Abstract
Significance: Wound healing requires a highly orchestrated coordination of processes that are not yet fully understood. Therefore, available clinical therapies are thus far limited in their efficacy in preventing and treating both chronic wounds and scars. Current gene-based therapeutics is largely based on our understanding of the protein-coding genome and proteins involved in known wound healing pathways.
Recent Advances: Noncoding RNAs such as microRNAs and long noncoding RNAs have recently been found to be significant modulators of gene expression in diverse cellular pathways. Research has now implicated noncoding RNAs in nearly every stage of the wound healing process, suggesting that they may serve as clinical therapeutic targets. Noncoding RNAs are critical regulators in processes such as angiogenesis and cutaneous cell migration and proliferation, including classically described biological pathways previously attributed to mostly protein constituents.
Critical Issues: The complexity and diversity of the interactions of noncoding RNAs with their targets and other binding partners require thorough characterization and understanding of their functions before they may be altered to modulate human wound healing pathways.
Future Directions: Research in the area of noncoding RNAs continues to rapidly expand our understanding of their potential roles in physiological and pathological wound healing. Coupled with improving technologies to enhance or suppress target noncoding RNA in vivo, these advances hold great promise in the development of new therapies for wound healing.
Keywords: : RNA, gene expression, lncRNA

Scope and Significance
Although there has been significant progress in the molecular understanding, therapeutic options, and prevention strategies related to wound healing, the exact mechanisms underlying the spectrum of pathologies remain unknown. Until very recently, most of these studies have been confined to the world of proteins, such as signaling and growth factors, and the genes that encode for them. However, recent large-scale analyses of the human genome have revealed that 98% of the genome does not, in fact, encode for proteins.1 Fascinatingly, much of the intergenic region is actively transcribed, and members of these classes of noncoding RNA have been found to have critical roles in diverse cellular processes, such as cell differentiation and cancer regulation. However, their specific functions are still largely unexplored and represent a relatively untapped potential source of targets in regenerative medicine and tissue engineering.2,3 Herein, we provide an overview of the research to date regarding the roles, significance, and potential therapeutic benefits of noncoding RNA in wound healing.
Translational Relevance
Traditional therapies for wound healing are often ineffective. Unfortunately, clinical trials of newer experimental biological therapies have also largely failed to demonstrate efficacy in the treatment of chronic wounds. The limited success of administration of factors known to be important in wound healing, such as keratinocyte growth factor and fibroblast growth factor (FGF), suggests that our current understanding of the process of wound healing and how we can modulate that process is still lacking.4 Recently, stem cell-based therapies have shown promise in improving wound healing, but these approaches typically require harvest and potential ex vivo culture, and are subject to significant regulatory hurdles.5 The clinician's limited existing arsenal in wound healing necessitates an improved understanding of the precise mechanisms that direct successful tissue repair and regeneration, and targeted therapies derived from them. Noncoding RNAs offer significant translational promise as therapeutic targets and have been demonstrated to have the potential to be powerful mediators of physiological and pathological pathways.
Clinical Relevance
The sequelae of pathologic wound healing impose a heavy clinical and economic burden. Underhealing, in the form of chronic wounds, is a snowballing public health concern. With the increase in comorbidities such as diabetes, vascular diseases, and obesity, the incidence of chronic, nonhealing wounds continues to rise. It is estimated that chronic wounds affect about 6.5 million patients, and an excess of $25 billion is spent annually for treatment in the United States.6 On the other end of the spectrum is pathologic overhealing in the form of hypertrophic scars and keloids. These challenging disorders can result in pain and itching; contracture and decreased mobility, especially when occurring in the pediatric population or across joints; and devastating psychosocial consequences, particularly when occurring in the face.7,8 Even normal scarring is undesirable, resulting in an estimated annual market for treatment of scarring of $12 billion in the United States alone.6
Background
Overview of the wound healing process
Wound healing is a complex physiological process involving multiple cell types in complex, coordinated interactions that have evolved to quickly protect tissues from further injury and infection at the cost of perfect regeneration. It is classically divided into three overlapping phases—inflammation, proliferation, and remodeling. Within these stages, angiogenesis, reepithelialization, and collagen deposition are critical to healing, and local and systemic factors are known to modulate cell recruitment, proliferation, migration, differentiation, and secretion.9,10 Successful completion of this process reproducibly results in the formation of a fibroproliferative scar. However, this process can proceed pathologically along a spectrum from underhealing to overhealing, both of which can have devastating consequences.11 At each step, the process requires sophisticated, accurate, and synchronized coordination of alterations in gene expression.
