Abstract
Mosquito-borne viruses, including Zika virus (ZIKV) and dengue virus (DENV), are global threats that continue to infect millions annually. Historically, efforts to combat the spread of these diseases have sought to eradicate the mosquito population. This has had limited success. Recent efforts to combat the spread of these diseases have targeted the mosquito population and the mosquito's ability to transmit viruses by altering the mosquito's microbiome. The introduction of particular strains of Wolbachia bacteria into mosquitos suppresses viral growth and blocks disease transmission. This novel strategy is being tested worldwide to reduce DENV and has early indications of success. The Wolbachia genus comprised divergent strains that are divided in major phylogenetic clades termed supergroups. All Wolbachia field trials currently utilize supergroup A Wolbachia in Aedes aegypti mosquitos to limit virus transmission. Here we discuss our studies of Wolbachia strains not yet used in virus control strategies but that show strong potential to reduce ZIKV replication. These strains are important opportunities in the search for novel tools to reduce the levels of mosquito-borne viruses and provide additional models for mechanistic studies.
Keywords: : arbovirus, Wolbachia, vector control, emerging viruses, Zika virus, dengue virus
Introduction
Mosquito-transmitted viruses are a global concern due to increasing incidence and geographic range. Although these viruses have been identified for decades, we still lack proper treatment and control. Dengue virus (DENV) cases have doubled every decade since 1900 and expanded geographically such that four DENV serotypes can be found throughout Europe, Asia, Africa, and the Americas (Messina et al., 2014; Sharp et al., 2017). Likewise, Chikungunya virus (CHIKV) has caused a greater rather than diminishing threat over time (Pybus et al., 2015). Recently, Zika virus (ZIKV) has shown us just how quickly a new outbreak of mosquito-transmitted disease can spread. ZIKV was introduced into Brazil in 2013 (Faria et al., 2016) and is now endemic throughout the Americas causing devastating birth defects (Fauci and Morens, 2016). DENV, CHIKV, and ZIKV are all transmitted by Aedes sp. mosquitos, including Aedes aegypti and Aedes albopictus. Our inability to stop the spread of these diseases emphasizes the need to control the disease vector in addition to virus-specific efforts.
Common Mosquito Control Strategies
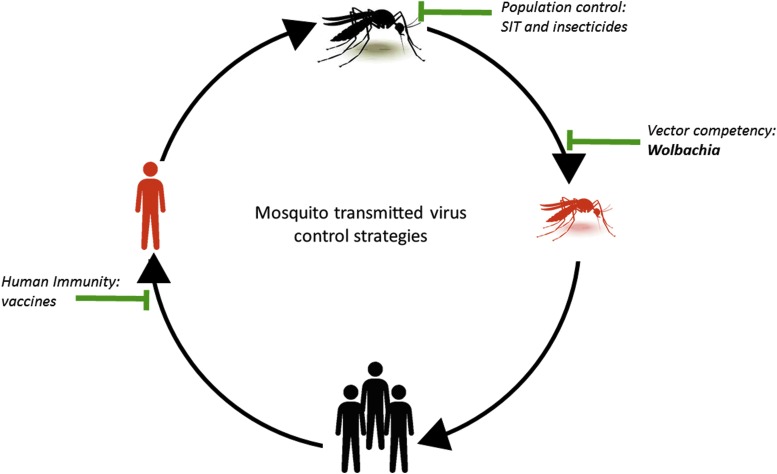
Targeting the mosquito vector by decreasing the mosquito population decreases disease spread without the timely cost of vaccination, appealing to the immediate need to stop these viruses. Strategies to reduce total mosquito populations include the use of insecticides or sterile insect technique (SIT) (Fig. 1). Insecticides are efficient chemicals to kill larvae and adult mosquitos but have high environmental and health costs. They also require regular innovation to counterbalance the emergence of resistance in insects [reviewed by Liu (2015) and Moyes et al. (2017)]. SIT sterilizes males or limits the development of offspring by irradiating males (Rodriguez et al., 2013; Yamada et al., 2014; Dandalo et al., 2017) or genetically modifies reproductive genes (Catteruccia et al., 2009), respectively. SIT requires costly annual release of sterile males to prevent populations from rebounding, suggesting alternative sustainable strategies are needed.
