Abstract
Background: Thyroid nodules are less common in pediatric patients (i.e., those ≤18 years) than they are in adults. The Bethesda System for Reporting Thyroid Cytopathology allows for individual risk stratification, but a significant number of nodules are indeterminate. Incorporating gene mutation panels and gene expression classifiers may aid in preoperative diagnosis. The overall aim of this study was to assess the prevalence of oncogene alterations in a representative pediatric population and across a broad-spectrum of thyroid tumor diagnoses.
Methods: This was a retrospective cross-sectional evaluation of 115 archived samples, including: 47 benign (29 follicular adenoma, 11 diffuse hyperplasia, four thyroiditis, and three multinodular goiter), six follicular thyroid carcinomas (FTC), 24 follicular variant of papillary thyroid carcinomas (fvPTC), 27 classic variant of PTC (cPTC), eight diffuse sclerosing variant of PTC (dsvPTC), and three other PTC. Molecular testing was performed by multiplex qualitative polymerase chain reaction followed by bead array cytometry. Oncogene results were analyzed for association with age, sex, histology, lymph node metastasis, and intrathyroidal spread.
Results: A mutation in one of the 17 molecular markers evaluated was found in: 2/6 (33%) FTC, 8/24 (33%) fvPTC, 17/27 (63%) cPTC, and 4/8 (50%) dsvPTC. Mutations in RAS or PAX8/PPARG were exclusive to FTC and fvPTC. BRAF was the most common mutation in cPTC (12/17; 71%), and RET/PTC was the only mutation associated with dsvPTC. Overall, a mutation was found in 32/68 (47%) malignant specimens, with a single follicular adenoma positive for PAX8/PPARG. The relative distribution of gene alterations in pediatric lesions was similar to adults. The presence of a BRAF mutation in pediatric cPTC did not predict a more invasive phenotype.
Conclusions: Of 33 nodules with genetic alterations, 32 were malignant. Mutations in RAS were most frequently associated with FTC, RET/PTC rearrangements with dsvPTC, and invasive fvPTC, and BRAF with cPTC. These results suggest a clinical role for mutational analysis of pediatric nodules to guide the surgical approach.
Keywords: : pediatric thyroid cancer, oncogene testing
Introduction
The incidence of thyroid nodules and differentiated thyroid cancer (DTC) in pediatric patients has increased over the last several decades (1,2). While the reason for this increase is not fully elucidated, several factors play a role, including an increase in incidental thyroid nodules discovered during non-thyroid-related head and neck imaging, augmented radiological surveillance for children at risk for thyroid malignancy (i.e., cancer survivors post neck radiation), and other confounding factors such as autoimmune thyroid disease, iodine insufficiency, and environmental ionizing radiation. In adults, the increasing incidence of DTC is related to identified subclinical disease (3–5), and an increase in disease is associated with regional and distant metastases (6). Compared to adults, pediatric patients demonstrate a fivefold increased risk of malignancy when a thyroid nodule is identified (25% in children vs. 5% in adults) (7–9). In adolescent girls aged 15–19 years, DTC remains the second most common malignancy (2) after Hodgkin's lymphoma, highlighting the importance of developing accurate protocols for the evaluation and management of pediatric patients with thyroid nodules.
The evaluation of a thyroid nodule in a child or adolescent should be based on individual risk factors and ultrasound features rather than the method of nodule detection. Patients are recommended to undergo fine-needle aspiration (FNA) based on ultrasound features of the nodule, including nodule composition, echogenicity, shape, margin, presence or absence of echogenic foci, and cervical lymphadenopathy (10,11). The FNA result is subsequently stratified based on The Bethesda Classification for Reporting Thyroid Cytopathology and classified as: (I) non-diagnostic, (II) benign, (III) atypia of undetermined significance/follicular lesion of undetermined significance, (IV) follicular neoplasm/suspicious for follicular neoplasm, (V) suspicious for malignancy, and (VI) malignant (12). Even with proper slide preparation and interpretation by pathologists with expertise in thyroid cytology, up to 35% of patients have indeterminate cytology (Bethesda categories III and IV), with a corresponding 10–30% estimated risk of malignancy (13).
Female sex, adolescent age, previous exposure to ionizing radiation for treatment of primary non-thyroid malignancy, and predisposing genetic syndromes (e.g., PTEN hamartoma syndrome, familial adenomatous polyposis, DICER1 pleuropulmonary blastoma syndrome, and others), are associated with an increased risk of developing a thyroid nodule and/or DTC (14–16). Given presumed higher risk of malignancy compared to adults, pediatric patients with a thyroid nodule are more frequently referred for surgical resection (lobectomy or total thyroidectomy). Yet, this may not always be beneficial, as data suggest lower incidence of histologically confirmed thyroid cancer after surgery for pediatric patients compared to adults (26% vs. 43%, respectively) (9,17,18). In addition, a higher surgical complication rate (e.g., hypoparathyroidism or recurrent laryngeal nerve damage) has been reported in pediatric patients, secondary to less frequent referral to a high-volume thyroid surgeon, defined as someone who performs >25–30 thyroid surgeries per year (1,19,20). Less than 25% of pediatric thyroid surgeries are performed at centers where the surgeon annually operates on >25 pediatric thyroid cases (1,20,21).
