Abstract
Background: Social support interventions can improve diabetes self-care, particularly for Latinos, but are time and resource intensive. Mobile health may overcome these barriers by engaging and training supporters remotely.
Methods: We conducted a randomized controlled feasibility trial of emergency department patients with diabetes to determine the feasibility of enrolling patients and supporters, acceptability of the intervention, and preliminary efficacy results to power a larger trial. All patients received an existing mHealth curriculum (TExT-MED). After identifying a supporter, patients were randomized to intervention: supporters receiving FANS (family and friends network support), a text message support curriculum synchronized to patient messages, or control: supporters receiving a mailed pamphlet of the same information. Participants followed up at 3 months. FANS intervention participants came to postintervention interviews as part of a qualitative analysis.
Results: We enrolled 44 patients (22 per arm) and followed up 36 at 3 months. Participants were positive about the program. FANS intervention improved HbA1c (intervention mean decreased from 10.4% to 9.0% vs. from 10.1% to 9.5%, delta −0.8%, confidence interval [CI] −0.4 to 2, P = 0.30), self-monitoring of glucose (intervention increased 1.6 days/week vs. control decreased 2 days/week, delta 2.3 days/week, CI 4–0.6, P = 0.02), and physical activity (mean Godin leisure time activity score improved 16.1 vs. decreased 9.6 for control, delta 25.7, CI 49.2–2.3, P = 0.10). In qualitative analysis, patients reported improved motivation, behaviors, and relationships. Supporters reported making healthier decisions for themselves.
Conclusions: mHealth is a feasible, acceptable, and promising avenue to improve social support and diabetes outcomes.
Keywords: : Diabetes mellitus, Social support, Text messaging, Latinos, Disease management.
Introduction
Low-income Latinos have higher rates of diabetes and complications than the national average.1,2 This is likely multifactorial, related to genetics and socioeconomic status, as well as to language barriers and difficulty with access to primary care.3–5 Mobile health (mHealth) interventions to improve diabetes self-care have promising results in several disparity populations, including low-income English- and Spanish-speaking Latinos.6 Automated text-message-based mHealth interventions offer an attractive solution to some of these barriers as they are relatively inexpensive, highly scalable and most low-income Latinos have mobile phones that are capable of receiving basic SMS (short-message service) text messages based on national estimates.7 However, mHealth interventions have great heterogeneity in outcomes, and most have had modest treatment effect.8–12 The TExT-MED trial previously conducted by our group is the only mHealth-based diabetes trial to focus on emergency department (ED) patients with poor access to primary care. We found improvements in glycemic control for Spanish-speaking participants in the trial, and modest overall improvement in patients receiving the TExT-MED intervention versus usual care.13 In postintervention focus groups, patients identified a need for more personalization and support, and they also identified family members as integral to diabetes management.14 Incorporating family members into the intervention could potentially increase its efficacy.
Social support interventions typically create a new support network among peers with a shared diagnosis or engage family members in providing disease-specific social support to improve health outcomes.15–20 Among Latino patients, social support interventions have successfully improved diabetes outcomes and have been viewed favorably; however, this has not been studied in ED-based interventions.21–23 Social support interventions tend to be time intensive for family members, requiring travel to clinics for training. Alternative strategies are to deploy diabetes educators or community health workers to a family member's home; however, this can be costly to healthcare systems. This can result in family members who live closest to the patient with the most available free time to travel participating in support interventions, rather than the potential supporters who might be more influential or helpful to the patient.
Merging these two types of interventions could generate a solution that has the ease and scalability of mobile technologies coupled with the personal touch of social support. mHealth-based social support interventions could reduce the need for physical presence and make social support interventions more accessible to populations in need. By increasing social support and educating family members about good diabetes self-management practices, we may increase activation for behavior change and decrease the barrier to healthy life choices for these patients. In this study, we conduct and analyze a randomized controlled feasibility trial to determine the acceptability, feasibility, and efficacy of a novel social support module integrated into an existing mHealth intervention for low-income Latinos with diabetes.
Methods
Trial design
This is a parallel, nonblinded, randomized control trial with a 1:1 allocation.
Patient population, study setting, and recruitment goals
The study was conducted in the ED of Los Angeles County + University of Southern California Medical Center (LAC + USC). This patient population (LAC + USC ED patients with diabetes) was previously studied by our group, and is predominately Latino, Spanish-speaking, and low-income. Our prior work indicates that diabetes-specific knowledge is low in this population, and that the average HbA1c is 10.9%. Prior work in this group shows that 80% of patients have a text-capable phone, and prior mHealth interventions in this population have had higher than 80% satisfaction ratings.13,24–26 The recruitment goal was to determine the number of patients that could feasibly be enrolled in a 6-week period; our high estimate of feasibility was 50 patient/family member pairs.
