Abstract
The link between autonomic stress reactivity and subjective urge/craving has been less systematically examined in behavioral addictions (i.e. problematic Internet use) than in substance use disorders. The present study investigated whether problematic Internet users (PU) show enhanced autonomic stress reactivity than non-PU, indexed by lower Heart Rate Variability (HRV) and higher Skin Conductance Level (SCL) reactivity during the Trier Social Stress Test (TSST), whether greater reactivity is related to stronger Internet craving, and whether problematic Internet usage is associated with some dysfunctional psychological features. Based on their Internet Addiction Test scores, participants were divided into PU (N = 24) and non-PU (N = 21). Their heart rate and skin conductance were continuously recorded during baseline, social stressors, and recovery. Craving for Internet usage were collected using a Likert scale before and after the TSST. The SDNN, an overall measure of HRV, was significantly lower in PU than non-PU during baseline, but not during and after stressful task. Furthermore, only among PU a significant negative correlation emerged between SDNN during recovery and craving ratings after the test. No group differences emerged for SCL. Lastly, PU endorsed more mood, obsessive-compulsive, and alcohol-related problems. Our findings suggest that problems in controlling one’s use of the Internet may be related to reduced autonomic balance at rest. Moreover, our results provide new insights into the characterization of craving in PIU, indicating the existence of a relationship between craving for Internet usage and reduced autonomic flexibility.
Introduction
In the last decade, the Internet has become part of our daily life, changing the way we work and communicate. Despite its advantages, many people spend more time than necessary on the Internet and sometimes a psychopathological condition may result. Despite the growing number of studies in this context, researchers are still not yet in agreement on the conceptualization of Internet-related psychological problems, and a specific diagnosis is not included in any established diagnostic system of mental disorders [1–3]. Psychological problems regarding Internet use were first described as Internet Addiction Disorder, defined as an impulse-control disorder that does not involve an intoxicant [4]. Since then, different labels have been used in the scientific literature to capture Internet-related problems, including Internet addiction, compulsive Internet use, and Problematic Internet Use (PIU) [5]. PIU refers to “Internet use that creates psychological, social, school, and/or work difficulties in a person’s life” [6,7]. The dominant perspective conceptualizes PIU as an addictive behavior [4,8,9], thus suggesting that PIU, similarly to substance use disorders (SUDs) and behavioral addictions (BA; e.g., pathological gambling), is characterized by the persistence of a specific behavior (Internet use) despite its adverse effects [10].
A number of different psychometric tools have been developed to assess PIU, among which the Assessment of Internet and Computer Game Addiction Scale (AICA-S, [11], the Compulsive Internet Use Scale (CIUS) [12], and the Young’s Internet Addiction Test (IAT) [4]. The IAT has been used in the majority of studies on PIU, although comparison among studies is made difficult by the fact that different cut-off scores have been used to classify participants [13,14].
Several psychopathological disorders have been found to be often comorbid with PIU. It has been recently reported that individuals with PIU are more likely to have psychiatric disorders or symptoms including SUDs, mood disorders, anxiety disorders, somatoform disorders, pathological gambling, Attention-Deficit Hyperactivity Disorder symptoms, sleep disturbances and suicide ideation [15], obsessive-compulsive symptoms [16], and alexithymia [17]. Such findings suggest that it is important to carefully assess the presence of such conditions in individuals with PIU.
In addition to assessing and managing comorbidities, identifying the factors that precipitate and maintain PIU is of fundamental importance. Studies on SUDs have shown that craving is a key factor in the maintenance of addictive behaviors [18]. Craving is defined as a subjective motivational state involving an intense urge to engage in a specific behavior, and is thought to reflect a conditioned response resulting from repeated pairing of stimuli with reward [19]. A recent study showed that, among individuals with PIU, exposure to Internet-related words was followed by an increase in craving ratings, suggesting that PIU may share similar underlying mechanisms with other addiction disorders [20].
Research has increasingly recognized the importance of understanding the relationship between PIU and stress, including the role of potential mediators and moderators [2]. Stress occurs when an individual perceives that environmental demands exceed his or her adaptive capacity [21]. Studies on addictions suggest that acute and/or chronic stress can determine the attribution of additional salience to reward-related stimuli [22,23], favoring the formation of conditioned responses without an appraisal of response consequences, i.e., habits [24]. Habits are believed to be at the basis of craving [25–27]. Specifically, Schwabe and colleagues [26–28] proposed that acute or chronic stress cause the release of several hormones, including glucocorticoids, noradrenaline and adrenaline, that facilitate striatum-dependent memory processes by favoring dorsolateral striatum-based habits.
The link between stress reactivity and craving has been less systematically examined in behavioral addictions than in SUDs. However, it can be hypothesized that Internet-related habits are potentiated and reinstated by stress, leading to craving symptoms and PIU establishment. In other words, an exaggerated reactivity to stressors may lead to an excessive engagement of habit processes in instrumental action, thus promoting conditioned habitual responses to Internet-related stimuli at the basis of compulsive Internet use [29,30].
Few studies have investigated the relationship between stress reactivity and PIU using self-report measures. Specifically, it has been shown that stressful life events were positively correlated with Internet addiction [31]. Furthermore, perceived stress has been found to be one of the predictors of PIU for sexual purposes [32], and PIU appears to be associated with stress, depression and anxiety symptoms [33]. No study to our knowledge has yet investigated stress reactivity in individuals with PIU using psychophysiological indices in addition to self-report instruments.
In the assessment of psychophysiological indices of the stress response, both the magnitude of response and the capacity to recover (i.e., the degree to which a psychophysiological response returns to pre-stress levels following a stressor) have been commonly considered as relevant parameters. Classical laboratory stress tasks used to investigate psychophysiological stress responses include public speaking and mental arithmetic, and the most commonly assessed indices include autonomic measures such as heart rate and heart rate variability (HRV) and skin conductance (SC).
HRV consists in the variations over time of the period between consecutive heartbeats (RR intervals). Such variations represent a fine tuning of the beat-to-beat control mechanisms by vagal and sympathetic activity directed to the sinus node of the heart [34]. High HRV reflects the autonomic nervous system (ANS) ability to adapt to changing circumstances, and it seems to be associated with goal-based control of emotions, context-appropriate responses and recovery after stressor [35]. In contrast, low HRV reflects the ANS inability to adapt to stressful events and is associated with delayed recovery from psychological stress [36].
The analysis of the spectral components of HRV allows to understand the modulatory effects of neural mechanisms on the sinus node. In particular, the high frequency (HF; .15-.4 Hz) component is mainly determined by efferent vagal activity, whereas the low frequency (LF; .04-.15 Hz) component is considered by some as a marker of sympathetic modulation and by others as a parameter that includes both sympathetic and vagal influences [34]. In response to stressors, an increase in sympathetic cardiac control, a decrease in parasympathetic control, or both, are often observed, as reflected by increase in LF, a decrease in HF power, and/or an increase in the LF/HF ratio [37].
Skin conductance (SC) is a non-invasive measure of the variations in electrical conductance of the skin depending on the changes in the levels of sweat in the ducts [38]. SC reflects only the activity of the sympathetic component of the ANS, due to the absence of parasympathetic innervation on eccrine sweat glands. SC has been largely measured to assess sympathetic activation during challenging situations [39,40].
The goals of the present study were to investigate (i) whether individuals with PIU show enhanced autonomic reactivity to a standardized psychosocial stress task; (ii) whether greater autonomic reactivity is related to higher craving ratings; and (iii) whether the presence of PIU is associated with high levels of anxiety, depression, impulsivity, alexithymia, obsessive-compulsive symptoms and more frequent use of alcohol and cannabis.
