Abstract
Background:
Associations between HIV-related stigma and reduced antiretroviral therapy (ART) adherence are widely established, yet the mechanisms accounting for this relationship are underexplored. There has been less attention to HIV-related stigma and its associations with ART initiation and current ART use. We examined pathways from HIV-related stigma to ART initiation, current ART use, and ART adherence among women living with HIV in Canada.
Methods:
We used baseline survey data from a national cohort of women living with HIV in Canada (n = 1425). Structural equation modeling using weighted least squares estimation methods was conducted to test the direct effects of HIV-related stigma dimensions (personalized, negative self-image, and public attitudes) on ART initiation, current ART use, and 90% ART adherence, and indirect effects through depression and HIV disclosure concerns, adjusting for sociodemographic factors.
Results:
In the final model, the direct paths from personalized stigma to ART initiation (β = −0.104, P < 0.05) and current ART use (β = −0.142, P < 0.01), and negative self-image to ART initiation (β = −0.113, P < 0.01) were significant, accounting for the mediation effects of depression and HIV disclosure concerns. Depression mediated the pathways from personalized stigma to ART adherence, and negative self-image to current ART use and ART adherence. Final model fit indices suggest that the model fit the data well [χ2(25) = 90.251, P < 0.001; comparative fit index = 0.945; root-mean-square error of approximation = 0.044].
Conclusions:
HIV-related stigma is associated with reduced likelihood of ART initiation and current ART use, and suboptimal ART adherence. To optimize the benefit of ART among women living with HIV, interventions should reduce HIV-related stigma and address depression.
Key Words: HIV stigma, women, antiretroviral therapy, adherence, depression, structural equation modeling
INTRODUCTION
The HIV care cascade encompasses stages between HIV acquisition to virological suppression.1 Engaging people living with HIV (PLWH) in the HIV care cascade is vital to reaching the UNAIDS 90-90-90 goals, which include 90% of PLWH diagnosed, 90% of persons diagnosed with HIV initiating antiretroviral therapy (ART), and 90% of people taking ART virally suppressed.2 Suboptimal adherence may contribute to virological failure—when ART treatment fails to suppress a person's viral load to less than 200 copies per millimeter—and death among PLWH3–6 and can contribute to virological resistance.7,8 Meta-analytic findings from 102 independent estimations of ART adherence reported over one-third of participants (n = 33,199) had less than 90% adherence—suggesting significant ongoing concerns with suboptimal adherence globally.9
Women account for one-fifth of PLWH in Canada10 and over half of PLWH worldwide.11 Sex disparities are well documented in access to HIV care and outcomes, even within contexts of universal access to health care, such as Canada.12,13 For example, a Canadian longitudinal study of PLWH who use injection drugs (n = 545) reported that female sex was associated with a 30% reduction in the odds of 95% adherence to ART, after adjusting for clinical characteristics and drug use patterns.12 These findings underscore the importance of examining factors that are associated with ART initiation, use, and adherence among women living with HIV (WLWH) in Canada.
HIV-related stigma and discrimination compromise access to the HIV care continuum among WLWH,14–17 including reduced access to HIV prevention, early access to treatment, and suboptimal ART outcomes.18 Stigma processes include labeling, loss of status, and discrimination in contexts of unequal power distribution.19 Stigma types include: anticipated, expecting or fearing discrimination, stereotyping, and prejudice from others in the future; perceived, perceptions of the existence and severity of stigmatizing attitudes in the community; enacted, experienced acts of violence, poor treatment, and discrimination from others; and internalized, acceptance of negative societal beliefs about PLWH by PLWH themselves.20–22
HIV-Related Stigma and ART Outcomes, Depression, and HIV Disclosure Concerns and Behaviors
There is a well-established negative association between HIV-related stigma and ART adherence.23–25 Katz et al's24 systematic review and metasynthesis assessed the relationship between HIV-related stigma and ART adherence. Results from 34 qualitative studies suggest that HIV-related stigma may compromise adherence through intrapersonal (reduced adaptive coping strategies) and interpersonal (disclosure practices, concealment, and reduced social support) processes.24 Quantitative study results (n = 41) assessed whether associations existed between HIV-related stigma and nonadherence. Most cross-sectional—but not longitudinal—studies confirmed associations between stigma and lower adherence. In a more recent review, Sweeney and Vanable23 reviewed quantitative studies (n = 38) to further explore the causal mechanisms accounting for the associations between HIV-related stigma and adherence. This review examined types of HIV-related stigma and adherence; most studies that assessed a single dimension (eg, anticipated) of stigma found associations with lower adherence. All studies that examined more than 1 stigma dimension (eg, anticipated, enacted, and internalized) found an association between at least 1 type of stigma and nonadherence.23 Scant studies in the review explored more than 1 stigma dimension with adherence. The review identified 2 empirically supported pathways between HIV-related stigma and adherence: the first pathway was from HIV-related stigma to increased depression and worse adherence, and the second was from HIV-related stigma to reduced self-efficacy and subsequently reduced adherence. One pathway emerged in qualitative findings but was not examined in quantitative studies: from HIV-related stigma, to HIV disclosure concerns, to reduced adherence.23 Although this lack of study may be because HIV disclosure concerns are often considered a manifestation or type of HIV-related stigma, as these authors suggest, further studies are needed to examine specific causal mechanisms, including potential mediators, between HIV-related stigma and reduced adherence. Moreover, a recent conceptual model described by Turan et al22 that explored causal pathways and potential mediators between different types of HIV-related stigma (enacted, community/perceived, anticipated, and internalized) and ART adherence identified nondisclosure as a potential mediator between HIV-related stigma and poor ART outcomes, and suggested nondisclosure is a manifestation of anticipated or perceived stigma. Another knowledge gap is that stigma dimensions have commonly been assessed in isolation (eg, only anticipated, perceived, enacted, or internalized stigma)22; the inclusion of multiple forms of stigma in 1 model would allow for estimation of the association between variables without measurement error.
Depression may play a mediating role in the relationship between HIV-related stigma and HIV care cascade outcomes. Depression is a widely established concern among PLWH,26 and is associated with both HIV-related stigma among PLWH27–29 and lower odds of retention in care30 and reduced adherence.31,32 Three studies have assessed mental health issues (broadly),33 depressive symptoms,34 and depression diagnoses,35 as a mediator between HIV-related stigma and poor ART adherence. Although Sayles et al33 found that mental health issues fully mediated the relationship between internalized HIV-related stigma and nonadherence in their multivariate logistic regression analysis, Rao et al34 found that depression partially mediated the association between HIV-related stigma (a composite measure of internalized and enacted stigma dimensions) and lower adherence among PLWH (n = 720) in their structural equation model. Studies by DiIorio et al35 and Sayles et al33 assessed 1 dimension of HIV-related stigma—internalized—precluding our understanding of the role of other stigma dimensions in this relationship.
