Abstract
Delivery of adeno-associated viral (AAV) vectors into the cerebrospinal fluid (CSF) can achieve gene transfer to cells throughout the brain and spinal cord, potentially making many neurological diseases tractable gene therapy targets. Identifying the optimal route of CSF access for intrathecal AAV delivery will be a critical step in translating this approach to clinical practice. We previously demonstrated that vector injection into the cisterna magna is a safe and effective method for intrathecal AAV delivery in nonhuman primates; however, this procedure is not commonly used in clinical practice. More routine methods of administration into the CSF are now being explored, including intracerebroventricular (ICV) injection and injection through a lumbar puncture. In this study, we compared ICV and intracisternal (IC) AAV administration in dogs. We also evaluated vector administration via lumbar puncture in nonhuman primates, with some animals placed in the Trendelenburg position after injection, a maneuver that has been suggested to improve cranial distribution of vector. In the dog study, ICV and IC vector administration resulted in similarly efficient transduction throughout the brain and spinal cord. However, animals in the ICV cohort developed encephalitis associated with a T-cell response to the transgene product, a phenomenon that was not observed in the IC cohort. In the nonhuman primate study, transduction efficiency was not improved by placing animals in the Trendelenburg position after injection. These findings illustrate important limitations of commonly used methods for CSF access in the context of AAV delivery, and will be important for informing the selection of a route of administration for first-in-human studies.
Keywords: : AAV, intrathecal, gene therapy, central nervous system, mucopolysaccharidosis, GUSB
Introduction
Many genetic disorders affect the CNS, making the brain and spinal cord critical target tissues for gene therapy. However, application of gene therapy to the CNS has been restricted by obstacles to effective gene delivery. The first critical obstacle was the need for vectors capable of safe, efficient, and durable gene transfer to neurons and glia. This challenge was addressed by the development of vectors based on adeno-associated viruses (AAVs). The viral coding sequences of these nonpathogenic, single-stranded DNA viruses can be entirely replaced by a therapeutic transgene, yielding vectors capable of stably transducing nondividing cells in vivo. An early clinical study for Parkinson's disease, using an AAV serotype 2 vector to deliver the aromatic amino acid decarboxylase gene to the putamen, showed evidence of stable gene expression for at least 4 years after injection.1,2 The first generation of AAV vectors based on AAV serotype 2 were too inefficient for many applications that require more widespread gene transfer in the brain; however, second-generation vectors, such as the human isolate AAV serotype 9, are substantially more efficient and show potential for expanding the applications of CNS gene transfer to diseases that impact the entire CNS.3
The second critical obstacle to effective gene therapy in the CNS is the method of vector delivery. AAV9 can cross the blood–brain barrier to transduce cells within the CNS after intravenous delivery, an approach that has already shown promise in infants with spinal muscular atrophy (SMA).4 However, while trans-blood–brain barrier (BBB) AAV9 delivery is efficient in mice, the inefficiency of this approach when scaled to larger animals necessitates extremely large vector doses. These doses result in high levels of transduction in peripheral organs with potential associated toxicity, and face manufacturing limitations that may preclude clinical applications beyond the treatment of infants.4 Direct vector administration to the CNS via intraparenchymal injections is far more efficient, achieving effective transduction near the injection site with comparatively low vector doses.5 This approach is promising for diseases that can be treated by gene transfer to a specific brain region, but many disorders require gene transfer to cells throughout the CNS. Intraparenchymal injection is less suitable for these applications, as gene transfer is restricted to the area surrounding the injection site.6 Studies in dogs and cats have required 4–8 needle tracks to achieve significant coverage of the brain, which would equate to more than 100 needle tracks in the brain of an adult human, presenting a significant barrier to the translation of this approach.5,6 More recently, many groups have demonstrated that delivery of AAV vectors into cerebrospinal fluid (CSF) can achieve transduction throughout the brain and spinal cord of large animals.7–13 The scalability and relatively noninvasive nature of this approach make it appealing for translation to the clinic, and, in fact, trials have already begun for intrathecal AAV9 delivery for giant axonal neuropathy (ClinicalTrials.gov identifier, NCT02362438).
