Supplemental Digital Content is available in the text.
Keywords: guideline adherence, intensive care units, prevention & control, ventilator-associated pneumonia
Abstract
Objectives:
The “Pneumonia Zero” project is a nationwide multimodal intervention based on the simultaneous implementation of a comprehensive evidence-based bundle measures to prevent ventilator-associated pneumonia in critically ill patients admitted to the ICU.
Design:
Prospective, interventional, and multicenter study.
Setting:
A total of 181 ICUs throughout Spain.
Patients:
All patients admitted for more than 24 hours to the participating ICUs between April 1, 2011, and December 31, 2012.
Intervention:
Ten ventilator-associated pneumonia prevention measures were implemented (seven were mandatory and three highly recommended). The database of the National ICU-Acquired Infections Surveillance Study (Estudio Nacional de Vigilancia de Infecciones Nosocomiales [ENVIN]) was used for data collection. Ventilator-associated pneumonia rate was expressed as incidence density per 1,000 ventilator days. Ventilator-associated pneumonia rates from the incorporation of the ICUs to the project, every 3 months, were compared with data of the ENVIN registry (April–June 2010) as the baseline period. Ventilator-associated pneumonia rates were adjusted by characteristics of the hospital, including size, type (public or private), and teaching (postgraduate) or university-affiliated (undergraduate) status.
Measurements and Main Results:
The 181 participating ICUs accounted for 75% of all ICUs in Spain. In a total of 171,237 ICU admissions, an artificial airway was present on 505,802 days (50.0% of days of stay in the ICU). A total of 3,474 ventilator-associated pneumonia episodes were diagnosed in 3,186 patients. The adjusted ventilator-associated pneumonia incidence density rate decreased from 9.83 (95% CI, 8.42–11.48) per 1,000 ventilator days in the baseline period to 4.34 (95% CI, 3.22–5.84) after 19–21 months of participation.
Conclusions:
Implementation of the bundle measures included in the “Pneumonia Zero” project resulted in a significant reduction of more than 50% of the incidence of ventilator-associated pneumonia in Spanish ICUs. This reduction was sustained 21 months after implementation.
The prevalence rates of ventilator-associated pneumonia (VAP) is a common indicator for safety and quality of care in critically ill patients admitted to the ICU (1, 2). Morbidity and mortality attributed to VAP have been extensively documented in the literature (3–6). Specific programs for the prevention of this infection have been included in national campaigns promoted in the United States with the objective to reduce mortality (The 100,000 lives campaign) (7, 8).
In Spain, healthcare-associated infection rates in critical care are collected in the database of the National ICU-Acquired Infection Surveillance Study (ENVIN-HELICS registry) in which clinical data of patients admitted to Spanish ICUs from April 1 to June 30 each year are captured. From 2000 to 2008, Spanish national VAP rates have remained stable at around 15 episodes per 1,000 ventilator days (9, 10). In recent years, benefits of the implementation of sets of different preventive measures or bundles to reduce healthcare-associated infections, such as catheter-related bloodstream infection (CRBSI) (11, 12) or VAP (13–22), have been reported. In Spain, a multifaceted intervention to prevent CRBSI was implemented during 2009–2010, the so-called “Bacteremia Zero” project, resulted in a 50% risk reduction of bloodstream infections (23). Based on the organizational structure of “Bacteremia Zero,” a new nationwide project, called “Pneumonia Zero,” was designed to implement a bundle of VAP-specific preventive measures (23). It was hypothesized that implementation of the intervention would result in a significant reduction of VAP rates. The main objective of this study was to assess the impact of the “Pneumonia Zero” project in reducing the rates of VAP in Spanish ICUs.
MATERIALS AND METHODS
Design and Setting
We designed a prospective, interventional, and multicenter study. Adult ICUs from the 17 HealthCare Regions of the Spanish National HealthCare System or from the private sector were invited to participate in the “Pneumonia Zero” project. The study period lasted 21 months, from April 1, 2011 to December 31, 2012. The annual Spanish registry of healthcare-associated infections in critically ill patients (ENVIN-HELICS registry) was used for data collection. The first 3 months matched the annual data collection time frame of the annual Spanish registry of healthcare-associated infections in critically ill patients (April 1, 2010, to June 30, 2010) and were considered the baseline period (9).
Participation in the study was voluntary and required provision of basic data (number of VAP episodes and number of days on mechanical ventilation) and adherence to the bundle recommendations for at least 6 months during the 21 months of the study period.
Structure and Organization
The Spanish Ministry of Health, Social Policy and Equality provided national supervision and program coordination in collaboration with the Regional Health Care Authorities, which were in charge of coordination at regional level and provided the necessary resources for implementation. The Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) and the Spanish Society of Intensive Nursing and Coronary Units were in charge of technical coordination of the project. An intensivist and a critical care nurse in each ICU were appointed to as local leaders.
