Abstract
Hydrogel and electrospun scaffold materials support cell attachment and neotissue development and can be tuned to structurally and mechanically resemble native extracellular matrix by altering either electrospun fiber or hydrogel properties. In this study, we examined meniscus tissue generation from different human cell sources including meniscus cells derived from vascular and avascular regions, human bone marrow-derived mesenchymal stem cells, synovial cells, and cells from the infrapatellar fat pad (IPFP). All cells were seeded onto aligned electrospun collagen type I scaffolds and were optionally encapsulated in a tricomponent hydrogel. Single or multilayered constructs were generated and cultivated in defined medium with selected growth factors for 2 weeks. Cell viability, cell morphology, and gene-expression profiles were monitored using confocal microscopy, scanning electron microscopy, and quantitative polymerase chain reaction (qPCR), respectively. Multilayered constructs were examined with histology, immunohistochemistry, qPCR, and for tensile mechanical properties. For all cell types, TGFβ1 and TGFβ3 treatment increased COL1A1, COMP, Tenascin C (TNC), and Scleraxis (SCX) gene expression and deposition of collagen type I protein. IPFP cells generated meniscus-like tissues with higher meniscogenic gene expression, mechanical properties, and better cell distribution compared to other cell types studied. We show proof of concept that electrospun collagen scaffolds support neotissue formation and IPFP cells have potential for use in cell-based meniscus regeneration strategies.
Keywords: : meniscus, biomimetic materials, adult stem cells
Introduction
Meniscus tears are the most frequently recorded orthopedic diagnosis and a common cause of knee impairment and dysfunction.1–4 Traumatic or degenerative meniscal lesions and tears that occur in the inner avascular region of meniscus are commonly more extensive, are more complex, and due to the lack of vascularity, possess limited self-healing properties.5 Such lesions and tears disrupt the fibrous architecture of the meniscus that impairs normal load transmission within the joint6 and can result in osteoarthritis.7 Partial or total meniscectomy is a common procedure in symptomatic patients; however, these procedures do little to prevent late onset secondary osteoarthritis.8–10
To overcome the disadvantages partial or total meniscectomy following degeneration or tear in the avascular region, alternative means to restore native tissue function may be achieved by improving surgical repair techniques (sutures and implants) and by encouraging cell growth and new reparative tissue formation. However, tears located in the avascular region or spanning vascular and avascular regions still pose a significant challenge. Tissue-engineered collagen constructs (without cells and growth factors), also known as the collagen meniscus implant,11 have been implanted in ∼200 patients with varying results.12,13
To address the lack of consistent clinical success with acellular approaches, a number of cell-based strategies have been tested to improve the healing of a torn meniscus using cultured meniscus cells or stem cells. Collagen scaffolds combined with human bone marrow-derived mesenchymal stem cell (hMSC) were used to sandwich constructs of two white-zone ovine meniscus discs.14 A swine chondrocyte-fibrin glue suspension was utilized as a biological glue to improve bonding between two meniscal slices obtained from swine menisci.15 VICRYL® mesh scaffold seeded with chondrocytes from different sources (articular, auricular, and costal) were implanted into a porcine bucket-handle lesion model; however, this mainly generated scar-like tissue.16,17 More relevant to meniscus regeneration, bovine MSCs seeded onto nanofibrous scaffolds comprised of poly(ɛ-caprolactone) (PCL)-generated multilayered constructs with promising collagen content and biomechanical properties.18 Bovine meniscal cells have also been cultured in collagen I gels to engineer meniscus-like tissue.19 These studies illustrate the potential of cell-based therapies to improve meniscus repair. However, none have been translated for clinical applications in part due to the lack of a clinically relevant source of cells.
Several cell sources have been tested for meniscus healing, along with meniscus fibrochondrocytes,20–22 chondrocytes,23–25 MSCs,26,27 synovium-derived stem cells,28,29 infrapatellar fat pad (IPFP) progenitors,28,30 and adipose-derived stem cells.31–33 However, it has not yet been determined which cell type is ideal for repair.
Hydrogel-based biomaterial systems have potential for tissue reconstruction by serving as temporary scaffolds and cell delivery vehicles for tissue engineering approaches. Hydrogels are biocompatible cross-linkable hydrophilic polymers,34 with a high water content and a low mechanical modulus, which make them attractive as soft-tissue engineering constructs.35 For creating nanofibrous scaffolds to emulate the structure of native tissue architectures like meniscus, electrospinning is an attractive method.36 Electrospun fibers are produced by applying voltage to charged polymer solutions.37,38 Electrospinning has attracted interest in tissue engineering with a fibrous structure, and mimics components of the extracellular matrix (ECM). Fiber diameters in the nanometer to micrometer range possess a high surface area-to-volume ratio and increase cell contact area.39,40 Electrospun materials can be tuned to structurally and mechanically resemble native ECM by controlling fiber diameter and fiber orientation. Nanofibers can also reinforce the poor mechanical properties of hydrogels; both can support cell attachment and proliferation.39,41 We previously demonstrated that polylactic acid electrospun scaffolds can be fabricated with anisotropic properties by altering the alignment of the electrospun fibers. These advantages of electrospun constructs support potential for meniscus tissue engineering.42
Gene expression is often used as an early and sensitive marker of tissue regeneration. However, genes specific for the meniscus phenotype are not clearly defined. The expression of COL1A1 and COMP are typically used as important matrix components.43 SOX9 is a major transcription factor regulating differentiation of mesenchymal cells.44,45 COL2A1 and ACAN are markers of chondrocytic matrix.46 In a microarray study of meniscal cell culture, we found that THY-1 is a marker of meniscus cell dedifferentiation and CHAD is a marker cell redifferentiation.47,48 Tenascin C (TNC) has been shown to be important for meniscus development,49–51 is one of the hallmarks of fibroblastic phenotype52,53 and has been shown to be upregulated during repair of meniscus injury.54 The tendon transcription factor, Scleraxis (SCX), is expressed by meniscus cells following chondrocyte growth factor stimulation.55
Our objective was to identify clinically relevant cell sources and culture conditions that generated tissue replicating the important features of the meniscus. Toward that objective, we utilized the electrospinning process to produce electrospun scaffolds comprised of collagen type I with a fiber arrangement that emulated circumferential meniscus fibers. We assessed cell morphology and density and assessed the quality of engineered tissue when seeded with either human meniscus cells harvested from vascular and avascular regions, MSCs, synovial cells, or stromal cells from IPFP. Finally, to demonstrate potential for tissue fabrication at a clinically relevant scale, we created multilayered constructs composed of electrospun scaffolds with each of the aforementioned human cells embedded in a tricomponent hydrogel.