Epigenetic regulation of gene expression
The central dogma of molecular biology dictates that the information encoded in DNA is transcribed into RNA, which is then processed and translated into functional proteins. Indeed, proteins such as signaling factors and growth factors are critical in appropriate wound healing. However, the appropriate, accurate, and timely synthesis of these proteins require the orchestration of numerous complex processes that extend beyond the simplicity of this core principle.
Epigenetic regulation refers to the mechanisms by which a cell's genomic message, encoded by its DNA, is modified to produce different phenotypes. Of these mechanisms, the most well characterized are those involving the posttranslational modifications of histone residues and the methylation of DNA. More recently, however, a new area of study in epigenetic regulation has emerged in the form of noncoding RNAs.
The world of noncoding RNAs
Noncoding RNAs, unlike the classic messenger RNA (mRNA) molecules in the central dogma, serve their functional purposes as RNA rather than as an intermediary encoding for functional proteins. Long neglected in favor of coding genes, it has only recently been discovered that these families of noncoding transcripts actually account for the vast majority of human and other mammalian genomes. Although we have only just begun to appreciate their functional roles in human health and disease, they have already been shown to have effects as significant and diverse as DNA localization, chromatin remodeling, RNA splicing, and gene silencing. Furthermore, noncoding RNAs come in a dizzying variety of forms: the familiar ribosomal RNA (rRNA) and transfer RNA (tRNA); the shorter small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), microRNA (miRNA), and short interfering RNA (siRNA); PIWI-interacting RNA (piRNA); and most recently, long noncoding RNA (lncRNA) and long intergenic noncoding RNA (lincRNA). Of these, miRNAs and, more recently, lncRNAs have garnered significant interest in their potential as therapeutic targets in treating human disease, and therefore are the focus of this review.
Discussion
miRNA: a window into a deeper understanding of wound healing
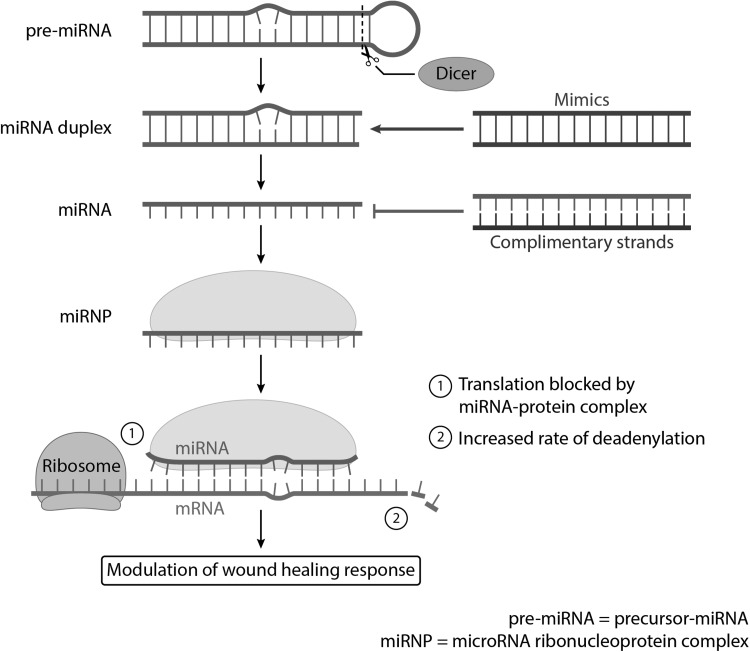
miRNAs are small endogenously expressed RNA molecules, ∼22 nucleotides in length, which can exert direct influence on mRNA. Following transcription in the nucleus and export to the cytoplasm of the cell, miRNAs interact with specific mRNA to cause cleavage, degradation, and transcriptional or translational repression of their targets (Fig. 1). Adding complexity to these relationships, however, is the fact that a single miRNA can regulate more than one gene, and conversely, a single gene can be acted upon by multiple miRNAs.12 Therefore, it is highly plausible that the effect of miRNAs in a genetic pathway can be parallel or cumulative in nature, and can thus have significant effects on a cell's phenotype. Of all classes of noncoding RNAs, miRNAs are thus far the most extensively studied and offer an intriguing glance into the extent of involvement of noncoding RNAs in wound healing.13
Figure 1.
miRNAs interact with specific mRNA to cause cleavage, degradation, and transcriptional or translational repression of their targets, resulting in modulation of the wound healing response. miRNA, microRNA.