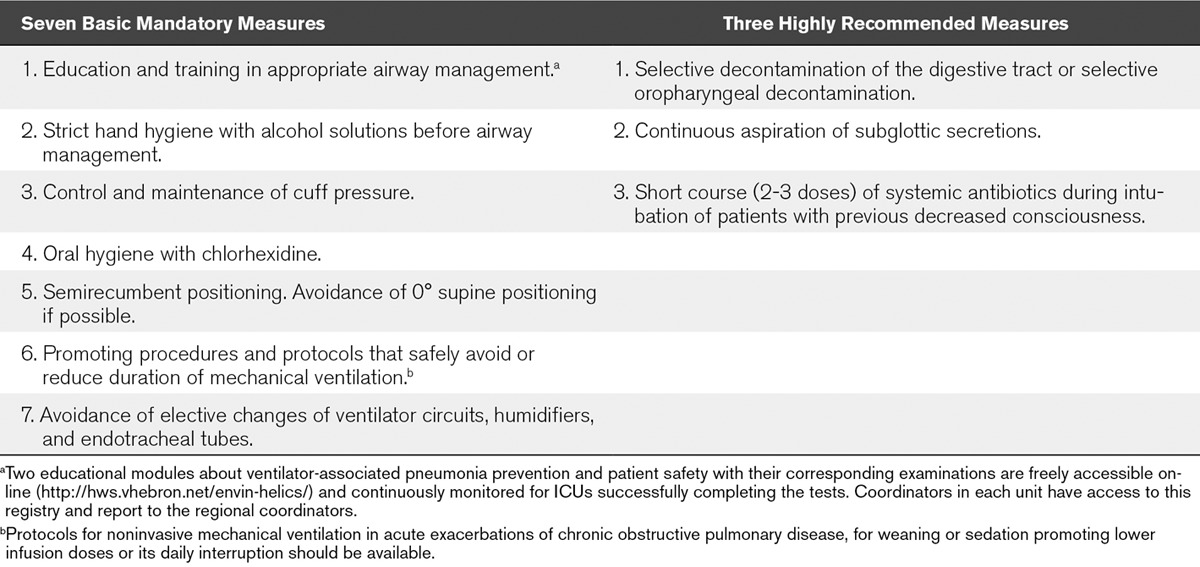
A detailed description of the VAP prevention bundle and tools developed for its implementation and control of adherence has been published previously (24). The bundle includes seven basic mandatory measures and three highly recommended measures all of which with proven efficacy in the prevention of VAP (24) (Table 1). The study protocol was approved by the Clinical Research Ethics Committee of Hospital del Mar, Barcelona (Spain). Also, the institutional review boards waived the need for informed consent.
TABLE 1.
Individual Components of the Ventilator-Associated Pneumonia Prevention Bundle
Patients
All patients admitted for more than 24 hours to the participating ICUs between April 1, 2011, and December 31, 2012, were included in the study. Patients who developed one or more episodes of VAP during their stay in the ICU were compared with those who developed VAP during the baseline period (April–June 2010). Criteria for the diagnosis of VAP in the ENVIN-HELICS registry (9) are in accordance with the European Centre for Disease Prevention and Control (25) and included the following: 1) two or more sequential chest x-rays or CT scans with a suggestive image of pneumonia for patients with underlying cardiac or pulmonary disease, or one definitive chest x-ray or CT scan in patients without underlying cardiac or pulmonary disease; 2) fever greater than 38°C and/or leukocytosis greater than or equal to 12,000 WBC/mm3 or leukopenia less than or equal to 4,000 WBC/mm3; and 3) at least one of the following: a) new onset purulent sputum or change in the characteristics of sputum; b) cough, dyspnea, or tachycardia; c) suggestive of auscultation (rales or bronchial breath sounds), ronchi, wheezing; or d) worsening gas exchange (e.g., oxygen desaturation or increased oxygen requirements or increased ventilation demand). For the diagnosis of a second pneumonia at least two of the following was required: 1) new suggestive signs and symptoms; 2) radiologic evidence or other diagnostic tests at least after 2 days of clinical resolution; and 3) isolation of a microorganism different from the causative pathogen of the first episode or an interval of 2 weeks between two samples in case of the same microorganism. VAP episodes were additionally stratified according to the microbiological sampling diagnostic method employed (9). Patients were followed up to 48 hours after discharge from the ICU.
Workshops were held in all regions in order to guarantee a homogeneous use of the definition of VAP. Prior to the beginning of the project, from April to June 2010, an audit of the ENVIN-HELICS registry (23) had established the adequacy of the collected diagnoses of VAP. Also, annual national meetings were held to provide updates, follow-up, description of barriers and to promote improvement strategies at local and regional levels.
Data Collection
The “ENVIN-HELICS” database (restricted access via password) was adapted to facilitate specific data collection for the “Pneumonia Zero” project (http://hws.vhebron.net/Neumonia-zero/). Monthly unit data were entered by local coordinators, including the number of ICU admissions, total patient days in the ICU and on mechanical ventilation. The device use ratio was calculated as days of mechanical ventilation divided by days of ICU stay.