Materials and Methods
Fabrication of electrospun collagen type I scaffolds
Bovine Collagen type I (Semed S, acid-soluble; DSM) at 16% (w/v) was dissolved in 20 × phosphate-buffered saline (PBS) in ethanol at a ratio of 1:1 v/v as described previously.56 The collagen solution was placed in a syringe, which was controlled by a syringe pump (KDS200; KD Scientific, Inc.) at a feeding rate of 0.1 mL/h. A Teflon tube (Dimensions: 2.15 mm, inner diameter × 3.25 mm, outer diameter; Scientific Commodities, Inc.) was used to connect the syringe and a 21-G needle. All collector surfaces were covered with aluminum foil. To spin the aligned fibers, a rotating drum (∼2400 rpm) was placed 12 cm from the needle tip that was tilted at 45° to the drum (DC90 portable type, size: Ø90 × W200, source: stainless steel-drum, PTFE-body; NanoNC). A voltage of 18 kV was applied using a voltage-regulated DC power supply (NNC-30 kV-2 mA portable type; NanoNC) to eject the polymer jet. Electrospinning was performed in a fume hood under clean room conditions. Surfaces and equipment was sterilized by alcohol. The drum was covered by aluminum foil. Collagen nanofibers were deposited on the aluminum foil and were collected in the form of a cylindrical sheet, which was removed from the drum and cut onto a flat sheet. Electrospun collagen scaffolds were cross-linked by soaking the mats in 0.25% glutaraldehyde (Sigma-Aldrich) in 1 × PBS for 1 h. After fixation, the scaffolds were washed thrice with ethanol for 10 min per wash and stored at 4°C. This process of chemically cross-linking the electrospun scaffolds also disinfected the scaffolds.
Tissues and cell isolation
Normal human meniscus (medial and lateral) was obtained from tissue banks (approved by the Scripps Institutional Review Board) from three donors (mean age: 34.6 ± 3.21; age range: 31–37 years; two men and one woman). A macroscopic and histologic grading system57 was used to select normal menisci: representative sagittal sections of menisci (0.5–1 cm thick) were fixed and processed for paraffin embedding followed by histological evaluations as described by Pauli et al.57 The outer 1/3 (vascular) portion of the meniscus and inner 2/3 (avascular) were separated and minced with a surgical blade and were enzymatically digested using collagenase (2 mg/mL; C5138; Sigma-Aldrich) in Dulbecco's Modified Eagle's Medium (DMEM) (Mediatech, Inc.) and 1% Penicillin-Streptomycin-Fungizone (Life Technologies) for 5–6 h. Digested tissues were filtered through 100 μm cell strainers (BD Biosciences) and were seeded in monolayer culture at a density of 2.5 × 105 cells per T175 cm2 flask in DMEM (Mediatech) supplemented with 10% calf serum (Omega Scientific, Inc.) and 1% Penicillin/Streptomycin/Gentamycin (Life Technologies). Meniscus cells were cultured for one passage before use in the scaffold seeding experiments.
Human MSCs were purchased from Lonza or were supplied by Texas A&M (Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott and White). MSCs were cultured in Lonza MSC medium (Lonza) and were used at passage four.
IPFP and synovial cells (from the suprapatellar pouch of the knee) were harvested and isolated from human knees obtained from tissue banks (IPFP donor mean age: 41 ± 12; age range: 29–52 years; two men and one woman, synovial donor mean age: 41 ± 30; age range: 22–75 years; two men and one woman) within 72 h after death. IPFP and synovial tissues were rinsed with sterilized 1 × PBS and, after mincing with a surgical blade, the fragments were placed for overnight digestion under constant rotation at 37°C with collagenase (2 mg/mL; C5138; Sigma-Aldrich) in DMEM (Mediatech) and 1% Penicillin-Streptomycin-Fungizone (Life Technologies). The digested tissues were filtered through a cell strainer with pore size of 100 μm (BD Biosciences) and the cells were washed in 1 × PBS in 50 mL conical tubes by centrifuging (1500 rpm for 5 min, three times, Beckman Coulter, Allegra X-15R Centrifuge [5250 g]). The isolated synovial and IPFP cells were suspended in expansion medium, DMEM (Mediatech) supplemented with 10% calf serum (Omega Scientific, Inc.) and 1% Penicillin/Streptomycin/Gentamycin (Life Technologies). Cells were expanded for two passages before seeding on scaffolds.
Single layer scaffold cell culturing
Cultured human meniscus cells derived from the vascular and avascular regions, MSCs, synovial cells, and IPFP cells were seeded onto discs (5 mm in diameter), were cut using a 5-mm dermal biopsy punch, at a final density of 0.25 × 106 per scaffold. Cell-seeded scaffolds were maintained in 3 mL of monolayer culture medium in six-well plates for 3 days for initial cell attachment and scaffold colonization. After 3 days, the culture medium was changed to serum-free ITS + medium (Sigma) supplemented with 10 ng/mL TGFβ1 (PeproTech), TGFβ3 (PeproTech), or no growth factor (controls), with medium changes every 3–4 days for 2 weeks.