Patient and supporter recruitment, enrollment, randomization, and follow-up
IRB approval was obtained before study initiation, and the trial was registered with clinicaltrials.gov (NCT01945996). Research assistant (RA) surveyed the ED electronic patient tracking system for patients with diabetes during daytime hours for 6 weeks. While in the ED, patients were screened for eligibility based on these criteria: having a text-capable mobile phone, being comfortable with sending and receiving texts, and having a glycosylated hemoglobin A1c (HbA1c) of ≥8, which falls into the ADA “Take Action” range. The HbA1c-based eligibility requirement was verified by using the Afinion AS-100 capillary point-of-care HbA1c meter. Patients were excluded if they were <18 years old, pregnant, or were unable to provide consent. During this initial screen, patients identified a family member or friend to act as a supporter. Patients were excluded if we could not reach the family member or friend within 1 week of patient screening. One supporter was enrolled per patient. At enrollment, we collected patients' self-reported race, ethnicity, age, language preference, and proficiency. Patients returned to the hospital for a baseline assessment. Once baseline assessment was complete, patients were randomized to receive the intervention condition (TExT-MED + FANS intervention) or control condition (TExT-MED with un-augmented social support and a pamphlet for supporters) by sequential closed envelope assignment. Envelopes were created before study initiation, and they were opened at baseline assessment by the RA enrolling the patient. Neither patients, supporters, nor research staff were blinded to allocation. Patients were contacted at 1 month to ensure that they were still receiving messages. Patients in both arms followed up in person at 3 months (the end of the message curriculum) to complete repeat assessment. Patients in the intervention arm were invited to stay after this visit to participate in a focus group interview. Supporters were contacted by phone or in person at 3 months to take a brief survey on change in texting habits and satisfaction with the intervention. Supporters in the FANS intervention arm were invited to a focus group interview for supporters only. Patients received a total of $100 in gift cards if they completed follow-up at 3 months.
Interventions
The intervention consisted of two curricula: one for patients and another for supporters. The patient messages were previously developed for TExT-MED, a uni-directional, fully automated, text-message-based program designed to increase knowledge, self-efficacy, and subsequent disease management and glycemic control. These twice-daily text messages for patients were derived from the National Diabetes Education Program (NDEP),25 and they consisted of: (1) educational/motivational messages (1/week), (2) medication reminders (3/week), (3) trivia questions (2/week), and (4) healthy living challenges (2/week).13 Patients in both trial arms received two messages daily: one educational or motivational message in the morning, and one message from one of the other three categories in the evening.
The FANS (family and friends network supporters) messages for supporters constituted a newly developed curriculum that mirrored the patient messages. Supporters in the FANS arm received one or two text messages a day: an educational or motivational message in the morning, and a trivia or support challenge in the evening, which corresponded to the patient messages. Challenge messages were modified to inspire social support for the patient. Trivia questions were identical to patient trivia questions. Supporters did not receive a message when the patient received a medication reminder. The pair of messages was sent to the patient and the supporter synchronously (see Fig. 1 for example of patient and supporter messages that would correspond). This synchronous message delivery was designed to promote conversation between the patient and the supporter, increasing the impact of the message. The FANS messages are based on the module of social support developed by Hinson-Langford et al., which recognizes four arenas of social support: (1) Instrumental support (tangible goods and actions), (2) Informational support (knowledge sharing), (3) Emotional support, and (4) Appraisal support (feedback on accuracy of beliefs and appropriateness of actions).27 To ensure that positive and appropriate support behaviors were emphasized in family members, the FANS curriculum included basic educational information, in addition to motivational messages and challenges to provide specific acts of support. The FANS curriculum was translated into Spanish by a professional translator, and it was back translated by two native Spanish speakers to ensure retention of meaning.
FIG. 1.
Examples of corresponding TExT-MED patient and FANS messages. FANS, family and friends network support.
In the intervention group, patients received the TExT-MED program, and their supporters received the FANS intervention daily for 3 months. In the control group, patients also received the TExT-MED program daily, but the supporters received a pamphlet mailed at the time of enrollment only with the same information as the FANS curriculum with instructions indicating when they should read each message to synchronize with the patient message.
Outcome measures
As this was a preliminary trial designed to inform future trials, we had several types of outcomes of interest: feasibility, acceptability, and preliminary efficacy.
Feasibility outcomes were the percent of eligible patients who opted to participate, percent of willing patients who were able to come to an enrollment visit and for whom we could contact a supporter by phone to enroll, follow-up rate for patients and supporters, and percent of invited patients and supporters who attended in-depth interviews at the end of the study. We collected these outcomes to be able to plan for the enrollment time frame needed for a larger trial. We also collected information related to whether supporters reported receiving either the text messages or mailed pamphlet, respective to the arm they were assigned to assess technical feasibility.
Acceptability outcomes were divided into patient-focused and supporter-focused outcomes. At the conclusion of the intervention, patients answered yes or no if they (1) believed the platform was a good way to learn about diabetes, (2) would recommend the program to friends or family members, and (3) wanted the messages to continue. Supporters answered yes or no whether they (1) liked being involved as a supporter, (2) would recommend the program to friends or family members, and (3) would want the messages to continue at the end of the trial (FANS intervention arm only). We also sought to record if and why any patients or supporters withdrew from the intervention.
Preliminary efficacy outcomes were split into (1) diabetes-specific outcomes, (2) social support outcomes, and (3) communication outcomes. These outcomes were measured at baseline assessment and at 3-month follow-up.
Diabetes-specific outcomes
We collected point-of-care HBA1c; self-efficacy, measured by diabetes empowerment scale—short form (DES-SF)28; diabetes-related quality of life, measured by the problem areas in diabetes (PAID) scale29; healthy behaviors, measured via the summary of diabetes self-care activities (SDSCA)30; and the Godin leisure time activity scale.31 We selected these measures as intermediary steps to glycemic control at the individual behavioral level (SDSCA and Godin scale), with potential relationships with behavioral activation/self-efficacy (DES-SF) and perceived barriers to healthy choices (PAID).
Social support outcomes
We collected measures of social support, measured by the Norbeck social support questionnaire, which is sub-scored as tangible and emotional support32; and social connectedness, measured by the social connected scale—revised.33
Communication outcomes
We also collected patient and supporter reports of the number of text messages exchanged with the supporter as well as the percent of messages about diabetes.
