Abstract
Objective: Electrospinning is a promising technology that provides biodegradable nanofiber scaffolds for cardiovascular tissue engineering. However, success with these materials has been limited, and the optimal combination of scaffold parameters for a tissue-engineered vascular graft (TEVG) remains elusive. The purpose of the present study is to evaluate the effect of bone marrow mononuclear cell (BM-MNC) seeding in electrospun scaffolds to support the rational design of optimized TEVGs.
Methods: Nanofiber scaffolds were fabricated from co-electrospinning a solution of polyglycolic acid and a solution of poly(ι-lactide-co-ɛ-caprolactone) and characterized with scanning electron microscopy. Platelet activation and cell seeding efficiency were assessed by ATP secretion and DNA assays, respectively. Cell-free and BM-MNC seeded scaffolds were implanted in C57BL/6 mice (n = 15/group) as infrarenal inferior vena cava (IVC) interposition conduits. Animals were followed with serial ultrasonography for 6 months, after which grafts were harvested for evaluation of patency and neotissue formation by histology and immunohistochemistry (n = 10/group) and PCR (n = 5/group) analyses.
Results: BM-MNC seeding of electrospun scaffolds prevented stenosis compared with unseeded scaffolds (seeded: 9/10 patent vs. unseeded: 1/10 patent, p = 0.0003). Seeded vascular grafts demonstrated concentric laminated smooth muscle cells, a confluent endothelial monolayer, and a collagen-rich extracellular matrix. Platelet-derived ATP, a marker of platelet activation, was significantly reduced after incubating thrombin-activated platelets in the presence of seeded scaffolds compared with unseeded scaffolds (p < 0.0001). In addition, reduced macrophage infiltration and a higher M2 macrophage percentage were observed in seeded grafts.
Conclusions: The beneficial effects of BM-MNC seeding apply to electrospun TEVG scaffolds by attenuating stenosis through the regulation of platelet activation and inflammatory macrophage function, leading to well-organized neotissue formation. BM-MNC seeding is a valuable technique that can be used in the rational design of optimal TEVG scaffolds.
Keywords: : tissue-engineered vascular graft (TEVG), bone marrow mononuclear cell (BM-MNC) seeding, nanofiber, electrospinning, biodegradable scaffold, stenosis
Introduction
Electrospinning has been increasingly explored as a fabrication method to create seamless biodegradable tubular scaffolds for vascular tissue engineering, with applications ranging from surgical reconstruction of congenital cardiac anomalies to small-diameter bypass grafting in atherosclerotic coronary artery disease.1–4 Electrospinning is a rapid, reproducible, and highly customizable technique that can be used to fabricate scaffolds of various geometries.5 Varying electrospinning process parameters can yield tissue-engineered scaffolds that are made of dry, nonwoven polymer fibers, ranging in diameter from 50 nm to 20 μm, smaller than what most other available fiber spinning techniques can produce.3 The nanoarchitecture of electrospun scaffolds is especially relevant in tissue engineering as it mimics native extracellular matrix (ECM) in scale, which is believed to encourage cellular ingrowth, ECM deposition, and neotissue formation.6 However, the sequential layering of nanofibers in the construction of tubular TEVG scaffolds produces constructs with three-dimensional pore sizes that are limited by fiber diameter. The effect of small pores (<10 μm) in TEVG scaffolds has been recently explored, and most published reports indicate that small pores inhibit cellular infiltration, delay degradation, and support chronic inflammation.7–11 The latter aspect hinders the immune-mediated process of neovessel formation,7,12,13 which, in turn, may support adverse remodeling such as calcification or chronic intimal hyperplasia. Despite the fact that many innovative electrospun homopolymers, copolymers, and polymer blends have been created in response to these challenges, there is no electrospun scaffold design that has been translated to clinical application.6
The first human clinical trial investigating the use of TEVGs in children with congenital heart defects began in 2001.14 Highly porous biodegradable polymeric scaffolds, comprising poly(L-lactide-co-ɛ-caprolactone) (PLCL) reinforced by polyglycolic acid (PGA) mesh,15 were seeded with bone marrow mononuclear cell (BM-MNC). There is now established clinical evidence to show that TEVGs are safe and effective to use in pediatric patients undergoing extracardiac total cavopulmonary connection procedure.16,17
In this article, we fabricated a co-electrospun scaffold from PGA and PLCL modeled after those materials recently reported in human trials.3,15–17 Due to reports of BM-MNC seeding preventing TEVG stenosis in other scaffold types,18 we investigated whether BM-MNC seeding before implant improved the performance of electrospun scaffolds in a murine venous interposition model over a 6-month time course.
Materials and Methods
Animal care and ethics statement
All animals received humane care in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the Research Institute at Nationwide Children's Hospital approved and monitored all animal procedures described in this article. Thirty-five C57BL/6 mice (17–20 g, 8–12 week old, female) were purchased from Jackson Laboratories.
Graft fabrication
To create the co-electrospun PGA and PLCL scaffolds, 10 wt% PGA was dissolved in hexafluoroisopropanol and 5 wt% PLCL was dissolved in hexafluoroisopropanol. Each solution was stirred via a magnetic stir bar for at least 3 h at room temperature. In separate syringes, the PGA solution was dispensed at a flow rate of 2.5 mL/h and the PLCL solution was dispensed at a flow rate of 5.0 mL/h to create grafts with a 1:1 PGA:PLCL weight ratio. Both solutions were simultaneously electrospun onto the mandrel that was positioned 20 cm from the needle tips and rotated at 30 RPM. A +25 kV charge was applied to each syringe tip, and electrospun nanofibers were deposited onto the grounded mandrel until the desired wall thickness of 0.1 mm was achieved. The electrospun scaffold was then removed from the mandrel, and the wall thickness was measured with a digital snap gauge by placing the scaffold between two glass slides. The PGA/PLCL tubes were cut into 5 mm lengths, and the inner diameter was 1 mm. The scaffolds were then packaged in Tyvek pouches and sterilized with 25 kGy of gamma irradiation.
