Abstract
Liver or other organ accumulation of drugs is one of the major problems that leads to toxicity and side effects in therapy using chemicals or other macromolecules. It has been shown that specially designed RNA nanoparticles can specifically target cancer cells, silence oncogenic genes, and stop cancer growth with little or no accumulation in the liver or other vital organs. It is well known that physical properties of nanoparticles such as size, shape, and surface chemistry affect biodistribution and pharmacokinetic profiles in vivo. This study examined how the hydrophobicity of chemicals conjugated to RNA nanoparticles affect in vivo biodistribution. Weaker organ accumulation was observed for hydrophobic chemicals after they were conjugated to RNA nanoparticles, revealing RNA's ability to solubilize hydrophobic chemicals. It was found that different chemicals conjugated to RNA nanoparticles resulted in the alteration of RNA hydrophobicity. Stronger hydrophobicity induced by chemical conjugates resulted in higher accumulation of RNA nanoparticles in vital organs in mice. This study provides new insights for handling drug insolubility, therapeutic toxicity, and organ clearance in drug development.
Keywords: : RNA nanoparticles, pRNA 3WJ motif, nanobiotechnology, biodistribution, nanoparticle properties
Introduction
Interactions of nanoparticles with their in vivo environment is an important factor and affects nanoparticle characteristics such as protein binding, toxicity, pharmacokinetics, and biodistribution.1–8 Accumulation of nanoparticles in healthy organs, such as the liver, kidneys, spleen, and lungs, can lead to toxicity and lower efficacy of the administered dose of nanoparticles. While researchers are attempting to overcome this challenge with a myriad of different nanoparticle systems, generally <2% of the administered dose reaches the treatment site, with the remaining dose sequestered by filtration organs, which leads to toxicity and off-target drug effects.4
Recently, RNA nanoparticles have seen increased use as an in vivo delivery system.9–16 Additionally, RNA-based nanoparticles have been engineered to diverse shapes and sizes to carry multiple functionalities with unique release mechanisms.17–30 RNA was once thought to have little potential for in vivo use due to biological and thermodynamic stability issues. However, these issues have been solved systematically by: (1) finding a thermodynamically stable three-way junction (3WJ) motif9; (2) findings that chemical modifications to RNA nanoparticles confer enzymatic stability in vivo31–34; and (3) finding that RNA nanoparticles exhibit little to no immunogenicity in vivo.19 RNA nanoparticles are also water soluble and anionic due to the charged phosphate backbone. RNA nanoparticles show no accumulation in healthy organs, navigating to tumors after 4 h of circulation.9–12,35–38
It is well known that a nanoparticle's size, shape, and physical properties affect their interactions and biodistribution in vivo, and will therefore play a large role in determining their pharmacokinetics and biodistribution.2,39,40 Thus, tremendous efforts have been made by nanoparticle engineers to overcome inherent downfalls in nanoparticle construction. For example, polyethylene glycol (PEG) is frequently used to increase water solubility of otherwise insoluble nanoparticles.41,42
Conjugation of chemicals, such as fluorophores, drugs, and targeting ligands, is a popular method to decorate nanoparticles with functional moieties. However, many studies using hydrophobic fluorophores show nanoparticle accumulation in vital organs such as the liver, kidneys, lung, and spleen.43–47 It is possible that the hydrophobic conjugates, in this case fluorophores, are promoting interaction with cell membranes and plasma proteins, causing accumulation of the nanoparticles in organs. In order for a nanoparticle to display low toxicity and low accumulation in vital organs, it is important to understand how conjugation of chemicals to nanoparticles affects their in vivo properties.
Previous in vivo studies using pRNA nanoparticles were carried out using charged and water-soluble fluorophores to make sure the properties of the nanoparticles were not changed drastically.9–11,48 However, as RNA nanotechnology progresses, it will be important to know to what extent an RNA nanoparticle can be decreased in water solubility before accumulating in vital organs. Many potential applications such as chemical drug delivery and ligand-based targeting involve conjugation of chemical groups to RNA nanoparticles, and many near-infrared fluorophores are hydrophobic, potentially causing organ accumulation.43–47 Additionally, toxicity and lower therapeutic effect of certain drugs, such as Taxol, has been correlated to their insolubility. When chemicals are conjugated externally and not encapsulated, the impact of the conjugate is likely to be more significant.