miRNA involvement in inflammation and proliferation
In the inflammatory phase of wound healing, leukocytes enter the wound through damaged blood vessels, a process mediated by chemoattractants such as transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α), platelet-derived growth factor (PDGF), and others.10 While the role of miRNAs in this phase is still poorly understood, miRNAs have now been implicated in nearly every other step of the wound healing process. Recently, for example, it has been suggested that miRNA-132 is a critical facilitator of the transition from the inflammatory to proliferative phases of wound healing.14 In response to TGF-β, miRNA-132 expression is upregulated in epidermal keratinocytes during the inflammatory phase and reaches its peak in the proliferative phase. Through the NF-κB and STAT/ERK pathways, it simultaneously inhibits leukocyte recruitment as it stimulates keratinocyte growth, thereby regulating the transition to the proliferative phase. Corresponding wound models suppressing miRNA-132 revealed delayed wound healing with increased inflammation and decreased keratinocyte proliferation.14
Next, the proliferative phase of wound healing is driven by the secretion of factors such as FGF, TGF-α and TGF-β, interleukin-1 (IL-1), and TNF-α.13 While the importance of these chemotactic factors have been well studied, the influence of noncoding RNAs reveals an entirely new layer of regulation and coordination. Migration and proliferation of keratinocytes during this phase are critical for reepithelialization and wound closure, and several miRNAs have been implicated in this process. For example, induction of miRNA-210 results in an impaired closure of ischemic wounds through a reduction in keratinocyte proliferation.15,16 In hypoxic environments such as chronic ischemic wounds, the transcription factor hypoxia-inducible factor (HIF) is known to upregulate vascular endothelial growth factor (VEGF), stimulating angiogenesis and vasculogenesis.17 However, studies have now also found that HIF is also responsible for the specific upregulation of miRNA-210 in response to hypoxia.18,19 Downstream, miRNA-210 targets the transcription factor E2F3, which in turn promotes keratinocyte proliferation, and thereby may contribute to the progression of chronic ischemic wounds through this mechanism.16
Our understanding of the influence of miRNAs in other classically well-described pathways continues to expand. The AKT/mTOR signaling pathway is known to be a critical mediator of cell migration and proliferation in wound healing, and has been shown to be dysfunctional in diabetic rat wounds.20 Jin et al. revealed that members of the miRNA-99 family (miRNA-99a, miRNA-99b, and miRNA-100) regulate the AKT/mTOR pathway by targeting and altering the expression of multiple genes within the pathway during wound healing.21 Finally, miR483-3p directs the final phase of reepithelialization by controlling the arrest of keratinocyte migration and proliferation, thereby signaling the end of wound closure.22 Furthermore, if miR483-3p is suppressed, keratinocyte proliferation continues despite wound closure.
miRNAs and angiogenesis
The vascularity of a wound is closely linked with its ability to heal. Successful angiogenesis in the wound bed is critical to wound healing, by providing nutrients to support tissue regeneration throughout the wound healing process.23 Much of the earliest work linking miRNAs to angiogenesis has been through in vivo studies utilizing the dicerex1/2 mouse model, which involves a disabling mutation in Dicer, a key enzyme that processes pre-miRNA into mature functional miRNA.24 In embryos with this mutation, blood vessels are less developed, and yolk sacs contain fewer, thinner, and less organized vessels.25 Furthermore, in vitro research has utilized siRNA to silence Dicer in postnatal human endothelial cells, finding decreased endothelial cell migration, capillary sprouting, and vascular tube forming.26–28 Interestingly, in mutant mice, VEGF was in fact found to be upregulated, suggesting that VEGF alone was not sufficient for angiogenesis when in the setting of disrupted miRNA biogenesis. This finding hints at the critical importance of miRNAs in successful angiogenesis.