To maximize the effectiveness of intrathecal AAV delivery, it will be critical to determine the optimal route of vector administration into the CSF. We previously reported that vector injection into the cisterna magna (cerebellomedullary cistern) by suboccipital puncture achieved effective vector distribution in nonhuman primates, whereas injection via lumbar puncture resulted in substantially lower transduction of the spinal cord and virtually no distribution to the brain, underscoring the importance of the route of administration.11 Others have suggested that vector delivery into the lateral ventricles, a common clinical procedure, results in effective vector distribution.14 It has also been reported that delivery via lumbar puncture can be improved by placing animals in the Trendelenburg position after injection to promote cranial vector distribution.13 In the current study, we compared intraventricular and intracisternal administration of an AAV9 vector expressing a green fluorescent protein (GFP) reporter gene in dogs. We found that both routes achieved effective distribution throughout the CNS, although intraventricular delivery may carry additional risks of a transgene-specific immune response. We also evaluated vector delivery by lumbar puncture in nonhuman primates (NHPs), and the impact of placing animals in the Trendelenburg position after injection. There was no clear effect of postinjection positioning. These findings should inform the selection of vector route of administration in future CNS-directed gene therapy clinical trials.
Materials and Methods
Vector production
The GFP vector consisted of an AAV serotype 9 capsid carrying an expression cassette comprising a chicken β-actin promoter with cytomegalovirus immediate-early enhancer, an artificial intron, the enhanced green fluorescent protein cDNA, a woodchuck hepatitis virus posttranscriptional regulatory element, and a rabbit β-globin polyadenylation sequence. The GUSB vector consisted of an AAV serotype 9 capsid carrying an expression cassette comprising a chicken β-actin promoter with cytomegalovirus immediate-early enhancer, an artificial intron, the canine GUSB cDNA, and a rabbit β-globin polyadenylation sequence. The vectors were produced by triple transfection of HEK 293 cells and purified on an iodixanol gradient as previously described.15
Animal experiments
All dogs were raised in the National Referral Center for Animal Models of Human Genetic Disease of the School of Veterinary Medicine of the University of Pennsylvania (NIH OD P40-010939) under National Institutes of Health and U.S. Department of Agriculture guidelines for the care and use of animals in research.
NHP study
This study included six cynomolgus monkeys between 9 and 12 years of age. Animals were between 4 and 8 kg at the time of injection. The vector (2 × 1013 genome copies [GC]) was diluted in 5 ml of Omnipaque (iohexol) 180 contrast material before injection. Injection of the vector via lumbar puncture was performed as previously described.11 Correct injection into the intrathecal space was verified by fluoroscopy. For animals in the Trendelenburg group, the head of the bed was lowered 30 degrees for 10 min immediately after injection. Euthanasia and tissue collection were performed as previously described.11
Dog study
This study included six 1-year-old mucopolysaccharidosis type I (MPS I) dogs, as well as a 2-month-old MPS VII dog. Baseline magnetic resonance imaging (MRI) was performed on all intracerebroventricular (ICV)-treated dogs to plan the injection coordinates. Intracisternal injection was performed as previously described.16 For ICV injection, dogs were anesthetized with intravenous propofol, endotracheally intubated, maintained under anesthesia with isoflurane, and placed in a stereotaxic frame. The skin was sterilely prepped, and an incision was made over the injection site. A single burr hole was drilled at the injection site, through which a 26-gauge needle was advanced to the predetermined depth. Placement was confirmed by CSF return. The vector (1.8 × 1013 GC in 1 ml) was slowly infused over 1–2 min. Euthanasia and tissue collection were performed as previously described.16
Histology
Brains were processed as described for evaluation of GFP expression.11 β-Glucuronidase (GUSB) enzyme stains and ganglioside GM3 stains were performed as previously described.8
ELISPOT
At the time of necropsy, blood was collected from vector-treated dogs into heparinized tubes. Peripheral blood mononuclear cells were isolated by Ficoll gradient centrifugation. T-cell responses to AAV9 capsid peptides and GFP peptides were evaluated by interferon-γ enzyme-linked immunospot (ELISPOT) assay. AAV9 and GFP peptide libraries were synthesized as 15-mers with 10-amino acid overlap (Mimotopes, Clayton, Victoria, Australia). The AAV9 peptide library was grouped in three pools: pool A from peptide 1 to 50, pool B from peptide 51 to 100, and pool C from peptide 101 to 146. The GFP peptide library was contained in a single pool. Phorbol 12-myristate 13-acetate plus ionomycin salt (PMA+ION) was used as positive control. Dimethyl sulfoxide (DMSO) was used as negative control. Cells were stimulated with peptide, and interferon-γ secretion was detected as described. A response was considered positive if it was both greater than 55 spot-forming units (SFU) per million lymphocytes and at least three times the DMSO negative control value.