To investigate this, three fluorophores of different hydrophobicities were conjugated to the 3WJ RNA nanoparticle to serve as model chemicals: Cyanine5.5 (C5.5), Sulfonated-Cyanine 5.5 (SC5.5), and Alexa Fluor® 700 (A700). All three fluorophores display similar excitation and emission spectra, despite minimal differences in their structures and solubility.49,50 High-performance liquid chromatography (HPLC) analysis demonstrated that conjugation of the fluorophores increased RNA nanoparticle hydrophobicity to differing extents. Hydrophobicity induced by chemical conjugates resulted in higher accumulation of RNA nanoparticles in the vital organs of mice. Additionally, weaker organ accumulation for hydrophobic chemicals was observed after they were conjugated to RNA nanoparticles, suggesting that RNA can increase the solubility of hydrophobic chemicals. To offer a physical explanation for in vivo results, predictive compound logP (ClogP) values of the fluorophores and 3WJs were compared to experimentally determined hydrophobicity scales of amino acids, which are used to predict transmembrane protein residues.51–54 This study offers insight into drug development concerning the reduction of organ accumulation and drug toxicity or side effects of nanoparticles that are modified with chemical conjugates such as targeting ligands, fluorophores for tracking, and chemical drugs.
Materials and Methods
RNA synthesis and fluorophore conjugation
RNA oligomers were chemically synthesized using phosphoramidite chemical synthesis on an automated oligo synthesizer. Sequences of the RNA oligomers used in biodistribution studies are as follows, listed in 5′ to 3′ orientation (“r” denotes 2′-OH base, “f” denotes 2′-F modified base). 3WJ-a: fUfUrGfUfCrAfUrGfUrGfUrAfUrG; 3WJ-b: fCrAfUrAfCfUfUfUrGfUfUrGrGfCfUrGrG; and 3WJ-c: fCfCrArGfCfCrArAfUfCrAfUrGrGfCrArA. Following synthesis, oligomers were deprotected and desalted using conventional methods.55 Each 3WJ strand was synthesized using 2′-fluorinated cytidine and uracil. 3WJ-c strand was 5′ modified with a primary amine (cat. no. 10-1947-90; Glen Research). Cyanine5.5-NHS ester and Sulfo-Cyanine5.5-NHS ester were purchases from Lumiprobe. AlexaFluor700-NHS Ester was purchased from Molecular Probes. Conjugation reactions were carried out by mixing a 1:10 molar ratio of primary amine labeled 3WJ-c: NHS Ester-Fluorophore in 0.1 M of sodium bicarbonate buffer, pH 8.5. The conjugation reactions were incubated at room temperature for 16 h while protected from light. Following incubation, the reactions were ethanol precipitated and washed twice with cold 75% ethanol to remove the majority of unreacted fluorophore, facilitating purification.
HPLC purification and analysis of RNA-fluorophore conjugates
Samples were purified using ion-pair reversed-phase (IPRP)-HPLC. Due to different hydrophobicities of each RNA-fluorophore conjugate, different HPLC gradient methods were used for each conjugate. Buffer A was 0.1 M triethylamine acetate (TEAA; Glen Research) in water, and buffer B was 0.1 M TEAA in 75% acetonitrile and 25% water. All purifications were performed using an Agilent 1260 HPLC and Agilent PLRP-S HPLC column (Agilent cat. no. PL1512-5500). A flow rate of 1.5 mL/min was used throughout all HPLC methods, and absorbance was monitored at 260 nm (RNA), 675 nm (C5.5, SC5.5), and 700 nm (A700). 3WJ-c-C5.5 was purified by a 5–100% gradient of buffer B over 15 min. 3WJ-c-SC5.5 was purified by 5–18% gradient of buffer B over 5 min followed by an 18–39% gradient over 15 min. 3WJ-c-A700 was purified by 5–18% gradient of buffer B over 5 min followed by an 18–38% gradient over 15 min. Fractions were collected when RNA absorbance and fluorophore absorbance eluted simultaneously. After fraction collection, RNA-fluor conjugates were dried to completion under vacuum.
For %ACN elution comparison, an identical gradient of 5–100% buffer B over 20 min was used to analyze 3WJ-fluorophore nanoparticles, 3WJ-c-fluorophore conjugates, and fluorophores. %ACN elution was calculated based on elution times of RNA oligoes or 3WJ complexes. Delay time was calculated by injection of an RNA sample in a high hydrophobic environment (100% acetonitrile) to prevent interaction with the column. The experimentally determined delay time was then subtracted from sample elution time, which was then used to calculate %ACN elution.
Chromatograms were plotted using OriginPro. Plots comparing 3WJ %ACN elution show 3WJ absorbance at 260 nm, 3WJ-Cy5.5 and 3WJ-SCy5.5 absorbance at 675 nm, and 3WJ-A700 absorbance at 700 nm. Comparison of %ACN elution of dye species were values taken from analysis chromatograms of dye species (Supplementary Fig. S1B–D; Supplementary Data are available online at www.liebertpub.com/hum). Values were plotted and then slope determined using a linear fit.