FIG. 1.
Mosquito-transmitted virus control strategies target multiple stages of viral transmission: vaccination of human host, suppression of mosquito population (SIT—insecticides and transgenesis), and vector competency. Several strains of the intracellular bacteria Wolbachia suppress viral growth in mosquitos, blocking their competency to transmit several arboviruses, including DENV and more recently ZIKV. Orange indicates virus-infected mosquito or human. DENV, dengue virus; SIT, sterile insect technique; ZIKV, Zika virus.
Wolbachia-Based Vector Control Approaches
Recent novel control strategies have focused on a self-sustaining method using the bacteria Wolbachia pipientis to restrict virus transmission (Fig. 1). Wolbachia-infected female mosquitos have reduced capacity to transmit pathogens [reviewed by Caragata et al. (2016)]. Wolbachia are maternally transmitted from mother to offspring suggesting this strategy could be widely effective. Some mosquitos, including A. albopictus (Armbruster et al., 2003), A. fluviatilis (Baton et al., 2013), and Culex pipiens (Hertig, 1936), are naturally infected with their own strains of Wolbachia. These native infections can limit virus replication (Glaser and Meola, 2010; Mousson et al., 2012; Raquin et al., 2015). However, A. aegypti, a prominent vector of DENV, CHIKV, and ZIKV is naturally devoid of Wolbachia. wMel native to Drosophila melanogaster has been successfully introduced into Aedes sp. to limit virus replication (Fragkoudis et al., 2009; Walker et al., 2011; Joubert et al., 2016). wMel has been described to inhibit DENV (Walker et al., 2011; Blagrove et al., 2012; Ye et al., 2015), ZIKV (Aliota et al., 2016a; Dutra et al., 2016), and CHIKV (Blagrove et al., 2013; Aliota et al., 2016b), demonstrating the broad reaching potential of Wolbachia-mediated virus control. As a result, A. aegypti mosquitos infected with wMel are being released in a worldwide effort to control arboviruses.
Maternal transmission of Wolbachia drives an intimate relationship between Wolbachia and its host resulting in coevolution (Shaikevich and Zakharov, 2014) and promoting high diversity in Wolbachia phylogeny. The wMel Wolbachia strain is part of a distinct clade termed supergroup A. Recent studies in A. aegypti mosquitos demonstrate that under natural cyclical heat stress, wMel has reduced maternal transmission and cytoplasmic incompatibility, a form of reproductive manipulation that favors infected females (Ross et al., 2017). This sensitivity of the wMel strain to cyclical heat stress reduces the ability of this strain to integrate into large populations in certain regions and jeopardizes the success of utilizing this strain for vector control in A. aegypti. In contrast, maternal transmission of supergroup B Wolbachia, such as wAlbB, was unaffected by cycling temperatures suggesting that it or another Wolbachia strain from supergroup B would be better sustained in a mosquito population and more likely to succeed in Wolbachia-based interventions.