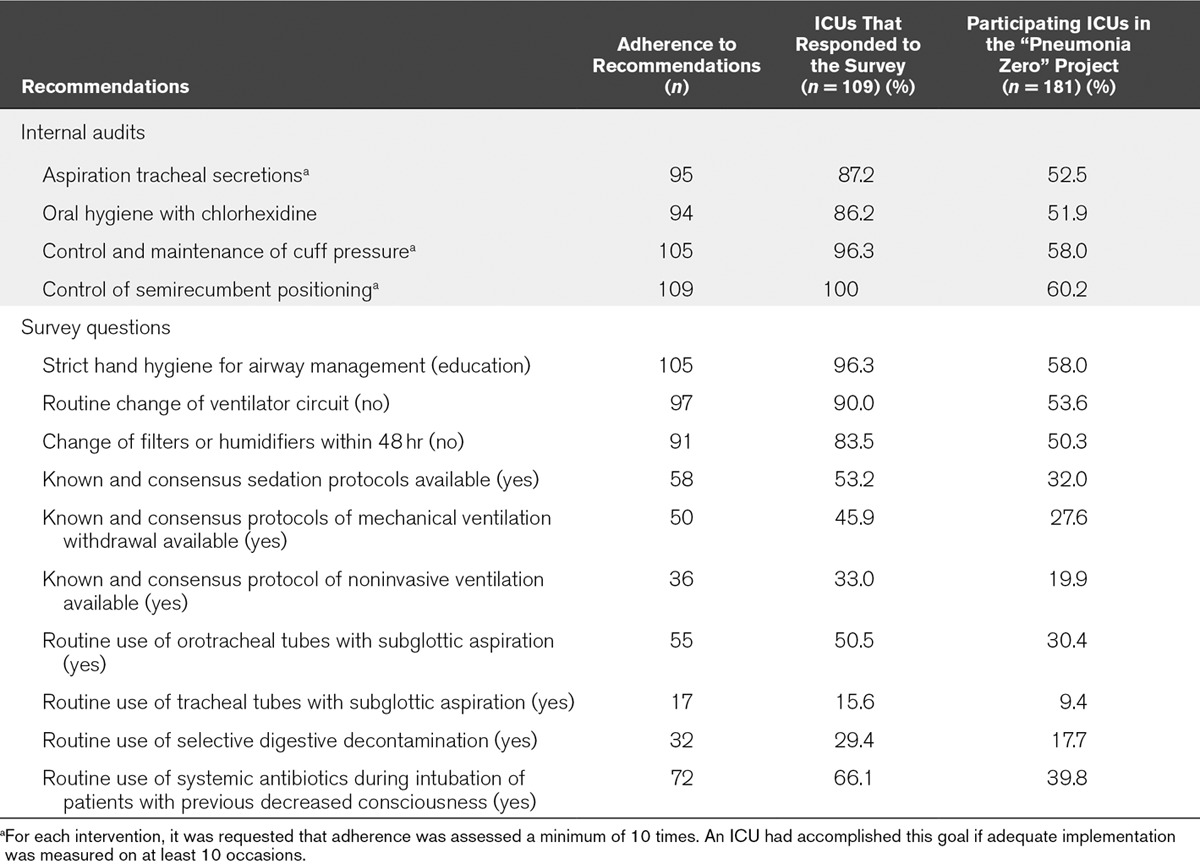
For patients developing one or more episodes of VAP, additional data recorded were as follows: demographics (age, sex), severity of illness on ICU admission using the Acute Physiology and Chronic Health Evaluation II score (26), comorbidities, risk factors for ICU-acquired infection, length of ICU stay, days of ICU stay before diagnosis of VAP was established, and mortality. VAP rates were compared between public and private hospitals, university-affiliated (undergraduate) and teaching (postgraduate) hospitals, and small (< 200 beds), medium (200–500 beds), and large (> 500 beds) hospitals. The degree of adherence to recommendations included in the bundle was recorded. Adherence was assessed using a survey. Questions in the survey were designed to capture daily activities reflecting compliance with components of the VAP prevention bundle. Questions asked were 1) whether an internal audit about the quality of adherence to four recommendations (aspiration of tracheal secretions, oral hygiene with chlorhexidine, control and maintenance of cuff pressure, and control of semirecumbent positioning) had been performed and 2) implementation or use the 10 recommendations in the ICU. The survey was sent by e-mail to the local physician intensivist leader of the project at each of the 181 participating ICUs, 109 of which responded. Adherence was expressed as a percentage of the number of responses received and the total number of participating units. We hypothesized that adherence varies between a maximum value, if calculated as the percentage of adherence expressed in responses, and a minimum value, if calculated as the percentage of adherence expressed in responses over total of surveys sent. The length of follow-up (duration of participation) for each participating ICU was also registered.
An online training course for healthcare professionals involved in the project was available on ENVIN-HELICS platform. The course included clinical aspects of VAP prevention and basic concepts of patient safety to learn from errors and promote teamwork. Furthermore, the number of staff completing the 6-hour online training course was recorded. Every ICU had online access to its own data and descriptive analysis. At regional and national levels, aggregated information was available.
Exposure and Outcomes
Exposure was defined as the implementation period of the study intervention (April 1, 2011, to December 31, 2012), rendering quarterly VAP rates for comparison with the baseline period rate obtained in the April to June 2010 ENVIN-HELICS study period (http://hws.vhebron.net/envin-helics/). The primary outcome was the quarterly rate of VAP. Secondary outcomes were the rates of VAP according to hospital features (type, teaching status, and size).
Statistical Analysis
The characteristics of patients with VAP diagnosed during the intervention period were compared with those of the baseline period. Quantitative variables were expressed as mean and sd or median and interquartile range (IQR) (25–75th percentile) as appropriate. Categorical variables are expressed as frequencies and percentages. Continuous data were compared using Student’s t test or Mann-Whitney U test, and categorical data with the chi-square test or the Fisher exact test according to distribution and size of the variables.