To date, the best approach for managing pediatric patients with indeterminate cytology has not been adequately assessed. In The Bethesda System for Reporting Thyroid Cytopathology, it is recommended that patients with class III indeterminate nodules undergo repeat FNA, whereas patients with class IV indeterminate nodules usually require surgical excision (lobectomy) for definite diagnosis. Compared to adults, the risk of malignancy on histologic follow-up is higher in pediatric patients: 28% versus 5–15% for Bethesda III, and up to 58% versus 15–30% for Bethesda IV. Histologically, there are no differences in microscopic appearance or diagnostic criteria for DTC between adult and pediatric patients. In pediatrics, DTC is most frequently comprised of classic (cPTC), follicular-variant (fvPTC), diffuse sclerosing variant (dsvPTC), and solid papillary thyroid cancer (sPTC), as well as minimally invasive follicular thyroid cancer (FTC). On a cellular level, both adult and pediatric DTC are associated with constitutive activation of the PI3K/AKT and MAPK signaling pathways. Yet, the prevalence of associated oncogene mutations is different, with a higher incidence of RET/PTC1 and RET/PTC3 in pediatric PTC (7,22). BRAF mutations, the most common mutation in adult PTC (40–50%), were previously reported in only 5% of pediatric PTC (23,24). However, recent data suggest that up to 40% of pediatric PTC may harbor a BRAF mutation (25–29). Furthermore, due to low incidence, oncogene mutations in pediatric FTC have not been adequately examined.
Commercially available confirmatory panels are available to predict benignity or malignancy in nodules from adult patients with indeterminate thyroid cytology. These panels may be equally effective and clinically relevant for adjunct testing in the pediatric population but have not been extensively studied to date. The aims of this study were to identify the prevalence of mutations in oncogenes across a broad range of thyroid pathology in children and adolescents, and to determine if the presence of an oncogene mutation or gene fusion correlated with thyroid malignancy. In patients with DTC, the results were also analyzed for an association of metastatic disease, as defined by the American Joint Committee on Cancer (AJCC) TNM classification. Lastly, the relative distributions of gene mutations and fusion transcripts in this pediatric study were compared to data previously reported for primary thyroid lesions from adult patients evaluated with the same molecular test.
Materials and Methods
Study protocol
A total of 129 archived surgical specimens collected from 115 patients at the Children's Hospital of Philadelphia (CHOP) between 1989 and 2012 were evaluated. Cases with available clinical data and residual material were selected to represent the most common benign and malignant conditions encountered in the pediatric population. All slides were reviewed by expert pathologists (L.S. and V.L.) to ensure adequacy of sample and accuracy of diagnosis. Slides with <50% tumor content were marked for enrichment by microdissection. Classification of DTC was based on standard histopathological criteria defined by the World Health Organization. Tumor classification was designated according to the seventh edition of the TNM system of the AJCC (30). All specimens were de-identified and blinded, and 10 × 5 μm slides labeled with a unique specimen identifier were provided to the molecular testing laboratory. The study's principal investigator (A.J.B.) reviewed the available medical records and entered clinical and molecular data into the database. No protected health information or other patient identifiers were released, and no molecular result from this investigation was used for patient management. The research protocol was approved by CHOP's Institutional Review Board.
The cohort was subsequently compared to a cohort of 257 adult surgical specimens collected under a research protocol approved by the Institutional Review Board of the University of Michigan, as previously described (31). Surgical diagnosis was established by a single expert pathologist (Thomas J. Giordano), and specimens were processed under the same procedures as the present study and tested in the same molecular laboratory, using the same pre-analytical and analytical methods and reagents.
Molecular analyses
Nucleic acid extraction and molecular testing were performed in Asuragen's clinical laboratory (Austin, TX), as previously described (31). Briefly, the presence of 17 genetic alterations, 14 single nucleotide substitutions (BRAF, HRAS, KRAS, and NRAS genes), and three fusion transcripts (RET/PTC1, RET/PTC3, and PAX8/PPARG) was assessed using 80 ng of total nucleic acids and multiplex polymerase chain reaction (PCR) for gene mutations or multiplex reverse transcription PCR for fusion transcripts. Following amplicon hybridization onto liquid bead array, labeling with streptavidin–phycoerythrin conjugate and detection of median fluorescence intensity signals by flow cytometry, qualitative molecular results were generated relative to validated positive/negative cutoff values. Specimen adequacy was assessed by co-amplification and co-detection of an internal endogenous control sequence in every reaction. Positive results were confirmed by retesting the same nucleic acid samples.
Data analyses
Statistical analyses were performed by comparing binary molecular results (positive or negative) relative to binary clinicopathologic parameters. Odds ratios (OR) and their confidence intervals (CI) were calculated using the Clopper–Pearson exact method for proportions or the method described by Armitage and Berry (32,33). p-Values were calculated using Fisher's exact test (where appropriate) and Welch's t-test for continuous variables, respectively. All reported p-values were two-sided, with a significance level of p ≤ 0.05.
Results
Study design and population
Characteristics of the study population and distribution of genetic alterations are shown in Tables 1 and 2, respectively. The study population consisted of 115 unique subjects aged 2–18 years, including 92 (80%) females. The relative proportion of females in the study population increased as a function of age (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy); females represented 71% of patients ≤14 years and 88% of patients >14 years (p = 0.03). Mutational status was determined in a total of 129 surgically resected thyroid lesions blinded to the molecular laboratory: 115 primary thyroid lesions (one from each study subject), 10 adjacent normal thyroid tissues from patients with DTC (one FTC and nine PTC), and lymph nodes from four different patients with metastatic DTC. Among the 115 primary thyroid lesions, 68 had a malignant histopathologic diagnosis, and 33 had a single genetic alteration detected (Table 1). There was no statistical difference in age or sex between the benign and malignant groups or the molecular negative and positive groups.