Statistical analysis
We generated descriptive statistics for patient demographics, feasibility outcomes, and acceptability outcomes by using STATA version 13.34 For the preliminary efficacy data, we generated descriptive statistics, and we used t tests or rank-sum tests as appropriate for variable type to compare outcomes between the groups. We also completed post hoc analysis of baseline characteristics of patients who completed follow-up versus those who did not, and outcome analysis for intervention group dyads who completed the study, based on supporter report of receiving the messages.
Postintervention qualitative analysis
At the conclusion of the 3-month trial, we conducted a series of group and individual interviews with only intervention group patients and intervention group supporters (half of the total participants) to assess acceptability to patients and family members and to understand the patient and supporter factors that might impact efficacy. The question guide (See Supplementary Data available online at http://online.liebertpub.com/doi/suppl/10.1089/dia.2017.0198) focused on how the intervention impacted behavior motivation, patients' perception of their disease, and the role that their supporter played in their diabetes management. Interviews were conducted on the same day as the 3-month follow-up visit, after survey instruments were administered. Experienced interviewers conducted 6 focus groups in Spanish and English with a total of 22 participants (14 patients and 8 family members). We imported verbatim transcripts into a computerized qualitative analysis program, Dedoose. A rigorous text-based, modified grounded theory approach was used.35 Transcripts were analyzed in an iterative process, reexamining the earlier transcripts with the new codes derived from each round of analysis until saturation was reached. Broad categorical key themes arose from the initial codes. We reviewed 297 pages of transcripts in the initial line-by-line process. Through three iterative rounds of co-coding, we developed a set of 32 codes and subcodes. Intercoder reliability was excellent (pooled Kappa 0.86).36
Results
Enrolled patient characteristics
We enrolled a total of 44 patients. Patients were predominantly Latino (80%), more often female (57%), and preferred Spanish to English at home (57%). Intervention and control group patients had similar baseline HbA1c, social support, social connectedness, diabetes-related self-efficacy, physical activity, and diabetes self-care behaviors (Table 1), with the exception of more frequent foot self-exams among control group patients.
Table 1.
Characteristics of TExT-MED + FANS Patients (N = 44)
| Intervention (n = 22) | Control (n = 22) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | n | % | n | % | P | ||
| Gender | 0.361 | ||||||
| Female | 11 | 50 | 14 | 64 | |||
| Male | 11 | 50 | 8 | 36 | |||
| Race/ethnicity | 0.670 | ||||||
| Non-Latino White | 1 | 5 | 1 | 5 | |||
| Latino | 17 | 77 | 18 | 82 | |||
| African American | 1 | 5 | 2 | 9 | |||
| Asian | 1 | 5 | 1 | 5 | |||
| Other | 2 | 9 | 0 | 0 | |||
| Language preference | 0.833 | ||||||
| Both equally | 1 | 5 | 1 | 5 | |||
| More English than Spanish | 4 | 18 | 2 | 9 | |||
| More Spanish than English | 3 | 14 | 4 | 18 | |||
| Only English | 2 | 9 | 2 | 9 | |||
| Only Spanish | 0 | 32 | 11 | 50 | |||
| Mean | 95% CI | Mean | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| HbA1c | 10.4 | 9.6 | 11.3 | 10.1 | 9.5 | 10.8 | 0.725 |
| Self-efficacy: DES-SF | 4.3 | 4.0 | 4.6 | 4.1 | 3.9 | 4.4 | 0.480 |
| Perception of problems: PAID | 49.0 | 35.8 | 62.3 | 45.9 | 33.0 | 58.9 | 0.742 |
| Self-care behaviors: SDSDCA | |||||||
| General diet | 3.8 | 2.8 | 4.8 | 4.0 | 2.8 | 5.2 | 0.670 |
| Specific diet | 4.3 | 3.4 | 5.2 | 3.9 | 3.1 | 4.8 | 0.532 |
| Exercise | 2.8 | 1.7 | 3.9 | 2.4 | 1.3 | 3.4 | 0.560 |
| Blood glucose | 3.7 | 2.5 | 4.8 | 5.3 | 4.2 | 6.4 | 0.037 |
| Foot care | 4.0 | 2.8 | 5.2 | 4.3 | 3.1 | 5.4 | 0.913 |
| Carb space | 2.7 | 1.8 | 3.7 | 3.4 | 2.2 | 4.5 | 0.552 |
| Godin leisure time activity | 37.5 | 23.0 | 52.1 | 38.3 | 23.7 | 52.9 | 0.897 |
| Social support: SSQ | |||||||
| Emotional | 14.9 | 13.7 | 16.1 | 15.5 | 15.0 | 16.0 | 0.382 |
| Tangible | 6.6 | 5.7 | 7.6 | 7.5 | 7.1 | 7.9 | 0.216 |
| Social connectedness: SCS | 18.9 | 13.9 | 23.9 | 16.8 | 12.1 | 21.5 | 0.470 |
| Texts sent per week by patient | 76.3 | 23.8 | 128.9 | 91.6 | 24.3 | 158.9 | 0.953 |
| Texts received per week by patient | 87.4 | 30.2 | 144.6 | 88.3 | 22.3 | 154.2 | 0.953 |
DES-SF, diabetes efficacy scale-short form; FANS, family and friends network support; PAID, problem areas in diabetes measure; SCS, social connectedness scale; SDSCA, summary of diabetes self-care activities measure; SSQ, Norbeck social support questionnaire.