The number of VAP episodes, days of mechanical ventilation, and incidence rates were expressed as median and IQR. Median monthly data were aggregated in quarters. The implementation period for each ICU was analyzed by quarters of participation starting on the date of inclusion in the study. The quarterly infection rate was calculated as the number of infections per 1,000 mechanical ventilation days for each 3-month period. To explore the exposure-outcome relationship, we used generalized linear mixed regression models with a Poisson distribution (27) to calculate the incidence rates, incidence rate ratio, and 95% CI, considering the ICU unit as random and the other factors as fixed effects. In the final regression analysis, period estimates were adjusted by hospital type, teaching status, and size. All tests of significance were two sided and set at p value of less than 0.05. We used R version 3.1.2 language for statistical analysis (R Foundation for Statistical Computing, Vienna, Austria, 2014; http://www.R-project.org).
RESULTS
A total of 181 ICUs participated in the “Pneumonia Zero” project, with a total of 171,237 admissions, 1,011,782 days of ICU stay, and a rate of artificial airway use of 50.5% (505,802 d of mechanical ventilation). Participation in the project during the whole 21-month study period occurred in 132 ICUs (72.9%). The duration of participation for each ICU is shown in Table 1-e (Supplemental Digital Content 1, http://links.lww.com/CCM/C886). ICUs from large hospitals (> 500 beds) accounted for 44.7% of the total participating units, ICUs from medium-size hospitals (200–500 beds) for 42.0%, and ICUs from small hospitals (< 200 beds) for 13.3%. Also, there were 119 (65.7%) university-affiliated hospitals, 171 (94.5%) public hospitals, and 157 (86.7%) were certified for postgraduate training. A total of 109 ICUs (60.2%) completed the adherence survey. The results of the survey are shown in Table 2.
TABLE 2.
Adherence to Recommendations Included in the Spanish Ventilator-Associated Pneumonia Bundle
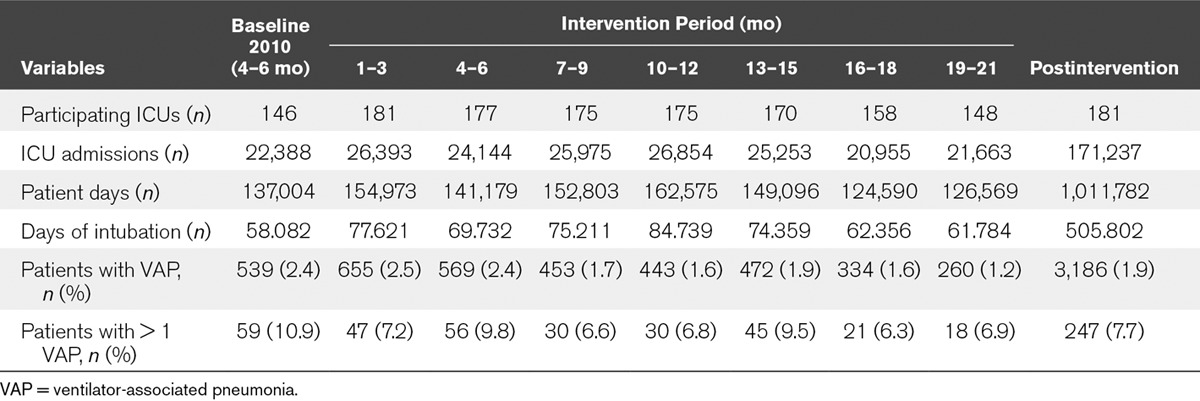
During the intervention period, a total of 3,474 episodes of VAP were diagnosed in 3,186 patients (6.87 episodes of VAP per 1,000 d of mechanical ventilation). The number of participating ICUs, admitted patients, days at risk (artificial airway), and patients with one or more episodes of VAP at baseline and per quarter are shown in Table 3. The percentage of patients with VAP decreased from 2.4% at baseline to 1.9% during the intervention period (p < 0.0001). ICUs with prolonged participation of 19–21 months achieved an even lower mean incidence of VAP of 1.2% (p < 0.0001). Fewer patients suffered from more than one episode of VAP during the intervention period compared with the baseline period (7.7% vs 10.9%; p = 0.037).
TABLE 3.
Characteristics of the Study Sample at Each Time Period
The characteristics of the 542 patients who developed VAP during the baseline period were similar to those of the 3,189 patients with VAP during the intervention period (Table 2-e, Supplemental Digital Content 1, http://links.lww.com/CCM/C886). Diagnostic sampling methods, associated systemic response, and etiology of VAP episodes are shown in Table 3-e (Supplemental Digital Content 1, http://links.lww.com/CCM/C886). During the intervention period, episodes of VAP occurred 2 days later than during the baseline period (median [IQR] 8.0 [4.0–16.0] vs 6.0 [3.0–13.0] d; p < 0.001).
The median incidence densities of VAP of those ICUs participating in both baseline and all subsequent quarters of exposure to the intervention are shown in Table 4-e (Supplemental Digital Content 1, http://links.lww.com/CCM/C886). The adjusted incidence density of VAP by hospital characteristics (Table 4) showed a 55.8% decrease, from a median (IQR) of 9.89 (8.42–11.48) to 4.34 (3.22–5.84) episodes per 1,000 days of mechanical ventilation in ICUs with 19–21 months of participation in the project. Also, a lower incidence density rate of VAP in large hospitals (> 500 beds) was observed. A reduction of the incidence density rates along the intervention period was observed, independently of hospital size (Fig. 1).
