Abstract
Background
Current retrospective evidence suggests similar clinical and superior hemodynamic outcomes of the Sorin Freedom Solo stentless aortic valve (SFS) (LivaNova PLC, London, UK) compared to the Carpentier Edwards Perimount stented aortic valve (CEP) (Edwards Lifesciences Inc., Irvine, California, USA). To date, no reports exist describing case-matched long-term outcomes and analysis for treatment of native valve endocarditis (NVE).
Methods
From 2004 through 2014, 77 consecutive patients (study group, 59.7% male, 68.9 ± 12.5 years, logEuroSCORE II 7.6 ± 12.3%) received surgical aortic valve replacement (SAVR) with the SFS. A control group of patients after SAVR with the CEP was retrieved from our database and matched to the study group regarding 15 parameters including preoperative endocarditis. Acute perioperative outcomes and follow-up data (mean follow-up time 48.7±29.8 months, 95% complete) were retrospectively analyzed.
Results
No differences in early mortality occurred during 30-day follow up (3/77; 3.9% vs. 4/77; 5.2%; p = 0.699). Echocardiographic findings revealed lower postprocedural transvalvular pressure gradients (max. 17.0 ± 8.2 vs. 24.5 ± 9.2 mmHg, p< 0.001/ mean pressure of 8.4 ± 4.1 vs. 13.1 ± 5.9 mmHg, p< 0.001) in the SFS group. Structural valve degeneration (SVD) (5.2% vs. 0%; p = 0.04) and valve explantation due to SVD or prosthetic valve endocarditis (PVE) (9.1% vs. 1.3%; p = 0.04) was more frequent in the SFS group. All-cause mortality during follow-up was 20.8% vs. 14.3% (p = 0.397). When patients were divided into subgroups of NVE and respective utilized bioprosthesis, the SFS presented impaired outcomes regarding mortality in NVE cases (p = 0.031).
Conclusions
The hemodynamic superiority of the SFS was confirmed in this comparison. However, clinical outcomes in terms of SVD and PVE rates, as well as survival after NVE, were inferior in this study. Therefore, we are reluctant to recommend utilization of the SFS for treatment of NVE.
Introduction
Surgical aortic valve replacement (SAVR) utilizing stentless bioprostheses has been reported a reasonable alternative to stented xenovalves. Main advantages of stentless valves are considered superior hemodynamic outcomes in terms of postoperative transprosthetic pressure gradients and effective orifice area (EOA). Especially in small aortic annuli stentless valves are able to generate adequate EOA and avoid patient-prosthesis mismatch due to the supra-annular implantation technique [1]. However, there is a lack of knowledge regarding clinical long-term outcomes compared to stented bioprostheses [2]. At least, there are few studies emphasizing the risk of premature structural valve deterioration (SVD) and high explantation rates of stentless bioprostheses [1; 3; 4]. Furthermore, there are only few reports for efficacy of treatment for native valve endocarditis (NVE) utilizing stentless bioprostheses in aortic position. NVE remains a life-threatening condition and surgical treatment of NVE is indicated when heart failure due to valve insufficiency or uncontrolled infection occurs, or when embolism due to persistent vegetations >15 mm is anticipated [5; 6]. Beside debridement of infected tissue and repair of cardiac structures utilizing pericardium, SAVR is the operative approach of choice for the infected aortic valve (AV) [7]. For SAVR mechanical and biological prostheses or homografts can be taken into consideration [8; 9]. Recently, SAVR with the Medtronic Freestyle (Medtronic Inc., Minneapolis, MN, USA) stentless bioprosthesis for extensive NVE with aortic root involvement and periannular abscess formation was described with good late survival and low rates of recurrence of prosthetic valve endocarditis (PVE) [10]. One of the most frequently utilized stentless valve is the bovine pericardial Sorin Freedom Solo (SFS) (LivaNova PLC, London, UK). The SFS was reported to present an equivalent safety profile compared to stented bioprostheses, while yielding superior mid- and long-term hemodynamic outcomes in a non-infective setting [11]. Furthermore, the SFS is considered to facilitate more rapid left ventricular (LV) reverse remodeling [12].
We hereby aimed to analyze long-term outcomes of the SFS with regards to mortality, rates of SVD, valve explantation, PVE, and hemodynamic outcomes compared with the Carpentier Edwards Perimount (CEP) stented bovine pericardial aortic valve (Edwards Lifesciences Inc., Irvine, California, USA) in a case-matched study design. Furthermore, a subgroup of patients treated for AV- NVE was evaluated.