Multilayer construct formation
We used two approaches to produce multilayer cell-seeded scaffolds: (i) cells encapsulated in a tricomponent hydrogel comprised of collagen type II, chondroitin sulfate, and hyaluronan or (ii) cells seeded directly on the scaffolds. The tricomponent hydrogel was prepared using soluble collagen type II (10 mg/mL; Innovative Research; dissolved in 10 mM acetic acid and then neutralized in NaCOH3 and Medium 199), chondroitin sulfate (2.8 mg/mL; Sigma-Aldrich), and hyaluronan (3 mg/mL; Supartz®, manufactured by Seikagaku Corporation, and distributed by Smith & Nephew Orthopaedics). Isolated human vascular and avascular meniscus cells, MSCs, synovial cells, and IPFP cells were suspended in the tricomponent hydrogel at 6 × 106 cells/mL (pink discs in Fig. 1A). Aligned collagen scaffolds were cut into discs (5 mm in diameter) (blue discs in Fig. 1A). Cells in tricomponent hydrogel were seeded onto each disc and the three discs were multilayered and encapsulated with 2% alginate (PRONOVA UP LVG; NovaMatrix) cross-linked in calcium chloride (120 mM; Sigma) for 20 min.42
FIG. 1.
Schematic and cell density of three-dimensional cultures on electrospun collagen scaffolds with or without hydrogel, and pictures of the constructs as representative. (A) Schematic representation of the multilayered constructs of collagen-aligned fibrous scaffolds with cells encapsulated within the tricomponent hydrogel with meniscus cells. (B) Schematic representation of the multilayered constructs of collagen-aligned fibrous scaffolds with cells excluding tricomponent hydrogel. (C) Macro top-view showing half a disc (scale in millimeters). (D) Macro side-view of half a disc of hMSCs was encapsulated in tricomponent hydrogel between electrospun collagen scaffold sheets. (E) Cell density in the multilayered collagen constructs with or without hydrogel. *p < 0.05, **p < 0.005, ***p < 0.0005; ++p < 0.05 compared to vas, avas, MSC, ##p < 0.05 compared to vas, avas, MSC, and synovial. §p < 0.05 compared to avas, #p < 0.05 compared to vas and avas, and +p < 0.05 compared to vas. hMSC, human bone marrow-derived mesenchymal stem cell.
In the second approach, cells were seeded directly onto aligned collagen scaffolds cut into discs (5 mm in diameter). Two cell-laden scaffold layers were stacked (pink discs in Fig. 1B) and covered by one acellular collagen layer (blue discs in Fig. 1B). To hold the multiple layers during culture, the constructs were covered by a layer of 2% alginate (PRONOVA UP LVG; NovaMatrix) and were cross-linked in calcium chloride (120 mM; Sigma) for 20 min.
Multilayered constructs (with or without tricomponent hydrogel) seeded with each cell type (meniscus cells, MSCs, synovial cells, or IPFP) were cultured in 3 mL of culture medium, DMEM (Mediatech) supplemented with 10% calf serum (Omega Scientific, Inc.) and 1% Penicillin/Streptomycin/Gentamycin (Life Technologies) for 3 days to permit cell attachment and scaffold colonization. After 3 days, the culture medium was changed to serum-free ITS + medium (Sigma) supplemented with 10 ng/mL TGFβ1 (PeproTech), TGFβ3 (PeproTech), or no growth factor (controls). The constructs were then cultured for 2 weeks with medium changes every 3–4 days.
Cell viability assessments
Viability was assessed after 2–3 days. The live/dead kit consisting of Calcein-AM and Ethidium Homodimer-1 (Life Technologies) was used for viability assessments using a laser confocal microscope (LSM-510; Zeiss).42
Cell density assessments
Cells density in multilayered constructs was calculated after 2 weeks of culture. Hematoxylin and eosin (H&E) and 4′, 6-diamidino-2-phenylindole (DAPI)-stained images were analyzed using ImageJ v1.48 (National Institutes of Health). Cell nuclei were counted in 20 randomly superimposed fields of view at 40 × magnification and cell density was calculated as the total number of cells divided by the total area of the fields of view.
Scanning electron microscopy
Scanning electron microscopy (SEM) was employed to observe high-resolution features of cell and matrix grown on a single layer of an electrospun collagen scaffold. After 2 weeks in culture, the cell-seeded substrates were washed with 1 × PBS and were fixed with 2.5% w/v glutaraldehyde (Sigma-Aldrich) in 1 × PBS for 1 h. After fixation, the samples were washed thrice with PBS for 10 min each wash. Following dehydration in a graded series of ethanol (50%, 70%, and 90%) for 30 min each, the samples were maintained in 100% ethanol for 24 h at 4°C. While in 100% ethanol, the samples were completely dried in a critical point dryer (Autosamdri-815, Series A; Tousimis, Inc.). The surfaces of the dried samples were metalized by sputter coating with iridium for SEM examination. The morphology of the samples and that of the adherent cells was observed by SEM (Philips XL30; FEI Co.).
Histology and immunohistochemistry
Multilayered constructs seeded with the different human cells were fixed in Z-Fix (ANATECH) and were embedded in paraffin. Sections (5–7 μm thick) were stained with H&E for the study of morphological details and with Safranin O-fast green to assess glycosaminoglycan distribution. For detection of collagen type I by immunohistochemistry (IHC), cut sections were treated with hyaluronidase for 2 h and were incubated with a primary antibody against collagen type I (Anti-col I: ab34710; Abcam) at 2 μg/mL.58 We have previously shown that this antibody only stains newly synthesized collagen and not the electrospun collagen fabricated from acid solubilized collagen powder.59 Secondary antibody staining and detection procedures were followed as previously described.60 An isotype control was used to monitor nonspecific staining.60 Cell nuclei were stained with VECTASHIELD® mounting medium containing DAPI (Vector Laboratories).