Determination of predictive ClogP values
ChemDraw Professional 16 was used to predict ClogP values of amino acids, fluorophores, and nucleotide-fluorophore conjugates. Amino acids, fluorophores, and nucleotide-fluorophore conjugates were drawn with charges analogous to their charged state at physiological pH 7.4. Only the side chains of the amino acids were used for predictive ClogP calculations. Predicted ClogP values of amino acid side chains were then plotted versus previously published hydrophobicity scales of amino acids.53,54,56,57 Four different hydrophobicity scales were chosen for comparison (Supplementary Fig. S2), and it was found that the Cornette hydrophobicity scale was the best match to predictive ClogP values. The Cornette scale is based on 28 published scales and demonstrates one of the best overall scales of amino acid hydrophobicity. High values indicate higher degree of hydrophobicity.
Next, ClogP values of C5.5, SC5.5, and mono-, di-, and trinucleotide derivatives of the fluorophores were predicted (Supplementary Table S1 and Supplementary Fig. S3). The structure of A700 is proprietary, and no structural information was available. However, SC5.5 and A700 demonstrate similar hydrophobicity based on IPRP-HPLC.
In vivo biodistribution
Male BALB/c mice, 5–6 weeks old (Taconic), were injected intravenously (i.v.) through the eye using retro-orbital injection.58 At 1, 4, and 8 h, mice were sacrificed, and their hearts, kidneys, livers, spleen, and lungs were collected and imaged for Cy5.5, SCy5.5, and A700 fluorescent signal using an In Vivo Imaging System (IVIS) imager (Caliper Life Sciences). Mice were administered phosphate-buffered saline (PBS) as a blank control. One hundred microliters of 20 μM of nanoparticle sample or dye sample were injected. It is important to note that the concentrations of dyes were kept consistent in all in vivo experiments. All animal experiments were housed and performed in accordance with the Subcommittee on Research Animal Care of the Ohio State University guidelines approved by the Institutional Review Board.
Atomic force microscopy imaging
Specially modified mica surfaces (APS mica) were used. The APS mica was obtained by incubation of freshly cleaved mica in 167 nM of 1-(3-aminopropyl) silatrane following a previously reported protocol.59 The RNA samples were diluted with 1 × tricaine mesylate buffer to a final concentration of 3–5 nM. Then, 5–10 μL was immediately deposited on APS mica. After 2 min of incubation on the surface, excess samples were washed with diethyl pyrocarbonate–treated water and dried under a flow of Argon gas. Atomic force microscopy (AFM) images in air were acquired using the MultiMode AFM NanoScope IV system (Veeco/Digital Instruments) operating in tapping mode. The 3WJ nanoparticles used for AFM imaging only included 60 base pair extensions in order to visualize the nanoparticles better. Please refer to sequence information listed in the RNA Synthesis methods section for sequence information of RNA nanoparticles used in biodistribution studies.
Results and Discussion
3WJ-fluorophore HPLC analysis
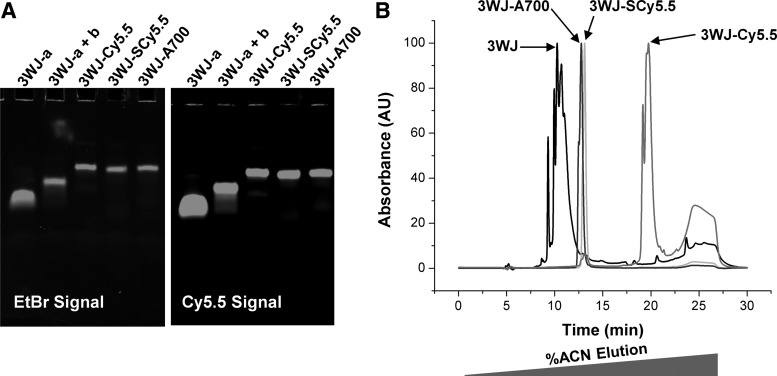
The 3WJ is composed of three component RNA oligomers: 3WJ-a, 3WJ-b, and 3WJ-c (Fig. 1A). Mixing equimolar amounts of each component strand at room temperature in physiological buffer yields homogeneous RNA nanoparticles (Fig. 1B).9 To label the 3WJ nanoparticles fluorescently, NHS-Ester derivatives of C5.5, SC5.5, and A700 were conjugated to primary amine labeled 3WJ-c strand (Fig. 1C). Fluorophore conjugated oligomers were purified from unlabeled RNA by IPRP-HPLC. Following assembly of fluorophore labeled 3WJ nanoparticles (3WJ-C5.5, 3WJ-SC5.5, and 3WJ-A700), polyacrylamide gel analysis was used to determine assembly efficiency. A decrease in migration rate of fully assembled 3WJ nanoparticles compared to monomer and dimer species indicates successful formation at high yield (Fig. 2A). Gels were stained with ethidium bromide (EB) for total RNA visualization followed by scanning for EB and fluorophore signal. Co-migration of EB and fluorophore signal indicates successful incorporation of fluorophore-labeled oligomers into 3WJ nanoparticles. Labeling only one of three strands was used to confirm that the particles were not dissociating in vivo. It has been shown previously that single-stranded RNA is eliminated rapidly through the kidneys after i.v. injection.60,61
Figure 1.