We hypothesized that PIU individuals would be characterized by lower HRV and higher SC level during the stress task as compared with individuals without PIU. Furthermore, we expected to observe an increase of craving ratings after the stress task in individuals with, but not in individuals without, PIU. Lastly, we expected that individuals with PIU would show higher scores on self-reports of anxiety, depression, impulsivity, alexithymia, obsessive-compulsive symptoms and use of alcohol and cannabis than individuals without PIU.
Materials and methods
Participants
Students of the University of Padua, Italy, were contacted informally at university facilities and asked to fill in an anonymous online version of the IAT [4; Italian version by 41]. The IAT is a 20-item questionnaire that measures six factors at the basis of PIU, i.e., compromised social and individual quality of life, careers, and time control, and excitatory/compensatory usage of the Internet. Based on Italian cut-off scores, Internet usage was defined as non problematic (scores 20–50), occasional or frequent problematic (scores 50–80), and severe problematic (scores 80–100) [42].
188 students filled in the online questionnaire. Twenty-four students who qualified as problematic Internet users (PU; 15 females; mean age = 23.04 ± 3.57; mean IAT score = 58 ± 7.2, range = 49–71), and 21 who qualified as non-problematic Internet users (non-PU; 17 females, mean age = 23.29 ± 2.87; mean IAT score = 31 ± 4.6, range = 23–39) accepted to participate in the study. No age, gender, sleep hours, and cigarettes consumption differences between groups were found.
Approval for the study was obtained from the Ethical Committee of Psychological Research, Area 17, University of Padova (prot. N. 1887).
Self-report measures
The Italian version of the Alcohol Use Disorders Identification Test (AUDIT) [43] was used to assess the frequency and quantity of alcohol consumption [44]. Score ranges from 0 to 40, with higher scores indicating more problematic alcohol use.
The Italian version of the Cannabis Abuse Screening Test (CAST) [45] was administered to assess cannabis use with reference to the past 12 months. Score ranges from 0 to 24. Cut-off score for problematic cannabis use is 7.
The Italian version of the Depression Anxiety Stress Scales-21 (DASS-21) [46] was administered to assess general distress through three separate subscales (i.e., anxiety, depression, and stress).
The Italian version of the Barratt Impulsiveness Scale (BIS-11) [47] was administered to assess impulsivity. The higher the total score (range = 30–120), the higher the impulsiveness level.
The Italian version of the short UPPS-P Impulsive Behaviour Scale [48] was administered to assess five components of impulsivity: positive urgency, negative urgency, lack of perseverance, lack of premeditation, and sensation seeking.
The Italian version of the Obsessive-Compulsive Inventory-Revised (OCI-R) [49] was used to measure obsessive-compulsive symptoms.
The Italian version of the Toronto Alexithymia Scale (TAS-20) [50] was used to assess alexithymia symptoms.
See data in S2 File.
Craving measure
To assess craving for Internet use, participants were asked to respond to a single question (“How much would you like to use the Internet now?”) using a Likert scale (range 1–5; 1 = not at all, 5 = very much). See data in S2 File.
Stress task
A modified version of the Trier Social Stress Test (TSST) [51] was employed. Participants were first invited to remain quiet (Phase 1; 3 minute-baseline). Then, they were asked to prepare an oral speech about their personal traits qualifying them for their “dream” job position (Phase 2; 3 minutes). In the following phase, they were asked to speak in front of a video camera (Phase 3; 5 minutes). Participants were informed that video camera was connected to a monitor in another room, where an evaluation commission would judge their performance. Then the experimenter invited participants to rest again for six minutes (Phase 4, 3-minute recovery; and Phase 5, 3-minute baseline). In the following phase (Phase 6, 5 minutes), participants were asked to start counting backwards in steps of 13, starting at 2011. Upon each error, the experimenter asked them to start over. Lastly, participants were invited to rest again for three minutes (Phase 7).
Autonomic measures
The electrocardiogram (ECG) and skin conductance (SC) were recorded continuously using a ProComp Infiniti system (Thought Technology; Montreal, Canada). To record the ECG, three disposable Ag/AgCl electrodes were placed on the participant's chest in a modified lead II configuration. The ECG signal was sampled at 256 Hz, band-pass filtered (1–100 Hz), and amplified. A digital trigger detecting R-waves was applied to the ECG signal to obtain inter-beat intervals (IBIs). All ECG data were visually examined and artifacts were corrected. Time domain and frequency domain indices of HRV were compute by Kubios HRV Analysis Software 2.0 (The Biomedical Signal Analysis Group, Department of Applied Physics, University of Kuopio, Finland). Fourier analysis was used to calculate frequency domain indices, i.e., low frequency power (LF: 0.04 to 0.15 Hz) in ms2, considered as an index of both ANS branches activity; High frequency power (HF: 0.15 to 0.40 Hz) in ms2, a HRV index of cardiac parasympathetic tone. As time domain indices, the standard deviation of all normal-to-normal intervals (SDNN) was calculated as an index of the total HRV, and the root mean square of successive difference of N-to-N intervals (rMSSD), expressed in ms, was calculated as an index of vagal control on the heart [34].
Skin Conductance Level (SCL) was recorded by two Ag/AgCl electrodes fixed to the medial phalanx surface of the first and middle finger of the nondominant hand Sampling rate was 256 Hz.
See data in S2 File.
Procedure
After participants provided a written informed consent, they were asked to rate their Internet craving using the Likert scale. Then, ECG and SC sensors were placed and participants were given instructions about the task. After completion of the task, participants were asked again to rate their Internet craving on the Likert scale and sensors were removed. After the experimental session, the participants were asked to fill-in the questionnaires. The entire procedure took about 40 min.
Statistical analysis
All statistical analyses were conducted on the mean values of SDNN, rMSSD, HF, LF, HF/LF ratio, and SCL calculated over the 3-min interval of Phases 1, 2, 4, 5 and 7, and the central 3 minutes in the 5-min Phases 3 and 6.
All analyses were performed using R software [52]. Specifically, Pearson’s r (R package: Hmisc) [53] was calculated to assess the strengths of correlations between self-report measures in both PU and non-PU.
To test autonomic reactivity during the TSST we estimated fifty mixed-models (Formulae A in S1 File by R package: lme4) [54] and the best-fitting model was selected using the AIC criteria [55,56], i.e., the model with the smallest AIC and the highest AIC weight is considered as the most appropriate model for reproducing the observed data. Mixed-effects models are considered as a powerful procedure for repeated-measures designs in psychophysiology [57]. Considering autonomic indices as dependent variables, the mixed-models were defined by starting from a simple model with individuals (i) random intercept only (Model 0; see Formulae A in S1 File: Yij = b0 + vi + eij, where Yij was the response for jth measurement of ith individual; b0 was the fixed intercept; vi was the random intercept fot the ith individual and eij was a Gaussian error term) and adding one fixed predictor to each subsequent model. Fixed predictors included Group (PU and non-PU), Phase (TSST phases), their interaction, and self-report measures that had been observed to be significantly reciprocally correlated in each Group. Hypothesized group differences in stress reactivity were fitted adding Group, Phase, and their interaction as fixed factors (Model 46; see Formulae A in S1 File) to Model 0. The maximum likelihood method was employed to analyze the contribution of parameters within the selected model (the modeling approach utilized data of all participants, except for SCL, for which one participant was excluded due to marked deviation from all other observations in the sample).
To assess whether Group (PU and non-PU), Time (before and after the TSST) and their interaction predict craving ratings (R package: MASS) [58] we estimated five nested ordinal logistic models and the AIC criteria were employed to select the model that more appropriately described our data [55,56].