Table 1.
Characteristics of Study Population
| Histopathology | Molecular Mutation | ||||
|---|---|---|---|---|---|
| Benign | Malignant | Negative | Positive | Overall | |
| Subject, n | 47 | 68 | 82 | 33 | 115 |
| Age range, years | 2–18 | 4–18 | 2–18 | 4–18 | 2–18 |
| Median age, years | 15 | 15 | 15 | 15 | 15 |
| Female, % | 87% | 75% | 82% | 76% | 80% |
Age and sex differences between histopathology or molecular groups were not statistically significant.
Table 2.
Distribution of Genetic Alterations in Primary Thyroid Lesions
| Histopathology | Lesions, n | Positive, % | BRAF, % | RAS, % | RET, % | PPARG, % |
|---|---|---|---|---|---|---|
| All benign | 47 | 1 (2%) | 1 (100%) | |||
| Follicular adenoma | 29 | 1 (3%) | 1 (100%) | |||
| Other benign | 18 | — | ||||
| All malignant | 68 | 32 (47%) | 12 (37.5%) | 6 (19%) | 12 (37.5%) | 2 (6%) |
| Follicular carcinoma | 6 | 2 (33%) | 2 (100%) | |||
| Papillary carcinoma | 62 | 30 (48%) | 12 (40%) | 4 (13%) | 12 (40%) | 2 (7%) |
| Classical variant | 27 | 17 (63%) | 12 (71%) | 5 (29%) | ||
| Follicular, encapsulated | 21 | 6 (29%) | 4 (67%) | 2 (33%) | ||
| Follicular, widely invasive | 3 | 2 (67%) | 2 (100%) | |||
| Diffuse sclerosing variant | 8 | 4 (50%) | 4 (100%) | |||
| Mix classic, follicular, solid | 2 | 1 (50%) | 1 (100%) | |||
| Oncocytic variant | 1 | — | ||||
| Total | 115 | 33 (29%) | 12 (36%) | 6 (18%) | 12 (36%) | 3 (9%) |
Distribution of molecular results
The 47 benign specimens consisted of 15 FA, 14 FA with hyperplastic/papillary changes (pFA), 11 diffuse hyperplasia (Graves' disease), three multinodular goiters, three chronic lymphocytic thyroiditis, and one infectious thyroiditis. A single PAX8/PPARG fusion transcript was detected in a pFA case, confirmed by retesting (Table 2). No other benign lesion had a mutation or fusion transcript detected.
The 68 malignant specimens consisted of six FTC and 62 PTC, with genetic alterations detected in 32 cases (Table 2). Among the six FTC, one out of five minimally invasive cases was positive for HRASQ61R and the single widely invasive case was positive for KRASG12V. Among the 62 PTC, 16 were positive for a point mutation, and 14 were positive for a fusion transcript. The distribution of genetic alterations varied across histopathological subtypes. BRAFV600E was detected exclusively in classical variants of PTC, and accounted for 40% (12/30) of the positive PTC cases. RAS mutations and PAX8/PPARG fusion transcripts were found only in fvPTC. Specifically, there were three NRASQ61R and two PAX8/PPARG in five encapsulated fvPTC and one NRASQ61R in a fvPTC with solid features. RET/PTC fusion transcripts were detected in 12/30 PTC (40%); 10 fusions involved the CCDC6 gene (PTC1), and two involved the NCOA4 gene (PTC3). RET/PTC was the only genetic alteration detected in the aggressive variants of PTC, present in 4/8 dsvPTC, 2/3 widely invasive fvPTC, and 1/2 mixed PTC (displaying classic papillary, follicular, and solid-growth patterns). In addition, no genetic alteration was detected in 10 normal thyroid tissues adjacent to 10 carcinomas positive by molecular testing (Supplementary Table S1). Among the four lymph node metastases (LNM) available for analysis, three were positive for the same alteration as the primary thyroid lesion (BRAFV600E).
Relationship between genetic alterations and clinical characteristics
As noted in Table 3, oncogenic gene alterations were detected in 47% of malignant and 2% of benign specimens (p < 0.001; OR = 41 [CI 5.3–3.14]). In malignant lesions, mutations and/or fusion transcripts were more frequent in PTC relative to FTC (48% vs. 33%) and in cPTC relative to fvPTC (63% vs. 33%; Table 2). However, these differences were not statistically significant. The presence of a genetic alteration did not correlate with age, sex, histologic tumor size, intrathyroidal spread, or extrathyroidal extension (ETE; p > 0.05 for all). In 30 patients with documented evidence of LNM, 63% of the primary carcinomas were positive by molecular testing, all for BRAFV600E or RET/PTC (p = 0.04; OR = 4.0 [CI 1.2–14]; Tables 3 and 4 and Supplementary Table S2).
Table 3.