Interpersonal factors, such as HIV disclosure concerns and HIV disclosure behavior, may mediate associations between HIV-related stigma and ART adherence.22,23 HIV disclosure, the act of a PLWH telling another person about their HIV diagnosis, continues to be feared and stressful,36 particularly if inadvertent.23 Qualitative studies suggest that a fear of inadvertently disclosing one's HIV-positive serostatus through discovery of ART use may present barriers to ART adherence.32,37–39 A qualitative study of pregnant and breastfeeding women who had initiated ART at the time of pregnancy (n = 57) described the mechanisms that women used to protect themselves from disclosure, such as throwing away ART containers.38
Disclosure behaviors may have positive or negative associations with ART outcomes. Some studies have demonstrated that WLWH are less likely to disclose than men40; this may be associated with sex inequity.41 Qualitative studies have shown that HIV disclosure increases a woman's risk of intimate partner violence after diagnosis, this may be particularly challenging for women in serodiscordant or unknown partner status couples.38,42 Women may also experience economic loss after disclosure.43 HIV-related stigma may thereby reduce likelihood of disclosure.41 For example, in a multisite observational cohort study of WLWH in Zambia, Thailand, and Brazil (n = 299), women who reported higher anticipated stigma had a 70% reduction in the odds of HIV disclosure to sexual partners.41 A meta-analysis exploring associations between HIV-related stigma, social support, and HIV disclosure found a small but consistent relationship between HIV-related stigma and reduced HIV disclosure.36 The associations between HIV-related stigma and HIV disclosure concerns or behavior, and between HIV disclosure concerns or behavior and adherence, have been cited within the literature, yet no peer-reviewed published studies were located that quantitatively tested HIV disclosure concerns or behavior as a mediator of the relationship between HIV-related stigma and ART adherence.23
Few studies have explored relationships between HIV-related stigma and HIV care cascade outcomes beyond ART adherence. This is particularly important in the context of test-and-treat approaches to ART initiation that recommend that all PLWH begin treatment on diagnosis.44 Patel et al's37 qualitative study with PLWH (n = 33) reported that HIV disclosure concerns presented a barrier to ART initiation. Moreover, participants reported that HIV-related stigma, including both anticipated and enacted stigma, prevented both HIV disclosure and initiation of ART. In another qualitative study,45 newly diagnosed WLWH described HIV-related stigma and fear of disclosure as concerns for ART initiation.
Gaps in the Literature, Study Objective, and Hypotheses
To meet the HIV care cascade goals, there needs to be a better understanding of the mechanisms by which HIV-related stigma influences ART initiation, use, and adherence.22 Our study objective was to test a conceptual model exploring associations between a multidimensional HIV-related stigma construct and ART initiation, current ART use, and >90% ART adherence among WLWH in Canada. We examined direct relationships between HIV-related stigma and these variables, and indirect associations through depression and HIV disclosure concerns as mediators.
METHODS
This analysis draws on baseline cross-sectional survey data collected as part of a national cohort study (Canadian HIV Women's Sexual & Reproductive Health Cohort Study [CHIWOS]) conducted in Ontario, QC, and British Columbia, Canada between August 2013 and May 2015.46,47 Data were collected by WLWH and/or women not living with HIV from highly affected communities (eg, transgender women) trained as peer research associates who recruited self-identified WLWH aged 16 years or older using purposive sampling methods (eg, word-of-mouth) and venue-based recruitment (eg, HIV clinics). Community advisory boards were formed to enhance targeted recruitment of women overrepresented in Canada's HIV epidemic, including transgender-specific and Indigenous community advisory boards. The 90–120 minutes of survey included questions about sociodemographic factors, health care access, physical/mental health outcomes, stigma, and discrimination. Surveys were administered by peer research associates using a web-based interface in a confidential location (eg, AIDS service organization, space in or near clinics, and women's home) or by telephone or Skype for some rural residents located in British Columbia's interior, and Northern Ontario and Quebec.48 All participants provided informed consent before commencing the interview, consistent with the ethics protocol approved by Women's College Hospital, University of Toronto (Ontario), Simon Fraser University and the University of British Columbia/Providence Health (British Columbia), and McGill University Health Centre (Quebec). Participants received a $50 Canadian honorarium for their participation.
Measures
Clinical outcomes included ART initiation, currently being on ART, and ART adherence. ART initiation was assessed dichotomously by “Have you ever taken Antiretroviral Medications (ARVs) for your own health” (Yes = 1, No = 0). Currently on ART was measured also dichotomously by “Are you currently on Antiretroviral Medications (ARVs)” (Yes = 1, No = 0). ART adherence was measured continuously by asking participants to provide their best estimate about how much medication they took in the past month from 0% to 100%. For the purpose of this study, we categorized this variable into 2 values: (1) ≥90% adherence; (0) <90% adherence.49
HIV-related stigma was measured with Wright's shortened 10-item version of Berger's HIV-Stigma Scale (score range: 0–100; Cronbach α = 0.85).50 For the purpose of this study, we included 3 subscales of this HIV-related scale: personalized (“I have been hurt by how people reacted to learning I have HIV”; “I have stopped socializing with some people because of their reactions of my having HIV”; and “I have lost friends by telling them I have HIV”), negative self-image (“Having HIV makes me feel unclean”; “I feel that I am not as good a person as others because I have HIV”; “Having HIV makes me feel that I'm a bad person”), public attitudes (“Most people think that a person with HIV is disgusting”; and “Most people with HIV are rejected when others find out”).50 We omitted the HIV disclosure subscale, as we measured this as a separate construct and potential mediator between HIV-stigma and ART outcomes, using a different measure (detailed below). Items of the HIV-related stigma scale were measured using a Likert scale (strongly agree, agree, neither agree nor disagree, disagree, and strongly disagree). For conceptual purposes, the personalized stigma subscale measures enacted HIV-related stigma, the negative self-image subscale measures internalized HIV-related stigma, and the public attitudes subscale measures perceived HIV stigma.
Potential mediators included depression and HIV disclosure concerns. Depression was assessed using the Center for Epidemiologic Studies Depression 10-item Scale (CES-D 10; eg, “How often in the past week were you bothered by things that don't usually bother you”)51,52 (score range: 0–30; Cronbach α = 0.87). Higher scores in the CES-D 10 indicate higher level of depression. Depression scale items were measured using a Likert scale (most or all the time, occasionally or a moderate amount of time, same or a little of the time, and rarely or none of the time). HIV disclosure concerns were measured with a HIV/AIDS quality of life (HAT-QOL) sub-scale, which contains 6 questions regarding disclosure practices among HIV-positive people (“I've limited what I tell others about myself”; “I have been afraid to tell other people that I have HIV”; “I have been worried about my family members finding out that I have HIV”; “I have been worried about people at my job/routine daily activities finding out that I have HIV”; “I have been worried that I will lose my source of income if other people find out that I have HIV”; and “I have been worried that I will lose access to health services or care if people find out that I have HIV”).53,54 Scores range from 0 to 24. Cronbach 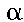 for the HIV disclosure scale was 0.95. Items of disclosure concerns were measured using a Likert scale as well (strongly agree, agree, neutral, disagree, or strongly disagree). Items were reverse coded and summed so that a higher score indicated more disclosure concerns. For all scales, ambiguous responses (ie, prefer not to say/don't know) and missing values were excluded from analyses.
for the HIV disclosure scale was 0.95. Items of disclosure concerns were measured using a Likert scale as well (strongly agree, agree, neutral, disagree, or strongly disagree). Items were reverse coded and summed so that a higher score indicated more disclosure concerns. For all scales, ambiguous responses (ie, prefer not to say/don't know) and missing values were excluded from analyses.