Scaffold degradation
Electrospun sheets were prepared from the same co-electrospinning setup as used to produce the tubes for this experiment, except the fibers were deposited onto a flat plate to a thickness of approximately 250 μm. The scaffold degradation samples were 250 μm thick, thus following the guidelines outlined in the American Society for Testing and Materials Standard Test Method for Tensile Properties of Plastics (ASTM D638). The difference in thickness does not affect the mechanics of the graft, as we report the ultimate tensile strength (UTS) in MPa, which is independent of scaffold thickness. The degradation rate is not affected by the scaffold thickness because the construct is completely porous. The thickness of every test sample was measured before testing. A digital caliper (VWR) with an accuracy of two decimal places was zeroed on two 3″ × 1″ × 1 mm glass microscope slides (VWR). Samples were placed between the glass slides, and the thicknesses were measured by following ASTM standard D6988-13. Three measurements were taken, one on the right, center, and left of each sample. The median of these three measurements was recorded in millimeters as the thickness of the sample.
Five tensile dogbones were cut from the electrospun sheets according to ASTM D638 Type V by using a universal testing machine (MultiTest 5-i; Mecmesin Corporation). For the in vitro degradation, a single tensile dogbone was placed in a glass vial with 25 mL of phosphate-buffered saline (PBS). Five samples per timepoint were placed in an incubator and held at a constant 37°C until they were withdrawn for mechanical testing. The ends of each dogbone sample were clamped to opposite arms of the testing machine and pulled apart until failure. The peak load from each sample was averaged and recorded in MPa as the UTS. The elongation to failure of each sample was also recorded as% of the original sample length. Young's modulus of each sample was determined from the slope of the linear regime of the resulting stress-strain curve and recorded in MPa.
Bone marrow harvest and graft seeding
Clinically, we have already used BM-MNCs as a cell source.16,17 As a large number of cells can be obtained from BM-MNCs without further in vitro culture, there are fewer steps in the preparation of tissue-engineered vascular grafts (TEVGs). This means a lower risk of contamination, less work, and less time needed to fabricate the TEVGs. In addition, these cells have the potential to differentiate into both smooth muscle cells and endothelial cells.15,19
Bone marrow donor mice (n = 5) were administered an overdose cocktail of ketamine (300 mg/kg) and xylazine (30 mg/kg). Their bone marrow was harvested, the MNC population was enriched, and scaffolds were seeded as previously described.18,20 Briefly, hind limbs were disarticulated in sterile fashion, muscle and tendon were dissected, and the femoral and tibial heads were removed. Bone marrow was collected by repeated flushing with 5.0 mL of RPMI 1640 (Sigma) + 1% penicillin/streptomycin (P/S; Sigma). The pooled bone marrow was filtered to remove bone spicules and macroaggregates (100 μm cell strainer; Fisher). The bone marrow aspirate was layered on Ficoll Histopaque solution (1083; Sigma) in a 1:1 ratio in volume. After density gradient centrifugation, the mononuclear cell layer was carefully collected, washed twice with 1x PBS, and resuspended at a concentration of 2.0 × 108 cells/mL in RPMI-1640 + 1%P/S. Cell concentrations were determined with Trypan blue exclusion by using a Countess™ automated cell counter (Invitrogen) as the mean of two separate cell counts. Before seeding, grafts were prewet by applying 5 μL of 1× PBS +1%P/S to the scaffold lumen for 5:00 min. Excess PBS was aspirated, and 5 μL of BM-MNCs (1.0 × 106 cells) was introduced to the scaffold lumen. After a 10 min incubation to allow for cell attachment, a 22G needle was threaded through the lumen before immersion in 1 mL of RPMI-1640 + 1%P/S in a 24-well plate. Seeded grafts were incubated overnight at 37°C 5% CO2 before implantation. Efficacy of cell seeding was verified with SEM and PicoGreen dsDNA assay.
Graft implantation
Seeded or unseeded scaffolds (n = 15/group) were implanted as infrarenal inferior vena cava (IVC) interposition grafts by following the standard microsurgical technique as previously described.18,20,21 Briefly, mice were administered a pre-anesthesia analgesic of ketoprofen (5 mg/kg, IP) and anesthetized with a cocktail of ketamine (100 mg/kg, IP) and xylazine (10 mg/kg, IP). Scaffolds (5.0 mm length) were interposed end-to-end with a 10-0 nylon suture in running fashion. After confirming patent anastomosis and ensuring hemostasis, the abdomen was closed in two layers with a 6-0 prolene suture in running fashion. Animals were followed for 6 months without anti-platelet or anti-coagulation therapy. There was no operative and graft-related mortality at any point during follow-up.
Ultrasound interrogation
Implanted grafts were monitored longitudinally until explant (2, 4, 8, 12, and 24 weeks after implantation) with high-frequency Doppler ultrasonography (Vevo® 2100; VisualSonics, Inc.). Anesthesia was induced with 1.5% inhaled isoflurane vaporized with 100% O2 at a rate of 1 L/min. Body temperature was maintained at 38°C, and vitals were maintained within normal limits for the duration of the ultrasound examination. Long-axis images were acquired with B-mode, pulse-wave, and color Doppler. ImageJ (NIH) was used to quantify lumen diameter of B-mode images, and graft patency was determined with pulse-wave and color Doppler images as previously described.12
Graft explant and tissue analysis
After 6-months of follow-up, animals were euthanized by administration of an overdose cocktail of ketamine (300 mg/kg) and xylazine (30 mg/kg) followed by induction of pneumothorax. Mice were systemically perfused with cold phosphate-buffered saline (1× PBS). Grafts intended for reverse transcription-quantitative polymerase chain reaction (RT-qPCR) experiments (n = 5/group) were explanted and embedded in OCT compound (optimal cutting temperature, Tissue-Tek, Sakura) on dry ice. Grafts intended for histologic and immunohistochemical analyses (n = 10/group) were perfusion fixed with 10% neutral buffered formalin (Fisher) before explant.