Feasibility outcomes
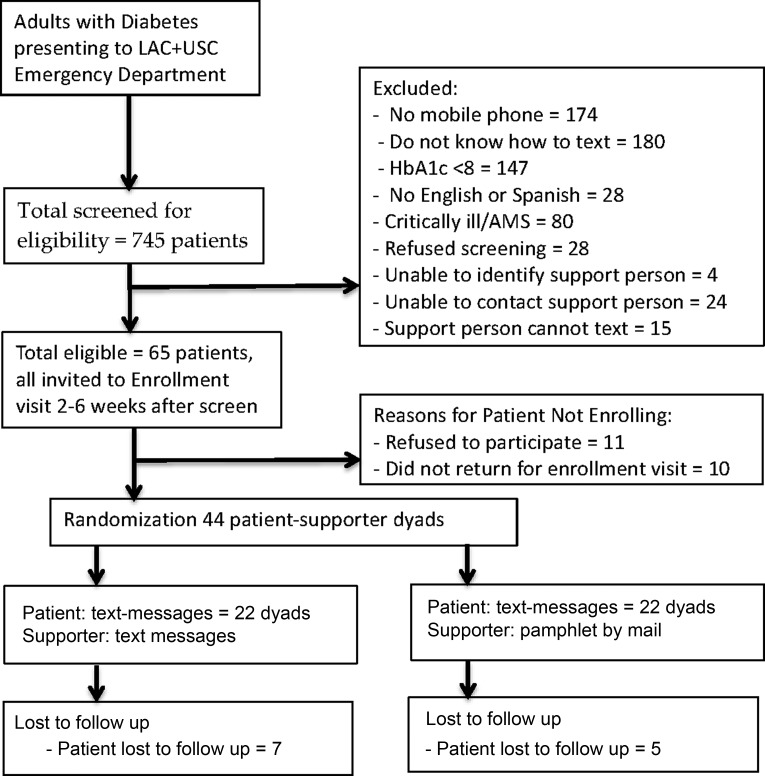
Enrollment and follow-up: (see Fig. 2 for consort-style diagram) We screened 745 ED patients with diabetes for eligibility. Out of them, 58 were eligible; reasons for ineligibility were: no mobile phone (174), did not know how to text (180), HbA1c in good control (147), did not speak English nor Spanish (28), critically ill (80), refused screening (28), no support person identified (4), unable to contact support person (24), and identified support person cannot text (15). Of these 65 eligible patients, 11 refused to participate, and 10 did not return for their enrollment visit, resulting in 44 enrolled patients (68% of eligible patients enrolled). At the conclusion of the study, 82% (36/44) of patients followed up at 3 months. We were able to follow up with 60% (26/44) of supporters. One hundred percent of patients reported receiving text messages. Ninety-four percentage of supporters in the FANS intervention arm reported receiving support messages, and 80% of supporters in the control mailed pamphlet arm reported receiving the pamphlet.
FIG. 2.
Diagram of screening, enrollment, randomization, and follow-up of TExT-MED + FANS patients.
Acceptability outcomes
One hundred percent of patients who followed up at the end of the intervention stated that text messages were a good way to teach about diabetes, 97% stated that they would want the messages to continue at the conclusion of the intervention, and 97% would recommend the program to a friend or family member with diabetes. One hundred percent of supporters stated that they liked being involved as a supporter, 88% stated that they would like the text messages to continue, and 93% stated that they would recommend the program to friends or family members. No patients or supporters requested to be dropped from the intervention nor opted out of messages.
Preliminary efficacy outcomes
For diabetes-specific outcomes, both groups experienced decreases in HbA1c at the 3-month follow-up. The intervention group experienced a greater drop in mean HbA1c (intervention patients' mean HbA1c decreased from 10.4% to 9.0%, delta 1.4 [95% confidence interval; CI 2.3–0.4] compared with control group patients' mean HbA1c decrease from 10.1% to 9.5%, delta 0.6 [95% CI 1.4 to −0.2], P = 0.296). FANS intervention patients also reported increased self-monitoring of glucose (intervention mean increased 1.6 days/week vs. control decreased 2 days/week, CI 4–0.6, P = 0.02) and physical activity (mean Godin leisure time activity score improved 16.1 vs. decreased 9.6 for control, delta 25.7, CI 49.2–2.3, P = 0.10). There were no differences between intervention and control patients' report of other self-care activities (SD-SCA), diabetes-related quality of life (PAID scale), and self-efficacy (DES-SF). See Table 2 for full results.
Table 2.