Materials and methods
Patients
All research was approved by the authors' Institutional Review Board (University Hospital Hamburg-Eppendorf). All clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki. Informed consent, was obtained from the participants. A consecutive series of 77 patients received SAVR using the stentless bovine pericardial SFS for treatment of severe symptomatic calcified aortic stenosis or aortic regurgitation in cases of NVE (study group). Of those 24.7% (19/77 pts.) suffered from NVE. Allocation of patients to SAVR followed current international recommendations after consensus of the local dedicated heart team [13]. For comparative assessment, a matched control group of 77 patients treated by SAVR using the stented bovine pericardial CEP was retrieved from our dedicated hospital database containing a total of 614 CEP patients. Follow-up was conducted by mail for patients and the treating physicians, respectively. In cases of missing answers, patients and/or physicians were contacted by phone.
Diagnostic work-up and study procedure
Diagnostics and procedures followed institutional routines: By routine, all patients received preoperative transthoracic echocardiography (TTE) and transesophageal echocardiography in cases of suspected NVE for evaluation of cardiac functional status, valve morphology and assessment of vegetations and/or involvement of the aortic annulus and surrounding structures in NVE. The SFS was preferred in aortic annuli with a diameter ≤ 20 mm; destroyed aortic annuli in NVE, and severly hypertrophed LV.
Operative technique
All operations were performed through a median sternotomy (in all NVE cases) or partial sternotomy (11.6%; 18/154 pts.) with CPB on the arrested heart using Bretschneider Cardioplegia. SFS valves were implanted in a supra-annular position with a single running Prolene (Ethicon Inc., Somerville, NJ, USA) suture line and interrupted u-stitches for the CEP. All patients received lifelong aspirin as antiplatelet inhibitor.
Statistics
Baseline, intraprocedural and follow-up data (mean follow-up time 48.7±29.8 months) were collected, entered into a dedicated standardized database and retrospectively analyzed. Primary clinical endpoints were (1) death, (2) re-operation, (3) PVE and (4) structural valve deterioration. Secondary endpoint was hemodynamic performance in terms of (1) peak/mean transprosthetic pressure gradients and (2) trans- and/or paravalvular leakage.
Data are presented as absolute numbers and percentages for categorical variables and mean values and standard deviation for continuous variables unless stated otherwise.
Matching was performed as previously described [14]: To evaluate the effect of a treatment in a non-randomized setting, 1:1 matching (drawing without replacement) was conducted by logistic regression and nearest neighbor matching as the measure of proximity. In a first step matching pairs of all complete cases from the treatment group were identified for the following 15 variables: age, gender, NVE, logEuroSCORE II, New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF), pulmonary hypertension, peripheral artery disease, creatinine at baseline, chronic obstructive pulmonary disease (COPD) > Gold II, previous sternotomy, previous stroke, coronary artery disease (CAD), diabetes mellitus and arterial hypertension. In consecutive steps, all remaining pairs were identified in case of missing data. All computation was carried out by the statistical software R and the R-package MatchIt [15; 16]. Due to the dependence structure of the matched pairs data, we used t-tests the for continuous data and McNemar`s tests for categorical data. A level of significance was set to two-tailed p < 0.05.
To further evaluate survival of the study and control group we performed a multivariate COX-regression including five different variables.
Results
Baseline demographics and matching results
77 consecutive patients (study group) received SAVR using the SFS valve (study group, 59.7% male, 68.9 ± 12.5 years, logEuroSCORE II 7.6 ± 12.3%). Matching yielded a control group of 77 patients receiving SAVR utilizing the CEP valve who were similar to the study group with regard to 15 important baseline parameters including endocarditis (24.7% vs. 20.1%; p = 0.53]), EuroSCORE II (7.6 ± 12.3 vs. 5.2 ± 6.3; p = 0.09) and Creatinine (1.14 ± 0.61 mg/dl vs. 1.02 ± 0.34 mg/dl; p = 0.08). No significant inter-group differences were present after matching. Detailed patient demographics are summarized in Table 1.
Table 1. Baseline demographics and matching results.
