Abstract
Background: This study sought to determine whether levothyroxine pharmacokinetics (PKs) are affected by age, weight, and sex.
Methods: A PK study was performed after administration of a tracer dose of carbon-13-labeled LT4 (13C-LT4). The study was conducted at an academic medical center. Adults of any age being treated with levothyroxine for hypothyroidism were enrolled in the study. A single dose of 13C-LT4 was administered. Eighteen serial plasma samples were collected. One sample was obtained before the 13C-LT4 dose, and the majority of the remaining samples were collected over the 120-hour period post dosing. 13C-LT4 concentration was quantified using liquid chromatography tandem mass spectrometry. PK analysis was conducted using a linear log trapezoidal non-compartmental analysis using Phoenix 6.4.
Results: Eight males and 33 females with a median age of 50 years (range 22–78 years) and median weight of 65.9 kg (range 50–150 kg) were enrolled in the study. The median 13C-LT4 dose administered was 100 μg (range 70–300 μg). The median oral clearance rate (CL/F), apparent volume of distribution (V/F), time to peak concentration (Tmax), and dose-normalized peak concentration (Cmax) of 13C-LT4 were estimated to be 0.712 L/h, 164.9 L, 4 h, and 7.5 ng/L/μg, respectively. The dose-normalized area under the concentration–time curve from time 0 to 120 hours and half-life of the terminal distribution phase were 0.931 ng.h/mL/μg and 172.2 h, respectively. There was no significant difference in any 13C-LT4 PK parameter between patients aged >60 years (n = 10) and patients aged ≤60 years (n = 31), nor was there a relationship between age as a continuous variable and 13C-LT4 PK parameters. Sex only affected CL/F, V/F, and dose-normalized Cmax in univariate analyses. However, after adjusting for weight, sex was no longer a significant covariate. Weight was a significant predictor for CL/F, V/F and dose-normalized Cmax of 13C-LT4 in multivariate analyses.
Conclusion: Prior studies suggest that patient age affects levothyroxine dose requirement. This study did not identify an effect of age and suggests that age-related changes in levothyroxine pharmacokinetics may be mediated by age-related weight differences. Physicians should consider a patient's weight, rather than age, for estimating levothyroxine dosage requirement.
Keywords: : levothyroxine, metabolism, clearance, aging, weight
Introduction
Hypothyroidism is a common condition resulting from deficiency of thyroid hormones (1,2). The prevalence of hypothyroidism varies according to the region being studied and whether overt or both overt and subclinical disease is being considered, but it is approximately 4.6% in the United States (2,3). In addition to the incidence of hypothyroidism being higher in females compared to males, some studies also suggest the incidence of hypothyroidism is higher in older age groups, starting at about 60 years of age (4), or at least that hypothyroidism is being treated more frequently in older age groups (5). Levothyroxine (LT4) is a synthetic thyroid hormone that is biochemically and physiologically indistinguishable from endogenous T4, and is indicated for the treatment of hypothyroidism. The goal of therapy is to bring thyroid hormones into the normal physiologic range and decrease the symptoms of hypothyroidism (1,2).
Although this seems to be an easy goal, previous studies have shown that approximately 20% of individuals with hypothyroidism, including those with low-risk thyroid cancer, are over-treated, and almost the same percentage are under-treated (5–12). Despite the generally held belief that iatrogenic thyroid disease may pose more of a risk for patients in older age groups (1,13), older patients seem to be at similar, or even greater risk, of being treated with larger doses of LT4 (14), or of not being treated to an appropriate thyrotropin (TSH) goal (5,7). There is also evidence that LT4 may be prescribed more frequently in the elderly (5,7), and that there is a trend over time to initiate LT4 treatment with lesser degrees of TSH elevation in patients of all age groups (11,15).
Achieving a serum TSH that is within the normal age-adjusted reference interval is desirable for all patients. However, the risks stemming from iatrogenic thyroid disease may be greater in patients falling into older age groups (e.g., >60 years, >65 years, >75 years, etc.) (13,16,17). Risks of over-treatment that are more pronounced in older age groups include decreased bone mineral density (18–21) and increased risk of cardiac arrhythmias (21,22). Risks of under-treatment include unresolved symptoms of hypothyroidism, dyslipidemia, cardiac dysfunction, and increased mortality (16,23). Avoidance of iatrogenic thyroid disease can be achieved by accurate estimation of initial dose requirement, appropriate regimens for taking LT4 replacement, and close monitoring of serum TSH values during therapy so early adjustments in LT4 can be made (1). With respect to initial estimation of LT4 dose, several factors are agreed upon as being important. These include the amount of residual endogenous thyroid function and patient weight or lean body mass (1,24–29). Other factors that have an influence on the required dose in some studies but not in others include patient sex (30,31) and age (32,33).