Histology
Formalin-fixed samples were dehydrated, paraffin embedded, and serially sectioned (4 μm thick). Histologic stains were performed in one batch by following the standard technique and included hematoxylin and eosin, Masson's Trichrome, von Kossa, Hart's, and Picro Sirius Red. Quantification of lumen diameter was performed with ImageJ by division of luminal circumference by π, and the incidence of stenosis was calculated as the ratio of patent to occluded grafts in each group. A graft was considered patent if the measured lumen diameter was ≥75% of the original lumen diameter of the scaffold at implantation.
Immunohistochemistry and immunofluorescence
For immunohistochemistry, slides were deparaffinized, rehydrated, and blocked for endogenous peroxidase activity (0.3% H2O2 in MeOH). Antigens were retrieved via the citrate buffer method (pH 6.0, 90°C for 15 min). Slides were blocked for nonspecific background staining (Background Sniper, BioCare Medical) before overnight incubation at 4°C with the following primary antibodies: F4/80 (1:1000; Abd Serotec), iNOS (1:200; Abcam), CD206 (1:100; Abcam), αSMA (1:500; Dako), CD31 (1:50; Abcam), and SM-MHC (1:1000; Abcam). For immunohistochemistry, sequential incubation with species-appropriate biotinylated secondary antibodies (1:300-500; Dako) and Horseradish Peroxidase Streptavidin (Dako) identified antibody binding. Chromogenic detection was performed by development with 3,3-diaminobenzidine (Vector). Nuclei were identified via hematoxylin counterstain (Gill's Formula, Vector). Immunofluorescence was performed by incubation with a cocktail of species-appropriate Alexa-Fluor®-647 or -488 secondary antibodies (1:300; Life Technologies) followed by nuclear counterstaining with 4′-6 diamidino-2-phenylindole (DAPI, Vector).
Reverse transcription-quantitative polymerase chain reaction
Frozen tissue blocks were sliced (30 μm thick sections) on a CM1950 cryostat (Leica Biosystems). Sections were washed in 1× PBS to remove excess OCT compound, and total RNA was extracted and purified by using the RNeasy mini kit (Qiagen). Reverse transcription was performed by using the High-Capacity RNA-to-cDNA Kit (Applied Biosystems) by following the manufacturer's instructions. Quantitative PCR assay was performed with the Step One Plus Real-Time PCR System by using the TaqMan Universal PCR Master Mix Kit (Applied Biosystems). Total reaction volume was 20 μL: 10 μL TaqMan Fast Advanced Master Mix (2 × ), 1 μL Taqman Gene Expression Assay (20 × ), and 7 μL nuclease-free H2O, 2 μL cDNA. All gene expression assays were obtained from Applied Biosystems (Nitric oxide synthase 3 and smooth muscle alpha 2 actin). All assays were performed in duplicate, and values were analyzed by using the comparative threshold cycle method and normalized to the expression of the endogenous reference genes HPRT or GAPDH. Gene expression in seeded samples is reported as a relative value (ΔΔCT) to that of unseeded samples (n = 5/group).
Scanning electron microscopy
For characterization of pore size and fiber diameter, a total of 10 scaffolds were cut open. After dehydration with hexamethyldisilazane, samples were prepared by gold sputter coating and 10 images of the luminal surface were obtained by scanning electron microscopy (SEM) with a Hitachi S-4800 Scanning Electron Microscope at 5.0–15.0 kV, for each scaffold. Pore size, which is the area surrounded by 3 or 4 nanofibers, and fiber diameter were determined by acquisition of 30 measurements with perimeters and diameter per SEM image using ImageJ (NIH).
In vitro analyses
DNA assay
The fluorometric Quant-iT™ PicoGreen® dsDNA Assay Kit (Life Technologies) was used following the manufacturer's protocol to monitor cell seeding (n = 5, seeded scaffolds). Fluorescence intensity measurements were acquired with a SpectraMax M5 Microplate reader (Excitation 488 nm, Emission 525 nm, Molecular Devices) and applied to a standard curve. The efficacy of cell seeding was determined by the total number of cells in each well divided by the surface area of the scaffold (cells/mm2) as previously described.18
Platelet isolation and ATP secretion assays
Whole blood was harvested by cardiac puncture by using a 25G needle and a 3 mL syringe pre-loaded with 100–200 μL of citrate-dextrose solution (C3821; Sigma), and platelets were enriched as previously described.22 Briefly, aspirated blood was centrifuged (120 g, 8 min) to separate erythrocytes and leukocytes from the platelet-rich plasma (PRP). PRP was aspirated and centrifuged (120 g, 3 min) to further enrich the platelet fraction. The supernatant was centrifuged (740 g, 10 min) to yield a pellet that was then re-suspended with 200 μL of 1× PBS. Platelet concentration was quantified by using an ABX Micros 60 hematology analyzer (Horiba), and it was determined as the mean of two separate measurements. Cells were resuspended in 1× PBS at a concentration of 1 × 104 cells/μL. Fifty microliters of platelet suspension (5.0 × 105 platelets) was added to the wells of a black polystyrene 96-well microplate (Corning Costar) containing either BM-MNC-seeded (n = 18) or -unseeded (n = 10) scaffolds under either thrombin-activated (0.1 U/mL) or resting conditions. Platelets were incubated at room temperature with gentle shaking for 1.0 h, after which grafts were removed from the wells. The concentration of platelet-derived ATP in each well was determined by the addition of 50 μL ChronoLume™ reagent (Chrono-Log Corp.) followed by luminescent detection with a LUMIstar Omega microplate luminometer (BMG Labtech) as previously described.23 Measured values were applied to a standard curve, and the degree of platelet ATP secretion in each condition was determined.