We included several sociodemographic factors in this study as covariates, including age (continuous), legal relationship status (single vs. married/common laws), immigration status (Canadian citizen, landed immigrant/permanent resident, refugee, and other), ethnicity (Indigenous, Black, white, and other), education (less than high school vs. high school or higher), injection drug use history (never used injection drugs, previous injection drug use but not current, and current injection drug use), and years of HIV diagnosis (continuous). Viral load was assessed with a self-reported measure that was validated among a subsample of participants from the CHIWOS study (n = 356) whose data could be linked with clinical data; 94% of women correctly self-reported an undetectable viral.55
Statistical Analyses
We first conducted descriptive analyses of all variables for the full sample. Unadjusted and adjusted logistic regression models were used to estimate the odds ratio (OR) of clinical outcomes (ART initiation, current ART use, and 90% ART adherence).
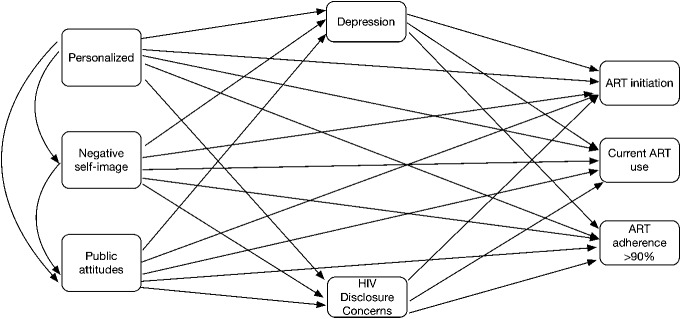
Structural equation modeling was conducted using weighted least square estimation methods to test the direct effects of HIV-related stigma dimensions (personalized, negative self-image, and public attitude) on HIV care cascade outcomes (ART initiation, current ART use, and 90% ART adherence), and the indirect effects through depression and HIV disclosure concerns, adjusting for sociodemographic factors. Model fit was assessed using: χ2, root-mean-square error of approximation (RMSEA), and comparative fit index (CFI). A score of <0.05 for RMSEA and a score greater than 0.90 for CFI indicate an acceptable fit.56 Figure 1 illustrates all the tested path models.
FIGURE 1.
Conceptualized pathways from HIV-related stigma to ART initiation, current ART use, and >90% ART adherence.
Statistical significance was set at the P < 0.05 level. Missing responses were excluded from the analyses. All statistical analyses were performed using STATA (version 14.0) and Mplus (version 1.40).
RESULTS
Participant Characteristics
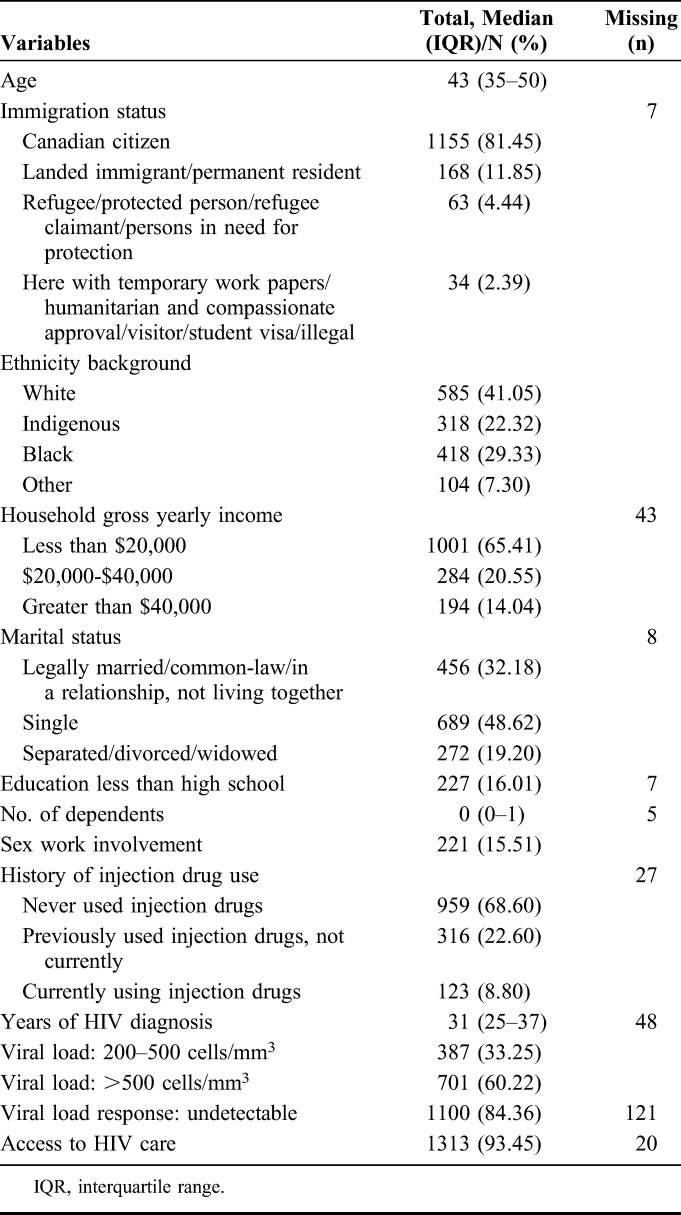
Table 1 reports sociodemographic characteristics for the whole sample (N = 1425). Participants were a median age of 43 years old (interquartile range = 35–50). Most of the sample (81.5%) reported being Canadian citizens. Approximately two-thirds (65.3%) of the participants received less than Canadian $20,000 annual household income. One-third (32.2%) of the participants were legally married/in a relationship/common law. Most participants (87.4%) had initiated ART treatment. Most participants reported currently taking ART (83.07%). More than four-fifths of participants (82.68%) reported >90% adherence.
TABLE 1.