TSH serves as a surrogate marker of euthyroidism. It is assumed to be an integrated measure of how the individual pituitary–thyroid axis responds to prevailing free thyroxine (fT4) levels. Although this may be a simplification, at least in animal models (34), it is assumed that all other clinical effects of thyroid hormone in patients receiving LT4 replacement parallel its effect on the hypothalamus, so that TSH can provide a quantitative measure of the adequacy of LT4 replacement. However, another key issue for thyroid hormone replacement adequacy is appropriate fT4 concentrations at the tissue level.
Given the altered drug metabolism in older age groups, the narrow therapeutic index of LT4 (1), and the possible risk associated with higher exposure to fT4 (35,36) or low TSH levels (18–22), it is important to study the pharmacokinetics (PKs) of LT4 across the age spectrum. Therefore, the objectives of this study were to (i) determine the PKs of LT4 in individuals of various ages, (ii) determine if LT4 PKs are different for older individuals, and (iii) determine if other patient demographics such as sex and weight affect the dose exposure relationship. This information could help physicians optimizing LT4 dosing in older patients.
Methods
Study overview
This study was a prospective, single-center, open-label, non-randomized study to evaluate the PKs of a stable carbon isotope labeled LT4 (13C6-LT4) following single-dose administration. The research protocol was approved by the Georgetown University Institutional Review Board and written informed consent was obtained from all study participants. All participants were recruited from Georgetown University Medical Center, and the study was conducted in the Georgetown Clinical Research Unit. The clinical trial registration number is NCT03102177.
Participants
Both men and women being treated for hypothyroidism were enrolled in the study. The inclusion criteria were age ≥21 years at the time of consent, euthyroidism while undergoing treatment with LT4, no other serious illness, and ability to give written informed consent. Participants were excluded from the study for any of the following reasons: baseline hematocrit <28.0%, TSH >4.5 mIU/L, hepatic dysfunction, kidney dysfunction, and concomitant use of drugs that may affect thyroidal axis interactions, including drugs that potentially alter TSH and thyroid hormone secretion, transport, or metabolism.
Drug, drug administration, and blood sampling for pharmacokinetics
The 13C6-LT4 derivative was synthesized for the purpose of this study and purchased from IsoSciences (King of Prussia, PA). The 13C6-LT4 was compounded in 70 and 100 μg capsules by a compounding pharmacy (Wells Pharmacy Network, Ocala, FL). The initial T4 content of the compounded capsules was verified by high-performance liquid chromatography spectrophotometry, and the capsules were also tested to exclude microbial contamination by Eagle Analytic Services (Houston, TX). The 13C6-LT4 capsules were kept at room temperature in the dark in a firmly closed desiccator until use. The capsules were stored and dispensed by the Georgetown Research Pharmacy. Quality control potency and stability testing were conducted biannually by Dynalabs (St. Louis, MO), an independently licensed quality control laboratory.
Participants were required to fast for at least eight hours before and two hours after ingesting the morning 13C6-LT4 dose. Patients did not take any other medication within two hours of ingesting 13C6-LT4 in order to prevent any interference with 13C6-LT4's rate of absorption. They were offered a simple breakfast two hours after ingesting the 13C6-LT4 dose. Each participant received capsules of 100 or 70 μg of 13C6-LT4 (the molar equivalent of 100 or 70 μg of unlabeled-LT4) depending on their usual prescribed LT4 dose. Participants whose usual daily dose exceeded multiples of 100 or 70 μg of LT4 received the difference between their usual dose and the 100 or 70 μg 13C6-LT4 dose as unlabeled LT4 in order to make up the difference. Thus, a patient's total daily dose was approximately unchanged on the study day. Blood samples were drawn at pre-dose (0 hours) and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 24, 48, 72, 96, 120, 144, and 312 hours post dose. All blood samples were drawn into 10 mL tubes without anticoagulant. After a 10 min centrifugation at 2500 rpm, serum aliquots were stored at −80°C before assay.
13C6-LT4 assay
Serum concentrations of 13C6-LT4 were measured using a validated, sensitive, and specific isotope dilution tandem mass spectrometry method at NMS laboratories based on methodology developed by Dr. Steven Soldin (37). The assay details have been prescribed in a previous work (38). The assay used 13C12-LT4 as the internal standard. The linear range of the assay was 0.050–10 ng/mL. The limit of quantification was 0.002 ng/mL. The assay demonstrated excellent precision and accuracy, with coefficients of variation as follows: 0.14 ng/mL, CV ∼3.38%, 1.4 ng/mL, CV ∼5.52%, and 7.0 ng/mL, CV ∼6.63%.
Pharmacokinetic methodology
PK assessments were conducted by a standard two-stage approach using non-compartmental techniques in Phoenix v6.4 (Pharsight Corporation, Mountain View, CA). Calculated PK parameters included peak concentration (Cmax), time to peak concentration (Tmax), area under the concentration–time curve from time 0 to infinity (AUC0–∞), AUC from time 0 to 120 hours (AUC0–t), oral clearance rate (CL/F; defined as the ratio of administered dose to AUC0–∞), the apparent plasma terminal rate constant (λ), apparent volume of distribution (V/F, estimated by CL/F/λ), and the half-life of the terminal disposition phase (t1/2) estimated by ln(2)/λ. A combination of linear trapezoidal approach during the ascending phase and log linear method during the descending phase was used for estimating AUC. Cmax and AUC were normalized for the dose of 13C6-LT4 administered.