RNA isolation and quantitative polymerase chain reaction
Total RNA was isolated from single and multilayered constructs with or without hydrogel containing human meniscus cells, MSCs, synovial cells, or IPFP cells cultured for 2 weeks (n = 3 donors, two replicates) using the RNeasy Mini Kit (Qiagen). The first strand cDNA was made according to the manufacturer's protocol (High-Capacity cDNA Reverse Transcription Kits; Applied Biosystems). Quantitative polymerase chain reaction was performed using TaqMan® gene expression reagents. COL1A1 (Hs00164004_m1; Applied Biosystems), COL2A1 (Hs00264051_m1; Applied Biosystems), ACAN (Hs00153936; Applied Biosystems), SOX9 (Hs00165814; Applied Biosystems), COMP (Hs01572837_g1; Applied Biosystems), THY-1 (Hs00174816; Applied Biosystems), CHAD (Hs00154382; Applied Biosystems), TNC (Hs01115665_m1; Applied Biosystems), SCX (Hs03052634_g1; Applied Biosystems), and GAPDH (4352934E; Applied Biosystems) were detected using Assays-on-Demand™ primer/probe sets (LightCycler® 480 Probes Master; Applied Biosystems). Gene expression was normalized relative to GAPDH expression using the ΔCt method.61
Mechanical properties of multilayered constructs
The tensile mechanical properties of multilayered constructs after 1 week in culture were measured under four conditions (n = 1 donor, nine replicates per group): (i) freshly constructed acellular multilayered constructs without hydrogel, which were multilayered constructs without cells 2 h after soaking in 1 × PBS, (ii) acellular multilayered constructs with hydrogel, which were multilayered constructs without cells, (iii) human avascular meniscus cells encapsulated in hydrogel (avascular meniscal cell-laden multilayered constructs), and (iv) human IPFP cell (IPFP cell-laden multilayered constructs). Electrospun scaffolds were cut into dog bone-shaped specimens with a gauge length of 8 mm and width of 2 mm as previously described.42 The thickness of each construct was measured using a digital caliper. The specimens were mounted in the grips of a uniaxial testing machine (Instron® Universal Testing Machine; 3342 Single Column Model) with a 500 N load cell and were tested for failure at a crosshead speed of 1 mm/min. Young's modulus was calculated from the slope of the linear segment of the stress–strain curve. Ultimate tensile strength was calculated at the maximum load before failure. Values were presented as mean ± standard deviation (SD).
Statistical analysis
Values are presented as mean ± SD. Kruskal–Wallis nonparametric tests were performed to determine significant differences in cell density, gene expression, and mechanical properties between groups. Post hoc Mann–Whitney tests with Bonferroni correction were used to analyze the statistical significance of pairwise differences. p-Values <0.05 were considered significant.
Results
Cell morphology and density
Human meniscus cells, MSCs, synovial cells, and IPFP were seeded on aligned scaffolds of electrospun collagen. Seeded cells were elongated in line with the direction of the fibers on confocal microscopy (after 2–3 days, Fig. 2F–J) and on SEM (after 14 days, Fig. 2A–E). Cell viability after 3 days in culture was >85% without significant differences between cell types (data not shown). To create thicker meniscus-like graft tissues, multilayer constructs of aligned electrospun collagen scaffolds were fabricated and seeded with cells with or without encapsulation in a tricomponent hydrogel (Fig. 1A–C). Encapsulating cells in tricomponent hydrogel increased cell density for all cell types (Fig. 1E). Cell type also affected cell density, with synovial and IPFP cells generating the highest density approximately twice the density of avascular meniscus cells.
FIG. 2.

Cellular response to single aligned collagen fibrous scaffolds. Scanning electron microscopy of (A) vascular, (B) avascular human meniscus cells, (C) MSCs, (D) synovial, and (E) IPFP cells cultivated on aligned electrospun collagen fibers (Mag. 625 × ; scale bar: 5 μm). (F) vascular, (G) avascular human meniscus cells, (H) MSCs, (I) synovial, and (J) IPFP cells on aligned scaffolds demonstrating viability (live/dead) and aligned cells cultivated on collagen scaffolds (Mag. 10 × ; scale bar: 200 μm in confocal images). White arrows indicate fiber direction on each image. IPFP, infrapatellar fat pad.
Multilayered collagen constructs support meniscus-like neotissue formation
Histological analysis of multilayered constructs created with or without tricomponent hydrogels revealed cells embedded in newly formed tissue between the electrospun layers consisting of ECM that was negative for Safranin O but positive for collagen type I (Figs. 3 and 4). Without hydrogel, the seeded cells remained between the layers and did not populate the electrospun scaffolds. Encapsulating cells in the hydrogel apparently induced migration of synovial and IPFP cells into the electrospun scaffolds. IPFP cells generated more ECM positive for collagen type I than the other cell types (Figs. 3C, D, and 4C, D) that were associated with higher cell density (Fig. 1E).
FIG. 3.
Histological analysis of three-dimensional cultures of human meniscus vascular and avascular cells, hMSCs, synovial, and IPFP cells on electrospun collagen scaffolds without hydrogel (A) H&E, (B) Safranin O fast green, (C) DAPI, and (D) IHC collagen type I stain of all cell types for multilayered constructs without hydrogel. (Mag. = 40 × , scale bar: 100 μm). H&E, hematoxylin and eosin; DAPI, 4′, 6-diamidino-2-phenylindole; IHC, immunohistochemistry.
FIG. 4.
Histological analysis of three-dimensional cultures of human meniscus vascular and avascular cells, hMSCs, synovial, and IPFP cells on electrospun collagen scaffolds embedded in the tricomponent hydrogel. (A) H&E, (B) Safranin O fast green, (C) DAPI, and (D) IHC collagen type I stain of all cell types encapsulated within hydrogel for multilayered constructs. (Mag. = 40 × , scale bar: 100 μm).
Modulation of meniscogenic genes
We measured gene expression of COL1A1, COL2A1, COMP, and ACAN for matrix proteins; SOX9, THY-1, and CHAD for mesenchymal differentiation; and TNC and SCX for meniscal growth and development. COL1A1, COMP, TNC, and SCX were significantly upregulated by culturing cells on scaffolds (Fig. 5). COL2A1, ACAN, SOX9, THY-1, and CHAD were not significantly changed relative to monolayer culture (data not shown).
FIG. 5.