Linear model analysis considering Group (PU and non-PU) as predictor was performed to compare scores on self-reports between groups. Bayes factor analysis was run to quantify the predictive success of linear models with Group predictor relative to an intercept-only model (R package: BayesFactor) [59].
Results
Autonomic measures
Descriptive statistics of autonomic indices are reported in Table 1.
Table 1. Descriptive statistics of autonomic measures.
| PU | non-PU | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TSST phases |
Index | mean | sd | median | range | mean | sd | median | Range |
| Phase1 | SDNN | 66.23 | 28.38 | 63.06 | 96 | 80.21 | 32.6 | 78.08 | 124.43 |
| rMSSD | 39.95 | 18.69 | 38.24 | 82.86 | 45.43 | 28.43 | 33.98 | 127.77 | |
| LF (ms2) | 915.43 | 455.43 | 901.08 | 1976.5 | 1317.8 | 1140.2 | 1023 | 4829.7 | |
| HF (ms2) | 932.06 | 957.74 | 783.96 | 4552.27 | 1296.5 | 2365.4 | 733.59 | 11135 | |
| SCL | 1.93 | 2.21 | 1.43 | 11.15 | 1.7 | 1.48 | 1.42 | 6.61 | |
| Phase 2 | SDNN | 58.55 | 16.63 | 54.19 | 56.19 | 56.24 | 20.59 | 50.75 | 81.52 |
| rMSSD | 40.44 | 17.73 | 38.2 | 72.39 | 42.28 | 23.61 | 35.42 | 104.71 | |
| LF (ms2) | 1058.05 | 847.64 | 694.28 | 3243.61 | 990.88 | 978.25 | 639.55 | 3730.8 | |
| HF (ms2) | 1025.46 | 1312.99 | 547.31 | 4473.59 | 905.71 | 949.02 | 570.17 | 3745.6 | |
| SCL | 3.54 | 3.28 | 2.54 | 15.57 | 3.06 | 2.33 | 2.3 | 8.52 | |
| Phase3 | SDNN | 54.61 | 18.83 | 53.82 | 81.13 | 53.06 | 20.63 | 50.87 | 68.88 |
| rMSSD | 32.41 | 13.98 | 31.5795 | 65.15 | 35.63 | 18.46 | 33.32 | 62.1 | |
| LF (ms2) | 1148 | 856.9 | 1098.42 | 2864.45 | 1381.6 | 1614 | 523.71 | 5144.3 | |
| HF (ms2) | 616.1 | 508.21 | 604.81 | 2456.82 | 685.93 | 914.53 | 466.46 | 4002.1 | |
| SCL | 4.53 | 4.17 | 2.96 | 20.51 | 3.97 | 3.34 | 2.84 | 11.46 | |
| Phase4 | SDNN | 57.56 | 16.79 | 55.48 | 58.72 | 64.36 | 22.91 | 59.26 | 79.27 |
| rMSSD | 37.35 | 20.78 | 30.04 | 80.83 | 41.33 | 27.3 | 33.41 | 116.83 | |
| LF (ms2) | 1365.97 | 1058.1 | 976.86 | 4271.03 | 1551.4 | 1035.4 | 1203.7 | 4106.5 | |
| HF (ms2) | 716.55 | 809.94 | 449.58 | 3326.49 | 1046.1 | 1694.9 | 426.96 | 7663.1 | |
| SCL | 4.22 | 4.58 | 2.91 | 22.45 | 3.61 | 3.28 | 2.24 | 10.12 | |
| Phase5 | SDNN | 60.12 | 22.02 | 54.86 | 94.8 | 61.95 | 17.08 | 63.11 | 69.26 |
| rMSSD | 47.51 | 30.69 | 39.5 | 143.84 | 49.72 | 22.12 | 46.64 | 101.12 | |
| LF (ms2) | 1253.989 | 1111.24 | 770.8 | 4259.27 | 1173.6 | 1351.2 | 697.82 | 6004.9 | |
| HF (ms2) | 1178.72 | 1400.36 | 750.63 | 6240.42 | 1367 | 1550.2 | 1010.7 | 6759.4 | |
| SCL | 3.92 | 4.67 | 2.74 | 22.8 | 3.34 | 3.40 | 1.75 | 11.52 | |
| Phase 6 | SDNN | 58.59 | 14.6 | 56.97 | 57.73 | 57.76 | 19.64 | 54.06 | 70.4 |
| rMSSD | 39.84 | 15.76 | 41.39 | 64.21 | 43.25 | 18.06 | 36.77 | 59.81 | |
| LF (ms2) | 1561.15 | 1087.48 | 1306.3 | 5123.18 | 1428.9 | 1693.9 | 949.61 | 7705.8 | |
| HF (ms2) | 1022.81 | 892.13 | 672.71 | 3046.49 | 963.51 | 840.33 | 778.6 | 3290.1 | |
| SCL | 5.51 | 4.99 | 3.89 | 23.97 | 4.66 | 3.83 | 3.22 | 12.23 | |
| Phase7 | SDNN | 62.35 | 18.51 | 59.86 | 74.84 | 62.94 | 22.40 | 64.72 | 93.05 |
| rMSSD | 41.86 | 23.86 | 38.17 | 106.44 | 43.75 | 27.1 | 38.97 | 125.59 | |
| LF (ms2) | 1761.95 | 1273.08 | 1580.55 | 4904.47 | 1818.2 | 1856.8 | 1336.7 | 7646.2 | |
| HF (ms2) | 1102.03 | 1543 | 591.42 | 7082.26 | 1040 | 1729.4 | 424.41 | 8063.5 | |
| SCL | 5.19 | 5.38 | 3.76 | 26.35 | 4.12 | 3.76 | 2.45 | 12.36 | |
Table 2 shows the AIC and AIC weights of fitted mixed-models for each considered autonomic index. The mixed-model with fixed Phase predictor (M48; see Formulae A in S1 File) resulted the preferred model to fit the rMSSD, LF, HF and SCL (see Table 2).
Table 2. The AIC model comparison analysis of the mixed-effects models (Mn).