| Freedom Solo (n = 77) |
Edwards Perimount (n = 77) |
p-value | |
|---|---|---|---|
| Age, y | 68.9 ± 12.5 | 67.1 ± 12.2 | 0.302 |
| Male gender, % (n) | 59.7 (46) | 68.8 (53) | 0.239 |
| Endocarditis, % (n) | 24.7 (19) | 20.1 (16) | 0.534 |
| EuroSCORE II, % | 7.6 ± 12.3 | 5.2 ± 6.3 | 0.093 |
| NYHA ≥ III, % (n) | 56.2 (44) | 58.4 (45) | 0.870 |
| PHT > 60 mmHg, % (n) | 16.9 (13) | 11.7 (9) | 0.836 |
| Extracardiac atheropathy, % (n) | 11.7 (9) | 7.8 (6) | 0.685 |
| - Peripheral artery disease | 2.6 (2) | 1.3 (1) | |
| - Carotid artery stenosis | 7.8 (6) | 6.5 (5) | |
| - Both | 1.3 (1) | 0.0 (0) | |
| Creatinine, mg/dl | 1.14 ± 0.61 | 1.02 ± 0.34 | 0.083 |
| COPD > GOLD II, % (n) | 9.1 (7) | 7.8 (6) | 0.772 |
| Previous cardiac surgery, % (n) | 9.1 (7) | 10.4 (8) | 0.786 |
| Prior stroke, % (n) | 3.9 (3) | 5.2 (4) | 0.690 |
| Coronary heart disease, % (n) | 36.4 (28) | 33.8 (26) | 0.962 |
| - 1-VD | 14.3 (11) | 14.3 (11) | |
| - 2-VD | 10.4 (8) | 10.4 (8) | |
| - 3-VD | 11.7 (9) | 9.1 (7) | |
| Diabetes, % (n) | 19.5 (15) | 13.0 (10) | 0.527 |
| Arterial hypertension, % (n) | 59.7 (46) | 62.3 (48) | 0.619 |
EuroSCORE: European System for Cardiac Operative Risk Evaluation, NYHA: New York Heart Association, PHT: Pulmonary hypertension, COPD: Chronic obstructive pulmonary disease, GOLD: Global Initiative For Chronic Obstructive Lung Disease, VD: Vessel disease
Perioperative data
There were no significant differences between SFS and CEP groups regarding baseline echocardiography parameters, with the exception of preoperative peak pressure gradients, which were significantly lower in the SFS group (55.5 ± 29.7 mmHg vs. 71.2 ± 29.3 mmHg, p = 0.004), preoperative EOA (1.0 ± 0.5 cm2 vs. 0.8 ± 0.5 cm2, p = 0.044) and diameter of the interventricular septum (IVS) (12.0 ± 2.2 mm vs. 14.0 ± 2.9 mm, p = 0.015). For detailed echocardiography values see Table 2.
Table 2. Preprocedural echocardiography.
| Freedom Solo (n = 77) |
Edwards Perimount (n = 77) |
p-value | |
|---|---|---|---|
| Peak gradient (mmHg) | 55.5 ± 29.7 | 71.2 ± 29.3 | 0.004 |
| Mean gradient (mmHg) | 33.9 ± 20.5 | 41.0 ± 18.0 | 0.052 |
| AVA (cm2) | 1.0 ± 0.5 | 0.8 ± 0.5 | 0.044 |
| Aortic valve regurgitation ≥ Grade III, % (n) | 23.4 (18) | 23.4 (18) | 0.805 |
| Mitral valve regurgitation ≥ Grade III, % (n) | 9.1 (7) | 11.7 (9) | 0.575 |
| LV EF < 45%, % (n) | 11.7 (9) | 15.6 (12) | 0.590 |
| Diameter (mm) | |||
| - LVEDD | 56.2 ± 9.5 | 53.2 ± 11.6 | 0.360 |
| - LVESD | 31.6 ± 5.9 | 38.4 ± 10.8 | 0.100 |
| LV hypertrophy, % (n) | 41.6 (32) | 55.8 (43) | 0.325 |
AVA: Aortic valve area, LVEF: Left ventricular ejection fraction, LV: Left ventricular, LVEDD: Left ventricular end diastolic diameter, LVESD: Left ventricular end systolic diameter, IVS: Interventricular septum, PW: Posterior wall
Rate of re-do surgery did not differ between SFS and CEP group. In the control group significant more patients presented an acute indication for SAVR (elective: 90.9%, acute: 2.6%, emergency: 6.5% vs. elective: 77.9%, acute: 14.3%, emergency: 7.8%, p = 0.029). Extracorporeal circulation (ECC) time (135.0 ± 48.7 min vs. 132.3 ± 50.4 min, p = 0.76), aortic cross clamp (ACC) time (90.6 ± 37.6 min vs. 90.1 ± 32.7 min, p = 0.94) and rate of concomitant procedures (42.9% vs. 42.9%, p = 1.0) showed no significant differences among groups. In the SFS group a longer hospital stay (12.0 ± 8.4 d vs. 6.2 ± 3.6 d, p< 0.001), a higher rate of blood transfusion (70.1% vs. 33.8%, p< 0.001), and a shorter ventilation time (8.8 ± 11.5 h vs. 14.9 ± 5.8 h, p< 0.001) was documented. Detailed periprocedural data are summarized in Table 3.