Biodistribution
At the time of necropsy, tissues for biodistribution were immediately frozen on dry ice. DNA isolation and quantification of vector genomes by TaqMan PCR was performed as described.17
GUSB enzyme assay
GUSB activity was measured in CSF as described.8
Results
Comparison of intracerebroventricular and intracisternal vector delivery in dogs
Our previous studies using a canine model of the lysosomal storage disease, mucopolysaccharidosis type I (MPS I), demonstrated that AAV9 injection into the cisterna magna could effectively target the entire brain and spinal cord.16 In this study, we compared distribution of an AAV9 vector expressing a GFP reporter gene administered into the cisterna magna or lateral ventricle of adult MPS I dogs. Three dogs were treated with a single 1-ml injection of the vector (1.8 × 1013 GC) into the cisterna magna. Three additional dogs received a single vector injection of the same vector into the lateral ventricle. For dogs treated by ICV injection, baseline MRI was performed to select the larger lateral ventricle for injection and to define the target coordinates. Injection was performed with a stereotaxic frame to accurately target the designated ventricle.
The three dogs treated by intracisternal (IC) vector injection appeared healthy throughout the study. They were euthanized 2 weeks after vector injection for evaluation of vector biodistribution and transgene expression. No gross or microscopic brain lesions were observed in any IC-treated dogs (Fig. 1). Measurement of vector genomes by quantitative PCR revealed vector deposition throughout all sampled regions of the brain and spinal cord (Fig. 2). Consistent with the distribution of vector genomes, robust transgene expression was detectable in most regions of the cerebral cortex as well as throughout the spinal cord (Fig. 3, Supplementary Fig. S1; supplementary data are available online at www.liebertpub.com/hum). Spinal cord histology was notable for strong transduction of alpha motor neurons, with a gradient of transduction favoring thoracic and lumbar segments.
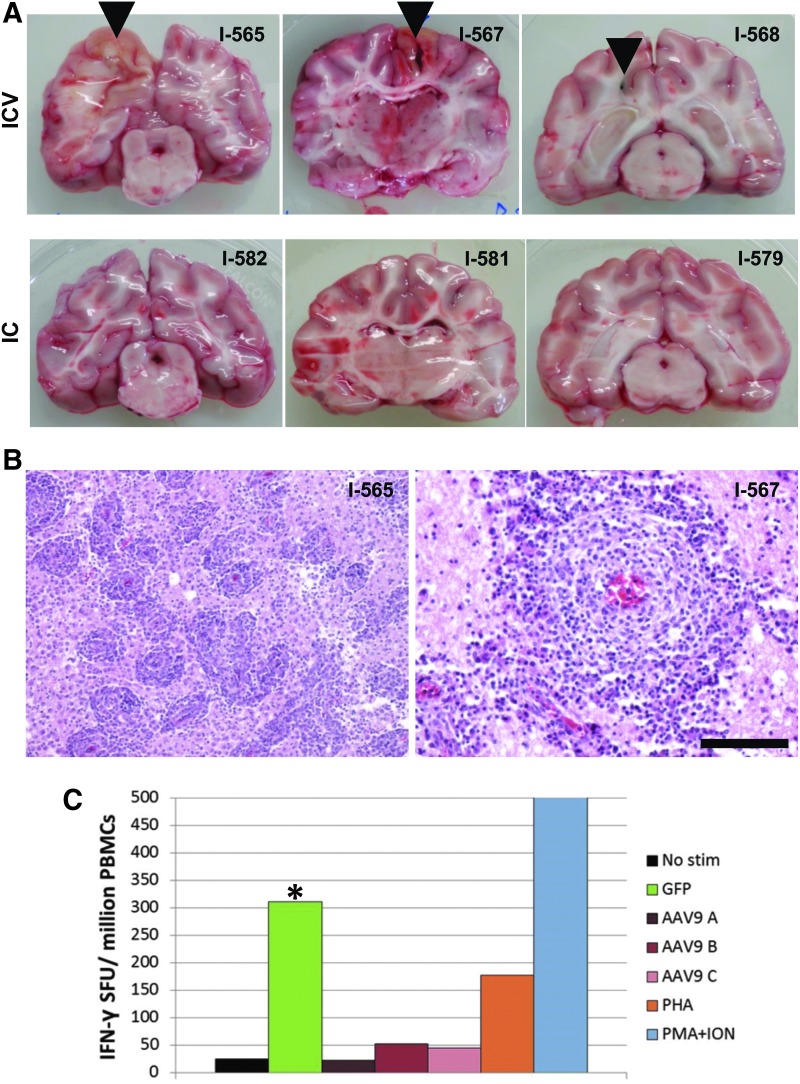
Figure 1.