Demographic Information Among Women With HIV in Canada (N = 1425)
Multivariate Logistic Regressions on Correlates of ART Initiation, Currently on ART and Medication Adherence
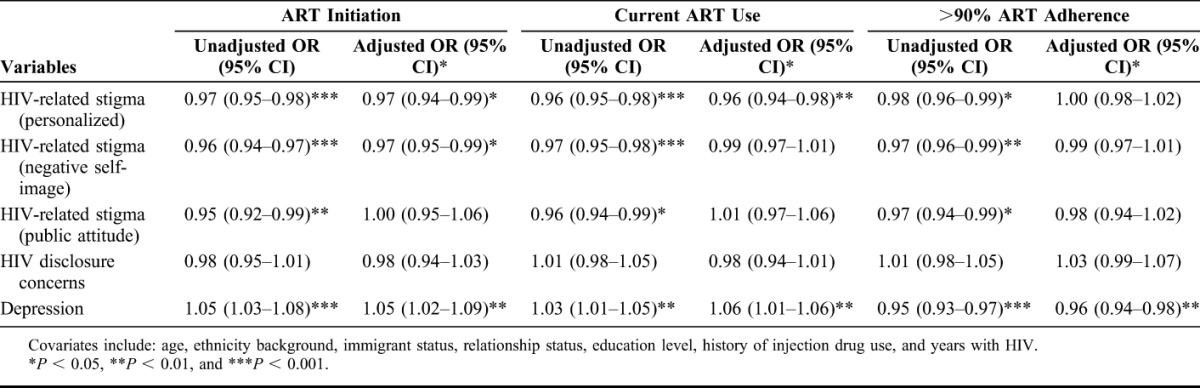
Table 2 illustrates the unadjusted and adjusted (A) OR for ART initiation, current ART use, and >90% ART adherence. All results were adjusted for age, ethnicity background, immigration status, relationship status, and education level. The odds of ART initiation were negatively associated with personalized HIV-related stigma [AOR: 0.97, 95% confidence interval (CI): 0.95 to 0.99], negative self-image (AOR: 0.97, 95% CI: 0.95 to 0.99), and positively associated with depression (AOR: 1.05, 95% CI: 1.02 to 1.09). The odds of currently taking ART were lower for participants with higher personalized HIV-related stigma (AOR: 0.96, 95% CI: 0.94 to 0.98), and higher for participants reporting depression (AOR: 1.06, 95% CI: 1.01 to 1.06). The likelihood of reporting >90% ART adherence was lower for participants reporting depression (AOR: 0.96, 95% CI: 0.94 to 0.98).
TABLE 2.
Unadjusted and Adjusted Logistic Regression of ART Initiation, Current ART Use, and >90% ART Adherence on HIV-Related Stigma Among Women With HIV in Canada (N = 1418)
Structural Equation Modeling
Structural equation modeling was conducted to examine the direct and indirect effects of HIV-related stigma dimensions (personalized, negative self-image, public attitudes) on HIV care cascade outcomes: ART initiation, currently on ART, and >90% ART adherence. Final model fit indices suggested that the model fit the data well [χ2(25) = 90.251, P < 0.001; CFI = 0.945; RMSEA = 0.044; weighted root-mean-square residual = 1.026]. Table 3 displays the results of the final model.
TABLE 3.
Final Path Model Parameter Estimates of ART Initiation, Current ART Use, and >90% ART Adherence Among Women With HIV in Canada (N = 1418)*
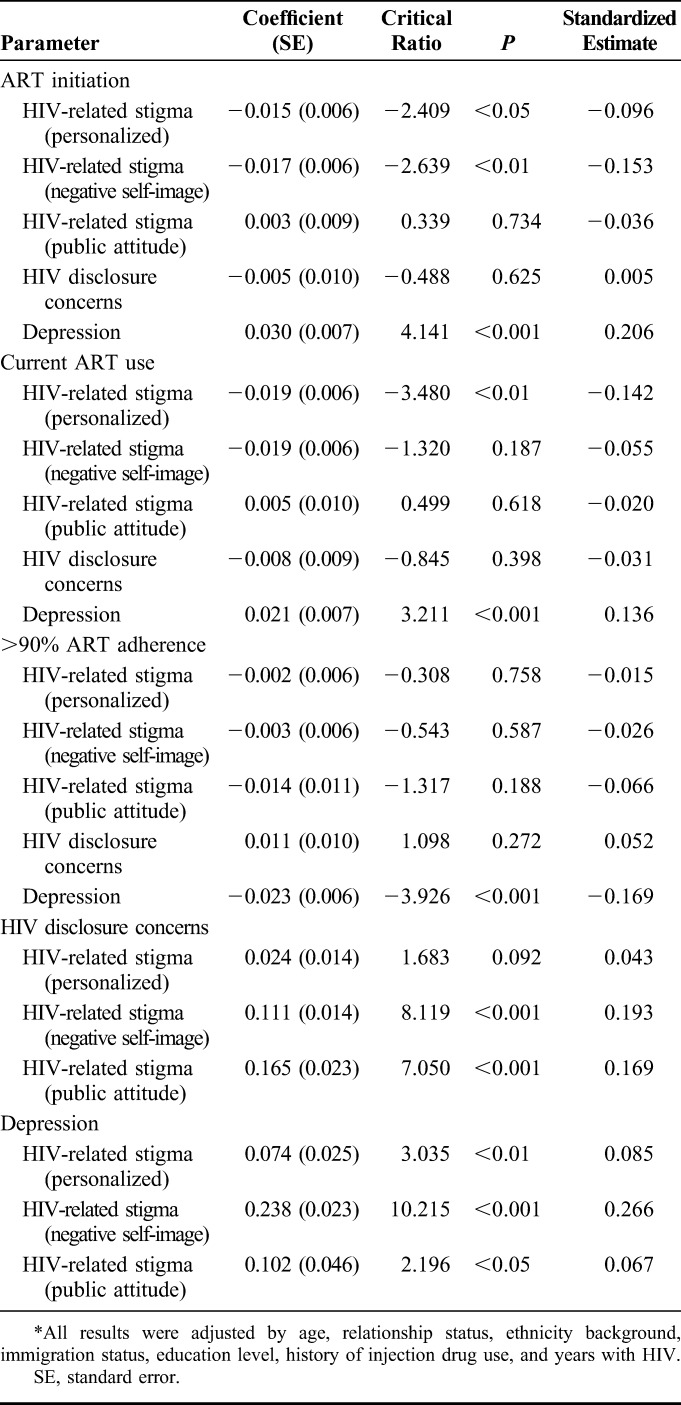
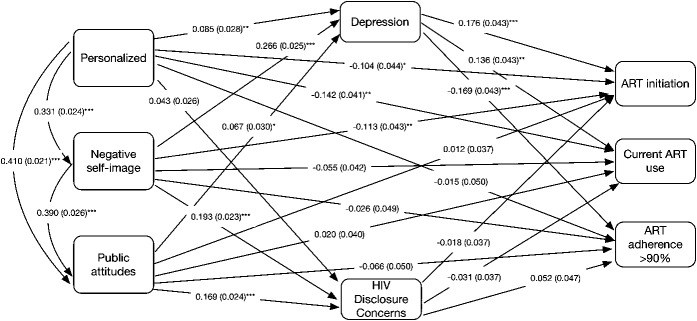
Figure 2 illustrates the model with standard coefficients and their significance levels of each pathway. The standardized coefficient indicated that with a SD increase of the independent variable, the dependent variable would increase by x SD, holding all other variables constant.57 Standard errors were included in parenthesis. In the final model, the direct paths from personalized HIV-related stigma to ART initiation (β = −0.104, P < 0.05: direct effect, β = 0.014, P < 0.05: indirect effect) and current ART use (β = −0.142, P < 0.01: direct effect, β = 0.010, P < 0.05: indirect effect), negative self-image to ART initiation (β = −0.113, P < 0.01: direct effect, β = 0.043, P < 0.01: indirect effect) were significant, accounting for the mediation effects of depression and HIV disclosure concerns. Depression fully mediated the pathway from personalized HIV-related stigma to >90% ART adherence (β = −0.014, P < 0.05), and the pathway from negative self-image HIV stigma to current ART use (β = 0.036, P < 0.01) and >90% ART adherence (β = −0.045, P < 0.001). Depression partially mediated the relationship between negative self-image and ART initiation (β = 0.047, P < 0.001), current ART use (β = 0.037, P < 0.01), and personalized HIV-related stigma and ART initiation (β = 0.015, P < 0.05), current ART use (β = 0.012, P < 0.05).
