxel size. Second, this study was based on the population who visited the hospital with neck or backpain, so this result is not indicated the normal value of healthy population. Nevertheless these limitations, this recent study provided useful information about the natural course of whole spine disc degeneration, and standard disc degeneration grade according to the ages. In the near future, the authors considered that large-scale and well-designed studies are required reviewing the topic of disc degeneration.
CONCLUSION
As a conclusion, disc degeneration is natural course after one’s 2nd decades, and its incidence and grade were increased with age. Disc degeneration affected by sexual difference was focused in the motional segments such as C3/4, L4/5, and L5/S1 until 5th decades, and it changed to the thoracic segments after 6th decades.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 2.Akyol S, Eraslan BS, Etyemez H, Tanriverdi T, Hanci M. Catabolic cytokine expressions in patients with degenerative disc disease. Turk Neurosurg. 2010;20:492–499. doi: 10.5137/1019-5149.JTN.3394-10.1. [DOI] [PubMed] [Google Scholar]
- 3.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976) 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cats-Baril WL, Frymoyer JW. Identifying patients at risk of becoming disabled because of low-back pain. The Vermont Rehabilitation Engineering Center predictive model. Spine (Phila Pa 1976) 1991;16:605–607. doi: 10.1097/00007632-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Chung SA, Khan SN, Diwan AD. The molecular basis of intervertebral disk degeneration. Orthop Clin North Am. 2003;34:209–219. doi: 10.1016/s0030-5898(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 6.Colombier P, Clouet J, Hamel O, Lescaudron L, Guicheux J. The lumbar intervertebral disc: from embryonic development to degeneration. Joint Bone Spine. 2014;81:125–129. doi: 10.1016/j.jbspin.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Erwin WM. The Notochord, Notochordal cell and CTGF/CCN-2: ongoing activity from development through maturation. J Cell Commun Signal. 2008;2:59–65. doi: 10.1007/s12079-008-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erwin WM, Ashman K, O’Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54:3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- 9.Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FW. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R215. doi: 10.1186/ar3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frith JE, Cameron AR, Menzies DJ, Ghosh P, Whitehead DL, Gronthos S, et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials. 2013;34:9430–9440. doi: 10.1016/j.biomaterials.2013.08.072. [DOI] [PubMed] [Google Scholar]
- 11.Gawri R, Antoniou J, Ouellet J, Awwad W, Steffen T, Roughley P, et al. Best paper NASS 2013: link-N can stimulate proteoglycan synthesis in the degenerated human intervertebral discs. Eur Cell Mater. 2013;26:107–119. doi: 10.22203/ecm.v026a08. [DOI] [PubMed] [Google Scholar]
- 12.Grunhagen T, Shirazi-Adl A, Fairbank JC, Urban JP. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42:465–477. vii. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;90:1261–1270. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- 14.Haeusler M, Schiess R, Boeni T. Evidence for juvenile disc herniation in a homo erectus boy skeleton. Spine (Phila Pa 1976) 2013;38:E123–128. doi: 10.1097/BRS.0b013e31827cd245. [DOI] [PubMed] [Google Scholar]
- 15.Kao PY, Chan D, Samartzis D, Sham PC, Song YQ. Genetics of lumbar disk degeneration: technology, study designs, and risk factors. Orthop Clin North Am. 2011;42:479–486. vii. doi: 10.1016/j.ocl.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Lee TH, Lim SM. Prevalence of disc degeneration in asymptomatic korean subjects. Part 1: lumbar spine. J Korean Neurosurg Soc. 2013;53:31–38. doi: 10.3340/jkns.2013.53.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Lee TH, Yi S. Prevalence of disc degeneration in asymptomatic korean subjects. Part 3: cervical and lumbar relationship. J Korean Neurosurg Soc. 2013;53:167–173. doi: 10.3340/jkns.2013.53.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MJ, Dettori JR, Standaert CJ, Brodt ED, Chapman JR. The natural history of degeneration of the lumbar and cervical spines: a systematic review. Spine (Phila Pa 1976) 2012;37(22 Suppl):S18–30. doi: 10.1097/BRS.0b013e31826cac62. [DOI] [PubMed] [Google Scholar]
- 19.Lee TH, Kim SJ, Lim SM. Prevalence of disc degeneration in asymptomatic Korean subjects. Part 2: cervical spine. J Korean Neurosurg Soc. 2013;53:89–95. doi: 10.3340/jkns.2013.53.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 21.Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine (Phila Pa 1976) 1992;17:1079–1082. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Okada E, Matsumoto M, Fujiwara H, Toyama Y. Disc degeneration of cervical spine on MRI in patients with lumbar disc herniation: comparison study with asymptomatic volunteers. Eur Spine J. 2011;20:585–591. doi: 10.1007/s00586-010-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 24.Rim DC. Quantitative Pfirrmann disc degeneration grading system to overcome the limitation of Pfirrmann disc degeneration grade. Korean J Spine. 2016;13:1–8. doi: 10.14245/kjs.2016.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 26.Roughley PJ, Melching LI, Heathfield TF, Pearce RH, Mort JS. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S326–332. doi: 10.1007/s00586-006-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takatalo J, Karppinen J, Taimela S, Niinimäki J, Laitinen J, Sequeiros RB, et al. Association of abdominal obesity with lumbar disc degeneration--a magnetic resonance imaging study. PLoS One. 2013;8:e56244. doi: 10.1371/journal.pone.0056244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeno K, Kobayashi S, Negoro K, Uchida K, Miyazaki T, Yayama T, et al. Physical limitations to tissue engineering of intervertebral disc cells: effect of extracellular osmotic change on glycosaminoglycan production and cell metabolism. Laboratory investigation. J Neurosurg Spine. 2007;7:637–644. doi: 10.3171/SPI-07/12/637. [DOI] [PubMed] [Google Scholar]
- 29.Williams FM, Sambrook PN. Neck and back pain and intervertebral disc degeneration: role of occupational factors. Best Pract Res Clin Rheumatol. 2011;25:69–79. doi: 10.1016/j.berh.2011.01.007. [DOI] [PubMed] [Google Scholar]