Table 3. Periprocedural data.
| Freedom Solo (n = 77) |
Edwards Perimount (n = 77) |
p-value | |
|---|---|---|---|
| Re-do surgery, % (n) | 9.1 (7) | 10.4 (8) | 0.786 |
| Urgency, % (n) | |||
| - Elective | 90.9 (70) | 77.9 (60) | 0.029 |
| - Acute | 2.6 (2) | 14.3 (11) | |
| - Emergency | 6.5 (5) | 7.8 (6) | |
| LOS ICU (d) | 3.8 ± 7.3 | 2.5 ± 1.8 | 0.160 |
| LOS Hospital (d) | 12.0 ± 8.4 | 6.2 ± 3.6 | < 0.001 |
| Procedure time (min) | 275.7 ± 64.0 | 281.9 ± 81.5 | 0.610 |
| Blood transfusion, % (n) | 70.1 (54) | 33.8 (26) | < 0.001 |
| Blood transfusion, n | 4.4 ± 6.4 | 3.2 ± 2.9 | 0.260 |
| Access, % (n) | |||
| - Median sternotomy | 64.9 (50) | 63.6 (49) | 0.083 |
| - Minimal invasive | 31.2 (24) | 29.9 (23) | |
| Procedure, % (n) | |||
| - Isolated | 57.1 (44) | 57.1 (44) | 1 |
| - Two or more procedures | 42.9 (33) | 42.9 (33) | |
| Mitral valve procedure, % (n) | |||
| - Valve repair | 13.0 (10) | 3.9 (3) | 0.137 |
| - Valve replacement | 2.6 (2) | 3.9 (3) | |
| Ventilation time (h) | 8.8 ± 11.5 | 14.9 ± 5.8 | < 0.001 |
| ECC (min) | 135.0 ± 48.7 | 132.3 ± 50.4 | 0.760 |
| ACC (min) | 90.6 ± 37.6 | 90.1 ± 32.7 | 0.940 |
LOS: Length of stay, ICU: Intensive care unit, ECC: Cardiopulmonary bypass, ACC: Aortic cross clamp
Echocardiographic follow-up
In the SFS group, peak and mean transvalvular gradients as determined by TTE prior to discharge decreased from 55.5 ± 29.7 mmHg to 17.0 ± 8.2 mmHg and 33.9 ± 20.5 mmHg to 8.4 ± 4.1 mmHg, respectively (both p<0.01). Effective orifice area (EOA) increased from 1.0 ± 0.5 cm2 to 2.16 ± 0.57 cm2 (p<0.01) compared to baseline values. Corresponding data in the CEP group were: decrease of peak and mean transvalvular gradients from 71.2 ± 29.3 mmHg to 24.5 ± 9.2 mmHg and 41.0 ± 18.0 mmHg to 13.1 ± 5.9 mmHg (both p<0.01), increase of EOA from 0.8 ± 0.5 cm2 to 2.07 ± 0.48 cm2 (p<0.01). The SFS group presented significant lower postoperative peak and mean pressure gradients compared to the CEP group (17.0 ± 8.2 mmHg vs. 24.5 ± 9.2 mmHg (p< 0.001) and 8.4 ± 4.1 mmHg vs. 13.1 ± 5.9 mmHg (p< 0.001)).
There was a significant higher rate of transvalvular leakage (TVL) = Grade I (26.0% vs. 3.9%, p< 0.001) in the SFS group. Rates of paravalvular leakage (PVL) ≥ Grade I were comparable among groups (7.8% vs. 3.9%, p = 0.521).
Early and late outcome
There were 3/77 deaths (3.9%) during the 30-day follow up in the SFS group and 4/77 (5.2%) in the CEP group (p = 0.699). For detailed acute 30-day outcome data see Table 4.
Table 4. Clinical 30-day outcome and echocardiographic results at discharge.
| Freedom Solo (n = 77) |
Edwards Perimount (n = 77) |
p-value | |
|---|---|---|---|
| Creatinine (mg/dl) | 1.2 ± 0.5 | 1.0 ± 0.4 | 0.036 |
| Creatinine peak (mg/dl) | 1.6 ± 1.1 | 1.3 ± 0.6 | 0.010 |
| AVR ≥ Grade II, % (n) | |||
| - Valvular | 0 (0) | 0 (0) | 0.867 |
| - Paravalvular leak | 2.6 (2) | 0 (0) | 0.521 |
| MVR ≥ Grade II, % (n) | 3.9 (3) | 9.1 (7) | 0.174 |
| AVA (cm2) | 2.16 ± 0.57 | 2.07 ± 0.48 | 0.782 |
| PPR (cm2/m2) | 1.1 ± 0.3 | 1.0 ± 0.2 | 0.600 |
| Peak gradient (mmHg) | 17.0 ± 8.2 | 24.5 ± 9.2 | < 0.001 |
| Mean gradient (mmHg) | 8.4 ± 4.1 | 13.1 ± 5.9 | < 0.001 |
| LVEF < 45%, % (n) | 5.2 (4) | 7.8 (6) | 0.557 |
| Cardiac tamponade, % (n) | 9.1 (7) | 15.6 (12) | 0.624 |