Change-Score Analysis for TExT-MED + FANS Patients Who Completed Follow-Up, n = 36
| Intervention (n = 17) | Control (n = 19) | Difference (C−I) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | P | |||
| HbA1c | −1.4 | −2.3 | −0.4 | −0.6 | −1.4 | 0.2 | 0.8 | −0.4 | 2.0 | 0.296 |
| Self-efficacy: DES-SF | 0.1 | −0.2 | 0.4 | 0.3 | −0.1 | 0.7 | 0.2 | −0.3 | 0.7 | 0.295 |
| Perception of Problems: PAID | −6.8 | −16.0 | 2.4 | −0.9 | −15.0 | 13.2 | 5.8 | −10.8 | 22.5 | 0.568 |
| Self-Care Behaviors: SDSDCA | ||||||||||
| General diet | 0.9 | −0.3 | 2.0 | 0.1 | −1.0 | 1.3 | −0.7 | −2.3 | 0.9 | 0.324 |
| Specific diet | 1.2 | 0.2 | 2.0 | 0.9 | −0.1 | 1.9 | −0.3 | −0.9 | 1.5 | 0.690 |
| Exercise | 0.4 | −0.6 | 1.3 | 0.7 | −0.6 | 2.0 | 0.3 | −1.3 | 1.9 | 0.588 |
| Blood glucose | 1.6 | 0.5 | 2.7 | −0.7 | −2.0 | 0.7 | −2.3 | −4.0 | −0.6 | 0.024 |
| Foot care | 1.0 | −0.5 | 2.6 | 0.9 | −0.3 | 2.1 | −0.1 | −2.0 | 1.8 | 0.870 |
| Carb space | 0.5 | −0.7 | 1.6 | 0.5 | −0.6 | 1.5 | 0.0 | −1.5 | 1.5 | 0.911 |
| Godin leisure time activity | 16.1 | 33.5 | −1.3 | −9.6 | 7.3 | −26.6 | −25.7 | −2.3 | −49.2 | 0.096 |
| Social support: SSQ | ||||||||||
| Emotional | −1.4 | −2.7 | 0.0 | −1.5 | −2.8 | −0.3 | −0.2 | −1.9 | 1.6 | 0.722 |
| Tangible | −0.2 | −1.2 | 0.7 | −0.4 | −1.2 | 0.4 | −0.1 | −1.3 | 1.1 | 0.709 |
| Social connectedness: SCS | 0.8 | −6.1 | 7.8 | −1.9 | −6.5 | 2.7 | 5.8 | −10.8 | 22.5 | 0.568 |
For social support outcomes, we found no difference in patients in the FANS intervention and the control group reported levels of tangible social support, emotional social support, and social connectedness; see Table 2 for full results.
For communication outcomes, intervention group patient-supporter dyads also reported an increased number of texts per week (51 more texts per week for FANS intervention group dyads compared with a decrease of two messages per week for control dyads, P = 0.06) but no change in the percent of text messages about diabetes, with a 26% (95% CI 0.76–52) increase in intervention dyads compared with a 30% (95% CI 11–49) increase in control intervention dyads, P = 0.82. The correlation between supporter and patient reports of number of text messages exchanged each week was moderate (r = 0.57).
Post hoc sub-analysis
We found no differences in baseline characteristics for patients who completed follow-up versus those who did not, nor in preliminary outcome measures for patients whose supporters received the messages versus those who did not. See Supplementary Tables S1 and S2.
Qualitative analysis
Through the qualitative analysis, we identified several key themes regarding the intervention's acceptability and impact on patients' motivation for behavior change and diabetes self-management (see Table 3 for exemplary quotes for the key themes identified). Foremost, intervention group participants in the interviews were overwhelmingly positive about the program. The most impactful messages were those that had specific calls for action such a dietary goal for a meal or physical activity challenge. Some patients also felt motivated by messages that called on them to stay healthy and honor their responsibilities. Both patients and family members noted that their communication had improved, and that they felt that the communication initiated by the FANS intervention had strengthened their relationships. Both patients and supporters believed that there could be more personalization of the messages, both in time of day delivered and in content tailored to their specific needs. Interestingly, supporters noted that they were more mindful of their own health decisions, and they believed that participation had improved their own health. The FANS curriculum was designed to improve social support behaviors from family members, but not specifically to improve the health choices that supporters made for themselves, whereas TExT-MED was designed to improve diabetes-related self-efficacy, specific self-care behaviors, and resultant glycemic control among patients.
Table 3.
Key Themes and Exemplary Quotes of Impact of TExT-MED FANS
| Theme | Quotes from Interview Participants |
|---|---|
| Positive regard for the TExT-MED FANS intervention | “I think it [TExT-MED+FANS] is very good. To motivate people. For example, the people, the messages that you send. They are good, as long as the people who receive them read them. Because it's no longer up to you. It depends on the people that receive them.” |
| Desire to be healthy and honor responsibilities | “I always knew that back in my mind, but didn't come forth… One of the last few texts that you guys sent, that, uh, you also wanna be there around your family. You know, your loved ones … I remember that. And I go, ‘yeah, that's the reason why I'm doing it.’” |
| Improved communication and emotional support | “I thank you very much for this, the messages, because that's how my husband would communicate with me. Let me tell you, he and I did not have a good relationship. But now, he is my support.” (Translated from Spanish) |
| “I think emotionally closer we became. Sometimes we would receive a text that would say, ‘tell them how much you appreciate them.’ And then, sometimes, you know, when I'm random and I just go up and I told her like why I love her and it makes her feel good about herself. Cause sometimes she goes in a state of like depression. I noticed that I'm like, whoa. Don't worry, it'll get better. I just try to tell her positive things to help her out, emotionally. I'm not really good at like expressing how I feel. So I think reminders, the text messages, help me.” | |
| Desire for more personalization | “I'm taking [medications] on a regular basis, the times where I should take them. Not just, forget in the morning. Examples like texting [medication reminders] at nine o'clock. For me, that's late. So, if I forgot because it was late. Nine o'clock is late for me. Nine o'clock is my break time.” |
| Change in supporters' health behaviors | “You have someone that is receiving the same information that you are and that it's making, even making the other person a little bit more self conscious about their own health.” |
| “[A Challenge] Message would say, ‘don't eat bread or sweets or carbohydrates’ or something like that, and ‘tell the person that you are doing the same thing’… it makes you conscious of your own diet and how screwed up it is, you know? Like that you really don't, if you're not being forced to actually take a look at what you're putting in your mouth you really don't really think about it.” |
Conclusions
Patient-centered mHealth interventions for diabetes are generally modestly effective at improving diabetes outcomes.11 Improving the quality and quantity of social support provided by a patient's family members also improves intentions and diabetes self-care behaviors,37–39 but it is difficult to scale up for larger populations. We created the TExT-MED + FANS intervention to add a mobile social support intervention to a previously successful patient-centered mHealth intervention, and we tested whether this could feasibly enhance clinical outcomes in a scalable manner. In this study, we found that such a novel mobile social support intervention was feasible, acceptable to patients and their family members, and produced promising results for diabetes-specific outcomes.