Covariate testing
The PK parameters CL/F, V/F, Tmax, dose-normalized Cmax, AUC, and t1/2 were compared in younger participants (age ≤60 years) versus older participants (age >60 years) using Wilcoxon signed-rank test with a significance level of α = 0.05. Additionally, these PK parameters were regressed against age (as a continuous covariate), sex (male or female), weight (as a continuous covariate), and body mass index (BMI; as a continuous covariate) using a stepwise linear regression approach. Briefly, this approach involved testing all covariates on specific PK parameters using univariate linear regression. The most significant covariate (i.e., lowest p-value of <0.05) was then kept in the linear model, and the remaining covariates were entered linearly one at a time. The most significant added covariate was then kept, and the process was repeated until no other significant covariate was identified. In all analyses, the significance level was identified as a p-value of <0.05.
Results
Eighty-one individuals inquired about the study. Thirty-two individuals were either not interested in participation or were ineligible. A further eight individuals did not complete their visit to receive the dose of stable isotope labeled LT4. Thus, PK data were obtained from 31 participants aged ≤60 years (25 females) and 10 participants aged >60 years (8 females) who completed the study between May 2011 and November 2013. The median (range) of age in each group was 43 years (22–59 years) and 69.5 years (61–78 years), respectively. A summary of the characteristics of all study participants is listed in Table 1.
Table 1.
Summary of Study Participants' Demographics
| Continuous characteristics | |
| Age (years) | 50 (22–78) |
| Weight (kg) | 65.9 (50–150) |
| BMI (kg/m2) | 24.8 (18.9–45.2) |
| 13C6-LT4 dose (μg) | 100 (70–300) |
| Categorical characteristics | |
| Male sex | 8 (19.5) |
| Female sex | 33 (80.5) |
| Age ≤60 years | 31 (75.6) |
| Age >60 years | 10 (24.4) |
Data shown are median (range) for continuous characteristics and n (%) for categorical characteristics.
BMI, body mass index.
The median 13C-LT4 dose administered was 100 μg (range 70–300 μg). The 13C-LT4 concentrations over time for the first 72 hours of the study are shown in Figure 1. A summary of the PK parameters of 13C6-LT4 after oral administration for the entire group are shown in Table 2. The PK parameters broken down into two groups of those aged ≤60 years and >60 years are also shown in Table 2. The median (range) of CL/F in the ≤60 years and >60 years groups were: 0.712 L/h (0.244–2.91 L/h) and 0.796 L/h (0.301–1.74 L/h), respectively. This represents a 12% increase in CL/F in the older age group. The median (range) of V/F in the ≤60 years and >60 years groups were: 174.7 L (34–504.2 L) and 157.4 L (100.7–587.5 L), respectively, with the decrease seen in V/F in the older age group being 10%. The median (range) of Tmax in the ≤60 years and >60 years groups were: 4 h (1.5–24 h) and 5 h (2.5–8 h), respectively. Thus, there was a 25% increase in Tmax in the older age group. The median (range) of dose-normalized Cmax in ≤60 years and >60 years groups were: 7.5 ng/L (2–20 ng/L) and 7.1 ng/L (2.3–13 ng/L), respectively. The resultant decrease in the older age group was therefore 5%. The median (range) of the dose-normalized AUC0–120 were 0.931ng/h.mL/μg (0.288–2.84 ng/h.mL/μg) and 1.01ng/h.mL/μg (0.339–1.99 ng/h.mL/μg) for the ≤60 years and >60 years groups, respectively (8% increase in the older group), while that for dose-normalized AUC0–∞ were 1.41ng/h.mL/μg (0.344–4.09 ng/h.mL/μg) and 1.36ng/h.mL/μg (0.576–3.33 ng/h.mL/μg) for the ≤60 years and >60 years groups (4% decrease in the older group). The median (range) of the t1/2 for the adult and older groups were 166 h (51.4–265.7 h) and 206.4 h (109.4–310.8 h), respectively. This represents a 24% increase in the older age group. There were no significant differences in CL/F, V/F, Tmax, dose-normalized Cmax, dose-normalized AUC, and t1/2 between the ≤60 years and >60 years groups (p > 0.05).
FIG. 1.
13C-T4 concentration over time after administration of 13C-LT4 dose.
Table 2.