Three-way junction (3WJ) introduction and dye conjugation. (A) pRNA-3WJ secondary structure and sequences. (B) Atomic force microscopy images of pRNA-3WJ with 60 base pair extensions from each helix to show overall shape. (C) Assembly scheme of fluorescently labeled 3WJ nanoparticles. Color images available online at www.liebertpub.com/hum
Figure 2.
3WJ assembly and high-performance liquid chromatography (HPLC) analysis. (A) Native polyacrylamide assembly gels of fluorescently labeled 3WJ nanoparticles. (B) Ion-pair reversed-phase HPLC chromatograms of fluorescently labeled nanoparticles.
3WJ particles were then analyzed by IPRP-HPLC to compare elution times and %ACN elution (Fig. 2B). Supplementary Table S2 shows a summary of nanoparticle elution times and %ACN elution. There is a strong correlation between the number of charged sulfate groups per fluorophore and the %ACN elution. 3WJ-C5.5 has the highest %ACN elution of 46.39, followed by 3WJ-SC5.5 at 22.72%, and finally 3WJ-A700 at 21.49%. 3WJ with no fluorophore eluted at 14.13 %ACN. HPLC analysis indicates that different chemicals conjugated to RNA nanoparticles will increase the nanoparticle's hydrophobicity to differing degrees.
RNA nanoparticles solubilize hydrophobic chemicals
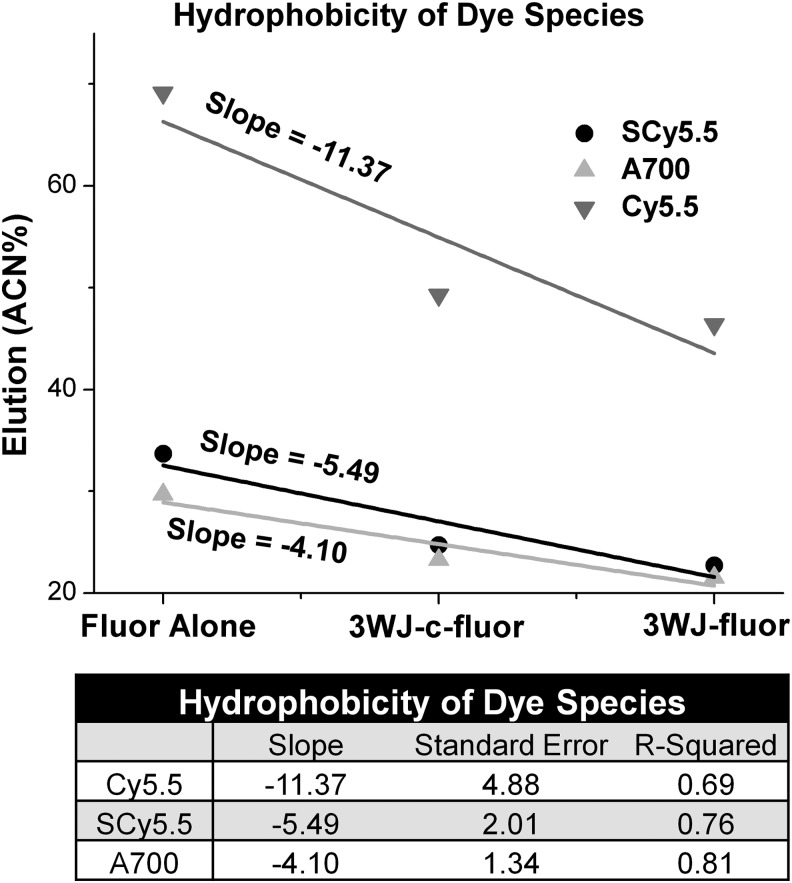
The hydrophilic property of RNA nanoparticles enables them to solubilize many hydrophobic chemicals. IPRP-HPLC demonstrates an increase in solubility (decrease in %ACN elution) from fluorophore alone to 3WJ-c-fluorophore to 3WJ-fluorophore (Supplementary Fig. S1B–D). Interestingly, a much larger decrease in %ACN elution is seen for the hydrophobic Cy5.5 fluorophore than for both hydrophilic fluorophores, indicated by more negative slope for Cy5.5 (Fig. 3). The effect of RNA nanoparticles increasing solubility is evidenced by decreased fluorescent signal in vital organs when comparing 3WJ-fluorophore to fluorophore alone (Fig. 4B). Especially strong evidence supporting this fact is observed when comparing fluorescence in lung and liver tissue between mice injected with fluorophore alone and 3WJ-fluorophore nanoparticles (Fig. 5). The fluorophores alone accumulate in vital organs after 4 h, whereas 3WJ-fluorophores display lower fluorescence, especially with C5.5 and A700.