| SDNN | rMSSD | LF | HF | SCL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | AIC | AICw | Mn | AIC | AICw | Mn | AIC | AICw | Mn | AIC | AICw | Mn | AIC | AICw |
| M46 | 2740.9 | 23.10% | M48 | 2573.0 | 38.47% | M48 | 5270.7 | 42.69% | M48 | 5208.7 | 37.04% | M48 | 1026.6 | 35.63% |
| M48 | 2741.2 | 19.38% | M47 | 2574.7 | 16.44% | M47 | 5272.6 | 16.61% | M47 | 5210.6 | 14.30% | M47 | 1028.6 | 13.22% |
| M47 | 2742.7 | 9.24% | M42 | 2574.8 | 15.39% | M42 | 5272.7 | 16.06% | M42 | 5210.6 | 14.26% | M42 | 1028.6 | 13.12% |
| M41 | 2742.7 | 9.21% | M44 | 2576.6 | 6.15% | M44 | 5274.6 | 6.12% | M44 | 5212.6 | 5.35% | M39 | 1029.2 | 9.89% |
| M42 | 2742.8 | 8.98% | M32 | 2576.9 | 5.43% | M27 | 5275.7 | 3.49% | M22 | 5213.2 | 4.02% | M32 | 1030.1 | 6.36% |
| M36 | 2743.2 | 7.30% | M39 | 2577.4 | 4.22% | M17 | 5276.2 | 2.72% | M32 | 5213.2 | 3.95% | M34 | 1030.4 | 5.42% |
| M32 | 2744.5 | 3.77% | M22 | 2578.1 | 3.02% | M34 | 5276.3 | 2.64% | M39 | 5213.4 | 3.63% | M44 | 1030.6 | 4.89% |
| M44 | 2744.5 | 3.68% | M27 | 2578.7 | 2.25% | M24 | 5276.4 | 2.50% | M0 | 5214.0 | 2.63% | M27 | 1032.1 | 2.34% |
| M31 | 2744.7 | 3.47% | M34 | 2578.9 | 2.00% | M19 | 5277.7 | 1.29% | M29 | 1032.4 | 1.99% | |||
| M39 | 2745.0 | 2.92% | M17 | 2578.9 | 1.95% | M12 | 5277.7 | 1.28% | M22 | 1032.7 | 1.68% | |||
| M27 | 2746.4 | 1.43% | M24 | 2579.9 | 1.21% | M14 | 5278.2 | 1.00% | M24 | 1033.5 | 1.12% | |||
| M34 | 2746.5 | 1.39% | M29 | 2580.7 | 0.83% | M46 | 5278.2 | 0.99% | M17 | 1034.3 | 0.78% | |||
| M26 | 2746.6 | 1.32% | M12 | 2580.7 | 0.81% | M7 | 5279.4 | 0.55% | M7 | 1034.4 | 0.72% | |||
| M22 | 2747.3 | 0.91% | M19 | 2580.9 | 0.73% | M0 | 5279.5 | 0.53% | M4 | 1034.6 | 0.66% | |||
| M21 | 2747.5 | 0.85% | M7 | 2582.4 | 0.34% | M2 | 1034.6 | 0.65% | ||||||
| M17 | 2748.1 | 0.61% | M14 | 2582.6 | 0.31% | M19 | 1035.3 | 0.47% | ||||||
| M16 | 2748.3 | 0.56% | M9 | 2584.3 | 0.13% | M9 | 1035.6 | 0.40% | ||||||
| M29 | 2748.4 | 0.53% | M2 | 2584.4 | 0.13% | M12 | 1036.3 | 0.29% | ||||||
| M24 | 2749.3 | 0.34% | M46 | 2585.8 | 0.06% | M14 | 1037.2 | 0.18% | ||||||
| M12 | 2750.1 | 0.23% | M4 | 2586.3 | 0.05% | M46 | 1039.2 | 0.06% | ||||||
| M19 | 2750.1 | 0.23% | M41 | 2587.7 | 0.02% | M36 | 1039.8 | 0.05% | ||||||
| M11 | 2750.3 | 0.21% | M36 | 2588.5 | 0.02% | M31 | 1041.0 | 0.03% | ||||||
| M7 | 2752.1 | 0.08% | M31 | 2590.0 | 0.01% | M41 | 1041.2 | 0.02% | ||||||
| M14 | 2752.1 | 0.08% | M21 | 2591.0 | 0.00% | M26 | 1043.0 | 0.01% | ||||||
| M6 | 2752.3 | 0.08% | M26 | 2591.7 | 0.00% | M21 | 1044.2 | 0.01% | ||||||
| M2 | 2754.0 | 0.03% | M16 | 2592.0 | 0.00% | M1 | 1045.2 | 0.00% | ||||||
| M9 | 2754.1 | 0.03% | M11 | 2593.7 | 0.00% | M16 | 1045.9 | 0.00% | ||||||
| M1 | 2754.1 | 0.03% | M6 | 2595.4 | 0.00% | M6 | 1046.3 | 0.00% | ||||||
| M4 | 2756.0 | 0.01% | M1 | 2597.4 | 0.00% | M11 | 1047.9 | 0.00% | ||||||
| M0 | 2771.1 | 0.00% | M0 | 2599.0 | 0.00% | M0 | 1212.1 | 0.00% | ||||||
Given 50 candidate mixed-effects models (Mn; see Formulae A in S1 File), the best fitting models are reported in terms of AIC and AICweight. Considering SDNN index of HRV, the best fitting model was our modeled expectations (Code M46, Formula: SDNN∼Phase * Group + (1|Individual)). Conversely, considering rMSSD, LF, HF indices of the HRV and SCL, M48 (Formula: Index∼Phase + (1|Individual)) was the best fitting model.
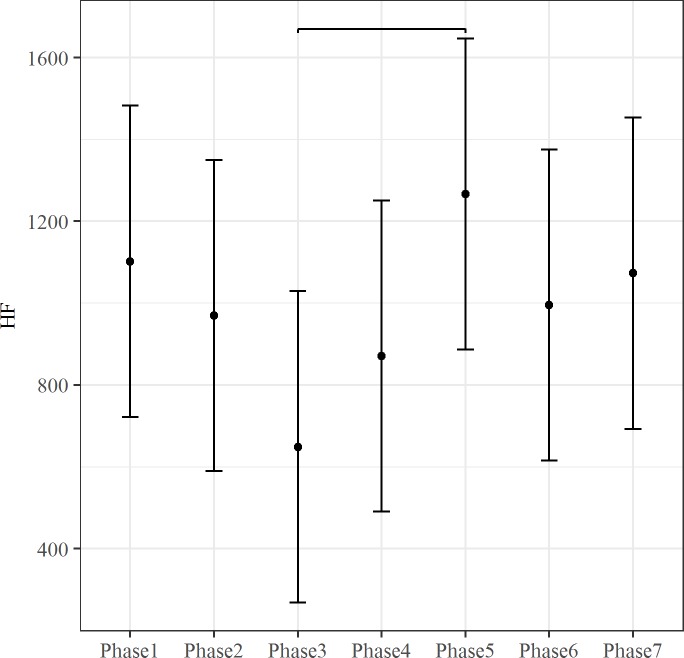
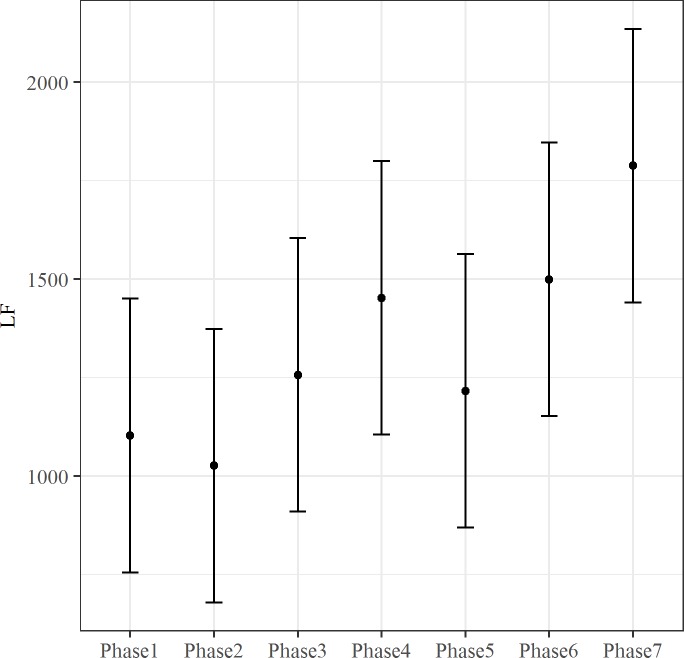
The effect of fixed predictor was tested by the maximum likelihood method. The inclusion of Phase predictor improved the fit of the model for rMSSD, LF, HF and SCL (rMSSD: ΔAIC = 26.03, X2 (6, N = 9) = 38.03, p < .001; LF: ΔAIC = 10.91, X2(6, N = 9) = 22.91 p < .01; HF: ΔAIC = 5.29, X2(6, N = 9) = 17.289 p < .01; SCL: ΔAIC = 185.51, X2(6, N = 9) = 197.51, p < .001). The Phase effect for these autonomic indices is showed in Figs 1–4. Both rMSSD and HF were lower during Phase 3 than Phase 5 (Figs 1 and 2, respectively). On the contrary, no significant differences between Phase levels were found for LF (Fig 3). Lastly, SCL was lower during Phase 1 than Phase 3 and Phase 4 (Fig 4).