Summary of Pharmacokinetic Parameters of 13C6-LT4 After Oral Administration
| Median (range) | |||
|---|---|---|---|
| Parameter | All ages | Age ≤60 years (n = 31) | Age >60 years (n = 10) |
| CL/F (L/h) | 0.712 (0.244–2.91) | 0.712 (0.244–2.91) | 0.796 (0.301–1.74) |
| V/F (L) | 164.9 (34.02–587.52) | 174.7 (34–504.2) | 157.4 (100.7–587.5) |
| Tmax (h) | 4 (1.5–24) | 4 (1.5–24) | 5 (2.5–8) |
| Dose-normalized Cmax (ng/L μg) | 7.5 (2–20) | 7.5 (2–20) | 7.1 (2.3–13) |
| Dose-normalized AUC0–120 (ng/h.mL/μg) | 0.931 (0.288–2.84) | 0.931(0.288–2.84) | 1.01 (0.339–1.99) |
| Dose-normalized AUC0–∞ (ng/h.mL/μg) | 1.41 (0.344–4.09) | 1.41(0.344–4.09) | 1.36 (0.576–3.33) |
| t1/2 (h) of the terminal distribution phase | 172.2 (51.36–310.8) | 166 (54.1–265.7) | 206.4 (109.4–310.8) |
CL/F, oral clearance rate; V/F, volume of distribution; Tmax, time to peak concentration; Cmax, peak concentration; AUC1–120, area under the concentration–time curve from time 0 to 120 hours; AUC0–∞, area under the concentration–time curve from time 0 to infinity; t1/2, the half-life of the terminal disposition phase.
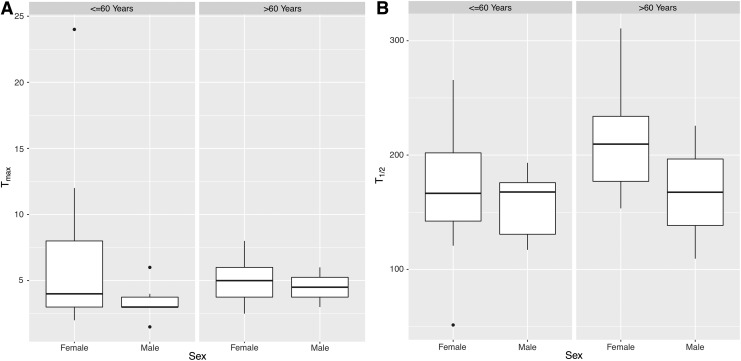
As previously described, stepwise linear regression covariate analyses were used to identify significant covariates affecting PK parameters. Tmax and t1/2 were unaffected by age, weight, and sex. Figure 2 shows the distribution of these PK parameters in the two age groups stratified by sex in box plot format. The box plots indicate the median, the first and third quartiles, and the interquartile range. The univariate linear regression analyses of PK parameters revealed both weight and sex but not age to be significant covariates affecting CL/F, V/F, dose-normalized Cmax, and dose-normalized AUC0–∞. Figure 3 shows the distribution of these four PK parameters in the two age groups stratified by sex.
FIG. 2.
(A) Box plot of time to peak concentration (Tmax) divided by age ≤60 years and age >60 years, and further divided by sex. (B) Box plot of the half-life of the terminal disposition phase (t1/2) divided by age ≤60 years and age >60 years, and further divided by sex.
FIG. 3.
(A) Box plot of the median oral clearance rate (CL/F) divided by age ≤60 years and age >60 years, and further divided by sex. (B) Box plot of the apparent volume of distribution (V/F) divided by age ≤60 years and age >60 years, and further divided by sex. (C) Box plot of dose-normalized peak concentration (Cmax) divided by age ≤60 years and age >60 years, and further divided by sex. (D) Box plot of dose-normalized area under the concentration–time curve from time 0 to infinity (AUC0–∞) divided by age ≤60 years and age >60 years, and further divided by sex.
However, the results of stepwise linear regression covariate analyses identified only weight as a significant covariate affecting CL/F, V/F, dose-normalized Cmax, and dose-normalized AUC0–∞. Thus, after adjusting for weight, sex was no longer a significant factor. Table 3 summarizes the results of the stepwise covariate linear regression analyses. For every kilogram increase in weight, there was a 2.54 L increase in V/F, a 0.0162 L/h increase in CL/F, and 0.0974 ng/L decrease in dose-normalized Cmax. Dose-normalized AUC0–120 and dose-normalized AUC0–∞ also significantly decreased with weight. A significance level of 0.05 without accounting for multiple statistical testing was used, as the sample size was small. Nonetheless, weight was a highly significant covariate affecting CL/F, even after the most stringent Bonferroni correction.
Table 3.
Summary of Covariate Analyses Showing Effect of 1 kg Increase in Weight on PK Parameters
| Parameter | Estimate ± SE | p-Value |
|---|---|---|
| V/F (L) | 2.54 ± 1.02 | 0.0171* |
| CL/F (L/h) | 0.016 ± 0.003 | 1.86e-05*** |
| Dose-normalized Cmax (ng/L) | –0.0974 ± 0.0351 | 0.00842** |
| Dose-normalized AUC0–120 (ng/h.mL/μg) | –0.012795 ± 0.003976 | 0.0026** |
| Dose-normalized AUC0–∞ (ng/h.mL/μg) | –0.019729 ± 0.006622 | 0.00495** |
, **, and *** indicate degrees of significance.