Statistical analysis
Lumen diameter measurements derived from serial ultrasound imaging were analyzed via two-way ANOVA with Tukey's correction for multiple comparisons. Histomorphometric quantifications of lumen diameter were compared with nonparametric unpaired two-tailed Mann–Whitney t-test, as the data were not normally distributed. The incidence of stenosis between both groups was compared with a two-sided Chi-square test. F4/80+ cell counts and SEM measurements of fiber diameter and pore size were analyzed with a parametric unpaired two-tailed t-test. iNOS and CD206 IHC and PCR, eNOS and ACTA2 PCR, and ATP secretion data were compared within and across groups by two-way ANOVA with Tukey's correction for multiple comparisons. p < 0.05 was considered statistically significant.
Results
PGA/PLCL scaffolds comprised nanofibers forming pores of <10 μm
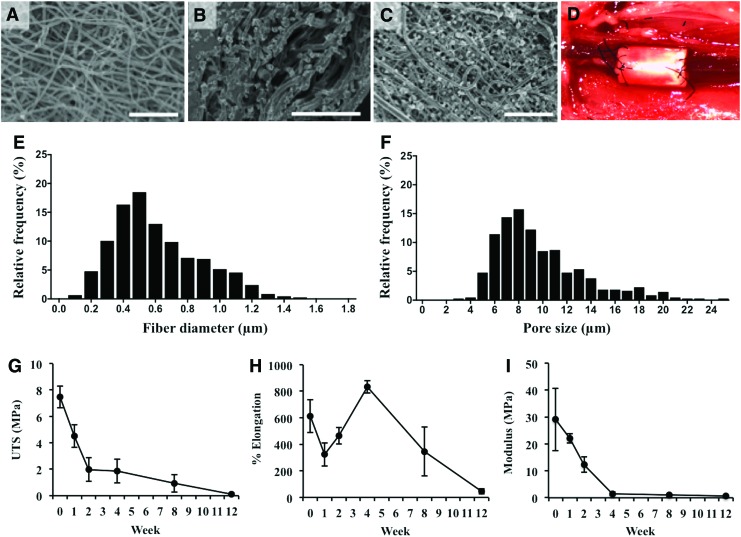
The mean fiber diameter and pore size of the PGA/PLCL electrospun scaffold for the lumen surface were 0.61 ± 0.27 μm and 9.71 ± 0.16 μm, respectively. SEM images of scaffold are shown in Figure 1A–C. The results of mechanical testing of in vitro degraded PGA/PLCL scaffolds are shown in Figure 1G–I. The average UTS of the scaffolds decreased from 7.47 ± 0.83 MPa to 1.96 ± 0.89 MPa in 2 weeks. After 12 weeks, the scaffolds' average UTS decreased to 0.11 ± 0.09 MPa. No material remained viable for testing at longer time periods. After 4 weeks, the scaffold's Young's Modulus decreased from 29.06 ± 11.65 MPa to 1.44 ± 0.66 MPa. The elongation to failure decreased to 324% ± 39% after 1 week, increased to 832% ± 20% after 4 weeks, and finally decreased to 46% ± 8% at 12 weeks (Fig. 1G–I).
FIG. 1.
Scaffold characterization. SEM images of luminal (A) and adventitial (B) surfaces of the electrospun scaffold. SEM of a BM-MNC-seeded scaffold luminal surface (C). Implantation of a seeded graft (D) in the murine infrarenal IVC interposition model. Fiber diameter (E) and pore size (F) for luminal surface (Fiber diameter: 0.61 ± 0.27 μm, pore size: 9.71 ± 0.16 μm). In vitro degradation mechanical values of UTS (G), percent elongation to failure (H), and Young's Modulus (I). Scale bar = 50 μm. BM-MNC, bone marrow mononuclear cell; IVC, inferior vena cava; UTS, ultimate tensile strength. Color images available online at www.liebertpub.com/tea
BM-MNC seeding of PGA/PLCL nanofiber scaffolds prevents acute graft narrowing and chronic stenosis
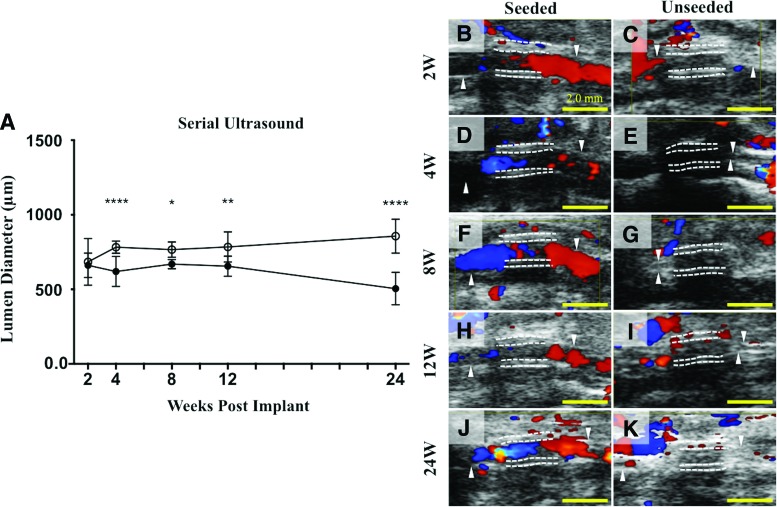
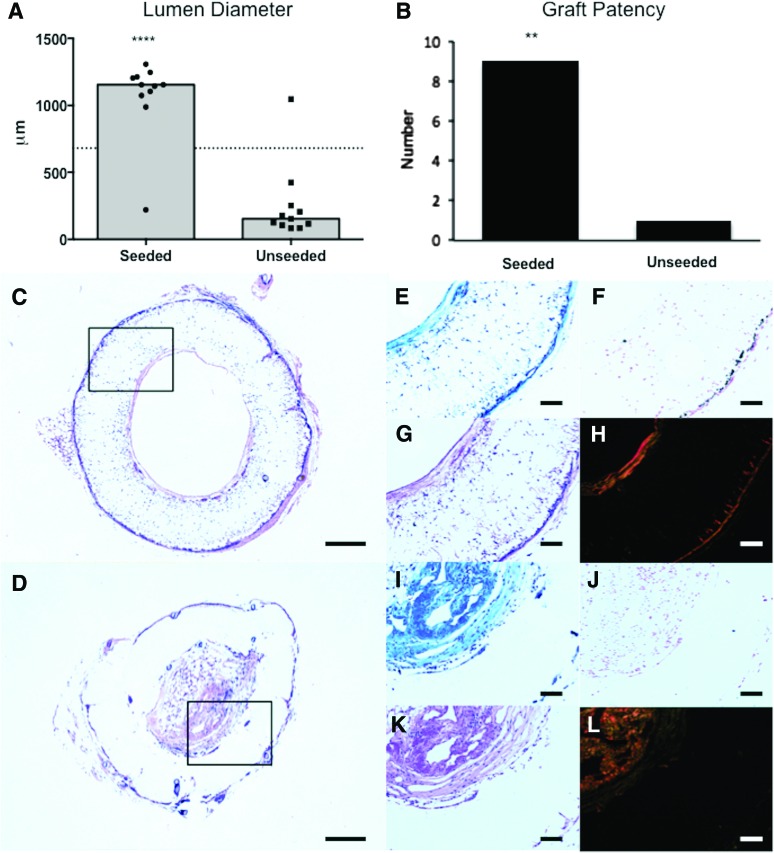
A serial ultrasound of TEVG implants performed at 2, 4, 8, 12, and 24 weeks after graft implantation indicated a significant difference in lumen diameter between the seeded and unseeded groups as early as 4 weeks after implantation (p < 0.001, Fig. 2A). This difference was maintained throughout the duration of follow-up. Histomorphometric analysis after explant confirmed that the lumen diameter and graft patency of seeded grafts were significantly greater than those of unseeded grafts (Lumen diameter: 1074 ± 295.8 μm in seeded vs. 252.3 ± 281.2 μm in unseeded; p < 0.0001, Fig. 3A; Graft patency: 90% in seeded vs. 10% in unseeded: p = 0.0003, Fig. 3B).