Fig 1. The effect of Phase on rMSSD.
The bars at each data point represent the confidence limits computed at .95. Asterisks and lines indicate significant differences between Phase levels.
Fig 4. The effect of Phase on SCL.
The bars at each data point represent the confidence limits computed at .95. Asterisks and lines indicate significant differences between Phase levels.
Fig 2. The effect of Phase on HF.
The bars at each data point represent the confidence limits computed at .95. Asterisks and lines indicate significant differences between Phase levels.
Fig 3. The effect of Phase on LF.
The bars at each data point represent the confidence limits computed at .95. Asterisks and lines indicate significant differences between Phase levels.
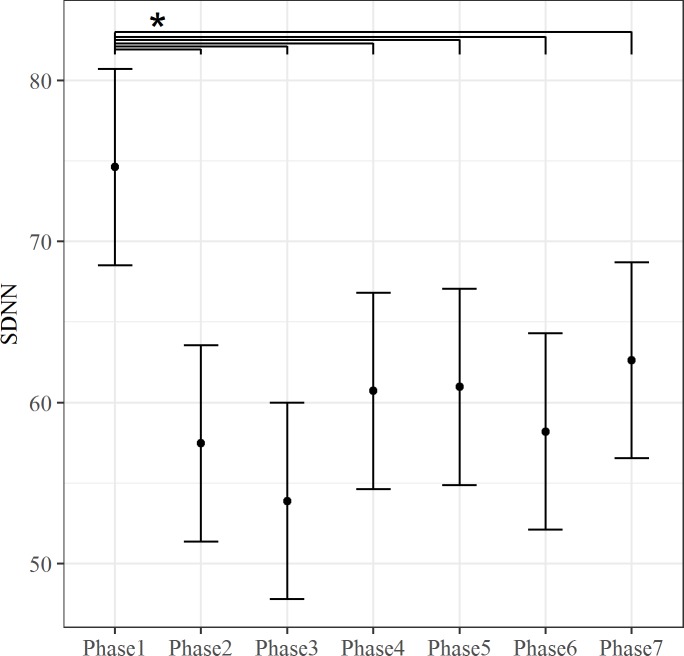
Different results were obtained considering SDNN. Our modeled expectations that considered Group, Phase and their interaction as fixed predictors (Model 46, see Formulae A in S1 File) resulted the best to describe the data. The inclusion of Phase predictor improved the fit of the model (ΔAIC = 29.91, X2(6, N = 10) = 41.91, p < .001), see Fig 5. SDNN was higher during Phase 1 than any other TSST phase.
Fig 5. The effect of Phase on SDNN.
The bars at each data point represent the confidence limits computed at .95. Asterisks and lines indicate significant differences between Groups for each level of the Phase predictor.
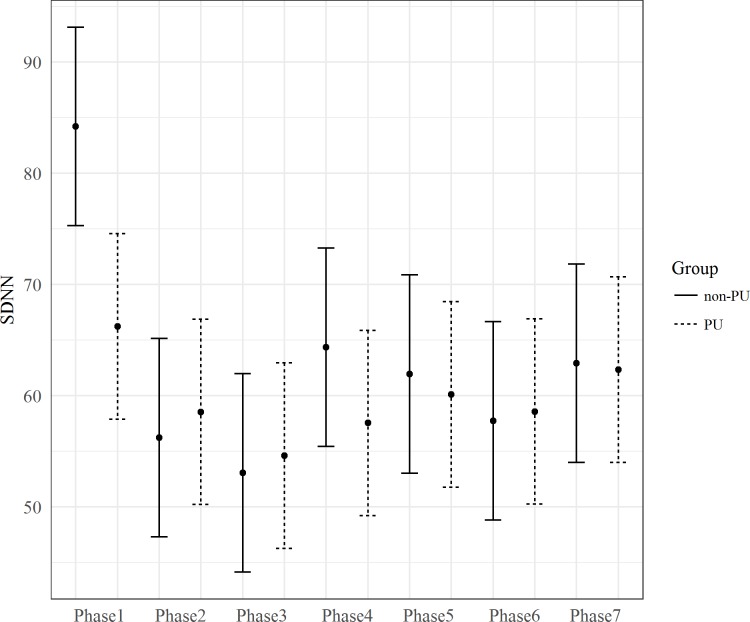
No improvement in the fit of the model was found when Group was included as a predictor, however including the Group × Phase interaction resulted in an improvement in the fit of the model (ΔAIC = 1.83, X2(6, N = 16) = 13.83, p = .03), i.e., Groups and Phase predictors interact. As shown in Fig 6, during the first rest period (Phase 1) SDNN was lower in PU than non-PU. Moreover, SDNN during Phase 1 was higher than during any other TSST phase only among non-PU.
Fig 6. The Phase*Group interaction for SDNN.
SDNN in each Phase and Group. The bars at each data point represent the confidence limits computed at .95. SDNN during Phase 1 was significantly greater in the non-PU than in the PU group. In the non-PU group, SDNN was greater during Phase 1 than in all the other Phases. No significant differences among Phases were found in the PU group.
Craving ratings
As indicated by the AIC selection method, the model without the Group × Time interaction term (L2; see Formulae B in S1 File) best fitted the data (Table 3).
Table 3. The AIC model comparison analysis of the ordinal logistic models (Ln).
| Ln | AIC | AICw |
|---|---|---|
| L2 | 191.5 | 47% |
| L4 | 192.2 | 34% |
| L1 | 193.5 | 18% |
| L3 | 206.2 | 0.3% |
| L0 | 206.4 | 0.3% |
Based on the AIC and the AICweight of the ordinal logistic models (Ln; see Formulae B in S1 File), L2 (Formula: Craving∼Time + Group) was the preferred model, indicating insufficient evidence to support a Group × Time interaction.
The ordinal logistic regression was significant only using Group to predict craving ratings: t = 3.89, p < .001, OR = 5.65, 95% CI = [0.88, 2.64]), indicating that PU were more likely to report higher craving ratings then non-PU. Time was found not to predict craving ratings (t = 1.62, p > .05, OR = 1.98, 95% CI = [-0.13, 1.52]).
Finally, the Pearson correlation between SDNN measured during Phase 7 and craving ratings after the TSST showed a strong negative correlation between SDNN and craving ratings only among PU (r(24) = —.53, p < .01), see Fig 7.
Fig 7. Correlation between craving ratings and SDNN after the stress task.

Self-report measures
Descriptive statistics of self-report measures are reported in Table 4.