Clinical Parameters Significantly Associated with Positive Molecular Results
| Alteration | Parameter | Positive, n (%) | p-Value | OR [CI] |
|---|---|---|---|---|
| Any | Malignant | 32 (47%) | <0.001 | 41 [5.3–314] |
| Benign | 1 (2%) | |||
| Any | LNM | 19 (63%) | 0.04 | 4.0 [1.2–14] |
| No LNM | 6 (30%) | |||
| BRAF | Classic PTC | 12 (44%) | <0.001 | 57 [3.2–103] |
| Other PTC | 0 (0%) | |||
| RET/PTC | dsvPTC/wifvPTC | 6 (55%) | <0.01 | 9.0 [2.1–39] |
| Other PTC | 6 (12%) | |||
| RET/PTC | LNM | 12 (40%) | <0.01 | 28 [1.5–501] |
| No LNM | 0 (0%) | |||
| RET/PTC | ITS | 9 (33%) | 0.02 | 5.3 [1.3–22] |
| No ITS | 3 (9%) |
OR, odds ratio; CI, confidence interval; LNM, lymph node metastasis; PTC, papillary thyroid carcinoma; dsvPTC, diffuse sclerosing variant of PTC; wifvPTC, widely invasive follicular variant of PTC; ITS, intrathyroidal spread.
Table 4.
Clinical Characteristics of 12 Papillary Thyroid Carcinomas Positive for RET/PTC
| Histopathology | Size, cm | TNM | Sex | Age, years | RET fusion gene |
|---|---|---|---|---|---|
| PTC, classical variant | 2.6 | pT3N1aM1 | Female | 9 | PTC1 (CCDC6) |
| PTC, classical variant | 0.9 | pT1aN1aM0 | Female | 12 | PTC3 (NCOA4) |
| PTC, classical variant | 2.8 | pT2N1aM0 | Female | 17 | PTC1 (CCDC6) |
| PTC, classical variant | 3.2 | pT2N1aM1 | Female | 17 | PTC1 (CCDC6) |
| PTC, classical variant | 1.9 | pT1bN1bM0 | Male | 18 | PTC1 (CCDC6) |
| PTC, diffuse sclerosing variant | Infa | pT?N1bMx | Female | 5 | PTC1 (CCDC6) |
| PTC, diffuse sclerosing variant | 2.2 | pT3N1bM0 | Female | 12 | PTC1 (CCDC6) |
| PTC, diffuse sclerosing variant | 5.6 | pT3N1bM1 | Female | 13 | PTC1 (CCDC6) |
| PTC, diffuse sclerosing variant | 1.1 | pT1bN1aM0 | Female | 16 | PTC1 (CCDC6) |
| PTC, follicular variant, widely invasive | Infa | pT4aN1bMx | Female | 11 | PTC1 (CCDC6) |
| PTC, follicular variant, widely invasive | 5 | pT3N1bM0 | Female | 13 | PTC1 (CCDC6) |
| PTC, mix classical, solid, follicular | Infa | pTxN1bM0 | Male | 4 | PTC3 (NCOA4) |
Infiltrative form of papillary thyroid carcinomas (PTC) limiting the ability and value of reporting the tumor size.
Further analysis by mutation type showed that BRAFV600E was strongly associated with cPTC (p < 0.001; OR = 57 [CI 3.2–103]; Table 3) but not with other clinicopathologic parameters. Among the 12 BRAF-positive cases (Supplementary Table S2), 75% had advanced disease (tumor >4 cm or cancer had spread to outside the thyroid and/or to the lymph nodes). Yet, the frequency of ETE or LNM was not statistically significant relative to BRAF-negative carcinomas. In contrast, RET/PTC was highly correlated with invasive disease, with 100% of the 12 positive cases displaying ETE or central and/or lateral LNM, and three patients with distant metastasis (Table 4). RET fusion transcripts were associated with aggressive variant of PTC (p < 0.01), intrathyroidal spread (p = 0.02), and LNM (p < 0.01), independent of age, sex, and tumor size (Table 3).
Three patients had a prior history of radiation treatment for a non-thyroid malignancy (ependymoma, neuroblastoma, and hematopoietic stem cell transplantation for juvenile myelomonocytic leukemia). Two of the three had fvPTC, with one of the two displaying a NRAS mutation, and one with cPTC with no identified mutation. The number of cases with individual KRAS (n = 1), HRAS (n = 1), NRAS (n = 4), or PPARG (n = 2) gene alterations was too low to assess statistical significance. No ETE or LNM was recorded for any of these eight patients.
Comparison with adult population
Molecular results were subsequently compared to data previously reported for 257 primary thyroid lesions from study subjects aged 14–85 years (72% female) evaluated with the same molecular test (31). The set consisted of representative benign lesions (n = 110), FTC (n = 38), fvPTC (n = 36), cPTC (n = 29), oncocytic (n = 2), and sclerosing variants of PTC (n = 1). All histopathologic subtypes are reported in Supplementary Table S3. Eight (3.1%) study subjects were ≤18 years of age. Comparative analyses performed using the entire adult cohort (257 specimens, median patient age 49 years) or after exclusion of the eight cases ≤18 years of age (249 specimens, median patient age 50 years) yielded the same statistical outcome (proportions and significance). As shown in Table 5, the performance of molecular testing in the adult and pediatric populations was very similar. In adults, gene alterations were detected in 23% of FA and 2% of other benign cases, corresponding to an overall specificity of 86%. Furthermore, the adult detection rates were 26–79% in FTC, fvPTC, or cPTC, with an overall sensitivity of 56%. The only statistically significant difference between adults and pediatrics was the higher rate of gene alterations in adult FA (23% vs. 3%; p = 0.03), resulting in a lower overall specificity (86% vs. 98%; p = 0.04).