Encephalitis and transgene-specific T-cell responses in dogs treated with ICV AAV9. One-year-old MPS I dogs were treated with a single ICV or IC injection of an AAV9 vector expressing GFP. All animals were sacrificed 14 days after injection, except for I-567, which was found dead 12 days after injection. (A) Brains were divided into coronal sections, which revealed gross lesions near the injection site (arrowheads) in ICV-treated animals. (B) Tissue sections from the brain regions surrounding the gross lesions were stained with hematoxylin and eosin. Representative images are shown from animals I-565 (left) and I-567 (right). Scale bar: 500 μm (left); 200 μm (right). (C) Peripheral blood mononuclear cells were collected from one ICV-treated dog (I-565) at the time of necropsy, and T-cell responses against the AAV9 capsid and GFP were measured by interferon-γ ELISPOT. T-cell responses to the GFP transgene product were measured using a single pool of overlapping 15-amino acid peptides covering the full GFP sequence. The peptides comprising the AAV9 capsid protein were divided into three pools (designated pools A–C). *Positive response, defined as >3-fold background (unstimulated cells) and greater than 55 spots per million cells. Phytohemagglutinin (PHA) and ionomycin with phorbol 12-myristate 13-acetate (PMA+ION) served as positive controls for T-cell activation. IC, intracisternal; ICV, intraventricular; MPS I, mucopolysaccharidosis type I; PBMCs, peripheral blood mononuclear cells.
Figure 2.
Vector biodistribution in dogs treated with ICV or IC AAV9. Dogs were sacrificed 14 days after receiving a single ICV or IC injection of an AAV9 vector expressing GFP, except for animal I-567, which was necropsied 12 days after injection. Vector genomes were detected in tissue samples by quantitative PCR. Values are expressed as vector genome copies per diploid cell (GC/diploid genome). Brain samples collected from the hippocampus or cerebral cortex are indicated as either the injected or uninjected hemisphere for the ICV-treated dogs; for the IC-treated animals, these are the right and left hemispheres, respectively. Samples were not collected for PCR from the injected cerebral hemisphere of animal I-567.
Figure 3.
GFP expression in brain and spinal cord of dogs treated with ICV or IC AAV9. GFP expression was evaluated by direct fluorescence microscopy of brain and spinal cord samples collected from dogs treated by ICV or IC injection of an AAV9 vector expressing GFP. Representative sections are shown for samples of frontal cortex, and from the anterior horn of the spinal cord collected at the cervical, thoracic, and lumbar levels. Scale bar: 200 μm.
The three dogs treated with vector injected ICV initially appeared healthy after the procedure. However, one animal (I-567) was found dead 12 days after injection. The other two animals survived to the designated day 14 necropsy time point, although one animal (I-565) became stuporous before euthanasia, and the other (I-568) began to exhibit weakness of facial muscles. These clinical findings correlated with significant gross brain lesions (Fig. 1A). Brains from all three animals exhibited discoloration surrounding the needle track, with associated hemorrhage in the animal that was found dead. Histological evaluation revealed severe lymphocytic inflammation in the region surrounding the injection site. Perivascular lymphocytic infiltration was also observed throughout the brain of each animal (Fig. 1B). Given this evidence for immunological toxicity, T-cell responses to both the AAV9 capsid protein and the GFP transgene were evaluated in peripheral blood samples collected from one of the ICV-treated dogs (I-565) at the time of necropsy. An interferon-γ ELISPOT showed a strong T-cell response directed against GFP, with no evidence of a response to capsid peptides (Fig. 1C). This suggests that the encephalitis observed was caused by a cell-mediated immune response against the transgene product.
Vector distribution in the ICV-treated animals was similar to that observed in the IC-treated group, although spinal cord transduction was somewhat greater in the IC cohort (Fig. 2). GFP expression was observed throughout the CNS regions examined in the ICV-treated animals (Fig. 3, Supplementary Fig. S1).