FIG. 2.
Lumen diameter over time by serial ultrasound. Serial ultrasound of implanted TEVGs performed at 2, 4, 8, 12, and 24 weeks after graft implantation indicated a significant difference in lumen diameter between the seeded and unseeded groups as early as 4 weeks after implantation that was maintained throughout the 6-month time course (A), ****p < 0.0001, *p < 0.05, **p < 0.005. Representative ultrasound images from each time point (B–K), respectively, demonstrating patent grafts characterized by color Doppler flow through the lumen (B, D, F, H, J) in the seeded group and occluded grafts in the unseeded group (C, E, G, I, K). Color Doppler signals in the unseeded group may indicate the presence of collateral vasculature surrounding stenotic TEVGs. The white dotted lines indicate the luminal and adventitial surfaces of the TEVGs. Scale bar = 2.0 mm. TEVG, tissue-engineered vascular graft. Color images available online at www.liebertpub.com/tea
FIG. 3.
Histological assessment of vascular neotissue formation after 6 months. Histomorphometric comparison of the lumen diameter measurements (A) and graft patency (B) between the seeded and unseeded groups demonstrates that BM-MNC seeding prevents occlusion in the nanofiber scaffold (****p < 0.0001, ***p = 0.0003). Histologic comparison between seeded and unseeded scaffolds demonstrated vascular neotissue formation in seeded grafts characterized by mature ECM on the luminal and adventitial graft surfaces, adequate cellular infiltration, and minimal vascular calcification. Representative photomicrographs for each group (Seeded: [C, E–H]; Unseeded: D, I–L) are shown for H&E (C, D), Masson's trichrome (E, I), Hart's (G, K), von Kossa (F, J), and Picrosirius red stains (H, L). Scale bar = 200 μm for (C) and (D), and 100 μm otherwise. ECM, extracellular matrix. Color images available online at www.liebertpub.com/tea
BM-MNC-seeded PGA/PLCL scaffolds demonstrate vascular neotissue formation and ECM deposition after 6 months
A histologic comparison between seeded and unseeded scaffolds demonstrated vascular neotissue formation in seeded grafts characterized by mature ECM on the luminal and adventitial graft surfaces (Masson's Trichrome and Picrosirius Red, Fig. 3E, H) and appreciable cellular infiltration (H&E, Fig. 3E). In unseeded grafts, stenotic tissue was characterized by excessive ECM deposition (Fig. 3I, L). Little to no elastin deposition occurred in either group (Hart's, Fig. 3G, K). Minimal vascular calcification was observed, despite the limited in vivo degradation of the scaffold (Fig. 3F, J).
BM-MNC seeding modulates host inflammation
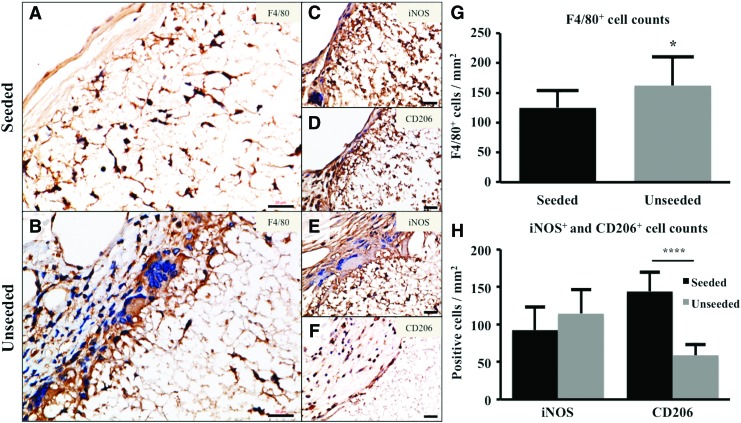
Immunohistochemical staining for F4/80+ macrophages demonstrated significantly less macrophage infiltration in seeded scaffolds at 6 months (Fig. 4A, B, G, p < 0.05). Regarding macrophage phenotype (Fig. 4C–F), there was a trend that the number of iNOS-positive cells in seeded scaffolds was smaller than that in unseeded scaffolds, which did not reach statistical difference (Fig. 4H, p = 0.14). The number of CD206-positive cells was significantly higher in seeded scaffolds (Fig. 4H, p < 0.0001).