Table 5.
Summary of Molecular Testing Results Comparing Adult (n = 257) to Pediatric (n = 115) Specimensa
| Proportion [CI] | |||
|---|---|---|---|
| Metric | Histopathology | Adult >19 years | Pediatric <18 years |
| Specificity | All benign | 86% [79–92%] | 98% [89–100%] |
| Follicular adenoma | 77% [65–87%] | 97% [82–100%] | |
| Other benign | 98% [89–100%] | 100% [89–100%] | |
| Sensitivity | All carcinomas | 56% [47–64%] | 47% [35–60%] |
| Follicular carcinoma | 26% [13–43%] | 33% [4–78%] | |
| PTC, classical variant | 79% [60–92%] | 63% [42–81%] | |
| PTC, follicular variant | 44% [28–62%] | 33% [16–55%] | |
| Other carcinomas | 75% [60–87%] | 45% [17–77%] | |
Based on the relative distribution of various histopathological categories reported in Giordano et al. for adults (31) and in the present study for pediatrics.
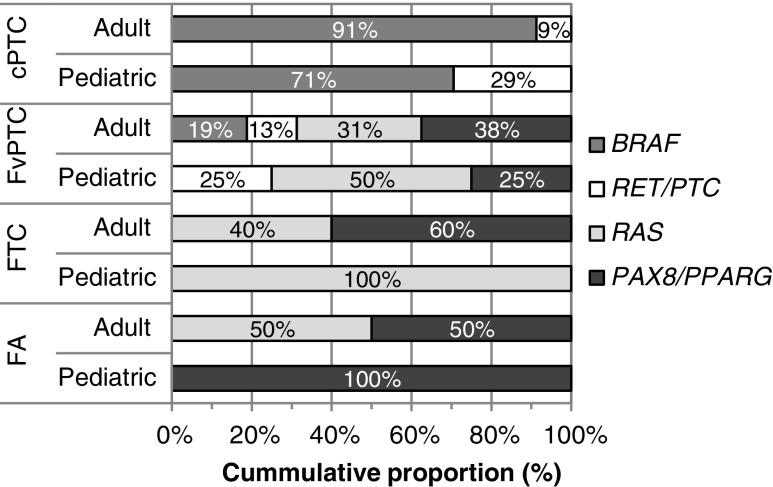
The relative distributions of gene mutations and fusion transcripts in adult and pediatric lesions were also very similar (Fig. 1; p > 0.05). In both populations, RET/PTC was detected only in PTC, while RAS mutations and PAX8/PPARG were found in fvPTC and follicular-pattern tumors. BRAFV600E was more frequent in adult cPTC (91% vs. 71%) and in adult fvPTC (19% vs. 0%). While not statistically significant, RAS mutations and PAX/PPARG fusion transcripts were present in both adult FA and FTC. A single FA was positive for PAX/PPARG, and two FTC were positive for RAS in pediatrics. Finally, the adult set also included one oncocytic variant of PTC positive for BRAF out of two cases (vs. a single negative case in pediatrics) and one dsvPTC positive for RET/PTC (vs. 4/8 positive for RET/PTC in the pediatric cohort; Supplementary Table S3).
FIG. 1.
Distribution of gene mutations and fusion transcripts in adult and pediatric thyroid lesions positive by molecular testing. The graph shows the relative proportion of each gene alteration in molecular-positive classical variant of papillary carcinoma (cPTC), follicular variant of papillary carcinomas (fvPTC), follicular thyroid carcinomas (FTC), and follicular adenomas (FA) based on Table 2 for pediatric cases and Supplementary Table S3 for adult cases. The differences between pediatrics and adults for BRAF, RAS, RET/PTC, or PAX8/PPARG proportions were not statistically significant (p > 0.05).
Discussion
In an effort to improve the management of thyroid nodules with indeterminate cytology, diagnostic molecular testing is being incorporated into clinical practice with increasing frequency. As gene expression classifiers and miRNA panels have not been validated in patients <21 years of age, oncogene analysis remains the only approach with tested clinical utility within the pediatric population (i.e., those ≤18 years). To date, the only study that included molecular analysis for both benign and malignant lesions was limited by the small number of samples tested (n = 66) and inclusion of subjects above the pediatric cutoff age (up to 21 years of age) (34). The present study is the largest pediatric study to evaluate mutations and translocations across the spectrum of common benign and malignant thyroid conditions. The study demonstrates that the presence of an oncogene mutation or fusion is highly correlated with thyroid malignancy and is uncommon in benign thyroid disease.
Single mutations or rearrangements were identified in 47% (32/68) of malignant samples. BRAFV600E was the most common genetic abnormality found in cPTC, while RET/PTC1 was most frequently associated with invasive forms of PTC, such as dsvPTC (4/8; 50%) and widely invasive fvPTC (2/3; 67%). In this selected sample set with confirmed histologic diagnosis, oncogene analysis demonstrated significant clinical utility with 98% specificity (46/47) and 97% positive predictive value (32/33 [CI 84–100%]). As the pretest probability of malignancy (cancer prevalence) impacts the estimation of a test's predictive value, ORs were also reported. ORs measure the strength of an association between exposure and outcome, independently from the pretest probability of the outcome; an OR of 41 for benign versus malignant should be interpreted as the odds of a correct molecular result (true positive or true negative) is 41 times higher than the odds of an incorrect result (false positive or false negative).