Other evidence for miRNA involvement in angiogenesis is derived from work examining miRNA expression levels in human umbilical vein endothelial cells.29 Of those miRNAs identified to be differentially expressed, miRNA-221 and miRNA-222 appear to block translation of the protein c-Kit, leading to impaired angiogenesis and wound healing in vitro.29 Additional miRNAs of interest include miRNA-15b and miRNA-16, which have been shown to be downregulated by hypoxia and to suppress expression of VEGF.30
The role of miRNAs in cutaneous remodeling
The remodeling phase of wound healing involves organized collagen deposition, ultimately resulting in formation and maturation of a scar. In an in vivo animal skin excisional wound model, Wang et al. found 33 miRNAs to be upregulated and 21 miRNAs to be downregulated during granulation tissue formation.31 Among those miRNAs with an increased expression during wound healing, miR-21 was found to be highly expressed in epithelial cells in the epidermis surrounding the wound and in mesenchymal cells in the dermis.31 To date, several studies have now demonstrated the potential significance of miR-21 in normal and impaired wound healing. For example, in loss-of-function experiments, wounds with an inhibited miR-21 expression exhibited a decreased early wound contraction, resulting in delayed healing.31 Meanwhile, miR-21 has also been implicated in venous disease and diabetes mellitus, two common causes of chronic nonhealing wounds. Murine studies comparing normal and diabetic wounds have revealed differential expression of the miRNA miR-21, with decreased expression during diabetic wound healing compared to normal wound healing, although the diabetic wounds originally exhibited a higher basal expression.32 Similarly, abnormal overexpression of miR-21 has also been found in human venous ulcers.33 Studies have now shown the involvement of miR-21 in enhancing fibroblast migration, as well as in accelerating reepithelialization in wound healing by promoting keratinocyte migration.32,34 Proposed mechanisms of action involve downregulation of their targets, leptin receptor and early growth response factor 3.33
Given these findings, it is not surprising that differential levels of several specific miRNAs have also been found when comparing normal fibroblasts and those in keloid scars.35 One of the most significant is miRNA-196a, which has subsequently been shown to decrease secretion of type I and type III collagens when overexpressed, and the reverse when knocked down.36 Thus, lowered levels of miRNA-196a appear to have a role in the overproduction of collagens characteristic of keloids, and may prove to be a potential method to reduce keloid scar formation clinically.
In addition, beyond normal physiological scar formation, research implicating miRNAs in scarless wound healing has also emerged. Early mammalian fetal wounds heal without a scar and then transition to a scar-forming phenotype during late gestation.37,38 Increased expression of miRNA-29b, miRNA-29c, and miRNA-192 from the early to late gestational stages suggests that these miRNAs may help facilitate this phenotypic change, although these associations are still preliminary.39
miRNAs regulate epithelial–mesenchymal transition
The epithelial–mesenchymal transition (EMT) drives cells from an epithelial to a mesenchymal phenotype, a process involved in both physiologic and pathologic circumstances such as embryologic development, cancer invasion, fibrosis, and wound healing.40 In the context of wound healing, EMT is driven by cytokines such as TGF-β and is necessary for sedentary proliferative keratinocytes to migrate toward the center of the wound. In normal wound healing, this process leads to reepithelialization; however, excess migration may lead to fibrosis and hypertrophic scarring. The miRNA-200 family and miRNA-205 regulate the EMT process in a signaling network with TGF-β and E-cadherin transcriptional repressors.41 Inhibition of these miRNAs induces EMT, while an enforced expression suppresses EMT in the presence of TGF-β, suggesting that modulation of this family of miRNAs may allow for the control of the degree to which EMT occurs in wound healing.
lncRNAs in wound healing
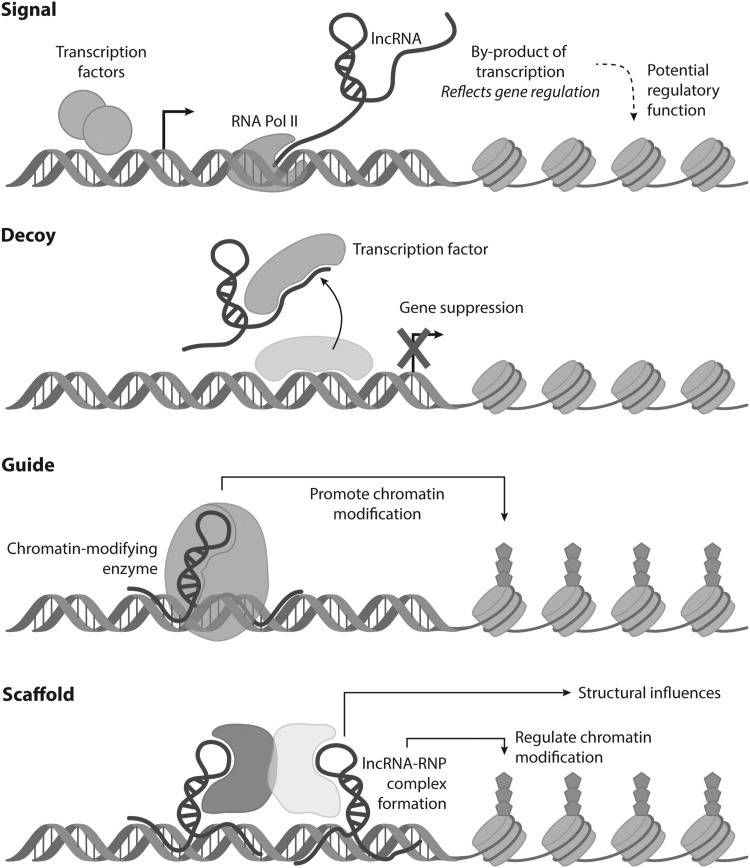
Defined as noncoding RNAs larger than 200 nucleotides in length, lncRNAs are a heterogeneous class of moderately conserved noncoding RNAs. With the capability of binding DNA, RNA, and proteins alike, their known mechanisms of action can be broadly categorized into several types: (1) molecular signal of transcriptional activity, (2) endogenous decoy to bind and reduce availability of other regulatory RNA or proteins, (3) guide to localize chromatin-modifying complexes to target DNA, and (4) structural scaffold for protein and transcript binding (Fig. 2).42,43
Figure 2.