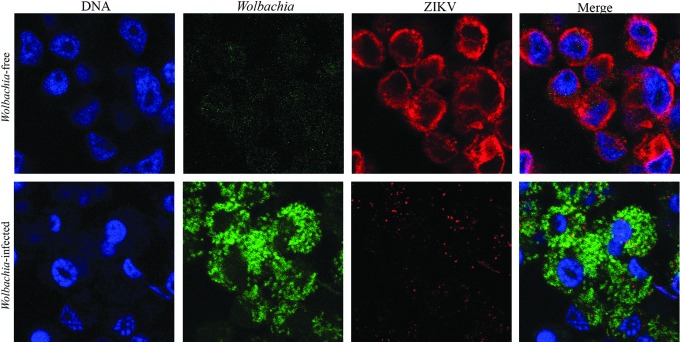
Most studies of Wolbachia suppression of ZIKV have previously been limited to supergroup A's wMel. However, recent work by Schultz et al. (2017) assessed supergroup B Wolbachia strain's potential to limit ZIKV. Two supergroup B Wolbachia wAlbB and wStri in A. albopictus cells were shown to reduce ZIKV growth. wAlbB, a native infection of these cells isolated from A. albopictus mosquitos, limited African ZIKV growth by 90% and Puerto Rican ZIKV growth by 99.9%. wStri, a strain isolated from Laodelphax striatellus (a leafhopper) and infected into A. albopictus cells to form a non-native infection reduced African and Puerto Rican ZIKV by greater than 99.99% below the limit of detection (Fig. 2). It was previously shown that introduction of a non-native Wolbachia infection promotes a stronger antiviral response (Bian et al., 2013). Furthermore, in A. albopictus mosquitos, wAlbB causes a moderate inhibition of DENV (Lu et al., 2012; Mousson et al., 2012), CHIKV (Raquin et al., 2015), and a pronounced repression of DENV in its non-native host: A. aegypti (Bian et al., 2010; Joubert et al., 2016). Thus, increased viral protection by wStri may be because it is not native to A. albopictus.
FIG. 2.
Wolbachia wStri-infected cells are resistant to ZIKV infection. Aedes mosquito cells infected with Wolbachia bacteria (stained in green) do not support ZIKV growth (shown in red).
Wolbachia density is also important to sustained virus protection in mosquitos. wAlbB repression of DENV has previously been shown to be dependent on wAlbB density (Lu et al., 2012). wStri-infected cells carried two to three times more Wolbachia- than wAlbB-infected cells (Schultz et al., 2017). Reduced wStri density resulted in increased viral growth suggesting a Wolbachia density-specific repression of ZIKV. Low Wolbachia titers have been suggested to be problematic in whole mosquitos, and superinfection of mosquitos with wMel and wAlbB has been shown to sustain overall Wolbachia titers in A. aegypti mosquitos while repressing DENV (Joubert et al., 2016). Further studies should investigate wStri concentration in A. aegypti mosquitos and if superinfection with wStri exacerbates antiviral phenotypes.
Mechanistic Insights into Wolbachia Viral Suppression
The expansion of the repertoire of Wolbachia strains available in cell lines has allowed for the development of multiple in vitro models to investigate the mechanism of Wolbachia-mediated viral repression. Early viral inhibition by different Wolbachia strains has been shown for three viruses. Consistent with alphavirus studies of Semliki Forest virus by Rainey et al. (2016) and Sindbis virus by Bhattacharya et al. (2017) in Drosophila cells, Schultz et al. (2017) showed ZIKV repression occurs at or before viral replication in mosquito cells. These data move the field forward toward a molecular mechanism of viral repression, involving entry, viral translation, or genome replication.
Immune priming by Wolbachia may stimulate innate defense (IMD, Toll, and small interfering RNAs) to repress viral replication (Rancès et al., 2012). Conflicting data negate (Rancès et al., 2013) or implicate (Pan et al., 2012) IMD and Toll-mediated virus protection by the stimulation of reactive oxygen species to repress virus growth. There is evidence that RNAi is not required for viral suppression (Hedges et al., 2012), but it may participate in enhancing the antiviral response (Terradas et al., 2017). Together, these studies show that innate immunity may promote protection from viruses but additional mechanisms are likely.