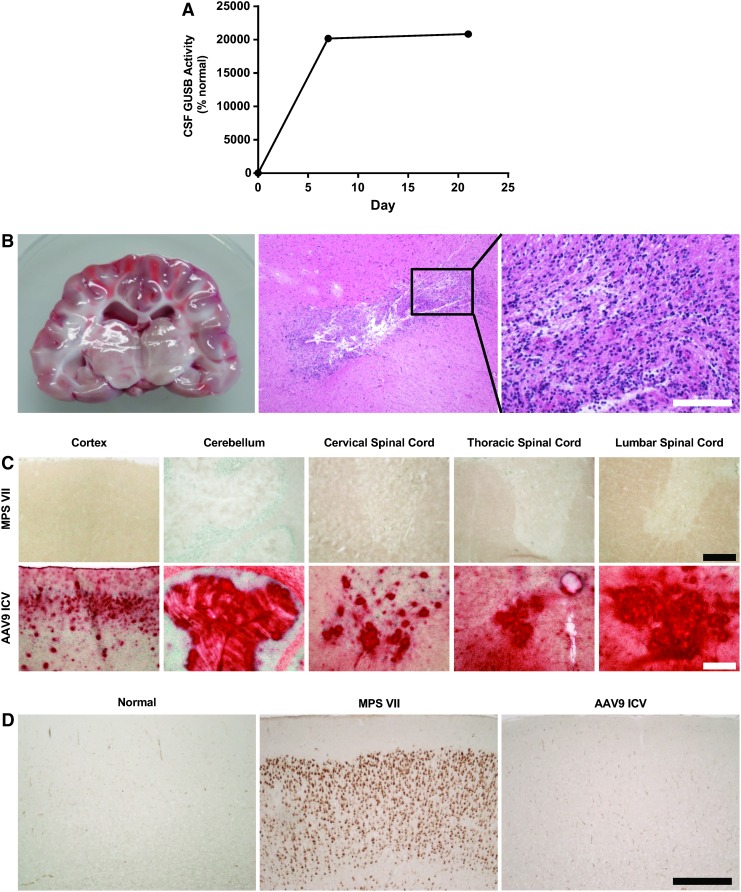
The toxicity associated with ICV administration of an AAV9 vector expressing GFP was consistent with an immune response against the transgene product. Such an immune response might be particularly severe because the GFP transgene is entirely foreign; animals may be more immunologically tolerant to a transgene that is similar to an endogenous protein. We evaluated this possibility in a canine model of the lysosomal storage disease, MPS VII. These animals carry a mutation in the gene encoding the lysosomal enzyme, β-glucuronidase (GUSB), resulting in absence of GUSB activity and intracellular accumulation of GUSB polysaccharide substrates that are normally degraded in the lysosome. The mutant enzyme expressed by these dogs differs from the normal enzyme by a single amino acid, potentially making these animals more tolerant to the normal GUSB protein than to a completely foreign protein such as GFP. Previous studies have demonstrated that these dogs do not develop an immune response to normal canine GUSB when treated with an IC or intravenous injection of an AAV vector expressing the enzyme.8 We treated a 2-month-old MPS VII dog with an ICV injection of AAV9 expressing canine GUSB. The dog exhibited no clinical abnormalities throughout the 3-week in-life phase of the study. GUSB enzyme activity was detectable in CSF at greater than 200-fold normal levels throughout the study (Fig. 4A). At necropsy, there were no gross brain lesions. Histopathology revealed no evidence of generalized encephalitis, although a single lesion with destruction of the brain parenchyma and lymphocytic infiltration was observed adjacent to the injection site, consistent with a needle track (Fig. 4B). GUSB enzyme distribution was evaluated in brain and spinal cord tissue samples using an activity stain, which demonstrated active GUSB (in red) throughout the cerebrum, cerebellum, and spinal cord (Fig. 4C). Purkinje cells of the cerebellum and alpha motor neurons of the spinal cord demonstrated particularly strong transgene expression. To evaluate the efficacy of gene transfer, brain sections were immunostained for the ganglioside GM3, which pathologically accumulates in neurons in MPS VII (Fig. 4D). The treated animal demonstrated complete clearance of cortical GM3.
Figure 4.