Only three of our patients with DTC had a previous history of radiation exposure for treatment of a non-thyroid malignancy. None of these tumors were found to harbor a gene fusion; one tumor had an NRAS point mutation, and no oncogene mutation was detected in the other two tumors. Thus, while radiation increases DNA breaks with a subsequent increased risk for formation of RET/PTC, NTRK, and BRAF gene fusions in radiation-induced PTC, gene fusions are also common in pediatric patients with sporadic PTC (28,35–39). In the present study, all of the cases with a RET/PTC fusion were sporadic, and all were associated with LNM, including 75% (12/16 cases) associated with lateral neck LNM (Table 4).
The incorporation of molecular testing to supplement malignancy evaluation in adult patients with indeterminate thyroid cytology is widely accepted and supported by professional society guidelines (40,41). Unfortunately, due to limited data, the incorporation of oncogene testing for pediatric patients with indeterminate thyroid cytology has received minimal support from published guidelines (42), and insurance companies often challenge approval on prior authorization requests. The present data, and others, support the clinical utility of performing oncogene testing in pediatric patients to identify thyroid malignancies, particularly given its low false-positive rate in benign tumors (25,28,29,43). Importantly, the present results demonstrate similar performance in detecting a thyroid malignancy for adult and pediatric thyroid samples prepared in a similar fashion and performed using the same oncogene profiling platform.
While the incidence and prevalence of thyroid nodules is lower in pediatric patients compared to adults, indeterminate cytology is present in up to 35% of pediatric cases. In addition, the pretest probability of malignancy is higher in pediatrics compared to adults (28% vs. 5–15% for Bethesda III and up to 58% vs. 15–30% for Bethesda IV) (12,13,34). Because of the increased risk of malignancy and unknown impact of long-term surveillance, the majority of pediatric patients with a thyroid nodule of indeterminate cytology are referred for surgical resection. For patients with unilateral thyroid nodules, lobectomy remains the recommended initial surgical approach, with completion thyroidectomy performed if invasive DTC is confirmed by pathology (42). Thus, in an effort to avoid missing thyroid malignancy, many children with benign nodules undergo unnecessary surgery, while children with thyroid malignancy are more likely to proceed to a second surgery if the diagnosis of malignancy is only established following lobectomy. It is predicted that with an increased post-test probability of malignancy, a positive oncogene analysis in a pediatric nodule displaying indeterminate cytology would have a high likelihood of accurate stratification for total thyroidectomy, reducing the need for completion thyroidectomy after diagnostic lobectomy in patients in whom removal of the whole gland seems indicated. As many nodules may not carry one of the evaluated oncogenic alterations, pediatric patients with a negative molecular result still benefit from close follow-up or repeat FNA, depending on the initial index of suspicion of malignancy prior to initial FNA.
This study has several limitations, including overall small sample size per DTC variant, prevalence selection bias and using an oncogene panel with a relatively restricted number of oncogenic markers. Furthermore, this study examined the distribution of gene mutations and rearrangements in surgical specimens from pediatric thyroid nodules. The samples were selected based on availability of tissue blocks across the full-range of thyroid diagnoses, and the data cannot be extrapolated to cytology specimens. Nonetheless, the data presented here represent the largest in pediatrics to date and a necessary first step. In an effort to address these limitations, the authors are currently pursuing a follow-up prospective study with an expanded number of samples using a comprehensive next-generation sequencing-based oncogene panel.
Future prospective studies are needed to confirm the utility of oncogene testing in pediatric thyroid nodules with indeterminate cytology. Detection of a mutation or fusion associated with an increased risk of malignancy would support proceeding to total thyroidectomy rather than lobectomy, at least in a subset of patients. This would be particularly true for BRAF mutation or RET/PTC fusion, given the increased risk of invasive disease. In addition, other studies have shown an association between invasive pediatric DTC and NTRK fusions (28,44) as well as the AGK/BRAF oncogene fusion (36). Based on available data, incorporating oncogene testing likely provides a useful framework to guide clinical practice (45).
Conclusions
Thyroid nodules from pediatric patients demonstrate a similar distribution of common thyroid oncogene mutations or fusions compared to adults. BRAF mutations and RET/PTC fusions are most common, and are highly correlated with thyroid malignancy. Only RET/PTC fusions correlated with lateral neck lymph node metastasis. Molecular testing may improve the surgical management of pediatric patients with indeterminate nodules in the future. Prospective studies with broader oncogene panels are needed to confirm the clinical utility of oncogene testing in order to guide management recommendations for surgery, adjuvant radioactive iodine, intensity of surveillance, and the individualized selection of systemic therapy for patients with refractory disease.
Supplementary Material
Acknowledgments
The authors wish to thank Rupali Shinde from Asuragen, Inc., for her excellent technical support. This study was supported by the National Cancer Institute (K07 CA166177; S.M.M.).
Author Disclosure Statement
S.B.H. and E.L. were employees of Asuragen, Inc., at the time of the study. None of the other authors have any conflicts of interest to declare.