The chief reason to conduct this study was to determine the time frame necessary to enroll patients in a fully powered trial and to determine the scalability of the intervention. The enrollment procedures for family members and the requirement for patients and family members to be able to text could limit eligibility and future scalability. Although these additional inclusion criteria (willing and available family member to serve as a supporter, owning a mobile phone, and knowing how to text) did limit the percent of eligible patients who were successfully enrolled, we were able to enroll 44 patients and their family members in a 6-week time frame, indicating that a fully powered trial is feasible in this setting. The primary limitation to enrollment was mobile phone ownership and use of text messaging. However, multiple studies have shown that cell phone ownership and use of text messaging by low-income Latino patients is increasing, so this potential limit to scalability is decreasing rapidly.24,40 In addition, there is value in an intervention that can change behavior for a segment of this underserved population, even if mobile phone ownership does not reach 100%. An additional issue for a few patients was the lack of a support person to enroll; this intervention is designed as an induction intervention, where we activate existing social connections, rather than an alteration intervention in which we would attempt to create new connections.41 We chose this kind of induction intervention, because although social support is generally believed to improve diabetes self-management, in some populations there is evidence that some family behaviors can be obstructive to good health decisions.42–45 To encourage positive support behaviors from family members, the FANS curriculum included basic educational information rather than solely “pushes” to provide specific acts of support. In larger iterations of this intervention, the intricacies of helpful and obstructive behaviors will need to be better elucidated. This intervention can increase support, but we must ensure that the support provided is appropriate and helpful.
We found that this kind of social support intervention was highly accepted by patients and family members, with overwhelming positive regard for the program. It was engaging, as no supporters or patients stopped the text messages and follow-up with patients was >80%. Through our qualitative analysis, we similarly found that patients and supporters were highly engaged in the program and enjoyed the experience. We also found evidence that an mHealth social support strategy may improve diabetes management compared with a more traditional pamphlet version. TExT-MED + FANS resulted in changes in self-efficacy compared with the control intervention. As this was a feasibility trial and not powered to find statistical differences in clinical outcomes, we did not anticipate finding differences in clinical outcomes. However, we found modestly better glycemic control among FANS intervention patients versus control intervention patients. Both groups significantly improved glycemic control from baseline, likely because the control group was not a “no-touch” group, but instead received a previously tested and successful patient-focused intervention with a basic social support component. Our novel approach of encouraging family members to engage via a mobile intervention has the potential to make lasting behavior changes, thus a longer follow-up period should be tested. The promising improvements in our FANS intervention patients indicate that this augmented intervention can improve diabetes self-care and resultant glycemic control. By creating this scalable intervention, we anticipate that we can reach a larger portion of these high-need patients and engage them in better self-care as well as relink them to diabetes specialists and/or primary care providers.
Prior reports of utilizing mHealth to augment, initiate, or improve social support for patients with diabetes consist primarily of pilot work, and comparisons between studies are limited by heterogeneous patient populations. Of four identified published studies, two attempted to create new social support ties by patients with diabetes with people they did not know and two studies recruited people from the patients' existing social support network. Of the two studies that matched patients to strangers, one study matched patients to other patients with diabetes,46 and another study matched patients to community volunteers47; both reported negative findings. Rather than trying to create new social bonds between strangers, our intervention builds on previously established social connections. Two other published studies examined the potential effects of recruiting a patient's loved one as a support person; however, they both reported difficulty in identifying and recruiting supporters, and less than half of the patients had a supporter enrolled.15,48 One study examined patient perspectives qualitatively only,48 and the other found that patients with a supporter showed better medication adherence, particularly among those patients under psychological distress.15 However, as patients who were able to recruit a loved one were compared with those who could not, this positive change could be due to differences in baseline social support moderating the effect of the intervention. Our strategy of only enrolling patients with a willing supporter decreases potential recruitment bias, and it better isolates the effect of augmented social support.
Our promising 3-month feasibility trial has several limitations that must be resolved before conducting larger and longer studies. Follow-up with supporters was only 60% at 3 months, and this could decline even further with longer trials. The high approval ratings from supporters could be inflated given that those who did not enjoy the program may be less likely to complete follow-up. Clarifying the expectations of supporters participating in the trial, regular engagement check with messages requesting a text-back, and improved incentives for supporter follow-up would likely improve this. However, no supporters opted out of messages, indicating that they continued in the intervention even if they were not available for follow-up. In addition, the focus of this intervention is the patients' health and behavior choices, so the suboptimal follow-up among supporters does not impact the main patient-centered objective of this study. We must also decide how to accurately and appropriately measure the quantity of patient/supporter communication. Correlation of self-report of text messaging between the patient and supporter dyads in which both members completed follow-up measures was moderate. Checking phones for the actual number of messages exchanged may be a more objective measure, but it invades privacy, erodes trust, and presumes that participants are not deleting messages due to memory restrictions on their mobile phone. The perception of social support has been shown to be more important than the actual provision of the support when examining outcomes.49 This potential issue in measuring the mechanism of action of the intervention does not negate the importance of our findings and the potential benefit of this kind of intervention. The participants in this trial likely have a higher level of social support at baseline than patients who did not have a family member available to serve as supporter, which limits the external validity of our findings. Both patients and supporters who came to the interviews are also more likely to be highly engaged users, and the qualitative analysis likely reflects a more positive view of the program than the study population at large. Lastly, although this brief trial was designed to test the feasibility of this complicated intervention, and was not powered to detect changes in diabetes outcomes, the positive findings are encouraging.