TABLE 4.
Univariate and Adjusted Multivariate Analyses for Ventilator-Associated Pneumonia
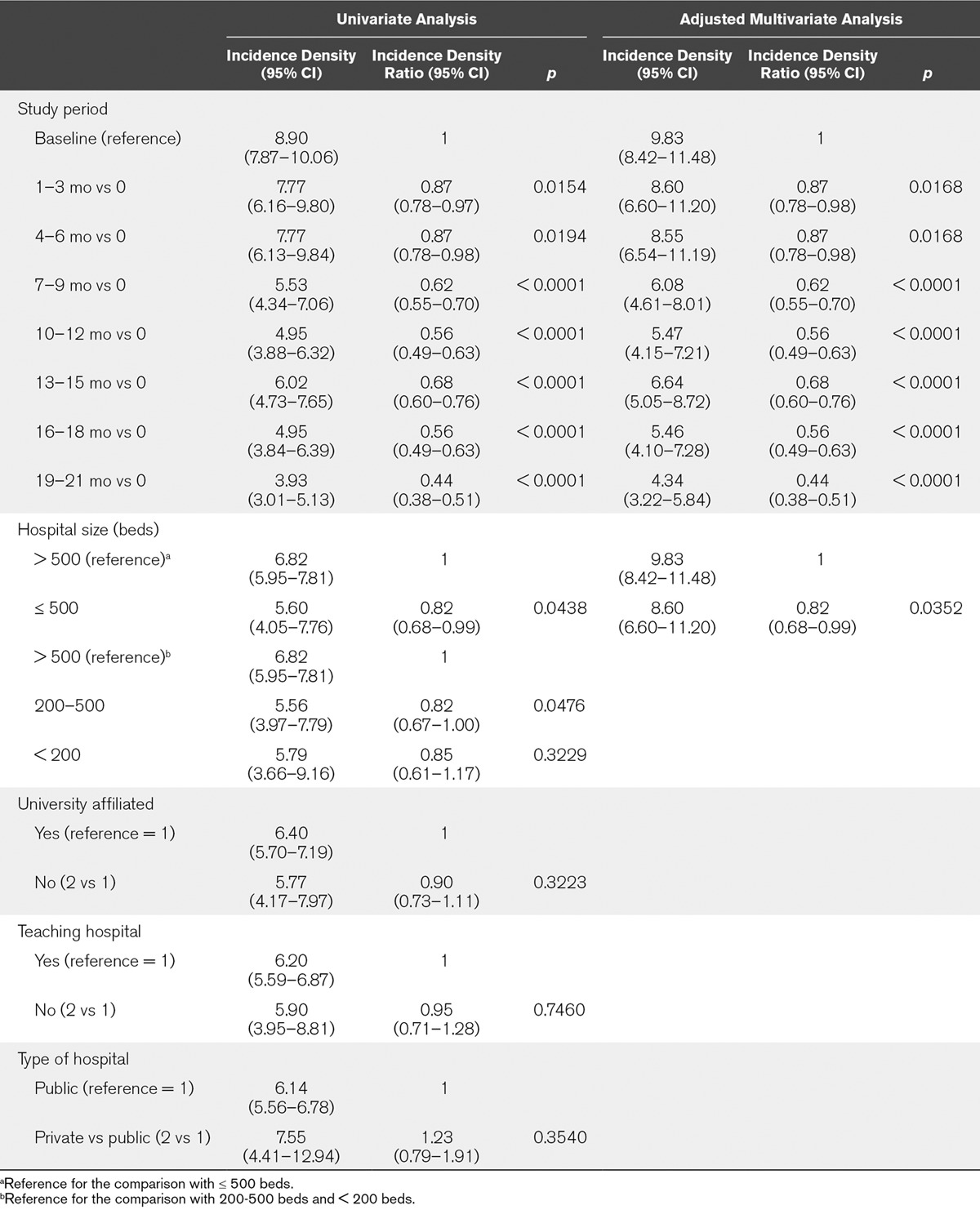
Figure 1.
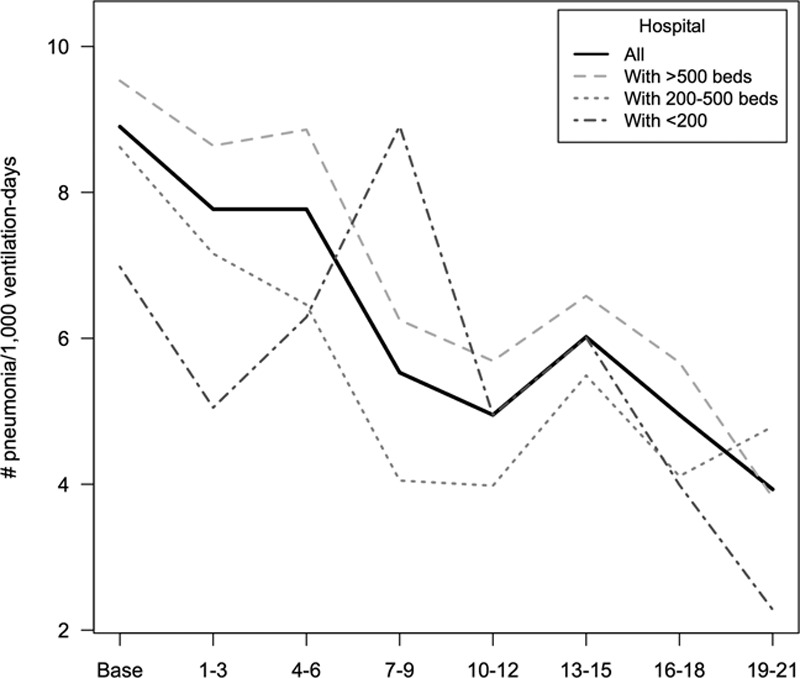
Changes of rates for ventilator-associated pneumonia for each time period of participation in the “Pneumonia Zero” project according to characteristics of the hospital (individual values of each curve are shown in Table 5-e, Supplemental Digital Content 1, http://links.lww.com/CCM/C886).