| Wound healing deficit, % (n) | 2.6 (2) | 1.3 (1) | 0.207 |
| MACCE, % (n) | 2.6 (2) | 0.0 (0) | 0.166 |
| - Stroke, TIA | 2.6 (2) | 0.0 (0) | |
| - Myocardial infarction | 0.0 (0) | 0.0 (0) | |
| Death, % (n) | 3.9 (3) | 5.2 (4) | 0.699 |
| Pacemaker, % (n) | 2.6 (2) | 0.0 (0) | 0.157 |
NYHA: New York Heart Association, AVR: Aortic valve regurgitation, MVR: Mitral valve regurgitation, AVA: Aortic valve area, PPR: Patient prosthesis ratio, LVEF: Left ventricular ejection fraction
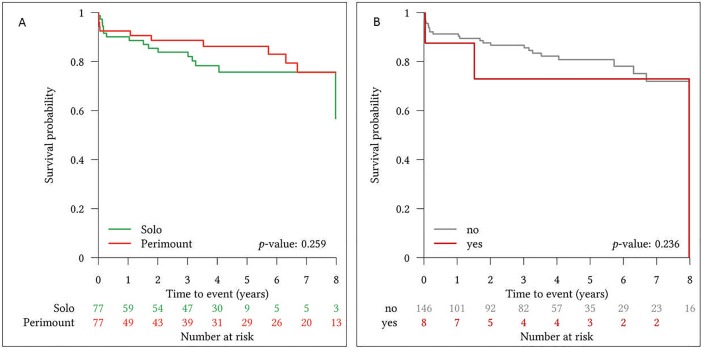
All-cause mortality during the mean follow-up of 48.7±29.8 months was 20.8% (16/77) in the SFS group and 14.3% (11/77) in the CEP group, showing no significant difference (p = 0.397). Kaplan-Meier curves for long-term survival showed no significant group differences among groups (p = 0.259) and are presented in Fig 1A.
Fig 1.
Kaplan-Meier survival curve for survival comparison of Sorin Freedom Solo and Carpentier Edwards Perimount bioprosthetic heart valves (A) and survival probability for patients with or without re-do surgery after index procedure [no = without re-do surgery; yes = with re-do surgery] (B).
SVD (2 cases of severe calcification with subsequent severe bioprosthetic stenosis, 2 cases of severe paravalvular leakage) (5.2% vs. 0%; p = 0.04) and valve explantation due to SVD and/or PVE (9.1% vs. 1.3%; p = 0.04) was more frequent in the SFS group. Re-do surgery due to SVD and PVE was not associated with a higher risk for death in follow-up (Fig 1B). No significant differences in postoperative stroke (5.2% vs. 2.6%, p = 0.507) or myocardial infarction rates (0% vs. 1.3%, p = 0.281) during follow-up were observed. Detailed follow-up data are summarized in Table 5.
Table 5. Long term follow-up.
| FreedomSolo (n = 77) |
Edwards Perimount (n = 77) |
p-value | |
|---|---|---|---|
| Events, % (n) | |||
| - Valve degeneration | 5.2 (4) | 0 (0) | 0.049 |
| - Thromboembolic | 1.3 (1) | 0 (0) | 0.341 |
| - Valve thrombosis | 0 (0) | 0 (0) | 0.571 |
| - Endocarditis | 5.2 (4) | 1.3 (1) | 0.211 |
| - Paravalvular leak | 2.6 (2) | 0 (0) | 0.176 |
| MACCE, % (n) | 5.2 (4) | 3.9 (3) | 0.456 |
| - Stroke | 5.2 (4) | 2.6 (2) | 0.507 |
| - Myocardial infarction | 0 (0) | 1.3 (1) | 0.281 |
| Explantation/ Re-Do, % (n) | 9.1 (7) | 1.3 (1) | 0.038 |
| - Structural valve deterioration | 5.2 (4) | 0 (0) | 0.285 |
| - Endocarditis | 3.9 (3) | 1.3 (1) | |
| Mortality, % (n) | |||
| - Survival | 79.2 (61) | 85.7 (66) | 0.397 |
| - Overall mortality | 20.8 (16) | 14.3 (11) | |
| Cause of death, % (n) | |||
| - Cardiac related | 3.9 (3) | 2.6 (2) | 0.937 |
| - Valve related | 6.5 (5) | 1.3 (1) | 0.211 |
| - Sepsis | 1.3 (1) | 1.3 (1) | 0.727 |
| - Multi organ failure | 1.3 (1) | 0 (0) | 0.420 |
| - Tumor | 3.9 (3) | 2.6 (2) | 0.937 |
| - Other | 0 (0) | 1.3 (1) | 0.197 |
| - Unknown | 3.9 (3) | 5.2 (4) | 0.780 |
MACCE: Major adverse cardiac and cerebrovascular events
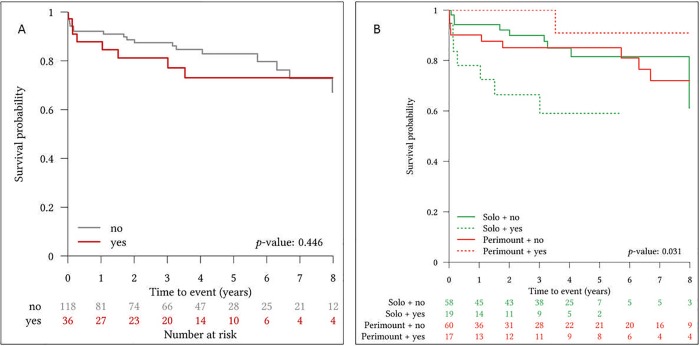
When comparing patients with or without preoperative NVE in survival analysis, no group differences in survival were present (Fig 2A). However, when patients were divided into subgroups of NVE and respective utilized bioprosthesis, the SFS presented impaired outcomes in NVE cases (p = 0.031) (Fig 2B).