Stable transgene expression and absence of encephalitis in an MPS VII dog treated with ICV AAV9 expressing β-glucuronidase (GUSB). A 6-week-old dog with genetic deficiency of GUSB (a model of MPS VII) was treated with a single ICV injection of an AAV9 vector expressing GUSB. (A) GUSB enzyme activity was measured in CSF samples collected at the time of injection and on days 7 and 21 after injection. GUSB activity is represented as the percentage of the mean activity in CSF samples from six normal controls. The dog was sacrificed 3 weeks after injection. (B) Gross and microscopic evaluation of the brain regions surrounding the injection site was performed. Scale bar: 500 μm (middle); 200 μm (right). (C) GUSB activity was detected in brain and spinal cord sections, using a substrate that produces a red product when cleaved by active GUSB. Representative sections are shown for samples of cerebral cortex, cerebellum, and the anterior horn of the spinal cord collected at the cervical, thoracic, and lumbar levels. Scale bars: 500 μm (cortex and cerebellum); 200 μm (spinal cord). (D) Sections of cerebral cortex collected from an untreated MPS VII dog, a normal dog, and the MPS VII dog treated with ICV AAV9 were stained for the ganglioside GM3, which pathologically accumulates in the brains of MPS VII dogs. Scale bar: 500 μm.
Impact of Trendelenburg position on CNS transduction after AAV9 administration by lumbar puncture in NHPs
We previously compared AAV9 injection into the cisterna magna or lumbar cistern of NHPs and found that the lumbar route was 10-fold less efficient for targeting the spinal cord and 100-fold less efficient for targeting the brain.11 Other investigators have since demonstrated better transduction using AAV9 administration by lumbar puncture, with improvements in cranial distribution of the vector achieved by placing animals in the Trendelenburg position after injection.13 In this approach, the vector is diluted into an excess volume of contrast material to increase the density of the solution and promote gravity-driven distribution while in the Trendelenburg position.
Six adult cynomolgus monkeys were treated with a single injection of AAV9 expressing GFP (2 × 1013 GC) in the L3–4 interspace. The vector was diluted to a final volume of 5 ml in iohexol 180 contrast material. Four of the animals were positioned with the head of the procedure table at a −30 degree angle for 10 min immediately after injection. After 10 min, fluoroscopic images were captured to verify contrast distribution in the CSF. Notably, with this large injection volume (approximately 40% of the total CSF volume of the animal)18 contrast material was rapidly distributed along the entire spinal subarachnoid space and into the basal cisterns even in animals that were not placed in the Trendelenburg position (Fig. 5).
Figure 5.
Contrast distribution after lumbar intrathecal injection in nonhuman primates (NHPs). Adult cynomolgus macaques received an intrathecal injection via lumbar puncture of an AAV9 vector diluted in 5 ml of iohexol 180. The distribution of contrast along the spinal cord was evaluated by fluoroscopy. Representative images of the thoracic (left) and cervical (right) regions are shown for an animal that was not placed in the Trendelenburg position. Contrast material (arrowheads) was visible along the entire length of the spinal cord within 10 min of injection in all animals.
Analysis of vector genome distribution by PCR (Fig. 6) and GFP expression (Fig. 7) demonstrated transduction throughout the brain and spinal cord. There was no apparent impact of postinjection positioning on the number or distribution of transduced cells.
Figure 6.
Vector biodistribution in NHPs treated with intrathecal AAV9. NHPs were sacrificed 14 days after intrathecal injection via lumbar puncture of an AAV9 vector diluted in 5 ml of iohexol 180. Four of the animals were placed in the Trendelenburg position for 10 min after injection. Vector genomes were detected in tissue samples by quantitative PCR. Values are expressed as vector genome copies per diploid cell (GC/diploid genome).
Figure 7.
GFP expression in brain and spinal cord of NHPs treated with intrathecal AAV9. GFP expression was evaluated by direct fluorescence microscopy of brain and spinal cord samples collected from NHPs treated by intrathecal injection of an AAV9 vector. The vector was administered by lumbar puncture. Four of the animals were placed in the Trendelenburg position for 10 min after injection. Representative sections are shown for samples of frontal cortex, and from the anterior horn of the spinal cord collected at the cervical, thoracic, and lumbar levels. Because of the presence of autofluorescent material in some NHP tissues, red channel images were captured to differentiate autofluorescence from GFP signal. Autofluorescence images are overlaid in magenta. Scale bar: 500 μm (cortex); 200 μm (spinal cord).