DISCUSSION
This study shows that the implementation of a bundle of measures to prevent VAP (24) in Spanish ICUs was associated with a highly significant reduction of VAP from 9.83 episodes during the baseline period to 4.34 episodes per 1,000 days of mechanical ventilation in the last 3 months of the intervention period (55.8% reduction). Furthermore, VAP occurred 2 days later when the bundle was applied. The magnitude of the reduction of VAP rates was progressive throughout the duration of participation, suggesting that sustained application of the bundle increases its clinical impact.
To our knowledge, this is the largest study demonstrating the effectiveness of implementing a VAP-specific prevention bundle at a national level. A total of 181 Spanish ICUs participated in the “Pneumonia Zero” project, accounting for approximately 75% of Spanish ICUs (28). These units had been involved annually since 1994 in reporting data of healthcare-related infections to a national registry (ENVIN, available at http://hws.vhebron.net/envin-helics/). It is the information available in this registry which allowed establishing baseline national incidence rates for VAP and to assess the effect of the intervention.
In recent years, a number of initiatives, mostly evidence-based guidelines for the prevention of VAP, have been published (29–36). In 2002, the Institute for Healthcare Improvement (IHI) launched “The 100k lives campaign,” which included a “ventilator bundle” to prevent VAP, with measures of peptic ulcer prophylaxis, deep vein thrombosis prophylaxis, elevation of the head of the bed, and a sedation vacation (7, 8). The impact of the implementation of the IHI bundle in 61 U.S. hospitals was associated with a 59% reduction in VAP rates in hospitals with a higher than 95% adherence (13). Similar experiences reported by other authors in different ICU settings have consistently shown a reduction of VAP rates (14–22).
Adherence to recommendations has been recognized as the main factor associated with reduction of VAP rates. Concanour et al (14) showed that VAP rates did not decrease with implementation of the ventilator bundle alone, but VAP decreased significantly after compliance with the ventilator bundle was audited daily, and weekly feedback was provided to the caregivers. Involvement of hospital managers together with continued education and feedback was crucial to maintain a low VAP rate (14). In another study, Hawe et al (37) reported a significant reduction of VAP rate from 19.2 to 7.5 per 1,000 ventilator days if a VAP prevention bundle was actively associated with a multimodal program incorporating staff education, process measurement, and outcome measurement, as well as feedback to staff and organizational change. In two surgical ICUs, Bird et al (18) showed a progressive decrease of the rate of VAP when compliance with the bundle was maintained. In our study, adherence to the project was documented by the completion of data required for calculation of rates (number of VAP episodes, days of ICU stay, and days of mechanical ventilation), with internal audits of the degree of compliance of four recommendations of the bundle.
The impact of the specific bundle on reduction of VAP rates was independent of the teaching status of the hospital and whether the hospital was of public or private nature. However, baseline and final VAP rates were higher in large hospitals with postgraduate and undergraduate training programs, probably due to differences in patient complexity and severity, as well as device use ratio, workload, and the increased presence of trainees in larger centers. It should be noted that ICU length of stay of patients with VAP increased significantly from baseline to the intervention period. A possible explanation may be the greater severity of VAP during the study period with an increased percentage of associated severe sepsis, septic shock, and increased cases of VAP caused by Gram-negative pathogens.
Implementation of measures for the prevention of VAP has been associated with training in safety, that is, our training module, which was completed during the implementation period by more than 18,000 healthcare workers, nurses in particular. These 18,000 healthcare workers accounted for approximately 83% of total workers in the participating ICUs. However, adherence to the use of the recommended tools was not measured. It should also be noted that one fourth of Spanish ICUs did not participate for economic reasons and possibly lack of motivation and leadership. Nonetheless, the total number of participating units is higher than that of previous national projects with the same organizational structure (“Bacteremia Zero” project) (23).
One of the consequences of the results presented here is that the reference quality indicator for VAP rate of the SEMICYUC needs to be adjusted. Every 5 years, the Society publishes quality reference standards which apply to Spanish ICUs, including VAP episodes per 1,000 ventilator days. For 2011, prior to the “Pneumonia Zero” program, the proposed rate was 12 episodes per 1,000 days of mechanical ventilation (data available at http://www.semicyuc.org/sites/default/files/quality_indicators_update_2011.pdf). Based on the results of implementing “Pneumonia Zero” program, the authors propose to readjust the acceptable national standard for VAP to seven episodes per 1,000 days of mechanical ventilation, with adjustments to hospital size.