Relative fold change in gene expression of human vascular and avascular meniscus cells, MSCs, synovial, and IPFP cells of single collagen scaffolds and multilayered collagen constructs without hydrogel or embedded in the tricomponent hydrogel. (A) Gene expression of different cell types on single collagen scaffolds, (B) of different cell types encapsulated without or (C) within the tricomponent hydrogel for multilayered constructs (n = 3 donors, with duplicates). Expression levels are relative to monolayer controls (dotted line). Each (i) COL1A1, (ii) COMP, (iii) TNC, and (iv) SCX gene expression of all different type of cells encapsulated within or without hydrogel for multilayered constructs (n = 3 donors, with duplicates). Line = p < 0.05 compared to different condition (CNT = without growth factors, TGFβ1, and TGFβ3); ++p < 0.05 compared to vas, avas, MSC, and synovial cells, ##p < 0.05 compared vas, avas, and MSC cells, lllp < 0.05 compared to vas, MSC, and synovial cells, *+p < 0.05 compared to vas, avas, and synovial cells, §+p < 0.05 compared to vas, MSC, and synovial cells, #+p < 0.05 compared to vas, and synovial cells, #$p < 0.05 compared to avas, and synovial cells, §§p < 0.05 compared to vas, and MSC cells, llp < 0.05 compared to avas, and MSC cells,**p < 0.05 compared to vas, and avascular cells, +p < 0.05 compared to synovial cells, §p < 0.05 compared to MSC cells #p < 0.05 compared to avascular cells, *p < 0.05 compared to vascular cells.
Differences between constructs
There were overall similarities in relative gene expression of cells in single or multilayered scaffolds. A notable exception was a relatively lower increase in TNC for MSC in multilayered constructs. Hydrogel encapsulation also did little to change relative gene expression. Almost all genes were upregulated in response to TGF-β, with little difference between the isoforms TGF-β1 or TGF-β3. COMP expression was the highest among the genes analyzed, for all cell types and conditions, often reaching a 1000-fold increase.
Differences between cell types
Gene expression was similar in cells from vascular or avascular regions of the meniscus, except for SCX, which was higher in avascular cells in multilayered constructs. Vascular and avascular meniscal cells also expressed the most SCX but the least TNC. MSC, synovial, and IPFP cells expressed higher levels of TNC compared to the meniscus cells. IPFP expressed the highest COL1A1, COMP, and TNC in multilayered constructs.
Tensile mechanical property of multilayered constructs dependent on existing cells or cell types
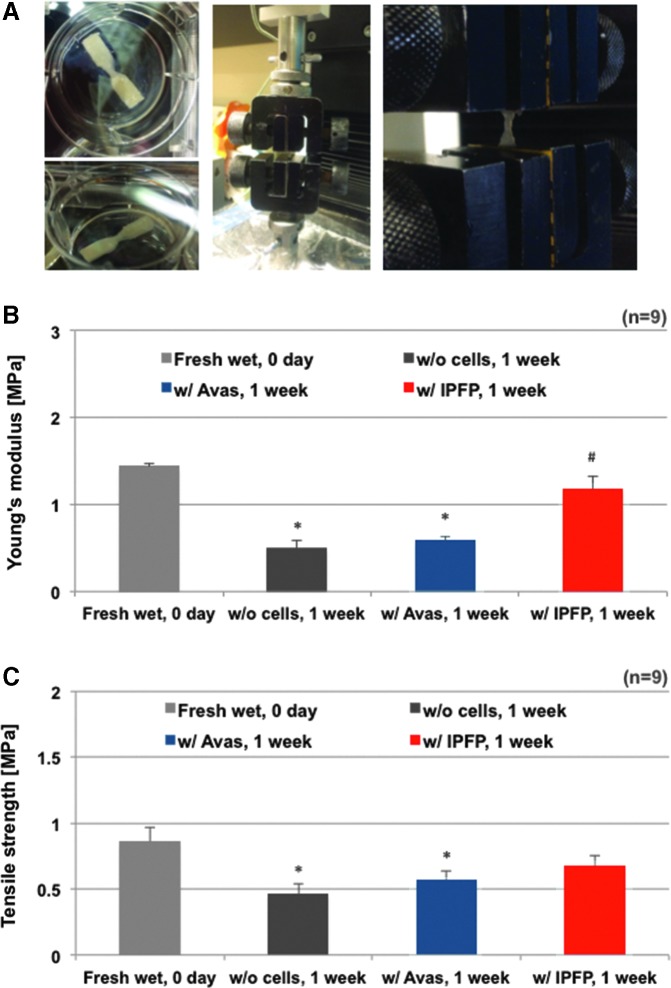
The gene expression and histology (especially the IHC) indicated that IPFP generated by far the most de novo type I collagen, which was the major structural protein in meniscal tissue. Therefore, we selected IPFP constructs for mechanical testing and compared to meniscal cells as positive controls. Tensile mechanical properties of human meniscus avascular and IPFP cell-seeded constructs were assessed over 1 week in culture (Fig. 6). The stiffness of all constructs decreased after 1 week in culture. However, IPFP cell-seeded constructs generated higher stiffness than meniscus avascular cells and acellular scaffolds (p < 0.05), which restored almost all the lost stiffness
FIG. 6.
Mechanical strength of three-dimensional cultures of human meniscus and IPFP cells on electrospun collagen scaffolds embedded in the tricomponent hydrogel. The mechanical properties of multilayered constructs were quantified via tensile testing (n = 1 donor, nine replicates group). (A) Images of the multilayered collagen construct specimen and the tensile strength testing process and (B) Young's modulus (p = 0.0007 among experimental groups), Kruskal–Wallis test; *p < 0.005 versus freshly wet acellular multilayered construct's Young's modulus, Mann–Whitney test; #p < 0.005 versus Young's modulus of IPFP-cell encapsulated within hydrogel on multilayered constructs cultured for 1 week, Mann–Whitney test and (C) ultimate tensile strength of three-dimensional constructs (p < 0.05 among experimental groups, Kruskal–Wallis test; *p < 0.05 vs. freshly wet multilayered constructs' tensile strength, Mann–Whitney test).