As previously reported, there was vector escape to the periphery and hepatic transduction after intrathecal AAV administration.11,14 The extent of liver transduction was dependent on the presence of preexisting neutralizing antibodies (NAbs) against AAV9. Four of six animals had no detectable baseline AAV9 NAbs (titer, <1:5), and two animals (4051 and 07-11) had detectable preexisting antibodies to AAV9, with a titer of 1:40. Consistent with previous results, preexisting antibodies blocked liver transduction, and resulted in increased vector distribution to the spleen,17 but had no impact on CNS transduction.7,14
Discussion
Gene therapy using AAV vectors has shown promise for the treatment of a wide range of diseases of the CNS in animal models, and initial human studies support the safety of this approach. Achieving optimal results in patients will ultimately require reconciling the most effective delivery approaches that can be identified in preclinical studies with methods that can be readily deployed in the clinic. Initially, we identified IC injection via suboccipital puncture as an ideal method of vector delivery in dog, cat, and primate models.9,11,16 However, because suboccipital puncture is not a common procedure in clinical practice, we continued to evaluate more routine sites of CSF access, including the lateral ventricle and the lumbar cistern. We previously evaluated AAV administration through a lumbar puncture in NHPs with limited efficacy; here, we evaluated an improved method reported in the literature, employing vector solutions with higher density and postinjection Trendelenburg positioning to improve vector distribution cranially from the lumbar cistern.
In the dog study, both IC and ICV vector injection yielded similarly effective vector distribution, but encephalitis occurred only in the ICV group. A T-cell response against the GFP transgene was detectable in the one ICV-treated dog evaluated, suggesting that the lymphocytic encephalitis observed in these animals was due to a transgene-specific immune response. Induction of a T-cell response to a new antigen requires two elements—recognition of an epitope from the protein by a naive T cell and an inflammatory “danger signal” that promotes activation of the T cell. AAV is believed to be capable of expressing foreign transgenes without eliciting immunity against the transgene product because it does not activate the innate immune system, thereby avoiding inflammatory signals and promoting tolerance, rather than immunity, when naive lymphocytes encounter the newly expressed antigen. Local inflammation caused by the trauma of penetrating the brain parenchyma, occurring at the same location that the foreign transgene product is expressed, may provide the danger signal needed to induce an immune response to the transgene product. This is supported by previous studies in MPS I dogs, which developed cell-mediated immune responses to an enzyme expressed from an AAV vector delivered by direct brain injection but not by IC injection.5,16 The potential for such an immune response will depend on whether the transgene product is recognized as foreign—for delivery of vectors expressing a protein that is also produced endogenously, even an inflammatory response caused by injection may not break tolerance to the self-protein. The results in the study of ICV vector delivery in the MPS VII dog support this concept, as the similarity of the transgene product to an endogenous protein was likely responsible for the absence of the type of T-cell response that was observed to GFP. The same may be true for patients with recessive diseases, who carry missense mutations that allow for production of a protein similar to the transgene product. Risk of immunity could, therefore, vary depending on patient population and transgene product, and in some cases immunosuppression may be necessary to prevent destructive T-cell responses to a transgene product. The present findings suggest that the risk of deleterious immune responses can likely be mitigated by using an IC rather than ICV route of administration.
Three important caveats to this study should be noted. The T-cell response to GFP could be evaluated in only one of the affected animals, and the presence of this response does not prove that it was the cause of the encephalitis observed in the dogs treated by ICV vector injection. Second, although this study demonstrated that ICV vector delivery is less prone to elicit anti-transgene immunity in the context of a GFP transgene, this is an unusually immunogenic protein in mammals, and it is possible that the greater immunogenicity of ICV administration will not be clinically relevant in the context of less immunogenic therapeutic proteins. Finally, the early sacrifice time points employed in this study may impact the findings in several ways. Transduction measured by GFP expression may be underestimated by collecting tissue before GFP levels have reached a stable plateau, whereas transduction measured by vector genome copies may be overestimated by the presence of vector particles that are retained in the tissue but do not represent stable nuclear vector DNA. Because of the early sacrifice time point, it is also not clear whether the T-cell response against GFP would have ultimately eliminated the transduced cell population.