FIG. 4.
BM-MNC seeding of electrospun TEVG modulated host inflammatory cell infiltration and phenotype. F4/80 staining of seeded (A) and unseeded (B) electrospun TEVGs. Phenotypic characterization of TEVG macrophages demonstrated iNOS+ (Seeded: C; Unseeded: E) and CD206+ (Seeded: [D]; Unseeded: [F]) cells. F4/80+ cells in the seeded vs. unseeded group at 6 months (G); seeded group revealed significantly less macrophage infiltration (*p < 0.05). iNOS+ and CD206+ cells in the seeded versus unseeded group at 6 months (H); unseeded group showed significantly less CD206+ cells (****p < 0.0001). Scale bar = 20 μm. Color images available online at www.liebertpub.com/tea
Cell-seeded nanofiber scaffolds support formation of well-organized luminal neotissue
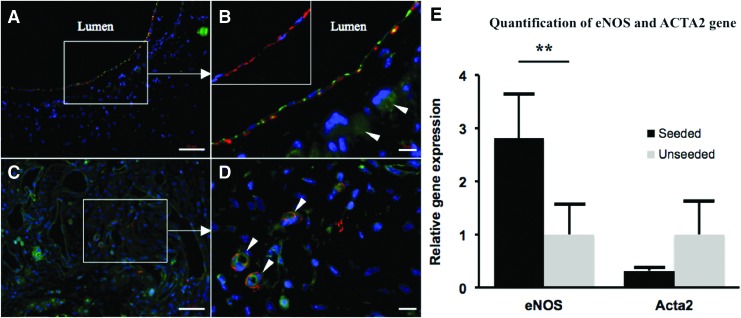
Immunofluorescent analysis of TEVG neotissue indicated the formation of well-organized neo-intima in the seeded group characterized by a medial α-SMA+ smooth muscle cell layer lined by a CD31+ endothelial cell monolayer (Fig. 5A, B). Subsequent staining for SM-MHC in the seeded group confirmed that the luminal smooth muscle cells were mature and contractile (Fig. 5B inset). As expected, the unseeded TEVG neotissue was characterized by an unorganized population of α-SMA+ and CD-31+ cells (Fig. 5C), and evidence of re-canalization was appreciated (Fig. 5D). RT-qPCR indicated less ACTA2 gene transcription and significantly more eNOS transcription in the seeded group (Fig. 5E, p < 0.005).
FIG. 5.
Cell-seeded nanofiber scaffolds are characterized by well-organized and mature vascular neotissue. Immunofluorescent staining of BM-MNC-seeded scaffolds indicated the formation of well-organized neo-intima characterized by a medial α-SMA+ (red) smooth muscle cell layer lined by CD31+ (green) endothelial cells (A, B, arrowheads denote background staining due to remaining scaffold material). SM-MHC staining (red) confirmed that the medial smooth muscle cells in the seeded group were mature and contractile (B, inset). Unseeded TEVG neotissue was characterized by an unorganized population of α-SMA+ and CD-31+ cells (C), with evidence of re-canalization (D, arrowheads indicate neovascularization of stenotic tissue). Quantification of eNOS and ACTA2 gene transcription by RT-qPCR (E); less ACTA2 and significantly more eNOS gene transcription in the seeded group (**p < 0.005). Scale bar = 50 μm for (A, C), 10 μm for (B, D). RT-qPCR, reverse transcription-quantitative polymerase chain reaction. Color images available online at www.liebertpub.com/tea
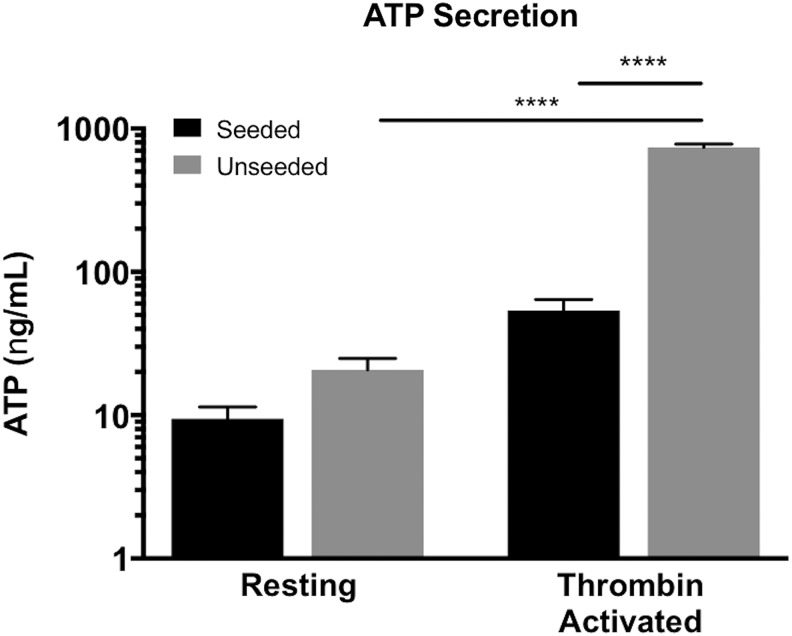
BM-MNC seeding of electrospun PGA/PLCL scaffolds attenuated platelet-derived ATP
Since the occlusive luminal narrowing of the unseeded TEVGs was observed as early as 4 weeks after implantation, we suspected thrombosis and, therefore, investigated the interaction of activated platelets (distinguished by increased ATP secretion) with seeded and unseeded scaffolds in vitro. Platelets are among the first immune cells to interact with an implanted vascular graft, and aberrant platelet activation could initiate a cascade involving thrombus formation, fibrin deposition, inflammatory cell recruitment, mesenchymal cell proliferation, and progressive neo-intimal hyperplasia. We found a significant difference in the quantity of measureable ATP from thrombin-activated platelets between seeded and unseeded scaffolds (Fig. 6, p < 0.001). Interestingly, although a significant difference in the amount of ATP secreted between thrombin-activated and resting platelets in the unseeded group (Fig. 6, p < 0.001) was found, no difference was appreciated in the seeded group, suggesting that BM-MNC seeding may attenuate adverse platelet function.