Limitations of our study results relate to interobserver variability, that is, risk of bias, in the diagnosis of VAP, which is difficult to avoid in a multicenter study with and voluntary participation. Performance of many training workshops aimed at standardizing the definition of VAP was organized to limit bias. In addition, results of an audit of patients included in the ENVIN-HELICS registry between June and April 2010 (9) confirmed the quality of the diagnoses of VAP. On the other hand, no external audit of the degree and quality of compliance with each of the recommendations was performed, although internal assessment of adherence of four of the recommendations was requested using a survey (unpublished data).
Finally, it is our unproven understanding that success of implementation of the VAP prevention bundle is due to the effect of three simultaneous factors: 1) a bundle composed of interventions with proven efficacy in the prevention of VAP; 2) a pyramid shape organizational structure with participation of the Spanish Ministry of Health, the regional health care authorities, intensivists who are leaders in control of ICU infections, and other healthcare workers at a local level; and 3) an associated certified educational program as the instrument for disseminating patient safety concepts and providing detailed information about the implementation of well-known VAP prevention measures.
CONCLUSIONS
The nationwide implementation of a VAP prevention bundle was associated with a significant reduction of VAP rates of more than 50% at a national level. These findings confirm the hypothesis that implementation of a comprehensive evidence-based bundle is effective in reducing VAP rates.
ACKNOWLEDGMENTS
We are grateful to all healthcare professionals, physicians, and nurses who participated in training, education, and implementation of recommendations, project leaders in each participating ICU, technical managers in the different HealthCare Regions, and technical and administrative staff of the Spanish Ministry of Health, Social Policy and Equality. We are indebted to Sonia Uriona, MD, and Susana Otero for their contribution in the administration and secretariat of the ENVIN-HELICS registry and to Marta Pulido, MD, for editing the article and editorial assistance.
Supplementary Material
Footnotes
*See also p. 324.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by the Spanish Ministry of Health, Social Policy and Equality through a contract (Number 2012/07/CM016).
Dr. Alvarez-Lerma’s institution received funding from the Spanish Ministry of Health, Social Policy, and Equality through a contract (Number 2012/07/CM016) to Spanish Society of Intensive and Critical Care Medicine and Coroanry Units (SEMICYUC). Dr. Sánchez-García received funding for speaker fees from Pfizer, Merck, Sharp & Dohme, Astra-Zeneca, Orion, and Cepheid; for consulting fees from Bayer, GlaxoSmithKline, Pfizer, and Masimo; and for research grants from the European Union, 7th Framework Programme IMI, H2020. Dr. Vázquez-Calatayud disclosed that the study was supported by the Spanish Ministry of Health, Social Policy and Equality through a minor (Number 2012/07/CMO16) and scientific advised by the SEMICYUC and Sociedad Española de Enfermería Intensiva y Unidades Coronarias. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Rhodes A, Moreno RP, Azoulay E, et al. Task Force on Safety and Quality of European Society of Intensive Care Medicine (ESICM): Prospectively defined indicators to improve the safety and quality of care for critically ill patients: A report from the Task Force on Safety and Quality of the European Society of Intensive Care Medicine (ESICM). Intensive Care Med 2012; 38:598–605. [DOI] [PubMed] [Google Scholar]
- 2.Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias (SEMICYUC): Quality Indicators in Critically Ill Patients, 2011. Available at: http://www.semicyuc.org/temas/calidad/indicadores-de-calidad. Accessed September 5, 2016
- 3.Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: A cohort study. Lancet Infect Dis 2011; 11:30–38. [DOI] [PubMed] [Google Scholar]
- 4.Melsen WG, Rovers MM, Koeman M, et al. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit Care Med 2011; 39:2736–2742. [DOI] [PubMed] [Google Scholar]
- 5.Agrafiotis M, Siempos II, Ntaidou TK, et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis. Int J Tuberc Lung Dis 2011; 15:1154–63, i. [DOI] [PubMed] [Google Scholar]
- 6.Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 2013; 13:665–671. [DOI] [PubMed] [Google Scholar]
- 7.Berwick DM, Calkins DR, McCannon CJ, et al. The 100,000 lives campaign: Setting a goal and a deadline for improving health care quality. JAMA 2006; 295:324–327. [DOI] [PubMed] [Google Scholar]
- 8.McCannon CJ, Schall MW, Calkins DR, et al. Saving 100,000 lives in US hospitals. BMJ 2006; 332:1328–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sociedad Española de Medicina Intensiva: Grupo de Trabajo de Enfermedades Infecciosas (SEMICYUC-GTEI). Estudio Nacional de Vigilancia de Infección Nosocomial en UCI (ENVIN-UCI). Informes de los años 2001–2010. Available at: http://hws.vhebron.net/envin-helics/. Accessed September 1, 2017
- 10.Olaechea PM, Insausti J, Blanco A, et al. [Epidemiology and impact of nosocomial infections]. Med Intensiva 2010; 34:256–267. [DOI] [PubMed] [Google Scholar]