Discussion
Obtaining sufficient meniscal cells clinically for tissue engineering is challenging. Our objective was to identify clinically relevant cell sources and culture conditions that generated tissue replicating the important features. We therefore explored the potential for electrospun collagen scaffolds seeded with human cells from different sources to generate tissue capable of repairing or regenerating meniscal tears. We studied the meniscogenic potential of mesenchymal cells, synovial cells, and cells from IPFP. Previously, we have shown the potential for tissue engineering using meniscus cells isolated from human meniscus tissue seeded in biodegradable electrospun scaffolds.42,59 Baker et al.62 also demonstrated that expansion and seeding of meniscal debris-derived cells onto nanofibrous biodegradable scaffolds resulted in engineered constructs with mechanical properties approaching native tissue levels. Tissue obtained during meniscectomy is a limited source of meniscal cells.63 It is therefore critical that alternative cell sources be investigated for meniscogenic potential. In this study, we investigated several cell types to assess their capacity for meniscus tissue formation. Previously, we demonstrated meniscogenesis by seeding in single-layered collagen electrospun collagen scaffolds.59 A single electrospun layer is too thin to emulate the tissue thickness desired for a clinically relevant meniscus graft. Therefore, we fabricated thicker constructs by layering up to three aligned collagen scaffolds as proof of concept in engineering fiber-reinforced meniscal tissues.
All the cell types we studied, readily attached to the collagen scaffolds with high initial cell viability and resulted in high cell density after 2 weeks of culture. As evidence of de novo ECM production, we observed high COL1A1 mRNA levels, and deposition of de novo collagen type I between the electrospun collagen fibers and throughout the multiple electrospun collagen layers within 2 weeks of culture. SOX9, ACAN, and COL2A1 gene expression remained low in all cells and corresponded with the lack of Safranin O stain, indicating low chondrocytic phenotype. SCX expression was relatively higher in avascular cells compared to vascular cells in keeping with another report on pellet cultures of meniscal cells.55 MSC, synovial, and IPFP cells expressed relatively more TNC than meniscal cells. TNC is a glycoprotein commonly found in tendons, articular cartilage, meniscus, and skin. Tenascin has been associated with the developing meniscus and could be a marker of early tissue generation.50 In multilayered gels with hydrogel, IPFP cells expressed among the highest COL1A1, TNC, and SCX gene expression.
Electrospinning is a convenient manufacturing technique for modulating the mechanical properties and anisotropy of tissue engineering scaffolds. We have generated fibrous scaffolds with a range of anisotropic behavior with different polymers and by modulating the alignment of the spun fibers.42,59 Culturing electrospun collagen fibers reduced the tensile stiffness within a week, presumably due to partial dissolution of collagen. However, IPFP cell-encapsulated multilayered constructs almost fully restored the mechanical properties by deposition of neotissue that was rich in collagen type I. The higher mechanical properties were related to the increase in relevant gene expression and were associated with a more even distribution of cell density throughout the engineered tissue.
Our cell-laden multilayered constructs also had higher tensile mechanical property compared to three-dimensional (3D) constructs reported in other studies. Kai et al.64 studied the mechanical properties of nanofiber-hydrogel composite by blending or co-axial electrospinning of two different materials: PCL and gelatin. The tensile properties of their blend and coaxial PCL/gelatin nanofibers after hydration was 0.13 ± 0.04 and 0.56 ± 0.09 MPa, respectively, which was substantially lower than the tensile properties of our multilayered constructs (Young's modulus: 1.18 ± 0.39 MPa). Our previous study of 3D methacrylated gelatin constructs fabricated using projection stereolithography also generated lower tensile modulus of 10.5 kPa.65 Xu et al.66 reported on multilayered constructs using a hybrid inkjet printing/PCL electrospinning system for cartilage tissue engineering applications and reported a tensile modulus of 1.76 MPa for their printed hybrid constructs. While this modulus is somewhat higher than ours, our fibrous scaffold was composed entirely of natural protein polymers and our hydrogel more closely mimicked the matrix components of native human meniscus avascular tissue.
We generated promising results from five different cell types: meniscus cells derived from vascular and avascular regions, MSCs, synovial cells, and IPFP cells. These results support the potential for tissue engineering by layering multiple collagen scaffolds. Our sample size was relatively low (n = 3 donors for gene expression and histology, and n = 1 donor for mechanical testing) and could be underpowered. However, the statistical significance of differences that we did find remained valid to support proof of concept. Several issues remain to be addressed to translate our approach to clinical application. The mechanical properties of the collagen scaffolds, while encouraging, were still lower than the optimal levels required to survive loading in vivo in the knee. We are developing methods to improve structural and mechanical properties of the construct. One approach is co-axial core/shell electrospinning using a synthetic biodegradable polymer as the core, to enhance the structural and mechanical properties with a protein shell to maintain cytocompatibility.
Summary
This study provides proof of concept for combining cells, fibrous scaffolds, and hydrogel to generate meniscus-like neotissue. Our data indicate that IPFP cells seeded onto electrospun collagen scaffolds with a tricomponent hydrogel resulted in the highest cell density, produced the most meniscus-like neotissues with a greater deposition of collagen type I, and generated the highest mechanical property compared to the meniscal cells, MSC, and synovial cells. Given the lack of readily available meniscus cells, the IPFP provides a more clinically relevant source of tissue for harvesting cells. Future studies to enhance mechanical properties of these constructs and in vivo validation are required before translation to the repair of human meniscal defects.
Acknowledgments
Funding provided by NIH/NIAMS P01 AG007996, NIH UL1 RR025774, and CIRM TR1-01216, by Donald and Darlene Shiley, and the Shaffer Family Foundation. We are grateful for article formatting and copyediting by Judy Blake. Some of the materials employed in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH, Grant # P40RR017447.