FIG. 6.
Cell seeding reduced measurable ATP from thrombin-activated platelets. The quantity of ATP derived from thrombin-activated platelets in the presence of BM-MNC-seeded scaffolds was significantly less than in the presence of unseeded scaffolds (****p < 0.001). In addition, after incubation with unseeded scaffolds, a significant difference in the amount of ATP derived from thrombin-activated and resting platelets was found (****p < 0.001), but no difference was observed in the seeded group, suggesting that BM-MNC seeding may regulate platelet function.
Discussion
The results of this study demonstrated the effect of BM-MNC seeding in a novel electrospun vascular graft. BM-MNC seeding of nanofiber scaffolds prevented stenosis, compared with unseeded scaffolds over 6 months. The patent vascular grafts showed concentric laminated smooth muscle cells, a confluent endothelial monolayer, and collagen-rich ECM. Platelet-derived ATP was significantly reduced after incubating thrombin-activated platelets in the presence of BM-MNC-seeded nanofiber scaffolds compared with -unseeded scaffolds. Macrophage infiltration into the cell-seeded grafts was significantly less than -unseeded grafts. These data suggest that BM-MNC seeding on nanofiber vascular grafts attenuated stenosis through the prevention of host macrophage infiltration and platelet function, which led to well-organized neotissue formation.
Cell seeding reduces monocyte/macrophage infiltration, expression of proinflammatory markers, and promotes TEVG patency and longevity.7,12,18 We have previously demonstrated that BM-MNC cell seeding effectively attenuates intimal hyperplasia in both murine7,12,18 and ovine24,25 models, and that this effect is dose responsive.18 However, the scaffolds used in previous reports consisted of a nonwoven PGA that was felt sealed with a polymer solution of PLCL,26 which yielded a microarchitecture permitting a relatively high degree of cell infiltration (pore size: 45.54 ± 17.6 μm), rapid degradation kinetics (loss of tensile strength by 8 weeks), with favorable growth and remodeling over the time course of implantation.27,28 The model scaffold examined in this article had a much smaller fiber diameter (0.61 ± 0.24 μm) and pore size (9.12 ± 3.15 μm) than previous studies, which represents many electrospun constructs examined in recent literature.3 Despite the much smaller pore size of the scaffold, the role of cell seeding to attenuate inflammation and prevent graft stenosis was similar. These data indicate that the beneficial effects of BM-MNC seeding may, at least to a certain degree, be independent of scaffold type.
Macrophage phenotype (M1/M2) plays an important role in remodeling. Recent studies have suggested that cell-free nanofiber scaffolds directed macrophages into the M1 phenotype.29,30 In our previous scaffolds, we have demonstrated that BM-MNCs seeded into TEVGs reduced the overall influx of macrophages and the magnitude of M1 activation. Moreover, macrophages that infiltrate cell-seeded grafts do not undergo M1 to M2 transition, as observed in unseeded grafts.12 It is noted by F4/80 staining that macrophage infiltration was present in both seeded and unseeded grafts at 6 months due to remaining nanofiber scaffolds; however, this was significantly less in the seeded grafts. Interestingly, by iNOS and CD206 staining, there was higher M2 macrophage infiltration and lower M1 macrophage infiltration noted in the seeded scaffolds compared with the unseeded scaffolds. Daley et al. reported wound-healing macrophages with features of both M1 and M2 activation.31 Therefore, macrophages that populate cell-seeded grafts assume a unique activation state promoting remodeling, which is yet to be defined. If we can find specific markers of macrophage subtypes that affect graft patency, these would be useful for predicting graft stenosis in the clinical setting.
More recently, the regulation of platelet activity by seeded BM-MNCs has been considered, and significant platelet activation, aggregation, and mural thrombus formation/fibrin deposition are believed to initiate an inflammatory cascade leading to progressive vessel occlusion,32,33 which may be the root cause of stenosis observed in our model. In 2007, Hashi et al. revealed the antithrombogenic property of bone marrow mesenchymal stem cells (MSCs) in nanofiber vascular grafts; cross-sectional staining showed platelet activation/aggregation and thrombus formation on the luminal surface of acellular grafts but not of MSC-seeded grafts.34 In this article, we demonstrated that BM-MNC seeding of our electrospun grafts potently reduced measurable platelet-derived ATP in vitro, a molecule co-secreted with ATP from activated platelets that is implicated in the aggregation of platelets to a growing thrombus in vivo.
Regarding the seeding technique, the traditional cell seeding approach for vascular grafts is known as static cell seeding, and it involves the manual pipetting of cells directly onto a graft. However, this technique is operator dependent, as we are using small lumen diameter grafts (1 mm) in a murine infrarenal IVC interposition model. An operator-independent seeding method such as vacuum seeding would be effective for standardization in the future.35
BM-MNC seeding for nanofiber vascular grafts led to the attenuation of inflammation and the prevention of graft stenosis, as demonstrated by high M2 and low M1 macrophage infiltration and reduction of measurable platelet-derived ATP in vitro.
Although the results of this study are promising and clearly encourage further investigation, this experiment was limited by the small animal model and seeding technique in this study. We utilized a murine model for vascular graft implantation; however, the typical lifespan of mice is a maximum of 1–1.5 years. It is difficult to perform long-term experiments in mice with nanofiber scaffolds.
Conclusion
BM-MNC seeding of electrospun TEVG scaffolds prevents stenosis by modulating host macrophage and platelet function. To our knowledge, this study is the first to report the prevention of platelet activation using BM-MNC-seeded nanofiber biodegradable scaffolds. The incorporation of BM-MNC seeding into novel electrospinning approaches for cardiovascular tissue engineering may improve patency in the venous circulation and allow for the rational design of an optimized electrospun TEVG.