Known mechanisms of lncRNAs include acting as signals, decoys, guides, and scaffolds to regulate cell behavior. Adapted from Wang et al.43 lncRNA, long noncoding RNA.
We have only begun to explore lncRNA roles in human health and disease, and there exists little research to date regarding lncRNAs in wound healing. However, in the past few years, lncRNAs have been demonstrated to have significant involvement in multiple related important processes in skin biology.44 For example, the lncRNA antidifferentiation noncoding RNA is required to maintain the undifferentiated progenitor cell state in the epidermis, and depletion using RNA interference results in induction of differentiation genes and regeneration of epidermal tissue.45 Another lncRNA, terminal differentiation-induced noncoding RNA, stabilizes differentiation mRNAs such as keratin 80, and ultimately controls human epidermal differentiation.46 Recently, Li et al. identified a set of lncRNAs with potential roles in scar progression.47 lncRNAs determined to be differentially expressed between regressive and mature human scars included lncRNA8975-1, AC097662.2, and RP11-586K2.1. These were associated with COL1A2, COL4A3, and MMP16 mRNA levels, respectively, suggesting roles in both collagen synthesis and matrix degradation.47
lncRNAs in angiogenesis
The functions of lncRNAs in angiogenesis were virtually unknown until recently, when technologies such as next-generation sequencing have allowed for more thorough analysis of the human transcriptome. Like their miRNA counterparts, several lncRNAs have also recently been found to be sensitive to hypoxia with proposed roles in angiogenesis. For example, the lncRNAs LINC00323, MIR503HG, and metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) are activated in response to hypoxia in human endothelial cells.48,49 Loss of LINC00323 and MIR503HG leads to impaired cell cycle control and capillary formation.48 MALAT1 is induced by TGF-β and has now been found to be an important mediator in endothelial cell proliferation.49,50 In both in vitro and in vivo experiments, inhibition of MALAT1 in endothelial cells decreases proliferation and results in defects in vascular growth.49 Other highly expressed lncRNAs in endothelial cells include taurine upregulated gene 1 (TUG1), maternally expressed 3 (MEG3), LINC00657, and LINC00493, although their functions remain unclear.49
Interestingly, recent studies have also found lncRNAs and miRNAs to be capable of binding to one another to regulate their counterpart's function in endothelial cells.42 This finding opens further possibilities for regulation of miRNA and lncRNA function.
lncRNAs in the EMT
Finally, several lncRNAs have also been strongly implicated in regulating the EMT, although nearly all of this research thus far has been in the context of tumor growth and metastasis. Briefly, examples include homeobox transcript antisense intergenic RNA (HOTAIR) and lincRNA-regulator of reprogramming (LincRNA-ROR), which are associated with invasion of carcinomas of different origins. Meanwhile, low expression in tumor (LET) suppresses metastasis, and conversely, downregulation of LET by HIF1α induces invasion.51 While it appears that these and other lncRNAs may have roles in the EMT, more work remains to be completed to elucidate the molecular differences between normal reepithelialization and pathologic states such as fibrosis and tumor invasion.