References
- 1.Raval MV, Bentrem DJ, Stewart AK, Ko CY, Reynolds M. 2010. Utilization of total thyroidectomy for differentiated thyroid cancer in children. Ann Surg Oncol 17:2545–2553 [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Groves FD, McLaughlin CC, Jemal A, Martin J, Chen VW. 2005. Cancer incidence patterns among adolescents and young adults in the United States. Cancer Causes Control 16:309–320 [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 4.Morris LG, Myssiorek D. 2010. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 200:454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wartofsky L. 2011. Increasing world incidence of thyroid cancer: increased detection or higher radiation exposure? Hormones (Athens, Greece) 9:103–108 [DOI] [PubMed] [Google Scholar]
- 6.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. 2017. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 317:1338–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarzab B, Handkiewicz-Junak D. 2007. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens, Greece) 6:200–209 [PubMed] [Google Scholar]
- 8.Na SJ, Yoo Ie R, O JH, Lin C, Lin Q, Kim SH, Chung SK. 2012. Diagnostic accuracy of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in differentiated thyroid cancer patients with elevated thyroglobulin and negative (131)I whole body scan: evaluation by thyroglobulin level. Ann Nucl Med 26:26–34 [DOI] [PubMed] [Google Scholar]
- 9.Niedziela M. 2006. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer 13:427–453 [DOI] [PubMed] [Google Scholar]
- 10.American Institute of Ultrasound in Medicine; American College of Radiology; Society for Pediatric Radiology; Society of Radiologists in Ultrasound 2013. AIUM practice guideline for the performance of a thyroid and parathyroid ultrasound examination. J Ultrasound Med 32:1319–1329 [DOI] [PubMed] [Google Scholar]
- 11.Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, Cronan JJ, Desser TS, Frates MC, Hamper UM, Middleton WD, Reading CC, Scoutt LM, Stavros AT, Teefey SA. 2015. Thyroid ultrasound reporting lexicon: white paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J Am Coll Radiol 12:1272–1279 [DOI] [PubMed] [Google Scholar]
- 12.Cibas ES, Ali SZ. 2009. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19:1159–1165 [DOI] [PubMed] [Google Scholar]
- 13.Smith M, Pantanowitz L, Khalbuss WE, Benkovich VA, Monaco SE. 2013. Indeterminate pediatric thyroid fine needle aspirations: a study of 68 cases. Acta Cytol 57:341–348 [DOI] [PubMed] [Google Scholar]
- 14.Kovalchik SA, Ronckers CM, Veiga LH, Sigurdson AJ, Inskip PD, de Vathaire F, Sklar CA, Donaldson SS, Anderson H, Bhatti P, Hammond S, Leisenring WM, Mertens AC, Smith SA, Stovall M, Tucker MA, Weathers RE, Robison LL, Pfeiffer RM. 2013. Absolute risk prediction of second primary thyroid cancer among 5-year survivors of childhood cancer. J Clin Oncol 31:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nose V. 2011. Familial thyroid cancer: a review. Mod Pathol 24:S19–33 [DOI] [PubMed] [Google Scholar]
- 16.Rio Frio T, Bahubeshi A, Kanellopoulou C, Hamel N, Niedziela M, Sabbaghian N, Pouchet C, Gilbert L, O'Brien PK, Serfas K, Broderick P, Houlston RS, Lesueur F, Bonora E, Muljo S, Schimke RN, Bouron-Dal Soglio D, Arseneau J, Schultz KA, Priest JR, Nguyen VH, Harach HR, Livingston DM, Foulkes WD, Tischkowitz M. 2011. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli–Leydig cell tumors. JAMA 305:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oertel YC, Miyahara-Felipe L, Mendoza MG, Yu K. 2007. Value of repeated fine needle aspirations of the thyroid: an analysis of over ten thousand FNAs. Thyroid 17:1061–1066 [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Schnadig V, Logrono R, Wasserman PG. 2007. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 111:306–315 [DOI] [PubMed] [Google Scholar]
- 19.Adam MA, Thomas S, Youngwirth L, Hyslop T, Reed SD, Scheri RP, Roman SA, Sosa JA. 2017. Is there a minimum number of thyroidectomies a surgeon should perform to optimize patient outcomes? Ann Surg 265:402–407 [DOI] [PubMed] [Google Scholar]
- 20.Sosa JA, Tuggle CT, Wang TS, Thomas DC, Boudourakis L, Rivkees S, Roman SA. 2008. Clinical and economic outcomes of thyroid and parathyroid surgery in children. J Clin Endocrionol Metab 93:3058–3065 [DOI] [PubMed] [Google Scholar]
- 21.Raab SS, Silverman JF, Elsheikh TM, Thomas PA, Wakely PE. 1995. Pediatric thyroid nodules: disease demographics and clinical management as determined by fine needle aspiration biopsy. Pediatrics 95:46–49 [PubMed] [Google Scholar]
- 22.Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. 2000. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrionol Metab 85:1170–1175 [DOI] [PubMed] [Google Scholar]
- 23.Penko K, Livezey J, Fenton C, Patel A, Nicholson D, Flora M, Oakley K, Tuttle RM, Francis G. 2005. BRAF mutations are uncommon in papillary thyroid cancer of young patients. Thyroid 15:320–325 [DOI] [PubMed] [Google Scholar]