Competition between Wolbachia and the virus for host factors such as amino acids, cholesterol (Caragata et al., 2014), and host lipids (Molloy et al., 2016) may also facilitate viral inhibition. Cholesterol and lipids are important for virus entry, replication, and assembly (Stiasny et al., 2003; Mazzon and Mercer, 2014), thus may be required by both organisms. In the model system, D. melanogaster, feeding flies with cholesterol has been shown to rescue Drosophila C virus (DCV) growth in the presence of Wolbachia implying that Wolbachia was sequestering cholesterol from DCV (Caragata et al., 2013). Schultz et al. (2017) tested if cholesterol was also playing a role in Wolbachia suppression of ZIKV in mosquito cells. Cholesterol supplementation rescues ZIKV growth supporting this hypothesis. However, this rescue was incomplete, again suggesting multiple mechanisms of repression of viruses by Wolbachia working simultaneously.
A third mechanism proposed is by modulation of methylation patterns. Wolbachia disrupts global methylation of its host genome and RNA (Ye et al., 2013). RNA methylation is a means to control viral translation and genome replication (Lichinchi et al., 2016). Dnmt2 is RNA methyltransferase dysregulated by Wolbachia. Dnmt2 has been shown to be upregulated by Wolbachia to repress Sindbis virus (an alphavirus similar to CHIKV) growth (Bhattacharya et al., 2017) in D. melanogaster. However, in A. aegypti, Dnmt2 has been shown to be downregulated by Wolbachia limiting the growth of DENV (Zhang et al., 2013). These contradicting results may be due to different methylation control of alphaviruses and flaviviruses suggesting a virus family-specific mechanism or due to different host organisms. Further studies are needed to elucidate the mechanisms of Wolbachia-mediated virus suppression.
Wolbachia release studies have shown promise in implementation (Hoffmann et al., 2011). Release strategies are currently focusing on optimizing when and how many Wolbachia-infected mosquitos to release to successfully incorporate Wolbachia into a population. Using small-scale releases, optimal release quantities have been determined (Schmidt et al., 2017). Future efforts should aim to test Wolbachia-mediated virus control by group B Wolbachia, specifically wStri in A. aegypti cells and in vivo. Further in vitro and in vivo studies to delineate the multifaceted mechanisms of Wolbachia-mediated virus suppression with different Wolbachia strains and hosts are needed. This knowledge will aid in the development of novel strategies to reduce transmission of pathogens by insects. The release of Wolbachia-infected females is not yet approved in the United States. Field studies showing repression of Wolbachia-infected mosquitos to control DENV will inform the U.S. approval of virus control efforts.
Disclosure Statement
No competing financial interests exist.
References
- Aliota M.T., Peinado S.A., Velez I.D., and Osorio J.E. (2016a). The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci Rep 6, 28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota M.T., Walker E.C., Yepes Uribe A., Velez I.D., Christensen B.M., and Osorio J.E. (2016b). The wMel strain of Wolbachia reduces transmission of Chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis 10, e0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster P., Damsky W.E., Jr., Giordano R., Birungi J., Munstermann L.E., and Conn J.E. (2003). Infection of new- and old-world Aedes albopictus (Diptera: Culicidae) by the intracellular parasite Wolbachia: implications for host mitochondrial DNA evolution. J Med Entomol 40, 356–360 [DOI] [PubMed] [Google Scholar]
- Baton L.A., Pacidonio E.C., Goncalves D.S., and Moreira L.A. (2013). wFlu: characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS One 8, e59619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya T., Newton I.L.G., and Hardy R.W. (2017). Wolbachia elevates host methyltransferase expression to block an RNA virus early during infection. PLoS Pathog 13, e1006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G., Xu Y., Lu P., Xie Y., and Xi Z. (2010). The endosymbiotic bacterium wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6, e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G., Zhou G., Lu P., and Xi Z. (2013). Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to dengue virus in a mosquito vector. PLoS Negl Trop Dis 7, e2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove M.S.C., Arias-Goeta C., Di Genua C., Failloux A.-B., and Sinkins S.P. (2013). A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits Chikungunya virus. PLoS Negl Trop Dis 7, e2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagrove M.S.C., Arias-Goeta C., Failloux A.-B., and Sinkins S.P. (2012). Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci 109, 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata E.P., Dutra H.L.C., and Moreira L.A. (2016). Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol 32, 207–218 [DOI] [PubMed] [Google Scholar]