Current clinical trials
As registered on clinicaltrials.gov, several clinical studies are underway to further examine the role of noncoding RNAs in human skin. One such study, entitled “Role of Tissue Oxygenation and the miR-210 Gene in Wound Healing” is currently recruiting and aims to determine the relationship between miRNA-210 expression and wound healing outcome in chronic venous ulcers. Several studies, such as “Microarray Analysis of microRNA Expression Profiles in Cutaneous Squamous Cell Carcinoma” and “miRNA Machinery in Melanoma, Melanoma Metastases and Benign Melanocytic Naevi,” have examined miRNA expression profiles in various human skin cancers. Still, others are now measuring miRNA expression changes during treatment of psoriasis.
Developments and challenges in noncoding RNA therapeutics
Several methods are available to modulate miRNA levels, whether the goal is to overexpress a beneficial miRNA or to downregulate a harmful miRNA.15 Overexpression can be achieved using mimics, synthetic double-stranded oligonucleotides. Meanwhile, complementary oligonucleotides can be synthesized to bind to target miRNAs, with options to either inhibit interactions with all downstream targets or only one specific target.52 However, to achieve therapeutic efficacy, chemical modifications are often necessary to deliver these constructs to their target tissues. Researchers are now making progress in developing more sophisticated methods for the in vivo delivery of these synthetic oligonucleotides. For example, in a mouse model of heart failure, Thum et al. conjugated anti-miRNA-21 to cholesterol and successfully delivered the oligonucleotide to fibroblasts, with the result of reduced interstitial fibrosis and cardiac dysfunction.53
lncRNAs may also be targeted for inhibition or delivered for exogenous expression. The binding of antisense oligonucleotides or siRNAs, depending on whether the lncRNA is primarily nuclear or cytoplasmic in distribution, may potentially be made more efficient by the prediction of lncRNA secondary structure.54 In addition, as with miRNA, in vivo delivery of lncRNA modulators can be challenging, although development of several types of delivery vehicles is under way.54 Finally, the new clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) system has already been utilized to generate knockouts of both lncRNAs and miRNAs in human cell lines, providing an efficient and precise means to target noncoding RNA with potential in therapeutic applications.55
Noncoding RNAs, with their ability to regulate multiple gene targets, offer the possibility of coordinated and robust modulation at multiple points simultaneously within a pathway rather than only a single gene. However, while this may prove to be a therapeutic advantage to conventional gene therapy, this complexity of noncoding RNA involvement in biological pathways necessitates further research to reach a more thorough understanding of precise effects on their targets. Of note, unlike miRNA, lncRNA has higher tissue specificity and may have fewer off-target effects.42 With additional work to elucidate the mechanisms of target noncoding RNAs and to establish their safety, they may become a safe and revolutionary class of wound healing therapies.
Summary
Recent research has shown that RNA is more than a simple intermediary between DNA and proteins, with an emerging appreciation of a vast population of nonprotein-coding RNAs capable of affecting critical changes in gene expression. Thus far, noncoding RNAs such as miRNA and lncRNA have demonstrated significant roles in cell differentiation, angiogenesis, and other wound healing processes. Although our understanding of these roles and their precise mechanisms is still far from complete, their promise as not only biomarkers but also as therapeutic targets has rapidly accelerated research to characterize their functions and interactions within traditional wound healing pathways. Meanwhile, advancements to enhance successful delivery of molecules to target noncoding RNAs in tissues in vivo continue to improve the practical ability to develop therapeutic drugs to modulate them. With these and future discoveries, we stand at a vast frontier of enormous opportunity to alter the processes of pathologic wound healing.
Take-Home Messages.
• Noncoding RNAs regulate gene expression and cell phenotype by binding to and acting upon DNA, proteins, and/or other RNA.
• Characterization of noncoding RNAs and their precise functions will be necessary to achieve a thorough understanding of the wound healing process.
• Noncoding RNAs such as miRNA and lncRNA have been found both in vitro and in vivo to have significant roles in angiogenesis, reepithelialization, matrix deposition, and other critical aspects of wound healing.
• Synthetic molecules that can overexpress or repress noncoding RNAs can be delivered in vivo to modulate activity of their target noncoding RNA.
• Noncoding RNAs offer the opportunity to affect changes in gene expression of higher complexity and sophistication than conventional gene therapy.
• More research regarding their specific mechanisms will need to be completed to ensure safety and minimize off-target effects.