FIGURE 2.
Final path analysis results for HIV-related stigma on ART initiation, current ART use, and ART adherence >90%. Standard coefficients are reported with the standard errors in parentheses. Statistical significance is noted with the following notations; *P < 0.05, **P < 0.01, ***P < 0.001. Covariates include age, ethnicity, immigration status, relationship status, education level, and years with HIV.
DISCUSSION
HIV-related stigma was associated with less engagement across the HIV care cascade in the form of reduced ART initiation, ART adherence, and ART use among this large cohort of WLWH. An intrapersonal (depression) level factor partially mediated these associations. Our findings offer insight into the role HIV-related stigma may play in limiting engagement in HIV care.
First, our findings corroborate studies that report negative associations between HIV-related stigma and ART outcomes such as adherence.23–25 We build on qualitative studies to provide quantitative evidence demonstrating that HIV-related stigma, and in particular, negative self-image, a measure of internalized HIV-related stigma, and personalized HIV-related stigma, a measure of enacted HIV-related stigma, are barriers to ART initiation.37,45 We contribute to the literature by examining these different dimensions of HIV-related stigma, in addition to negative public attitudes (a measure of perceived HIV-related stigma) in relation to multiple ART outcomes. Specifically, we found that enacted HIV-related stigma influenced both ART initiation and current ART use, and internalized HIV-related stigma influenced ART initiation, accounting for the mediation effects of depression. Interestingly, perceived HIV-related stigma was not associated with any ART outcome. These findings corroborate theorizing by Turan et al22 who suggest that different types of HIV-related stigma may be differentially associated with ART adherence and indicate a need for multilevel interventions. Structural interventions could focus on reducing enacted HIV-related stigma, and intrapersonal interventions could address internalized HIV-related stigma.
These findings also suggest that depression is a particularly important underlying mechanism of suboptimal ART outcomes for WLWH. First, consistent with much research studies, depression was significantly and positively associated with all HIV-related stigma dimensions27 and negatively associated with ART adherence.31,32 Moreover, depression mediated the pathways between enacted and internalized stigma dimensions and ART adherence. These findings underscore the importance of addressing intrapersonal (internalized HIV-related stigma and resultant depression) and structural (enacted HIV-related stigma) factors to improve ART adherence for WLWH. However, depression only partially mediated the relationship between enacted and internalized HIV-related stigma and ART initiation and current ART use, underscoring the important role of HIV-related stigma as a driver of health.22
Interestingly, we also found that depression was positively associated with ART initiation and current use. This finding is consistent with emerging research; for example, a longitudinal study conducted in Kenya (n = 162) similarly found that depressive symptom severity was associated with greater odds of ART initiation.58 It is plausible that WLWH may seek health care because of depression, promoting ART initiation and maintenance, even while acting as a barrier to optimal ART adherence. Depression is common among people newly diagnosed with HIV59 and may persist over time due in part to HIV-related stigma. Depression interventions may have limited or brief impacts on adherence60,61 if not coupled with evidence-based HIV-stigma reduction interventions that address both depression and social contexts (eg, stigma) that contribute to depression.62,63
We found that all HIV-related stigma dimensions were associated with higher HIV disclosure concerns, although disclosure concerns were not associated with ART outcomes and did not mediate the association between HIV-related stigma and ART outcomes. Future studies should seek to measure disclosure behaviors, the context under which disclosure occurs (eg, purposely, inadvertently; by self, by others),22 and disclosure outcomes, which still constitute a gap in the literature.23 Studies focused on understanding HIV disclosure must take into account the legal and sexed contexts within which disclosure occurs for WLWH, and the particular concerns related to violence and economic insecurity for women after disclosure.41,42 Most research studies on disclosure among WLWH has emerged from low- and middle-income contexts. More research studies are necessary about HIV disclosure practices among WLWH in Canada, a country with one of the strictest laws of criminalization of HIV nondisclosure globally.64 A systematic review of studies assessing the impact of the criminalization of HIV nondisclosure on HIV testing and diagnosis, linkage and retention in care, and ART adherence65 found only 2 studies focused on women.
Our study has limitations. The cross-sectional design limits the ability to assess causation, and reverse causation is possible. For example, some studies have found an increase in HIV-related stigma and depression after ART initiation.66 This could be better understood using a longitudinal design. The use of a purposive, nonrandom sampling strategy may have resulted in oversampling WLWH already engaged in care. Women engaged in HIV care may differ from those not engaged in care across key sociodemographics, in addition to levels of marginalization. However, we oversampled WLWH experiencing intersecting forms of marginalization including substance use and sex work to reduce this sampling bias.46 Data were self-reported, and therefore subject to recall and social desirability bias, including the ART outcome variables. Other indicators, such as unannounced pill counts or viral load, may be more robust. Similarly, a systematic review of studies (n = 77) that used self-report measures of ART adherence found that self-reported adherence was significantly related to adherence as assessed by other measures, such as electronic drug measure and pill count in 79% of studies comparing measurement approaches.67 Because of the differing national roll out of test and treat,68 a proportion of those who have not initiated or are not currently using ART may have not initiated because of lack of provider recommendation. However, regardless of ART recommendation practices, HIV-related stigma is important to consider across the cascade and is associated with the range of ART outcomes.
Despite these limitations, our study has several strengths. First, we enhance understanding of pathways between HIV-related stigma dimensions and ART adherence, and this can inform tailored adherence strategies. Previous qualitative37,45 and conceptual23 work have theorized a pathway between HIV-related stigma and ART adherence mediated by HIV disclosure concerns and behaviors, and we contribute to this evidence base by testing this pathway using a large sample size and robust structural equation model analyses. Second, our use of a multidimensional measure of HIV-related stigma including personalized stigma, negative self-image, and negative public attitudes, provides a comprehensive measure of multiple stigma dimensions that may shape health behaviors. Finally, we broaden the focus from HIV-related stigma and ART adherence to other components that are critical to the HIV care cascade: ART initiation and currently taking ART. To the best of our knowledge, we are among the first quantitative studies to examine these relationships—this can inform interventions and programs that span the cascade.
Antiretroviral uptake and adherence remain suboptimal globally9 and among WLWH in our sample. Future research should draw on evidence-based HIV-stigma reduction strategies62,63 and expand on these interventions to target multiple stigma dimensions, as these are likely to contribute to the UNAIDS 90-90-90 goals. Until a concerted effort is made to address the drivers of HIV-related stigma at individual, interpersonal, and structural levels, WLWH will experience barriers to engaging across the HIV care cascade that will compromise their health and well-being.