We previously compared the IC route of vector administration with a lumbar puncture approach in nonhuman primates and found that IC delivery much more efficiently targeted the brain and spinal cord.11 A subsequent report demonstrated that the efficiency of the lumbar puncture approach could be improved by placing animals in the Trendelenburg position after injection.13 In contrast to this report, we found no additional benefit of placing animals in the Trendelenburg position after lumbar vector injection. The reason for this discrepancy is not clear. Both studies used an AAV9 vector suspended in iodinated contrast material, and both were performed in cynomolgus macaques. However, the previous report was performed in 1-year-old animals with an average body mass of 2 kg, and it is possible that rostral vector spread from a lumbar injection is more efficient in these smaller animals.
Together, these findings support vector administration at the level of the cisterna magna, as this approach achieves more efficient vector distribution than administration via lumbar puncture and appears to carry less risk of immunity to the transgene product than ICV administration. Vector delivery to the cisterna magna could be carried out clinically by the suboccipital puncture approach that was used in preclinical studies. In addition, injection into the subarachnoid space between the first and second cervical vertebra, using a lateral approach (C1–2 puncture), is likely to produce similar vector distribution given the proximity of the injection site to the cisterna magna. The C1–2 approach has the additional advantage that, unlike suboccipital puncture, it is more widely used clinically for CSF access, particularly for intrathecal contrast administration for myelography.
Supplementary Material
Acknowledgments
The authors acknowledge the support of the Vector Core and Immunology Core at the Gene Therapy Program (University of Pennsylvania). The authors also thank Jennifer Stewart for editorial assistance with this manuscript. This work was supported by a grant from REGENXBIO (J.M.W.) and NIH grants 5R01DK054481 (M.L.C.) and 5P40OD010939 (C.H.V.).
Author Disclosure
J.M. Wilson is an advisor to REGENXBIO, Dimension Therapeutics, and Solid Gene Therapy, and is a founder of, holds equity in, and has a sponsored research agreement with REGENXBIO and Dimension Therapeutics; in addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. M.E.H. is a stockholder of BioMarin Pharmaceuticals. The remaining co-authors do not have any conflicts of interest to disclose.
References
- 1.Hadaczek P, Eberling JL, Pivirotto P, et al. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther 2010;18:1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittermeyer G, Christine CW, Rosenbluth KH, et al. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum Gene Ther 2012;23:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao GP, Vandenberghe LH, Alvira MR, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray SJ, Matagne V, Bachaboina L, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 2011;19:1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciron C, Desmaris N, Colle MA, et al. Gene therapy of the brain in the dog model of Hurler's syndrome. Ann Neurol 2006;60:204–213 [DOI] [PubMed] [Google Scholar]
- 6.Vite CH, McGowan JC, Niogi SN, et al. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol 2005;57:355–364 [DOI] [PubMed] [Google Scholar]
- 7.Gray SJ, Kalburgi SN, McCown TJ, et al. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther 2013;20:450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurda BL, De Guilhem De Lataillade A, Bell P, et al. Evaluation of AAV-mediated gene therapy for central nervous system disease in canine mucopolysaccharidosis VII. Mol Ther 2016;24:206–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinderer C, Bell P, Gurda BL, et al. Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol Ther 2014;22:2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passini MA, Bu J, Richards AM, et al. Translational fidelity of intrathecal delivery of self-complementary AAV9-survival motor neuron 1 for spinal muscular atrophy. Hum Gene Ther 2014;25:619–630 [DOI] [PubMed] [Google Scholar]
- 11.Hinderer C, Bell P, Vite CH, et al. Widespread gene transfer in the central nervous system of cynomolgus macaques following delivery of AAV9 into the cisterna magna. Mol Ther Methods Clin Dev 2014;1:14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucher T, Dubreil L, Colle MA, et al. Intracisternal delivery of AAV9 results in oligodendrocyte and motor neuron transduction in the whole central nervous system of cats. Gene Ther 2014;21:522–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer K, Ferraiuolo L, Schmelzer L, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose–response study in mice and nonhuman primates. Mol Ther 2015;23:477–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haurigot V, Marco S, Ribera A, et al. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest 2013;123:3254–3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lock M, Alvira M, Vandenberghe LH, et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther 2010;21:1259–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinderer C, Bell P, Louboutin JP, et al. Neonatal systemic AAV induces tolerance to CNS gene therapy in MPS I dogs and nonhuman primates. Mol Ther 2015;23:1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LL, Calcedo R, Bell P, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 2011;22:1389–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieselbach RE, Chiro GD, Freireich EJ, et al. Subarachnoid distribution of drugs after lumbar injection. N Engl J Med 1962;267:1273–1278 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.