Table 4. Descriptive statistics of self-report questionnaires.
| PU | Non-PU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | mean | sd | median | range | n | mean | sd | median | range | |
| AUDIT | 24 | 8.08 | 4.26 | 8 | 20 | 21 | 4.48 | 4.24 | 3 | 19 |
| CAST | 24 | 0.75 | 2.25 | 0 | 11 | 21 | 0.67 | 1.6 | 0 | 7 |
| DASS-21 | 24 | 21.88 | 11.67 | 17 | 39 | 21 | 12 | 6.83 | 12 | 24 |
| BIS-11 | 24 | 61.75 | 9.43 | 63.5 | 31 | 21 | 54.95 | 6.5 | 56 | 23 |
| NU | 24 | 11.04 | 2.36 | 11 | 8 | 21 | 10.14 | 2.99 | 10 | 9 |
| PU | 24 | 10.04 | 2.37 | 10 | 11 | 21 | 9.1 | 2.59 | 8 | 9 |
| LoPRE | 24 | 8.63 | 3.46 | 8.5 | 12 | 21 | 6.33 | 1.83 | 6 | 5 |
| LoPER | 24 | 9.25 | 3.19 | 9.5 | 11 | 21 | 5.9 | 2.21 | 5 | 7 |
| SS | 24 | 10.21 | 2.96 | 10.5 | 11 | 21 | 8.86 | 2.87 | 9 | 11 |
| OCI.R | 24 | 16.67 | 7.04 | 16.5 | 27 | 21 | 8.24 | 3.67 | 8 | 17 |
| TAS-20 | 24 | 47.67 | 10.37 | 46.5 | 45 | 21 | 38.1 | 8.94 | 35 | 35 |
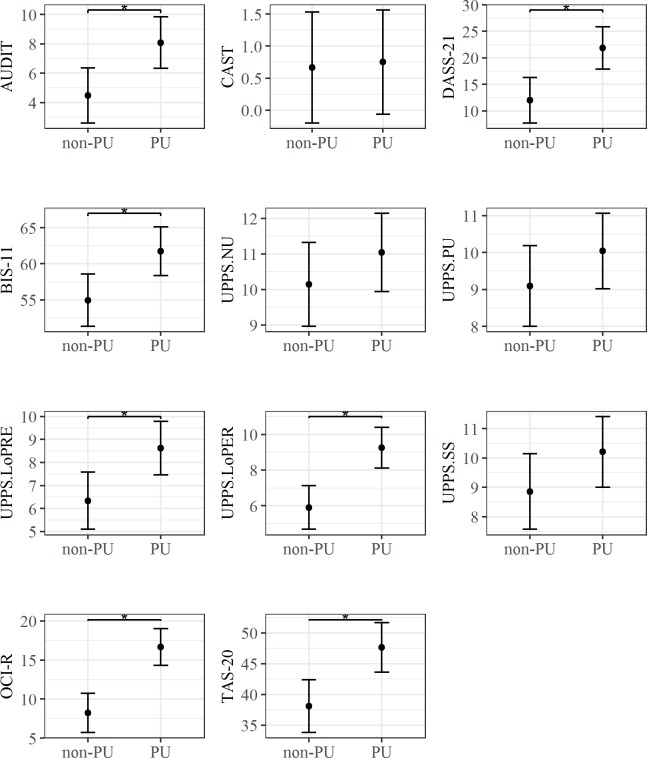
As shown in Fig 8, linear model analysis revealed higher scorings in the PU than the non-PU group in the AUDIT (F(1) = 8.06, p < .01, R2 = .16, BF = 6.57), the DASS-21 total score (F(1) = 11.54, p < .01, R2 = .21, BF = 22.62 ±0%), the BIS-11 (F(1) = 7.7, p < .01, R2 = .15, BF = 5.74 ±0%); the lack of premeditation (F(1) = 7.39, p < .01, R2 = .15, BF = 5.13 ±0%) and lack of perseverance (F(1) = 16.22, p < .001, R2 = .27, BF = 108.86 ±0%) components of the UPPS, the OCI-R (F(1) = 24.28, p < .001, Multiple R2 = .36, BF = 1308.96 ±0) and the TAS-20 (F(1) = 10.84, p< .01, R2 = .2, BF = 17.69 ±0%).
Fig 8. Significant group differences for self-report measures.
The bars at each data point represent the confidence limits computed at .95. Asterisks and lines indicate significant differences between groups.
Discussion and conclusions
This is the first study to our knowledge to investigate the relationship between autonomic stress reactivity and Internet craving in PIU. Specifically, we wanted to investigate (i) whether individuals with PIU show enhanced autonomic reactivity (i.e., lower HRV and higher SCL) to a standardized psychosocial stress task, (ii) whether greater autonomic reactivity is related to higher craving ratings, and (iii) whether PIU is associated with dysfunctional psychological features.
Contrary to our expectations, we did not find any group difference during the stress tasks. It may be hypothesized that the version of the TSST used in this study was not stressful enough to highlight possible differences in autonomic reactivity between individuals with vs without PIU. Moreover, the participants with PIU were recruited using the cut-off scores of the IAT, and were classified as occasional or frequent problematic Internet users. As such, they may not be fully representative of problematic Internet users. Future studies should include participants with severe problematic Internet usage to better elucidate autonomic stress reactivity in PIU.
We found that SDNN, that reflects the activity of all the cyclic components responsible for HRV [34], was lower in PU than non-PU before, but not during and after, the stress task. Lower HRV before the stress task suggests that, in PIU, reduced autonomic flexibility and impaired control of emotions may represent a stable condition, that is evidenced even in non-stressful conditions. Studies on SUDs suggest that regular and chronic use of drugs is associated with adaptations in stress-related brain pathways (specifically, the hypothalamic-pituitary-adrenal axis and autonomic nervous system pathways) [60]. It might be hypothesized that, similarly to substance addictions, behavioral addictions (including PIU) adversely impact autonomic functioning, reducing HRV at rest. On the other hand, low HRV in PU might be a vulnerability factor that underlies difficulty in self-regulation and inhibitory capacity [61], leading to problems in controlling one’s use of the Internet. Future research aimed at preventing and treating PIU should investigate whether low HRV represents a risk factor or a consequence of PIU.
The fact that we found group differences only for SDNN, reflecting both sympathetic and parasympathetic activity, but neither for other HRV indices nor for SCL, suggests that PIU is associated with an overall autonomic unbalance, rather than a specific dysregulation related to the sympathetic or the parasympathetic nervous system.
As regards the second research question, self-reported craving for Internet usage was higher in individuals with PIU than those without PIU, both before and after the stress task. Furthermore, after the stressful task, higher craving ratings were related to lower HRV only in PU. These findings support our hypothesis about the relationship between lower HRV and higher craving for Internet usage, suggesting that lower HRV in PU may be related to reduced capacity for self-regulation and ability to inhibit craving. Of note, these results fit with previous research showing that lower resting-state HRV predicted higher craving in alcohol dependent outpatients [62]. Overall, our findings generate new insight into the study of PIU by adding further support to the existence of a relationship between HRV and craving. However, the nature of the relationship between these variables is not currently understood. Future studies should further investigate the nature of this relationship in both behavioral and substance addictions.
Lastly, we found that PU endorsed more mood, obsessive and compulsive, and alcohol-related problems. Overall, these results are in line with previous findings showing that Internet addiction is associated with depression, anxiety, and stress [33,63], problematic alcohol use [64]; and obsessive-compulsive symptoms [16].
In addition to the above-mentioned limitations related to the task and to the criteria employed for sample selection, a further limitation of the current study is represented by the fact that we employed a single-item scale to collect Internet craving ratings [65,66]. Although this is considered as a sensitive method to measure craving, the combination with a questionnaire that explores the construct of craving through multiple items would improve the accuracy of the measure [67].
In conclusion, our findings provide new insights into the relationship between stress reactivity and craving in PIU, by supporting the existence of a relationship between reduced autonomic flexibility and Internet craving. Finally, our results confirm the previously reported associations of PIU with mood, obsessive-compulsive, and alcohol-related problems.