Fig 2.
Survival probability for patients with or without NVE prior to index procedure [no = no preoperative endocarditis; yes = preoperative endocarditis] (A) and survival probability for patients treated with Sorin Freedom Solo or Carpentier Edwards Perimount bioprosthetic heart valves with or without preoperative native valve endocarditis [NVE] [no = no NVE; yes = NVE] (B).
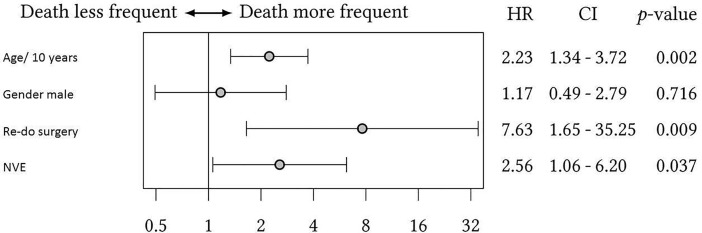
Multivariate COX-analysis revealed a pronounced impact of re-do surgery on survival (HR: 7.63, CI: 1.65–35.25, p = 0.009). Also, age and preoperative NVE were connected with an increased risk of death during follow up with hazard ratios of 2.23 and 2.56, respectively.
Details of multivariate COX-analysis are depicted by Forest-plot (Fig 3).
Fig 3. Forest-plot of multivariate COX-analysis with inclusion of four parameters; NVE native valve endocarditis.
Discussion
Main findings
The hemodynamic superiority of the SFS in terms of postoperative transprosthetic pressure gradients compared to the CEP valve was confirmed in this case-matched comparison. However, clinical outcomes in terms of SVD and PVE rates were inferior in this study. Furthermore, the SFS stentless aortic valve showed impaired outcomes regarding mortality in a subgroup of patients who presented with NVE.
A current multicenter study including 565 patients provided with the SFS, reported postoperative transprosthetic pressure gradients of peak/mean 17.7 ± 9.1/ 9.9 ± 5.4 mmHg and a reasonable safety profile with freedom from SVD and re-operation of 90.8% and 87.3% during follow-up [17]. In this analysis the subgroup of patients provided with SAVR for NVE was not further reviewed. While our study confirms the excellent hemodynamic outcomes of the SFS, already known from multiple reports [18; 19], we demonstrated impaired outcomes of patients with NVE treated with the SFS. Reports of surgery for NVE utilizing stentless pericardial valves are scarce and present limited patient numbers with or without group comparison [20; 21]. Explanations for superiority of a stented pericardial valve for AV-NVE are only speculative. Most centers prefer mechanical or stented bioprostheses for AV-NVE surgery [22; 23] and also international guidelines recommend utilization of those prostheses [6] due to best documented results. However, stentless valves may be advantageous in root involvement due to the option of supra-annular implantation or root replacement which is for instance feasible with the Medtronic Freestyle valve. In our institution we followed this approach during the study period of this work. Although we saw more frequent postoperative PVE in the SFS group, the difference between the two groups was not significant and occurrence of more frequent PVE in the SFS group may be due to utilization in severely destroyed aortic annuli. Due to higher re-do rates compared to the utilized stented bioprosthesis and other reports of early SVD [1; 3], this strategy has to be reconsidered.
Patients undergoing re-do surgery of the aortic valve for SVD or PVE present an elevated risk for periprocedural mortality and morbidity [24; 25]. We herein also showed a HR of 7.63 for patients undergoing AV re-do surgery for SVD or PVE. Since the SFS presented a significant higher re-do rate, it should be selected carefully, especially in young patients in which a second operation can be anticipated. On the other hand the effect of re-do surgery in the COX analysis may be due to the small patient number and is worth further investigation. Nevertheless, the SFS should still be considered for small aortic annuli due to the excellent hemodynamics and to avoid patient-prosthesis mismatch. Also, patients with extensive destruction of the aortic annulus in NVE may benefit from the supra-annular implantation technique. Accordingly, SAVR in an infective setting should follow a tailored approach for each individual patient.