Author Contributions
J.B., S.P.G., S.J., and D.D.D. were responsible for the overall experimental design. J.B., S.P.G. and D.D.D. wrote the article in close collaboration with the other authors. J.B. and S.J. designed and conducted electrospinning and scanning electron microscopy. J.B., S.S., and W.C. conducted cell culture studies. J.B. and S.S. conducted RT-PCR and histology. J.B. and D.D.D. designed and performed mechanical tests. All authors discussed the results and approved the final version of the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Sweigart M.A., and Athanasiou K.A. Toward tissue engineering of the knee meniscus. Tissue Eng 7, 111, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Baker P., Coggon D., Reading I., Barrett D., McLaren M., and Cooper C. Sports injury, occupational physical activity, joint laxity, and meniscal damage. J Rheumatol 3, 557, 2002 [PubMed] [Google Scholar]
- 3.Boyd K.T., and Myers P.T. Meniscus preservation; rationale, repair techniques and results. Knee 10, 1, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Poulsen M.R., and Johnson D.L. Meniscal injuries in the young, athletically active patient. Phys Sportsmed 39, 123, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Setton L.A., Guilak F., Hsu E.W., and Vail T.P. Biomechanical factors in tissue engineered meniscal repair. Clin Orthop Relat Res 367(Suppl), S254, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Jones R.S., Keene G.C.R., Learmonth D.J.A., Bickerstaff D., Nawana N.S., Costi J.J., et al. Direct measurement of hoop strains in the intact and torn human medial meniscus. Clin Biomech 11, 295, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Roos H., Lauren M., Adalberth T., Roos E.M., Jonsson K., and Lohmander L.S. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum 41, 687, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Garrett W.E., Swiontkowski M.F., Weinstein J.N., Callaghan J., Rosier R.N., Berry D.J., et al. American board of orthopaedic surgery practice of the orthopaedic surgeon: part-II, certification examination case mix. J Bone Joint Surg Am 88, 660, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Englund M., Roos E.M., Roos H.P., and Lohmander L.S. Patient-relevant outcomes fourteen years after meniscectomy: influence of type of meniscal tear and size of resection. Rheumatology (Oxford) 40, 631, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Lohmander L.S., Englund P.M., Dahl L.L., and Roos E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries. Am J Sports Med 35, 1756, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Reguzzoni M., Manelli A., Ronga M., Raspanti M., and Grassi F.A. Histology and ultrastructure of a tissue-engineered collagen meniscus before and after implantation. Appl Biomater 74B, 808, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Vaquero J., and Forriol F. Meniscus tear surgery and meniscus replacement. Muscles Ligaments Tendons J 6, 71, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaffagnini S., Grassi A., Marcheggiani Muccioli G.M., Bonanzinga T., Nitri M., Raggi F., et al. MRI evaluation of a collagen meniscus implant: a systematic review. Knee Surg Sports Traumatol Arthrosc 23, 3228, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Pabbruwe M.B., Kafienah W., Tarlton J.F., Mistry S., Fox D.J., and Hollander A.P. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials 31, 2583, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Peretti G., Scotti C., Pozzi A., Mangiavini L., Vitari F., Domeneghini C., et al. Bonding of meniscal tissue: a nude mouse repair model. Sports Sci Health 3, 47, 2008 [Google Scholar]
- 16.Weinand C., Peretti G., Adams S., Jr., Randolph M., Savvidis E., and Gill T. Healing potential of transplanted allogeneic chondrocytes of three different sources in lesions of the avascular zone of the meniscus: a pilot study. Arch Orthop Trauma Surg 126, 599, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Weinand C., Peretti G.M., Adams S.B.J., Bonassar L.J., Randolph M.A., and Gill T.J. An allogenic cell-based implant for meniscal lesions. Am J Sports Med 34, 1779, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Fisher M.B., Henning E.A., Söegaard N., Bostrom M., Esterhai J.L., and Mauck R.L. Engineering meniscus structure and function via multi-layered mesenchymal stem cell-seeded nanofibrous scaffolds. J Biomech 48, 1412, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puetzer J., and Bonassar L. Physiologically distributed loading patterns drive the formation of zonally organized collagen structures in tissue-engineered meniscus. Tissue Eng Part A 22, 907, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Ibarra C., Jannetta C., Vacanti C.A., Cao Y., Kim T.H., Upton J., et al. Tissue engineered meniscus: a potential new alternative to allogeneic meniscus transplantation. Transplant Proc 29, 986, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Hidaka. C., Ibarra. C., Hannafin J.A., Torzilli P.A., Quitoriano M., Jen S.-S., et al. Formation of vascularized meniscal tissue by combining gene therapy with tissue engineering. Tissue Eng 8, 93, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Martinek V., Ueblacker P., Bräun K., Nitschke S., Mannhardt R., Specht K., et al. Second generation of meniscus transplantation: in-vivo study with tissue engineered meniscus replacement. Arch Orthop Trauma Surg 126, 228, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Peretti G.M., Caruso E.M., Randolph M.A., and Zaleske D.J. Meniscal repair using engineered tissue. J Orthop Res 19, 278, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Peretti G.M., Gill T., Xu J.-W., Randolph M.A., Morse K.R., and Zaleske D.J. Cell-based therapy for meniscal repair: a large animal study. Am J Sports Med 32, 146, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Weinand C., Randolph M., Peretti G., Adams S., and Gill T. Cellular repair of meniscal tears in avascular region (Abstract). Trans Orthop Res Soc 2004 [Google Scholar]
- 26.Dave L.Y.H., Nyland J., McKee P.B., and Caborn D.N.M. Mesenchymal stem cell therapy in the sports knee: where are we in 2011? Sports Health 4, 252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferris D., Frisbie D., Kisiday J., and McIlwraith C.W. In vivo healing of meniscal lacerations using bone marrow-derived mesenchymal stem cells and fibrin glue. Stem Cells Int 2012, 691605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsano A., Millward-Sadler S.J., Salter D.M., Adesida A., Hardingham T., Tognana E., et al. Differential cartilaginous tissue formation by human synovial membrane, fat pad, meniscus cells and articular chondrocytes. Osteoarthritis Cartilage 15, 48, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa Y., Muneta T., Kondo S., Mizuno M., Takakuda K., Ichinose S., et al. Synovial mesenchymal stem cells promote healing after meniscal repair in microminipigs. Osteoarthritis Cartilage 23, 1007, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Oda S., Otsuki S., Kurokawa Y., Hoshiyama Y., Nakajima M., and Neo M. A new method for meniscus repair using type I collagen scaffold and infrapatellar fat pad. J Biomater Appl 29, 1439, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Ibán M.Á., Díaz-Heredia J., García-Gómez I., Gonzalez-Lizán F., Elías-Martín E., and Abraira V. The effect of the addition of adipose-derived mesenchymal stem cells to a meniscal repair in the avascular zone: an experimental study in rabbits. Arthroscopy 27, 1688, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Pak J., Lee J.H., and Lee S.H. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. BioMed Res Int 2014, 436029, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi Y., Yang Z., Ding Q., Zhao T., Huang Z., and Feng G. Targeted transplantation of iron oxide-labeled, adipose-derived mesenchymal stem cells in promoting meniscus regeneration following a rabbit massive meniscal defect. Exp Ther Med 11, 458, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashyap N., Kumar N., and Kumar M.N. Hydrogels for pharmaceutical and biomedical applications. Crit Rev Ther Drug Carrier Syst 22, 107, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Peppas N.A., Hilt J.Z., Khademhosseini A., and Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater 18, 1345, 2006 [Google Scholar]