Supporting information
Formulae A. Mixed-effects models. Legend: Index = HRV indices (i.e., SDNN; rMSSD; LF(ms2); HF(ms2)) and SCL; Phase = the Phase predictor (i.e. 1, 2, 3, 4, 5, 6 and 7 phases of the TSST); Group = the Group predictor (non-PU and PU); Individual = participants; AUDIT = the AUDIT ratings; DASS.D, DASS.A, and DASS.S = depression, anxiety, and stress subscales of the DASS-21 respectively; DASS.T = the DASS total score; LoPER and LoPRE = the lack of perseverance and lack of premeditation components of the UPPS-P, respectively; OCI = the OCI-R total score and TAS = the TAS score. Formulae B. Ordinal logistic regression models. Legend: Craving = Craving ratings; Time = Time predictor (i.e., before and after TSST); Group = Group Predictor (i.e., non-PU and PU).
(DOCX)
(ZIP)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
No external funding source for this study has been received.
References
- 1.Chamberlain SR. Behavioural addiction—a rising tide? Eur Neuropsychopharmacol. 2015;0;26(5): 841–855. doi: 10.1016/j.euroneuro.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 2.Li D, Zhang W, Li X, Zhou Y, Zhao L, Wang Y. Stressful life events and adolescent Internet addiction: The mediating role of psychological needs satisfaction and the moderating role of coping style. Comput Human Behav. 2016;63: 408–15. [Google Scholar]
- 3.Kardefelt-Winther D. Conceptualizing Internet use disorders: Addiction or coping process? Psychiatry Clin Neurosci. 2016. [DOI] [PubMed] [Google Scholar]
- 4.Young K. Internet addiction: The emergence of a new clinical disorder. CyberPsychology Behav. 1998;1(3): 237–44. [Google Scholar]
- 5.Cash H, Rae CD, Steel a H, Winkler a. Internet Addiction: A Brief Summary of Research and Practice. Vol. 8, Curr Psychiatry Rev. 2012;8(4): 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard K, Wolf E. Modification in the proposed diagnostic criteria for Internet addiction. CyberPsychology Behav. 2001;4(3): 377–383. [DOI] [PubMed] [Google Scholar]
- 7.Van Rooij AJ, Prause N. A critical review of “Internet addiction” criteria with suggestions for the future. J Behav Addict. 2014;3(4): 203–13. doi: 10.1556/JBA.3.2014.4.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potenza MN. Should addictive disorders include non-substance-related conditions? Vol. 101, Addiction. 2006;101(s1): 142–151. [DOI] [PubMed] [Google Scholar]
- 9.Yau YHC, Crowley MJ, Mayes LC, Potenza MN. Are Internet use and video-game-playing addictive behaviors? Biological, clinical and public health implications for youths and adults. Vol. 53, Minerva Psichiatrica. 2012;53(3): 153. [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgenson A, Hsiao R, Yen C. Internet addiction and other behavioral addictions. Adolesc Psychiatr Clin North America. 2016;25(3): 509–520. [DOI] [PubMed] [Google Scholar]
- 11.Wölfling K, Müller K, Beutel M. Diagnostic measures: scale for the assessment of Internet and computer game addiction (AICA-S). Prev diagnostics, Ther Comput. 2010; 212–215. [Google Scholar]
- 12.Meerkerk G-J, Van Den Eijnden RJJM, Vermulst a a, Garretsen HFL. The Compulsive Internet Use Scale (CIUS): some psychometric properties. Cyberpsychol Behav. 2009;12(1): 1–6. doi: 10.1089/cpb.2008.0181 [DOI] [PubMed] [Google Scholar]
- 13.Kuss DJ, Lopez-Fernandez O. Internet addiction and problematic Internet use: A systematic review of clinical research. World J Psychiatry. 2016;6(1):143–76. doi: 10.5498/wjp.v6.i1.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuss DJ, Griffiths MD, Karila L, Billieux J. Internet addiction: A systematic review of epidemiological research for the last decade. Curr Pharm Des. 2014;20(25): 1–27. [DOI] [PubMed] [Google Scholar]
- 15.Kim B-S, Chang SM, Park JE, Seong SJ, Won SH, Cho MJ. Prevalence, correlates, psychiatric comorbidities, and suicidality in a community population with problematic Internet use. Psychiatry Res. 2016;244: 249–56. doi: 10.1016/j.psychres.2016.07.009 [DOI] [PubMed] [Google Scholar]
- 16.Stavropoulos V, Gentile D, Motti-Stefanidi F. A multilevel longitudinal study of adolescent Internet addiction: The role of obsessive–compulsive symptoms and classroom openness to experience. Eur J Dev Psychol. 2016;13(1): 99–114. [Google Scholar]
- 17.Taylor RN, Koerber R, Parker JDA, Maitland SB. Alexithymia and internet abuse in young adults. Pers Individ Dif. 2014;60, Supple: S50. [Google Scholar]
- 18.Tiffany ST, Wray JM. The clinical significance of drug craving. Vol. 1248, Annals of the New York Academy of Sciences. 2012;1248(1): 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Vol. 36, Brain Research Reviews. 2001;36(2): 129–138. [DOI] [PubMed] [Google Scholar]
- 20.Niu GF, Sun XJ, Subrahmanyam K, Kong FC, Tian Y, Zhou ZK. Cue-induced craving for Internet among Internet addicts. Addict Behav. 2016;62: 1–5. doi: 10.1016/j.addbeh.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Kessler RC, Gordon LU. Strategies for measuring stress in studies of psychiatric and physical disorders In: Measuring stress: A guide for health and social scientists; 1995; 3–26. [Google Scholar]
- 22.Sinha R, Fox H, Hong K, Bergquist K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. 2009;34(5): 1198–1208. doi: 10.1038/npp.2008.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantsch J, Baker D, Funk D, Lê A. Stress-induced reinstatement of drug seeking: 20 years of progress. 2016;41(1): 335–356. doi: 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balleine BW, JP O’Doherty. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35(1): 48–69. doi: 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl). 2000;152(2): 140–8. [DOI] [PubMed] [Google Scholar]
- 26.Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol. 2011;19(1): 53–63. doi: 10.1037/a0022212 [DOI] [PubMed] [Google Scholar]
- 27.Schwabe L, Tegenthoff M, Höffken O. Simultaneous glucocorticoid and noradrenergic activity disrupts the neural basis of goal-directed action in the human brain. J Neurosci. 2012; 32(30): 10146–10155. doi: 10.1523/JNEUROSCI.1304-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwabe L, Schächinger H, Kloet E de. Corticosteroids operate as a switch between memory systems. J Cogn. 2010;22(7): 1362–1372. doi: 10.1162/jocn.2009.21278 [DOI] [PubMed] [Google Scholar]
- 29.LaRose R. The problem of media habits. Commun Theory. 2010;20(2): 194–222. [Google Scholar]
- 30.LaRose R. The psychology of interactive media habits. Psychol Commun Technol. 2015; 365–383. [Google Scholar]
- 31.Li W, O’Brien JE, Snyder SM, Howard MO. Diagnostic criteria for problematic internet use among U.S. university students: A mixed-methods evaluation. PLoS One. 2016;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velezmoro R, Lacefield K, Roberti J. Perceived stress, sensation seeking, and college students’ abuse of the Internet. Comput Human Behav. 2010; 26(6): 1526–1530. [Google Scholar]
- 33.Akin A, Iskender M. Internet addiction and depression, anxiety and stress. Int Online J Educ. 2011;3(1): 138–148. [Google Scholar]
- 34.Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93: 1043–65. [PubMed] [Google Scholar]