Study limitations
Typical limitations for a retrospective, single-center study with limited patient numbers apply [13]: Patients were not randomized to the respective treatment groups and even though analysis of baseline patient characteristics did not reveal statistically significant inter-group differences results may have been biased by hidden confounders. Furthermore, the choice of the respective bioprosthesis was left to the surgeon’s discretion. Although the SFS was the preferred valve for treatment of NVE during the study period at our institution, this can lead to a certain selection bias.
Moreover, there may be a bias regarding experience with the two different bioprostheses, since the CEP was used more frequent during the study period.
Conclusions
In this case-matched analysis the SFS stentless pericardial valve presented impaired outcomes in NVE as well as higher rates of re-do surgery, which is connected with a pronounced decrease in freedom from death. Therefore, we are reluctant to recommend utilization of this particular bioprosthesis in patients with infective aortic valve endocarditis or in young patients, due to the anticipated early SVD. The SFS could still be considered for small aortic annuli and severe destruction of the aortic annulus in NVE.
Abbreviations
- ACC
Aortic cross clamp
- AV
Aortic valve
- CAD
Coronary artery disease
- CEP
Carpentier Edwards Perimount
- COPD
Chronic obstructive pulmonary disease
- CT
Computed tomography
- ECC
Extracorporal circulation
- EOA
Effective orifice area
- IVS
Interventricular septum
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- NVE
Native valve endocarditis
- NYHA
New York Heart Association
- PVE
Prosthetic valve endocarditis
- PVL
Paravalvular leakage
- SAVR
Surgical aortic valve replacement
- SFS
Sorin Freedom Solo
- SVD
Structural valve deterioration
- TTE
Transthoracic echocardiography
- TVL
Transvalvular leakage
Data Availability
The data underlying this study is available in the following public repository: https://doi.org/10.6084/m9.figshare.5755926.v1.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sponga S, Barbera MD, Pavoni D, Lechiancole A, Mazzaro E, Valente M et al. Ten-year results of the Freedom Solo stentless heart valve: excellent haemodynamics but progressive valve dysfunction in the long term. Interact Cardiovasc Thorac Surg. 2017. February 23. [DOI] [PubMed] [Google Scholar]
- 2.Carrel T. Mid-term haemodynamic is important after implantation of a tissue valve but long-term durability is what makes the difference. Eur J Cardiothorac Surg 2016;49:1180–1. doi: 10.1093/ejcts/ezv284 [DOI] [PubMed] [Google Scholar]
- 3.Stanger O, Bleuel I, Reineke S, Banz Y, Erdoes G, Tevaearai H et al. Pitfalls and premature failure of the Freedom SOLO stentless valve. Eur J Cardiothorac Surg. 2015. October;48(4):562–70. doi: 10.1093/ejcts/ezu473 [DOI] [PubMed] [Google Scholar]
- 4.Stanger O, Bleuel I, Gisler F, Göber V, Reineke S, Gahl B et al. The Freedom Solo pericardial stentless valve: Single-center experience, outcomes, and long-term durability. J Thorac Cardiovasc Surg. 2015. July;150(1):70–7. doi: 10.1016/j.jtcvs.2015.01.060 [DOI] [PubMed] [Google Scholar]
- 5.Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016. February 27;387(10021):882–93. doi: 10.1016/S0140-6736(15)00067-7 [DOI] [PubMed] [Google Scholar]
- 6.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F et al. 2015. ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015 November 21;36(44):3075–128. doi: 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 7.Meszaros K, Nujic S, Sodeck GH, Englberger L, König T, Schönhoff F et al. Long-term results after operations for active infective endocarditis in native and prosthetic valves. Ann Thorac Surg. 2012. October;94(4):1204–10. doi: 10.1016/j.athoracsur.2012.04.093 [DOI] [PubMed] [Google Scholar]
- 8.Musci M, Weng Y, Hubler M, Amiri A, Pasic M, Kosky et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: twenty-year single-center experience. J Thorac Cardiovasc Surg 2010;139:665–673. doi: 10.1016/j.jtcvs.2009.07.026 [DOI] [PubMed] [Google Scholar]
- 9.Klieverik LM, Yacoub MH, Edwards S, Bekkers JA, Roos-Hesselink JW, Kappetein AP et al. Surgical treatment of active native aortic valve endocarditis with allografts and mechanical prostheses. Ann Thorac Surg. 2009. December;88(6):1814–21. doi: 10.1016/j.athoracsur.2009.08.019 [DOI] [PubMed] [Google Scholar]