Abbreviations and Acronyms
- CRISPR
clustered regularly interspaced short palindromic repeats
- DNA
deoxyribonucleic acid
- EMT
epithelial–mesenchymal transition
- FGF
fibroblast growth factor
- HIF
hypoxia-inducible factor
- IL
interleukin
- LET
low expression in tumor
- lincRNA
long intergenic noncoding RNA
- lncRNA
long noncoding RNA
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- mRNA
messenger RNA
- miRNA
microRNA
- PDGF
platelet-derived growth factor
- piRNA
PIWI-interacting RNA
- RNA
ribonucleic acid
- rRNA
ribosomal RNA
- siRNA
short interfering RNA
- snoRNA
small nucleolar RNA
- snRNA
small nuclear RNA
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- tRNA
transfer RNA
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
M.T.L. was supported by NIH grants U01 HL099776, R01 DE021683-03, R21 DE024230-02, the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Gunn/Olivier Fund. D.C.W. was supported by NIH grant 1K08 DE024269-01, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Anna Luan, MD, MS, is a resident physician in Plastic and Reconstructive Surgery at Stanford University and a research fellow in the Hagey Laboratory for Regenerative Medicine. Michael S. Hu, MD, MPH, MS, Tripp Leavitt, MD, and Elizabeth A. Brett, MS, are research fellows in the Hagey Laboratory for Regenerative Medicine. Kevin C. Wang, MD, PhD, is an Assistant Professor in Dermatology at Stanford University School of Medicine. Michael T. Longaker, MD, MBA, is the Dean P. & Louise Mitchell Professor and Vice Chair of the Department of Surgery at Stanford University School of Medicine, Co-Director of the Institute for Stem Cell Biology & Regenerative Medicine at Stanford University, and Professor by courtesy in Bioengineering and Materials Science and Engineering. Derrick C. Wan, MD, is an Associate Professor in the Department of Surgery, Division of Plastic and Reconstructive Surgery, at Stanford University School of Medicine. He is also the Director of Maxillofacial Surgery at Lucile Packard Children's Hospital, Endowed Faculty Scholar of the Child Health Research Institute at Stanford University, and Hagey Family Faculty Scholar in Stem Cell Research and Regenerative Medicine.
References
- 1.Alexander RP, Fang G, Rozowsky J, Snyder M, Gerstein MB. Annotating non-coding regions of the genome. Nat Rev Genet 2010;11:559–571 [DOI] [PubMed] [Google Scholar]
- 2.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011;12:861–874 [DOI] [PubMed] [Google Scholar]
- 3.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009;10:155–159 [DOI] [PubMed] [Google Scholar]
- 4.Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg 2003;90:133–146 [DOI] [PubMed] [Google Scholar]
- 5.Hu MS, Leavitt T, Malhotra S, et al. Stem cell-based therapeutics to improve wound healing. Plast Surg Int 2015;2015:383581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 2008;61:1049–1058 [DOI] [PubMed] [Google Scholar]
- 8.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 2011;17:113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey C. Wound healing. Orthop Nurs 2005;24:143–157; quiz 158–149 [DOI] [PubMed] [Google Scholar]
- 10.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstruct Surg 2006;117:12S-34S [DOI] [PubMed] [Google Scholar]
- 11.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 12.Shilo S, Roy S, Khanna S, Sen CK. MicroRNA in cutaneous wound healing: a new paradigm. DNA Cell Biol 2007;26:227–237 [DOI] [PubMed] [Google Scholar]
- 13.Sand M, Gambichler T, Sand D, Skrygan M, Altmeyer P, Bechara FG. MicroRNAs and the skin: tiny players in the body's largest organ. J Dermatol Sci 2009;53:169–175 [DOI] [PubMed] [Google Scholar]
- 14.Li D, Wang A, Liu X, et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J Clin Invest 2015;125:3008–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee J, Chan YC, Sen CK. MicroRNAs in skin and wound healing. Physiol Genomics 2011;43:543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas S, Roy S, Banerjee J, et al. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci U S A 2010;107:6976–6981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaluz S, Kaluzova M, Stanbridge EJ. Regulation of gene expression by hypoxia: integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin Chim Acta 2008;395:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res 2009;69:1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol 2007;27:1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, Cui W, Qiu W, et al. Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats. J Dermatol Sci 2015;79:241–251 [DOI] [PubMed] [Google Scholar]
- 21.Jin Y, Tymen SD, Chen D, et al. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One 2013;8:e64434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertero T, Gastaldi C, Bourget-Ponzio I, et al. miR-483-3p controls proliferation in wounded epithelial cells. FASEB J 2011;25:3092–3105 [DOI] [PubMed] [Google Scholar]