- 35.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2): 747–56. doi: 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 36.Weber C, Thayer J, Rudat M, Wirtz P. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J. 2010;109(2): 201–211. [DOI] [PubMed] [Google Scholar]
- 37.Berntson GG, Cacioppo JT. Heart Rate Variability: Stress and Psychiatric Conditions. Dyn Electrocardiogr. 2004; 56–63. [Google Scholar]
- 38.Cacioppo J, Tassinary LG, Berntson GG. The Handbook of Psychophysiology. Vol. 44, Dreaming. 2007. [Google Scholar]
- 39.Lazarus RS, Speisman JC, Mordkoff AM. The Relationship Between Autonomic Indicators of Psychological Stress: Heart Rate and Skin Conductance. Psychosom Med. 1963;25(1): 19–30. [Google Scholar]
- 40.Jacobs S, Friedman R, Parker J, Tofler G. Use of skin conductance changes during mental stress testing as an index of autonomic arousal in cardiovascular research. Am Hear. 1994;128(6): 1170–1177. [DOI] [PubMed] [Google Scholar]
- 41.Ferraro G, Caci B, D’Amico A, Di Blasi M. Internet addiction disorder: An Italian study. Cyberpsychol Behav. 2007;10(2): 170–5. doi: 10.1089/cpb.2006.9972 [DOI] [PubMed] [Google Scholar]
- 42.Poli R, Agrimi E. Internet addiction disorder: Prevalence in an Italian student population. Nord J Psychiatry. 2012;66(1): 55–9. doi: 10.3109/08039488.2011.605169 [DOI] [PubMed] [Google Scholar]
- 43.Struzzo P, Faccio S De, Moscatelli E, Scafato E. Identificazione precoce dei bevitori a rischio in Assistenza Primaria in Italia: adattamento del questionario AUDIT e verifica dell’efficacia d’uso dello short-AUDIT. 2006; 29: 20–25. [Google Scholar]
- 44.Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test (AUDIT): a review of recent research. Alcohol Clin Exp Res. 2002;26(2): 272–9. [PubMed] [Google Scholar]
- 45.Bastiani L, Siciliano V, Curzio O, Luppi C, Gori M. Optimal scaling of the CAST and of SDS Scale in a national sample of adolescents. Addict Behav. 2013; 38(4): 2060–2067. doi: 10.1016/j.addbeh.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 46.Bottesi G, Ghisi M, Altoè G, Conforti E, Melli G. The Italian version of the Depression Anxiety Stress Scales-21: Factor structure and psychometric properties on community and clinical samples. Compr Psychiatry. 2015;60: 170–81. doi: 10.1016/j.comppsych.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 47.Fossati A, Di Ceglie A, Acquarini E. Psychometric properties of an Italian Version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J Clin Psychol. 2001;57(6): 815–28. [DOI] [PubMed] [Google Scholar]
- 48.D’Orta I, Burnay J, Aiello D, Niolu C. Development and validation of a short Italian UPPS-P Impulsive Behavior Scale. Addict Behav Rep. 2015; 2: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sica C, Ghisi M, Altoè G, Chiri LR, Franceschini S, Coradeschi D, et al. The Italian version of the Obsessive Compulsive Inventory: Its psychometric properties on community and clinical samples. Vol. 23, J Anxiety Disord. 2009;23(2): 204–11. [DOI] [PubMed] [Google Scholar]
- 50.Bressi C, Taylor G, Parker J, Bressi S. Cross validation of the factor structure of the 20-item Toronto Alexithymia Scale: an Italian multicenter study. J Psychosom Res. 1996;41(6): 551–9. [DOI] [PubMed] [Google Scholar]
- 51.Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”—a tool for investigating psychobiological stress responses in a labor atory setting. Neuropsychobioloy. 1993;28(1–2): 76–81. [DOI] [PubMed] [Google Scholar]
- 52.R Development Core Team. R: A Language and Environment for Statistical Computing. R Found Stat Comput; Vienna Austria: 2016. [Google Scholar]
- 53.Harrell FJ, with contributions from Dupont C many others. Hmisc: Harrell Miscellaneous. R package version 4.0–0. 2016.
- 54.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. 2014;1–51. [Google Scholar]
- 55.Wagenmakers E-J, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev. 2004;11: 192–6. [DOI] [PubMed] [Google Scholar]
- 56.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24(3): 127–35. doi: 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 57.Bagiella E, Sloan R, Heitjan D. Mixed‐effects models in psychophysiology. Psychophysiology. 2000; 37(1): 13–20. [PubMed] [Google Scholar]
- 58.Venables WN, Ripley BD. Modern Applied Statistics with S. Issues of Accuracy and Scale. 2002. [Google Scholar]
- 59.Morey RD, Rouder JN, Jamil T. BayesFactor: Computation of Bayes factors for common designs. R Packag version 09. 2014;8. [Google Scholar]
- 60.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141(1): 105–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thayer JF, Lane RD, Benarroch EE, Benarroch EE, Berg WK, Donohue RL, et al. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3): 201–16. [DOI] [PubMed] [Google Scholar]
- 62.Quintana DS, Guastella AJ, McGregor IS, Hickie IB, Kemp AH. Heart rate variability predicts alcohol craving in alcohol dependent outpatients: Further evidence for HRV as a psychophysiological marker of self-regulation. Drug Alcohol Depend. 2013;132(1–2):395–8. doi: 10.1016/j.drugalcdep.2013.02.025 [DOI] [PubMed] [Google Scholar]
- 63.Shapira NA, Goldsmith TD, Keck PE, Khosla UM, McElroy SL. Psychiatric features of individuals with problematic internet use. J Affect Disord. 2000;57(1–3): 267–72. [DOI] [PubMed] [Google Scholar]
- 64.Ko C, Yen J-Y, Yen C, Chen C, Weng C, Chen C. The Association between Internet Addiction and Problematic Alcohol Use in Adolescents: The Problem Behavior Model. CyberPsychol Behav. 2008;11(5): 571–6. doi: 10.1089/cpb.2008.0199 [DOI] [PubMed] [Google Scholar]
- 65.Cano M, Lam C, Chen M, Adams C. Positive smoking outcome expectancies mediate the association between negative affect and smoking urge among women during a quit attempt. Exp Clin Psychopharmacol. 2014;22(4): 332–40. doi: 10.1037/a0036749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawkins L, Munafò M, Christoforou G. The effects of e-cigarette visual appearance on craving and withdrawal symptoms in abstinent smokers. Psychol Addict Behav. 2016;30(1): 101–5. doi: 10.1037/adb0000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davey HM, Barratt AL, Butow PN, Deeks JJ. A one-item question with a Likert or Visual Analog Scale adequately measured current anxiety. J Clin Epidemiol. 2007;60(4): 356–60. doi: 10.1016/j.jclinepi.2006.07.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Formulae A. Mixed-effects models. Legend: Index = HRV indices (i.e., SDNN; rMSSD; LF(ms2); HF(ms2)) and SCL; Phase = the Phase predictor (i.e. 1, 2, 3, 4, 5, 6 and 7 phases of the TSST); Group = the Group predictor (non-PU and PU); Individual = participants; AUDIT = the AUDIT ratings; DASS.D, DASS.A, and DASS.S = depression, anxiety, and stress subscales of the DASS-21 respectively; DASS.T = the DASS total score; LoPER and LoPRE = the lack of perseverance and lack of premeditation components of the UPPS-P, respectively; OCI = the OCI-R total score and TAS = the TAS score. Formulae B. Ordinal logistic regression models. Legend: Craving = Craving ratings; Time = Time predictor (i.e., before and after TSST); Group = Group Predictor (i.e., non-PU and PU).
(DOCX)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.