Figure 3.
RNA nanoparticles solubilize hydrophobic chemicals. Three species of each fluorophore were plotted: fluorophore alone, 3WJ-c-fluorophore, and 3WJ-fluorophore for SulfoCy5.5 (circles), AlexaFluor700 (triangles), and Cy5.5 (inverted triangles).
Figure 4.
Biodistribution in mice. (A) Whole-body fluorescent images of mice injected with fluors and 3WJ-fluors. (B) Organ images of mice injected intravenously with fluors and 3WJ-fluors. Color images available online at www.liebertpub.com/hum
Figure 5.
Fluor only versus 3WJ-fluor tissue comparison. Comparison of lung (Lu) and liver (Li) tissue collected from mice injected with fluorophores only or 3WJ-fluor conjugates after 4 and 8 hours. Color images available online at www.liebertpub.com/hum
However, despite the high overall hydrophilicity, both increased %ACN elution and accumulation with hydrophobic cell membranes are still seen when using a hydrophobic conjugate. This once again leads to the belief that the conjugate itself is interacting with cell membranes, and if the interaction of the conjugate with the cell membrane is strong enough, it can overcome the hydrophilic nature of the RNA nanoparticle and the anionic property of RNA. Thus, not only is the overall hydrophilicity of the nanoparticle important, but so are the specific surface properties of the nanoparticle.
Fluorophore hydrophobicity versus biodistribution
Following HPLC analysis, the fluorophores were injected retro-orbitally into mice, and fluorescent signal was whole-body imaged 1, 4, and 8 h post injection. Mice were then sacrificed, and their organs were collected and imaged. Fluorescent imaging from whole-body images show low signal for A700 and SC5.5 and higher signal for C5.5 (Fig. 4A). Organ images of the mice injected with the fluorophores all show fluorescent signal after 8 h (Fig. 4B). These observations correlate well with the increased hydrophobicity of C5.5 over both SC5.5 and A700.
3WJ-fluorophore hydrophobicity versus biodistribution
The in vivo properties of the 3WJ-fluorophore nanoparticles were analyzed by testing their biodistribution profiles in mice. Whole-body images indicate faster clearance of 3WJ-SC5.5 and 3WJ-A700 compared to 3WJ-C5.5 (Fig. 4). 3WJ-C5.5 showed high-fluorescent signal in the organs compared to mice injected with PBS as a blank, primarily accumulating in the liver and kidneys after 8 h (Fig. 4B). 3WJ-SC5.5 does show fluorescence in organs after 8 h, albeit at a much lower intensity than observed for 3WJ-C5.5. 3WJ-A700 shows no fluorescence in organs after 8 h. These in vivo results demonstrate a strong correlation between the increased hydrophobicity of the fluorophore and increased accumulation of nanoparticles in organs. Furthermore, these results show the ability of the 3WJ nanoparticle to increase the solubility of the fluorophores, as less accumulation in vital organs of 3WJ-fluor nanoparticles is seen when compared to fluorophores. This is evidenced when examining differences in lung and liver tissue between mice injected with fluors alone and with 3WJ-fluorophore (Fig. 5).
Physical basis for hydrophobicity effect on biodistribution
After in vivo results, a physical explanation was sought for the observations. One likely explanation for accumulation of the nanoparticles is interaction with proteins and cell membranes in vivo. It is hypothesized that the increased hydrophobicity of the fluorophores conjugated to the 3WJ nanoparticles initiates interaction with the hydrophobic regions of proteins and cell membranes, causing the nanoparticles to accumulate in organs. Many studies have been done on the hydrophobicity of proteins and how the amino acid arrangement creates pockets of hydrophobicity and hydrophilicity.51–54,56,57 These studies have generated amino acid hydrophobicity scales, which are used to predict hydrophobic regions in proteins and determine transmembrane protein regions.
It was hypothesized that the increased hydrophobicity of 3WJ-C5.5 was increasing the strength of the interactions between the hydrophobic regions of proteins and cell membranes and the nanoparticles. Because the RNA nanoparticles are extremely hydrophilic with a hydrophobic fluorophore attached, they are expected to exhibit an amphipathic property. This is akin to transmembrane proteins, which are amphipathic to cross-cell membranes.
A logP (partition coefficient) value is the ratio of a compound's solubility in two immiscible solvents, normally octanol:water, and is a good indication of hydrophobicity. Additionally, logP values are useful in estimating the biodistribution of drugs. More hydrophobic logP values generally indicate accumulation of drugs in hydrophobic areas such as the lipid bilayers of cells, while more hydrophilic logP values indicate accumulation of drugs in hydrophilic regions such as blood serum.62 A ClogP value uses experimentally determined logP values of small fragments, and then adds these values together with correction factors to obtain a ClogP value.63 When dealing with complex molecules, such as those containing aromaticity, ClogP values tend to be quite accurate.