SE, standard error.
Discussion
Age-related physiological and PK changes, as well as the presence of comorbidity and polypharmacy, often complicate drug therapy in elderly patients. As LT4 has a narrow therapeutic index, precise dosing is critical. Accurate determination of LT4 PKs in different age groups provides an underpinning for optimizing dosing in older populations, as there have been suggestions of altered PKs in older patients due to slower clearance with aging.
To the authors' knowledge, this is the first study that addresses the issue of adequate LT4 replacement therapy across the age spectrum utilizing a unique 13C6-LT4 tracer method to characterize the PK of 13C6-LT4 in different age groups following a single dose. A specific and accurate tandem mass spectrometry assay was used that permits differentiation between 13C6-LT4 and LT4 analytes. Given that LT4 and endogenous T4 are pharmacologically identical, the ability to differentiate between each compound allows the PK parameters of LT4 to be estimated accurately without interference of endogenous T4. Previous studies from Blakesley et al. have shown that endogenous concentrations contribute significantly to the T4 AUC, even after administration of a pharmacologic dose of 600 μg. Inability to correct for this endogenous concentration can lead to incorrect conclusions regarding bioequivalence. For example, without correction for endogenous levels, two products that are as different as 33% with respect to their dosage strength may be found to be bioequivalent (39). A formula to determine baseline correction has also been generated from simulation studies and verified using data from Blakesley et al. (40). Patients in this study were also fasted for eight hours, such that absorption was likely to be optimized. This was an advantage of the study, but could also imply that the results may be less generalizable to non-fasting patients.
The primary pathway of thyroid hormone metabolism is through sequential de-iodination. In addition to de-iodination, thyroid hormones are metabolized through conjugation, including glucuronidation, and then excreted directly into the bile and the gut where they undergo enterohepatic recirculation (41,42). The excretion in feces is substantial, with very small amounts of excretion by the kidneys in normal circumstances. These elimination pathways are physiologically reduced in normal aging (43–46). Altered metabolism of T4 with advancing age could also potentially be secondary to altered free fractions of thyroid hormone associated with alterations in thyroxine-binding globulin. However, although early studies suggested a reduction in thyroxine-binding globulin concentrations with age, especially in older females aged approximately >60 years (47,48), newer studies have not supported age-related changes in thyroxine-binding globulin (49,50). Based on the prior data regarding decreased glucuronidation (45), decreased de-iodination (43), and potentially decreases in other conjugation reactions with age (44,46), a statistically and clinically significant difference in oral clearance with advancing age was expected in this study.
The data, however, suggest similar T4 clearance for the ≤60 years and >60 years age groups. It should be noted that the apparent oral clearance (CL/F) is a function of both total body clearance (intravenous clearance) and bioavailability. Therefore, for the drug oral clearance to remain the same, despite reduction of total body clearance, the bioavailability needs to be decreased to the same extent. LT4 absorption is reduced somewhat with age (51), an effect that could be speculated to be due to decreased absorption of LT4 with age as a result of lower gastric acidity (52). In previous studies, it has been shown that impaired gastric acid secretion in patients with hypothyroidism results in increased LT4 dose requirements in order to normalize TSH adequately (53,54). It is therefore postulated that any decreased metabolism/excretion of LT4 with age is compensated for by decreased LT4 absorption, such that oral clearance remains unchanged.
The estimated oral clearance at 0.712 L/h was considerably faster (>10-fold) than the range reported in previous studies (55,56). In these studies, clearance was 1.3 L/day (55) and 0.84 L/day (56), respectively. These studies determined the PKs of T4 using radioactive 131I or 125I-labeled LT4 administered intravenously, rather than using orally administered 13C-LT4. However, the difference in estimated clearance cannot be attributed solely to the bioavailability, as LT4 has a reported range of bioavailability of 70–80% (57–59). However, there were significant differences in methodology, other than the route of LT4 administration, the isotope, and the T4 assays that were available at the time. In one study, patients were blocked with cold iodine prior to the study, and study participants included euthyroid volunteers, untreated hypothyroid patients, and untreated hyperthyroid patients (55). In the other study, euthyroid volunteers were studied before and after administration of 300 μg of oral LT4 daily for 7–9 days, and the dose of labeled LT4 was 4 μg (56). A combination of these differences could account for a difference in oral clearance, although admittedly the magnitude of difference seen was unexpected and cannot be fully explained.
13C6-LT4 was slowly absorbed, with a median Tmax of 4 h. The median Tmax was 1 h longer in the >60 years group, but was not statistically significantly different from the ≤60 years group. Slow absorption of 13C6-LT4 is consistent with a transporter-mediated process rather than diffusion. Several thyroid hormone transporters have been identified in humans, and they are known to have a tissue-specific distribution and are essential for transporting thyroid hormone into specific cells. These include the organic anion transporting polypeptides (OATP) and the monocarboxylate transporter (MCT) family, among others. Human organic anion transporters may not be involved in intestinal transport of thyroid hormones, as, for example, OATP-E is only slightly expressed in intestinal cells (60), and although OATP2B1 is found in intestinal cells, it does not appear to transport thyroid hormone (61). However, MCT10 has been localized throughout the intestine and has been speculated to be possibly involved in the absorption of LT4 (62).