TExT-MED + FANS is an entirely automated, text-message-based, diabetes self-education and self-management intervention augmented with mobile instigation of social support. In this feasibility trial in low-income ED patients with diabetes, we found that mHealth is a feasible, acceptable, and promising avenue to increase diabetes self-care and resultant glycemic control. Interestingly, although the intervention was focused on patients, the family members believed that they made healthier decisions in their own lives. A fully powered trial is necessary to determine whether there are significant changes in diabetes self-care behaviors and resultant glycemic control. Instigating improved social support through this scalable mechanism may be an important key to reaching populations who suffer diabetes disparities and instigating long-term behavior change and improved diabetes outcomes.
Supplementary Material
Acknowledgments
Dr. Burner's time and the project were supported by a KL2 from the SC CTSI (NIH/NCRR/NCATS grant no. KL2TR000131). Dr. Burner's time was also supported by an NIH grant no. 1K23DK106538.
Author Disclosure Statement
The intellectual property rights to the original TExT-MED program have been purchased from the University of Southern California by Agile Health, LLC. Drs. Arora and Menchine consult for Agile Health, LLC. Agile Health did not participate in the design of the FANS curriculum. No other authors reported disclosures.
References
- 1.Geiss LS, Wang J, Cheng YJ, et al. : Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 2.Beckles GL, Chou CF: Disparities in the prevalence of diagnosed diabetes—United States, 1999–2002 and 2011–2014. MMWR Morb Mortal Wkly Rep 2016;65:1265–1269 [DOI] [PubMed] [Google Scholar]
- 3.Piccolo RS, Subramanian SV, Pearce N, et al. : Relative Contributions of socioeconomic, local environmental, psychosocial, lifestyle/behavioral, biophysiological, and ancestral factors to racial/ethnic disparities in type 2 diabetes. Diabetes Care 2016;39:1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caballero AE: Diabetes in the Hispanic or Latino population: genes, environment, culture, and more. Curr Diab Rep 2005;5:217–225 [DOI] [PubMed] [Google Scholar]
- 5.Daniulaityte R: Making sense of diabetes: cultural models, gender and individual adjustment to Type 2 diabetes in a Mexican community. Soc Sci Med 2004;59:1899–1912 [DOI] [PubMed] [Google Scholar]
- 6.Fortmann AL, Gallo LC, Garcia MI, et al. : Dulce Digital: an mHealth SMS-based intervention improves glycemic control in Hispanics with type 2 diabetes. Diabetes Care 2017;40:1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livingston G, Minushkin S, Cohn D: Hispanics and Healthcare in the United States: Access, Information and Knowledge, 2008: A Joint Pew Hispanic Center and Robert Wood Johnson Foundation Research Report. Pew Research Center; 2008 [Google Scholar]
- 8.Bell AM, Fonda SJ, Walker MS, et al. : Mobile phone-based video messages for diabetes self-care support. J Diabetes Sci Technol 2012;6:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn CC, Shardell MD, Terrin ML, et al. : Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011;34:1934–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seto E, Leonard KJ, Cafazzo JA, et al. : Mobile phone-based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res 2012;14:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou C, Carter B, Hewitt J, et al. : Do mobile phone applications improve glycemic control (HbA1c) in the self-management of diabetes? A systematic review, meta-analysis, and GRADE of 14 randomized trials. Diabetes Care 2016;39:2089–2095 [DOI] [PubMed] [Google Scholar]
- 12.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, et al. : Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev 2012;12:Cd007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora S, Peters AL, Burner E, et al. : Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med 2014;63:745–754.e6. [DOI] [PubMed] [Google Scholar]
- 14.Burner ER, Menchine MD, Kubicek K, et al. : Perceptions of successful cues to action and opportunities to augment behavioral triggers in diabetes self-management: qualitative analysis of a mobile intervention for low-income Latinos with diabetes. J Med Internet Res 2014;16:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aikens JE, Trivedi R, Aron DC, Piette JD: Integrating support persons into diabetes telemonitoring to improve self-management and medication adherence. J Gen Intern Med 2015;30:319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin J, Wong R, Au S, et al. : Effects of providing peer support on diabetes management in people with type 2 diabetes. Ann Fam Med 2015;13 Suppl 1:S42–S49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trief PM, Fisher L, Sandberg J, et al. : Health and psychosocial outcomes of a telephonic couples behavior change intervention in patients with poorly controlled type 2 diabetes: a randomized clinical trial. Diabetes Care 2016;39:2165–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang TS, Funnell MM, Sinco B, et al. : A randomized controlled trial in an African American community. Ann Fam Med 2015;13 Suppl 1:S27–S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safford MM, Andreae S, Cherrington AL, et al. : Peer coaches to improve diabetes outcomes in rural Alabama: a cluster randomized trial. Ann Fam Med 2015;13 Suppl 1:S18–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayala GX, Ibarra L, Cherrington AL, et al. : Puentes hacia una mejor vida (Bridges to a Better Life): outcome of a diabetes control peer support intervention. Ann Fam Med 2015;13 Suppl 1:S9–S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teufel-Shone NI, Drummond R, Rawiel U: Developing and adapting a family-based diabetes program at the U.S.-Mexico border. Prev Chronic Dis 2005;2:A20. [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson JR, Horton C, Flores C: Advancing diabetes self-management in the Mexican American population: a community health worker model in a primary care setting. Diabetes Educ 2007;33 Suppl 6:159 s–165 s [DOI] [PubMed] [Google Scholar]