The Cornette53 hydrophobicity scale was chosen, as the best correlation was observed between predicted ClogP values and Cornette values (Fig. 6A). A dotted line represents a neutral value of 0. The ClogP values were then plotted alongside ClogP values of amino acids (Fig. 6B). Interestingly, C5.5 displays a high ClogP value, while SC5.5 displays an extremely low ClogP value. Only when C5.5 is in trinucleotide form does the ClogP value reduce to near zero. ClogP values beyond trinucleotide form could not be predicted due to the increased number of atoms and software limitations. However, it was expected that as more nucleotides are added (54 in one 3WJ nanoparticle), the ClogP value would drastically decrease.
Figure 6.
Hydrophobicity comparison. (A) Plots of Cornette hydrophobicity scale values, along with predicted ClogP values of the amino acids. (B) ClogP values were predicted for SulfoCy5.5 and their mono- and dinucleotide derivatives, Cy5.5 and their mono- and dinucleotide derivatives, and mono-, di-, and trinucleotides.
Importantly, comparison of the predicted ClogP values of the amino acids with the fluorophores and their nucleotide derivatives provides some insights into the effect of hydrophobicity on cell membrane interaction. A highly correlated trend is seen between high ClogP values and organ accumulation in vivo. A highly correlated trend is also seen between high ClogP values and high values in the Cornette hydrophobicity scale. This shows that conjugates with high predicted ClogP values conjugated to a nanoparticle may cause interaction with the cell membrane, much like transmembrane proteins.
While this study uses fluorophores to demonstrate how hydrophobicity affects nanoparticles in vivo, it is expected to extend to other conjugates such as chemical drugs or targeting ligands. For future use, it will be beneficial to determine beforehand if a conjugate will or will not cause nanoparticles to accumulate non-specifically in vivo. It is possible that using a screening method, such as the one presented in this study, will greatly benefit nanoparticle engineers looking to optimize their nanoparticle's physical properties. Specifically, for RNA nanoparticles, it could be beneficial to use encapsulation methods to protect the hydrophobic molecules from strong interactions with cell membranes or other proteins.17
Conclusions
This study on RNA nanoparticles demonstrates a strong correlation between increased hydrophobicity of an external conjugate and increased accumulation of nanoparticles in vital organs. Comparison of ClogP values of C5.5, SC5.5, and A700 to amino acids showed a strong correlation between hydrophobic dyes, which accumulate in vivo, to amino acids that are commonly seen in hydrophobic regions of transmembrane proteins. The results demonstrate a method to prescreen nucleic acid–based nanoparticle conjugates for their hydrophobic properties using a common method of HPLC analysis. As shown here, careful consideration must be taken when choosing to conjugate chemical drugs, fluorophores, or targeting ligands externally to water soluble nanoparticles, as their effect on water solubility, and in turn in vivo biodistribution, could be detrimental to the safety of patients in future clinical settings. This study offers some insight into drug development concerning the reduction of organ accumulation of nanoparticles and reduction of drug toxicity and side effects.
Supplementary Material
Acknowledgments
The research in P.G.'s lab was supported by NIH grants R01EB019036, and U01CA207946. We thank Mario Vieweger and Daniel W. Binzel for their insight in manuscript preparation.
Author Disclosure
P.G.'s Sylvan G. Frank Endowed Chair position in Pharmaceutics and Drug Delivery is funded by the CM Chen Foundation. P.G. is the consultant of Oxford Nanopore Technologies and Nanobio Delivery Pharmaceutical Co. Ltd, as well as the cofounder of Shenzhen P&Z Bio-medical Co. Ltd and its subsidiary US P&Z Biological Technology LLC. No competing financial interests exist for the remaining authors.