The major limitation of this study is the small sample size, particularly with respect to the older age group and the inclusion of men. Given the small and variable effect size, a larger group would be much better able to detect differences. Thus, the conclusions of this study can only be tentative and need to be fully confirmed in a future larger, better-powered study. Another limitation of this study might be that only the total T4 concentration was measured, and fT4 was not measured. However, T4 was measured using tandem mass spectrometry, which is known to be an accurate and reliable method for measuring thyroid hormones. Furthermore, tandem mass spectrometry measurement of total T4 is more accurate than fT4 measured by immunoassay. In addition, the mass spectrometry method allowed the 13C6-LT4 to be detected, thus allowing single-dose PK studies uncontaminated by any baseline or previously ingested LT4.
The stepwise covariate analysis revealed that weight was the only significant covariate affecting CL/F, V/F, and dose-normalized Cmax. This finding supports LT4 dosing adjustment based on body weight and is in agreement with the current recommendations for dosing LT4 (1). In summary, stable isotope labeled LT4 was used to conduct PK studies in both younger and older men and women. The current work supports LT4 dosing adjustment based on weight, with no indication for additional adjusting based on age or sex. Future work could confirm these findings in a larger, better-powered study, and could also focus on evaluation of fT4 PKs using labeled 13C6-LT4 isotope in treated hypothyroid individuals across the age span.
Acknowledgments
This study was supported by the National Institutes of Aging (R01AG033867) and the National Center for Advancing Translational Sciences (UL1TR001409).
Author Disclosure Statement
I.Y. and M.A. are affiliated with the United States Food and Drug Administration, Silver Spring, Maryland. However, contributions to the article were made in a private capacity. No official support or endorsement by the Food and Drug Administration is intended or should be inferred. J.J., K.D.B, and O.P.S. have no relevant disclosures.
References
- 1.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM. 2014. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 24:1670–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaker L, Bianco AC, Jonklaas J, Peeters RP. 2017. Hypothyroidism. Lancet 390:1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 4.Flynn RW, MacDonald TM, Morris AD, Jung RT, Leese GP. 2004. The thyroid epidemiology, audit, and research study: thyroid dysfunction in the general population. J Clin Endocrinol Metab 89:3879–3884 [DOI] [PubMed] [Google Scholar]
- 5.Somwaru LL, Arnold AM, Cappola AR. 2011. Predictors of thyroid hormone initiation in older adults: results from the cardiovascular health study. J Gerontol A Biol Sci Med Sci 66:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 7.Mammen JS, McGready J, Oxman R, Chia CW, Ladenson PW, Simonsick EM. 2015. Thyroid hormone therapy and risk of thyrotoxicosis in community-resident older adults: findings from the Baltimore Longitudinal Study of Aging. Thyroid 25:979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parle JV, Franklyn JA, Cross KW, Jones SR, Sheppard MC. 1993. Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract 43:107–109 [PMC free article] [PubMed] [Google Scholar]
- 9.Ross DS, Daniels GH, Gouveia D. 1990. The use and limitations of a chemiluminescent thyrotropin assay as a single thyroid function test in an out-patient endocrine clinic. J Clin Endocrinol Metab 71:764–769 [DOI] [PubMed] [Google Scholar]
- 10.Martins de Almeida JF, Goncalves Tsumura W, Vaisman M, Montalli Assumpcao LV, Ward LS. 2012. Current recommendations for levothyroxine treatment of differentiated thyroid cancer patients are not properly implemented in clinical practice. J Endocrinol Invest 35:901–904 [DOI] [PubMed] [Google Scholar]
- 11.Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, Hamilton W, Okosieme O, Panicker V, Thomas SL, Dayan C. 2014. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med 174:32–39 [DOI] [PubMed] [Google Scholar]
- 12.Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. 2009. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab 94:1342–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laurberg P, Andersen S, Bulow Pedersen I, Carle A. 2005. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging 22:23–38 [DOI] [PubMed] [Google Scholar]
- 14.Young RE, Jones SJ, Bewsher PD, Hedley AJ. 1984. Age and the daily dose of thyroxine replacement therapy for hypothyroidism. Age Ageing 13:293–303 [DOI] [PubMed] [Google Scholar]