- 10.Schneider AW, Hazekamp MG, Versteegh MI, Bruggemans EF, Holman ER, Klautz RJ et al. Stentless bioprostheses: a versatile and durable solution in extensive aortic valve endocarditis. Eur J Cardiothorac Surg. 2016. June;49(6):1699–704. doi: 10.1093/ejcts/ezv463 [DOI] [PubMed] [Google Scholar]
- 11.van der Straaten EP, Rademakers LM, van Straten AH, Houterman S, Tan ME, Soliman Hamad MA. Mid-term haemodynamic and clinical results after aortic valve replacement using the Freedom Solo stentless bioprosthesis versus the Carpentier Edwards Perimount stented bioprosthesis. Eur J Cardiothorac Surg. 2016. April;49(4):1174–80. doi: 10.1093/ejcts/ezv255 [DOI] [PubMed] [Google Scholar]
- 12.Grubitzsch H, Wang S, Matschke K, Glauber M, Heimansohn D, Tan E et al. Clinical and haemodynamic outcomes in 804 patients receiving the Freedom SOLO stentless aortic valve: results from an international prospective multicentre study. Eur J Cardiothorac Surg. 2015. March;47(3):e97–104. doi: 10.1093/ejcts/ezu471 [DOI] [PubMed] [Google Scholar]
- 13.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H et al. ESC Committee for Practice Guidelines (CPG); Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1–S44. doi: 10.1093/ejcts/ezs455 [DOI] [PubMed] [Google Scholar]
- 14.Conradi L, Schaefer A, Seiffert M, Schirmer J, Schaefer U, Schön G et al. Transfemoral TAVI without pre-dilatation using balloon-expandable devices: a case-matched analysis. Clin Res Cardiol. 2015. September;104(9):735–42. doi: 10.1007/s00392-015-0836-1 [DOI] [PubMed] [Google Scholar]
- 15.R Core Team (2014). R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- 16.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28. [Google Scholar]
- 17.Repossini A, Fischlein T, Santarpino G, Schäfer C, Claus B, Passaretti B et al. Pericardial Stentless Valve for Aortic Valve Replacement: Long-Term Results. Ann Thorac Surg. 2016. December;102(6):1956–1965. doi: 10.1016/j.athoracsur.2016.05.080 [DOI] [PubMed] [Google Scholar]
- 18.Heimansohn D, Roselli EE, Thourani VH, Wang S, Voisine P, Ye J et al. North American trial results at 1 year with the Sorin Freedom SOLO pericardial aortic valve. Eur J Cardiothorac Surg. 2016. February;49(2):493–9. doi: 10.1093/ejcts/ezv169 [DOI] [PubMed] [Google Scholar]
- 19.Thalmann M, Kaiblinger J, Krausler R, Pisarik H, Veit F, Taheri N et al. Clinical experience with the freedom SOLO stentless aortic valve in 277 consecutive patients. Ann Thorac Surg. 2014. October;98(4):1301–7. doi: 10.1016/j.athoracsur.2014.05.089 [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer S, Santarpino G, Fischlein T. Stentless pericardial valve for acute aortic valve endocarditis with annular destruction. J Cardiovasc Med (Hagerstown). 2015. April;16(4):318–9. [DOI] [PubMed] [Google Scholar]
- 21.Miceli A, Croccia M, Simeoni S, Varone E, Murzi M, Farneti PA et al. Root replacement with stentless Freestyle bioprostheses for active endocarditis: a single centre experience. Interact Cardiovasc Thorac Surg. 2013. January;16(1):27–30. doi: 10.1093/icvts/ivs438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrotta S, Jeppsson A, Fröjd V, Svensson G. Surgical Treatment for Infective Endocarditis: A Single-Centre Experience. Thorac Cardiovasc Surg. 2016. April 13. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Rouzé S, Flécher E, Revest M, Anselmi A, Aymami M, Roisné A et al. Infective Endocarditis With Paravalvular Extension: 35-Year Experience. Ann Thorac Surg. 2016. August;102(2):549–55. doi: 10.1016/j.athoracsur.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 24.Ejiofor JI, Yammine M, Harloff MT, McGurk S, Muehlschlegel JD, Shekar PS et al. Reoperative Surgical Aortic Valve Replacement Versus Transcatheter Valve-in-Valve Replacement for Degenerated Bioprosthetic Aortic Valves. Ann Thorac Surg. 2016. November;102(5):1452–1458. doi: 10.1016/j.athoracsur.2016.05.086 [DOI] [PubMed] [Google Scholar]
- 25.Silaschi M, Wendler O, Seiffert M, Castro L, Lubos E, Schirmer J et al. Transcatheter valve-in-valve implantation versus redo surgical aortic valve replacement in patients with failed aortic bioprostheses. Interact Cardiovasc Thorac Surg. 2017. January;24(1):63–70. doi: 10.1093/icvts/ivw300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study is available in the following public repository: https://doi.org/10.6084/m9.figshare.5755926.v1.