References
- 1.Gustafson HH, Holt-Casper D, Grainger DW, et al. Nanoparticle uptake: the phagocyte problem. Nano Today 2015;10:487–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobrovolskaia MA, Shurin M, Shvedova AA. Current understanding of interactions between nanoparticles and the immune system. Toxicol Appl Pharmacol 2016;299:78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadauskas E, Danscher G, Stoltenberg M, et al. Protracted elimination of gold nanoparticles from mouse liver. Nanomedicine 2009;5:162–169 [DOI] [PubMed] [Google Scholar]
- 4.Wilheml S, Tavares AJ, Dai Q, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater 2016;1:1–12 [Google Scholar]
- 5.Kim ST, Saha K, Kim C, et al. The role of surface functionality in determining nanoparticle cytotoxicity. Acc Chem Res 2013;46:681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyano DF, Goldsmith M, Solfiell DJ, et al. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc 2012;134:3965–3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Li L, Liu T, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011;5:5390–5399 [DOI] [PubMed] [Google Scholar]
- 8.Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A 2008;105:11613–11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu D, Shu Y, Haque F, et al. Thermodynamically stable RNA three-way junctions for constructing multifuntional nanoparticles for delivery of therapeutics. Nat Nanotechnol 2011;6:658–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binzel D, Shu Y, Li H, et al. Specific delivery of miRNA for high efficient inhibition of prostate cancer by RNA nanotechnology. Mol Ther 2016;24:1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu D, Li H, Shu Y, et al. Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. ACS Nano 2015;9:9731–9740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui D, Zhang C, Liu B, et al. Regression of gastric cancer by systemic injection of RNA nanoparticles carrying both ligand and siRNA. Sci Rep 2015;5:10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu Y, Haque F, Shu D, et al. Fabrication of 14 different RNA nanoparticles for specific tumor targeting without accumulation in normal organs. RNA 2013;19:766–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afonin KA, Viard M, Koyfman AY, et al. Multifunctional RNA nanoparticles. Nano Lett 2014;14:5662–5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh YH, Deng JZ, Dreaden EC, et al. A multi-RNAi microsponge platform for simultaneous controlled delivery of multiple small interfering RNAs. Angew Chem Int Ed Engl 2016;55:3347–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JB, Hong J, Bonner DK, et al. Self-assembled RNA interference microsponges for efficient siRNA delivery. Nat Mater 2012;11:316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khisamutdinov EF, Jasinski DL, Li H, et al. Fabrication of RNA 3D nanoprism for loading and protection of small RNAs and model drugs. Advanced Materials 2016;28:10079–10087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khisamutdinov EF, Jasinski DL, Guo P. RNA as a boiling-resistant anionic polymer material to build robust structures with defined shape and stoichiometry. ACS Nano 2014;8:4771–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khisamutdinov E, Li H, Jasinski D, et al. Enhancing immunomodulation on innate immunity by shape transition among RNA triangle, square, and pentagon nanovehicles. Nucleic Acids Res 2014;42:9996–10004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasinski D, Khisamutdinov EF, Lyubchenko YL, et al. Physicochemically tunable poly-functionalized RNA square architecture with fluorogenic and ribozymatic properties. ACS Nano 2014;8:7620–7629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afonin KA, Desai R, Viard M, et al. Co-transcriptional production of RNA–DNA hybrids for simultaneous release of multiple split functionalities. Nucleic Acids Res 2014;42:2085–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afonin KA, Viard M, Martins AN, et al. Activation of different split functionalities on re-association of RNA–DNA hybrids. Nat Nanotechnol 2013;8:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabow WW, Jaeger L. RNA self-assembly and RNA nanotechnology. Acc Chem Res 2014;47:1871–1880 [DOI] [PubMed] [Google Scholar]
- 24.Grabow WW, Zakrevsky P, Afonin KA, et al. Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett 2011;11:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Severcan I, Geary C, Chworos A, et al. A polyhedron made of tRNAs. Nat Chem 2010;2:772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afonin KA, Bindewald E, Yaghoubian AJ, et al. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat Nanotechnol 2010;5:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chworos A, Severcan I, Koyfman AY, et al. Building programmable jigsaw puzzles with RNA. Science 2004;306:2068–2072 [DOI] [PubMed] [Google Scholar]
- 28.Osada E, Suzuki Y, Hidaka K, et al. Engineering RNA–protein complexes with different shapes for imaging and therapeutic applications. ACS Nano 2014;8:8130–8140 [DOI] [PubMed] [Google Scholar]
- 29.Boerneke MA, Dibrov SM, Hermann T. Crystal-structure-guided design of self-assembling RNA nanotriangles. Angew Chem Int Ed Engl 2016;55:4097–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dibrov SM, McLean J, Parsons J, et al. Self-assembling RNA square. Proc Natl Acad Sci U S A 2011;108:6405–6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Guo S, Cinier M, et al. Fabrication of stable and RNase-resistant RNA nanoparticles active in gearing the nanomotors for viral DNA packaging. ACS Nano 2011;5:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veedu RN, Wengel J. Locked nucleic acids: promising nucleic acid analogs for therapeutic applications. Chem Biodivers 2010;7:536–542 [DOI] [PubMed] [Google Scholar]