- 15.Delemer B, Aubert JP, Nys P, Landron F, Bouee S. 2012. An observational study of the initial management of hypothyroidism in France: the ORCHIDEE study. Eur J Endocrinol 167:817–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biondi B, Cooper DS. 2008. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- 17.Jones CM, Boelaert K. 2015. The endocrinology of ageing: a mini-review. Gerontology 61:291–300 [DOI] [PubMed] [Google Scholar]
- 18.Stall GM, Harris S, Sokoll LJ, Dawson-Hughes B. 1990. Accelerated bone loss in hypothyroid patients overtreated with L-thyroxine. Ann Intern Med 113:265–269 [DOI] [PubMed] [Google Scholar]
- 19.Bauer DC, Ettinger B, Nevitt MC, Stone KL; Study of Osteoporotic Fractures Research Group 2001. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med 134:561–568 [DOI] [PubMed] [Google Scholar]
- 20.Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY. 1996. Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab 81:4278–4289 [DOI] [PubMed] [Google Scholar]
- 21.Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. 2010. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 95:186–193 [DOI] [PubMed] [Google Scholar]
- 22.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D'Agostino RB. 1994. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med 331:1249–1252 [DOI] [PubMed] [Google Scholar]
- 23.Akirov A, Gimbel H, Grossman A, Shochat T, Shimon I. 2017. Elevated TSH in adults treated for hypothyroidism is associated with increased mortality. Eur J Endocrinol 176:57–66 [DOI] [PubMed] [Google Scholar]
- 24.Santini F, Pinchera A, Marsili A, Ceccarini G, Castagna MG, Valeriano R, Giannetti M, Taddei D, Centoni R, Scartabelli G, Rago T, Mammoli C, Elisei R, Vitti P. 2005. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab 90:124–127 [DOI] [PubMed] [Google Scholar]
- 25.Mistry D, Atkin S, Atkinson H, Gunasekaran S, Sylvester D, Rigby AS, England RJ. 2011. Predicting thyroxine requirements following total thyroidectomy. Clin Endocrinol (Oxf) 74:384–387 [DOI] [PubMed] [Google Scholar]
- 26.Del Duca SC, Santaguida MG, Brusca N, Gatto I, Cellini M, Gargano L, Verga Falzacappa C, Frattaroli FM, Virili C, Centanni M. 2015. Individually-tailored thyroxine requirement in the same patients before and after thyroidectomy: a longitudinal study. Eur J Endocrinol 173:351–357 [DOI] [PubMed] [Google Scholar]
- 27.Sukumar R, Agarwal A, Gupta S, Mishra A, Agarwal G, Verma AK, Mishra SK. 2010. Prediction of LT4 replacement dose to achieve euthyroidism in subjects undergoing total thyroidectomy for benign thyroid disorders. World J Surg 34:527–531 [DOI] [PubMed] [Google Scholar]
- 28.Olubowale O, Chadwick DR. 2006. Optimization of thyroxine replacement therapy after total or near-total thyroidectomy for benign thyroid disease. Br J Surg 93:57–60 [DOI] [PubMed] [Google Scholar]
- 29.Cunningham JJ, Barzel US. 1984. Lean body mass is a predictor of the daily requirement for thyroid hormone in older men and women. J Am Geriatr Soc 32:204–207 [DOI] [PubMed] [Google Scholar]
- 30.Devdhar M, Drooger R, Pehlivanova M, Singh G, Jonklaas J. 2011. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid 21:821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonklaas J. 2010. Sex and age differences in levothyroxine dosage requirement. Endocr Pract 16:71–79 [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum RL, Barzel US. 1982. Levothyroxine replacement dose for primary hypothyroidism decreases with age. Ann Intern Med 96:53–55 [DOI] [PubMed] [Google Scholar]
- 33.Sawin CT, Herman T, Molitch ME, London MH, Kramer SM. 1983. Aging and the thyroid. Decreased requirement for thyroid hormone in older hypothyroid patients. Am J Med 75:206–209 [DOI] [PubMed] [Google Scholar]
- 34.Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla S, Wittmann G, Lechan RM, Gereben B, Bianco AC. 2015. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest 125:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeap BB, Alfonso H, Hankey GJ, Flicker L, Golledge J, Norman PE, Chubb SA. 2013. Higher free thyroxine levels are associated with all-cause mortality in euthyroid older men: the Health In Men Study. Eur J Endocrinol 169:401–408 [DOI] [PubMed] [Google Scholar]
- 36.Yeap BB, Manning L, Chubb SA, Hankey GJ, Golledge J, Almeida OP, Flicker L. 2017. Reference ranges for thyroid-stimulating hormone and free thyroxine in older men: results from the Health In Men Study. J Gerontol A Biol Sci Med Sci 72:444–449 [DOI] [PubMed] [Google Scholar]
- 37.Soldin OP, Mendu DM, Soldin SJ. Development of a method for the simultaneous measurement of stable isotope C13- and C12-thyroxine in human serum or plasma. Thyroid 18:S84–S85 [Google Scholar]