Acknowledgments
The Morphology Core at Nationwide Children's Hospital performed the paraffin embedding, sectioning, and all histologic stains, including: H&E, Masson's Trichrome, von Kossa, Hart's, and Picrosirius Red. This study was supported by grants from the NIH Center for Accelerated Innovations: Technology Development Program (1UH54HL119810-01) (Dr. Hibino), Thrasher Research Fund Early Career Awards (Dr. Hibino), and by electrospun nanofiber TEVGs from Nanofiber Solutions, Inc.
Disclosure Statement
Drs. Breuer and Shinoka receive research support from Gunze Ltd. (Kyoto, Japan) and Cook Regentec (Indianapolis, IN). Dr. Breuer is on the Scientific Advisory Board of Cook Medical (Bloomington, IN). Dr. Hibino receives research support from Secant Medical (Telford, PA). Jed Johnson is a co-founder of Nanofiber Solutions, Inc. (Columbus, OH). The remaining authors have no conflicts of interest to disclose.
References
- 1.Pham Q.P., Sharma U., and Mikos A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng 12, 1197, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Cleary M.A., Geiger E., Grady C., Best C., Naito Y., and Breuer C. Vascular tissue engineering: the next generation. Trends Mol Med 18, 394, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Rocco K.A., Maxfield M.W., Best C.A., Dean E.W., and Breuer C.K. In vivo applications of electrospun tissue-engineered vascular grafts: a review. Tissue Eng Part B Rev 20, 628, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Tara S., Rocco K.A., Hibino N., et al. Vessel bioengineering. Circ J 78, 12, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Rathore A., Cleary M., Naito Y., Rocco K., and Breuer C. Development of tissue engineered vascular grafts and application of nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 4, 257, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Barnes C.P., Sell S.A., Boland E.D., Simpson D.G., and Bowlin G.L. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev 59, 1413, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Roh J.D., Sawh-Martinez R., Brennan M.P., et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 107, 4669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tara S., Kurobe H., Rocco K.A., et al. Well-organized neointima of large-pore poly(L-lactic acid) vascular graft coated with poly(L-lactic-co-epsilon-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(L-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis 237, 684, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khosravi R., Best C.A., Allen R.A., et al. Long-Term Functional Efficacy of a Novel Electrospun Poly(Glycerol Sebacate)-Based Arterial Graft in Mice. Ann Biomed Eng 44, 2402, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiura T., Tara S., Nakayama H., et al. Fast-degrading bioresorbable arterial vascular graft with high cellular infiltration inhibits calcification of the graft. J Vasc Surg 2016, pii: ; DOI: 10.1016/j.jvs.2016.05.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Y., Yi T., Shinoka T., et al. Pilot Mouse Study of 1 mm Inner Diameter (ID) Vascular Graft Using Electrospun Poly(ester urea) Nanofibers. Adv Healthc Mater 5, 2427, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibino N., Yi T., Duncan D.R., et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25, 4253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibino N., Mejias D., Pietris N., et al. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB J 29, 2431, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin'oka T, Imai Y., and Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 344, 532, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Matsumura G., Hibino N., Ikada Y., Kurosawa H., Shin'oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials 24, 2303, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Shin'oka T., Matsumura G., Hibino N., et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 129, 1330, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hibino N., McGillicuddy E., Matsumura G., et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139, 431, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Lee Y.U., Mahler N., Best C.A., et al. Rational design of an improved tissue-engineered vascular graft: determining the optimal cell dose and incubation time. Regen Med 11, 159, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurobe H., Maxfield M.W., Breuer C.K., and Shinoka T. Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med 1, 566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y.U., Yi T., Tara S., et al. Implantation of inferior vena cava interposition graft in mouse model. J Vis Exp 2014. DOI: 10.3791/51632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goyal A., Wang Y., Su H., et al. Development of a model system for preliminary evaluation of tissue-engineered vascular conduits. J Pediatr Surg 41, 787, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Tara S., Kurobe H., de Dios Ruiz Rosado J., et al. Cilostazol, not aspirin, prevents stenosis of bioresorbable vascular grafts in a venous model. Arterioscler Thromb Vasc Biol 35, 2003, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun B., Tandon N.N., Yamamoto N., Yoshitake M., and Kambayashi J. Luminometric assay of platelet activation in 96-well microplate. Biotechniques 31, 1174, 1176, 1178 passim, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Brennan M.P., Dardik A., Hibino N., et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg 248, 370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurobe H., Tara S., Maxfield M.W., et al. Comparison of the biological equivalence of two methods for isolating bone marrow mononuclear cells for fabricating tissue-engineered vascular grafts. Tissue Eng Part C Methods 21, 597, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roh J.D., Nelson G.N., Brennan M.P., et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 29, 1454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito Y., Williams-Fritze M., Duncan D.R., et al. Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells Tissues Organs 195, 60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khosravi R., Miller K.S., Best C.A., et al. Biomechanical diversity despite mechanobiological stability in tissue engineered vascular grafts two years post-implantation. Tissue Eng Part A 21, 1529, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Cui Y., Wang J., et al. The effect of thick fibers and large pores of electrospun poly(epsilon-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 35, 5700, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Garg K., Pullen N.A., Oskeritzian C.A., Ryan J.J., and Bowlin G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 34, 4439, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daley J.M., Brancato S.K., Thomay A.A., Reichner J.S., and Albina J.E. The phenotype of murine wound macrophages. J Leukoc Biol 87, 59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz J.A., Obi A.T., Myers D.D. Jr., et al. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol 32, 556, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabinovich A., Cohen J.M., Cushman M., Kahn S.R., and BioSOX Investigators. Association between inflammation biomarkers, anatomic extent of deep venous thrombosis, and venous symptoms after deep venous thrombosis. J Vasc Surg Venous Lymphat Disord 3, 347, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Hashi C.K., Zhu Y., Yang G.Y., et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A 104, 11915, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soletti L., Nieponice A., Guan J., Stankus J.J., Wagner W.R., and Vorp D.A. A seeding device for tissue engineered tubular structures. Biomaterials 27, 4863, 2006 [DOI] [PubMed] [Google Scholar]