- 33.Watts JK, Deleavey GF, Damha MJ. Chemically modified siRNA: tools and applications. Drug Discovery Today 2008;13:842–855 [DOI] [PubMed] [Google Scholar]
- 34.Somasunderam A, Thiviyanathan V, Tanaka T, et al. Combinatorial selection of DNA thioaptamers targeted to the HA binding domain of human CD44. Biochemistry 2010;49:9106–9112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee TJ, Haque F, Shu D, et al. RNA nanoparticles as a vector for targeted siRNA delivery into glioblastoma mouse model. Oncotarget 2015;6:14766–14776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Leonard M, Shu Y, et al. Overcoming tamoxifen resistance of human breast cancer by targeted gene silencing using multifunctional pRNA nanoparticles. ACS Nano 2017;11:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pi F, Zhang H, Li H, et al. RNA nanoparticles harboring annexin A2 aptamer can target ovarian cancer for tumor-specific doxorubicin delivery. Nanomedicine 2016;13:1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee TJ, Yoo JY, Shu D, et al. RNA nanoparticle-based targeted therapy for glioblastoma through inhibition of oncogenic miR-21. Mol Ther 2017. January 18 [Epub ahead of print]; DOI: 10.1016/j.ymthe.2016.11.016 [DOI] [PMC free article] [PubMed]
- 39.Canton I, Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev 2012;41:2718–2739 [DOI] [PubMed] [Google Scholar]
- 40.Arvizo RR, Miranda OR, Moyano DF, et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS One 2011;6:e24374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Q, Zhang Z, Gao F, et al. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small 2011;7:271–280 [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Hu Y, Huang L. Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials 2011;35:3027–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Owens EA, Hyun H, Dost TL, et al. Near-infrared illumination of native tissues for image-guided surgery. J Med Chem 2016;59:5311–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porcu EP, Salis A, Gavini E, et al. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol Adv 2016;34:768–789 [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Yang K, Wang Z, et al. Combined image guided monitoring the pharmacokinetics of rapamycin loaded human serum albumin nanoparticles with a split luciferase reporter. Nanoscale 2016;8:3991–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huynh AS, Estrella V, Stark VE, et al. Tumor targeting and pharmacokinetics of a near-infrared fluorescent-labeled delta-opioid receptor antagonist agent, Dmt-Tic-Cy5. Mol Pharm 2016;13:534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira P, Correia A, Gama FM. In vivo imaging of glycol chitosan-based nanogel biodistribution. Macromol Biosci 2016;16:432–440 [DOI] [PubMed] [Google Scholar]
- 48.Li H, Zhang K, Pi F, et al. Controllable self-assembly of RNA tetrahedrons with precise shape and size for cancer targeting. Adv Mater 2016;28:7501–7507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lumiprobe. Cyanine dyes. www.lumiprobe.com/tech/cyanine-dyes (last accessed March31, 2017)
- 50.Berlier JE, Rothe A, Buller G, et al. Quantitative comparison of long-wavelength Alexa Fluor dyes to Cy dyes: fluorescence of the dyes and their bioconjugates. J Histochem Cytochem 2003;51:1699–1712 [DOI] [PubMed] [Google Scholar]
- 51.Silverman BD. Hydrophobicity of transmembrane proteins: spatially profiling the distribution. Protein Sci 2003;12:586–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rees DC, DeAntonio L, Eisenberg D. Hydrophobic organization of membrane proteins. Science 1989;245:510–513 [DOI] [PubMed] [Google Scholar]
- 53.Cornette JL, Cease KB, Margalit H, et al. Hydrophobicity scales and computational techniques for detecting amphipathic structures in proteins. J Mol Biol 1987;195:659–685 [DOI] [PubMed] [Google Scholar]
- 54.Eisenberg D, Schwarz E, Komaromy M, et al. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol 1984;179:125–142 [DOI] [PubMed] [Google Scholar]
- 55.Usman N, Ogilvie KK, Jiang MY, et al. The automated chemical synthesis of long oligoribuncleotides using 2′-O-silylated ribonucleoside 3′-O-phosphoramidites on a controlled-pore glass support: synthesis of a 43-nucleotide sequence similar to the 3′-half molecule of an Escherichia coli formylmethionine tRNA. J Am Chem Soc 1987;109:7845–7854 [Google Scholar]
- 56.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol 1982;157:105–132 [DOI] [PubMed] [Google Scholar]
- 57.Engelman DM, Steitz TA, Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem 1986;15:321–353 [DOI] [PubMed] [Google Scholar]
- 58.Yardeni T, Eckhaus M, Morris HD, et al. Retro-orbital injections in mice. Lab Animal 2011;40:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyubchenko YL, Gall AA, Shlyakhtenko LS, et al. Atomic force microscopy imaging of double stranded DNA and RNA. J Biomol Struct Dyn 1992;10:589–606 [DOI] [PubMed] [Google Scholar]
- 60.Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides 2008;18:305–319 [DOI] [PubMed] [Google Scholar]
- 61.Behlke MA. Progress towards in vivo use of siRNAs. Mol Ther 2006;13:644–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shargel L, Susanna W, Yu A. Physiological drug distribution and protein binding. In Applied Biopharmaceutics and Pharmacokinetics, 6th ed. New York: McGraw-Hill Medical, 2012:211 [Google Scholar]
- 63.Hansch C, Leo A. Calculation of Octonal-water partition coefficients from fragments, etc. In Substituent Constants for Correlation Analysis in Chemistry and Biology. New York: John Wiley, 1979 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.