- Caragata E.P., Rancès E., Hedges L.M., Gofton A.W., Johnson K.N., O'Neill S.L., et al. (2013). Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9, e1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata E.P., Rances E., O'Neill S.L., and McGraw E.A. (2014). Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb Ecol 67, 205–218 [DOI] [PubMed] [Google Scholar]
- Catteruccia F., Crisanti A., and Wimmer E.A. (2009). Transgenic technologies to induce sterility. Malaria J 8 Suppl 2, S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandalo L.C., Kemp A., Koekemoer L.L., and Munhenga G. (2017). Effect of ionising (gamma) radiation on female Anopheles arabiensis. Trans R Soc Trop Med Hyg 111, 38–40 [DOI] [PubMed] [Google Scholar]
- Dutra H.L., Rocha M.N., Dias F.B., Mansur S.B., Caragata E.P., and Moreira L.A. (2016). Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host & Microb 19, 771–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria N.R., Azevedo R., Kraemer M.U.G., Souza R., Cunha M.S., Hill S.C., et al. (2016). Zika virus in the Americas: early epidemiological and genetic findings. Science 352, 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S., and Morens D.M. (2016). Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 374, 601–604 [DOI] [PubMed] [Google Scholar]
- Fragkoudis R., Attarzadeh-Yazdi G., Nash A.A., Fazakerley J.K., and Kohl A. (2009). Advances in dissecting mosquito innate immune responses to arbovirus infection. J Gen Virol 90, 2061–2072 [DOI] [PubMed] [Google Scholar]
- Glaser R.L., and Meola M.A. (2010). The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 5, e11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L.M., Yamada R., O'Neill S.L., and Johnson K.N. (2012). The small interfering RNA pathway is not essential for Wolbachia-mediated antiviral protection in Drosophila melanogaster. Appl Environ Microbiol 78, 6773–6776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertig M. (1936). The Rickettsia, Wolbachia pipientis (gen. et sp.n.) and associated inclusions of the mosquito, Culex pipiens. Parasitology 28, 453–486 [Google Scholar]
- Hoffmann A.A., Montgomery B.L., Popovici J., Iturbe-Ormaetxe I., Johnson P.H., Muzzi F., et al. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457 [DOI] [PubMed] [Google Scholar]
- Joubert D.A., Walker T., Carrington L.B., De Bruyne J.T., Kien D.H.T., Hoang N.L.T., et al. (2016). Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog 12, e1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichinchi G., Zhao B.S., Wu Y., Lu Z., Qin Y., He C., et al. (2016). Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microb 20, 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N. (2015). Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol 60, 537–559 [DOI] [PubMed] [Google Scholar]
- Lu P., Bian G., Pan X., and Xi Z. (2012). Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis 6, e1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon M., and Mercer J. (2014). Lipid interactions during virus entry and infection. Cell Microbiol 16, 1493–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina J.P., Brady O.J., Scott T.W., Zou C., Pigott D.M., Duda K.A., et al. (2014). Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 22, 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy J.C., Sommer U., Viant M.R., and Sinkins S.P. (2016). Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl Environ Microbiol 82, 3109–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousson L., Zouache K., Arias-Goeta C., Raquin V., Mavingui P., and Failloux A.-B. (2012). The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl Trop Dis 6, e1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes C.L., Vontas J., Martins A.J., Ng L.C., Koou S.Y., Dusfour I., et al. (2017). Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis 11, e0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhou G., Wu J., Bian G., Lu P., Raikhel A.S., et al. (2012). Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci 109, E23–E31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pybus O.G., Tatem A.J., and Lemey P. (2015). Virus evolution and transmission in an ever more connected world. Proc Biol Sci 282, 20142878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey S.M., Martinez J., McFarlane M., Juneja P., Sarkies P., Lulla A., et al. (2016). Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathog 12, e1005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancès E., Johnson T.K., Popovici J., Iturbe-Ormaetxe I., Zakir T., Warr C.G., et al. (2013). The Toll and Imd pathways are not required for Wolbachia-mediated dengue virus interference. J Virol 87, 11945–11949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancès E., Ye Y.H., Woolfit M., McGraw E.A., and O'Neill S.L. (2012). The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8, e1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raquin V., Moro Valiente C., Saucereau Y., Tran F.-H., Potier P., and Mavingui P. (2015). Native Wolbachia from Aedes albopictus blocks Chikungunya virus infection in cellulo. PLoS One 10, e0125066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez S.D., Brar R.K., Drake L.L., Drumm H.E., Price D.P., Hammond J.I., et al. (2013). The effect of the radio-protective agents ethanol, trimethylglycine, and beer on survival of X-ray-sterilized male Aedes aegypti. Parasit Vect 6, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P.A., Wiwatanaratanabutr I., Axford J.K., White V.L., Endersby-Harshman N.M., and Hoffmann A.A. (2017). Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog 13, e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T.L., Barton N.H., Rašić G., Turley A.P., Montgomery B.L., Iturbe-Ormaetxe I., et al. (2017). Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol 15, e2001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M.J., Isern S., Michael S.F., Corley R.B., Connor J., and Frydman H.M. (2017). Variable inhibition of Zika virus replication by different Wolbachia strains in mosquito cell cultures. J Virol 91, e00339–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikevich E.V., and Zakharov I.A. (2014). [Coevolution of symbiotic bacteria Wolbachia and host mtDNA in Russian populations of the Culex pipiens mosquito complex]. Genetika 50, 1390–1393 [PubMed] [Google Scholar]
- Sharp T.M., Tomashek K.M., Read J.S., Margolis H.S., and Waterman S.H. (2017). A new look at an old disease: recent insights into the global epidemiology of dengue. Curr Epidemiol Rep 4, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiasny K., Koessl C., and Heinz F.X. (2003). Involvement of lipids in different steps of the flavivirus fusion mechanism. J Virol 77, 7856–7862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradas G., Joubert D.A., and McGraw E.A. (2017). The RNAi pathway plays a small part in Wolbachia-mediated blocking of dengue virus in mosquito cells. Sci Rep 7, 43847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T., Johnson P.H., Moreira L.A., Iturbe-Ormaetxe I., Frentiu F.D., McMeniman C.J., et al. (2011). The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453 [DOI] [PubMed] [Google Scholar]
- Yamada H., Parker A.G., Oliva C.F., Balestrino F., and Gilles J.R. (2014). X-ray-induced sterility in Aedes albopictus (Diptera: Culicidae) and male longevity following irradiation. J Med Entomol 51, 811–816 [DOI] [PubMed] [Google Scholar]
- Ye Y.H., Carrasco A.M., Frentiu F.D., Chenoweth S.F., Beebe N.W., van den Hurk A.F., et al. (2015). Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl Trop Dis 9, e0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y.H., Woolfit M., Huttley G.A., Rancès E., Caragata E.P., Popovici J., et al. (2013). Infection with a virulent strain of Wolbachia disrupts genome wide-patterns of cytosine methylation in the mosquito Aedes aegypti. PLoS One 8, e66482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Hussain M., O'Neill S.L., and Asgari S. (2013). Wolbachia uses a host microRNA to regulate transcripts of a methyltransferase, contributing to dengue virus inhibition in Aedes aegypti. Proc Natl Acad Sci 110, 10276–10281 [DOI] [PMC free article] [PubMed] [Google Scholar]