- 11.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355:2725–2732. [DOI] [PubMed] [Google Scholar]
- 12.Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis 2010; 29:1173–1177. [DOI] [PubMed] [Google Scholar]
- 13.Resar R, Pronovost P, Haraden C, et al. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf 2005; 31:243–248. [DOI] [PubMed] [Google Scholar]
- 14.Cocanour CS, Peninger M, Domonoske BD, et al. Decreasing ventilator-associated pneumonia in a trauma ICU. J Trauma 2006; 61:122–129. [DOI] [PubMed] [Google Scholar]
- 15.Youngquist P, Carroll M, Farber M, et al. Implementing a ventilator bundle in a community hospital. Jt Comm J Qual Patient Saf 2007; 33:219–225. [DOI] [PubMed] [Google Scholar]
- 16.Unahalekhaka A, Jamulitrat S, Chongsuvivatwong V, et al. Using a collaborative to reduce ventilator-associated pneumonia in Thailand. Jt Comm J Qual Patient Saf 2007; 33:387–394. [DOI] [PubMed] [Google Scholar]
- 17.Marra AR, Cal RG, Silva CV, et al. Successful prevention of ventilator-associated pneumonia in an intensive care setting. Am J Infect Control 2009; 37:619–625. [DOI] [PubMed] [Google Scholar]
- 18.Bird D, Zambuto A, O’Donnell C, et al. Adherence to ventilator-associated pneumonia bundle and incidence of ventilator-associated pneumonia in the surgical intensive care unit. Arch Surg 2010; 145:465–470. [DOI] [PubMed] [Google Scholar]
- 19.Al-Tawfiq JA, Abed MS.Decreasing ventilator-associated pneumonia in adult intensive care units using the Institute for Healthcare Improvement bundle. Am J Infect Control 2010; 38:552–556. [DOI] [PubMed] [Google Scholar]
- 20.Berenholtz SM, Pham JC, Thompson DA, et al. Collaborative cohort study of an intervention to reduce ventilator-associated pneumonia in the intensive care unit. Infect Control Hosp Epidemiol 2011; 32:305–314. [DOI] [PubMed] [Google Scholar]
- 21.Sinuff T, Muscedere J, Cook DJ, et al. Canadian Critical Care Trials Group: Implementation of clinical practice guidelines for ventilator-associated pneumonia: A multicenter prospective study. Crit Care Med 2013; 41:15–23. [DOI] [PubMed] [Google Scholar]
- 22.Eom JS, Lee MS, Chun HK, et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: A multicenter study. Am J Infect Control 2014; 42:34–37. [DOI] [PubMed] [Google Scholar]
- 23.Palomar M, Álvarez-Lerma F, Riera A, et al. Bacteremia Zero Working Group: Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: The Spanish experience. Crit Care Med 2013; 41:2364–2372. [DOI] [PubMed] [Google Scholar]
- 24.Álvarez Lerma F, Sánchez García M, Lorente L, et al. Sociedad Española de Medicina Intensiva; Sociedad Española de Enfermería Intensiva: Guidelines for the prevention of ventilator-associated pneumonia and their implementation. The Spanish “Zero-VAP” bundle. Med Intensiva 2014; 38:226–236. [DOI] [PubMed] [Google Scholar]
- 25.Surveillance of nosocomial infections in Intensive Care Units: Hospital in Europe Link for Infection Control through Surveillance (HELICS) (Versión 6.1. September 2004). Available at: http://ecdc.europa.eu/en/publications/Publications/HAI-Net-ICU-protocol-v2.2.pdf. Accessed September 1, 2017
- 26.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med 1985; 13:818–829. [PubMed] [Google Scholar]
- 27.Molenberghs G, Verbeke G.Models for Discrete Longitudinal Data. 2005New York, NY, Springer Science+Business Media, [Google Scholar]
- 28.Martín MC, León C, Cuñat J, et al. [Intensive care services resources in Spain]. Med Intensiva 2013; 37:443–451. [DOI] [PubMed] [Google Scholar]
- 29.Tablan OC, Anderson LJ, Besser R, et al. Healthcare Infection Control Practices Advisory Committee: Guidelines for preventing health-care–associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004; 53:1–36. [PubMed] [Google Scholar]
- 30.Dodek P, Keenan S, Cook D, et al. Canadian Critical Care Trials Group; Canadian Critical Care Society: Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med 2004; 141:305–313. [DOI] [PubMed] [Google Scholar]
- 31.Masterton RG, Galloway A, French G, et al. Guidelines for the management of hospital-acquired pneumonia in the UK: Report of the working party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2008; 62:5–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muscedere J, Dodek P, Keenan S, et al. VAP Guidelines Committee and the Canadian Critical Care Trials Group: Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: Prevention. J Crit Care 2008; 23:126–137. [DOI] [PubMed] [Google Scholar]
- 33.Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol 2008; 29 (Suppl 1):S31–S40. [DOI] [PubMed] [Google Scholar]
- 34.Cason CL, Tyner T, Saunders S, et al. Centers for Disease Control and Prevention: Nurses’ implementation of guidelines for ventilator-associated pneumonia from the Centers for Disease Control and Prevention. Am J Crit Care 2007; 16:28–36; discussion 37; quiz 38. [PubMed] [Google Scholar]
- 35.Torres A, Ewig S, Lode H, et al. European HAP working group: Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med 2009; 35:9–29. [DOI] [PubMed] [Google Scholar]
- 36.Venkatram S, Rachmale S, Kanna B.Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care 2010: 25:174.e11–e18. [DOI] [PubMed] [Google Scholar]
- 37.Hawe CS, Ellis KS, Cairns CJ, et al. Reduction of ventilator-associated pneumonia: Active versus passive guideline implementation. Intensive Care Med 2009; 35:1180–1186. [DOI] [PubMed] [Google Scholar]