ACKNOWLEDGMENTS
The CHIWOS Research Team thanks women living with HIV for their contributions to this study. They also thank the national team of co-investigators, collaborators, and Peer Research Associates and acknowledge the national Steering Committee, our three provincial Community Advisory Boards, the National CHIWOS Aboriginal Advisory Board, the BC Centre for Excellence in HIV/AIDS for data support and analysis, and all our partnering organizations for supporting the study. Listed here are all research team members and affiliated institutions; all those not listed by name on the title page are to be hyperlinked as authors:
The CHIWOS Research Team: British Columbia: Aranka Anema (University of British Columbia), Denise Becker (Positive Living Society of British Columbia), Lori Brotto (University of British Columbia), Allison Carter (British Columbia Centre for Excellence in HIV/AIDS and Simon Fraser University), Claudette Cardinal (Simon Fraser University), Guillaume Colley (British Columbia Centre for Excellence in HIV/AIDS), Erin Ding (British Columbia Centre for Excellence), Janice Duddy (Pacific AIDS Network), Nada Gataric (British Columbia Centre for Excellence in HIV/AIDS), Robert S. Hogg (British Columbia Centre for Excellence in HIV/AIDS and Simon Fraser University), Terry Howard (Positive Living Society of British Columbia), Shahab Jabbari (British Columbia Centre for Excellence), Evin Jones (Pacific AIDS Network), Mary Kestler (Oak Tree Clinic, BC Women's Hospital and Health Centre), Andrea Langlois (Pacific AIDS Network), Viviane Lima (British Columbia Centre for Excellence in HIV/AIDS), Elisa Lloyd-Smith (Providence Health Care), Melissa Medjuck (Positive Women's Network), Cari Miller (Simon Fraser University), Deborah Money (Women's Health Research Institute), Valerie Nicholson (Simon Fraser University), Gina Ogilvie (British Columbia Centre for Disease Control), Sophie Patterson (Simon Fraser University), Neora Pick (Oak Tree Clinic, BC Women's Hospital and Health Centre), Eric Roth (University of Victoria), Kate Salters (Simon Fraser University), Margarite Sanchez (ViVA, Positive Living Society of British Columbia), Jacquie Sas (CIHR Canadian HIV Trials Network), Paul Sereda (British Columbia Centre for Excellence in HIV/AIDS), Marcie Summers (Positive Women's Network), Christina Tom (Simon Fraser University, BC), Lu Wang (British Columbia Centre for Excellence), Kath Webster (Simon Fraser University), and Wendy Zhang (British Columbia Centre for Excellence in HIV/AIDS). Ontario: Rahma Abdul-Noor (Women's College Research Institute), Jonathan Angel (Ottawa Hospital Research Institute), Fatimatou Barry (Women's College Research Institute), Greta Bauer (University of Western Ontario), Kerrigan Beaver (Women's College Research Institute), Anita Benoit (Women's College Research Institute), Breklyn Bertozzi (Women's College Research Institute), Sheila Borton (Women's College Research Institute), Tammy Bourque (Women's College Research Institute), Jason Brophy (Children's Hospital of Eastern Ontario), Ann Burchell (Ontario HIV Treatment Network), Allison Carlson (Women's College Research Institute), Lynne Cioppa (Women's College Research Institute), Jeffrey Cohen (Windsor Regional Hospital), Tracey Conway (Women's College Research Institute), Curtis Cooper (Ottawa Hospital Research Institute), Jasmine Cotnam (Women's College Research Institute), Janette Cousineau (Women's College Research Institute), Annette Fraleigh (Women's College Research Institute), Brenda Gagnier (Women's College Research Institute), Claudine Gasingirwa (Women's College Research Institute), Saara Greene (McMaster University), Trevor Hart (Ryerson University), Shazia Islam (Women's College Research Institute), Charu Kaushic (McMaster University), Logan Kennedy (Women's College Research Institute), Desiree Kerr (Women's College Research Institute), Maxime Kiboyogo (McGill University Health Centre), Gladys Kwaramba (Women's College Research Institute), Lynne Leonard (University of Ottawa), Johanna Lewis (Women's College Research Institute), Carmen Logie (University of Toronto), Shari Margolese (Women's College Research Institute), Marvelous Muchenje (Women's Health in Women's Hands), Mary (Muthoni) Ndung'u (Women's College Research Institute), Kelly O'Brien (University of Toronto), Charlene Ouellette (Women's College Research Institute), Jeff Powis (Toronto East General Hospital), Corinna Quan (Windsor Regional Hospital), Janet Raboud (Ontario HIV Treatment Network), Anita Rachlis (Sunnybrook Health Science Centre), Edward Ralph (St. Joseph's Health Care), Sean Rourke (Ontario HIV Treatment Network), Sergio Rueda [Centre for Addiction and Mental Health (CAMH)], Roger Sandre (Haven Clinic), Fiona Smaill (McMaster University), Stephanie Smith (Women's College Research Institute), Tsitsi Tigere (Women's College Research Institute), Wangari Tharao (Women's Health in Women's Hands), Sharon Walmsley (Toronto General Research Institute), Wendy Wobeser (Kingston University), Jessica Yee (Native Youth Sexual Health Network), and Mark Yudin (St-Michael's Hospital). Quebec: Dada Mamvula Bakombo (McGill University Health Centre), Jean-Guy Baril (Université de Montréal), Nora Butler Burke (University Concordia), Pierrette Clément (McGill University Health Center), Janice Dayle (McGill University Health Centre), Danièle Dubuc (McGill University Health Centre), Mylène Fernet (Université du Québec à Montréal), Danielle Groleau (McGill University), Aurélie Hot (COCQ-SIDA), Marina Klein (McGill University Health Centre), Carrie Martin (Native Women's Shelter of Montreal), Lyne Massie, (Université de Québec à Montréal), Brigitte Ménard, (McGill University Health Centre), Nadia O'Brien (McGill University Health Centre and Université de Montréal), Joanne Otis (Université du Québec à Montréal), Doris Peltier (Canadian Aboriginal AIDS Network), Alie Pierre (McGill University Health Centre), Karène Proulx-Boucher (McGill University Health Centre), Danielle Rouleau (Centre Hospitalier de l'Université de Montréal), Édénia Savoie (McGill University Health Centre), Cécile Tremblay (Centre Hospitalier de l’Université de Montréal), Benoit Trottier (Clinique l'Actuel), Sylvie Trottier (Centre Hospitalier Universitaire de Québec), and Christos Tsoukas (McGill University Health Centre). Other Canadian provinces or international jurisdictions: Jacqueline Gahagan (Dalhousie University), Catherine Hankins (University of Amsterdam), Renee Masching (Canadian Aboriginal AIDS Network), and Susanna Ogunnaike-Cooke (Public Health Agency of Canada).
All other CHIWOS Research Team Members who wish to remain anonymous.
Footnotes
Supported by Canadian Institutes of Health Research (MOP-111041), the CIHR Canadian HIV Trials Network (CTN 262), the Ontario HIV Treatment Network (OHTN), and the Academic Health Science Centres (AHSC) Alternative Funding Plans (AFP) Innovation Fund. Funders played no role in study design, data collection, analysis, or interpretation. C.H.L.'s contributions were also supported by an Ontario Ministry of Research and Innovation Early Researcher Award.