- 24.Xing M. 2005. BRAF mutation in thyroid cancer. Endocr Relat Cancer 12:245–262 [DOI] [PubMed] [Google Scholar]
- 25.Ballester LY, Sarabia SF, Sayeed H, Patel N, Baalwa J, Athanassaki I, Hernandez JA, Fang E, Quintanilla NM, Roy A, Lopez-Terrada DH. 2016. Integrating molecular testing in the diagnosis and management of children with thyroid lesions. Pediatr Dev Pathol 19:94–100 [DOI] [PubMed] [Google Scholar]
- 26.Givens DJ, Buchmann LO, Agarwal AM, Grimmer JF, Hunt JP. 2014. BRAF V600E does not predict aggressive features of pediatric papillary thyroid carcinoma. Laryngoscope 124:E389–393 [DOI] [PubMed] [Google Scholar]
- 27.Henke LE, Perkins SM, Pfeifer JD, Ma C, Chen Y, DeWees T, Grigsby PW. 2014. BRAF V600E mutational status in pediatric thyroid cancer. Pediatr Blood Cancer 61:1168–1172 [DOI] [PubMed] [Google Scholar]
- 28.Picarsic JL, Buryk MA, Ozolek J, Ranganathan S, Monaco SE, Simons JP, Witchel SF, Gurtunca N, Joyce J, Zhong S, Nikiforova MN, Nikiforov YE. 2016. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next-generation sequencing assay. Pediatr Dev Pathol 19:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikita ME, Jiang W, Cheng SM, Hantash FM, McPhaul MJ, Newbury RO, Phillips SA, Reitz RE, Waldman FM, Newfield RS. 2016. Mutational analysis in pediatric thyroid cancer and correlations with age, ethnicity, and clinical presentation. Thyroid 26:227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. (eds) 2010. AJCC Cancer Staging Manual. Seventh edition. Springer, New York, NY, pp 87–96 [Google Scholar]
- 31.Giordano TJ, Beaudenon-Huibregtse S, Shinde R, Langfield L, Vinco M, Laosinchai-Wolf W, Labourier E. 2014. Molecular testing for oncogenic gene mutations in thyroid lesions: a case-control validation study in 413 postsurgical specimens. Hum Pathol 45:1339–1347 [DOI] [PubMed] [Google Scholar]
- 32.Armitage P, Berry G, Matthew J. 2001. Statistical Methods in Medical Research. Fourth edition. Wiley-Blackwell, Malden, MA [Google Scholar]
- 33.Newcombe RG. 1998. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17:857–872 [DOI] [PubMed] [Google Scholar]
- 34.Monaco SE, Pantanowitz L, Khalbuss WE, Benkovich VA, Ozolek J, Nikiforova MN, Simons JP, Nikiforov YE. 2012. Cytomorphological and molecular genetic findings in pediatric thyroid fine-needle aspiration. Cancer Cytopathol 120:342–350 [DOI] [PubMed] [Google Scholar]
- 35.Cordioli MI, Moraes L, Bastos AU, Besson P, Alves MT, Delcelo R, Monte O, Longui C, Cury AN, Cerutti JM. 2017. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid 27:182–188 [DOI] [PubMed] [Google Scholar]
- 36.Cordioli MI, Moraes L, Carvalheira G, Sisdelli L, Alves MT, Delcelo R, Monte O, Longui CA, Cury AN, Cerutti JM. 2016. AGK–BRAF gene fusion is a recurrent event in sporadic pediatric thyroid carcinoma. Cancer Med 5:1535–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumagai A, Namba H, Saenko VA, Ashizawa K, Ohtsuru A, Ito M, Ishikawa N, Sugino K, Ito K, Jeremiah S, Thomas GA, Bogdanova TI, Tronko MD, Nagayasu T, Shibata Y, Yamashita S. 2004. Low frequency of BRAFT1796A mutations in childhood thyroid carcinomas. J Clin Endocrinol Metab 89:4280–4284 [DOI] [PubMed] [Google Scholar]
- 38.Lehman CE, Dillon LW, Nikiforov YE, Wang YH. 2017. DNA fragile site breakage as a measure of chemical exposure and predictor of individual susceptibility to form oncogenic rearrangements. Carcinogenesis 38:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, Heguy A, Viale A, Bogdanova T, Thomas GA, Mason CE, Fagin JA. 2013. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest 123:4935–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, Paschke R, Valcavi R, Vitti P; AACE/ACE/AME Task Force on Thyroid Nodules 2016. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr Pract 22:622–639 [DOI] [PubMed] [Google Scholar]
- 41.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID, Luster M, Parisi MT, Rachmiel M, Thompson GB, Yamashita S. 2015. Management guidelines for children with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer. Thyroid 25:716–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buryk MA, Monaco SE, Witchel SF, Mehta DK, Gurtunca N, Nikiforov YE, Simons JP. 2013. Preoperative cytology with molecular analysis to help guide surgery for pediatric thyroid nodules. Int J Pediatr Otorhinolaryngol 77:1697–1700 [DOI] [PubMed] [Google Scholar]
- 44.Prasad ML, Vyas M, Horne MJ, Virk RK, Morotti R, Liu Z, Tallini G, Nikiforova MN, Christison-Lagay ER, Udelsman R, Dinauer CA, Nikiforov YE. 2016. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer 122:1097–1107 [DOI] [PubMed] [Google Scholar]
- 45.Bauer AJ. 2017. Molecular genetics of thyroid cancer in children and adolescents. Endocrinol Metabo Clin North Am 46:389–403 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.