- 23.Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med 2001;125:67–71 [DOI] [PubMed] [Google Scholar]
- 24.Jaskiewicz L, Filipowicz W. Role of Dicer in posttranscriptional RNA silencing. Curr Top Microbiol Immunol 2008;320:77–97 [DOI] [PubMed] [Google Scholar]
- 25.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 2005;280:9330–9335 [DOI] [PubMed] [Google Scholar]
- 26.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 2007;100:1164–1173 [DOI] [PubMed] [Google Scholar]
- 27.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 2007;101:59–68 [DOI] [PubMed] [Google Scholar]
- 28.Suarez Y, Fernandez-Hernando C, Yu J, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A 2008;105:14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood 2006;108:3068–3071 [DOI] [PubMed] [Google Scholar]
- 30.Hua Z, Lv Q, Ye W, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 2006;1:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T, Feng Y, Sun H, et al. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol 2012;181:1911–1920 [DOI] [PubMed] [Google Scholar]
- 32.Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J 2012;9:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastar I, Khan AA, Stojadinovic O, et al. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem 2012;287:29324–29335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Wang J, Guo SL, et al. miR-21 promotes keratinocyte migration and re-epithelialization during wound healing. Int J Biol Sci 2011;7:685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X, Li Z, Chan MT, Wu WK. microRNA deregulation in keloids: an opportunity for clinical intervention? Cell Prolif 2015;48:626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashiyama K, Mitsutake N, Matsuse M, et al. miR-196a downregulation increases the expression of type I and III collagens in keloid fibroblasts. J Invest Dermatol 2012;132:1597–1604 [DOI] [PubMed] [Google Scholar]
- 37.Dang C, Ting K, Soo C, Longaker MT, Lorenz HP. Fetal wound healing current perspectives. Clin Plast Surg 2003;30:13–23 [DOI] [PubMed] [Google Scholar]
- 38.Beanes SR, Hu FY, Soo C, et al. Confocal microscopic analysis of scarless repair in the fetal rat: defining the transition. Plast Reconstruct Surg 2002;109:160–170 [DOI] [PubMed] [Google Scholar]
- 39.Cheng J, Yu H, Deng S, Shen G. MicroRNA profiling in mid- and late-gestational fetal skin: implication for scarless wound healing. Tohoku J Exp Med 2010;221:203–209 [DOI] [PubMed] [Google Scholar]
- 40.Barriere G, Fici P, Gallerani G, Fabbri F, Rigaud M. Epithelial mesenchymal transition: a double-edged sword. Clin Transl Med 2015;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593–601 [DOI] [PubMed] [Google Scholar]
- 42.Ballantyne MD, McDonald RA, Baker AH. lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol Ther 2016;99:494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011;43:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan DC, Wang KC. Long noncoding RNA: significance and potential in skin biology. Cold Spring Harb Perspect Med 2014;4:a015404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kretz M, Webster DE, Flockhart RJ, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev 2012;26:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kretz M, Siprashvili Z, Chu C, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 2013;493:231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Long W, Li Q, et al. Distinct expression profiles of lncRNAs between regressive and mature scars. Cell Physiol Biochem 2015;35:663–675 [DOI] [PubMed] [Google Scholar]
- 48.Fiedler J, Breckwoldt K, Remmele CW, et al. Development of long noncoding RNA-based strategies to modulate tissue vascularization. J Am Coll Cardiol 2015;66:2005–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalik KM, You X, Manavski Y, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 2014;114:1389–1397 [DOI] [PubMed] [Google Scholar]
- 50.Singh KK, Matkar PN, Quan A, et al. Investigation of TGFbeta1-induced long noncoding RNAs in endothelial cells. Int J Vasc Med 2016;2016:2459687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhamija S, Diederichs S. From junk to master regulators of invasion: lncRNA functions in migration, EMT and metastasis. Int J Cancer 2016;139:269–280 [DOI] [PubMed] [Google Scholar]
- 52.Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther 2010;125:92–104 [DOI] [PubMed] [Google Scholar]
- 53.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–984 [DOI] [PubMed] [Google Scholar]
- 54.Lavorgna G, Vago R, Sarmini M, Montorsi F, Salonia A, Bellone M. Long non-coding RNAs as novel therapeutic targets in cancer. Pharmacol Res 2016;110:131–138 [DOI] [PubMed] [Google Scholar]
- 55.Ho TT, Zhou N, Huang J, et al. Targeting non-coding RNAs with the CRISPR/Cas9 system in human cell lines. Nucleic Acids Res 2015;43:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]