Poster presentation for the 26th Annual Canadian Conference on HIV/AIDS Research; April 6–9, 2017; Montreal, Québec, Canada.
The authors have no funding or conflicts of interest to disclose.
Contributor Information
Collaborators: Aranka Anema, Denise Becker, Lori Brotto, Allison Carter, Claudette Cardinal, Guillaume Colley, Erin Ding, Janice Duddy, Nada Gataric, Robert S. Hogg, Terry Hosward, Shahab Jabbari, Evin Jones, Mary Kestler, Andrea Langlois, Viviane Lima, Elisa Lloyd-Smith, Melissa Medjuck, Cari Miller, Deborah Money, Valerie Nicholson, Gina Ogilvie, Sophie Patterson, Neora Pick, Eric Roth, Kate Salters, Margarite Sanchez, Jacquie Sas, Paul Sereda, Marcie Summers, Christina Tom, Lu Wang, Kath Webster, Wendy Zhang, Rahma Abdul-Noor, Jonathan Angel, Fatimatou Barry, Greta Bauer, Kerrigan Beaver, Anita Benoit, Breklyn Bertozzi, Sheila Borton, Tammy Bourque, Jason Brophy, Ann Burchell, Allison Carlson, Lynne Cioppa, Jeffrey Cohen, Tracey Conway, Curtis Cooper, Jasmine Cotnam, Janette Cousineau, Annette Fraleigh, Brenda Gagnier, Claudine Gasingirwa, Saara Greene, Trevor Hart, Shazia Islam, Charu Kaushic, Logan Kennedy, Desiree Kerr, Maxime Kiboyogo, Gladys Kwaramba, Lynne Leonard, Johanna Lewis, Carmen Logie, Shari Margolese, Marvelous Muchenje, Mary Ndung'u, Kelly O'Brien, Charlene Ouellette, Jeff Powis, Corinna Quan, Janet Raboud, Anita Rachlis, Edward Ralph, Sean Rourke, Sergio Rueda, Roger Sandre, Fiona Smaill, Stephanie Smith, Tsitsi Tigere, Wangari Tharao, Sharon Walmsley, Wendy Wobeser, Jessica Yee, Mark Yudin, Jean-Guy Baril, Nora Butler Burke, Pierrette Clément, Janice Dayle, Danièle Dubuc, Mylène Fernet, Danielle Groleau, Aurélie Hot, Marina Klein, Carrie Martin, Lyne Massie, Brigitte Ménard, Nadia O'Brien, Joanne Otis, Doris Peltier, Alie Pierre, Karène Proulx-Boucher, Danielle Rouleau, Édénia Savoie, Cécile Tremblay, Benoit Trottier, Sylvie Trottier, Christos Tsoukas, Jacqueline Gahagan, Catherine Hankins, Renee Masching, and Susanna Ogunnaike-Cooke
REFERENCES
- 1.Mugavero MJ, Amico KR, Horn T, et al. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57:1164–1171. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS. 90–90–90-An ambitious treatment target to help end the AIDS epidemic. 2014;1–33. [Google Scholar]
- 3.Giordano TP, Gifford AL, White AC, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. [DOI] [PubMed] [Google Scholar]
- 4.Horstmann E, Brown J, Islam F, et al. Retaining HIV-infected patients in care: where are we? Where do we go from here? Clin Infect Dis. 2010;50:752–761. [DOI] [PubMed] [Google Scholar]
- 5.Mugavero MJ, Lin HY, Allison JJ, et al. Racial disparities in HIV virologic failure: do missed visits matter? J Acquir Immun Defic Syndr. 2009;50:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner EM, Hullsiek KH, Telzak EE, et al. Antiretroviral medication adherence and class- specific resistance in a large prospective clinical trial. AIDS. 2010;24:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbloom DI, Hill AL, Rabi SA, et al. Antiretroviral dynamics determines HIV evolution and predicts therapy outcome. Nat Med. 2012;18:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15:1381–1396. [DOI] [PubMed] [Google Scholar]
- 10.Public Health Agency of Canada. Summary: estimates of HIV incidence, prevalence, and proportion undiagnosed in Canada, 2014. 2015;1–10. [Google Scholar]
- 11.UN Women. Facts and figures: HIV and AIDS 2016. Available at: http://www.unwomen.org/en/what-we-do/hiv-and-aids/facts-and-figures. Accessed May 1, 2017.
- 12.Tapp C, Milloy MJ, Kerr T, et al. Female gender predicts lower access and adherence to antiretroviral therapy in a setting of free healthcare. BMC Infect Dis. 2011;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cescon A, Patterson S, Chan K, et al. Gender differences in clinical outcomes among HIV-positive individuals on antiretroviral therapy in Canada: a multisite cohort study. PLoS One. 2014;8:e83649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandelowski M, Barroso J, Voils CI. Gender, race/ethnicity, and social class in research reports on stigma in HIV-positive women. Health Care Women Int. 2009;30:273–288. [DOI] [PubMed] [Google Scholar]
- 15.Herek GM, Mitnick L, Burris S, et al. Workshop report: AIDS and stigma: a conceptual framework and research agenda. AIDS Public Policy J. 1998;13:36–47. [PubMed] [Google Scholar]
- 16.Logie CH, James L, Tharao W, et al. HIV, gender, race, sexual orientation, and sex work: a qualitative study of intersectional stigma experienced by HIV-positive women in Ontario, Canada. PLoS Med. 2011;8:e1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orza L, Bewley S, Logie CH, et al. How does living with HIV impact on women's mental health? Voices from a global survey. J Int AIDS Soc. 2015;18:20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Soc Sci Med. 2003;57:13–24. [DOI] [PubMed] [Google Scholar]
- 19.Link BG, Phelan JC. Conceptualizing stigma. Annu Rev Sociol. 2001;27:363–385. [Google Scholar]
- 20.Earnshaw VA, Chaudoir SR. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS Behav. 2009;13:1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyblade LC. Measuring HIV stigma: existing knowledge and gaps. Psychol Health Med. 2006;11:335–345. [DOI] [PubMed] [Google Scholar]
- 22.Turan B, Hatcher AM, Weiser SD, et al. Framing mechanisms linking HIV-related stigma, adherence to treatment, and health outcomes. Am J Public Health. 2017;107:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney SM, Vanable PA. The association of HIV-related stigma to HIV medication adherence: a systematic review and synthesis of the literature. AIDS Behav. 2016;20:29–50. [DOI] [PubMed] [Google Scholar]
- 24.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16:18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;4:939–941. [DOI] [PubMed] [Google Scholar]
- 26.Lowther K, Selman L, Harding R, et al. Experience of persistent psychological symptoms and perceived stigma among people with HIV on antiretroviral therapy (ART): a systematic review. Int J Nurs Stud. 2014;51:1171–1189. [DOI] [PubMed] [Google Scholar]
- 27.Logie C, Gadalla TM. Meta-analysis of health and demographic correlates of stigma towards people living with HIV. AIDS Care. 2009;21:742–753. [DOI] [PubMed] [Google Scholar]
- 28.Hatzenbuehler ML, O'Cleirigh C, Mayer KH, et al. Prospective associations between HIV-related stigma, transmission risk behaviors, and adverse mental health outcomes in men who have sex with men. Ann Behav Med. 2011;42:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herek GM, Saha S, Burack J. Stigma and psychological distress in people with HIV/AIDS. Basic Appl Social Psychol. 2013;35:41–54. [Google Scholar]
- 30.Pecoraro A, Royer-Malvestuto C, Rosenwasser B, et al. Factors contributing to dropping out from and returning to HIV treatment in an inner city primary care HIV clinic in the United States. AIDS Care. 2013;25:1399–1406. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez JS, Penedo FJ, Antoni MH, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychol. 2004;23:413–418. [DOI] [PubMed] [Google Scholar]