- 23.Two Feathers J, Kieffer EC, Palmisano G, et al. : Racial and Ethnic Approaches to Community Health (REACH) Detroit partnership: improving diabetes-related outcomes among African American and Latino adults. Am J Public Health 2005;95:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arora S, Abramson T, Ruiz R, et al. : Describing the mobile health capacity of Inner City Latino Emergency Department Patients: are national estimates accurate? Acad Emerg Med 2013;20:187 [Google Scholar]
- 25.Arora S, Marzec K, Gates C, Menchine M: Diabetes knowledge in predominantly Latino patients and family caregivers in an urban emergency department. Ethn Dis 2011;21:1–6 [PubMed] [Google Scholar]
- 26.Arora S, Ford K, Terp S, et al. : Describing the evolution of mobile technology usage for Latino patients and comparing findings to National mHealth Estimates. J Am Med Inform Assoc 2016;23:979–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langford CP, Bowsher J, Maloney JP, Lillis PP. Social support: a conceptual analysis. J Adv Nurs 1997;25:95–100 [DOI] [PubMed] [Google Scholar]
- 28.Anderson RM, Fitzgerald JT, Gruppen LD, et al. : The Diabetes Empowerment Scale-Short Form (DES-SF). Diabetes Care 2003;26:1641–1642 [DOI] [PubMed] [Google Scholar]
- 29.Welch GW, Jacobson AM, Polonsky WH: The Problem Areas in Diabetes Scale: an evaluation of its clinical utility. Diabetes Care 1997;20:760–766 [DOI] [PubMed] [Google Scholar]
- 30.Toobert DJ, Hampson SE, Glasgow RE: The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 31.Godin G, Shephard R: Godin leisure-time exercise questionnaire. Med Sci Sports Exerc 1997;29:S36 [Google Scholar]
- 32.Norbeck JS, Lindsey AM, Carrieri VL: The development of an instrument to measure social support. Nurs Res 1981;30:264–269 [PubMed] [Google Scholar]
- 33.Lee RM, Robbins SB: Measuring belongingness: The Social Connectedness and the Social Assurance scales. J Counsel Psychol 1995;42:232 [Google Scholar]
- 34.STATA: Stata Statistical Software. College Station, TX: StataCorp LP, 2013 [Google Scholar]
- 35.Charmaz K: Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. Thousand Oaks, CA: Sage Publications Limited; 2006 [Google Scholar]
- 36.De Vries H, Elliott MN, Kanouse DE, Teleki SS: Using pooled kappa to summarize interrater agreement across many items. Field Methods 2008;20:272–282 [Google Scholar]
- 37.Kirk JK, Ebert CN, Gamble GP, Ebert CE: Social support strategies in adult patients with diabetes: a review of strategies in the USA and Europe. Expert Rev Endocrinol Metab 2013;8:379–389 [DOI] [PubMed] [Google Scholar]
- 38.Mansyur CL, Rustveld LO, Nash SG, Jibaja-Weiss ML: Social factors and barriers to self-care adherence in Hispanic men and women with diabetes. Patient Educ Couns 2015;98:805–810 [DOI] [PubMed] [Google Scholar]
- 39.Strom JL, Egede LE: The impact of social support on outcomes in adult patients with type 2 diabetes: a systematic review. Curr Diab Rep 2012;12:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livingston G: Latinos and Digital Technology, 2010. Pew Hispanic Center, web page, 2011;9 [Google Scholar]
- 41.Valente TW: Network interventions. Science 2012;337:49–53 [DOI] [PubMed] [Google Scholar]
- 42.Mayberry LS, Egede LE, Wagner JA, Osborn CY: Stress, depression and medication nonadherence in diabetes: test of the exacerbating and buffering effects of family support. J Behav Med 2015;38:363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayberry LS, Osborn CY: Family support, medication adherence, and glycemic control among adults with type 2 diabetes. Diabetes Care 2012;35:1239–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayberry LS, Osborn CY: Family involvement is helpful and harmful to patients' self-care and glycemic control. Patient Educ Couns 2014;97:418–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayberry LS, Rothman RL, Osborn CY: Family members' obstructive behaviors appear to be more harmful among adults with type 2 diabetes and limited health literacy. J Health Commun 2014;19(Supp 2):132–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotheram-Borus MJ, Tomlinson M, Gwegwe M, et al. : Diabetes buddies peer support through a mobile phone buddy system. Diabetes Educ 2012;38:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sylvetsky AC, Nandagopal R, Nguyen TT, et al. : Buddy Study: partners for better health in adolescents with type 2 diabetes. World J Diabetes 2015;6:1355–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayberry LS, Berg CA, Harper KJ, Osborn CY: The design, usability, and feasibility of a family-focused diabetes self-care support mHealth intervention for diverse, low-income adults with type 2 diabetes. J Diabetes Res 2016;2016:7586385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDowell TL, Serovich JM: The effect of perceived and actual social support on the mental health of HIV-positive persons. AIDS Care 2007;19:1223–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.