- 38.Soldin OP, Soldin SJ, Vinks AA, Younis I, Landy HJ. 2010. Longitudinal comparison of thyroxine pharmacokinetics between pregnant and nonpregnant women: a stable isotope study. Ther Drug Monit 32:767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blakesley V, Awni W, Locke C, Ludden T, Granneman GR, Braverman LE. 2004. Are bioequivalence studies of levothyroxine sodium formulations in euthyroid volunteers reliable? Thyroid 14:191–200 [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg M, Samuels M, DiStefano JJ., 3rd 2006. L-T4 bioequivalence and hormone replacement studies via feedback control simulations. Thyroid 16:1279–1292 [DOI] [PubMed] [Google Scholar]
- 41.Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ. 2005. Alternate pathways of thyroid hormone metabolism. Thyroid 15:943–958 [DOI] [PubMed] [Google Scholar]
- 42.Visser TJ. 1996. Pathways of thyroid hormone metabolism. Acta Med Austriaca 23:10–16 [PubMed] [Google Scholar]
- 43.Strich D, Karavani G, Edri S, Gillis D. 2016. TSH enhancement of fT4 to fT3 conversion is age dependent. Eur J Endocrinol 175:49–54 [DOI] [PubMed] [Google Scholar]
- 44.Robbins J. 1981. Factors altering thyroid hormone metabolism. Environ Health Perspect 38:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro AB, Vargens DD, Barros Filho Mde C, Bulzico DA, Kowalski LP, Meirelles RM, Paula DP, Neves RR, Pessoa CN, Struchine CJ, Suarez-Kurtz G. 2014. Effect of UGT1A1, UGT1A3, DIO1 and DIO2 polymorphisms on L-thyroxine doses required for TSH suppression in patients with differentiated thyroid cancer. Br J Clin Pharmacol 78:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregerman RI, Gaffney GW, Shock NW, Crowder SE. 1962. Thyroxine turnover in euthyroid man with special reference to changes with age. J Clin Invest 41:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braverman LW, Dawber NA, Ingbar SH. 1966. Observations concerning the binding of thyroid hormones in sera of normal subjects of varying ages. J Clin Invest 45:1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipson A, Nickoloff EL, Hsu TH, Kasecamp WR, Drew HM, Shakir R, Wagner HN., Jr 1979. A study of age-dependent changes in thyroid function tests in adults. J Nucl Med 20:1124–1130 [PubMed] [Google Scholar]
- 49.Kojima N, Sakata S, Nakamura S, Kamikubo K, Okuyama M, Miura K. 1983. Age- and sex-related differences of serum thyroxine binding globulin (TBG) in healthy subjects. Acta Endocrinol (Copenh) 104:303–306 [DOI] [PubMed] [Google Scholar]
- 50.Masuda Y, Masuoka T, Okawa H. 1982. [Normal range of thyroxine binding globulin (TBG)—relationship to aging chronological change in TBG] (author's translation). Radioisotopes 31:152–154 [PubMed] [Google Scholar]
- 51.Hays MT, Nielsen KR. 1994. Human thyroxine absorption: age effects and methodological analyses. Thyroid 4:55–64 [DOI] [PubMed] [Google Scholar]
- 52.Liwanpo L, Hershman JM. 2009. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab 23:781–792 [DOI] [PubMed] [Google Scholar]
- 53.Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, Annibale B. 2006. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med 354:1787–1795 [DOI] [PubMed] [Google Scholar]
- 54.Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, Kim PJ. 2007. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract 13:345–349 [DOI] [PubMed] [Google Scholar]
- 55.Nicoloff JT, Low JC, Dussault JH, Fisher DA. 1972. Simultaneous measurement of thyroxine and triiodothyronine peripheral turnover kinetics in man. J Clin Invest 51:473–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braverman LE, Vagenakis A, Downs P, Foster AE, Sterling K, Ingbar SH. 1973. Effects of replacement doses of sodium L-thyroxine on the peripheral metabolism of thyroxine and triiodothyronine in man. J Clin Invest 52:1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenzel KW, Kirschsieper HE. 1977. Aspects of the absorption of oral L-thyroxine in normal man. Metabolism 26:1–8 [DOI] [PubMed] [Google Scholar]
- 58.Read DG, Hays MT, Hershman JM. 1970. Absorption of oral thyroxine in hypothyroid and normal man. J Clin Endocrinol Metab 30:798–799 [DOI] [PubMed] [Google Scholar]
- 59.Hasselstrom K, Siersbaek-Nielsen K, Lumholtz IB, Faber J, Kirkegaard C, Friis T. 1985. The bioavailability of thyroxine and 3,5,3′-triiodothyronine in normal subjects and in hyper- and hypothyroid patients. Acta Endocrinol (Copenh) 110:483–486 [DOI] [PubMed] [Google Scholar]
- 60.Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, Onogawa T, Suzuki T, Asano N, Tanemoto M, Seki M, Shiiba K, Suzuki M, Kondo Y, Nunoki K, Shimosegawa T, Iinuma K, Ito S, Matsuno S, Abe T. 2001. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology 142:2005–2012 [DOI] [PubMed] [Google Scholar]
- 61.Hagenbuch B. 2007. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Pract Res Clin Endocrinol Metab 21:209–221 [DOI] [PubMed] [Google Scholar]
- 62.Friesema EC, Jansen J, Jachtenberg JW, Visser WE, Kester MH, Visser TJ. 2008. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol 22:1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]