- 32.Rao D, Kekwaletswe TC, Hosek S, et al. Stigma and social barriers to medication adherence with urban youth living with HIV. AIDS Care. 2007;19:28–33. [DOI] [PubMed] [Google Scholar]
- 33.Sayles JN, Wong MD, Kinsler JJ, et al. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24:1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao D, Feldman BJ, Fredericksen RJ, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS Behav. 2012;16:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiIorio C, McCarty F, Depadilla L, et al. Adherence to antiretroviral medication regimens: a test of a psychosocial model. AIDS Behav. 2009;13:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith R, Rossetto K, Peterson B. A meta-analysis of disclosure of one's HIV-positive status, stigma and social support. AIDS Care. 2008;20:1266–1275. [DOI] [PubMed] [Google Scholar]
- 37.Patel RC, Odoyo J, Anand K, et al. Facilitators and barriers of antiretroviral therapy initiation among HIV discordant couples in Kenya: qualitative insights from a pre-exposure prophylaxis implementation study. PLoS One. 2016;11:e0168057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buregyeya E, Naigino R, Mukose A, et al. Facilitators and barriers to uptake and adherence to lifelong antiretroviral therapy among HIV infected pregnant women in Uganda: a qualitative study. BMC Pregnancy Childbirth. 2017;17:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ware NC, Wyatt MA, Tugenberg T. Social relationships, stigma and adherence to antiretroviral therapy for HIV/AIDS. AIDS Care. 2006;18:904–910. [DOI] [PubMed] [Google Scholar]
- 40.Bachanas P, Medley A, Pals S, et al. Disclosure, knowledge of partner status, and condom use among HIV-positive patients attending clinical care in Tanzania, Kenya, and Namibia. AIDS Patient Care STDS. 2013;27:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ojikutu BO, Pathak S, Srithanaviboonchai K, et al. Community cultural norms, stigma and disclosure to sexual partners among women living with HIV in Thailand, Brazil and Zambia (HPTN 063). PLoS One. 2016;11:e0153600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulrenan C, Colombini M, Howard N, et al. Exploring risk of experiencing intimate partner violence after HIV infection: a qualitative study among women with HIV attending postnatal services in Swaziland. BMJ Open. 2015;5:e006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porter L, Hao L, Bishai D, et al. HIV status and union dissolution in sub-Saharan Africa: the case of Rakai, Uganda. Demography. 2004;41:465–482. [DOI] [PubMed] [Google Scholar]
- 44.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. [DOI] [PubMed] [Google Scholar]
- 45.Mbonye M, Seeley J, Nalugya R, et al. Test and treat: the early experiences in a clinic serving women at high risk of HIV infection in Kampala. AIDS Care. 2016;28:33–38. [DOI] [PubMed] [Google Scholar]
- 46.Loutfy M, de Pokomandy A, Carter A, et al. Cohort profile: the Canadian HIV Women's Sexual and Reproductive Health Cohort Study (CHIWOS). PLoS One. 2017;12:e0184708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loutfy M, Greene S, Kennedy VL, et al. Establishing the Canadian HIV Women's Sexual and Reproductive Health Cohort Study (CHIWOS): operationalizing community-based research in a large national quantitative study. BMC Med Res Methodol. 2016;16:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abelsohn K, Benoit AC, Conway T, et al. “Hear(ing) new voices”: peer reflections from community-based survey development with women living with HIV. Prog Community Health Partnersh. 2015;9:561–569. [DOI] [PubMed] [Google Scholar]
- 49.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–277. [DOI] [PubMed] [Google Scholar]
- 50.Wright K, Naar-King S, Lam P, et al. Stigma scale revised: reliability and validity of a brief measure of stigma for HIV+ youth. J Adolesc Health. 2007;40:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, O'Brien N, Forrest JI, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One. 2012;7:e40793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 53.Holmes W, Shea J. Performance of a new, HIV/AIDS-targeted quality of life (HAT-QoL) instrument in asymptomatic seropositive individuals. Qual Life Res. 1997;6:561–571. [DOI] [PubMed] [Google Scholar]
- 54.Holmes WC, Shea JA. A new HIV/AIDS-targeted quality of life (HAT-QoL) instrument: development, reliability, and validity. Med Care. 1998;36:138–154. [DOI] [PubMed] [Google Scholar]
- 55.Carter A, de Pokomandy A, Loutfy M, et al. Validating a self-report measure of HIV viral suppression: an analysis of linked questionnaire and clinical data from the Canadian HIV Women's Sexual and Reproductive Health Cohort Study. BMC Res Notes. 2017;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller RO, Hancock GR. Best practices in structural equation modeling. In: Best Practices in Quantitative Methods. Thousand Oaks, CA: Sage Publications; 2008:488–508. [Google Scholar]
- 57.Long JS, Freese J. Regression Models Categorical Dependent Variables Using Stata. College Station, TX: Stata Press; 2006. [Google Scholar]
- 58.Velloza J, Celum C, Haberer JE, et al. Depression and ART initiation among HIV serodiscordant couples in Kenya and Uganda. AIDS Behav. 2017;21:2509–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatia R, Hartman C, Kallen MA, et al. Persons newly diagnosed with HIV infection are at high risk for depression and poor linkage to care: results from the Steps Study. AIDS Behav. 2011;15:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Safren SA, O'Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai AC, Karasic DH, Hammer GP, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: a randomized controlled trial. Am J Public Health. 2013;103:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nyblade L, Stangl A, Weiss E, et al. Combating HIV stigma in health care settings: what works? J Int AIDS Soc. 2009;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stangl AL, Lloyd JK, Brady LM, et al. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc. 2013;16:18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mykhalovskiy E, Betteridge G. Who? what? where? when? and with what consequences?: an analysis of criminal cases of HIV non-disclosure in Canada. Can J L Soc. 2012;27:31–53. [Google Scholar]
- 65.Patterson SE, Milloy MJ, Ogilvie G, et al. The impact of criminalization of HIV non-disclosure on the healthcare engagement of women living with HIV in Canada: a comprehensive review of the evidence. J Int AIDS Soc. 2015;18:20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearson CR, Micek MA, Pfeiffer J, et al. One year after ART initiation: psychosocial factors associated with stigma among HIV-positive Mozambicans. AIDS Behav. 2009;13:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simoni JM, Kurth AE, et al. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Normand J, Montaner J, Fang CT, et al. HIV: seek, test, treat, and retain. J Food Drug Anal. 2013;21:S4–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]