- 36.Sill T.J., and von Recum H.A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29, 1989, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Chew S.Y., Wen Y., Dzenis Y., and Leong K.W. The role of electrospinning in the emerging field of nanomedicine. Curr Pharm Des 12, 4751, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murugan. R., and Ramakrishna S. Nano-featured scaffolds for tissue engineering—a review of spinning methodologies. Tissue Eng 12, 435, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Liang D., Hsiao B.S., and Chu B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv Drug Deliv Rev 59, 1392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoo H.S., Kim T.G., and Park T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev 61, 1033, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Lee K.Y., and Mooney D.J. Hydrogels for tissue engineering. Chem Rev 101, 1869, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Baek J., Chen X., Sovani S., Jin S., Grogan S.P., and D'Lima D.D. Meniscus tissue engineering using a novel combination of electrospun scaffolds and human meniscus cells embedded within an extracellular matrix hydrogel. J Orthop Res 33, 572, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunja N.J., and Athanasiou K.A. Passage and reversal effects on gene expression of bovine meniscal fibrochondrocytes. Arthritis Res Ther 9, R93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panda D.K., Miao D., Lefebvre V., Hendy G.N., and Goltzman D. The Transcription factor SOX9 regulates cell cycle and differentiation genes in chondrocytic CFK2 cells. J Biol Chem 276, 41229, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Frith J., and Genever P. Transcriptional control of mesenchymal stem cell differentiation. Transfus Med Hemother 35, 216, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sive J.I., Baird P., Jeziorsk M., Watkins A., Hoyland J.A., and Freemont A.J. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol 55, 91, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grogan S.P., Duffy S., Pauli C., Das S., Lotz M.K., and D'Lima D.D. Microarray analysis of human meniscus tissue and cultured cells: a guide for meniscus regeneration (Abstract). Trans 59th Orthop Res Soc 2013 [Google Scholar]
- 48.Grogan S.P., Pauli C., Lotz M.K., and D'Lima D.D. Relevance of meniscal cell regional phenotype to tissue engineering. Connect Tissue Res 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koyama Y.-I., Norose K., Kusubata M., Irie S., and Kusakabe M. Differential expression of tenascin in the skin during hapten-induced dermatitis. Histochem Cell Biol 106, 263, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Pacifici M., Iwamoto M., Golden E.B., Leatherman J.L., Lee Y.-S., and Chuong C.-M. Tenascin is associated with articular cartilage development. Dev Dyn 198, 123, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Koyama E., Leatherman J.L., Shimazu A., Nah H.-D., and Pacifici M. Syndecan-3, tenascin-C, and the development of cartilaginous skeletal elements and joints in chick limbs. Dev Dyn 203, 152, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Cheng T., Maddox N.C., Wong A.W., Rahnama R., and Kuo A.C. Comparison of gene expression patterns in articular cartilage and dedifferentiated articular chondrocytes. J Orthop Res 30, 234, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Lee C.H., Shah B., Moioli E.K., and Mao J.J. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 120, 3340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leal M.F., Arliani G.G., Astur D.C., Franciozi C.E., Debieux P., Andreoli C.V., et al. Comprehensive selection of reference genes for expression studies in meniscus injury using quantitative real-time PCR. Gene 584, 60, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Furumatsu T., Kanazawa T., Yokoyama Y., Abe N., and Ozaki T. Inner meniscus cells maintain higher chondrogenic phenotype compared with outer meniscus cells. Connect Tissue Res 52, 459, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Dong B., Arnoult O., Smith M.E., and Wnek G.E. Electrospinning of collagen nanofiber scaffolds from benign solvents. Macromol Rapid Commun 30, 539, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Pauli C., Grogan S.P., Patil S., Otsuki S., Hasegawa A., Koziol J., et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage 19, 1132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts S., Menage J., Sandell L.J., Evans E.H., and Richardson J.B. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee 16, 398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baek J., Sovani S., Glembotski N.E., Du J., Jin S., Grogan S.P., et al. Repair of avascular meniscus tears with electrospun collagen scaffolds seeded with human cells. Tissue Eng Part A 22, 436, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grogan S.P., Miyaki S., Asahara H., D'Lima D.D., and Lotz M.K. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther 11, R85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin I., Jakob M., Schäfer D., Dick W., Spagnoli G., and Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage 9, 112, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Baker B.M., Nathan A.S., Huffman G.R., and Mauck R.L. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthritis Cartilage 17, 336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scotti C., Hirschmann M.T., Antinolfi P., Martin I., and Peretti G.M. Meniscus repair and regeneration: review on current methods and research potential. Eur Cell Mater 26, 150, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Kai D., Prabhakaran M.P., Stahl B., Eblenkamp M., Wintermantel E., and Ramakrishna S. Mechanical properties and in vitro behavior of nanofiber-hydrogel composites for tissue engineering applications. Nanotechol 23, 095705, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Grogan S.P., Chung P.H., Soman P., Chen P., Lotz M.K., Chen S., et al. Digital micromirror device projection printing system for meniscus tissue engineering. Acta Biomater 9, 7218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu T., Binder K.W., Albanna M.Z., Dice D., Zhao W., Yoo J.J., et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 5, 015001, 2013 [DOI] [PubMed] [Google Scholar]